Abstract
Parkinson's disease is characterized by tremor, rigidity, bradykinesia, and postural abnormalities ascribed to the loss of nigrostriatal dopamine (DA). Symptoms similar to the human condition can be produced in the rat by DA-depleting 6-hydroxydopamine injections made into the nigrostriatal system. After a unilateral lesion, the rat symptoms include sensory and motor impairments and turning biases reflecting motor abnormalities to the contralateral-to-depletion side of the body. In addition, a number of studies on skilled reaching report impairments in the use of the ipsilateral limb. It is suggested that the ipsilateral deficit is secondary to the contralateral motor impairments however. Here we re-examine how rats with unilateral DA depletion use their ipsilateral limb for skilled reaching for food. We provide the first description of an impairment on the ipsilateral-to-depletion side of the body of the rat and the first demonstration of amelioration of the defect using behavioral therapy. Video analysis of rats reaching for single pellets of food with the ipsilateral limb revealed that, although limb advancement and food grasping were normal, paw supination and food release to the mouth were impaired. Consequently, the animals were unable to transport a grasped food pellet to the mouth. Behavioral therapy, consisting of training in a simpler reaching task, strikingly lessened the impairment and improved reaching movements to the point that the rats could transport the food to the mouth. The results are discussed in relation to possible causes of the ipsilateral impairment, its treatment, and to relevant research on human Parkinson patients, indicating that they display bilateral improvements after unilateral treatments.
Keywords: dopamine depletion, dopamine and skilled movement, 6-hydroxydopamine, nigrostriatal lesion, Parkinson analog rat, Parkinson's disease, Parkinson's disease rat model, skilled reaching, Parkinson's therapy, rehabilitation and Parkinson's disease
Introduction
Parkinson's disease is a neurological disorder that affects human movement, balance, and fine motor control, impairments that are related to the degeneration of dopaminergic (DA) neurons in the substantia nigra (Martin, 1967;Duvoisin, 1978). The human condition can be modeled by reducing striatal DA levels in nonhuman animals with the injection of the neurotoxin 6-hydroxydopamine (6-OHDA). The injections are made unilaterally and affect the sensorimotor behavior of the contralateral side of the body, still allowing the animals to eat and drink and care for themselves (Schultz, 1982; Robinson et al., 1990; Schwarting and Huston, 1996; Cenci et al., 2002). The DA-depleted rats display abnormalities in turning biases, impairments in locomotion and posture, and impairments in skilled limb movements that are analogous to human deficits, so they are useful in assessing therapeutic measures.
A method used to evaluate symptoms and treatments in the rat analog is skilled reaching, in which animals reach with a forepaw for food (for review, see Whishaw et al., 1986; Montoya et al., 1990; Whishaw et al., 1997; Nikkhah, 1993). Reaching consists of a number of movement subcomponents that include adjusting posture, aiming the limb, pronating the paw over the food, grasping, and supinating the paw during withdrawal to present the food to the mouth. After unilateral DA depletion, there is reduced success accompanied by abnormalities in the way that movements are performed, including changes in posture, shortened reaches, and loss of pronation and supination (Miklyaeva et al., 1994; Whishaw et al., 1997; Metz et al., 2001).
A puzzling finding is that, although severe symptoms are found contralateral to unilateral dopamine depletions, there are some reports of an ipsilateral impairment. A chronic impairment occurs when rats reach for single pellets (Miklyaeva et al., 1994), but the impairment is acute when they reach into a tray for food (Whishaw et al., 1986,1992). An ipsilateral impairment has been reported in some studies using the staircase test (Dunnett et al., 1988; Montoya et al., 1990, 1991; Olsson et al., 1995; Whishaw et al., 1997; Henderson et al., 1999; Döbrössy et al., 2000) but not in others (Abrous et al., 1993a,b; Nikkhah et al., 1993;Lee et al., 1996; Barnéoud et al., 2000; Glavan et al., 2001; Jeyasingham et al., 2001; Moore et al., 2001). In a study examining lesions given at different ages, an ipsilateral impairment is reported in rats given lesions at 3 d of age but not in rats given lesions at 7 and 21 d of age (Abrous et al., 1993b). An ipsilateral impairment is also reported after unilateral ibotenic or quinolinic acid lesions of the striatum (Montoya et al., 1990; Fricker et al., 1996; Jeyasingham et al., 2001) and brain infarction (Grabowski et al., 1993). Finally, there are also reports that therapies including grafts of embryonic tissue (Montoya et al., 1990) and subthalamic nucleus lesions (Henderson et al., 1999) improve the ipsilateral but not the contralateral impairment, whereas treatment with quinpirole aggravates the deficit (Olsson et al., 1995).
Although the ipsilateral impairment has been interpreted as being secondary to postural abnormalities on the side of the body contralateral to the lesion, there has been no previous direct investigation of the cause of the ipsilateral impairment. Given the number of reports of an ipsilateral impairment and the potential for its remediation, this was the purpose of the present study.
Materials and Methods
Subjects
Twenty female Long–Evans rats (University of Lethbridge vivarium) weighing 250–310 gm at the time of surgery were used. The rats were housed in groups of four to six animals in a 12 hr light/dark cycle, with lights on at 8 A.M. and with water available ad libitum. For the skilled-reaching task, the animals were food-deprived for 2 weeks before training or testing began. Each day, the rats received supplemental food to maintain their body weight at 95% of the initial body weight. All procedures were approved by the University of Lethbridge animal care committee.
Surgery
Thirty minutes before surgery, the rats received desmethylimipramine (25 mg/kg, i.p.; Sigma, St Louis, MO). The rats were then anesthetized with 60 mg/kg sodium pentobarbital. Twelve rats received 6-OHDA lesions (Ungerstedt, 1971), and eight received no additional procedures. The neurotoxic lesions of the nigrostriatal bundle were performed with injections of 6-OHDA hydrobromide (2 μl of 4 mg/ml in 0.9% saline with 0.02% ascorbic acid) (Whishaw et al., 1986; Miklyaeva et al., 1995) at the following coordinates: 4.0 mm posterior to bregma, 1.5 mm lateral to the midline, and 8.5 mm ventral to the skull surface, with the skull flat between the lambda and bregma, according to coordinates outlined by Paxinos and Watson (1998). Infusion took place over 5 min, with 5 min for diffusion.
Video recording
Video records were made with a Sony (Tokyo, Japan) Video 8 CCD VII portable camera with a shutter speed of 1/2000 sec. Illumination for high-shutter-speed filming was provided by a two-arm Nikon (Tokyo, Japan) MII cold light source. Frame-by-frame analysis at 30–60 frames per second was provided by a Sony Video 8 recorder or through a computer-based frame grabber (Whishaw and Pellis, 1990).
Skilled reaching
Two types of test boxes were used (Fig.1). Food tray boxes (10 × 18 × 10 cm high) were constructed of Plexiglas with a face consisting of 2 mm bars spaced 9 mm edge to edge. A 4-cm-wide and 5-cm-deep tray containing granules of food (20–40 mg of chick feed) was mounted in front of each box and extended for the length of the box. To obtain food, the rat had to reach through the bars, grasp the food, and retract it. The floor was made of grids so that if a rat dropped the food, it fell through the grids and was lost (Whishaw et al., 1986).
Fig. 1.
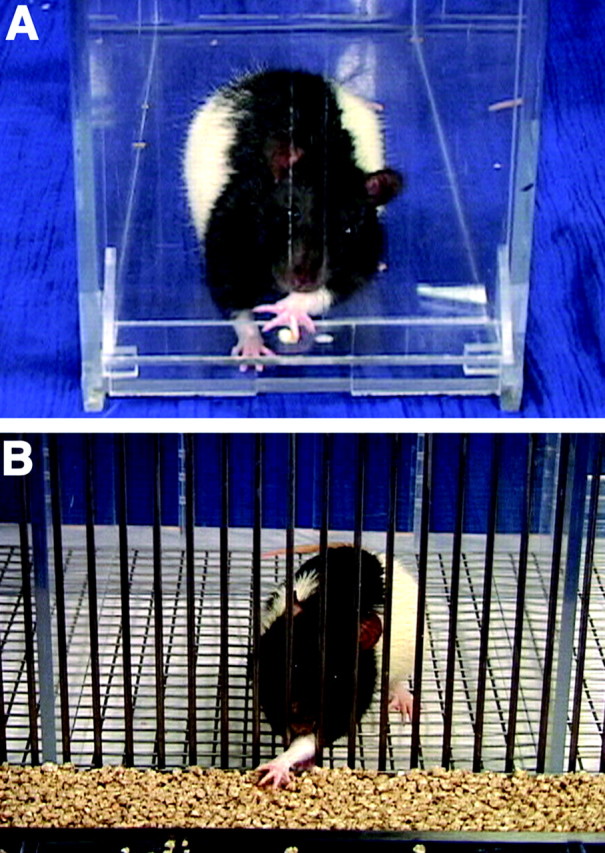
Tasks used to assess skilled reaching.A, In the single-pellet task, a rat was required to reach for a single food pellet. The floor of the cage is Plexiglas; therefore, lost food could be retrieved. B, In the food-tray task, the rat could reach through a slot to grasp food from a tray that was filled with food. If the rat dropped the food, it was lost through the floor of the cage.
Single-pellet boxes were made of clear Plexiglas so that the rats could be filmed from any perspective (Whishaw and Pellis, 1990; Whishaw, 2000). A box was 25 × 35 × 30 cm high. Five centimeters from the side of each front wall was a 1-cm-wide slit that extended from the floor to a height of 15 cm. On the outside of the wall, in front of the slit, mounted 3 cm above the floor, was a 2-cm-wide by 4-cm-long shelf. Food pellets (90 mg of Rodent Chow food pellets; Bioserve Inc., Frenchtown, NJ) were placed in one of two small indentations on the floor of the shelf. The indentations were 2 cm away from the inside wall of the box and were centered on the edges of the slit through which the rats reached. For each rat, food was placed in the indentation contralateral to the limb with which the rat reached. Training was administered in such a way that when a rat made a successful reach, a short pause preceded presentation of the next food pellet, during which another food pellet was dropped into the back of the box. This was done to ensure that a rat left the food aperture after each reach and repositioned itself at the food aperture for the next food pellet.
Scoring reaching success
Reaching performance was scored by counting misses and successful reaches for each limb. If a rat made a reaching movement in which a paw was inserted through the bars/aperture of the cage, the movement was scored as a “reach.” If the rat obtained a piece of food and then consumed it, the reach was scored as a “hit.” For each rat, two success scores were obtained: (1) success on first reach: if a rat obtained the food pellet on the initial limb advance, this reach was scored as a hit. If the rat made more than one limb advance before obtaining the food, this was not counted as a hit. (2) Total success: if a rat obtained a piece of food either on the first limb advance or after a number of limb advances, the reach was counted as a hit. Success scores were computed as follows: success percentage = (number of hits/number of reaches) × 100.
Qualitative reaching analysis
Reaching movements were analyzed using a rating scale derived from an Eshkol–Wachmann movement notation (Eshkol and Wachmann, 1958; Miklyaeva et al., 1994) analysis of reaching. Five reaches for each limb by each rat were rated for qualitative features of the movement (Miklyaeva et al., 1994). The movement rating scale is shown in Table 1, and 10 component movements of a reach were rated. (1) Digits to the midline: Using primarily the upper arm, the reaching limb is lifted from the floor so that the tips of the digits are aligned with the midline of the body. (2) Digits flexed: As the limb is lifted, the digits are flexed, the paw is supinated, and the wrist is partially flexed. (3) Elbow in: Using an upper arm movement, the elbow is adducted to the body midline and the tips of the digits retain their alignment with the midline of the body. (4) Advance: The limb is advanced directly through the slot toward the food target. (5) Digits extend: During the advance, the digits extend so that the digit tips are pointing toward the target. (6) Arpeggio: When the paw is over the target, the paw pronates from digit 5 (the outer digit) through to digit 2, and at the same time, the digits open. (7) Grasp: The digits close and flex over the food, with the paw remaining in place, and the wrist is slightly extended to lift the food. (8) Supination I: As the paw is withdrawn, the paw supinates by almost 90°. (9) Supination II: Once the paw is withdrawn from the slot to the mouth, the paw further supinates by ∼45° to place the food in the mouth. (10) Release: The mouth contacts the paw, and the paw opens to release the food.
Table 1.
Skilled reaching rating scale
| Behavior | Normal | Impaired1-a |
|---|---|---|
| (1) Digits to the midline | 0 | 1 |
| (2) Digits flexed | 0 | 1 |
| (3) Elbow in | 0 | 1 |
| (4) Advance | 0 | 1 |
| (5) Digits extend | 0 | 1 |
| (6) Arpeggio (pronate) | 0 | 1 |
| (7) Grasp | 0 | 1 |
| (8) Supination I | 0 | 1 |
| (9) Supination II | 0 | 1 |
| (10) Release | 0 | 1 |
| Total | 10 |
A mild impairment is indicated by a score of 0.5.
Each of the movements was rated on a two point scale. If the movement was performed normally, a score of 0 was given. If the movement was abnormal, a score of 1 was given. In cases in which there was some ambiguity concerning the occurrence of a movement, or if the impairment was mild, a score of 0.5 was given.
Reaching posture
On each reach, the posture used by the rat was rated on a two point scale (Miklyaeva et al., 1994). If a rat supported itself on the contralateral-to-reaching paw forelimb and its diagonal hindlimb as the reach was initiated, a score of 0 was given. If a rat failed to use this supporting posture, a score of 1 was given.
Cylinder test
The cylinder (30 cm high, 16 cm inner diameter) was made of 3-mm-thick Plexiglas. The cylinder was mounted on a glass table beneath which was positioned an inclined mirror (Bland et al., 2001) for filming. A rat was placed in the cylinder and left to explore for 5 min. Contacts of the forepaws with the wall of the cylinder were counted on a replay of the videotape. If a rat contacted the wall when it reared, a score of 1 was assigned each time a paw was used for support. Each rat's score was calculated as the percentage of preference for the paw ipsilateral to the lesion or, for the control rats, the paw that was used for reaching: preference = [ipsilateral paw/(ipsilateral paw + contralateral paw)] × 100.
Apomorphine-induced rotation
The animals were placed individually into 39-cm-diameter round rotometer bowls for a period of 30 min. A cuff was wrapped around the trunk of the rat, and this was connected to a lead and swivel, which in turn was connected to a computer. A custom computer program recorded the turns in the direction ipsilateral and contralateral to the lesion in 5 min intervals. Apomorphine-hydrochloride (0.05 mg/kg, s.c.) was dissolved in 0.9% saline solution with 2% ascorbic acid. Ipsilateral and contralateral turns were counted via a microcomputer and, using previous criteria, the turning bias was used as one of the measures of lesion size (Marshall and Ungerstedt, 1977; Hefti et al., 1980)
Histology
After behavioral testing, animals were anesthetized and perfused through the heart with a 0.9% sodium chloride solution and picric acid (Lana's fix). The brains were cut in 50 μm sections on a vibratome and mounted on gelatin-coated slides. For tyrosine hydroxylase (TH) immunocytochemistry, the sections were washed in 1 mphosphate buffer and then incubated overnight at room temperature with anti-TH monoclonal antiserum (1:10,000; Sigma). The sections were processed by the ABC method (Vectory, Vectastain; Vector Laboratories, Burlingame, CA) with anti-mouse antiserum IgG and horse serum and reacted with 3,3′-diaminobenzidine tetrahydrochloride (0.06%), hydrogen peroxide (0.03%), and nickel solution. Some sections were processed to control for either monoclonal antiserum or antibody stain (Lee et al., 1996).
Statistical analysis
Statistical analysis was performed using ANOVA with follow-up Newman–Keuls tests (Abacus Concepts, Calabasas, CA). Differences in between-group comparisons were assessed with unpairedt tests, with p < 0.05 set as the significance level.
Procedure
The rats were deprived of food for 2 weeks before the commencement of training in the single-pellet task. Training continued until the animals were consistently reaching. Thereafter, each rat reached for at least 20 food pellets each day throughout the testing period, with the exception of designated rest days. Once the rats were consistently reaching for food and achieving daily scores of at least 50% (10 of 20 pellets), 12 of the 20 rats received 6-OHDA lesions ipsilateral to their preferred paw. The following testing and training procedures were then given to eight 6-OHDA rats and eight control rats:
Single-pellet reaching. The rats were tested each day for 21 d (days 1–21; 20 pellets per day) on the single-pellet reach task. The performance of the rats was video-recorded before surgery, periodically throughout testing, and on day 21. Each rat was also tested in the cylinder test on days 7, 14, and 21.
Tray reaching. The rats were placed in the food-tray apparatus each day for 14 d (days 22–36; 30 min/d). On day 36, reach performance was video-recorded, and performance was evaluated from the video record.
Single-pellet test. On day 37, the rats were returned to the single-pellet testing apparatus and underwent a 20 pellet reaching test that was video-recorded. The rats were also tested in the cylinder test on day 37.
Single-pellet retest. On days 38–43, the rats remained in their home cage and received no testing. On day 44, the rats were returned to the single-pellet testing apparatus and underwent a 20 pellet reaching test that was video-recorded.
Rotation tests. At the completion of the behavioral testing, the rats received a rotational test after the administration of apomorphine.
Single-pellet training control group. Four 6-OHDA rats comprised a supplementary group that received all of the training described above, except that they continued to receive single-pellet training/testing in the period during which the other rats were reaching in the tray task. After day 36, their treatment was the same as that given to the rats that received the tray-task experience.
Results
Histology
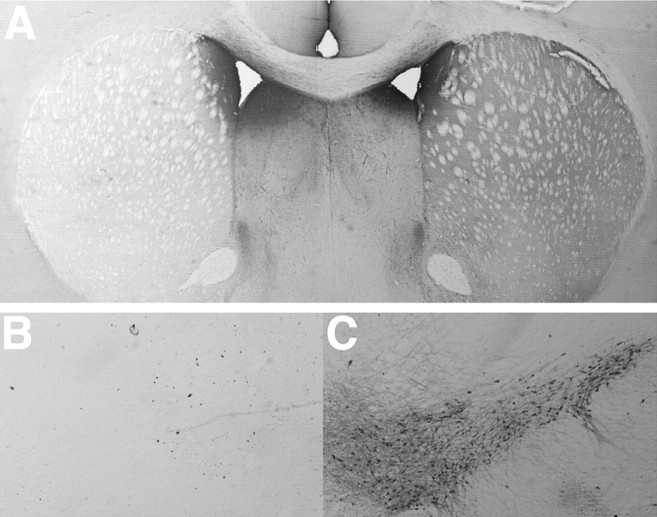
TH immunostaining indicated that the nigrostriatal 6-OHDA injections reduced the number of dopaminergic cells in the substantia nigra and the terminals and fibers in the caudate putamen. Figure2 illustrates a section through the mesencephalon of a lesion animal with representative lesion extent. The lesion resulted in an almost complete unilateral loss of nigrostriatal neurons and the loss of TH-positive fibers throughout the substantia nigra pars compacta. In most rats, cells in the lateral portions of the ventral tegmental area were also absent. A cell-count analysis revealed a significant reduction of TH-positive cells in the lesion mesencephalon in all areas compared with the intact hemisphere (p < 0.001). Figure 2 also illustrates a section through the forebrain showing a marked reduction of TH fiber stain in the caudate putamen of the lesion versus the intact hemisphere.
Fig. 2.
Coronal photomicrographs of the striatum (top) and substantia nigra (bottom) on the side of 6-OHDA depletion (A,B) and on the nonlesion side (C) as revealed by TH immunohistochemistry. Note the loss of TH staining in the caudate putamen and in the substantia nigra.
Apomorphine-induced rotation
After apomorphine injections, the rats with 6-OHDA lesions displayed an asymmetry in rotation, turning predominantly in the direction contralateral to the lesion. ANOVA indicated a significant group effect (F(1,14) = 10.5;p < 0.001), with the lesion apomorphine group displaying pronounced rotation and the control group displaying few rotations. The groups also differed in the direction of rotation (F(1,14) = 22.1; p < 0.001), with the 6-OHDA group rotating predominantly contralateral to the lesion (>6 rotations per minute), indicating an extensive DA depletion (Marshall and Ungerstedt, 1977; Hefti et al., 1980).
Cylinder test
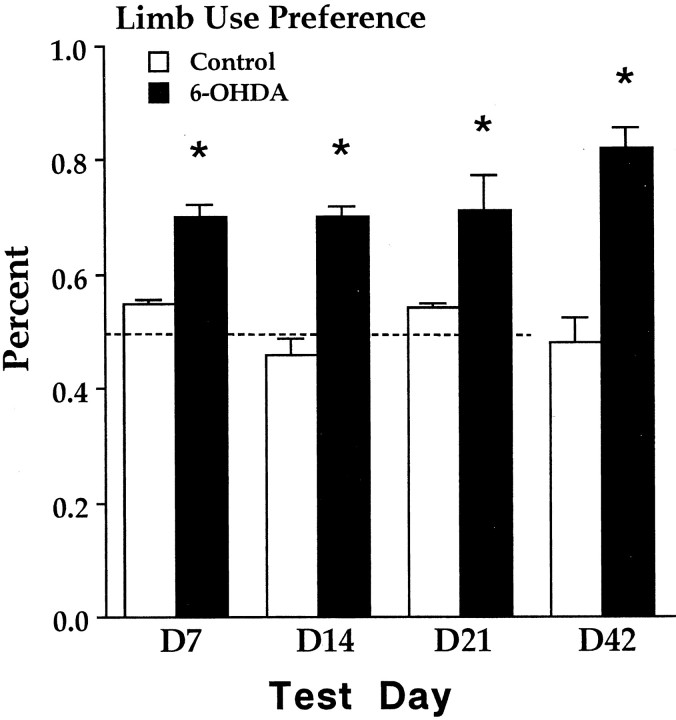
Figure 3 illustrates the rat's percentage of limb preference for the ipsilateral paw in the cylinder test on days 7, 14, 21, and 35 (the test given after 14 d of training in the food-tray task) after lesion. A repeated-measures ANOVA gave a significant group difference (F(1,14) = 33; p < 0.0.001). The performance of the rats was similar across test days (F(3,42) = 1.6; p > 0.5), and the groups remained different across test days (F(3,42) = 2.3; p > 0.05).
Fig. 3.
Relative percentage of paw preference of the ipsilateral paw at different time points in the cylinder test in rats with 6-OHDA lesion relative to control rats. Note that the 6-OHDA-treated rats display a significant preference in the use of the paw ipsilateral to the lesion (good paw). *p < 0.5. The dotted line equals chance.
Single-pellet skilled reaching
Figure 4 indicates performance on the skilled-reaching tests for total-reach success scores (Fig. 4,top) and single-reach success scores (Fig. 4,bottom). ANOVAs gave similar results on both measures; the two groups were significantly different (F(1,14) > 11; p < 0.001). The effect of test days (F(5,60) = 2.7; p <0.01) was significant, as was the group-by-test-day interaction (F(5,60) = 3.1; p < 0.001). Follow-up tests (Newman–Keuls; p <0.05) indicated that the performance of the lesion group was inferior to that of the control group on all of the postoperative test days. Nevertheless, after 2 weeks of training in the tray-reaching task, the lesion group showed a significant improvement in performance on the single-pellet task on tests given both immediately after the tray-reach training and on a test given 1 week after the tray-reach training (p < 0.5).
Fig. 4.
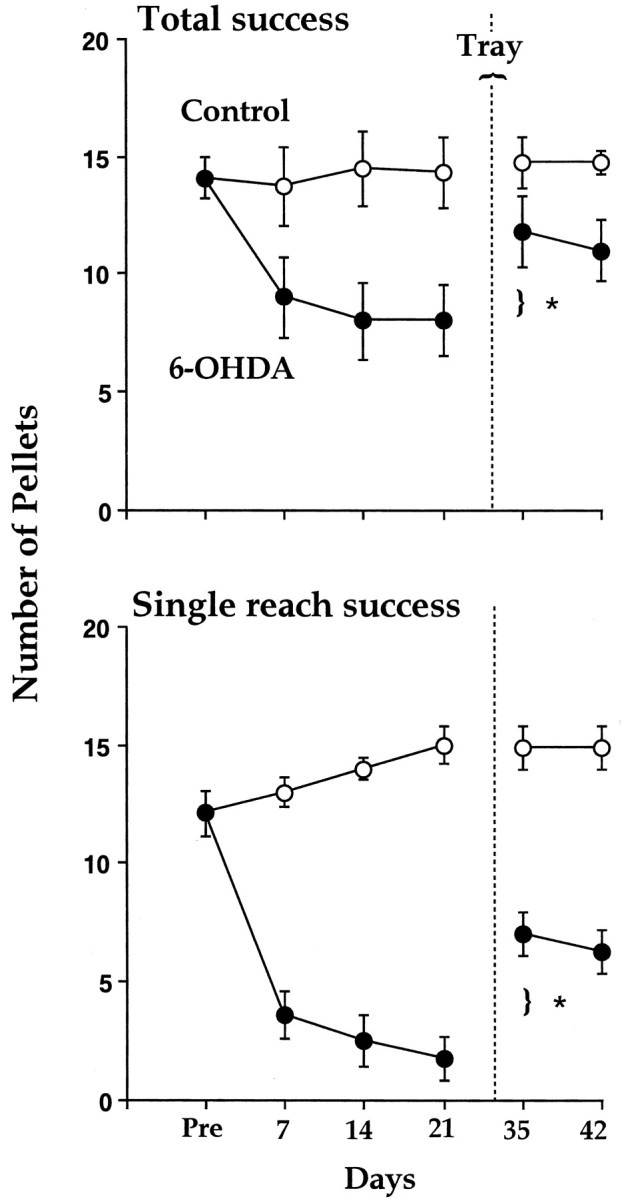
Reaching success (number of pellets obtained out of 20; mean ± SE) by control rats and rats with 6-OHDA lesion in the single-pellet task before and after training on the tray task (after rehabilitation; vertical dotted line).Top, Total success equals the number of pellets obtained of the 20. Bottom, Number of pellets obtained of the 20 on the first reach attempt. Note that the 6-OHDA group is impaired on both measures and displays significant improvement after the tray-reach (rehabilitation) experience.
Tray-task skilled reaching
The rats were allowed to reach for food in the tray task for 30 min each day for 14 d. On day 15, their performance was filmed and evaluated for 5 min of reaching. Rats in both groups used only the paw used in the single-pellet task, which, in the case of the 6-OHDA group, was the paw ipsilateral to the lesion. Overall, there were no differences in the number of reaches made by the two groups [t(1,14) = 0.1; p > 0.05; control, 124.3 ± 20.8; 6-OHDA, 124.13 ± 19.9 (mean ± SE)]. There were also no differences in reaching success percentage (t(1,14) = 0.2;p > 0.05; control, 62.8 ± 13.2; 6-OHDA, 61.9 ± 28.7).
Qualitative evaluation of single reach movement
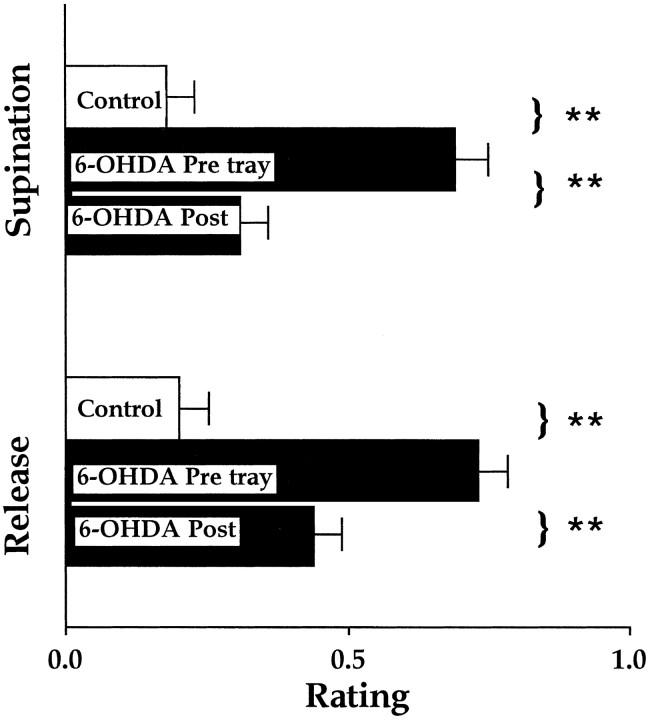
At least five reaches from each rat were scored for tests given on days 21, 37, and 44 after surgery. Analysis of the rats' movement scores indicated that the lesion group had significantly higher scores on all of the tests than did the control group (F(1,14) = 14.9; p < 0.001), but there was also a significant group-by-movement component interaction (F(1,126) = 4.2;p < 0.05). Follow-up analyses indicated that 2 of the 10 movement components were impaired in the lesion group (Supination II and Release). As is illustrated in Figure 5, comparisons of the rats' scores on Supination II and Release on day 21 versus day 36 indicated that the 6-OHDA group displayed significant improvement. Figure6illustrates the reaching movements made by a rat before surgery, after the 6-OHDA lesion, and after rehabilitation training on the tray-reaching task. Before surgery, the rat grasps the food, supinates the paw to withdraw the food through the slot (Supination I), supinates the paw further to place the food in its mouth (Supination II), and releases the food (Release). After the 6-OHDA lesion, the rat successfully grasps the food, displays a partial Supination I to withdraw the food through the slot, but fails to display Supination II to bring the food to the mouth. Rather, the paw adducts so that the rat's mouth comes in contact with the dorsal surface of the wrist/paw. Eventually the paw contacts the table and the food is released onto the floor of the test apparatus. After tray training, the rat displays a complete Supination I and Supination II and releases the food into the mouth.
Fig. 5.
Scores for supination and food release (mean ± SE) in control rats and in rats with 6-OHDA lesion before and after tray-reach training (rehabilitation). Note that performance after tray reach is significantly (**p < 0.01) improved compared with performance before rehabilitation.
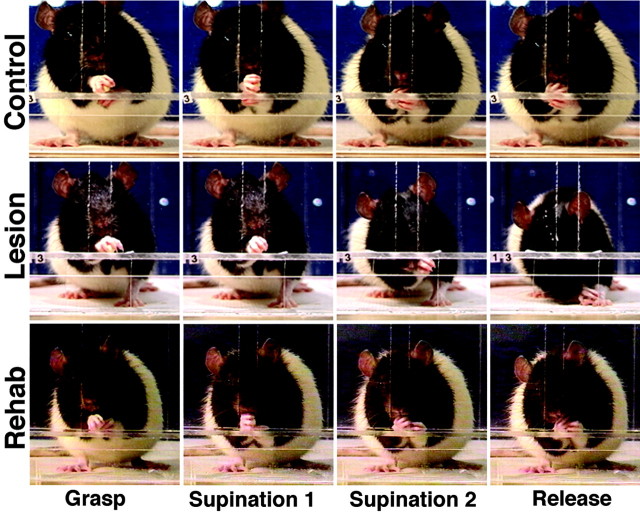
Fig. 6.
Illustration of supination and release by a rat before 6-OHDA lesion (Control), after the lesion (Lesion), and after training on the tray task (Rehab). Note that after the lesion, a failure to supinate the paw results in adduction of the paw, with the mouth contacting the outer surface of the wrist/paw. The paw eventually contacts the floor, and the food pellet is dropped. After rehabilitation, the rat is able to supinate the paw and eat the food while remaining in a sitting posture but uses a slight compensatory head tilt to retrieve the food pellet.
Although the rats appeared to have recovered the ability to make the Supination II movement, we were concerned that the movement may not have been independent, but rather assisted with the bad paw. Therefore, the tapes were carefully examined to determine whether Supination II was performed independently or was assisted. This analysis indicated that Supination II was completed before the contralateral paw was brought toward the mouth. In addition, all of the rats displayed reaches in which the contralateral paw was not used at all to assist eating. Thus, Supination II did appear to have recovered as an independent movement. When the rats were allowed a 7 d no-reach period after the day 36 test, a retest on day 42 indicated that the rats were still able to supinate and bring the paw to the mouth successfully.
Although tray-reach training appeared to serve as rehabilitative experience for all of the rats, a careful observation of the rats' movements indicated that a subtle deficit persisted. The 6-OHDA rats, although still able to bring the food to their mouths, tilted their heads slightly to the ipsilateral side, with their snouts directed slightly medially to assist in taking the food from the paw. This subtle difference suggested that there was a residual deficit in supinating the paw despite the rehabilitation experience.
Posture
The rat's posture was scored on days 21 and 36. There was a significant group difference (F(1,12)= 5.6; p < 0.01) but no group-by-day interaction. The control rats typically supported their weight during a reach on the forepaw contralateral to the reaching paw and on the ipsilateral hindpaw. During limb withdrawal and eating, the weight was supported relatively symmetrically on both hindpaws. Weight support by the 6-OHDA group was more obviously shifted to the ipsilateral hindpaw both during limb transport to the food and during limb withdrawal and eating.
Single-pellet-training control group
Because the improvement in performance produced by reaching in the tray task was unexpected, an additional group of DA-depleted rats received training only on the single-pellet task. All of the rats received daily training for 5 d each week for 45 d. Their performance did not improve over this period of training, and their success scores were 6.2 ± 2 on day 22 and 5.4 ± 3 on day 43, a difference that was not significant. Thus, these rats showed no improvement as a function of training on the single-pellet task. Inspection of the video records of the rats' performance indicated that across all testing they displayed the same impairment in supination displayed by the previously tested DA-depleted group, except that they did not show improvements as did the previous group in response to their additional experience on the single-pellet task.
Discussion
The objective of the present experiment was to determine why rats display a skilled-reaching impairment on the side of the body ipsilateral to the DA depletion. A quantitative evaluation of skilled reaching for single food pellets confirmed that the DA-depleted rats displayed reduced success in retrieving food pellets with the paw ipsilateral to the lesion. A qualitative analysis indicated that the deficit was attributable to a failure to supinate the paw and to release the food to the mouth. After rehabilitative training in a simpler reaching task, the rats displayed substantial improvement on the single-pellet task, and the impairment in supination was significantly reduced. The results clarify the nature of the unilateral skilled-reaching impairment and also demonstrate that rehabilitative procedures can lessen the impairment.
A number of studies have reported that unilateral DA-depleted rats display impairment in skilled reaching not only with the paw contralateral to the lesion but also with the paw ipsilateral to the lesion. On the expectation that deficits should be displayed only on the contralateral side of the body, Montoya et al. (1990) have suggested that the ipsilateral impairment may be secondary to “a nonspecific postural deviation rather than a specific impairment in skilled motor use of the ipsilateral limb.” Similarly, Miklyaeva et al. (1994) ascribe the ipsilateral impairment to compensatory postural adjustments for postural abnormalities in which the DA-depleted rats relied excessively on the ipsilateral hindlimb for support and movement. In neither study was a specific examination undertaken of the way that the ipsilateral limb is used.
We confirm that unilateral DA-depleted rats have an impairment in their success in retrieving food pellets using the ipsilateral limb on a demanding criterion, in which a success was counted only if the food was retrieved on a single reach, and a less demanding criterion, in which all successes were counted. A video analysis of the reaching movements indicated that the rats displayed an impairment in supinating the paw to bring food to the mouth. Rather than supinating, the paw was adducted across the snout so that the mouth contacted the upper surface of the wrist/paw. Food was lost because the paw was eventually placed on the floor of the cage. Thus, the impairment appeared to be referable to the proximal musculature of the body that might assist in supinating and holding the limb in a midline position to present food to the mouth.
The finding that unilateral DA depletion in the rat produces an ipsilateral impairment is consistent with findings in human Parkinson patients. Patients with unilateral posteroventral medial pallidotomy display improvements in ipsilateral bradykinesia and finger tapping (Lozano and Lang, 1995; Lang et al., 1997). In addition, patients receiving unilateral deep-brain stimulation experience improvement in the ipsilateral symptoms of tremor and rigidity (Kumar et al., 1999;Merello et al., 1999; Obwegeser et al., 2001). The neural pathways mediating either deficits or improvements are not known in the rat or in humans.
There are a number of potential explanations of the ipsilateral impairment. First, ∼5–10% of DA fibers are crossed (for review, seeFallon and Loughlin, 1995; Heimer et al., 1995), so damage to this crossed projection may be related to the impairment in the ipsilateral paw. Second, rats with unilateral amino acid neurotoxic lesions of the caudate putamen display an ipsilateral reaching impairment (Montoya et al., 1990; Jeyasingham et al., 2001), which could be taken as evidence that it is the uncrossed DA projections to the striatum that may be related to the ipsilateral reaching deficit. Third, DA depletion may indirectly impair some of the uncrossed projections of the extrapyramidal system to the spinal cord, or crossed projections within basal ganglia circuitry (Parent and Hazrati, 1995). Fourth, the deficit may be related to musculoskeletal asymmetry, such as scoliosis, which reportedly follows unilateral DA depletions (Herrera-Marschitz et al., 1990). With respect to this point, however, it is noteworthy that the limb impairment is manifested immediately after the lesion, before scoliosis might be expected to be severe. In addition, the brief period of rehabilitation that the rats received in the tray task would have been unlikely to have remediated scoliosis. Fifth, the DA-depleted rats obviously displayed postural abnormalities in relying excessively on the ipsilateral hind limb for support, which may have contributed to the limb impairment. However, rehabilitation ameliorated the impairment in limb use but not in postural support, which weakens this possibility. In addition, after unilateral pyramidal tract lesions at the medullary pyramids, postural changes similar to those observed after DA depletions occur, but the animals do not display an ipsilateral deficit in skilled reaching (Whishaw and Metz, 2002).
Because previous studies have shown that there is improved performance with training on the simpler tray task (Whishaw et al., 1986), the rats in the present study were trained on the tray task for 2 weeks. At the end of this training, the control and DA-depleted groups performed equally well. Surprisingly, when returned to the single-pellet task, the rats with 6-OHDA lesion displayed significant improvement in success. The rats also displayed significant improvement in supinating the limb and in releasing the food to the mouth. Thus, training on the simpler task provided a rehabilitative experience. The rats retained the benefits conferred by the rehabilitative experience over a number of tests given over a couple of weeks. Other rats tested during the same period in only the single-pellet task displayed no recovery.
The deficit in supination was not completely ameliorated, however. A careful inspection of the movements of the rats after rehabilitation indicated that they were sometimes clumsy and that they also had slight impairment in supination. They compensated for the impairment by turning the head toward the paw to retrieve the food pellet.
The tray-reaching task may be rehabilitative in that a tray full of food provides easier access to the food. The grids comprising the floor of the cage may also assist postural support by providing the animals with purchase for their hind feet. The task may also be ameliorative by being negatively reinforcing, in that releasing the food results in its loss through the floor of the cage (in the single-pellet task, the food can be retrieved once released). We were concerned that rehabilitation may simply have been attributable to a compensatory process on the good side of the body, such as using the bad paw to assist the good paw. Although the DA-depleted rats did use the bad paw to assist in holding retrieved food, an inspection of the videotapes indicated that rats successfully supinated the paw before the bad paw was recruited. The precise way in which the task is rehabilitative can be examined in future work. Nevertheless, the finding that rehabilitation can ameliorate a DA-related impairment opens the possibility that other impairments may also be lessened through similar rehabilitative training.
Previous work has suggested that restraint of the good limb, forcing use of the limb contralateral to the lesion, can lessen DA-related impairments, possibly by reducing the lesion size (Tillerson et al., 2001). It is unlikely that the therapeutic effects of the rehabilitative experience given here are produced in the same way. First, the rats were given a test of limb asymmetry in which contacts by the forepaws with the walls of a cylinder were measured (Tillerson et al., 2001), and on this test, the asymmetry was similar both before and after rehabilitative training. Because this test provides a measure of lesion size, the result suggests that lesion size was not affected by the testing/training procedures. In addition, the apomorphine test of rotation given at the end of the training/testing procedures and the histological procedures suggested that the DA depletions were relatively complete.
In conclusion, there is growing realization that the impairments produced by DA depletion are complex and vary in their sensitivity to therapy (Metz et al., 2001). Some deficits (e.g., bradykinesia) can be improved by therapies such as brain grafts andl-3,4-dihydroxyphenylalanine (Schallert et al., 1979; Olsson, 1995), whereas other behaviors (e.g., skilled reaching with the contralateral paw) seem more resistant to therapy (Dunnett et al., 1987; Olsson, 1995; Metz et al., 2001; but see Nikkhah et al., 1993; Glavan et al., 2001). Not only do the results of the present study suggest that unilateral DA depletion does affect some movements ipsilateral to the side of DA depletion, but the results also suggest that rehabilitative training can ameliorate this DA-related impairment. This finding is novel and important and adds to previous reports that ipsilateral but not contralateral skilled-reaching impairments are improved by grafts of embryonic DA tissue (Montoya et al., 1990) and subthalamic nucleus lesions (Henderson et al., 1999). At present, there is some doubt concerning the efficacy of physiotherapy in human patients with Parkinson's disease (Dean et al., 2001; de Goede et al., 2001), but the finding here that a specific treatment can have a specific beneficial effect could be considered in the design of future programs for human physiotherapy.
Footnotes
This study was supported by the Canadian Institute for Health Research. We thank Bogdan Gorny and Paul A. Whishaw for assistance with the video production.
Correspondence should be addressed to Dr. Ian Q. Whishaw, Canadian Centre for Behavioural Neuroscience, University of Lethbridge, 4401 University Drive, Lethbridge, Alberta, Canada, T1K 3M4. E-mail:whishaw@uleth.ca.
References
- 1.Abrous DN, Shaltot ARA, Torres EM, Dunnett SB. Dopamine-rich grafts in the neostriatum and/or nucleus accumbens: effects on drug-induced behaviours and skilled paw-reaching. Neuroscience. 1993a;53:187–197. doi: 10.1016/0306-4522(93)90297-s. [DOI] [PubMed] [Google Scholar]
- 2.Abrous DN, Torres EM, Dunnett SB. Dopaminergic grafts implanted into the neonatal or adult striatum: comparative effects on rotation and paw reaching deficits induced by subsequent unilateral nigrostriatal lesions in adulthood. Neuroscience. 1993b;54:657–668. doi: 10.1016/0306-4522(93)90237-a. [DOI] [PubMed] [Google Scholar]
- 3.Barnéoud P, Descombris E, Aubin N, Abrous DN. Evaluation of complex sensorimotor behaviours in rats with a partial lesion of the dopaminergic nigrostriatal system. Eur J Neurosci. 2000;12:322–336. doi: 10.1046/j.1460-9568.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- 4.Bland ST, Pillai RN, Aronowski J, Grotta JC, Schallert T. Early overuse and disuse of the affected forelimb after moderately severe intraluminal suture occlusion of the middle cerebral artery in rats. Behav Brain Res. 2001;126:33–41. doi: 10.1016/s0166-4328(01)00243-1. [DOI] [PubMed] [Google Scholar]
- 5.Cenci MA, Whishaw IQ, Schallert T. Animal models of neurological deficits: how relevant is the rat? Nat Neurosci Rev. 2002;13:574–579. doi: 10.1038/nrn877. [DOI] [PubMed] [Google Scholar]
- 6.Dean KH, Jones D, Playford ED, Ben-Shlomo Y, Clarke CE. Physiotherapy for patients with Parkinson's disease: a comparison of techniques. Cochrane Database Syst Rev. 2001;3:CD002817. doi: 10.1002/14651858.CD002817. [DOI] [PubMed] [Google Scholar]
- 7.de Goede CJ, Keus SH, Kwakkel G, Wagenaar RC. The effects of physical therapy in Parkinson's disease: a research synthesis. Arch Phys Med Rehabil. 2001;82:509–515. doi: 10.1053/apmr.2001.22352. [DOI] [PubMed] [Google Scholar]
- 8.Döbrössy MD, LeMoal M, Montaron M-F, Abrous N. Influence of environment on the efficacy of intrastriatal dopaminergic grafts. Exp Neurol. 2000;165:172–183. doi: 10.1006/exnr.2000.7462. [DOI] [PubMed] [Google Scholar]
- 9.Dunnett SB, Whishaw IQ, Rogers DC, Jones GH. Dopamine-rich grafts ameliorate whole body motor asymmetry and sensory neglect but not independent limb use in rats with 6-hydroxydopamine lesions. Brain Res. 1987;415:630–678. doi: 10.1016/0006-8993(87)90269-1. [DOI] [PubMed] [Google Scholar]
- 10.Dunnett SB, Isacson O, Sirinathsinghji DJS, Clarke DJ, Björklund A. Striatal grafts in rats with unilateral neostriatal lesions. III. Recovery from dopamine-dependent motor asymmetry and deficits in skilled paw reaching. Neuroscience. 1988;24:813–820. doi: 10.1016/0306-4522(88)90069-3. [DOI] [PubMed] [Google Scholar]
- 11.Duvoisin RC. Parkinson's disease. New York; Raven: 1978. [Google Scholar]
- 12.Eshkol N, Wachmann A. Movement notation. Weidenfeld and Nicholson; London: 1958. [Google Scholar]
- 13.Fallon JH, Loughlin SE. Substantia nigra. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 215–232. [Google Scholar]
- 14.Fricker RA, Annett LE, Torres EM, Dunnett SB. The locus of a striatal ibotenic acid lesion affects the direction of drug-induced rotation and skilled forelimb use. Brain Res Bull. 1996;41:409–416. doi: 10.1016/s0361-9230(96)00083-4. [DOI] [PubMed] [Google Scholar]
- 15.Glavan A, Floran B, Erlij D, Aceves J. Intrapallidal dopamine restores motor deficits induced by 6-hydroxydopamine in the rat. J Neural Transm. 2001;108:153–166. doi: 10.1007/s007020170085. [DOI] [PubMed] [Google Scholar]
- 16.Grabowski M, Brundin P, Johansson BB, Kontos HA. Paw reaching, sensorimotor and rotational behavior after brain infarction in rats. Stroke. 1993;24:889–895. doi: 10.1161/01.str.24.6.889. [DOI] [PubMed] [Google Scholar]
- 17.Hefti F, Melamed E, Shakian BJ, Wurtman RJ. Circling behavior in rats with partial unilateral nigrostriatal lesions: affect of amphetamine, apomorphine, and dopa. Pharmacol Biochem Behav. 1980;12:185–188. doi: 10.1016/0091-3057(80)90353-6. [DOI] [PubMed] [Google Scholar]
- 18.Heimer L, Zahm DS, Alheid GA. Basal ganglia. In: Paxinos G, editor. The rat nervous system. Academic; San Diego: 1995. pp. 579–614. [Google Scholar]
- 19.Henderson JM, Annett LE, Ryan LJ, Chiang W, Hidaka S, Torres EM, Dunnett SB. Subthalamic nucleus lesions induce deficits as well as benefits in the hemiparkinsonian rat. Eur J Neurosci. 1999;11:2749–2757. doi: 10.1046/j.1460-9568.1999.00692.x. [DOI] [PubMed] [Google Scholar]
- 20.Herrera-Marschitz M, Utsumi H, Ungerstedt U. Scoliosis in rats with experimentally-induced hemiparkinsonism: dependence upon striatal dopamine denervation. J Neurol Neurosurg Psychiatry. 1990;53:39–43. doi: 10.1136/jnnp.53.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jeyasingham RA, Baird AL, Meldrum A, Dunnett SB. Differential effects of unilateral striatal and nigrostriatal lesions on grip strength, skilled paw reaching and drug-induced rotation in the rat. Brain Res Bull. 2001;55:541–548. doi: 10.1016/s0361-9230(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 22.Kumar R, Lozano AM, Sime E, Halket E, Lang AE. Comparative effects of unilateral and bilateral subthalamic nucleus deep brain stimulation. Neurology. 1999;11:561–566. doi: 10.1212/wnl.53.3.561. [DOI] [PubMed] [Google Scholar]
- 23.Lang AE, Lozano AM, Montgomery E, Duff J, Tasker R, Hutchinson W. Posteroventral medial pallidotomy in advanced Parkinson's disease. N Engl J Med. 1997;337:1036–1042. doi: 10.1056/NEJM199710093371503. [DOI] [PubMed] [Google Scholar]
- 24.Lee CS, Sauer H, Björklund A. Dopaminergic neuronal degeneration and motor impairments following axon terminal lesion by intrastriatal 6-hydroxydopamine in the rat. Neuroscience. 1996;27:641–653. doi: 10.1016/0306-4522(95)00571-4. [DOI] [PubMed] [Google Scholar]
- 25.Lozano AM, Lang AE. Reports on a study of the effect of globus pallidus (Gpi) on the motor functions in Parkinson's disease patients. Lancet. 1995;346:1383–1387. doi: 10.1016/s0140-6736(95)92404-3. [DOI] [PubMed] [Google Scholar]
- 26.Marshall JF, Ungerstedt U. Supersensitivity to apomorphine following destruction of the ascending dopamine neurons: quantification using the rotational model. Eur J Pharmacol. 1977;41:361–367. doi: 10.1016/0014-2999(77)90256-4. [DOI] [PubMed] [Google Scholar]
- 27.Martin JP. The basal ganglia and posture. Pittman; London: 1967. [Google Scholar]
- 28.Merello M, Nouzeilles MI, Kuzis G, Commarota A, Sabe L, Betti O, Starkstein S, Leiguarda R. Unilateral radiofrequency lesion versus electrostimulation of posteroventral pallidum: a prospective randomized comparison. Mov Disord. 1999;14:50–56. doi: 10.1002/1531-8257(199901)14:1<50::aid-mds1010>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 29.Metz GAS, Ballermann M, Whishaw IQ. Chronic levodopa therapy does not improve skilled reach accuracy or reach range on a pasta matrix reaching task in 6-OHDA dopamine depleted (hemiparkinson analogue) rats. Eur J Neurosci. 2001;14:27–37. doi: 10.1046/j.0953-816x.2001.01615.x. [DOI] [PubMed] [Google Scholar]
- 30.Miklyaeva EI, Castañeda E, Whishaw IQ. Skilled reaching deficits in unilateral dopamine-depleted rats: impairments in movement and posture and compensatory adjustments. J Neurosci. 1994;14:7148–7158. doi: 10.1523/JNEUROSCI.14-11-07148.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miklyaeva EI, Martens DJ, Whishaw IQ. Impairments and compensatory adjustments in spontaneous movement after unilateral dopamine depletion in rats. Brain Res. 1995;681:23–40. doi: 10.1016/0006-8993(95)00277-w. [DOI] [PubMed] [Google Scholar]
- 32.Montoya CP, Astell S, Dunnett SB. Effects of nigral and striatal grafts on skilled forelimb use in the rat. Prog Brain Res. 1990;82:459–466. doi: 10.1016/s0079-6123(08)62634-5. [DOI] [PubMed] [Google Scholar]
- 33.Montoya CP, Campbell-Hope KD, Dunnett SB. The “staircase test”: a measure in independent forelimb reaching and grasping abilities in rats. J Neurosci Methods. 1991;36:219–228. doi: 10.1016/0165-0270(91)90048-5. [DOI] [PubMed] [Google Scholar]
- 34.Moore AE, Cicchetti F, Hennen J, Isacson O. Parkinsonian motor deficits are reflected by proportional A9/A10 dopamine neuron degeneration in the rat. Exp Neurol. 2001;172:363–376. doi: 10.1006/exnr.2001.7823. [DOI] [PubMed] [Google Scholar]
- 35.Nikkhah G, Duan W-M, Knappe U, Jökicke A, Björklund A. Restoration of complex sensorimotor behavior and skilled forelimb use by a modified nigral cell suspension transplantation approach in the rat Parkinson model. Neuroscience. 1993;56:33–43. doi: 10.1016/0306-4522(93)90559-x. [DOI] [PubMed] [Google Scholar]
- 36.Obwegeser AA, Utti RJ, Witte RJ, Lucas JA, Turk MF, Wharen RE., Jr Quantitative and qualitative outcome measures after thalamic deep brain stimulation to treat disabling tremors. Neurosurgery. 2001;48:281–284. doi: 10.1097/00006123-200102000-00004. [DOI] [PubMed] [Google Scholar]
- 37.Olsson M, Nikkhah G, Bentlage C, Björklund A. Forelimb akinesia in the rat Parkinson model: differential effects of dopamine agonists and nigral transplants as assessed by a new stepping test. J Neurosci. 1995;15:3863–3875. doi: 10.1523/JNEUROSCI.15-05-03863.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 39.Paxinos G, Watson C. The rat brain. Academic; New York: 1998. [Google Scholar]
- 40.Robinson TE, Castañeda E, Whishaw IQ. Compensatory changes in striatal dopamine neurons following recovery from injury induced by 6-OHDA or methamphetamine: a review of evidence from microdialysis studies. Can J Psychol. 1990;44:253–275. doi: 10.1037/h0084241. [DOI] [PubMed] [Google Scholar]
- 41.Schallert T, De Ryck M, Whishaw IQ, Ramirez VD, Teitelbaum P. Excessive bracing reactions and their control by atropine and l-DOPA in an animal analog of Parkinsonism. Exp Neurol. 1979;64:33–43. doi: 10.1016/0014-4886(79)90003-7. [DOI] [PubMed] [Google Scholar]
- 42.Schultz Q. Depletion of dopamine in the striatum as an experimental model of Parkinsonism: direct effects and adaptive mechanisms. Prog Neurobiol. 1982;8:121–166. doi: 10.1016/0301-0082(82)90015-6. [DOI] [PubMed] [Google Scholar]
- 43.Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioural brain research analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 44.Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ungerstedt U. Adipsia and aphagia after 6-hydroxydopamine induced degeneration of the nigro-striatal dopamine system. Acta Scand. 1971;82 [Suppl 367]:96–122. doi: 10.1111/j.1365-201x.1971.tb11001.x. [DOI] [PubMed] [Google Scholar]
- 46.Whishaw IQ. Loss of the innate cortical engram for action patterns used in skilled reaching and the development of behaviour compensation following motor cortex lesions in the rat. Neuropharmacology. 2000;39:788–805. doi: 10.1016/s0028-3908(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 47.Whishaw IQ, Metz GA. Absence of impairments or recovery mediated by the uncrossed pyramidal tract in the rat versus enduring deficits produced by the crossed pyramidal tract. Behav Brain Res. 2002;134:323–326. doi: 10.1016/s0166-4328(02)00051-7. [DOI] [PubMed] [Google Scholar]
- 48.Whishaw IQ, Pellis SM. The structure of skilled forelimb reaching in the rat: a proximally driven movement with a single distal rotatory component. Behav Brain Res. 1990;41:49–59. doi: 10.1016/0166-4328(90)90053-h. [DOI] [PubMed] [Google Scholar]
- 49.Whishaw IQ, O'Connor WT, Dunnett SB. The contributions of motor cortex, nigrostriatal dopamine, and caudate-putamen to skilled forelimb use in the rat. Brain. 1986;109:805–843. doi: 10.1093/brain/109.5.805. [DOI] [PubMed] [Google Scholar]
- 50.Whishaw IQ, Castañeda E, Gorny BP. Dopamine and skilled limb use in the rat: more severe bilateral impairments follow substantia nigra than sensorimotor cortex 6-hydroxydopamine injection. Behav Brain Res. 1992;47:89–92. doi: 10.1016/s0166-4328(05)80255-4. [DOI] [PubMed] [Google Scholar]
- 51.Whishaw IQ, Woodward NC, Miklyaeva E, Pellis SM. Analysis of limb use by control rats and unilateral DA-depleted rats in the Montoya staircase test: movements, impairments, and compensatory strategies. Behav Brain Res. 1997;89:167–177. doi: 10.1016/s0166-4328(97)00057-0. [DOI] [PubMed] [Google Scholar]