Abstract
In this study, we investigate how neurosteroid sensitivity of GABAA receptors (GABAARs) is regulated. We examined this issue in neurons of the supraoptic nucleus (SON) of the rat and found that, during parturition, the GABAARs become insensitive to the neurosteroid allopregnanolone attributable to a shift in the balance between the activities of endogenous Ser/Thr phosphatase and PKC. In particular, a constitutive endogenous tone of oxytocin within the SON after parturition suppressed neurosteroid sensitivity of GABAARs via activation of PKC. Vice versa before parturition, during late pregnancy, application of exogenous oxytocin brings the GABAARs from a neurosteroid-sensitive mode toward a condition in which the receptors are not sensitive. This indicates that there may be an inverse causal relationship between the extent to which the GABAAR or one of its interacting proteins is phosphorylated and the neurosteroid sensitivity of the GABAAR. Neurosteroid sensitivity was not affected by changes in subunit composition of GABAARs known to occur concurrently in these cells.
Keywords: neurosteroid, GABAA receptor, supraoptic nucleus, PKC, phosphatase, oxytocin
Introduction
The neurosteroid allopregnanolone [(5α-pregnan-3α-ol-20-one (3α-OH-DHP)] is a potent endogenous allosteric modulator of GABAA receptors (GABAARs) (Twyman and MacDonald, 1992) acting via a specific binding domain (Turner et al., 1989; Lambert et al., 1995). Variation in the efficacy of neurosteroids to modulate GABAARs, as observed in different types of neurons (Harrison et al., 1987; Zhu et al., 1996; Brussaard et al., 1997, 1999; Cooper et al., 1999), has been proposed to arise from differences in subunit composition of the GABAARs. Despite previous attempts to identify the specific GABAAR subunits that mediate neurosteroid effects, no adequate explanation for the apparent correlation between diversity in receptor subtype and neurosteroid sensitivity has been put forward (Belelli et al., 1996; Zhu et al., 1996; Brussaard et al., 1997; Davies et al., 1997; Smith et al., 1998a,b). Moreover, recent studies indicate that phosphorylation may affect neurosteroid modulation of recombinant (Leidenheimer and Chapell, 1997) and native (Fancsik et al., 2000) GABAARs. The latter study suggested that PKC-mediated phosphorylation is a prerequisite for neurosteroid regulation by showing that, in supraoptic nucleus (SON) neurons, the G-protein blocker GDP-β-S and the PKC antagonist bisindolylmaleimide both prevented an effect of subsequent 3α-OH-DHP treatment on the decay of IPSCs.
However, additional research is necessary because (1) the pharmacological agents used by Fancsik et al. (2000) to manipulate phosphorylation had an effect on the IPSC decay in the absence of neurosteroid, (2) pharmacological activation of G-protein signaling or PKC activity did not potentiate the effect of 3α-OH-DHP on GABAARs (Fancsik et al., 2000), and (3) hitherto it remained unclear what mechanism underlies the occurrence of neurosteroid-insensitive GABAARs in oxytocin (OT) neurons in the SON after parturition and of GABAARs being highly sensitive to neurosteroid during late pregnancy (Brussaard et al., 1997). The latter issue may be relevant because robust regulation of the endogenous PKC activity in SON neurons is likely to occur after, but not before, parturition (Brussaard et al., 2000) attributable to increased levels of oxytocin (Lambert et al., 1994; Leng et al., 1999). In addition, the oxytocin neurons in the SON were shown to shift between a major role for α1- during late pregnancy toward α2-containing GABAARs after parturition (Brussaard et al., 1997, 1999). Thus, the exact role of PKC activity, or of any other intracellular enzyme, in conditioning the neurosteroid sensitivity of GABAARs in the SON is still unclear.
Therefore, using the SON during the female reproduction cycle as a model, we aimed at explaining the endogenous shift between different modes of neurosteroid sensitivity of GABAAR activity. We also used a transgenic α1−/− knock-out mouse (Sur et al., 2001), which excluded a prominent role for changes in GABAAR subunit composition in the modulation of neurosteroid sensitivity. Instead, we provide a cellular mechanism that explains how GABAARs may switch their mode of neurosteroid sensitivity and that is also fully consistent with all observations on neurosteroid sensitivity of GABAARs that have been reported previously in the SON under natural conditions (Brussaard and Herbison, 2000).
Materials and Methods
Recombinant expression of GABAARs
Transfection and membrane preparation. Expression vectors for the α, β, and γ2L subunits from rat were transfected in triple combination into human embryonic kidney cells (HEK 293; American Type Culture Collection CRL 1573) as described previously (Lüddens and Korpi, 1995). For optimal receptor expression, final concentrations (in μg of vector DNA per 15 cm tissue culture plate) were as follows: 5 α1, 12 α2, 25 β2, and 0.5 γ2S. The γ2S variant is abbreviated γ2 in the remainder of the text. Cells were washed 40 hr after transfection with PBS, pH 7.4, at 37°C, harvested in ice-cold PBS, and centrifuged at 500 rpm. Cell pellets were homogenized in an Ultraturrax homogenizer for 15 sec, pelleted at 20,000 rpm, frozen at −20°C, and recentrifuged. The membrane pellets were resuspended in 50 mm Tris–citrate buffer, pH 7.3.
Binding assays. Resuspended cell membranes (50–200 μg of protein per tube) were incubated in a final volume of 0.5 ml of 50 mm Tris–citrate buffer, pH 7.3, supplemented with 0.2 m NaCl for [35S]t-butylbicyclo-phosphothionate (TBPS) (2–6 nm) binding. GABA was diluted from a 100 mm solution in H2O. Nonspecific binding was determined by 20 μm picrotoxin. After 90 min at room temperature, the assay mixtures were rapidly diluted to 5 ml with ice-cold 10 mm Tris–HCl, pH 7.4, filtered through glass fiber filters and washed once with 5 ml of 10 mm Tris–HCl, pH 7.4. Filters were immersed in 4 ml of Packard Ultima Gold scintillation fluid, and the radioactivity was determined in a Beckman Instruments (Fullerton, CA) liquid scintillation counter using external standardization. Statistical calculations were performed using the Graph Pad Prism program (GraphPad Software, San Diego, CA).
Receptor expression in Xenopus oocytes.Expression, drug application, and two-electrode voltage-clamp experiments were performed as described previously (Zwart and Vijverberg, 1997). Rat GABAAR α1, α2, β2, and γ2L subunit cDNAs, ligated into the VMT expression vector, were kindly provided by Dr. Paul Whiting (Merck, Sharp, and Dohme, Harlow, UK). Plasmids coding for the α, β, and γ subunits of GABAARs, dissolved in distilled water at a 1:1:1 molar ratio, were coinjected into the nuclei of stage VI oocytes. Approximately 1 ng of each plasmid containing α, β, and γ cDNA was injected in a total injection volume of 15–20 nl/oocyte. Experiments were performed on oocytes after 2–5 d of incubation in modified Barth's solution. Oocytes were voltage clamped at −80 mV. Aliquots of freshly prepared, concentrated stock solutions of GABA in distilled water and of 3α-OH-DHP in dimethylsulfoxide (DMSO) were added to the recording solution immediately before the experiments. Drug applications were alternated by 5 min of superfusion with agonist-free saline to allow the receptors to recover from desensitization.
Mouse model and quantitative PCR
Generation of α1 −/− mice. The GABAAR α1 gene-targeting vector was constructed from the genomic 129/SvEv λ fixII clone, which has been used for the introduction of the α1H101R mutation. However, for the complete gene knock-out, exon 4 was disrupted at the MscI restriction site by cloning the 1.2 kb BstBI+MscI and the 7 kbEcoRV+BamHI DNA fragment blunt ended into the targeting vector (Sur et al., 2001). For this study, the F5 generation was used.
Quantative PCRs. Primers for quantitative-PCR (Q-PCR) analysis of various GABAAR subunit mRNAs were designed using the Primer Express program (version 1.0; Applied Biosystems, Foster City, CA). Total RNA was isolated from SON punches obtained from slices of individual wild-type (WT) and α1 −/− animals after the recording session, reverse transcribed into cDNA, and used as template in a Q-PCR experiment. β-Actin mRNA levels were measured as an internal standard. The ABI 7700 Sequence Detection System (Applied Biosystems) was used with cyber green as the reporter dye.
Whole-cell voltage-clamp recordings of SON neurons of mice and rats
For this study, 202 neurons were recorded from the dorsomedial SON, which contains mostly oxytocin cells (Hou-Yu et al., 1986). In experiments involving oxytocin application, all cells responded to the peptide. After parturition, all cells recorded under control conditions were neurosteroid insensitive (Brussaard et al., 1997), possibly indicative of an oxytocinergic nature. Adult female WT and α1 −/− mice, as well as juvenile male (21–24 d postnatal) or adult female Wistar rats, either after 20 d of pregnancy (P20), or on postparturition day 1 (PPD1) were decapitated, and 400-μm-thick coronal hypothalamus slices incorporating the middle portion of the SON were prepared using a Leica (Nussloch, Germany) vibratome slicer. The external solution contained the following (in mm): 125 NaCl, 25 NaHCO3, 3 KCl, 1.2 NaH2PO4·H2O, 2.4 CaCl2·2H2O, 1.3 MgSO4·7H2O 1.3, and 10d(+)-glucose (304 mOsm, carboxygenated in 5% CO2–95% O2, pH 7.4). DNQX and AP-5 (both at 20 μm; Research Biochemicals, Natick, MA) were continuously present. The pipette solution contained the following (in mm): 140.5 CsCl, 10 HEPES, 2 MgATP, and 0.1 GTP (acid free), adjusted to pH 7.2 using CsOH (296 mOsm) and no EGTA or Ca2+ (i.e., “unbuffered” pipette medium), unless mentioned otherwise. Spontaneous GABAergic synaptic currents (sIPSCs) were recorded at room temperature (20°C), unless mentioned otherwise, at a holding potential of −70 mV with an Axopatch 200A amplifier (Axon Instruments, Foster City, CA) in the whole-cell voltage-clamp mode. For recording criteria for selection of cells, see Brussaard et al. (1996). Recorded sIPSCs were not sensitive to TTX or nominal zero extracellular Ca2+, and bicuculline (20 μm; Research Biochemicals) blocked all remaining synaptic activity (Brussaard et al., 1996, 1999). The sIPSCs had a decay that was best fitted with a single-exponential function. Applications were performed via a home-made so-called Y-tube microperfusion system (Brussaard et al., 1996). Oxytocin effects were effectively blocked by a specific oxytocin antagonist,d(CH2)5-OVT (dOVT) (Bachem, Bubendorf, Switzerland). 3α-OH-DHP was obtained from Research Biochemicals, dissolved in DMSO at 10 mm, and further diluted in external solution before application (10 μm). The lipophylicity of this substance prevents accurate estimates of the final concentration of 3α-OH-DHP at the site of action, as has been extensively discussed previously (Brussaard et al., 1997, 1999). Recorded cells were in the second layer of neurons below the slice surface (∼50 μm deep). The effect of 3α-OH-DHP in all recordings was determined during a 3 min period of recording starting 2 min after neurosteroid application. 12-O-Tetradecanoylphorbol-13-acetate (TPA) (PMA is phorbol 12-myristate 13-acetate), okadaic acid, microcystin, and chelerythrine were all from Research Biochemicals; C2-ceramide (N-acetyl-d-erythro-sphingosine) was obtained from Calbiochem (La Jolla, CA). For experiments using okadaic acid, microcystin, and chelerythrine, which were in the pipette solution only, 3α-OH-DHP was applied after 8 min of “pretreatment” recording. TPA and C2-ceramide were applied extracellularly 3 min after the whole-cell configuration was established and after this 8 min pretreatment period 3α-OH-DHP was applied.
Analysis of synaptic currents
Digital detection of sIPSCs, amplitude analysis, and curve-fitting procedures to quantify the decay rate of individual sIPSCs have all been described previously (Brussaard et al., 1996,1997, 1999). Per experiment and per condition, an unimodal lognormal function was fitted to histograms of binned data (either amplitude or decay time constants) obtained from a minimum of 250 sIPSCs per histogram. Average values thus obtained for control–pretreatment conditions and after neurosteroid application were compared using the paired t test. Control and pretreatment conditions of different experiments were compared using the unpaired ttest. In some experiments, nonparametric testing was performed using the Mann–Whitney U test. In all summary figures in which overall changes in sIPSC decay have been quantified, statistical significance has been indicated using *p < 0.05 and **p < 0.01. Data are reported as the mean ± SEM.
Results
Recombinant expression of GABAARs
To compare the sensitivity of α1- versus α2-containing GABAARs we expressed recombinant GABAARs in HEK 293 cells and in oocytes
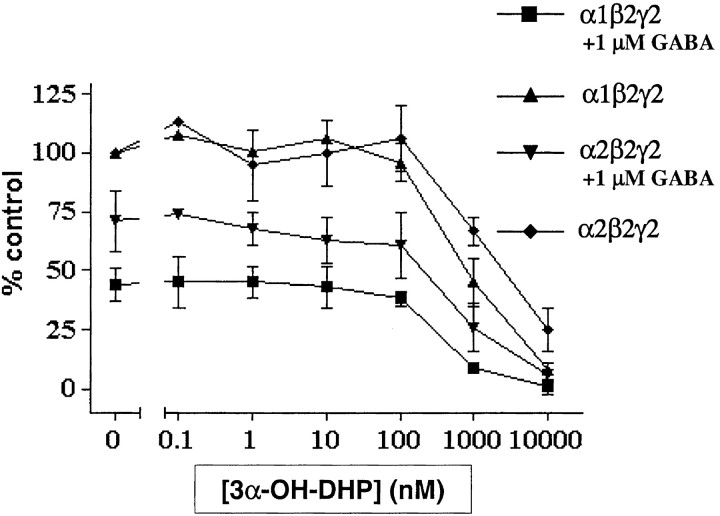
In HEK 293 cells, we found that GABA at 1 μm is slightly more potent in inhibiting the binding of [35S]TBPS to α1β2γ2L than to α2β2γ2L receptors (reduction to 45 ± 7 and 70 ± 13% of control [35S]TBPS binding, respectively) (Fig. 1), which is in line with previous results (Lüddens and Korpi, 1995). In addition, the potency of 3α-OH-DHP to block [35S]TBPS binding differed between the two receptor subtypes in the absence of exogenous GABA (Kd of 820 ± 220 and 2460 ± 160 nm respectively; p = 0.024). The latter result suggests that the affinity of α1-containing GABAARs for allosteric modulation with 3α-OH-DHP is significantly higher than that of α2-containing receptors. However, to study 3α-OH-DHP modulation under more physiological conditions, we also measured the potency of 3α-OH-DHP to block [35S]TBPS binding in the presence of 1 μm exogenous GABA. Under these conditions, no significant difference was observed between the effects of 3α-OH-DHP on the two receptor subtypes (390 ± 110 and 610 ± 260 nm, respectively;p = 0.4) (Fig. 1).
Fig. 1.
Neurosteroid affinity of recombinant GABAARs containing α1 or α2 subunit. Two distinct receptor compositions of the rat GABAAR were tested for their apparent affinity for 3α-OH-DHP using a [35S]TBPS binding assay. 3α-OH-DHP inhibited binding of the radioactive ligand in a concentration-dependent manner. In the absence of GABA (top 2 traces), the apparent affinity for 3α-OH-DHP was significantly higher for α1-containing receptors. However, in the presence of GABA (1 μm;bottom 2 traces), there was no such difference. All data were normalized to the control [35S]TBPS binding in the absence of GABA and 3α-OH-DHP.
To test the effects of 3α-OH-DHP on GABAAR ion channel activity, we expressed the same receptor subtypes inXenopus oocytes. In these tests, no difference was detected between the two receptor subtypes in the potentiation of GABA-induced (10 μm) whole-cell ionic currents by 100 nm 3α-OH-DHP (14-fold increase in both cases;n = 4; data not shown). These data suggest that rat GABAARs containing either the α1 or the α2 subunit are similarly sensitive to the neurosteroid allopregnanolone.
GABAARs in α1 subunit knock-out mice
To conclusively define whether in vivo the α1 subunit does contribute to a higher affinity for neurosteroids than the α2 subunit, the effect of neurosteroids on GABAARs needs to be studied within the context of its subcellular environment (i.e., the postsynaptic density at the GABA synapse) (Kneussel and Betz, 2000). To this end, we studied the effects of allopregnanolone on GABAARs in the dorsomedial part of the SON of WT and α1 knock-out (α1 −/−) mice (Sur et al., 2001).
Real-time PCR analysis of α1 −/− versus WT SON samples showed a complete lack of α1 subunit mRNA and no significant compensatory regulation of any of the other α subunits (Fig.2A). As a result, the relative contribution of α2 and α3 is larger in the SON of α1 −/− animals than in WT animals (Fig. 2B). Hence, in α1 −/− mice, the α2 and α3 subunits may have a prominent role in GABAAR formation. In situwhole-cell voltage-clamp recordings of dorsomedial SON neurons in adult female mice showed that the decay time constant of sIPSCs in α1 −/− mice was 35% longer than in WT mice (Fig.2C,F). This is in line with observations on cerebellar neurons in a different α1 −/− mouse (Vicini et al., 2001). We found that both WT and α1 −/− mouse SON neurons express neurosteroid-sensitive GABAARs, i.e., in both WT and α1 −/− animals, 3α-OH-DHP significantly increased sIPSC decay time constants (Fig.2D,E,G). Thus, in mouse SON neurons, lack of α1 subunit does not reduce the efficacy of allosteric modulation of GABAARs by 3α-OH-DHP.
Fig. 2.
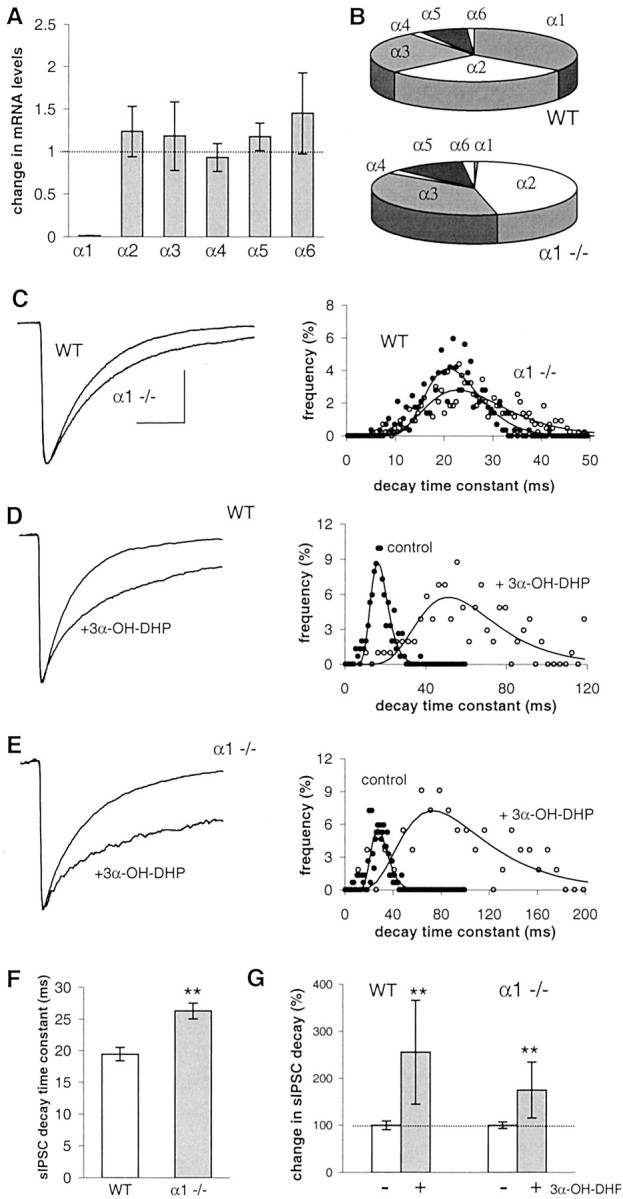
Effect of 3α-OH-DHP in transgenic mice lacking GABAAR α1 subunit. A, α subunit mRNA expression levels in the SON of α1 −/− animals, normalized for the expression of β-actin mRNA and plotted relative to the expression of the WT levels (n = 4). None of the subunits in α1 −/− mice was significantly different from WT. B, Relative contribution of α1 to α6 to the total α subunit mRNA expression in α1 −/− and WT mice. C,Left, Average sIPSCs obtained in SON neurons from WT and α1 −/− mice. Traces are averages of 100 sIPSCs per recording. The decay of sIPSCs was fitted with a single-exponential function. Right, Histogram of the sIPSC decay time constants of experiments shown on the left.D, Averaged sIPSCs recorded from WT in the absence and presence of 3α-OH-DHP and decay time constant histogram showing a significant shift to the right in the presence of 3α-OH-DHP.E, Average sIPSCs in the absence and presence of 3α-OH-DHP and histogram of sIPSC decay from an α1 −/− recording showing a similarly large increase of decay by 3α-OH-DHP.F, Summary graph illustrating difference in sIPSC decay time constants between WT and α1 −/− mice (WT, 19.5 ± 1.1 msec, n = 14; α1 −/−, 26.3 ± 1.3 msec,n = 15; p < 0.01; unpairedt test). G, Summary graph showing 3α-OH-DHP effects on the decay time constants (WT mice, significant effect in 6 of 6 cells, Mann–Whitney U test,p < 0.01, 256 ± 111% of control; α1 −/− mice, significant effect in 5 of 5 cells, Mann–WhitneyU test, p < 0.01, 181 ± 52% of control). In none of these conditions was the sIPSC amplitude significantly affected (data not shown). All other traces of average sIPSCs were plotted normalized to the control average inC. Calibration (of the control trace), 20 msec, 40 pA.
Neurosteroid sensitivity is dependent on signal transduction pathways
If subunit composition of GABAARs cannot explain the endogenous shift toward neurosteroid insensitivity in the SON, alternatively, signal transduction pathways may play a substantial role. Two changes in signal transduction pathways occur around parturition that can affect GABAARs. First, in contrast to the pregnant stage, at parturition and during lactation, a rise in the intracellular calcium concentration may occur (Lambert et al., 1994) as a result of endogenous oxytocin release (Neumann et al., 1995). This may have numerous effects on postsynaptic signaling routes targeted to GABAARs, which possibly involve Ca2+-dependent kinases and phosphatases (De Koninck and Mody, 1996). For instance, PKC was shown to affect GABAAR activity in SON cells after parturition (Brussaard et al., 2000). Second, the role of serine/threonine (Ser/Thr) phosphatases 1, 2A, and 2B (Price and Mumby, 1999), which are logical counterparts of PKC in regulating GABAAR properties, may be larger during pregnancy than after parturition.
To address the first issue, we manipulated the free intracellular calcium concentration and tested the effect on neurosteroid sensitivity. Next, given the second issue, we also manipulated endogenous Ser/Thr phosphatase activity to test a presumed prerequisitional role of this activity for conditional neurosteroid sensitivity of GABAARs.
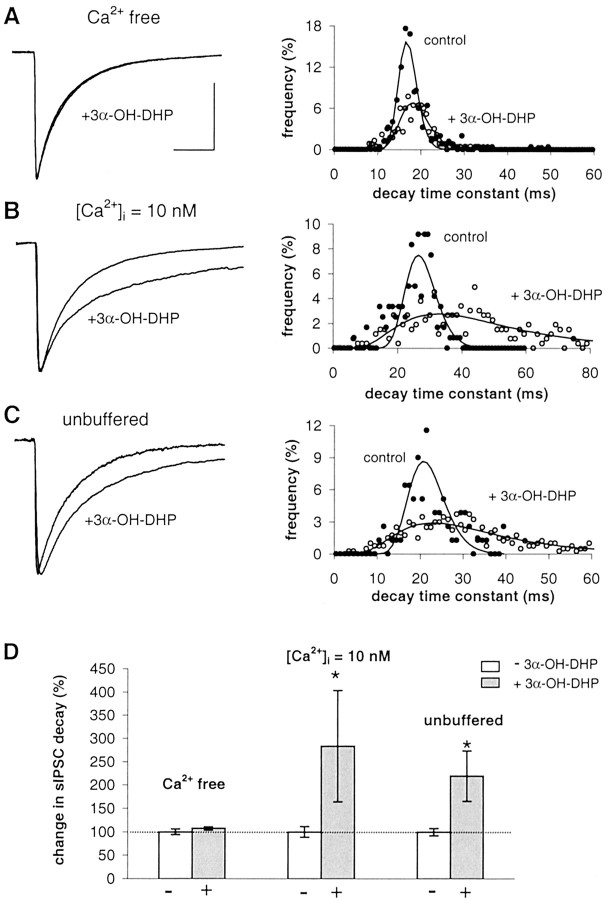
The role of Ca2+ was studied in juvenile (i.e., nonreproductive) male rats. For ethical reasons, we did not use reproductive adult rats in these initial experiments. We titrated the free intracellular calcium concentration, [Ca2+ ]i, to desired values by altering the concentrations of EGTA/Ca2+ ratio in the pipette solution. When recording from dorsomedial SON neurons, in the absence of exogenous Ca2+ buffer in the pipette (i.e., the unbuffered condition: 0 mm EGTA, 0 mm Ca2+), the average decay time constant of sIPSCs was 18.9 ± 1.5 msec, and 3α-OH-DHP significantly increased the sIPSC decay time constant (Fig.3C,D). Remarkably, when [Ca2+]i was nominal zero by adding 1 mm EGTA (0 mm Ca2+), which by itself did not affect the decay of sIPSC, there was no effect of 3α-OH-DHP on the decay time of IPSCs (Fig.3A,D). In contrast, when [Ca2+]i was set at 10 nm (11 mm EGTA, 1 mm Ca2+), 3α-OH-DHP strongly increased sIPSC decay time constants (Fig.3B,D). Also, in the latter condition, the sIPSC decay before application of 3α-OH-DHP was not different from control recordings in the unbuffered recording condition. Thus, neurosteroid sensitivity of GABAARs in SON neurons appeared to be dependent on [Ca2+]i.
Fig. 3.
Effect of 3α-OH-DHP is dependent on [Ca2+]i in juvenile male rats (postnatal days 21–27). A, Left, Superimposed average sIPSCs obtained in SON neurons with [Ca2+]i at nominal zero before and after 3α-OH-DHP application. Right, sIPSCs decay time constant histogram of this experiment. B, Average sIPSCs obtained in [Ca2+]i held at 10 nm in the absence and presence of 3α-OH-DHP and histogram of sIPSC decay time constants. C, Average sIPSCs recorded using unbuffered [Ca2+]i in the absence and presence of 3α-OH-DHP and histogram of the sIPSC decay time constants. D, Summary graph illustrating the relative effects of 3α-OH-DHP on sIPSC decay time constants at three dissimilar Ca2+ concentrations. Decay plotted as percentage of control values. White bars, Controls;gray bars, 3α-OH-DHP. Relative neurosteroid effects are as follows: [Ca2+]i at nominal zero, 108 ± 3% of control, p > 0.05,n = 5; [Ca2+]i at 10 nm, 284 ± 119% of control, p< 0.05, n = 6; unbuffered [Ca2+]i, 220 ± 54% of control, p < 0.05, n = 7. In none of these conditions was the sIPSC amplitude significantly affected (data not shown). All other traces of average sIPSCs were plotted normalized to the control average in A. Calibration (of the control trace), 20 msec, 100 pA.
Next, we recorded from SON neurons in nonreproductive rats while Ser/Thr phosphatases were blocked using okadaic acid and microcystin (specific blockers of phosphatases 1 and 2A) in the pipette solution. These two drugs had no effect on decay time constants by themselves. Unlike the endogenous condition of GABAARs at this stage (Fig. 4A), okadaic acid (50 nm) and microcystin (100 nm) rendered GABAARs insensitive to subsequent neurosteroid treatment (Fig.4B,C,E). This implies that basal levels of Ser/Thr phosphatase activity are of crucial importance for modulation of GABAARs by 3α-OH-DHP. In contrast, when PKC was blocked using the specific PKC inhibitor chelerythrine (1 μm) in the pipette, sIPSC decay time constants were increased during neurosteroid application to the same extent as in the control condition (Fig.4D,E). PKC inhibition did not reduce neurosteroid sensitivity at physiological temperature (33°C) either (data not shown; decay time 252 ± 58% of control;n = 8).
Fig. 4.
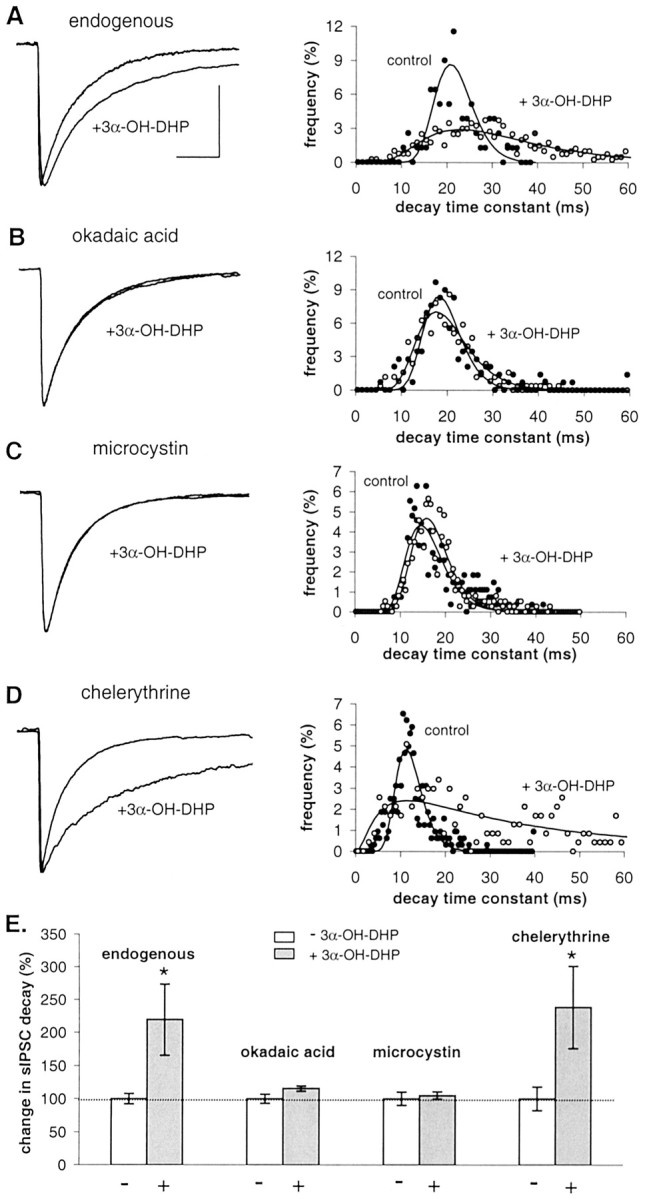
Effect of 3α-OH-DHP is dependent on Ser/Thr phosphatase activity in juvenile male rats (postnatal days 21–27). B, C, Average sIPSCs in the absence and presence of 3α-OH-DHP and decay time constant histogram showing that inhibition of phosphatase activity by 50 nm okadaic acid (A) or 100 nm microcystin (B) prevents the effect of 3α-OH-DHP. D, Average sIPSCs and histogram of sIPSC decay time constants obtained in the presence of 1 μm chelerythrine showing that PKC inhibition does not prevent the effect of 3α-OH-DHP. E, Summary graph illustrating the relative effects of 3α-OH-DHP on sIPSC decay time constants in the four experimental conditions. Decay plotted as percentage of control values. White bars, Controls;gray bars, 3α-OH-DHP. Relative neurosteroid effects are as follows: endogenous condition, 220 ± 54% of control,p < 0.05, n = 7; 50 nm okadaic acid, 115 ± 4% of control,p > 0.05, n = 7; 100 nm microcystin, 105 ± 6% of control,p > 0.05, n = 5; 1 μm chelerythrine, 239 ± 63% of control,p < 0.05, n = 5. Okadaic acid (104 ± 3%; n = 7), microcystin (97 ± 2%; n = 10), and chelerythrine (99 ± 8%;n = 5) by themselves had no effect on sIPSC decay (unpaired t test; p > 0.05). In none of these conditions was the sIPSC amplitude significantly affected (data not shown). All other traces of average sIPSCs were plotted normalized to the control average in A. Calibration (of the control trace), 20 msec, 100 pA.
Ser/Thr phosphatases determine neurosteroid sensitivity during late pregnancy
Because GABAARs at the juvenile stage become insensitive to 3α-OH-DHP when endogenous Ser/Thr phosphatases were blocked, we hypothesized that a relative shift toward more phosphorylation would also reduce neurosteroid responsiveness of GABAARs in the adult rat SON during parturition.
To test this, we first mimicked such a shift at P20 by inhibiting phosphatase activity (Fig. 5). GABAARs at P20 are very sensitive to neurosteroids when using unbuffered pipette medium, as shown by the 196 ± 31% increase in decay time constant induced by 3α-OH-DHP (Fig. 5A,D), which is in line with previous results (Brussaard et al., 1997). This effect was completely absent during a pretreatment with 100 nm okadaic acid (n = 6) (Fig.5B,D). The efficacy of this treatment was dose dependent, i.e., 50 nm okadaic acid reduced the effect of 3α-OH-DHP in only three of six cells (data not shown). Thus, at late pregnancy, as in juveniles, the constitutive activity of endogenous phosphatases is a prerequisite for neurosteroid sensitivity of GABAARs.
Fig. 5.
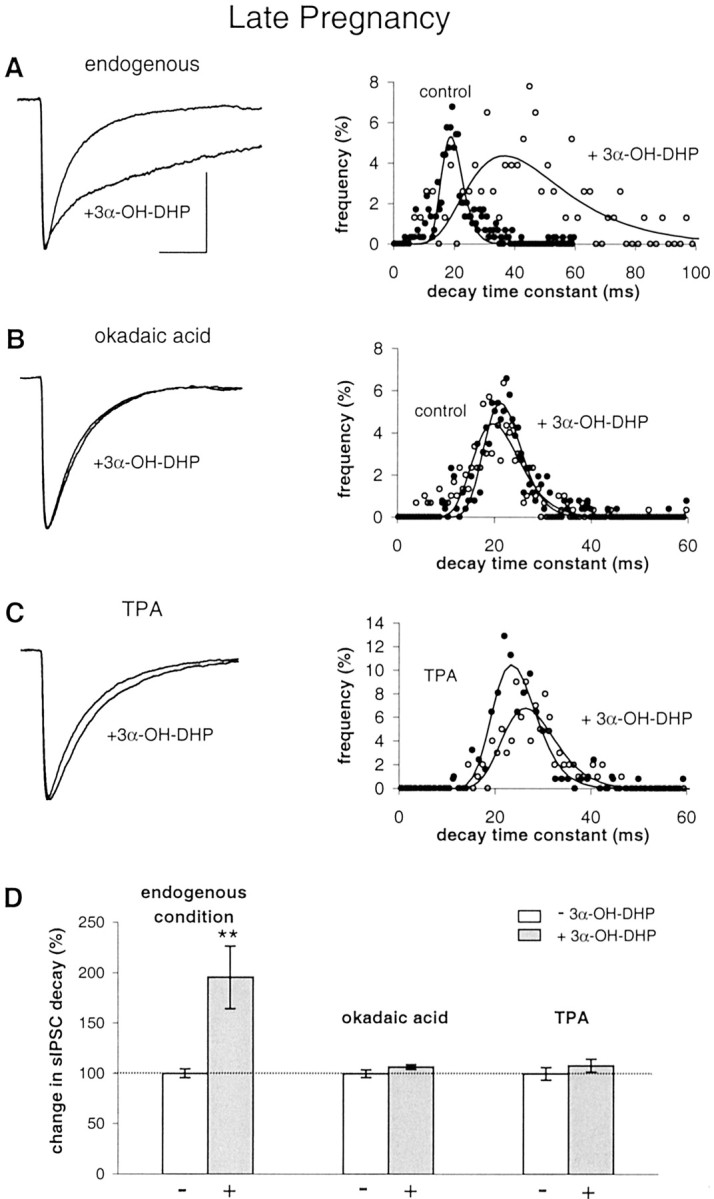
Experimental induction of neurosteroid resistance at late pregnancy. A, Left, Average sIPSCs obtained in SON neurons at P20 showing a large 3α-OH-DHP effect under endogenous conditions using unbuffered pipette medium.Right, Histogram of the sIPSC decay time constants of experiment shown on the left. B, Average sIPSCs in the absence and presence of 3α-OH-DHP and histogram showing that phosphatase inhibition prevents 3α-OH-DHP effect on sIPSC decay.C, Average sIPSCs in the absence and presence of 3α-OH-DHP and histogram showing that PKC activation also prevents 3α-OH-DHP effect on sIPSC decay. D, Summary graph illustrating the relative effects of 3α-OH-DHP on sIPSC decay time constants under the endogenous condition (to 196 ± 31% of control; p < 0.01; n = 6), after phosphatase inhibition (107 ± 2%; p > 0.05; n = 6), and after PKC activation (108 ± 6% compared with during TPA pretreatment; p > 0.05; n = 7). TPA application did not affect sIPSC decay itself (104 ± 3% of control; p > 0.05; n = 6). However, it did significantly suppress the average sIPSC amplitude to 65 ± 9% of control values (p < 0.05; n = 6), which corresponds to previous findings in this cell system (Brussaard et al., 2000). sIPSC decay was not different in okadaic acid (n = 6) compared with in experiments without the drug (n = 22; 108 ± 2%; unpairedt test; p > 0.05). All other traces of average sIPSCs were plotted normalized to the control average inA. Calibration (of the control trace), 20 msec, 100 pA.
Subsequently, we tested whether, at P20, 3α-OH-DHP insensitivity of GABAARs could also be induced by boosting endogenous PKC activity instead of by suppressing phosphatase activity. To this end, we used the phorbol ester TPA, which was applied to the outside of the cell 3 min after the whole-cell configuration was established. TPA (25 nm) by itself induced a reduction of the average sIPSC amplitude to 65 ± 9% of control, in line with previous findings (Brussaard et al., 2000), but had no effect on the sIPSC decay rate. More importantly, 3α-OH-DHP no longer affected sIPSC decay time constants after 8 min of TPA pretreatment, showing also that an increase in endogenous phosphorylation activity renders GABAARs insensitive to neurosteroids (Fig.5C,D).
Neurosteroid-insensitive mode of GABAARs during parturition
Our next step was to test whether, after parturition, stimulating dephosphorylation would restore 3α-OH-DHP sensitivity of GABAARs, to show that the determinant mechanism for neurosteroid effects on GABAARs is not endogenous shifts in subunit composition but rather a conditional alteration in signal transduction pathways. At PPD1, the decay of the sIPSCs was 24.6 ± 1.1 msec (n = 16) compared with 19.8 ± 0.8 msec (n = 22) at P20 (p < 0.01; unpaired t test with Welch correction). This is in line with previous work and is explained by the idea that at parturition a subunit switch from α1 to α2 occurs (Brussaard et al., 1997).
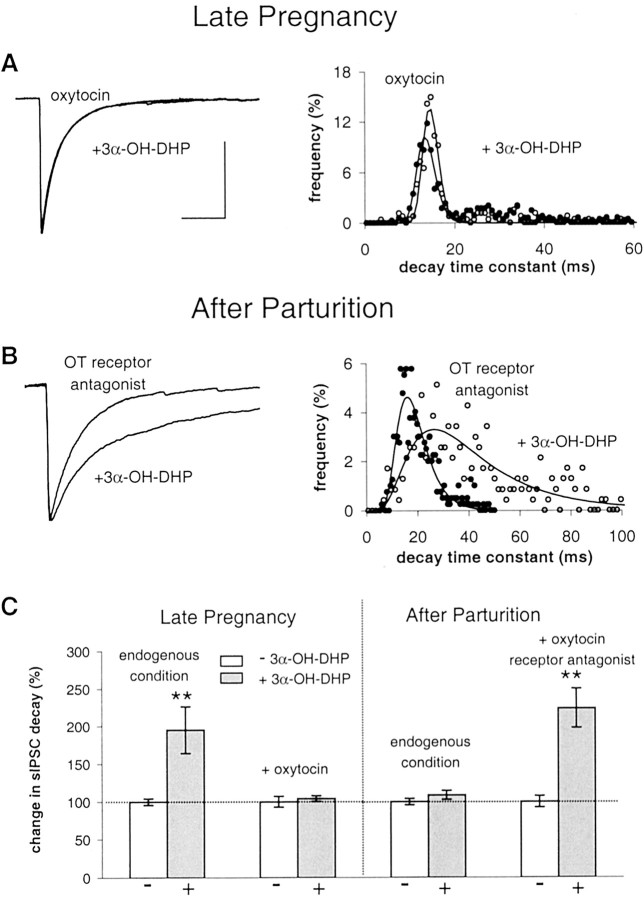
Treatment with 3α-OH-DHP had no effect on sIPSC decay at PPD1 (Fig.6A,E) (in line with Brussaard et al., 1997). To test whether we could restore neurosteroid sensitivity, we first stimulated endogenous Ser/Thr phosphatases in SON neurons with C2-ceramide (10 μm), an activator of phosphatases 2A and 1 (Yang, 2000). Before 3α-OH-DHP treatment, we applied C2-ceramide for 8 min to allow dephosphorylation to take place at GABA synapses. There was no effect of C2-ceramide on IPSC decay. However, 3α-OH-DHP did potentiate GABA current decay after C2-ceramide pretreatment, as shown by an increase to 190 ± 45% of control values (Fig.6B,E). Thus, by activation of endogenous phosphatases in the SON after parturition, the GABAAR responsiveness to 3α-OH-DHP can be restored.
Fig. 6.
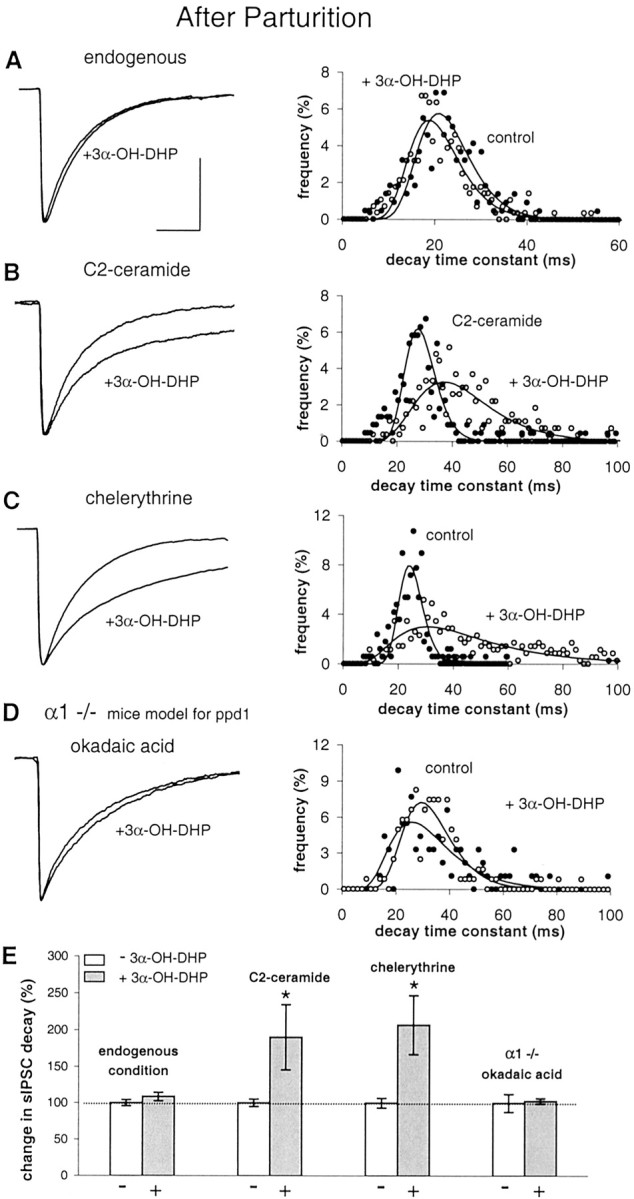
Experimental rescue of neurosteroid sensitivity after parturition. A, Left, Average sIPSCs obtained in SON neurons at PPD1 showing no effect of 3α-OH-DHP under endogenous conditions. Right, Histogram of the sIPSC decay time constants of this experiment.Filled circles are used for controls, and open circles are for 3α-OH-DHP. B, Average sIPSCs in the absence and presence of 3α-OH-DHP and decay time constant histogram showing shift to the right with 3α-OH-DHP after activation of phosphatases by C2-ceramide. C, Average sIPSCs in the absence and presence of 3α-OH-DHP and histogram showing that PKC inactivation by chelerythrine rescues 3α-OH-DHP effect.D, Recordings in α1 −/− mice as a model for the postpartum stage in rats. Average sIPSCs and histogram from experiment with okadaic acid showing that 3α-OH-DHP sensitivity (compare with Fig. 2E) is dependent on dephosphorylation. E, Summary graph illustrating the relative effects of 3α-OH-DHP on IPSC decay time constants under the endogenous condition (109 ± 6% of control; p > 0.05; n = 7), after activation of phosphatases (190 ± 45% compared with during C2-ceramide pretreatment; p < 0.05;n = 9), and after PKC inhibition (207 ± 40% of control; p < 0.05; n = 7). In α1 −/− mice neurons treated with okadaic acid, the neurosteroid effect on average was 103 ± 4% of controls (p > 0.05; in 4 of 4 cells). We observed no significant effect on the sIPSC decay of C2-ceramide (104 ± 2% of control; p > 0.05; n = 9). The sIPSC amplitude was unaffected by chelerythrine [275 ± 36 pA endogenous condition (n = 21), 320 ± 64 pA in chelerythrine (n = 7); p > 0.05]. The latter indicates that the constitutive release of OT within the SON in not high enough to induce a suppression of the sIPSC amplitude as was previously reported to occur with high concentrations of synthetic OT (Brussaard et al., 1996). sIPSC decay was not different in chelerythrine (n = 7) compared with in experiments without the drug (n = 16) (unpaired t test; p > 0.05). All sIPSC traces were normalized to the control amplitude level. In none of these conditions was the sIPSC amplitude significantly affected (data not shown). All other traces of average sIPSCs were plotted normalized to the control average in A. Calibration (of the control trace), 20 msec, 100 pA.
Next, we tested whether, at PPD1, PKC inhibition would also rescue neurosteroid sensitivity. Chelerythrine (1 μm), did not affect sIPSC amplitude or decay but restored GABAAR responsiveness to 3α-OH-DHP (Fig.6C,E), as shown by the 207 ± 40% increase in decay time constant. This implied that, when PKC is blocked, basal endogenous phosphatase activity suffices to rescue 3α-OH-DHP sensitivity. This indicated that, under physiological conditions, an increased level of phosphorylation activity is the causal factor to determine 3α-OH-DHP insensitivity in the SON during parturition.
Subsequently, we went back to record from α1 −/− mice SON neurons. These mice have a putative receptor subunit composition in the SON (Fig. 2B) that can be compared with that of rats at the postpartum state (Brussaard et al., 1997). However, they do not show neurosteroid resistance and are in fact very sensitive to 3α-OH-DHP (Fig. 2E,G). We argued that, as in rats at the juvenile or adult pregnant stage, also in α1 −/− mice a relatively higher level of endogenous dephosphorylation activity may be responsible for the neurosteroid sensitivity. Hence, we recorded SON neurons in α1 −/− mice while blocking Ser/Thr phosphatases with okadaic acid. This treatment induced GABAAR insensitivity to 3α-OH-DHP (Fig.6D,E). This conclusively showed that, regardless of the GABAAR subunit composition, i.e., with or without α1, it is the balance between phosphorylation and dephosphorylation that determines their sensitivity to neurosteroid.
Oxytocin causes neurosteroid-insensitive state of GABAAR
To find the physiological trigger that caused the shift in the balance between phosphatase and PKC activity at parturition, we tested whether the neurosteroid-resistant mode of GABAARs of oxytocin neurons in the SON is brought about by activation of oxytocin autoreceptors. We applied oxytocin (5 μm) at P20 and the peptidergic receptor antagonist dOVT (4 μm) at PPD1 during an 8 min pretreatment period. Application of OT in experiments on late pregnant rats by itself decreased sIPSC amplitudes as expected (Brussaard et al., 1996, 2000) but had no significant effect on sIPSC decay (data not shown). However, after oxytocin pretreatment, the normally observed effect of 3α-OH-DHP on sIPSCs was prevented (Fig.7A,C). Application of receptor antagonist dOVT during sIPSC recordings in PPD1 by itself had no significant effects on either sIPSC amplitude or decay (Fig. 7 legend). Subsequent application of 3α-OH-DHP increased decay time constants (Fig. 7B), which is in sharp contrast to the endogenous condition of the GABAAR neurosteroid sensitivity at this stage (Fig. 7C). This implied that constitutive OT release within the SON (Leng et al., 1999) after parturition prevents 3α-OH-DHP potentiation of GABA currents via activation of the oxytocin receptor. This effect occurred already at basal extracellular concentrations of OT after parturition and was therefore independent of sIPSC amplitude reduction observed only at higher concentrations of OT (Brussaard et al., 1996).
Fig. 7.
Oxytocin autoregulation renders GABAARs insensitive to neurosteroid. A,Left, Superimposed average sIPSCs before and after 3α-OH-DHP application obtained in SON neurons at P20 pretreated with OT. Right, Decay time constant histogram of this experiment. B, Average sIPSCs in the absence and presence of 3α-OH-DHP and decay time constant histogram showing large 3α-OH-DHP effect at PPD1 after block of oxytocin autoreceptors.C, Summary graph illustrating the dependence of the neurosteroid effect on oxytocin receptor activity. Decay plotted as percentage of controls. White bars, Control; gray bars, 3α-OH-DHP. Notice the differences with the endogenous condition at both stages. After OT pretreatment, 3α-OH-DHP did not affect sIPSC decay time constants (104 ± 5% compared with during OT pretreatment; p > 0.05; n = 6). OT by itself had a small but nonsignificant effect on sIPSC decay time constants (114 ± 7% of control; p > 0.05; n = 8). As expected, OT decreased sIPSC amplitudes (68 ± 13% of control; p < 0.05;n = 8). At PPD1, 3α-OH-DHP increased sIPSC decay time constants after OT antagonist (224 ± 26% compared with during OT antagonist pretreatment; p < 0.05;n = 5). OT antagonist by itself had no effect on sIPSC decay (109 ± 5% of control; p > 0.05;n = 5). OT antagonist had no effect on sIPSC amplitude (96 ± 19%; n = 5). All other traces of average sIPSCs were plotted normalized to the control average in A. Calibration (of the control trace), 20 msec, 100 pA.
Discussion
We propose that the naturally occurring reduction in neurosteroid sensitivity of the GABAAR in SON neurons after parturition is brought about by increased levels of phosphorylation and not, as we proposed previously (Brussaard and Herbison, 2000), by subunit composition of the GABAAR. Our data indicate that the extent of oxytocin receptor activation is the main determinant of PKC activity and GABAAR sensitivity to allopregnanolone in the SON. In lactating females that normally have high oxytocin levels and allopregnanolone-resistant GABAARs, we were able to restore neurosteroid sensitivity by pretreating the SON with an oxytocin receptor antagonist. Conversely, before parturition, when oxytocin levels in the SON are low (Leng et al., 1999) and GABAARs are sensitive to neurosteroid (Brussaard et al., 1997), application of oxytocin rendered GABAARs completely insensitive to allopregnanolone within minutes. These results are fully consistent with the functional significance of GABAAR plasticity in the oxytocin neuron (Brussaard and Herbison, 2000).
Neurosteroid sensitivity of GABAARs in the SON does not depend on subunit composition
Previously, it was reported that α1-containing GABAARs are sixfold to 10-fold more sensitive to allopregnanolone than α2-containing receptors in oocytes (Belelli et al., 1996). However, neurosteroids do bind with high specificity to the α2 subunit (Rick et al., 1998). Similarly, inclusion of α6 appeared to increase neurosteroid sensitivity (Hauser et al., 1995), whereas the α4 subunit is associated with a decrease in both neurosteroid and benzodiazepine sensitivity (Smith et al., 1998a,b). In addition, the reduced sensitivity of native GABAARs to neurosteroids during neonatal development of granule neurons in culture was partly attributed to a increase of δ subunit expression (Zhu et al., 1996). Also, our group has put forward the idea of subunit composition dependence of GABAAR neurosteroid sensitivity (Brussaard and Herbison, 2000). Nonetheless, at this moment, there is no unifying theory of the involvement of GABAAR subunit composition to explain the physiological regulation of allosteric receptor modulation by neurosteroids. Here we exclude a role for α1–α2 subunit switching, which is known to occur in the SON after parturition and which correlates with a transient decrease in receptor neurosteroid sensitivity (Brussaard et al., 1999). Our measurements in α1 knock-out animals show that receptor subunit switching of native receptors is very unlikely to be the direct cause of a reduction in neurosteroid sensitivity of the GABAAR in SON neurons.
Phosphorylation events determine neurosteroid sensitivity of GABAARs in the SON
Most likely the sensitivity of GABAARs to allopregnanolone in SON neurons during late pregnancy is brought about by a constitutive high level of endogenous phosphatase activity. Vice versa, after parturition, when GABAARs are insensitive to neurosteroids, inhibition of PKC or stimulation of endogenous phosphatases restored allopregnanolone sensitivity. Moreover, a constitutive level of oxytocin appears to be maintained under basal conditions at this stage, because blocking putative oxytocin effects with an antagonist readily brought about the normal sensitivity of GABAARs to allopregnanolone.
This implies that phosphatases and PKC may have converging effects, possibly acting on the same Ser/Thr residue of the β2, β3, or γ2 GABAAR subunit (McDonald and Moss, 1997). Alternatively, PKC and phosphatases may act on different phosphorylation sites or even on different proteins of the postsynaptic density of the GABA synapse (Kneussel and Betz, 2000), including the GABAAR-interacting protein RACK-1 (receptor for activated C kinase-1) (Brandon et al., 1999; Feng et al., 2001). In addition, phosphatase-targeting proteins like spinophilin (Hsieh-Wilson et al., 1999) or the scaffold protein AKAP79 (A kinase anchor protein 79) that binds both PKC and phosphatase 2B (Klauck et al., 1996) may be involved in the local regulation of phosphorylation cascades.
The putative role of the above proteins in or near the postsynaptic density may explain the discrepancy between the regulation of allopregnanolone sensitivity described in native synapses and of GABAARs expressed in oocytes (Leidenheimer and Chapell, 1997). In addition, in oocyte experiments, the effect of neurosteroids is on slow desensitization of GABAAR activity rather than on ion channel mechanisms involved in the decay of synaptic currents, i.e., receptor deactivation and fast desensitization (Jones and Westbrook, 1995). Because fast desensitization of the postsynaptic GABAAR channels can keep the receptor protein in an agonist-bound state, thereby slowing the rate of final ion channel closure during the synaptic decay, it is very likely that neurosteroids slow the recovery of the GABAAR from the desensitized state and in this way prolong synaptic current decays (Zhu and Vicini, 1997). Hence, we cannot exclude that distinct neurosteroid effects on GABAARs do occur that are different in their sensitivity to endogenous shifts in the balance of phosphorylation.
In addition to a putative phosphorylation-dependent regulation of the effect of an allosteric modulator, direct effects of phosphorylation of residues of β and γ subunit on desensitization properties of GABAAR channels have been reported previously (Krishek et al., 1994). Decay time constants of sIPSCs may be affected by alterations in protein kinase or phosphatase activity, such as reported for hippocampal neurons (Jones and Westbrook, 1997; Poisbeau et al., 1999).
Specificity of experimental interference with balance of PKC and phosphatase activity
It was proposed previously that PKC activation rather than PKC inhibition is required for allopregnanolone potentiation of the synaptic currents in the juvenile SON (Fancsik et al., 2000). However, we would argue that these previous experiments depended to some extent on the specificity of one PKC inhibitor, bisindolylmaleimide. This substance enhanced sIPSC decay already in the absence of neurosteroid (132.5% of control) (Fancsik et al., 2000), complicating the interpretation of the effect of subsequent neurosteroid treatment. We also observed that bisindolylmaleimide at a concentration of 500 nm, in the absence of neurosteroid, significantly potentiates the sIPSC decay (data not shown).
Instead, we used chelerythrine (1 μm), which did not affect the sIPSC decay in SON neurons by itself. In the neurosteroid-resistant condition after parturition, PKC inhibition by chelerythrine clearly shifted the GABAARs toward the neurosteroid-sensitive mode (Fig. 6). In contrast, in neurosteroid-sensitive nonreproductive animals, chelerythrine did not reduce neurosteroid effects, at neither room temperature (Fig. 4) nor 33°C (Fig. 4 legend).
To further substantiate our findings that PKC inhibition, rather than activation, renders GABAARs in the SON sensitive to allopregnanolone, we also performed the reverse experiment and blocked endogenous phosphatase activity in both juvenile and late pregnant rats. Again, we first tested the specificity of the phosphatase inhibitors. Both okadaic acid (50–100 nm) and microcystin (100 nm) did not affect the sIPSC decay in the absence of neurosteroid, whereas at high concentrations such blockers might affect GABA currents in other brain regions (Nusser et al., 2001). Subsequently, as expected from the PKC inhibition results, we found that, before parturition, both inhibitors shifted the neurosteroid-sensitive GABAARs toward the neurosteroid-insensitive mode (Figs. 4, 5). Moreover, activation of endogenous phosphatase activity after parturition, using C2-ceramide, rescued neurosteroid sensitivity of GABAARs after parturition (Fig. 6).
A model of regulation of GABAAR activity before and after parturition
During pregnancy, allopregnanolone levels are high (Concas et al., 1998), there is little oxytocin secretion (Leng et al., 1999), and basal oxytocin levels are low attributable to significant breakdown by aminopeptidases (Kombian et al., 1997). GABAARs at that stage are occupied by endogenous allopregnanolone, which results in a slower decay of synaptic current responses (Brussaard et al., 1997), and prevents putative PKC modulation (Brussaard et al., 2000). Intracellular [Ca2+] under these conditions is ∼43 nm (Lambert et al., 1994), which apparently favors endogenous phosphatase activity over PKC activity (this report). Thus, all conditions in the SON during pregnancy are aimed at keeping oxytocin neurons silent.
Before parturition, allopregnanolone levels abruptly drop (Concas et al., 1998), and the somatodendritic release of oxytocin goes up dramatically. Oxytocin receptor activation then results in increased PKC activity attributable to activation of the phospholipase C–IP3–DAG pathway, and release from allopregnanolone modulation will allow GABAARs to be influenced by this PKC activity (Brussaard et al., 2000). Moreover, an additional increase in [Ca2+] may be expected from the increase in firing rate and the concomitant transient calcium flux via voltage-gated calcium channels. Thus, altered conditions around parturition allow the SON system to be relieved from inhibition by at least two complementary cellular mechanisms: (1) reduced allosteric GABAAR potentiation attributable to removal of endogenous allopregnanolone, and (2) reduced GABAAR sensitivity toward allopregnanolone attributable to increased phosphorylation at the postsynaptic density.
Physiological significance of allosteric GABAAR modulation
Although the important functions of allopregnanolone become particularly clear during the female reproduction cycle, levels of allopregnanolone are also known to rise transiently in relation to acute stress (Purdy et al., 1991). Neurosteroid modulation of GABAARs has been associated with phenomena such as memory formation, anxiety, sleep, depression, seizure-like activity (Majewska, 1992), postpartum blues (Nappi et al., 2001), and mood swings during the menstrual cycle (Smith et al., 1998a,b). Thus, studying the molecular mechanism underlying regulation of neurosteroid sensitivity of postsynaptic GABAARs is important and may lead to the further development of neurosteroid-related compounds for use as improved anxiolytic drugs with a reduced risk of tolerance as an alternative to benzodiazepines (Rupprecht and Holsboer, 1999). In addition, GABAAR susceptibility to ethanol is altered in transgenic mice lacking particular PKC isomers (Hodge et al., 1999). Hence, metabotropic control over allosteric modulation of GABAARs may be a general phenomenon that regulates the efficacy of GABA synapses throughout the brain.
Footnotes
We thank N. Burnashev, W. P. Geraerts (Vrije Universiteit Amsterdam, Amsterdam, The Netherlands), H. D. Mansvelder (Columbia University, New York, NY), K. Wafford, R. McKernan, and P. Whiting (Merck Sharp & Dohme, Harlow, UK) for their comments on previous versions of this manuscript, and J. C. Lodder and T. Buse for technical support. This research was supported by Nederlandse Organisatie voor Wetenschappelijk Onderzoek–Aard en Levenswetenschappen Grant 809.67.011.
Correspondence should be addressed to Arjen Brussaard at the above address. E-mail: brssrd@bio.vu.nl.
R. Zwart's present address: Eli Lilly and Company, Lilly Research Centre, Erl Wood Manor, Sunninghill Road, Windlesham, Surrey, GU20 6PH, UK.
References
- 1.Belelli D, Lambert JJ, Peters JA, Gee KW, Lan NC. Modulation of human recombinant GABAA receptors by pregnanediols. Neuropharmacology. 1996;35:1223–1231. doi: 10.1016/s0028-3908(96)00066-4. [DOI] [PubMed] [Google Scholar]
- 2.Brandon NJ, Uren JM, Kittler JT, Wang H, Olsen R, Parker PJ, Moss SJ. Subunit-specific association of protein kinase C and the receptor for activated C kinase with GABA type A receptors. J Neurosci. 1999;19:9228–9234. doi: 10.1523/JNEUROSCI.19-21-09228.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brussaard AB, Herbison AE. Long-term plasticity of postsynaptic GABAA-receptor function in the adult brain: insights from the oxytocin neurone. Trends Neurosci. 2000;23:190–195. doi: 10.1016/s0166-2236(99)01540-4. [DOI] [PubMed] [Google Scholar]
- 4.Brussaard AB, Kits KS, de Vlieger TA. Postsynaptic mechanism of depression of GABAergic synapses by oxytocin in the supraoptic nucleus of immature rat. J Physiol (Lond) 1996;497:495–507. doi: 10.1113/jphysiol.1996.sp021783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brussaard AB, Kits KS, Baker RE, Willems WP, Leyting-Vermeulen JW, Voorn P, Smit AB, Bicknell RJ, Herbison AE. Plasticity in fast synaptic inhibition of adult oxytocin neurons caused by switch in GABA(A) receptor subunit expression. Neuron. 1997;19:1103–1114. doi: 10.1016/s0896-6273(00)80401-8. [DOI] [PubMed] [Google Scholar]
- 6.Brussaard AB, Devay P, Leyting-Vermeulen JL, Kits KS. Changes in properties and neurosteroid regulation of GABAergic synapses in the supraoptic nucleus during the mammalian female reproductive cycle. J Physiol (Lond) 1999;516:513–524. doi: 10.1111/j.1469-7793.1999.0513v.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brussaard AB, Wossink J, Lodder JC, Kits KS. Progesterone-metabolite prevents protein kinase C-dependent modulation of gamma-aminobutyric acid type A receptors in oxytocin neurons. Proc Natl Acad Sci USA. 2000;97:3625–3630. doi: 10.1073/pnas.050424697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Concas A, Mostallino MC, Porcu P, Follesa P, Barbaccia ML, Trabucchi M, Purdy RH, Grisenti P, Biggio G. Role of brain allopregnanolone in the plasticity of gamma-aminobutyric acid type A receptor in rat brain during pregnancy and after delivery. Proc Natl Acad Sci USA. 1998;95:13284–13289. doi: 10.1073/pnas.95.22.13284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cooper EJ, Johnston GAR, Edwards FA. Effects of a naturally occurring neurosteroid on GABAA IPSCs during development in rat hippocampal or cerebellar slices. J Physiol (Lond) 1999;521:437–449. doi: 10.1111/j.1469-7793.1999.00437.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davies PA, Hanna MC, Hales TG, Kirkness EF. Insensitivity to anaesthetic agents conferred by a class of GABA(A) receptor subunit. Nature. 1997;385:820–823. doi: 10.1038/385820a0. [DOI] [PubMed] [Google Scholar]
- 11.De Koninck Y, Mody I. The effects of raising intracellular calcium on synaptic GABAA receptor-channels. Neuropharmacology. 1996;35:1365–1374. doi: 10.1016/s0028-3908(96)00063-9. [DOI] [PubMed] [Google Scholar]
- 12.Fancsik A, Linn DM, Tasker JG. Neurosteroid modulation of GABA IPSCs is phosphorylation dependent. J Neurosci. 2000;20:3067–3075. doi: 10.1523/JNEUROSCI.20-09-03067.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feng J, Cai X, Zhao J, Yan Z. Serotonin receptors modulate GABA(A) receptor channels through activation of anchored protein kinase C in prefrontal cortical neurons. J Neurosci. 2001;21:6502–6511. doi: 10.1523/JNEUROSCI.21-17-06502.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harrison NL, Vicini S, Barker JL. A steroid anesthetic prolongs inhibitory postsynaptic currents in cultured rat hippocampal neurons. J Neurosci. 1987;7:604–609. doi: 10.1523/JNEUROSCI.07-02-00604.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hauser CA, Chesnoy-Marchais D, Robel P, Baulieu EE. Modulation of recombinant alpha 6 beta 2 gamma 2 GABAA receptors by neuroactive steroids. Eur J Pharmacol. 1995;289:249–257. doi: 10.1016/0922-4106(95)90101-9. [DOI] [PubMed] [Google Scholar]
- 16.Hodge CW, Mehmert KK, Kelley SP, McMahon T, Haywood A, Olive MF, Wang D, Sanchez-Perez AM, Messing RO. Supersensitivity to allosteric GABA(A) receptor modulators and alcohol in mice lacking PKC epsilon. Nat Neurosci. 1999;2:997–1002. doi: 10.1038/14795. [DOI] [PubMed] [Google Scholar]
- 17.Hou-Yu A, Lamme AT, Zimmerman EA, Silverman AJ. Comparative distribution of vasopressin and oxytocin neurons in the rat brain using a double-label procedure. Neuroendocrinology. 1986;44:235–246. doi: 10.1159/000124651. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh-Wilson LC, Allen PB, Watanabe T, Nairn AC, Greengard P. Characterization of the neuronal targeting protein spinophilin and its interactions with protein phosphatase-1. Biochemistry. 1999;38:4365–4373. doi: 10.1021/bi982900m. [DOI] [PubMed] [Google Scholar]
- 19.Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–191. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- 20.Jones MV, Westbrook GL. Shaping of IPSCs by endogenous calcineurin activity. J Neurosci. 1997;17:7626–7633. doi: 10.1523/JNEUROSCI.17-20-07626.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klauck TM, Faux MC, Labudda K, Langeberg LK, Jaken S, Scott JD. Coordination of three signaling enzymes by AKAP79, a mammalian scaffold protein. Science. 1996;271:1589–1592. doi: 10.1126/science.271.5255.1589. [DOI] [PubMed] [Google Scholar]
- 22.Kneussel M, Betz H. Clustering of inhibitory neurotransmitter receptors at developing postsynaptic sites: the membrane activation model. Trends Neurosci. 2000;23:429–435. doi: 10.1016/s0166-2236(00)01627-1. [DOI] [PubMed] [Google Scholar]
- 23.Kombian SB, Mouginot D, Pittman QJ. Dendritically released peptides act as retrograde modulators of afferent excitation in the supraoptic nucleus in vitro. Neuron. 1997;19:903–912. doi: 10.1016/s0896-6273(00)80971-x. [DOI] [PubMed] [Google Scholar]
- 24.Krishek BJ, Xie X, Blackstone C, Huganir RL, Moss SJ, Smart TG. Regulation of GABAA receptor function by protein kinase C phosphorylation. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 25.Lambert JJ, Belelli D, Hill-Venning C, Peters JA. Neurosteroids and GABAA receptor function. Trends Pharmacol Sci. 1995;16:295–303. doi: 10.1016/s0165-6147(00)89058-6. [DOI] [PubMed] [Google Scholar]
- 26.Lambert RC, Dayanithi G, Moos FC, Richard PH. A rise in the intracellular Ca2+ concentration of isolated rat supraoptic cells in response to oxytocin. J Physiol (Lond) 1994;478:275–288. doi: 10.1113/jphysiol.1994.sp020249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leidenheimer NJ, Chapell R. Effects of PKC activation and receptor desensitization on neurosteroid modulation of GABA(A) receptors. Brain Res Mol Brain Res. 1997;52:173–181. doi: 10.1016/s0169-328x(97)00255-6. [DOI] [PubMed] [Google Scholar]
- 28.Leng G, Brown CH, Russell JA. Physiological pathways regulating the activity of magnocellular neurosecretory cells. Prog Neurobiol. 1999;57:625–655. doi: 10.1016/s0301-0082(98)00072-0. [DOI] [PubMed] [Google Scholar]
- 29.Lüddens H, Korpi ER. GABA antagonists differentiate between recombinant GABAA/benzodiazepine receptor subtypes. J Neurosci. 1995;15:6957–6962. doi: 10.1523/JNEUROSCI.15-10-06957.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Majewska MD. Neurosteroids: endogenous bimodal modulators of the GABAA receptor. Mechanism of action and physiological significance. Prog Neurobiol. 1992;38:379–385. doi: 10.1016/0301-0082(92)90025-a. [DOI] [PubMed] [Google Scholar]
- 31.McDonald BJ, Moss SJ. Conserved phosphorylation of the intracellular domains of GABA(A) receptor beta2 and beta3 subunits by cAMP-dependent protein kinase, cGMP-dependent protein kinase protein kinase C and Ca2+/calmodulin type II-dependent protein kinase. Neuropharmacology. 1997;36:1377–1385. doi: 10.1016/s0028-3908(97)00111-1. [DOI] [PubMed] [Google Scholar]
- 32.Nappi RE, Petraglia F, Luisi S, Polatti F, Farina C, Genazzani AR. Serum allopregnanolone in women with postpartum “blues.”. Obstet Gynecol. 2001;97:77–80. doi: 10.1016/s0029-7844(00)01112-1. [DOI] [PubMed] [Google Scholar]
- 33.Neumann I, Pittman QJ, Landgraf R. Release of oxytocin within the supraoptic nucleus. Mechanisms, physiological significance and antisense targeting. Adv Exp Med Biol. 1995;395:173–183. [PubMed] [Google Scholar]
- 34.Nusser Z, Naylor D, Mody I. Synapse-specific contribution of the variation of transmitter concentration to the decay of inhibitory postsynaptic currents. Biophys J. 2001;80:1251–1261. doi: 10.1016/S0006-3495(01)76101-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poisbeau P, Cheney MC, Browning MD, Mody I. Modulation of synaptic GABAA receptor function by PKA and PKC in adult hippocampal neurons. J Neurosci. 1999;19:674–683. doi: 10.1523/JNEUROSCI.19-02-00674.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Price NE, Mumby MC. Brain protein ser/thr phosphatases. Curr Opin Neurobiol. 1999;9:336–342. doi: 10.1016/s0959-4388(99)80049-x. [DOI] [PubMed] [Google Scholar]
- 37.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rick CE, Ye Q, Fin SE, Harrison NL. Neurosteroids act on the GABA(A) receptor at sites on the N-terminal side of the middle of TM2. NeuroReport. 1998;9:1–5. doi: 10.1097/00001756-199802160-00004. [DOI] [PubMed] [Google Scholar]
- 39.Rupprecht R, Holsboer F. Neuropsychopharmacological properties of neuroactive steroids. Steroids. 1999;64:83–91. doi: 10.1016/s0039-128x(98)00101-9. [DOI] [PubMed] [Google Scholar]
- 40.Smith SS, Gong QH, Hsu FC, Markowitz RS, Ffrench-Mullen JM, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 41.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu FC. Withdrawal from 3α-OH-3α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sur C, Wafford KA, Reynolds DS, Hadingham KL, Bromidge F, Macaulay A, Collinson N, O'Meara G, Howell O, Newman R, Myers J, Atack JR, Dawson GR, McKernan RM, Whiting PJ, Rosahl TW. Loss of the major GABAA receptor subtype in the brain is not lethal in mice. J Neurosci. 2001;21:3409–3418. doi: 10.1523/JNEUROSCI.21-10-03409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Turner DM, Ransom RW, Yang JS, Olsen RW. Steroid anesthetics and naturally occurring analogs modulate the gamma-aminobutyric acid receptor complex at a site distinct from barbiturates. J Pharmacol Exp Ther. 1989;248:960–966. [PubMed] [Google Scholar]
- 44.Twyman RE, MacDonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vicini S, Ferguson C, Prybylowski K, Kralic J, Morrow AL, Homanics GE. GABA(A) receptor alpha1 subunit deletion prevents developmental changes of inhibitory synaptic currents in cerebellar neurons. J Neurosci. 2001;21:3009–3016. doi: 10.1523/JNEUROSCI.21-09-03009.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yang SN. Ceramide-induced sustained depression of synaptic currents mediated by ionotropic glutamate receptors in the hippocampus: an essential role of postsynaptic protein phosphatases. Neuroscience. 2000;96:253–258. doi: 10.1016/s0306-4522(99)00582-5. [DOI] [PubMed] [Google Scholar]
- 47.Zhu WJ, Vicini S. Neurosteroid prolongs GABAA channel deactivation by altering kinetics of desensitized states. J Neurosci. 1997;17:4022–4031. doi: 10.1523/JNEUROSCI.17-11-04022.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhu WJ, Wang JF, Krueger KE, Vicini S. Delta subunit inhibits neurosteroid modulation of GABAA receptors. J Neurosci. 1996;16:6648–6656. doi: 10.1523/JNEUROSCI.16-21-06648.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zwart R, Vijverberg HP. Potentiation and inhibition of neuronal nicotinic receptors by atropine: competitive and noncompetitive effects. Mol Pharmacol. 1997;52:886–895. doi: 10.1124/mol.52.5.886. [DOI] [PubMed] [Google Scholar]