Abstract
Glutamatergic neurotransmission via AMPA receptors has been an important focus of studies investigating neuronal plasticity. AMPA receptor glutamate receptor 1 (GluR1) subunits play a critical role in long-term potentiation (LTP). Because LTP is thought to be the cellular substrate for learning, we investigated whether mice lacking the GluR1 subunit [gria1 knock-outs (KO)] were capable of learning a simple cue–reward association, and whether such cues were able to influence motivated behavior. Both gria1 KO and wild-type mice learned to associate a light/tone stimulus with food delivery, as evidenced by their approaching the reward after presentation of the cue. During subsequent testing phases,gria1 KO mice also displayed normal approach to the cue in the absence of the reward (Pavlovian approach) and normal enhanced responding for the reward during cue presentations (Pavlovian to instrumental transfer). However, the cue did not act as a reward for learning a new behavior in the KO mice (conditioned reinforcement). This pattern of behavior is similar to that seen with lesions of the basolateral nucleus of the amygdala (BLA), and correspondingly,gria1 KO mice displayed impaired acquisition of responding under a second-order schedule. Thus, mice lacking the GluR1 receptor displayed a specific deficit in conditioned reward, suggesting that GluR1-containing AMPA receptors are important in the synaptic plasticity in the BLA that underlies conditioned reinforcement. Immunostaining for GluR2/3 subunits revealed changes in GluR2/3 expression in the gria1 KOs in the BLA but not the central nucleus of the amygdala (CA), consistent with the behavioral correlates of BLA but not CA function.
Keywords: learning, Pavlovian association, conditioned reinforcement, second order conditioning, Pavlovian to instrumental transfer, Pavlovian approach, AMPA receptor, GluR-A subunit, GluR1 subunit, GluR2 subunit, amygdala
Introduction
The ability of environmental cues associated with rewarding events to influence or maintain behavior in the absence of the primary reward is important in many aspects of motivated behavior. Thus, cues associated with drug taking may play important roles in initiating drug craving or drug seeking in the abstaining addict, and treatments for drug abuse recommend removing the individual from environments associated with drug use (O'Brien et al., 1998). Understanding the neural mechanisms underlying the response of organisms to reward-paired cues is thus of practical as well as theoretical interest.
In rodent models, stimuli paired with food have been shown to influence behavior in a number of different ways. First, cues conditioned to primary rewards are typically approached when presented (Pavlovian approach) (Tomie et al., 1999); second, such cues energize responding for the primary reinforcer, an effect often studied as an enhancement of operant responding for a primary reinforcer [Pavlovian to instrumental transfer (PIT)] (Dickinson, 1994); and third, conditioned cues act as reinforcers in their own right, supporting the acquisition of novel instrumental responses [conditioned reinforcement (CR)] (Mackintosh, 1974). All three properties arise through Pavlovian conditioning and may contribute in different ways to the propensity to seek the primary reward. Nevertheless, the three properties of Pavlovian-conditioned cues can be dissociated in terms of the underlying neural mechanisms on which they depend. Lesions of the basolateral nucleus of the amygdala (BLA) impair responding for conditioned reinforcement but leave conditioned approach and PIT intact (Everitt et al., 2000). Conversely, lesions of the central nucleus of the amygdala (CA) impair conditioned approach and PIT but do not affect responding for conditioned reinforcement (Everitt et al., 2000).
Synaptic plasticity underlying the formation of Pavlovian conditioned associations depends on increased expression and/or redistribution of glutamatergic AMPA receptors (Lledo et al., 1998; Nayak et al., 1998). In the hippocampus, most AMPA receptors are hetero-oligomers composed of glutamate receptor 1/2 (GluR1/2) subunits or GluR2/3 subunits (Shi et al., 2001), with GluR1/2 receptors being added to synapses during plasticity (whereas GluR2/3 are inserted during normal receptor turnover). In keeping with this idea, mice bearing targeted deletions of the gria1 gene (encoding the GluR1 subunit of AMPA receptors) do not show long-term potentiation (LTP) in hippocampal pathways (Zamanillo et al., 1999), whereas conditional restoration ofgria1 is sufficient to reinstate LTP (Mack et al., 2001). In the hippocampus, gria1 knock-outs (KOs) also demonstrate aberrant distribution of GluR2 protein, with reduced levels of GluR2 in dendritic regions, consistent with the impaired insertion of GluR2 subunits into synapses in the absence of coassembly with GluR1 subunits (Zamanillo et al., 1999).
Considerably less is understood about the functional significance of GluR1 subunits in the amygdala. GluR1 subunits are richly represented in amygdala nuclei, although the mechanisms of synaptic plasticity in the amygdala do not always resemble those in the hippocampus and may differ among different amygdala nuclei. In particular, different mechanisms underlie synaptic plasticity in the BLA and CA (Chapman and Chattarji, 2000), so it is likely that different synaptic mechanisms underlie the different properties of conditioning of environmental cues to primary rewards.
Therefore, we have examined the formation of a Pavlovian association, and its consequences for motivated behavior, in mice with targeted deletions of the gria1 gene; we also studied the extent to which such mutations affect the distribution of GluR2 subunits in amygdala nuclei.
Materials and Methods
Animals. gria1 Ko and wild-type (WT) littermates were bred at the University of Sussex from heterozygous parents obtained from P. H. Seeburg (MaxPlanck Institut für Molecular Biologie, Heidelberg, Germany) (Zamanillo et al., 1999). PCR was used to establish the genotype of offspring. Mice were housed two or three to a cage under a 12 hr light/dark schedule (lights on at 7:00 A.M.) and weighed 20–27 gm at the beginning of the experiment. Between five and eight WT and gria1 KO mice were used in each phase of the experiment. Except where specified, food and water were available ad libitum in a room with a controlled temperature (21 ± 2°C) and humidity (50 ± 10%). Initial training, in which the mice learned to associate stimulus presentation with food delivery, was performed overnight in 16 hr sessions. Subsequently, all testing took place during the light phase between 9:00 A.M. and 5:00 P.M. All experiments were approved by the institutional ethics committee and were performed under United Kingdom legislation on animal experimentation (Animal Scientific Procedures Act, 1986).
Pavlovian conditioning. Mice were food-restricted to ∼85% of baseline body weight. During 16 hr sessions, mice were placed into mouse-operant chambers (MedAssociates, Georgia, VT) with the levers removed, and food was delivered at random intervals [mean, 2 min; variable interval (VI)120 sec schedule], preceded by the cue, which consisted of the illumination of two flashing lights (1 Hz) located above each lever, and the onset of a tone (2.9 kHz; 5 dB above background). The cue was presented for 10 sec, and the associated reward, consisting of 0.01 ml of 30% condensed milk solution, was presented for the final 5 sec of this period. Training consisted of 12 sessions, each of which was split into eight 1 hr training blocks with intervening 1 hr pauses. Infrared detectors across the entrance to the food magazine allowed the latency between cue onset and reward retrieval to be measured.
Pavlovian approach. To assess the ability of the cue to elicit conditioned approach, one of the lights was removed from the chamber, and infrared detectors were placed across the entrance to the remaining light, which was relocated beneath the tone source. During a 1 hr test session, the light cue was presented every 2 min for 60 sec, and nose-pokes toward the cue were recorded.
Conditioned reinforcement. To assess the ability of the cue to act as a conditioned reward, two levers were introduced into the operant chambers. Responding on one lever (CR lever) resulted in a brief 1 sec presentation of the cue (cue locations and conditions exactly as during Pavlovian conditioning phase), whereas responding on the alternative lever [nonconditioned reinforcement (NCR) lever] had no consequences. Responses on each lever were recorded during a 15 hr session.
Instrumental responding. A separate group of mice was trained to respond for 15% condensed milk solution on a fixed rate (FR)1 schedule. After acquisition, mice were tested on four additional concentrations of condensed milk for 3 d each. The order of testing was randomized, and all sessions lasted for 180 min.
PIT. To assess PIT, mice were retrained to respond for 30% condensed milk solution on a variable-interval 120 sec schedule, in which the activation of a lever led to the delivery of a reward at random intervals of 120 sec mean duration; the first lever press after the random interval had elapsed produced a reward. This provided a stable, low rate of responding from which to assess the effects of cue presentation. Both WT and KO mice responded at stable rates on this schedule, although the overall rate of responding was higher in KO mice. Subsequently, during a 60 min test session using the VI120 sec schedule, the cue was presented at 300 sec intervals for 60 sec (cue locations and conditions exactly as during the Pavlovian conditioning phase). The rate of responding was then compared during the presence of the cue [conditioned stimulus-positive (CS+)] and the absence of the cue (CS−).
Second-order operant responding. Mice were trained to perform an operant lever-pressing task to obtain 30% condensed milk solution. Initially, food was delivered for each lever press, and then for every 2nd, 4th, and 10th lever press. Each food delivery was preceded and accompanied by presentation of the cue (cue locations and conditions exactly as during the Pavlovian conditioning phase). At this point, the schedule was advanced to a second-order schedule in which every xth lever press resulted in the presentation of the cue (FRx:S); every 10th cue presentation was accompanied by food delivery, a so-called FR10(FRx:S) schedule. Initially, mice were tested on consecutive days with x increasing daily according to the schedule: 1, 2, 4, 8, 16, 32, and 64. Subsequently,x was reduced to 4, and mice were allowed up to 10 sessions to reach a criterion. Animals that succeeded in reaching a criterion of obtaining the first reward within 5 min on each schedule then progressed to an FR10(FR8:S) schedule, subsequently to an FR10(FR16:S) schedule, and finally an FR10(FR32:S) schedule, in which 320 lever presses were required to obtain a single food delivery.
Immunohistochemistry. For immunohistochemical analysis of GluR2/3, adult mice were anesthetized with Avertin (20 mg/kg) and transcardially perfused with 4% paraformaldehyde. Brains were removed and stored in 4% paraformaldehyde for 24 hr, before placement in 0.1m phosphate buffer containing 30% sucrose for 48 hr. Brains were then frozen in isopentane at −45°C and stored at −80°C until sectioning. Coronal sections (30 μm) were taken using a cryostat, and sections were washed in PBS. Endogenous peroxidase was quenched by immersion in 0.3% hydrogen peroxide, and sections were washed in PBS before blocking in 1.5% normal donkey serum (SC-2044; Autogen Bioclear UK Ltd, Calne, UK). After additional washing in PBS, sections were incubated in 0.2 μg/ml anti-GluR2/3 [anti-GluR2 (C20) SC-7610; Autogen] overnight. Sections were then washed in PBS and incubated in a 1:400 dilution of biotinylated secondary antibody (SC-2042; Autogen) for 60 min before being washed again. Sections were subsequently incubated in ABC complex (Vectastain ABC elite kit: PK6100; Vector Laboratories, Peterborough, UK) and washed in PBS; staining was visualized using the nickel–DAB glucose (D-5637 and G-2133; Sigma Aldrich, Gillingham, UK) method. Sections were slide-mounted, dehydrated, and coverslipped before analysis. For analysis of sections, images were captured using a Sony (Tokyo, Japan) DSC-S75 digital camera mounted on a Zeiss (Oberkochen, Germany) Axioskop 2 microscope.
Statistical analysis. Two measures were analyzed for Pavlovian conditioning. First, the latency between cue onset and reward retrieval was compared between genotypes; second, the percentage of total food magazine entries occurring during the CS presentation was compared. Two-way ANOVA was performed, with training session (within subjects) and genotype (between subjects) as factors. Post hoc analysis was performed using independent-samples ttests. For analysis of Pavlovian approach, nose-poke rates toward the CS were assessed during the CS (CS+) and compared with rates when the CS was not presented (CS−). Two-way ANOVA was performed, with CS state (within subjects) and genotype (between subjects) as factors.Post hoc comparisons were performed using repeated-measurest tests. An independent-samples t test was also performed on data corrected for the overall rate of nose-poking (rate during CS+ divided by rate during CS−). For analysis of conditioned reward, responses on the CR lever were compared with responses on the NCR lever. Two-way ANOVA was performed with lever (within subjects) and genotype (between subjects) as factors. Post hoc comparisons were performed using repeated-measures t tests comparing responding on the CR lever with responding on the NCR lever. Analysis of response rates for the unconditioned stimulus (US) was also performed using two-way ANOVA.
Response rates during the test for PIT were collapsed into 10 sec time bins, and the rates were compared for the 60 sec before and after the CS with the rates during the 60 sec CS presentation. Additional comparisons were performed using one-way ANOVA, with response rates during the 60 sec CS being compared with response rates during the 60 sec periods before and after the CS presentation. Responding on a second-order schedule was initially analyzed using the time to obtain the first reinforcer, and total reinforcers obtained, as the dependent variables. The cumulative percentage of mice reaching a criterion of obtaining the first reinforcer within 5 min was also examined for each session; however, because of the relatively low subject numbers (n = 7 and 6 for WT andgria1 KOs, respectively), nonparametric analysis on contingency tables was not performed on these data.
Analysis of GluR2/3 immunoreactivity was performed by counting the number of GluR2/3-positive soma within a 130 × 170 μm region of the BLA and CA. The regions selected are indicated in Figure5C, and represent regions from within the basolateral amygdaloid nucleus and the central amygdaloid nucleus, respectively, as defined by Franklin and Paxinos (1997). Two-way ANOVA was then performed with region (within subjects) and genotype (between subjects) as factors. Post hoc comparisons were performed using independent samples t tests for each region.
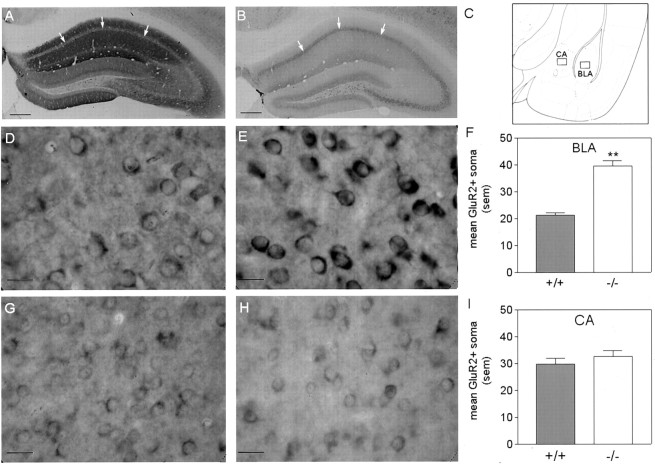
Fig. 5.
GluR2/3 immunoreactivity in WT(+/+) andgria1 KO(−/−) mice. A, B, Immunoreactivity in WT (left) and gria1KO (right) mice within the hippocampus observed at low magnification. Arrows indicate granule cell layer. gria1 KO mice display reduced immunoreactivity in dendritic areas of CA1, CA2, and CA3. Scale bars, 250 μm.C, Amygdaloid regions in which quantitative analysis of GluR2/3-positive soma was conducted. This image was modified fromFranklin and Paxinos (1997); it represents a coronal section at the bregma −1.22 mm. D, E, High-power magnifications of GluR2/3 immunoreactivity in the BLA of WT (left) andgria1 KO (right) mice. Scale bars, 20 μm. G, H, High-power magnifications of GluR2/3 immunoreactivity in the CA of WT (left) andgria1 KO (right) mice. Scale bars, 20 μm. F, I, Quantitative analysis of mean GluR2/3-positive soma in a 130 × 170 μm region of the BLA (top) and CA (bottom). **p < 0.01 compared with WT. Error bars indicate SEM.
Results
Pavlovian conditioning
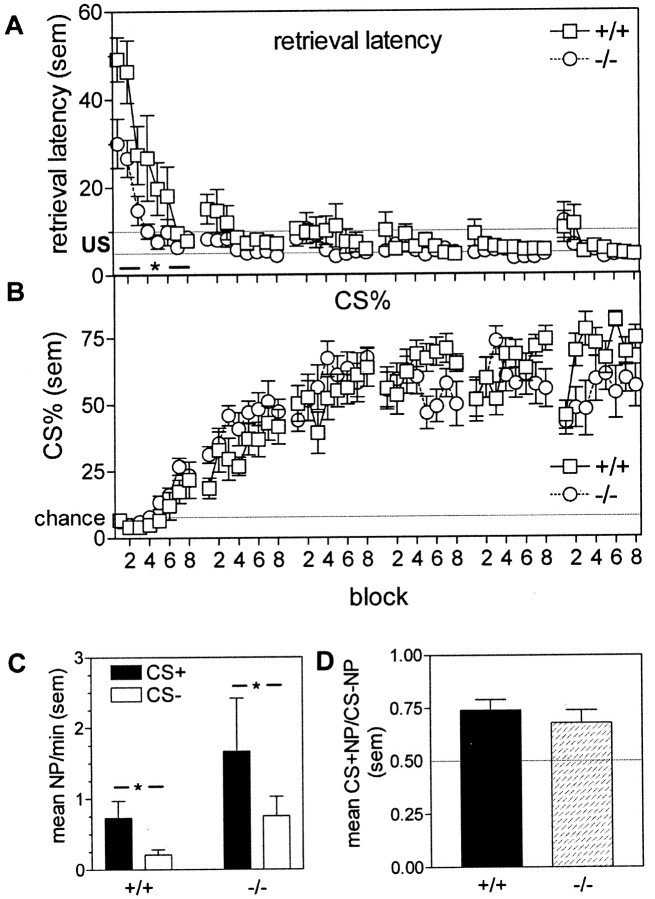
Figure 1Aindicates that when trained to associate a tone/light cue with delivery of a food reward, by presenting the cue immediately before the randomly timed delivery of reward, both WT and KO mice learned the association between cue presentation and food delivery, as indicated by a reduction over the first six training sessions in the latency with which they approached the food source when the cue was presented (main effect of session; F(5,70) = 42.9;p < 0.01). Although KO mice appeared to acquire the association more rapidly, as evidenced by a decreased latency in session 1 (session × genotype interaction:F(5,70) = 9.38; p < 0.01; t test for session 1:t(14) = 2.88; p < 0.05), this may be attributable to the general tendency of KO mice to nose-poke at faster rates than WTs (data not shown, but see results from Pavlovian approach for similar effect). Analysis of the percentage of total nose-pokes into the food magazine during the CS presentation (Fig. 1B) also indicated acquisition of the CS–US association, with a main effect of session (F(5,70) = 104.8; p < 0.01). There was also a significant session × genotype interaction (F(5,70) = 5.41;p < 0.01), although post hoc tests for each session indicated no significant between-genotype differences.
Fig. 1.
Pavlovian conditioning and Pavlovian approach in WT(+/+) and gria1 KO(−/−) mice. A, Reinforcer retrieval latency (seconds) after cue onset. Reward (30% condensed milk solution) presentation occurred between 5 and 10 sec. Data are shown for the first six training sessions (each containing 8 blocks) only. *p < 0.05 compared with WTs during session. B, Percentage of total nose-pokes into the food magazine occurring during the CS presentation. The chance level (i.e., equal rates of nose-poking during the CS and between CS presentations) is indicated by the solid line. Data are shown for the first six training sessions (each containing 8 blocks) only.C, Conditioned approach toward the cue in WT(+/+) andgria1 KO(−/−) mice. Data show mean nose-pokes (NP) per minute toward the cue light during the cue presentation (CS+) and intervening periods (CS−). The cue was presented every 2 min for 60 sec. *p < 0.05 between cue conditions D, Mean nose-poke rates toward the cue light expressed as a ratio of total nose-pokes. Solid line indicates random nose-poke behavior. Error bars indicate SEM.
Pavlovian approach
That conditioning itself was unimpaired in the KOs was confirmed by the animal's normal approach to the cue when its source was relocated in the wall opposite the food magazine, within a recess whose entrance was fitted with infrared beams to detect head entries. Figure1C shows that illumination of the cue light resulted in an increased approach to the cue (Pavlovian approach) in both WT and KO mice (main effect of CS presentation;F(1,14) = 8.76; p < 0.01). Rates of entry remained constant across the session (data not shown). Consistent with the heightened activity in gria1 KO mice (Vekovischeva et al., 2001), there was a tendency for them to approach the cue at higher rates than WT mice. However, this was also true for periods when the cue light was not illuminated. Figure1D corrects for this overall elevated rate of nose poking by the gria1 KO mice by expressing nose-poke rates during cue presentations and outside these periods as a ratio. No genotype differences were present (t(14) = 1.33; NS).
Conditioned reinforcement
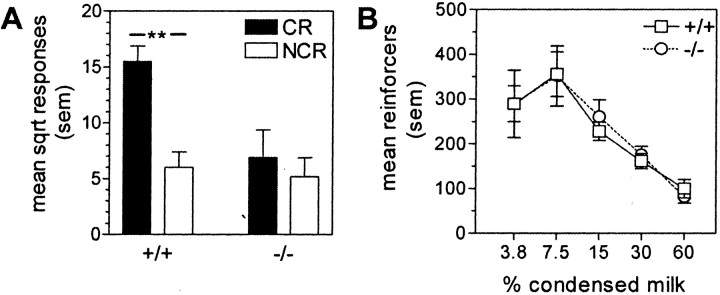
Despite similarities in conditioning in the WT andgria1 KO mice, a clear difference was seen when levers were introduced into the cage and the mice were able to activate the cue by operating one of the levers. Figure2A shows that WT mice performed a greater number of lever presses on the lever programmed to deliver the cue than on an alternative lever whose activation was not reinforced (t(7) = 8.69; p < 0.01), indicating that the cue had acquired conditioned rewarding properties as a result of being paired with the food primary reward. However, the gria1 KO mutants operated both levers at low rates, comparable with the rate at which the WT mice operated the ineffective lever (genotype × lever interaction:F(1,14) = 27.57; p < 0.01; t test for KOs: t(7)= 1.72; NS). This low rate was not attributable simply to impaired lever-pressing ability per se in the KO mice because in a separate experiment WT and KO mice responded on a lever at similar rates to obtain a food reward (Fig. 2B) (no main effect of genotype or concentration × genotype interaction).
Fig. 2.
Ability of the cue to act as a conditioned reinforcer in WT(+/+) and gria1 KO(−/−) mice.A, Mean square-root (sqrt) responses on a lever resulting in the cue presentation (CR) and on a control lever with no consequences (NCR) during a 15 hr session. **p < 0.01 compared with responding on the NCR lever. B, Ability of the primary reward to act as a reinforcer. Data show mean presentations obtained of different concentrations of condensed milk solution during a 3 hr session. Error bars indicate SEM.
PIT
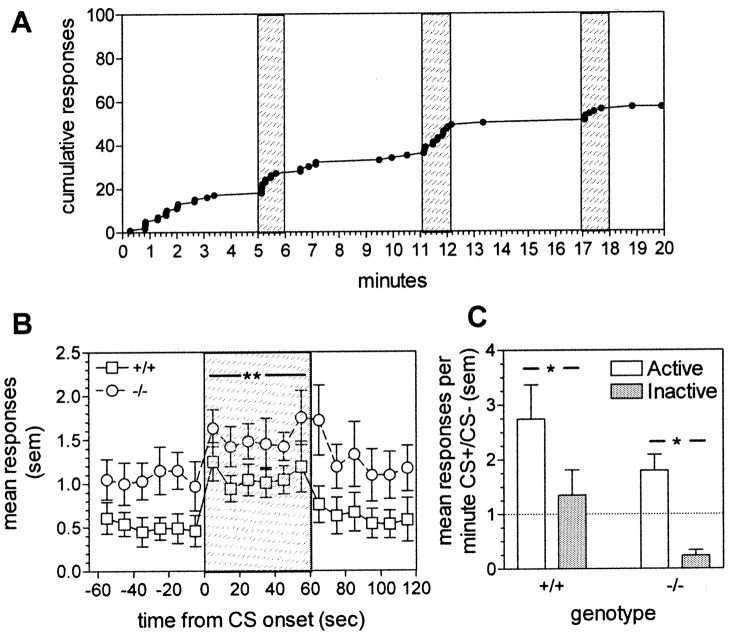
Stimuli conditioned to primary rewards are also known for a third property, their ability to energize behavior directed toward obtaining the primary reward (PIT) (Dickinson, 1994). We tested this property in the gria1 KO mice by training them and their WT littermates to perform an operant lever-press response to obtain food (condensed milk solution), using a variable interval schedule (during which responding is rewarded at unpredictable, variable intervals, thus ensuring steady, low rates of responding). Figure 3A illustrates that a 60 sec presentation of the cue increased the rate of responding in a WT mouse during its presentation, without carryover to the subsequent period when the cue was not present. Pooled data for the WT group and for KO mice are shown in Figure 3B (main effect of cue presentation: F(2,24) = 18.12;p < 0.01; main effect of genotype:F(1,12) = 4.86; p < 0.05). Cue presentation resulted in a dramatic increase in response rates on the active lever (whose operation delivered food on the VI schedule), but not on the second, inactive lever, whose operation had no consequences (Fig. 3C) (main effect of lever: F(1,12) = 5.74;p < 0.05). The lack of genotype differences indicated that PIT is intact in the KO mice (cue presentation × genotype interaction: F(2,24) = 0.28; NS).
Fig. 3.
PIT in WT(+/+) and gria1 KO(−/−) mice. A, Representative cumulative response record showing active-lever responses between cue presentations and during cue presentations (shaded areas) in a WT mouse.B, Mean active-lever responses in 10 sec time bins before, during (shaded area), and after the cue presentation. **p < 0.01 compared with response rates before and after the CS for both genotypes. C, Mean responses per minute during the cue presentation expressed as a ratio of total responses on active and inactive levers. Thesolid line indicates equal rates of lever pressing during the presence of the cue and the absence of the cue. *p < 0.05. Error bars indicate SEM.
Second-order operant responding
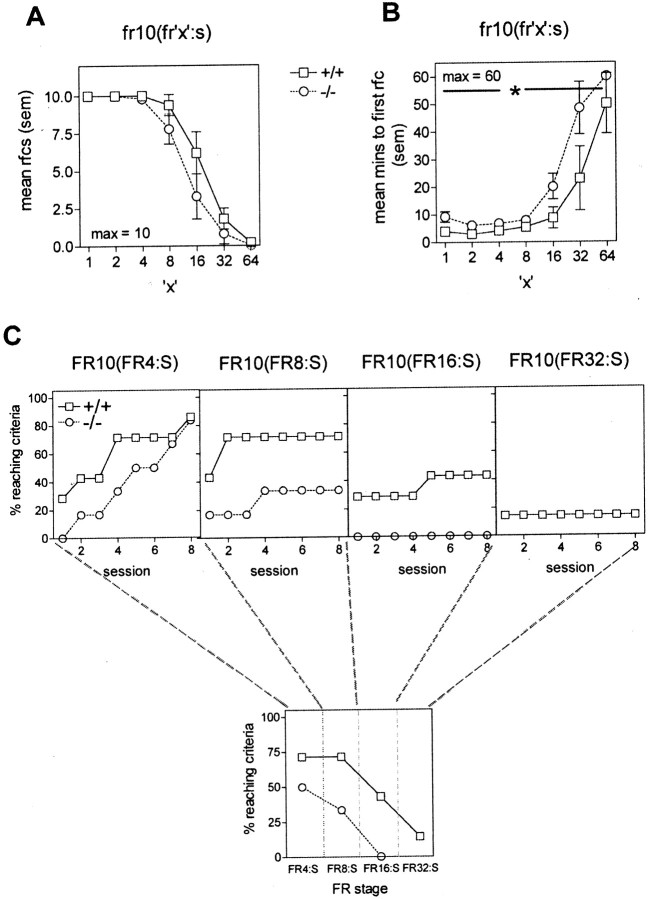
To test whether deletion of the gria1 gene impairs this behavior, we trained KO and WT mice to perform an operant lever-pressing task to obtain food on a second-order schedule. When tested on increasing second-order schedules on consecutive days, KO mice took longer to obtain the first reinforcer than WT controls (Fig.4B) (main effect of genotype: F(1,9) = 5.70;p < 0.05) but showed no deficit in total reinforcers obtained in the 60 min session (Fig. 4A) (main effect of genotype: F(1,9) = 2.62; NS).Gria1 KO mice performed the operant response to obtain food at rates comparable with those seen for WT mice during initial training. However, when allowed up to 10 sessions to reach the criterion of obtaining the first reinforcer within 5 min, Figure4C shows that they were less competent in acquiring responding on the second-order schedule, with only 50% of the mice reaching the criterion within five sessions when four responses were required for each cue presentation (FR4:S phase) and only 33% reaching the criterion when eight responses were required for each cue presentation (FR8:S phase).
Fig. 4.
Responding under a second-order schedule of reinforcement in WT(+/+) and gria1 KO(−/−) mice.A, Mean number of reinforcers (rfcs) obtained during a 60 min session under each schedule. Sessions were conducted on consecutive days. B, Mean time required to obtain the first reinforcer (rfc) under each schedule. Sessions were conducted on consecutive days. *p < 0.05 for all schedules compared with WT mice. C, Top, Percentage of mice reaching the criterion at each schedule, over the course of eight sessions, during the acquisition phase of the second-order schedule. Bottom, Percentage of mice from each genotype that reached the criterion within five sessions. Error bars indicate SEM. The vertical dotted lines separate the phases of the second-order training.
Immunohistochemistry
Figure 5A,B shows the distribution of staining for GluR2/3 protein in the hippocampus. Figure5A illustrates the distribution of GluR2/3 immunostaining in a WT mouse; Figure 5B illustrates staining in thegria1 KO. In the WT mouse, there is only faint staining in the granule cell body layer of the CA1 region, but there is denser immunoreactivity in areas containing dendrites. In contrast, thegria1 KO mouse shows dense staining of GluR2/3 in the cell body layer but poor staining in non-cell body areas. These patterns suggest that in the absence of GluR1 subunits, GluR2/3 protein is not transported away from the cell body, so gria1 KOs may have deficits in AMPA receptors as a result of both the absence of GluR1 and the failure of GluR2/3 to be inserted into functional receptors.
In the BLA, the gria1 KOs showed more GluR2/3-containing cell bodies (Fig. 5E) than the WTs (Fig.5D), although there were no similar changes in the CA (Fig.5G,H). Quantification of GluR2/3-positive soma in the BLA and CA confirmed these observations (Figs. 5F,I) (region × genotype interaction:F(1,8) = 13.67; p < 0.01; t test on BLA staining;t(8) = 8.99;p < 0.01).
Discussion
In the present experiments, we demonstrate that targeted deletion of the gria1 gene encoding GluR1 subunits of AMPA receptors leads to a subtle deficit in Pavlovian appetitive conditioning. Although gria1 KO mice showed an unaltered ability to associate a tone/light cue with food delivery, the CS retained its ability to act as a conditional stimulus for approach to the food cup (Pavlovian conditioning), to elicit approach (Pavlovian approach), and to facilitate instrumental responding to obtain food reward (PIT); in contrast to their WT littermates, the KO mice did not learn a novel response to obtain presentations of the conditioned cue (conditioned reinforcement). Failure to learn to respond to obtain a cue previously paired with a primary reward might be attributable to the animal's failure to form an association between the two events or to a failure of the animal to attribute incentive properties to the cue. Because the KOs were unimpaired in other measures of Pavlovian association and in their ability to learn the same instrumental response to obtain a milk reward, their failure to perform for a conditioned reinforcer indicates that in these animals, the cue had not acquired reinforcing properties of its own. Thus, the deficit in responding for the cue (conditioned reinforcer) is attributable to the gria1 KO mice not having attributed affective properties to the cue.
This pattern of results, with deficits in conditioned reward but not in discriminated approach, Pavlovian approach, or PIT, is similar to that found in rats with excitotoxic lesions of the BLA (Everitt et al., 2000), an area rich in AMPA receptors containing the GluR1 subunit (McDonald, 1996). In contrast, Pavlovian approach and PIT remain intact after BLA lesions but are impaired by lesions of the CA (Everitt et al., 2000). Lesions of the BLA also result in impairments of performance on second-order schedules of reinforcement (Everitt et al., 1989; Hatfield et al., 1996; Whitelaw et al., 1996), in which instrumental responding is maintained for lengthy periods, during which the primary reward is delayed, by periodic presentations of a reward-paired cue. Such a schedule mimics the maintenance of appetitive or seeking behavior in the presence of cues, indicating the eventual availability of the primary reward. The gria1 KOs also exhibited a deficit in performing a second-order schedule of this type. These data are consistent with the KOs having a specific deficit in neurotransmission within the BLA but not the CA, although the possibility that mice differ from rats in the brain circuitry used by rats and mice in performance of these behavioral tasks must be acknowledged.
In the BLA, AMPA receptors mediate fast EPSPs in response to the activation of glutamatergic inputs from both cortical and subcortical regions (Rainnie et al., 1991; Gean and Chang, 1992). The BLA contains two major classes of neuron: (1) spiny pyramidal projection neurons and (2) sparsely spined, nonpyramidal local circuit neurons, most of which are GABAergic (McDonald, 1992). Marked GluR1 immunoreactivity is found in nonpyramidal neurons, whereas pyramidal cells exhibit only light GluR1 immunoreactivity (McDonald, 1996). Although GluR2/3 immunoreactivity has been reported in some interneurons, it is primarily limited to pyramidal neurons (McDonald, 1994, 1996; He et al., 1999). He et al. (1999), using a selective GluR2 antibody, conjecture that many AMPA receptors on interneurons may not contain GluR2. This interpretation is consistent with electrophysiological evidence indicating that although the AMPA component of the synaptic current at inputs to pyramidal cells is independent of calcium (the underlying receptors thus contain GluR2 subunits), in contrast, AMPA receptors on inhibitory interneurons show high permeability to calcium, indicating a low representation of GluR2 (Mahanty and Sah, 1998). Because GluR1 subunits represent by far the major component of AMPAergic receptors in the GABAergic interneurons, it is likely that targeted deletion of gria1 resulted in a profound reduction in their excitability, with a consequent disruption of firing patterns of BLA pyramidal output neurons to which they normally provide an inhibitory control.
In the absence of GluR2 subunits in most receptors, the high calcium permeability of AMPA receptors in synaptic contacts onto BLA interneurons may make such synapses especially sensitive to plastic modification. Tetanic stimulation of inputs to BLA inhibitory neurons results in increased synaptic efficacy, which is independent of NMDA receptor activation and is reflected in an increase in GABAergic inhibitory currents in pyramidal neurons (Mahanty and Sah, 1998). Thus, deletion of the gria1 gene encoding GluR1 subunits can be expected not only to reduce the extent to which the inhibitory interneurons modulate pyramidal cell activity but also to remove the substrate whereby plastic changes in the inhibitory control of pyramidal cell excitatory outputs (including those to accumbens) (Kelley et al., 1982; Brog et al., 1993; Wright et al., 1996) occur during learning. In principle, this action may account for the loss of conditioned reinforcement and impairment of second-order instrumental responding reported here.
Current theories hold that the BLA functions to allow animals to use cues associated with primary reinforcers to assess the current motivational properties of the primary reinforcer and to use that representation to alter their behavioral response (Baxter and Murray, 2002; Cardinal et al., 2002). According to the model of Cardinal et al. (2002), the affective value of the CS is processed by the BLA, but the consequences for behavioral output depend on the information being conveyed to the accumbens (Cador et al., 1989; Everitt et al., 1989; Setlow et al., 2002). Thus, an alternative account of our findings might be that deletion of gria1 leads to an impairment of the glutamatergic input from BLA to the ventral striatum (Brog et al., 1993; Wright et al., 1996) or orbitofrontal cortex (Gallagher et al., 1999; Baxter et al., 2000), because the medium spiny neuron targets of this amygdala–accumbens pathway also express GluR1 subunit-containing AMPA receptors (Bernard et al., 1997).
The gria1 KOs showed intact Pavlovian approach and PIT, behaviors that are disrupted by lesions of the CA (Gallagher et al., 1990; Hall et al., 2001). The CA differs markedly from the BLA in its cytoarchitecture and in its outputs (Swanson and Petrovich, 1998) as well as in the mechanisms underlying LTP (Chapman and Chattarji, 2000). Whereas the major output component of the BLA consists of glutamatergic pyramidal neurons, that of the central nucleus is made up of GABAergic projections, especially, but not limited to, the hypothalamus, midbrain, and brainstem (Kapp et al., 1992). Furthermore, in contrast to synapses on the BLA inhibitory neurons, LTP in the CA appears to depend on NMDA receptor-based mechanisms (Shindou et al., 1993;Watanabe et al., 1995a), and LTP in medial, but not lateral, amygdala nuclei is blocked by inhibitors of nitric oxide synthase (Watanabe et al., 1995b) and facilitated by nitric oxide donors (Abe et al., 1996). A likely explanation of the current set of behavioral observations, then, is that in the absence of AMPA receptors containing GluR1, synaptic plasticity is disrupted in the basolateral nucleus and/or its projections (disrupting conditioned reinforcement and second-order instrumental responding) but not in the CA (allowing Pavlovian approach and PIT to remain normal).
In the present experiments, evidence that gria1 deletion does indeed have different consequences for the function of BLA and CA is provided by immunostaining for GluR2/3 protein in thegria1 KOs. In the BLA, the gria1 KOs showed an increased number of GluR2/3-positive neuronal cell bodies, whereas there were no changes in the CA. It is very likely that the increases observed in the BLA are attributable to increased staining in interneurons, because GluR2 subunits in pyramidal cells are associated with dendritic processes rather than cell bodies (He et al., 1999). The mechanism whereby the absence of GluR1 leads to increased numbers of neurons expressing GluR2/3 is unclear. An obvious possibility is compensatory overexpression of GluR2/3 in the absence of GluR1. Although such an overexpression of GluR2/3 might compensate for some actions of GluR1 homomers, the low calcium flux through GluR2-containing receptors (Hollmann et al., 1991) would nevertheless alter the ability of the synapse to show plasticity and would be expected to disrupt learning. However, other mechanisms are possible. In the hippocampus, most AMPA receptors are hetero-oligomers composed of GluR1/2 subunits (Shi et al., 2001), with GluR1/2 receptors being added to synapses during plasticity (whereas GluR2/3 are inserted during normal receptor turnover). In the absence of GluR1, GluR2 subunits are not inserted into the membrane; therefore, they may accumulate in cell bodies (Zamanillo et al., 1999) (Fig.5A,B). It seems possible that the increased number of neurons positive for GluR2/3 immunoreactivity reflects a similar buildup of GluR2 protein in the cytoplasm in BLA neurons.
Our results have interesting implications for understanding the mechanisms underlying drug addiction and relapse to drug abuse. The BLA is thought to influence goal-directed instrumental behavior through its projections to the orbital prefrontal cortex and ventral striatum (Pitkanen, 2000). Secondary rewards (such as drug paraphernalia or situations in which drugs are experienced) may maintain or initiate drug-seeking behavior in addicts even when the drug itself is not immediately available (Carter and Tiffany, 1999). Blocking AMPA receptors in the BLA disrupts responding for a cue conditioned to amphetamine (Hitchcott and Phillips, 1997), and lesions of the BLA prevent drug-associated cues from reinstating lever-pressing for cocaine after extinction in an animal model of relapse to drug taking in abstaining addicts (Meil and See, 1997). The present experiments suggest an important and specific role of AMPA receptors containing the GluR1 subunit in regulation of these BLA projections. The development of specific antagonists that block GluR1-containing AMPA receptors may offer an approach to disrupting drug-seeking behavior maintained by cues conditioned to drug taking, without disrupting other aspects of learning and memory. In that context, it is of interest that stimuli associated with drug self-administration, such as the paraphernalia of drug taking, activate the amygdala and connections of the BLA in particular in human addicts (Childress et al., 1999).
Footnotes
This work was supported by funding from the United Kingdom Biotechnology and Biological Sciences Research Council. We thank Dr. S. Rulten and Dr. L. Mayne for genotyping and Prof. Peter Seeburg and Dr. Rolf Sprengel for donating gria1 breeding stock.
Correspondence should be addressed to Dr. David N. Stephens, Laboratory of Experimental Psychology, University of Sussex, Brighton BN1 9QG, UK. E-mail: dns@biols.susx.ac.uk.
References
- 1.Abe K, Watanabe Y, Saito H. Differential role of nitric oxide in long-term potentiation in the medial and lateral amygdala. Eur J Pharmacol. 1996;297:43–46. doi: 10.1016/0014-2999(95)00829-2. [DOI] [PubMed] [Google Scholar]
- 2.Baxter MG, Murray EA. The amygdala and reward. Nat Rev Neurosci. 2002;3:563–573. doi: 10.1038/nrn875. [DOI] [PubMed] [Google Scholar]
- 3.Baxter MG, Parker A, Lindner CC, Izquierdo AD, Murray EA. Control of response selection by reinforcer value requires interaction of amygdala and orbital prefrontal cortex. J Neurosci. 2000;20:4311–4319. doi: 10.1523/JNEUROSCI.20-11-04311.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernard V, Somogyi P, Bolam JP. Cellular, subcellular, and subsynaptic distribution of AMPA-type glutamate receptor subunits in the neostriatum of the rat. J Neurosci. 1997;17:819–833. doi: 10.1523/JNEUROSCI.17-02-00819.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brog JS, Salyapongse A, Deutch AY, Zahm DS. The patterns of afferent innervation of the core and shell in the “accumbens” part of the rat ventral striatum: immunohistochemical detection of retrogradely transported fluoro-gold. J Comp Neurol. 1993;338:255–278. doi: 10.1002/cne.903380209. [DOI] [PubMed] [Google Scholar]
- 6.Cador M, Robbins TW, Everitt BJ. Involvement of the amygdala in stimulus-reward associations: interaction with the ventral striatum. Neuroscience. 1989;30:77–86. doi: 10.1016/0306-4522(89)90354-0. [DOI] [PubMed] [Google Scholar]
- 7.Cardinal RN, Parkinson JA, Hall J, Everitt BJ. Emotion and motivation: the role of the amygdala, ventral striatum, and prefrontal cortex. Neurosci Biobehav Rev. 2002;26:321–352. doi: 10.1016/s0149-7634(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 8.Carter BL, Tiffany ST. Meta-analysis of cue-reactivity in addiction research. Addiction. 1999;94:327–340. [PubMed] [Google Scholar]
- 9.Chapman P, Chattarji S. Synaptic plasticity in the amygdala. In: Aggleton J, editor. The amygdala, Ed 2. Oxford UP; New York: 2000. pp. 117–153. [Google Scholar]
- 10.Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP. Limbic activation during cue-induced cocaine craving. Am J Psychiatry. 1999;156:11–18. doi: 10.1176/ajp.156.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dickinson A. Instrumental conditioning. In: Mackintosh N, editor. Animal learning and cognition. Academic; London: 1994. pp. 45–78. [Google Scholar]
- 12.Everitt BJ, Cador M, Robbins TW. Interactions between the amygdala and ventral striatum in stimulus-reward associations: studies using a second-order schedule of sexual reinforcement. Neuroscience. 1989;30:63–75. doi: 10.1016/0306-4522(89)90353-9. [DOI] [PubMed] [Google Scholar]
- 13.Everitt B, Cardinal R, Hall J, Parkinson J, Robbins T. Differential involvement of amygdala subsystems in appetitive conditioning and drug addiction. In: Aggleton J, editor. The amygdala, Ed 2. Oxford UP; New York: 2000. pp. 353–390. [Google Scholar]
- 14.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. Academic; San Diego: 1997. [Google Scholar]
- 15.Gallagher M, Graham PW, Holland PC. The amygdala central nucleus and appetitive Pavlovian conditioning: lesions impair one class of conditioned behavior. J Neurosci. 1990;10:1906–1911. doi: 10.1523/JNEUROSCI.10-06-01906.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gallagher M, McMahan RW, Schoenbaum G. Orbitofrontal cortex and representation of incentive value in associative learning. J Neurosci. 1999;19:6610–6614. doi: 10.1523/JNEUROSCI.19-15-06610.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gean PW, Chang FC. Pharmacological characterization of excitatory synaptic potentials in rat basolateral amygdaloid neurons. Synapse. 1992;11:1–9. doi: 10.1002/syn.890110102. [DOI] [PubMed] [Google Scholar]
- 18.Hall J, Parkinson JA, Connor TM, Dickinson A, Everitt BJ. Involvement of the central nucleus of the amygdala and nucleus accumbens core in mediating Pavlovian influences on instrumental behaviour. Eur J Neurosci. 2001;13:1984–1992. doi: 10.1046/j.0953-816x.2001.01577.x. [DOI] [PubMed] [Google Scholar]
- 19.Hatfield T, Han JS, Conley M, Gallagher M, Holland P. Neurotoxic lesions of basolateral, but not central, amygdala interfere with Pavlovian second-order conditioning and reinforcer devaluation effects. J Neurosci. 1996;16:5256–5265. doi: 10.1523/JNEUROSCI.16-16-05256.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He Y, Janssen WG, Morrison JH. Differential synaptic distribution of the AMPA-GluR2 subunit on GABAergic and non-GABAergic neurons in the basolateral amygdala. Brain Res. 1999;827:51–62. doi: 10.1016/s0006-8993(99)01264-0. [DOI] [PubMed] [Google Scholar]
- 21.Hitchcott PK, Phillips GD. Amygdala and hippocampus control dissociable aspects of drug-associated conditioned rewards. Psychopharmacology (Berl) 1997;131:187–195. doi: 10.1007/s002130050283. [DOI] [PubMed] [Google Scholar]
- 22.Hollmann M, Hartley M, Heinemann S. Ca2+ permeability of KA-AMPA-gated glutamate receptor channels depends on subunit composition. Science. 1991;252:851–853. doi: 10.1126/science.1709304. [DOI] [PubMed] [Google Scholar]
- 23.Kapp BS, Whalen PJ, Supple WF, Pascoe JP. Amygdaloid contribution to conditioned arousal and sensory processing. In: Aggleton J, editor. The amygdala. Wiley-Liss; New York: 1992. pp. 229–254. [Google Scholar]
- 24.Kelley A, Domesick V, Nauta W. The amygdalostriatal projection in the rat: an anatomical study by anterograde and retrograde tracing methods. Neuroscience. 1982;7:615–630. doi: 10.1016/0306-4522(82)90067-7. [DOI] [PubMed] [Google Scholar]
- 25.Lledo PM, Zhang X, Sudhof TC, Malenka RC, Nicoll RA. Postsynaptic membrane fusion and long-term potentiation. Science. 1998;279:399–403. doi: 10.1126/science.279.5349.399. [DOI] [PubMed] [Google Scholar]
- 26.Mack V, Burnashev N, Kaiser KM, Rozov A, Jensen V, Hvalby O, Seeburg PH, Sakmann B, Sprengel R. Conditional restoration of hippocampal synaptic potentiation in GluR-A-deficient mice. Science. 2001;292:2501–2504. doi: 10.1126/science.1059365. [DOI] [PubMed] [Google Scholar]
- 27.Mackintosh N. The psychology of animal learning. Academic; London: 1974. [Google Scholar]
- 28.Mahanty NK, Sah P. Calcium-permeable AMPA receptors mediate long-term potentiation in interneurons in the amygdala. Nature. 1998;394:683–687. doi: 10.1038/29312. [DOI] [PubMed] [Google Scholar]
- 29.McDonald AJ. Projection neurons of the basolateral amygdala: a correlative Golgi and retrograde tract tracing study. Brain Res Bull. 1992;28:179–185. doi: 10.1016/0361-9230(92)90177-y. [DOI] [PubMed] [Google Scholar]
- 30.McDonald AJ. Neuronal localization of glutamate receptor subunits in the basolateral amygdala. NeuroReport. 1994;6:13–16. doi: 10.1097/00001756-199412300-00005. [DOI] [PubMed] [Google Scholar]
- 31.McDonald AJ. Localization of AMPA glutamate receptor subunits in subpopulations of non-pyramidal neurons in the rat basolateral amygdala. Neurosci Lett. 1996;208:175–178. doi: 10.1016/0304-3940(96)12585-4. [DOI] [PubMed] [Google Scholar]
- 32.Meil WM, See RE. Lesions of the basolateral amygdala abolish the ability of drug associated cues to reinstate responding during withdrawal from self-administered cocaine. Behav Brain Res. 1997;87:139–148. doi: 10.1016/s0166-4328(96)02270-x. [DOI] [PubMed] [Google Scholar]
- 33.Nayak A, Zastrow DJ, Lickteig R, Zahniser NR, Browning MD. Maintenance of late-phase LTP is accompanied by PKA-dependent increase in AMPA receptor synthesis. Nature. 1998;394:680–683. doi: 10.1038/29305. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien CP, Childress AR, Ehrman R, Robbins SJ. Conditioning factors in drug abuse: can they explain compulsion? J Psychopharmacol. 1998;12:15–22. doi: 10.1177/026988119801200103. [DOI] [PubMed] [Google Scholar]
- 35.Pitkanen A. Connectivity of the rat amygdaloid complex. In: Aggleton J, editor. The amygdala, Ed 2. Oxford UP; New York: 2000. pp. 353–390. [Google Scholar]
- 36.Rainnie DG, Asprodini EK, Shinnick-Gallagher P. Excitatory transmission in the basolateral amygdala. J Neurophysiol. 1991;66:986–998. doi: 10.1152/jn.1991.66.3.986. [DOI] [PubMed] [Google Scholar]
- 37.Setlow B, Gallagher M, Holland PC. The basolateral complex of the amygdala is necessary for acquisition but not expression of CS motivational value in appetitive Pavlovian second-order conditioning. Eur J Neurosci. 2002;15:1841–1853. doi: 10.1046/j.1460-9568.2002.02010.x. [DOI] [PubMed] [Google Scholar]
- 38.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 39.Shindou T, Watanabe S, Yamamoto K, Nakanishi H. NMDA receptor-dependent formation of long-term potentiation in the rat medial amygdala neuron in an in vitro slice preparation. Brain Res Bull. 1993;31:667–672. doi: 10.1016/0361-9230(93)90139-3. [DOI] [PubMed] [Google Scholar]
- 40.Swanson LW, Petrovich GD. What is the amygdala? Trends Neurosci. 1998;21:323–331. doi: 10.1016/s0166-2236(98)01265-x. [DOI] [PubMed] [Google Scholar]
- 41.Tomie A, Brooks W, Zito B. Sign-tracking: the search for reward. In: Klein SB, Mower RR, editors. Contemporary learning theories: Pavlovian conditioning and the status of traditional learning theory. Lawrence Erlbaum; Hillsdale, NJ: 1999. pp. 191–226. [Google Scholar]
- 42.Vekovischeva OY, Zamanillo D, Echenko O, Seppala T, Uusi-Oukari M, Honkanen A, Seeburg PH, Sprengel R, Korpi ER. Morphine-induced dependence and sensitization are altered in mice deficient in AMPA-type glutamate receptor-A subunits. J Neurosci. 2001;21:4451–4459. doi: 10.1523/JNEUROSCI.21-12-04451.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Watanabe Y, Ikegaya Y, Saito H, Abe K. Roles of GABAA, NMDA, and muscarinic receptors in induction of long-term potentiation in the medial and lateral amygdala in vitro. Neurosci Res. 1995a;21:317–322. doi: 10.1016/0168-0102(94)00867-f. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe Y, Saito H, Abe K. Nitric oxide is involved in long-term potentiation in the medial but not lateral amygdala neuron synapses in vitro. Brain Res. 1995b;688:233–236. doi: 10.1016/0006-8993(95)00563-6. [DOI] [PubMed] [Google Scholar]
- 45.Whitelaw RB, Markou A, Robbins TW, Everitt BJ. Excitotoxic lesions of the basolateral amygdala impair the acquisition of cocaine-seeking behaviour under a second-order schedule of reinforcement. Psychopharmacology (Berl) 1996;127:213–224. [PubMed] [Google Scholar]
- 46.Wright CI, Beijer AV, Groenewegen HJ. Basal amygdaloid complex afferents to the rat nucleus accumbens are compartmentally organized. J Neurosci. 1996;16:1877–1893. doi: 10.1523/JNEUROSCI.16-05-01877.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]