Abstract
Neurosteroids are a class of steroids synthesized de novo in the brain, several of which are potent modulators of GABAA receptor function. In developing brain GABAA receptor, stimulation plays a trophic role. Cortical levels of the GABAergic neurosteroid 3α-hydroxy-5α-pregnan-20-one (3α,5α-THP) vary dramatically across development; during the second week of life, elevated levels of 3α,5α-THP are associated with decreased GABAA receptor function. To determine whether alteration of endogenous 3α,5α-THP levels during development alters GABAergic interneurons in prefrontal cortex (PFC) at maturity, rat pups were exposed to 3α,5α-THP (10 mg/kg) on postnatal day 1 (P1), P2, and P5. On P80, frontal cortex tissue was assayed for GABAergic cell localization (parvalbumin and calbindin immunoreactivity), agonist-dependent [3H] dizocilpine (MK-801) binding to NMDA receptors in cortical homogenates, muscimol-mediated 36Cl− influx into synaptoneurosomes, and 3α,5α-THP levels. The localization of parvalbumin-labeled cells was markedly altered; the ratio of cell number in the deep layers (V-VI) versus superficial layers (I-III) of adult PFC increased twofold in animals exposed to 3α,5α-THP on P1 or P5. Relative microtubule-associated protein-2 and calbindin immunoreactivity were not altered by perinatal 3α,5α-THP administration. Agonist-dependent [3H]MK-801 binding was decreased in PFC but not parietal cortex homogenates, whereas muscimol-mediated 36Cl− influx and 3α,5α-THP levels were unchanged in frontal cortex of adult males exposed to 3α,5α-THP on P5. These data are consistent with a change in the distribution of a subset of interneurons in response to neurosteroid exposure and suggest that GABAergic neurosteroids are critical for normal development of GABAergic systems in the PFC.
Keywords: allopregnanolone, calbindin, parvalbumin, schizophrenia, cortical development, stress
Introduction
GABA is the principle inhibitory neurotransmitter in the adult mammalian CNS. However, early in development, GABAA receptor-mediated signaling is excitatory; GABA functions as a chemoattractant, serves as a neurotrophic factor (Lauder et al., 1998), and facilitates neurite outgrowth through GABAA receptor mechanisms (Barbin et al., 1993; Ben-Ari et al., 1997; Behar et al., 1998). Exposure to GABAA receptor modulators such as benzodiazepines may alter the course of neuronal development, resulting in enhanced seizure susceptibility (Bitran et al., 1991b) and sex-specific behavioral deficits at maturity (Frieder et al., 1984;Kellogg et al., 1991). The progesterone metabolite 3α-hydroxy,5α-pregnan-20-one (3α,5α-THP; allopregnanolone) is one of the most potent known endogenous modulators of GABAA receptor function (Majewska et al., 1986;Morrow et al., 1987). 3α,5α-THP potentiates GABAA receptor function in assays of channel kinetics, Cl− flux, and GABA-dependent behavior (Majewska et al., 1986; Bitran et al., 1991a; Twyman and Macdonald, 1992). The enzymes necessary to manufacture 3α,5α-THPde novo are present in the brain (Baulieu, 1981); however, normal operating levels of cortical 3α,5α-THP are likely maintained via a combination of brain-derived synthesis and metabolism of peripherally derived precursors, the regulation of which is only now being understood (Mellon and Griffin, 2002). 3α,5α-THP levels vary widely across both development (Kellogg and Frye, 1999) and the estrous cycle (Concas et al., 1999), and they increase with stress (Purdy et al., 1991). Because 3α,5α-THP administration is anxiolytic (Bitran et al., 1991a), it has the potential to participate in a negative feedback loop or act as an endogenous coping mechanism. Accordingly, increased brain levels of allopregnanolone provide feedback on peripheral measures of stress such as cortisol and CRF production (Owens et al., 1992) and regulate CNS stress responses such as dopamine metabolism (Grobin et al., 1992; Motzo et al., 1996).
Potential functional and structural significance of these hormonal variations includes suppression of GABAAreceptor-mediated Cl− influx (Grobin and Morrow, 2001) and changes in GABAA receptor density and subunit expression (Concas et al., 1999; Grobin and Morrow, 2000). Moreover, withdrawal from progesterone metabolites results in functional and structural changes in GABAAreceptors (Smith et al., 1998a,b). Interestingly, maternal exposure to stress or ethanol during pregnancy (both known to elevate endogenous neurosteroid levels) (Purdy et al., 1991; VanDoren et al., 2000) results in behavioral deficits in adult offspring such as decreased ultrasonic vocalizations and decreased prepulse inhibition (Gruen et al., 1995; Zimmerberg and McDonald, 1996; Ellenbroek and Cools, 2000). Recent studies suggest GABAergic neuroactive steroids may control aspects of neuronal development in vitro. Under certain conditions, 3α,5α-THP inhibits neurite extension (Brinton, 1994), and chronically high 3α,5α-THP levels cause cell death through a Ca2+-sensitive GABAAreceptor mechanism (Xu et al., 2000). However, in the absence of GABA, neurite extension can be reinitiated by application of 3α,5α-THP (Maric et al., 2001).
The cortical GABAergic system plays a crucial role in refining and focusing signal processing in the mammalian cerebral cortex; localization of different types of interneurons relative to one another and to principal cells influences cortical function. However, molecular and chemical determinants of cortical interneuron cell placement during development are not well understood. Unlike pyramidal cells that follow an inside-out development pattern, GABAergic interneurons migrate tangentially from the thalamus (Marin and Rubenstein, 2001). They display a distinctive protracted developmental pattern from embryonic day 16 (E16) to postnatal day 10 (P10) at both the neuron and synapse levels (Micheva and Beaulieu, 1997) and thus may be preferentially vulnerable.
GABAergic neurotransmission is a potential site of considerable developmental regulation, yet very little is known about the role of endogenous effectors at GABAA receptors. The current studies were performed to determine whether developmentally altered neurosteroid levels influence the development of GABAergic interneurons in the prefrontal cortex (PFC). After administering pharmacologically relevant doses of 3α,5α-THP to neonatal rats, they were allowed to survive to maturity. The effects of perinatal 3α,5α-THP administration on PFC GABAergic interneuron localization were determined. The functional sequelae were explored by determination of frontal cortical [3H] dizocilpine (MK-801) binding, GABAAreceptor-mediated [36Cl−] uptake, and endogenous 3α,5α-THP levels.
Materials and Methods
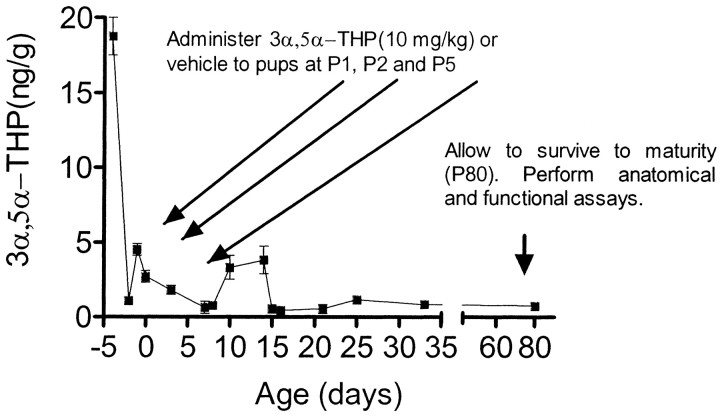
Animals. All animals were obtained, housed, and killed in accordance with approved Institutional Animal Care and Use Committee protocols. Timed pregnant females (Sprague Dawley rats) were obtained from Charles River (Raleigh, NC), singly housed, and allowed food and water ad libitum. They were closely watched, and on the day of birth (designated day 0), mothers were removed from the cage and litters were culled to eight pups, two per treatment group (vehicle, P1, P2, and P5). Neonatal 3α,5α-THP levels are at their nadir during the first week (Fig.1); therefore, the first week of life was chosen for the exogenous administration of 3α,5α-THP to optimally model the effects of neurosteroid fluctuation on GABAergic interneuron development as concentrations that could be observed after severe stress (Purdy et al., 1991). Pups received 3α,5α-THP (10 mg/kg, in-phase), 3α,21-dihydroxy-5α-pregnane-20-one [tetrahydrodeoxycorticosterone (THDOC); 8 mg/kg, in-phase], or vehicle (20% β-cyclodextrins). This dose of neurosteroid was chosen as the maximal dose possible without sedation. A similar dose (8 mg/kg) in adult animals raises cortical 3α,5α-THP levels to the range observed with swim stress (Vallée et al., 2000). Pups were immediately returned to their mother after injections, weaned, and separated into male and female cages at 22 d of age. Because cortical GABA systems are slow to develop, particularly the most anterior parts of the cortex, and because many behaviors that are stress-sensitive appear only after adolescent maturational events, measures of GABAergic neurotransmission were examined in adult tissue. Therefore, animals were allowed to survive until 80 d of age (P80).
Fig. 1.
Experimental design. In rat brain, cortical levels of 3α,5α-THP vary widely across development, showing a secondary elevation during the second week of life (line graph). To mimic potential consequences of a severe stress event, 3α,5α-THP was exogenously administered to rat pups on P1, P2, and P5 (long arrows). Animals were allowed to survive to maturity (P80), at which point brain tissue was collected for analysis (short arrow).
Tissue preparation. At maturity (P80), developmentally treated and littermate control animals (n = 6–8) were deeply anesthetized and transcardially perfused with 100 ml of warm saline followed by 250 ml of 4% paraformaldehyde. Brains were removed and postfixed for 4 hr in 4% paraformaldehyde and then blocked. Six sets of sequential 40 μm sections from the anterior pole through the genu of the corpus callosum were cut on a vibratome. For [3H]MK-801 binding and neurosteroid assays, animals were killed, the brain was removed, and regions of interest were rapidly dissected and stored at −80°C until time of assay. For chloride flux assays, the anterior third of cortical tissue was dissected away, kept cold on ice, and used immediately.
Immunohistochemistry. To control for potential processing bias, one set of free-floating sections from each condition was simultaneously processed for each antibody (Ab). Sections were rinsed in PBS, and then incubated (1 hr) in blocking buffer containing 0.5% detergent (Triton X-100) and 3% normal serum (goat or donkey, depending on the species of the secondary Ab). Sections were exposed to primary antibody overnight at 4°C with gentle agitation. Parvalbumin and calbindin were chosen for study because calcium-binding proteins are frequently colocalized with GABA, and parvalbumin- and calbindin-labeled cells together comprise a large portion of the GABAergic population (DeFelipe, 1993). Microtubule associated protein-2 (MAP2) was used as a marker for dendritic processes. Primary antibodies against calbindin (1:1000), parvalbumin (1:2000), and MAP2 (1:500) were obtained from Sigma (St. Louis, MO) and used at empirically determined dilutions. After primary antibody exposure, sections were rinsed in PBS, incubated with biotinylated IgG (secondary Ab) for 1 hr, washed again, and exposed to ABC solution (Elite Kit; Vector Laboratories, Burlingame, CA) for 1 hr. Finally, sections were washed in PBS and reacted with diaminobenzidine plus hydrogen peroxide to form a visible (brown) reaction product. After rinsing in PBS, sections were mounted on adhesive slides, counterstained with cresyl violet, dehydrated, and coverslipped. The specificity of the antibody was confirmed on adjacent sections by omitting incubation with the primary antibody; no immunoreactivity was observed.
Areas of interest of equivalent anteroposterior coordinates between vehicle- and neurosteroid-treated tissue were identified. Sections were chosen for analysis if they contained the infralimbic portion of the PFC; every sixth section ≤800 μm anterior of the genu of the corpus callosum was systematically selected to control for sampling consistency. The infralimbic portion of the PFC was defined as a band 900 μm wide extending from the pial surface laterally to white matter 200 μm dorsal of olfactory ventricle ependyma. Separate sets of sections were analyzed for parvalbumin and calbindin immunoreactivity under 200× magnification using an ocular grid (0.30 mm2); this area was examined for cell body staining (four grid areas were sampled per animal, 7–10 animals per group, for a total of 8–12 mm2 per condition). Laminar assignments were defined using cytoarchitectural features revealed by counterstain according to the atlas of Swanson (1992). To control for a possible change in total cell number and to obtain a gross measure of relative cell density, unbiased by potential differences in cortical thickness, data were expressed as a ratio of deep to superficial cell placement. Immunoreactive cells in layers I-III were counted as superficial expression; layers V and VI were counted as deep. Laminar cell counts were expressed as a percentage of total immunoreactive cells relative to intraexperimental controls. A volume estimation of the sampling area was made by multiplying the grid area (0.30 mm2) by section thickness (0.04 mm). A separate set of sections was stained with cresyl violet (Nissl) and counted independently using a 0.06 mm2 counting frame. All stained cells wholly within the grid or touching the top or left edge were counted. A fourth set of sections was analyzed for MAP2 immunoreactivity using image analysis software (Micro Computer Imaging Device; Imaging Research, St. Catherine, Ontario, Canada) to determine optical density of staining for MAP2 in each grid area.
[3H]MK-801 binding.Well-washed membranes were prepared from tissue homogenate of prefrontal and parietal cortex of adult males using methods described previously (Goodnough and Hawkinson, 1995) and stored at −80°C for 1–3 d. On the day of assay, pellet was resuspended in Tris-acetate (50 mm, 5 mm EDTA, pH 7.4) and washed twice; and final pellet was resuspended to a final concentration of 0.5–1 mg protein/ml. Single-point [3H]MK-801 (10 nm) binding was performed in the presence of coactivators (10 μm glutamate, 10 μmglycine, 50 μmMg2+). Nonspecific binding was determined in the presence of 100 μm MK-801. Binding reaction was terminated by rapid filtration after a 90 min incubation period. Radioactivity on polyethyleneimine presoaked filters was measured by liquid scintillation counting. Protein determinations were made using the Lowry method, and data are expressed as maximal binding in picomoles per milligram of protein.
Chloride ion influx assay. Synaptoneurosomes were prepared as described previously (Morrow et al., 1987). The final pellet was resuspended in 2.4 vol of ice-cold assay buffer for a final protein concentration of ∼5 mg/ml. The homogenate (200 μl) was aliquoted per assay tube and preincubated at 30°C for 12 min. Muscimol-gated Cl− influx was initiated by addition of 0.2 μCi of [36Cl−] in the presence of various concentrations of muscimol (0.5–100 μm), and uptake was terminated after 5 sec by addition of 4 ml of ice-cold assay buffer containing 100 μm picrotoxin. Basal chloride uptake was measured in the absence of muscimol and subtracted from all tubes to determine muscimol-gated chloride influx. Protein determinations were made using the Lowry method. Data were expressed as net [36Cl−] uptake or potentiation in nanomoles per milligram of protein. Concentration–response curves obtained in [36Cl−] influx assays were evaluated using computerized nonlinear regression, and the resultant Emax and EC50 values for each experiment were compared by ANOVA.
Neurosteroid assay. Cortical 3α,5α-THP levels were determined as described previously (Janis et al., 1998). Briefly, cerebral cortex samples were spiked with [3H]3α,5α-THP, digested, and extracted with ethyl acetate. Extracts were partially purified over a silica column, dried, and redissolved in RIA buffer. A sheep polyclonal antibody (1:1000; gift from CoCensys, Inc., Irvine, CA) raised against 3α,5α-THP was used in the RIA. Samples were compared with known standards.
Data analysis. All data were analyzed using a commercially available statistical program (Prism; Graph Pad, San Diego CA), using ANOVA with post hoc analysis (Tukey's or Student's t test) where appropriate.
Results
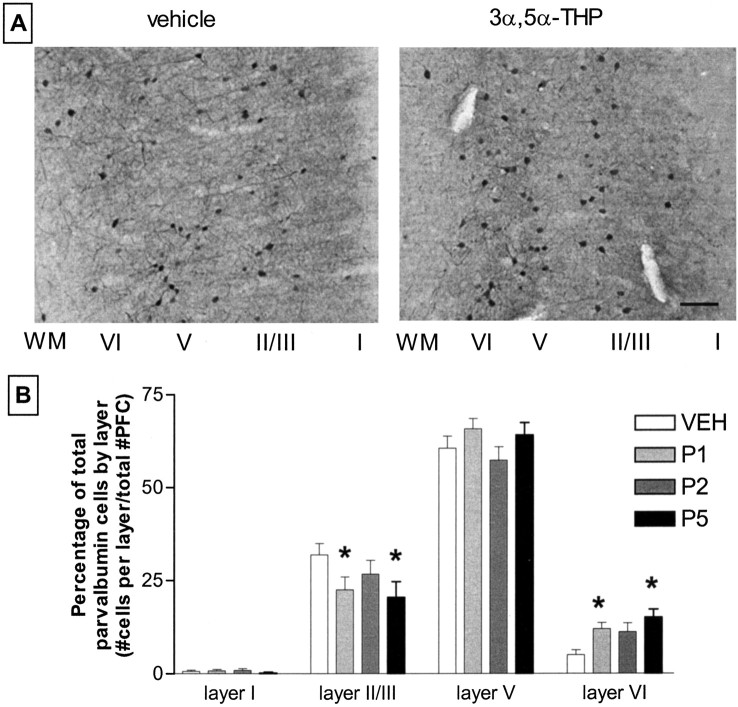
Cortical parvalbumin immunoreactivity is altered in adult animals that were administered 3α,5α-THP during the first week of postnatal development. Perinatal 3α,5α-THP administration on P1 and P5 decreases parvalbumin immunoreactivity in superficial layers of PFC with a corresponding increase in parvalbumin immunoreactivity in the deep layers (Fig. 2). The developmental effects of neurosteroid administration were dependent on the postnatal day of 3α,5α-THP administration. Perinatal neurosteroid administration on P2 did not result in altered parvalbumin-immunoreactive cell localization in the adult. These effects were observed in both male and female rats, with no significant difference in parvalbumin immunoreactivity across gender; therefore, data are collapsed.
Fig. 2.
Perinatal neurosteroid administration alters parvalbumin-immunoreactive cell localization in prefrontal cortex.A, top, Representative photomicrographs of stained PFC from animals treated with vehicle or 3α,5α-THP (10 mg/kg, i.p.) at P5. Cortical layers are labeled (I-VI) right toleft, with white matter (WM) oriented to the far left. Scale bar, ∼100 μm.B, bottom, A laminar analysis of the effect of 3α,5α-THP administration at different time points after birth. Pups receiving 3α,5α-THP on P1 and P5 developed into adults with altered parvalbumin-immunoreactive cell localization, whereas those administered 3α,5α-THP on P2 did not. Data are mean ± SEM the percentage of total parvalbumin-labeled cells in cortical layers I, II/III, V, and VI from bilateral coronal 40 μmsections of infralimbic PFC. ANOVA, p = 0.009 Dunnett's post hoc analysis; *p < 0.001; n = 7–10 per group. VEH, Vehicle.
To determine whether there is an overall difference in the total number of parvalbumin-immunoreactive cells in PFC, the number of parvalbumin-immunoreactive cells (regardless of layer assignment) was divided by a volume estimation of the total sampling area (in cubic millimeters). Total parvalbumin-immunoreactive or Nissl-stained cell densities in PFC are not different across groups (Table 1). However, the relative placement of parvalbumin-immunoreactive cells within the PFC is altered with neonatal 3α,5α-THP administration. The ratio of deep (V and VI) to superficial layer (I-III) parvalbumin-immunoreactive cell number increases from 2.41 ± 0.26 in vehicle-exposed animals to 5.27 ± 0.8 and 5.72 ± 1.03 in P1 and P5 3α,5α-THP-exposed animals, respectively (p< 0.01; overall ANOVA, p = 0.0086). Animals that received 3α,5α-THP on P2 exhibit an intermediate deep/superficial parvalbumin expression ratio of 3.46 ± 0.6 (p > 0.05). Laminar analysis of parvalbumin immunoreactivity shows that neonatal 3α,5α-THP administration results in a specific increase in labeling density in layer VI with a concomitant decrease in layer II/III (Fig. 2). There is also a trend for more parvalbumin immunoreactivity in the deeper portions of layer V, relative to the superficial regions (data not shown). In two of seven animals receiving 3α,5α-THP on P5, parvalbumin-immunoreactive cells are observed in the underlying white matter of the frontal cortex (Fig. 3). The demonstration of altered parvalbumin immunoreactivity in layers II/III and VI supports the conclusion that GABAergic interneuron localization is altered, and that data are not artifacts of cortical compression.
Table 1.
Numerical densities (number of neurons per cubic millimeter) of parvalbumin-immunoreactive and Nissl-stained cells in PFC
| Treatment | Parvalbumin | Nissl |
|---|---|---|
| VEH | 4672 ± 546 | 106500 ± 11520 |
| P1 | 4505 ± 434 | 110200 ± 7341 |
| P2 | 4551 ± 338 | 106000 ± 8343 |
| P5 | 4479 ± 373 | 110600 ± 5878 |
Total parvalbumin-immunoreactive cell density in PFC was not different across groups. To determine whether there was an overall difference in the total number of parvalbumin-immunoreactive cells in PFC, the number of parvalbumin-immunoreactive cells (regardless of layer assignment) was divided by a volume estimation of the sampling area made by multiplying the grid area (0.30 mm2) by section thickness (0.04 mm). A separate set of sections was stained with cresyl violet (Nissl) and counted independently. A 0.06 mm2 counting frame was used. All stained cells wholly within the grid or touching the top or left edge were counted.
Fig. 3.
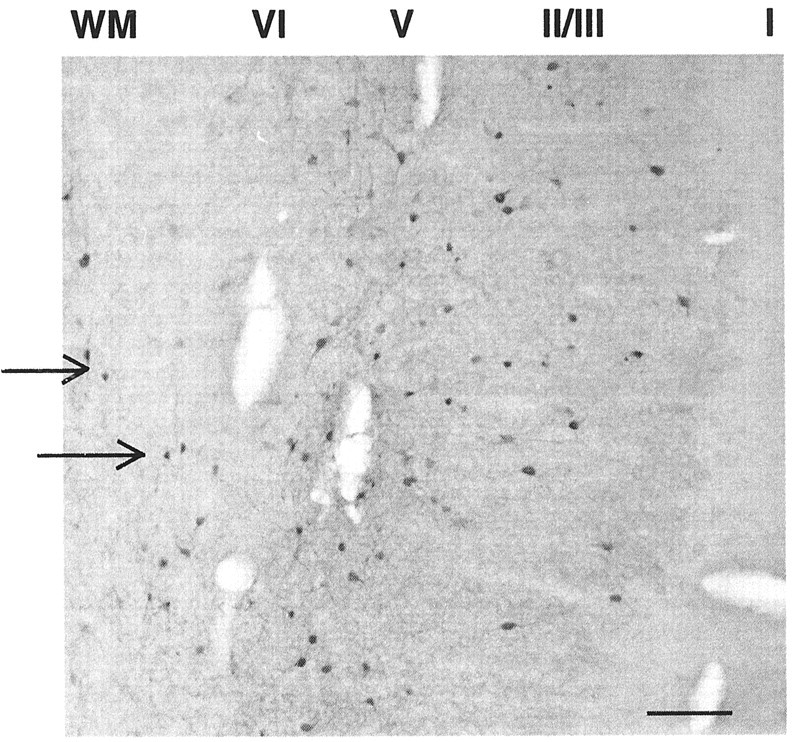
Developmental neurosteroid insult elicits aberrant parvalbumin-immunoreactive cell expression in white matter (WM). Parvalbumin-immunoreactive cells are present in white matter underlying the PFC (arrows) in two of seven animals receiving 3α,5α-THP (10 mg/kg) on P5. Cortical layers are labeled (I–VI)right to left with white matter oriented to the far left. Scale bar, ∼100 μm
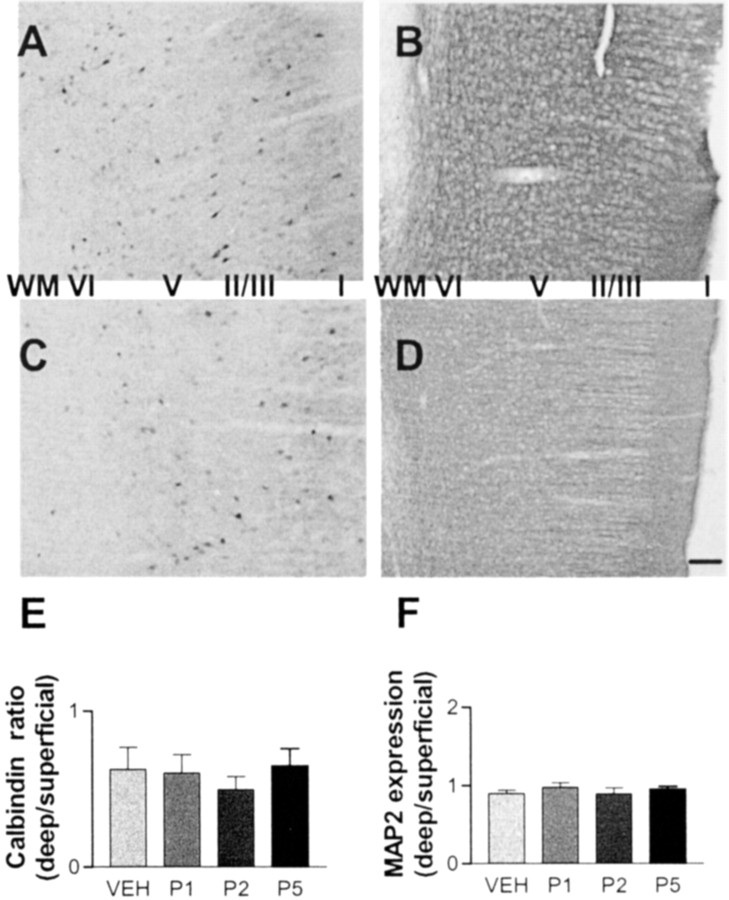
To determine the specificity of perinatal neurosteroid insult for affecting GABAergic interneurons, additional markers were examined. GABAergic interneurons in the cortex can be subdivided by differential expression of calcium-binding proteins. Calbindin-expressing interneurons are distinct from parvalbumin-positive cells. MAP2 immunoreactivity identifies neuronal processes in general, including pyramidal cells in the cortex. Only certain populations of interneurons are affected by early postnatal 3α,5α-THP administration; perinatal neurosteroid insult does not alter calbindin cell localization or MAP2 immunoreactivity density (Fig. 4). No difference between groups in total number or laminar placement of calbindin-immunoreactive cells is observed (ANOVA; p = 0.76). Densitometry does not reveal a shift in MAP2 immunoreactivity between layers in PFC (ANOVA; p = 0.47). As with parvalbumin immunoreactivity, no gender difference is observed in MAP2 or calbindin immunoreactivity.
Fig. 4.
Perinatal neurosteroid insult does not alter calbindin or MAP2 immunoreactivity. GABAergic interneurons in the cortex can be subdivided by differential expression of calcium-binding proteins. A–D, P5 rat pups received vehicle (A, B) or 10 mg/kg 3α,5α-THP (C, D) and were allowed to survive to maturity. Fixed 40 μm sections through PFC and anterior cingulate were processed for calbindin (A,C) and MAP2 (B,D) immunoreactivity. Cortical layers are labeled (I-VI) right toleft, with white matter (WM) oriented to thefar left. Scale bar, ∼100 μm. E, No difference between groups in total number or laminar placement of calbindin-immunoreactive cells was observed. F, Densitometry did not reveal a shift in MAP2 immunoreactivity between layers in PFC. VEH, Vehicle.
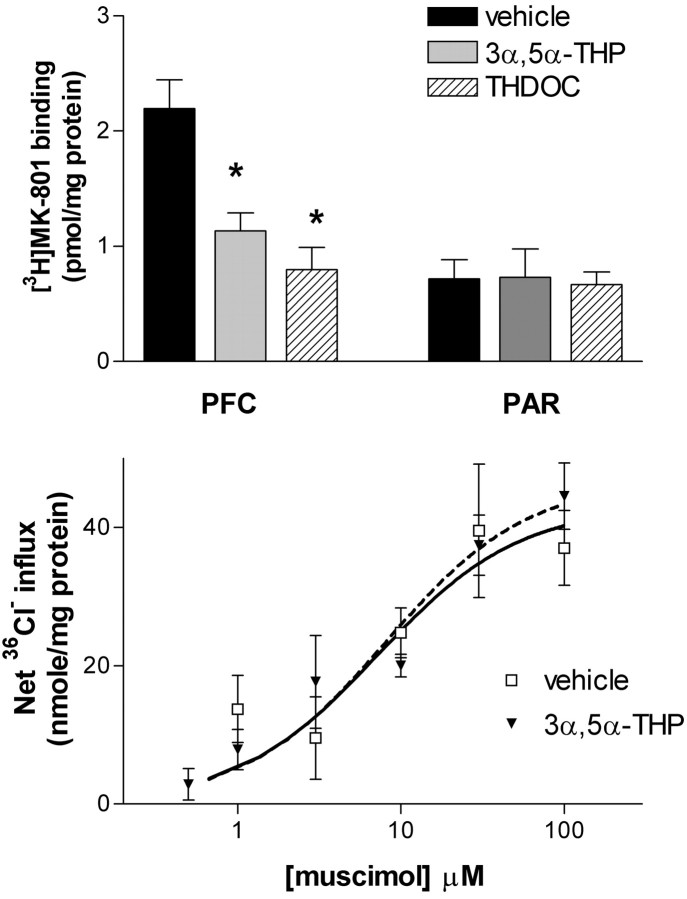
To explore the functional significance of the observed alterations in GABAergic interneuron cell localization in PFC, indices of glutamatergic and GABAergic transmission were examined. Only male tissue was used, because GABA and NMDA receptor function can vary with stage of estrous in females (Wilson, 1992; Bi et al., 2001). Because [3H]MK-801 binds to the activated state of the NMDA receptor complex, it is used as a functional assay in vitro (Foster and Wong, 1987). To assess the anatomical specificity of an effect at a gross level, single-point binding was performed on tissue from discrete regions of cortex (prefrontal and parietal). Perinatal neurosteroid (3α,5α-THP and THDOC) exposure reduced agonist-dependent [3H]MK-801 binding (10 nm) in PFC from 2.2 ± 0.2 to 1.1 ± 0.2 (3α,5α-THP) and 0.8 ± 0.2 (THDOC) pmol/mg protein (Fig. 5). In contrast, agonist-dependent [3H]MK-801 binding in tissue from parietal cortex was not altered by perinatal neurosteroid exposure.
Fig. 5.
Perinatal neurosteroid administration alters agonist-dependent [3H]MK-801 binding to NMDA receptors but not GABAA receptor-mediated Cl− uptake in adult PFC. A,Top, [3H]MK-801 binding is reduced in adult PFC of male rats after perinatal neurosteroid administration. [3H]MK-801 (10 nm) binding was determined in well washed membranes prepared from PFC and parietal cortex (PAR) of adult male rats perinatally administered vehicle, 3α,5α-THP (10 mg/kg), or THDOC (8 mg/kg) on P5. Data are means ± SEM. Overall, ANOVA, p = 0.0017; *p < 0.05 compared with vehicle (n = 4–5). B,Bottom, GABAA receptor function of adult frontal cortex tissue is not affected by perinatal neurosteroid administration. Muscimol (0.5–100 μm) was added to synaptoneurosomes prepared from anterior one-third of cortex from P80 male rats that had received 3α,5α-THP (10 mg/kg) or vehicle on P5. Data are means ± SEM (n = 5–6).
To determine whether perinatal 3α,5α-THP exposure alters GABAergic neurotransmission, GABAA receptor-mediated36Cl− influx was measured in synaptoneurosomes from adult frontal cortex of vehicle- or 3α,5α-THP-exposed animals. Previous studies have demonstrated that GABAA receptor activity is sensitive to developmental diazepam exposure (Kellogg, 1999). Perinatal 3α,5α-THP administration did not alter the potency (EC50) or maximal effect (Emax) of GABA-mediated Cl− uptake in adult male rats (Fig.5).
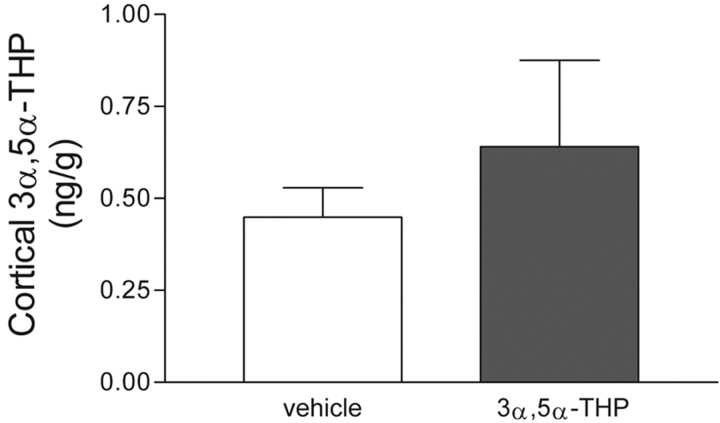
GABAA receptor function could be desensitized because of the presence of increased concentrations of GABA or modulator. Therefore, to determine whether perinatal neurosteroid exposure alters adult levels of endogenous modulator, cortical 3α,5α-THP levels were measured in adult male rats that were developmentally exposed to 3α,5α-THP. Cortical 3α,5α-THP levels are not different between groups (Fig.6).
Fig. 6.
Cortical 3α,5α-THP levels are unchanged in adult male rats treated perinatally with neurosteroid. Steroids were extracted from anterior one-third of cortex from P80 males that had received 3α,5α-THP (10 mg/kg) or vehicle on P5; 3α,5α-THP levels were then determined using a RIA. Data are means ± SEM of five to six determinations.
Discussion
The main finding of this study is a demonstration that developing rat forebrain interneurons are sensitive to perturbation by neuroactive steroids. The observed decrease in parvalbumin-immunoreactive cells in adult PFC layer II/III, and concomitant increase in layer VI without an overall change in parvalbumin-immunoreactive cell number, suggests a change in the localization of a subset of interneurons in response to neurosteroid. Alternatively, 3α,5α-THP may be acting in situ to change parvalbumin expression patterns (see below). A shift in relative parvalbumin immunoreactivity toward increased expression in deep cortical layers and anomalous expression in the underlying white matter is consistent with altered PFC development. Parvalbumin-immunoreactive cells are usually distributed across layers in agranular cortex; PFC displays enhanced neuropil and cellular immunolabeling in layer III (Uylings and van Eden, 1990). Presumably, this pattern reflects the integrative role played by this class of neurons in cortical circuitry. Altered localization of parvalbumin-labeled GABAergic interneurons could result in altered cortical connectivity and processing. The observed decrease in [3H]MK-801 binding in PFC is consistent with this possibility. Similar alterations in perinatal neurosteroid levels in the brain could occur in response to severe stress (Purdy et al., 1991; Vallée et al., 2000) and may be a relevant model for the understanding of the role of stress-induced steroids on PFC neurodevelopment.
Because measurements of parvalbumin immunoreactivity are relative, several steps were taken to reduce the possibility of artifact in these analyses. First, parvalbumin expression levels are expressed in both absolute and relative terms, thus controlling for potential overall changes in cell density or number. Second, tissue was processed simultaneously; every experiment contained tissue from every treatment condition, reducing the possibility of artifact. It is possible that the change in parvalbumin staining is a selective change in the proportion of neurons that express parvalbumin. However, the lack of change in overall parvalbumin-immunoreactive cell number (Table1) argues against such a scenario. Thus, the altered localization of parvalbumin-immunoreactive cells in PFC likely reflects an altered distribution of a distinct subset of cortical GABAergic interneurons.
The ability of an exogenously delivered increase in perinatal cortical neurosteroid levels to affect a change in the distribution of a subset of cortical interneurons suggests that normal fluctuations in cortical neurosteroid levels may play a role in normal cortical maturation. Clearly, GABA plays a role in normal neuronal development by regulating proliferation in the developing neocortex (LaMantia, 1995). However, less is known about how endogenous GABAA receptor modulators affect neuronal development. Recent data show that in the absence of GABA synthesis, neurite outgrowth of cortical plate neurons is attenuated, but can be reinitiated by exposure to 3α,5α-THP (Maric et al., 2001). Thus, there are neurosteroid-sensitive GABAA receptor pathways that could contribute to normal cortical maturation. Recent studies of developing GABAergic neurons show that neocortical interneurons originate in the ventricular zone of the subpallium and migrate tangentially rather than radially through the telencephalon (Marin and Rubenstein, 2001). Expression of homeobox genes has been identified as a key component of the lateral to medial gradient of these tangential migratory pathways (Anderson et al., 1997). However, mechanisms modulating laminar placement of cortical GABA interneurons are not well documented. The genetic null mutant rodents used to study tangential migration are generally embryonic lethal; therefore postnatal development events are difficult to study. It is possible that endogenous neurosteroids such as 3α,5α-THP modulate the localization of a subset of cortical interneurons during lamination. Alternatively, increases in cortical 3α,5α-THP during perinatal development may result in a latent vulnerability that is not expressed until the completion of other maturational events. For example, perinatal exposure to other GABAA modulators, such as diazepam, results in apparently normal juvenile offspring that exhibit behavioral deficits as adults (Kellogg, 1999).
Positive modulation of GABAA receptor function is one potential mechanism by which 3α,5α-THP could alter cortical development. However, there are other possibilities not addressed by these data. 3α,5α-THP regulates steroid levels via the hypothalamic–pituitary–adrenal axis (Owens et al., 1992), PFC dopamine levels (Grobin et al., 1992), and GABAAreceptor structure (Sundstrom-Poromaa et al., 2002). Thus, stress axis modulation or disruption of naturally occurring steroid levels could influence cell migration and/or cell placement. Normal developmental processes may rely on specific increases in neurosteroid levels as signals. Because a peak in endogenous cortical 3α,5α-THP levels normally occurs at approximately P10–P15 (Grobin and Morrow, 2001), the exogenous administration of 3α,5α-THP on P1 or P5 may represent an early signal that changes parvalbumin-labeled cell localization. Ablation of the steroid level peaks previously observed late in gestation or during the second week of life may help elucidate the normal developmental role of neurosteroids in the frontal cortex. Alternatively, neurosteroid-altered dopamine levels could influence cortical development (Porter et al., 1999). More work is needed to address these questions.
Altered location of a subpopulation of neurons (parvalbumin-labeled interneurons) in PFC may affect the ability of PFC to function properly. No change in GABAAreceptor-mediated Cl− influx without a change in parvalbumin-positive cell number is consistent with the idea that connectivity or communication between classes of neurons, rather than molecular structure, may be affected by perinatal neurosteroid administration. The observation that altered GABAergic cell localization is accompanied by a decrease in [3H]MK-801 binding with no change in GABA-mediated Cl− uptake across the entire PFC is consistent with altered cortical connectivity. [3H]MK-801 binds to NMDA receptors found on both pyramidal cells and GABAergic interneurons in PFC, and there are many more interneurons than pyramidal cells; thus, decreased [3H]MK-801 binding may reflect a decrease in NMDA receptor density on GABAergic interneurons. Alternatively, because MK-801 binds only to the activated state of NMDA receptors, decreased [3H]MK-801 binding may indicate depressed NMDA receptor-mediated function (Foster and Wong, 1987). Either interpretation, combined with regional specificity (no effect in parietal cortex), suggests a change in PFC circuits that are considered important for executive information processing and cognitive performance. Such a change is consistent with alterations in cortical connectivity resulting from altered GABAergic cell localization; however, because studies of MK-801 binding and GABAA receptor-mediated Cl− uptake were performed in tissue taken from the entire PFC, it is impossible to know whether these indices reflect cellular function of all interneurons or only the subset of displaced interneurons. Thus, it is likely that analysis of discrete circuits in superficial versus deep layers of cortex will be required to understand the functional sequelae of these alterations in GABAergic interneuron localization.
The timing of neurosteroid exposure may be an important component of the development of abnormal cortical morphology. Data presented herein suggest that parvalbumin-immunoreactive neurons are sensitive to neurosteroid perturbation during discrete time points. Animals exposed at P2 were not affected to the same extent as animals exposed at P1 or P5. This may reflect a lack of statistical power, and additional studies with more subjects may demonstrate that similar changes are found with animals exposed at P2. However, it is possible that differential sensitivity to the effects of neurosteroids results from the temporal characteristics of native neurosteroid levels. Normal cortical 3α,5α-THP levels at P2 decline after having peaked at E18 and before nadir at P6 (Grobin and Morrow, 2001). Because GABAA receptor structure and function are altered by withdrawal of high levels of 3α,5α-THP (Smith et al., 1998b), it is possible that P2 is a uniquely insensitive time point because it is situated between 3α,5α-THP withdrawal and 3α,5α-THP level nadir. It is also possible that late-maturation events are key to the expression of 3α,5α-THP-induced alterations in cortical morphology. In fact, parvalbumin immunoreactivity is not expressed in rodent brain until the second week of life (Uylings and van Eden, 1990). Hence, the combination of these factors may explain the apparent window of vulnerability to perinatal neurosteroid administration.
These data are potentially relevant to stress-sensitive developmental disorders such as schizophrenia. Prenatal stress is a predisposing factor for the development of schizophrenia, and perinatal 3α,5α-THP administration to rats is developmentally comparable with third trimester stress-induced increases in neurosteroid levels on human PFC neurodevelopment (Lewis, 2000). Abnormal development of GABAergic interneurons is one hypothesized locus of schizophrenia etiopathophysiology (Benes, 2000). Various findings in schizophrenia postmortem studies suggest alterations of GABAergic circuitry in the PFC, including reduction of neuropil (Ohnuma et al., 1999), decreased GABA synthesis (Akbarian et al., 1995) with compensatory increased GABAA receptor binding (Benes et al., 1992,1996), deficits in layer III/IV parvalbumin-positive cell populations (Lewis et al., 2001), and the presence of NADPH–diaphorase-positive cells in white matter underlying the PFC (Akbarian et al., 1996). The shift in parvalbumin immunoreactivity from superficial to deep layers of the PFC and the presence of parvalbumin-immunoreactive cells in the underlying white matter in perinatally 3α,5α-THP-treated rats are remarkably similar to several findings in schizophrenia postmortem studies described above. Thus, if GABAergic neurotransmission within selected cortical regions is compromised in the brain of schizophrenic patients, it is tempting to speculate that endogenous effectors of this system (neurosteroids) are plausible candidate molecules in the regulation of neurodevelopment leading to increased risk for schizophrenia.
These studies demonstrate that an endogenously produced GABAergic neurosteroid has the capacity to alter the localization of a subset of GABAergic interneurons when administered perinatally. The temporal specificity of this effect and its limited expression to parvalbumin-positive cells suggests that endogenous neurosteroids may play a specific role in the developing frontal cortex. Decreased agonist-dependent [3H]MK-801 binding suggests a functional alteration in cortical circuitry that could explain behavioral effects observed after maturity. Moreover, these data have important implications for the development of vulnerability to multiple stress-sensitive diseases including depression, alcoholism, and schizophrenia.
Footnotes
This work was supported by National Institute on Alcohol Abuse and Alcoholism Grant AA10564 to A.L.M., the Mental Health and Neuroscience Clinical Research Center Grant MH33127 to J.A.L., and the Foundation of Hope (Raleigh, NC). We gratefully acknowledge Todd O'Buckley for excellent technical assistance and Jean Lauder, Anthony LaMantia, and Ariel Deutch for helpful discussions regarding manuscript preparation.
Correspondence should be addressed to A. Chistina Grobin, Department of Psychiatry, CB 7160, University of North Carolina at Chapel Hill, Chapel Hill, NC 27599-7178. E-mail: grobinac@med.unc.edu.
References
- 1.Akbarian S, Kim JJ, Potkin SG, Hagman JO, Tafazzoli A, Bunney WE, Jr, Jones EG. Gene expression for glutamic acid decarboxylase is reduced without loss of neurons in prefrontal cortex of schizophrenics. Arch Gen Psychiatry. 1995;52:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 2.Akbarian S, Kim JJ, Potkin SG, Hetrick WP, Bunney WE, Jr, Jones EG. Maldistribution of interstitial neurons in prefrontal white matter of the brains of schizophrenic patients. Arch Gen Psychiatry. 1996;53:425–436. doi: 10.1001/archpsyc.1996.01830050061010. [DOI] [PubMed] [Google Scholar]
- 3.Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- 4.Barbin G, Pollard H, Gaiarsa JL, Ben-Ari Y. Involvement of GABAA receptors in the outgrowth of cultured hippocampal neurons. Neurosci Lett. 1993;152:150–154. doi: 10.1016/0304-3940(93)90505-f. [DOI] [PubMed] [Google Scholar]
- 5.Baulieu EE. Steroid hormone regulation of the brain. In: Fuxe K, Gustafsson JA, Wetterber L, editors. Proceedings of International Symposium at Wenner–Gren Center, Stockholm. Pergamon; Oxford: 1981. pp. 3–14. [Google Scholar]
- 6.Behar TN, Schaffner AE, Scott CA, O'Connell C, Barker JL. Differential response of cortical plate and ventricular zone cells. J Neurosci. 1998;18:6378–6387. doi: 10.1523/JNEUROSCI.18-16-06378.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ben-Ari Y, Khazipov R, Leinekugel X, Caillard O, Gaiarsa JL. GABAA, NMDA and AMPA receptors: a developmentally regulated “menage a trois.”. Trends Neurosci. 1997;20:523–529. doi: 10.1016/s0166-2236(97)01147-8. [DOI] [PubMed] [Google Scholar]
- 8.Benes FM. Emerging principles of altered neural circuitry in schizophrenia. Brain Res Brain Res Rev. 2000;31:251–269. doi: 10.1016/s0165-0173(99)00041-7. [DOI] [PubMed] [Google Scholar]
- 9.Benes FM, Vincent SL, Alsterberg G, Bird ED, SanGiovanni JP. Increased GABAA receptor binding in superficial layers of cingulate cortex in schizophrenics. J Neurosci. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benes FM, Vincent SL, Marie A, Khan Y. Up-regulation of GABAA receptor binding on neurons of the prefrontal cortex in schizophrenic subjects. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 11.Bi R, Foy MR, Vouimba RM, Thompson RF, Baudry M. Cyclic changes in estradiol regulate synaptic plasticity through the MAP kinase pathway. Proc Natl Acad Sci USA. 2001;98:13391–13395. doi: 10.1073/pnas.241507698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bitran D, Hilvers RJ, Kellogg CK. Anxiolytic effects of 3α-hydroxy-5α[β]-pregnan-20-one: endogenous metabolites of progesterone that are active at the GABAA receptor. Brain Res. 1991a;561:157–161. doi: 10.1016/0006-8993(91)90761-j. [DOI] [PubMed] [Google Scholar]
- 13.Bitran D, Primus RJ, Kellogg CK. Gestational exposure to diazepam increases sensitivity to convulsants that act at the GABA/benzodiazepine receptor complex. Eur J Pharmacol. 1991b;196:223–231. doi: 10.1016/0014-2999(91)90434-r. [DOI] [PubMed] [Google Scholar]
- 14.Brinton RD. The neurosteroid 3α-hydroxy-5α-pregnan-20-one induces cytoarchitectural regression in cultured fetal hippocampal neurons. J Neurosci. 1994;14:2763–2774. doi: 10.1523/JNEUROSCI.14-05-02763.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Concas A, Follesa P, Barbaccia ML, Purdy RH, Biggio G. Physiological modulation of GABAA receptor plasticity by progesterone metabolites. Eur J Pharmacol. 1999;375:225–235. doi: 10.1016/s0014-2999(99)00232-0. [DOI] [PubMed] [Google Scholar]
- 16.DeFelipe J. Neocortical neuronal diversity: chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- 17.Ellenbroek BA, Cools AR. The long-term effects of maternal deprivation depend on the genetic background. Neuropsychopharmacology. 2000;23:99–106. doi: 10.1016/S0893-133X(00)00088-9. [DOI] [PubMed] [Google Scholar]
- 18.Foster AC, Wong EH. The novel anticonvulsant MK-801 binds to the activated state of the N-methyl-d-aspartate receptor in rat brain. Br J Pharmacol. 1987;91:403–409. doi: 10.1111/j.1476-5381.1987.tb10295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Frieder B, Epstein S, Grimm VE. The effects of exposure to diazepam during various stages of gestation or during lactation on the development and behavior of rat pups. Psychopharmacology (Berl) 1984;83:51–55. doi: 10.1007/BF00427422. [DOI] [PubMed] [Google Scholar]
- 20.Goodnough DB, Hawkinson JE. Neuroactive steroid modulation of [3H]muscimol binding to the GABAA receptor complex in rat cortex. Eur J Pharmacol. 1995;288:157–162. doi: 10.1016/0922-4106(95)90190-6. [DOI] [PubMed] [Google Scholar]
- 21.Grobin AC, Morrow AL. 3α-Hydroxy-5α-pregnan-20-one exposure reduces GABAA receptor α4 subunit mRNA levels. Eur J Neurosci. 2000;409:R1–R2. doi: 10.1016/s0014-2999(00)00797-4. [DOI] [PubMed] [Google Scholar]
- 22.Grobin AC, Morrow AL. 3α-Hydroxy-5α-pregnan-20-one levels and GABAA receptor-mediated 36Cl− flux across development in rat cerebral cortex. Brain Res Dev Brain Res. 2001;131:31–39. doi: 10.1016/s0165-3806(01)00242-5. [DOI] [PubMed] [Google Scholar]
- 23.Grobin AC, Roth RH, Deutch AY. Regulation of the prefrontal cortical dopamine system by the neuroactive steroid 3α,21-dihydroxy-5α-pregnane-20-one. Brain Res. 1992;578:351–356. doi: 10.1016/0006-8993(92)90270-j. [DOI] [PubMed] [Google Scholar]
- 24.Gruen RJ, Wenberg K, Elahi R, Friedhoff AJ. Alterations in GABAA receptor binding in the prefrontal cortex following exposure to chronic stress. Brain Res. 1995;684:112–114. doi: 10.1016/0006-8993(95)00441-r. [DOI] [PubMed] [Google Scholar]
- 25.Janis GC, Devaud LL, Mitsuyama H, Morrow AL. Effects of chronic ethanol consumption and withdrawal on the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in male and female rats. Alcohol Clin Exp Res. 1998;22:2055–2061. [PubMed] [Google Scholar]
- 26.Kellogg CK. Sex differences in long-term consequences of prenatal diazepam exposure: possible underlying mechanisms. Pharmacol Biochem Behav. 1999;64:673–680. doi: 10.1016/s0091-3057(99)00137-9. [DOI] [PubMed] [Google Scholar]
- 27.Kellogg CK, Frye CA. Endogenous levels of 5 alpha-reduced progestins and androgens in fetal versus adult rat brains. Brain Res Dev Brain Res. 1999;115:17–24. doi: 10.1016/s0165-3806(99)00041-3. [DOI] [PubMed] [Google Scholar]
- 28.Kellogg CK, Primus RJ, Bitran D. Sexually dimorphic influence of prenatal exposure to diazepam on behavioral responses to environmental challenge and on γ-aminobutyric acid (GABA)-stimulated chloride uptake in the brain. J Pharmacol Exp Ther. 1991;256:259–265. [PubMed] [Google Scholar]
- 29.LaMantia AS. The usual suspects: GABA and glutamate may regulate proliferation in the neocortex. Neuron. 1995;15:1223–1225. doi: 10.1016/0896-6273(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 30.Lauder JM, Liu JP, Devaud LL, Morrow AL. GABA as a trophic factor for developing monoamine neurons. Perspect Dev Neurobiol. 1998;5:247–259. [PubMed] [Google Scholar]
- 31.Lewis DA. Is there a neuropathology of schizophrenia? The Neuroscientist. 2000;6:208–218. [Google Scholar]
- 32.Lewis DA, Cruz DA, Melchitzky DS, Pierri JN. Lamina-specific deficits in parvalbumin-immunoreactive varicosities in the prefrontal cortex of subjects with schizophrenia: evidence for fewer projections from the thalamus. Am J Psychiatry. 2001;158:1411–1422. doi: 10.1176/appi.ajp.158.9.1411. [DOI] [PubMed] [Google Scholar]
- 33.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 34.Maric D, Liu Q-Y, Maric I, Chaudry S, Chang Y-H, Smith SV, Sieghart W, Fritschy J-M, Barker JL. GABA expression dominates neuronal lineage progression in the embryonic rat neocortex and facilitates neurite outgrowth via GABAA autoreceptor/Cl− channels. J Neurosci. 2001;21:2343–2360. doi: 10.1523/JNEUROSCI.21-07-02343.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marin O, Rubenstein JL. A long, remarkable journey: tangential migration in the telencephalon. Nat Rev Neurosci. 2001;2:780–790. doi: 10.1038/35097509. [DOI] [PubMed] [Google Scholar]
- 36.Mellon SH, Griffin LD. Neurosteroids: biochemistry and clinical significance. Trends Endocrinol Metab. 2002;13:35–43. doi: 10.1016/s1043-2760(01)00503-3. [DOI] [PubMed] [Google Scholar]
- 37.Micheva KD, Beaulieu C. Development and plasticity of the inhibitory neocortical circuitry with an emphasis on the rodent barrel field cortex: a review. Can J Physiol Pharmacol. 1997;75:470–478. [PubMed] [Google Scholar]
- 38.Morrow AL, Suzdak PD, Paul SM. Steroid hormone metabolites potentiate GABA receptor-mediated chloride ion flux with nanomolar potency. Eur J Pharmacol. 1987;142:483–485. doi: 10.1016/0014-2999(87)90094-x. [DOI] [PubMed] [Google Scholar]
- 39.Motzo C, Porceddu ML, Maira G, Flore G, Concas A, Dazzi L, Biggio G. Inhibition of basal and stress-induced dopamine release in the cerebral cortex and nucleus accumbens of freely moving rats by the neurosteroid allopregnanolone. J Psychopharmacol. 1996;10:266–272. doi: 10.1177/026988119601000402. [DOI] [PubMed] [Google Scholar]
- 40.Ohnuma T, Augood SJ, Arai H, McKenna PJ, Emson PC. Measurement of GABAergic parameters in the prefrontal cortex in schizophrenia: focus on GABA content, GABAA receptor α-1 subunit messenger RNA and human GABA transporter-1 (HGAT-1) messenger RNA expression. Neuroscience. 1999;93:441–448. doi: 10.1016/s0306-4522(99)00189-x. [DOI] [PubMed] [Google Scholar]
- 41.Owens MJ, Ritchie JC, Nemeroff CB. 5α-Pregnane-3α,21-diol-20-one (THDOC) attenuates mild stress-induced increases in plasma corticosterone via a nonglucocorticoid mechanism: comparison with alprazolam. Brain Res. 1992;573:353–355. doi: 10.1016/0006-8993(92)90788-b. [DOI] [PubMed] [Google Scholar]
- 42.Porter LL, Rizzo E, Hornung J-P. Dopamine affects parvalbumin expression during cortical development in vitro. J Neurosci. 1999;19:8990–9003. doi: 10.1523/JNEUROSCI.19-20-08990.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Purdy RH, Morrow AL, Moore PH, Jr, Paul SM. Stress-induced elevations of gamma-aminobutyric acid type A receptor-active steroids in the rat brain. Proc Natl Acad Sci USA. 1991;88:4553–4557. doi: 10.1073/pnas.88.10.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Smith SS, Gong QH, Hsu F-C, Markowitz RS, Ffrench-Mullen JMH, Li X. GABAA receptor α4 subunit suppression prevents withdrawal properties of an endogenous steroid. Nature. 1998a;392:926–930. doi: 10.1038/31948. [DOI] [PubMed] [Google Scholar]
- 45.Smith SS, Gong QH, Li X, Moran MH, Bitran D, Frye CA, Hsu F. Withdrawal from 3α-OH-5α-pregnan-20-one using a pseudopregnancy model alters the kinetics of hippocampal GABAA-gated current and increases the GABAA receptor α4 subunit in association with increased anxiety. J Neurosci. 1998b;18:5275–5284. doi: 10.1523/JNEUROSCI.18-14-05275.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sundstrom-Poromaa I, Smith DH, Gong QH, Sabado TN, Li X, Light A, Wiedmann M, Williams K, Smith SS. Hormonally regulated alpha(4)beta(2)delta GABA(A) receptors are a target for alcohol. Nat Neurosci. 2002;5:721–722. doi: 10.1038/nn888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swanson LW. Brain maps: structure of the rat brain. Elsevier; Amsterdam: 1992. [Google Scholar]
- 48.Twyman RE, Macdonald RL. Neurosteroid regulation of GABAA receptor single-channel kinetic properties of mouse spinal cord neurons in culture. J Physiol (Lond) 1992;456:215–245. doi: 10.1113/jphysiol.1992.sp019334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Uylings HB, van Eden CG. Qualitative and quantitative comparison of the prefrontal cortex in rat and in primates, including humans. Prog Brain Res. 1990;85:31–62. doi: 10.1016/s0079-6123(08)62675-8. [DOI] [PubMed] [Google Scholar]
- 50.Vallée M, Rivera JD, Koob GF, Purdy RH, Fitzgerald RL. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal Biochem. 2000;287:153–166. doi: 10.1006/abio.2000.4841. [DOI] [PubMed] [Google Scholar]
- 51.VanDoren MJ, Matthews DB, Janis GC, Grobin AC, Devaud LL, Morrow AL. Neuroactive steroid 3α-hydroxy-5α-pregnan-20-one modulates electrophysiological and behavioral actions of ethanol. J Neurosci. 2000;20:1982–1989. doi: 10.1523/JNEUROSCI.20-05-01982.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson MA. Influences of gender, gonadectomy, and estrous cycle on GABA/BZ receptors and benzodiazepine responses in rats. Brain Res Bull. 1992;29:165–172. doi: 10.1016/0361-9230(92)90022-p. [DOI] [PubMed] [Google Scholar]
- 53.Xu W, Cormier R, Fu T, Covey DF, Isenberg KE, Zorumski CF, Mennerick S. Slow death of postnatal hippocampal neurons by GABAA receptor overactivation. J Neurosci. 2000;20:3147–3156. doi: 10.1523/JNEUROSCI.20-09-03147.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zimmerberg B, McDonald BC. Prenatal alcohol exposure influences the effects of neuroactive steroids on separation-induced ultrasonic vocalizations in rat pups. Pharmacol Biochem Behav. 1996;55:541–547. doi: 10.1016/s0091-3057(96)00281-x. [DOI] [PubMed] [Google Scholar]