Abstract
Although neurokinin 1 (NK1) receptors contribute to hyperalgesia, and their expression is increased in the spinal cord during peripheral inflammation, little is known regarding the signaling molecules and the second messenger pathways that they activate in regulating the expression of the NK1 receptor gene. Because the promoter region of the NK1 receptor contains a cAMP response element (CRE), we tested the hypothesis that calcitonin gene-related peptide (CGRP) regulates the expression of NK1 receptors via a pathway involving activation of the transcription factor cAMP response element binding protein (CREB). Experiments were conducted on primary cultures of neonatal rat spinal neurons. Treatment of cultures with CGRP for 8–24 hr increased125I-substance P binding on spinal neurons; the increase in binding was preceded by an elevation in NK1 receptor mRNA. The CGRP-induced change in 125I-substance P binding was concentration-dependent and was inhibited by the antagonist CGRP8–37. CGRP increased phosphorylated CREB immunoreactivity and CRE-dependent transcription in neurons, indicating the involvement of the transcription factor CREB. Evidence that CGRP increased cAMP levels in spinal neurons and that the protein kinase A inhibitor H89 attenuated CGRP-induced CRE-dependent transcription suggests that the intracellular pathway stimulated by CGRP leads to activation of protein kinase A. Collectively these data define a role for CGRP as a signaling molecule that induces expression of NK1 receptors in spinal neurons. The data provide evidence that a neuropeptide receptor controls gene expression in the CNS and add another dimension to understanding the cotransmission of substance P and CGRP by primary afferent neurons.
Keywords: CGRP, spinal cord, neurokinin receptor, CREB, substance P, prostaglandin E2
Introduction
Tachykinins, endogenous ligands of the neurokinin 1 (NK1) receptor, induce hyperalgesia after administration to the spinal cord. (Sweeney and Sawynok, 1986; Yashpal et al., 1993). Dynorphin (Laughlin et al., 1997) and brain-derived neurotrophic factor (BDNF; Groth and Aanonsen, 2002) have similar effects. Evidence of increased endogenous levels of these neuromodulators in the spinal cord during peripheral inflammation [tachykinins (Mapp et al., 1993; Galeazza et al., 1995), dynorphin (Ruda et al., 1988), and BDNF (Cho et al., 1997)] as well as increased expression of NK1 receptors (Abbadie et al., 1996; Honore et al., 1999) and trkB receptors, which bind BDNF (Mannion et al., 1999), suggests a role for these neuromodulators in hyperalgesia. The increases in peptides and receptor proteins occur in conjunction with increased levels of mRNA in primary afferent neurons [tachykinin (Donaldson et al., 1992) and BDNF (Mannion et al., 1999)] or spinal neurons [NK1 receptor (Schafer et al., 1993; McCarson and Krause, 1994) and dynorphin (Ruda et al., 1988)], indicating that increases in gene expression contribute to the changes in protein levels. However, little is known about the transmembrane signals that initiate intracellular signaling that leads to changes in gene expression in spinal neurons.
Induction and activation of transcription factors that regulate gene expression occur in the spinal cord as a consequence of peripheral inflammation. After the initial report of c-fos induction in spinal neurons (Hunt et al., 1987), increased expression of c-jun (Messersmith et al., 1998) as well as phosphorylation of cAMP response element binding protein (CREB) (Ji and Rupp, 1997; Messersmith et al., 1998;Anderson and Seybold, 2000) and nuclear factor κ-B (Chan et al., 2000) have been described. Changes in activity of transcription factors indicate that intracellular signaling pathways are mediating changes in protein expression. For example, cAMP activates protein kinase A, which phosphorylates CREB, and the promoter region of the NK1 receptor gene has a binding site for CREB (Gerard et al., 1991; Hershey et al., 1991).
A potential signaling molecule for regulating expression of NK1 receptors by spinal neurons would be released after peripheral injury and would activate intracellular signaling pathways leading to activation of CREB. We tested the hypothesis that calcitonin gene-related peptide (CGRP) is a transmembrane signaling molecule that regulates the expression of NK1 receptors in spinal neurons. CGRP is released centrally from primary afferent neurons in response to noxious stimuli (Morton and Hutchison, 1989), and its expression is increased in primary afferent neurons in models of peripheral inflammation (Donaldson et al., 1992; Mapp et al., 1993; Galeazza et al., 1995). However, the physiological relevance of CGRP receptors in the spinal cord has been elusive. We predicted that CGRP would increase the expression of NK1 receptors, because activation of CGRP receptors increases cAMP in spinal neurons (Parsons and Seybold, 1997), and the expression of NK1 receptors increases in spinal neurons in response to treatment with analogues of cAMP (Abrahams et al., 1999). Because a cAMP response element (CRE) site occurs within the promoter region of the NK1 receptor gene, we also tested whether CGRP activates CRE-dependent gene expression in spinal neurons.
Materials and Methods
Preparation of primary cell cultures. A model of primary cultures of dissociated neonatal rat spinal cord cells was used in these studies. Under normal conditions, NK1 receptor binding is restricted to neurons in these cultures (Seybold and Abrahams, 1995), and the receptor is functional (Parsons et al., 1995). Cultures of neonatal rat spinal cord were prepared as described previously (Seybold and Abrahams, 1995). Briefly, spinal cords were removed from 1- to 2-d-old neonatal Sprague Dawley rats using a protocol that was approved by the Animal Care and Use Committee of the University of Minnesota. Spinal cords were minced, digested in trypsin, and mechanically dissociated by trituration. The resulting cell suspension was centrifuged, and the recovered cells were resuspended in nutrient medium consisting of DMEM, 10% heat-inactivated horse serum, 100 U/ml penicillin, 100 μg/ml streptomycin, and 0.25 μg/ml amphotericin B.
Cell suspensions were aliquoted into laminin-coated wells of 24-well Costar (Cambridge, MA) plates. Because non-neuronal cells attach to the substrate more rapidly than neuronal cells (Bray, 1991), the cell suspension was incubated in the prepared tissue culture wells for 45 min, removed, and replated on fresh laminin-coated wells at 160,000 cells per well. This procedure produces cultures of non-neuronal cells from the initial plating and neuron-enriched cultures from the later plating (Seybold and Abrahams, 1995). Cells were grown in DMEM, 10% heat-inactivated horse serum (HS), 10% fetal bovine serum, and the antibiotics listed above, and the medium was changed once per week. Neuron-enriched cultures were used between 11 and 14 d in vitro. Non-neuronal cultures were used after 3–4 weeks in culture, at which time the density of non-neuronal cells and amount of protein were comparable with those of neuron-enriched cultures.
Treatment of cultures with agonists and inhibitors. Cell cultures were treated with CGRP and prostaglandin E2 (PGE2) for various periods before analyses of treatment effects. All treatments were performed at 37°C with an atmosphere of 90% O2and 10% CO2. Treatment was initiated by replacing the medium with fresh medium containing agonists. Tetrodotoxin (TTX, 0.5 μm) was included in all incubations to minimize secondary effects of agonists caused by release of other transmitters in the cultures. In some experiments, the CGRP1 receptor antagonist CGRP8–37 was used to test for receptor-mediated events. For measures of substance P (SP) binding and production of cAMP, treatments occurred in DMEM and 10% HS. To mimic persistent activation of receptors in some experiments, aliquots of agonist (10 μl) were added to the culture medium at 2 hr intervals to repeat the same final concentration as the initial treatment.
When determining effects of CGRP on CRE-dependent transcription, treatments were administered in DMEM and 1% HS. Some wells were treated with CGRP8–37 (1 hr) or the protein kinase A inhibitors H89 and 8-bromo (Br)-Rp-cAMPS (30 min) before the addition of agonist. The medium was then removed, and CGRP with or without inhibitor was added to the wells. Two hours later, an aliquot (10 μl) of agonist with or without the antagonist was added to yield the same final concentration as the first treatment. After 1 hr, the volume in each well was brought up to 1 ml with DMEM and 1% HS. The cells were returned to the incubator, and CRE-luciferase activity was measured the following day.
125I-Bolton Hunter-substance P binding assay.NK1 receptors can be quantified by measuring the binding of125I-SP. The protocol for measurement of high affinity binding of 125I-Bolton Hunter-SP (125I-SP, ∼2000 Ci/mmol) on primary cell cultures has been described in detail previously (Seybold and Abrahams, 1995). Unless otherwise noted, specific binding of125I-SP was measured 24 hr after initiation of treatment with agonist, and analyses were conducted at 4°C to decrease the uptake of 125I-SP into the cells. Briefly, before incubation with ligand, cultures were rinsed in binding buffer (10 mm HEPES, 150 mmNaCl, 5 mm KCl, 1 mmMgCl2, 2 mmCaCl2, and 0.1% bovine serum albumin, pH 7.4, plus 40 μg/ml bacitracin, 4 μg/ml leupeptin, and 2 μg/ml chymostatin). Cultures were then incubated with125I-SP (50 pm) for 105 min; nonspecific binding was determined in the presence of 100 nm SP. After washing to minimize nonspecific binding, cells were collected in 0.1 m PBS and 1% Triton X-100, and bound radiolabel was measured using a gamma counter. Unless otherwise noted, binding data are expressed as percentage of average specific binding measured in untreated wells within each experiment. Treatment with vehicles for the agonists had no effect on specific binding. To monitor the quality of the ligand, the percentage of specific binding of the ligand was calculated for total and nonspecific binding using a standard preparation of tissue. Under these conditions, the mean ± SEM percentage of specific binding of125I-SP was 89 ± 1% for 21 preparations of the ligand. Therefore, differences in binding in response to agonist treatment are not likely attributable to variability in the quality of the ligand used to measure NK1 receptors.
Total cellular protein per well was determined on randomly selected wells within each culture preparation using the Bradford protein assay (Bradford, 1976).
Measurement of cAMP. The formation of cAMP in the cultures was determined after agonist stimulation. Production of cAMP was stopped 10 min after the last addition of agonist by adding ice-cold 0.8 m HClO4 to each well. Samples were sonicated for 5 sec, transferred to 12 × 75 mm glass test tubes, and stored at –80°C until assayed. On the day of the assay, the samples were slowly thawed on ice, neutralized with ice-cold 0.8 N K2CO3, mixed, and centrifuged at 2500 × g at 4°C for 15 min. Aliquots of the final supernatant were acetylated, and the acetylated samples were assayed for cAMP using a commercial radioimmunoassay kit.
Measurement of mRNA for the NK1 receptor. To obtain sufficient mRNA for the measurement of NK1 receptor mRNA, the neuron-enriched cell suspension of neonatal rat spinal cord was plated in 9.5 cm2 wells at a density of 760,000 cells per well. After treatment of cultures for 4 hr with CGRP, total cellular RNA was extracted using the guanidinium-acid-phenol method (Chomczynski and Sacchi, 1987). Three wells from each treatment were pooled to obtain ∼10 μg of total mRNA. Total RNA samples were assayed for NK1 receptor and β-actin mRNAs using solution hybridization-nuclease protection assays as described previously (McCarson and Krause, 1994, 1995).
Three different plasmid constructs were used for the analysis of RNA in this study (McCarson and Krause, 1994, 1995). For the analysis of NK1 receptor mRNA, an NK1 receptor 3′ coding region plasmid [pSPR(+1213 → +1800)] and a full coding region plasmid [pSPR(+577 → +1800)] were used. For the analysis of β-actin mRNA, a β-actin coding region plasmid [pBS-rβA210] was used. The NK1 receptor and β-actin plasmids were linearized using restriction enzymes, and antisense cRNA probes were generated using T3 or T7 RNA polymerases and [α-32P]UTP (3000 Ci/mmol). Samples of total RNA were assayed for NK1 receptor (∼9.5 μg of total RNA/sample) or β-actin (∼0.4 μg of total RNA/sample) using the solution hybridization-nuclease protection assay. The nuclease digestion reaction products were precipitated and electrophoresed on urea-containing polyacrylamide gels, which were fixed, dried, and exposed to phosphor plates for 16–24 hr. Densitometric images were generated and analyzed as described previously (McCarson and Krause, 1994, 1995). The amount of NK1 receptor mRNA within each sample was normalized to the amount of β-actin mRNA within the same sample.
Immunocytochemistry. Cells were treated for 30 min with CGRP in DMEM and 1% HS with 0.5 μm TTX. After treatment, the cells were washed once with 0.1 m PBS, pH 7.4, and immediately fixed in 4% paraformaldehyde for 10 min at room temperature. After three 15 min rinses in PBS, cells were permeabilized with a solution of PBS, 0.1% Na azide, 10% normal donkey serum, and 0.3% Triton X-100 for 2 hr at room temperature. The cells were then incubated with a mixture of rabbit anti-pCREB (1:100) and mouse anti-β-tubulin III (1:200) antibodies overnight at 4°C. β-Tubulin-like immunoreactivity was used as a positive marker for neurons within the cultures (Lee et al., 1990). The next day, cells were rinsed three times with PBS and then incubated in a mixture of rhodamine red X-donkey anti-rabbit (1:100) and cyanine 2-donkey anti-mouse (1:100) antibodies for 1 hr at room temperature. After three 15 min rinses in PBS, cultures were coverslipped in PBS/glycerin (1:3).
β-Tubulin- and pCREB-like immunoreactivities were differentially visualized using a fluorescence microscope. Images of immunoreactivity were collected using a 20× objective and consistent camera settings for each antigen. The average pixel intensity of pCREB immunofluorescence was measured within the region of the nucleus of β-tubulin-immunoreactive cells using Image1 software (black = 0). The level of fluorescence in the cytoplasm of non-neuronal cells within each culture under illumination of rhodamine was defined as background and was subtracted from the value of pCREB-immunofluorescence to obtain a value for pCREB-specific immunofluorescence. Data were pooled from three culture preparations.
CRE-luciferase. Cultured cells were transfected with CRE-luciferase DNA to test for CRE-dependent gene expression in response to CGRP. After 10–11 d in vitro, cultured spinal neurons were transiently transfected with a CRE-luciferase reporter construct (1 μg of DNA/well) using the calcium phosphate method as described previously (Xia et al., 1996). After transfection, cells were washed with DMEM before replacing their original medium and were used in experiments the following day. Cells were lysed ∼48 hr after transfection and assayed for luciferase using a commercial light assay kit. Fluorescence was quantified using a luminometer.
Statistical comparisons. Each experiment was conducted at least three times on cultures prepared from different litters. Unless otherwise noted, experimental values were normalized to untreated samples that were included in each iteration of an experiment to reduce variability in data among culture preparations. Unless otherwise noted, data are summarized as the mean ± SEM for wells within each treatment group. One or two-way ANOVAs with Tukey's multiple comparisons test were used to compare results within and between groups. Student's t test was used when appropriate. Differences between means were considered significant atp < 0.05. Fisher's exact test was used to determine whether the proportion of pCREB-immunoreactive neurons was altered by treatment with CGRP.
Materials. Timed pregnant Sprague Dawley rats were obtained from Sasco Inc. (Omaha, NE). Bacto-Trypsin was purchased from Difco (Detroit, MI), and sera and antibiotics included in culture media were purchased from Invitrogen (Grand Island, NY). DMEM, TTX, and enzyme inhibitors were obtained from Sigma (St. Louis, MO). α-CGRP (rat), α-CGRP8–37 (human), and SP were purchased from Peninsula (San Carlos, CA), and PGE2 was purchased from Cayman Chemical Co. (Ann Arbor, MI). H89 and 8-Br-Rp-cAMPS were obtained from Calbiochem (San Diego, CA). Amersham Biosciences (Arlington Heights, IL) was the source for the125I-SP, and NEN (Boston, MA) was the source for the cAMP radioimmunoassay kit. RNA polymerases were purchased from Roche Molecular Biochemicals (Indianapolis, IN). The CRE-luciferase reporter was a gift of E. K. Heist (Stanford University, Stanford, CA). The Tropix kit used for the luciferase assay was purchased from Applied Biosystems (Atlanta, GA). The rabbit anti-pCREB antibody was obtained from Upstate Biotechnology (Lake Placid, NY), and the mouse anti-β-tubulin III antibody was obtained from Sigma. The secondary antibodies and normal donkey sera were purchased from Jackson ImmunoResearch (West Grove, PA).
Stock solutions of CGRP (100 μm), CGRP8–37 (100 μm and 1 mm), and TTX (1 mm) were prepared in 0.02m acetic acid. The stock solution of PGE2 (10 mm) was prepared in dimethyl sulfoxide. The stock solution of H89 (1 mm) was prepared in H2O.
Results
CGRP increased 125I-SP binding in primary cultures of neonatal rat spinal cord
Initial studies addressed whether CGRP modulates125I-SP binding directly. When CGRP (100 nm) was included with 125I-SP as a condition for ligand binding, there was no effect on specific binding of 125I-SP (control, 100 ± 0.1%; CGRP, 97 ± 0.1% relative to the mean of the control value within a culture preparation; n = 9 wells for each condition). Therefore, CGRP does not affect125I-SP binding by an allosteric mechanism, and long-term effects of CGRP on125I-SP binding cannot be attributed to residual CGRP in the binding conditions.
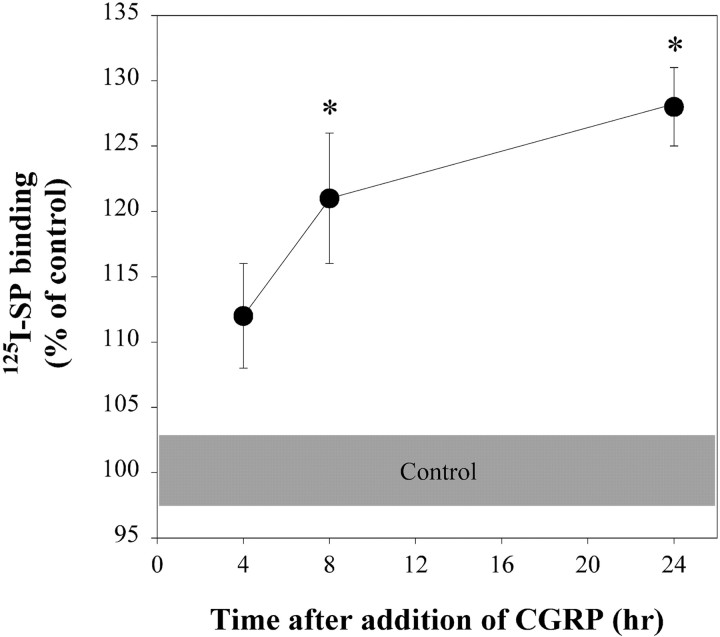
Detection of effects of CGRP on 125I-SP binding required long incubation times (Fig.1). These experiments used a high concentration of CGRP (1 μm) to compensate for catabolism of CGRP during the long treatment periods. No increases in125I-SP binding occurred at incubation periods up to 4 hr; however, binding was greater 8 and 24 hr after addition of CGRP compared with control. The long lag period before an increase in 125I-SP binding occurred is consistent with the effect of exogenous cAMP in this model (Abrahams et al., 1999).
Fig. 1.
CGRP increased 125I-SP binding to neonatal rat spinal neurons in a time-dependent manner. Ligand binding was measured after treatment with CGRP (1 μm) for 4–24 hr. The shaded area represents the average range of the SEM for the untreated control cultures. Data were normalized to the average for control binding within each experiment. *Significantly different from the control group at the same treatment period atp < 0.05 (2-way ANOVA with Tukey's multiple comparisons test). n = 8–9 wells per treatment period (2–3 wells per treatment period per experiment, 3–4 experiments per treatment period).
The increase in 125I-SP binding after treatment with CGRP occurred predominately on neurons in cultures from neonatal rat spinal cord (Table 1). The amount of 125I-SP binding was 10-fold greater in neuron-enriched cultures compared with cultures of non-neuronal cells. Under control conditions, total binding of125I-SP in cultures of non-neuronal cells was not significantly different from nonspecific binding of the ligand. Treatment with CGRP caused a small increase in specific binding in cultures of non-neuronal cells, but the increase in neuron-enriched cultures was approximately fourfold greater.
Table 1.
The effect of CGRP on 125I-SP binding occurred predominately on neurons
| Type of culture | Treatment | 125I-SP binding (fmol/mg of protein) | Protein (μg/well) |
|---|---|---|---|
| Neuron-enriched | Control | 11.2 ± 1.2 (4)* | 56 ± 3 (4) |
| CGRP (1 μm) | 13.5 ± 1.8 (4)*,1-160 | ||
| Non-Neuronal | Control | 0.9 ± 0.4 (3) | 49 ± 10 (3) |
| CGRP (1 μm) | 1.5 ± 0.3 (3)1-160 |
Specific binding of 125I-SP was determined 24 hr after treatment. Numbers in parentheses are numbers of experiments.
Significantly different from same treatment of non-neuronal cells; p < 0.001 (two-way ANOVA with Tukey's multiple comparisons test).
F1-160: Significantly different, p < 0.05 (Student's t test, paired) relative to control within the same culture preparation.
Receptors mediated the effect of CGRP on125I-SP binding
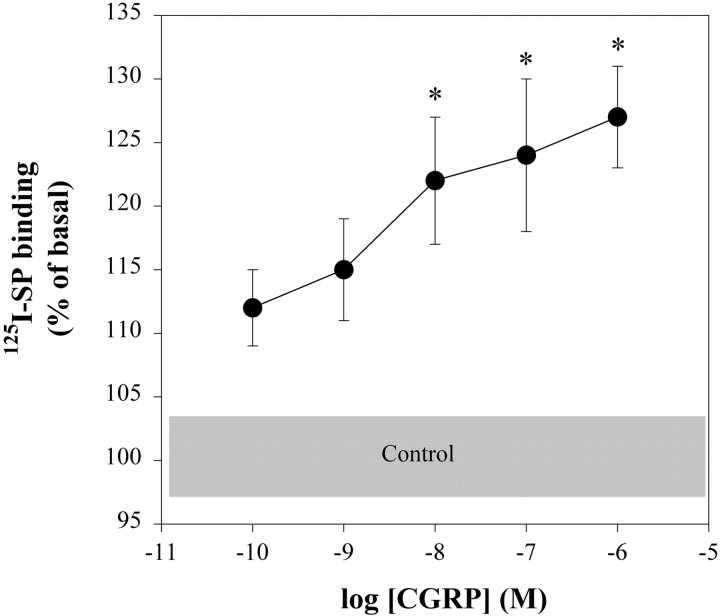
When neuron-enriched cultures of neonatal spinal cord cells were treated for 24 hr with concentrations of CGRP that ranged from 0.1 to 1000 nm, changes in125I-SP binding were concentration-dependent (Fig. 2). Concentrations higher than 1 μm were not tested, because they did not induce a greater increase in the level of cAMP (Parsons and Seybold, 1997). A concentration of 10 nm CGRP was the lowest concentration that increased125I-SP binding. Importantly, the CGRP receptor antagonist CGRP8–37 (3 μm) blocked the CGRP-induced increase in125I-SP binding measured 24 hr after initiation of treatment (3 × 100 nm CGRP, 117 ± 5% of basal, n = 12; CGRP plus CGRP8–37, 97 ± 5,n = 6; p < 0.05; Student's t test). Together these results indicate that the effect of CGRP on 125I-SP binding was receptor-mediated.
Fig. 2.
CGRP increased 125I-SP binding to neonatal rat spinal neurons in a concentration-dependent manner. Ligand binding was measured after treatment with CGRP for 24 hr. IBMX (50 μm) was included in all of the treatments, and the average amount of specific binding in the presence of IBMX alone within an experiment was subtracted from all values for that experiment. Data were normalized to the average for control binding within each experiment. *Significantly different from the control group atp < 0.05 (1-way ANOVA with Tukey's multiple comparisons test). n = 11–24 wells per concentration (2–4 wells per concentration per experiment, 4–8 experiments per concentration).
Not all receptors that couple to adenylyl cyclase increased125I-SP binding
Measurement of cAMP in spinal cord cultures after treatment with CGRP confirmed that CGRP increased cAMP levels in the cells (Table2). The effect of CGRP on cAMP was inhibited by CGRP8–37 but not completely blocked at the concentration of 3 × 100 nm CGRP. The CGRP receptor, however, is not the only receptor that couples to the stimulation of adenylyl cyclase. The prostaglandin EP2 receptor, activated by PGE2, also stimulates adenylyl cyclase (Narumiya et al., 1999), and exogenous PGE2 increases gene expression in peripheral tissue (Ohnishi et al., 2000; Lin et al., 2001). Whereas PGE2 acutely increased cAMP levels in the neuron-enriched cultures of neonatal spinal cord,125I-SP binding was inhibited 24 hr later (Table 3).
Table 2.
CGRP receptors mediated the CGRP-induced increase of cAMP in neuron-enriched cultures
| Treatment | cAMP (pmol/mg protein) | Effect of CGRP8–37 on cAMP |
|---|---|---|
| Control | 19 ± 1 (30) | 23 ± 3 (15) |
| CGRP (1 × 100 nm) | 44 ± 4 (7) | 7 ± 1 (7) |
| CGRP (2 × 100 nm) | 112 ± 7 (12)* | 28 ± 2 (12) |
| CGRP (3 × 100 nm) | 230 ± 15 (7)* | 68 ± 2 (7)2-160 |
CGRP8–37 was used at 3 μm. Multiple treatments occurred at 2 hr intervals. Samples were collected 10 min after the last addition of agonist. Control groups for multiple addition of CGRP were not different from each other, so these groups were pooled. Numbers in parentheses are numbers of wells per treatment (two or three wells per treatment per experiment, three to five experiments per treatment).
p < 0.001 compared with control within the same group and cAMP production in the presence of antagonist.
F2-160: p < 0.05 compared with control within the same group (two-way ANOVA with Tukey's multiple-comparisons test).
Table 3.
Treatment with PGE2 did not increase125I-SP binding
| Treatment | cAMP (pmol/mg of protein) | 125I-SP binding (% of control) |
|---|---|---|
| Control | 17 ± 3 (20) | 100 ± 3 (17) |
| PGE2 (1 × 1 μm) | 72 ± 11 (9)3-150 | 98 ± 5 (10) |
| PGE2 (3 × 1 μm) | 147 ± 5 (9)3-150 | 84 ± 3 (6)3-160 |
Multiple treatments occurred at 2 hr intervals. Samples were collected 10 min after the last addition of PGE2 for determination of cAMP, and 125I-SP binding was determined 24 hr after initiation of treatment. 125I-SP binding was normalized to the mean of samples for control binding within the same culture preparation. Control groups for multiple additions of PGE2 were not different from each other so these groups were pooled. Numbers in parentheses are numbers of wells per treatment (two or three wells per treatment per experiment, three or four experiments per treatment with the exception of SP-binding after 3 × 1 μm PGE2, which was replicated once.
F3-150: p < 0.001 compared with all other groups within the measure.
F3-160: p < 0.05 compared with control within the measure (one-way ANOVA with Tukey's multiple-comparisons test).
An increase in NK1 receptor mRNA preceded the CGRP-induced increase in 125I-SP binding
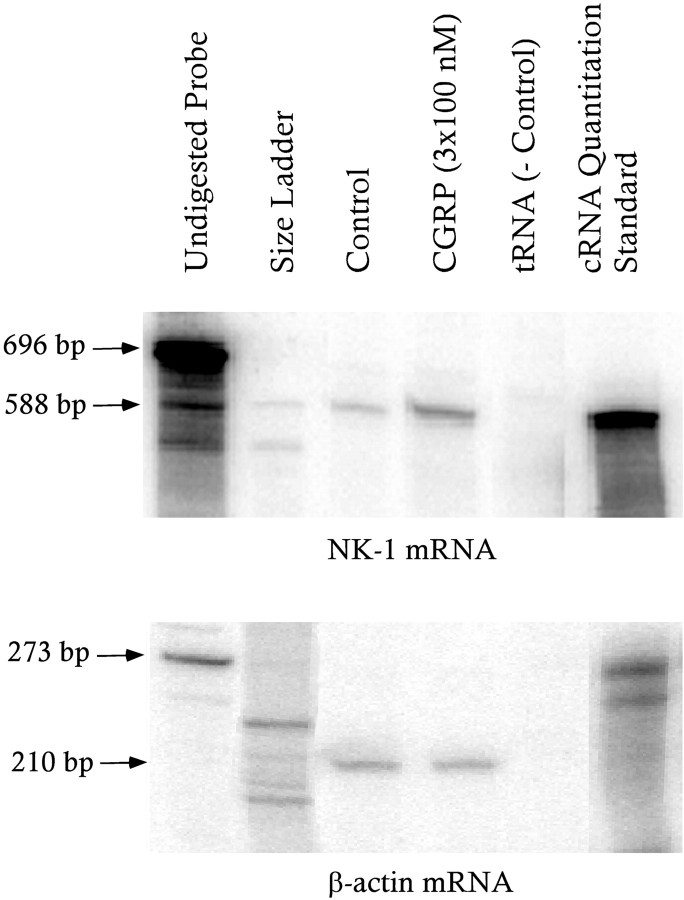
The lag in detection of increased125I-SP binding after treatment with CGRP may reflect time for increased transcription of the gene encoding the NK1 receptor. If this is true, we predicted that treatment with CGRP would increase levels of mRNA for the NK1 receptor before the increase in 125I-SP binding occurred. Treatment of primary cultures with a cell-permeant analogue of cAMP increases mRNA for the NK1 receptor within 4 hr (Abrahams et al., 1999). One or three treatments with CGRP (100 nm) for the same time also increased NK1 receptor mRNA (Table 4). Representative autoradiograms of mRNA detected in the samples are shown in Figure 3.
Table 4.
CGRP increased levels of NK1 receptor mRNA in neuron-enriched spinal cord cultures
| Treatment | NK1 mRNA (pg/ng of β-actin mRNA) |
|---|---|
| Control | 0.40 ± 0.03 (6) |
| CGRP (1 × 100 nm) | 0.98 ± 0.06 (3)4-150 |
| CGRP (3 × 100 nm) | 1.43 ± 0.17 (3)4-150,4-160 |
Multiple treatments occurred at 2 hr intervals. Samples were collected 4 hr after the last addition of CGRP. Numbers in parentheses are numbers of samples per treatment, one sample per culture preparation.
F4-150: p < 0.01 compared with control.
F4-160: p < 0.05 compared with one dose (one-way ANOVA with Tukey's multiple-comparisons test).
Fig. 3.
Representative images of autoradiograms from solution hybridization-nuclease protection assays measuring NK1 receptor (top gel) and β-actin (bottom gel) mRNAs in control and CGRP-treated samples. Each sample for measurement of NK1 receptor mRNA contained 90% of the total RNAs isolated from the sample (for description, see Materials and Methods); each sample for measurement of β-actin mRNA contained the remainder. Note that treatment with CGRP increased the amount of NK1 receptor mRNA. The arrows show the sizes of the undigested receptor antisense RNA probe (696 and 273 bases, respectively) and the RNA species protected by mRNAs or message-sense cRNAs (588 and 210 bases, respectively).
CGRP induced CREB phosphorylation
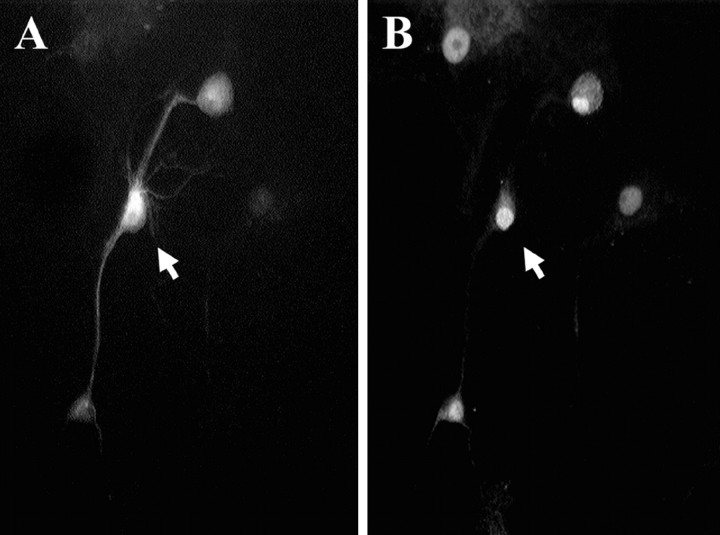
Comparisons of biochemical data for cultures enriched in neurons versus non-neuronal cells provide indirect evidence that CGRP increases gene expression in neurons. To confirm a direct effect of CGRP on neurons, levels of pCREB-like immunoreactivity were measured in β-tubulin-immunoreactive cells after treatment with CGRP (Fig.4). Treatment of cultures with CGRP (300 nm) increased the average pixel intensity of pCREB immunofluorescence within the nucleus of β-tubulin-immunoreactive cells [CGRP, 41 ± 2 relative light units (RLU),n = 191 neurons; control, 30 ± 2 RLU,n = 167; p < 0.002; Student'st test].
Fig. 4.
Representative images illustrating the identification of a spinal neuron in culture by the presence of β-tubulin immunoreactivity (A) and the neuron also exhibiting pCREB-immunofluorescence (B). Images are of the same field under conditions for the visualization of different fluorophores; the arrows mark the same neuron.
The increase in pCREB immunoreactivity was also reflected in an increase in the number of pCREB-positive neurons. In this analysis, a neuron was defined as pCREB-positive if the average level of fluorescence in the nucleus was the mean fluorescence + 1 SD or greater for the culture treatment defined as basal within each experiment (Anderson and Seybold, 2000). By this criterion, 20% of the neurons (n = 167) were pCREB-positive under basal conditions. Treatment with CGRP increased the number of pCREB-positive neurons to 31% (n = 191; p = 0.02; Fisher's exact test).
CGRP increases CRE-dependent gene expression
To establish a more direct link between CGRP receptor activation and gene expression dependent on activation of CREB protein, neuron-enriched spinal cord cultures were transiently transfected with a gene construct in which expression of luciferase was dependent on a CRE-promoter. Treatment with CGRP increased expression of luciferase activity threefold over basal activity in these cultures (Table5), and the increase was preceded by a 20-fold increase in cAMP 2 hr after initiation of treatment. Coincubation of CGRP with the CGRP receptor antagonist CGRP8–37 decreased the effect of CGRP on luciferase expression by 50%, indicating that some luciferase expression was dependent on CGRP receptor-mediated activation of CREB. The luciferase activity in the presence of CGRP plus CGRP8–37 was not different from that in the presence of the antagonist alone but was associated with a residual increase in cAMP.
Table 5.
CGRP increased CRE-dependent gene activity in neuron-enriched spinal cord cultures
| Treatment | cAMP (pmol/mg of protein) | Luciferase activity (% of basal) |
|---|---|---|
| Basal | 16 ± 1 (15) | 98 ± 9 (15) |
| CGRP (2 × 300 nm) | 367 ± 22 (17)5-150 | 330 ± 29 (17)5-150 |
| CGRP8–37 (3 μm) | 20 ± 10 (17) | 140 ± 17 (14) |
| CGRP + CGRP8–37 | 118 ± 10 (17)5-160 | 212 ± 19 (17) |
Multiple treatments occurred at 2 hr intervals. Samples for cAMP were collected 10 min after the last addition of the drug. For determination of CRE-dependent gene expression, treatments were initiated 24 hr after transfection with CRE-luciferase DNA, and samples were collected for assay approximately 21 hr after initiation of treatment. Luciferase activity was normalized to basal levels within the same culture preparation. Numbers in parentheses are numbers of wells per treatment (five or six wells per treatment per experiment; three experiments per treatment). Values were deleted from the data set for luciferase activity if they were <2 SDs below the mean for basal luciferase activity or >2 SDs above the mean for a treatment group. No more than four values were deleted from any treatment group, and the deleted values were evenly distributed between these two conditions.
F5-150: p < 0.001 compared with all other groups within the measure.
F5-160: p < 0.05 compared with antagonist alone (one-way ANOVA with Tukey's multiple-comparisons test).
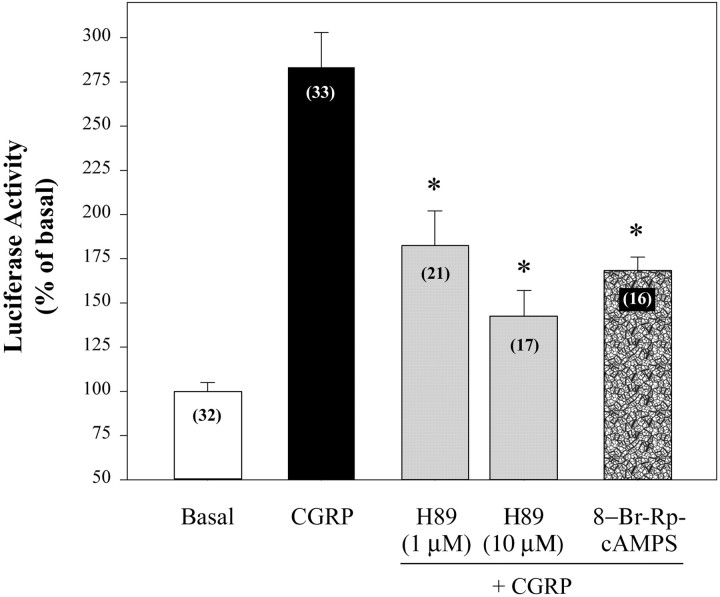
Cotreatment of CGRP with inhibitors of protein kinase A inhibited the effect of CGRP on expression of luciferase up to 80% (Fig.5), further implicating a cAMP signaling pathway in CGRP regulation of CRE-dependent gene expression. The protein kinase A inhibitor H89 had a concentration-dependent effect on CGRP-evoked luciferase activity but had no effect on its own (117 ± 5% of basal luciferase activity at 1 μm;n = 14). The competitive inhibitor of cAMP, 8-Br-Rp-cAMPS, inhibited the response to CGRP by 60%, confirming participation of protein kinase A in CGRP regulation of luciferase expression.
Fig. 5.
CGRP increased CRE-dependent gene activity in neuron-enriched spinal cord cultures through protein kinase A. Primary cultures of neonatal rat spinal neurons were transfected with CRE-luciferase DNA and 24 hr later were treated with CGRP (2 × 300 nm at an interval of 2 hr). Inhibitors were added 30 min before CGRP. 8-Br-Rp-cAMPS was used at 500 μm. Samples were collected for assay ∼21 hr after initiation of CGRP treatment. Luciferase activity was normalized to basal levels within the same culture preparation. Numbers inparentheses are the number of wells per treatment (5–6 wells per treatment per experiment, 3–6 experiments). *p < 0.001 compared with CGRP (1-way ANOVA with Tukey's multiple comparisons test). Values were deleted from the data set for luciferase activity if they were <2 SD below the mean for basal luciferase activity or >2 SD above the mean for a treatment group. No more than three values were deleted from any treatment group, and the deleted values were evenly distributed between these two conditions.
Studies conducted in cultures of non-neuronal cells in parallel with neuron-enriched cultures provided evidence that CGRP-induced expression of CRE-dependent luciferase activity was mediated by neurons in the cultures. Although cultures of non-neuronal cells were grown to the same cellular density as neuron-enriched cultures and transfected with CRE-luciferase under the same conditions, the basal level of luciferase activity was 10-fold less than that in neuronal cultures (Table6). The low basal level of enzyme activity is consistent with using a protocol that favored transfection of neurons over glia (Xia et al., 1996). Importantly, CGRP had no significant effect on luciferase activity in cultures of non-neuronal cells (Table 6).
Table 6.
CRE-dependent expression of luciferase
| Type of culture | Treatment | Luciferase activity (RLU) |
|---|---|---|
| Neuron-enriched | Control | 16.5 ± 1.4 (18)6-150 |
| CGRP (2 × 300 nm) | 42.9 ± 6.0 (18)6-150,6-160 | |
| Non-neuronal | Control | 1.3 ± 0.5 (18) |
| CGRP (2 × 300 nm) | 3.5 ± 1.0 (17) |
Cultures were treated 24 hr after transfection with CRE-luciferase DNA. Multiple treatments with CGRP occurred at 2 hr intervals. Luciferase activity was measured approximately 21 hr after initiation of treatment with CGRP and is reported as RLU per 1000 per well. Numbers in parentheses are numbers of wells per treatment (five or six wells per treatment per experiment, three experiments per treatment). One value was deleted from the CGRP-treated group of non-neuronal cells because it was >2 SDs of the mean for the group.
F6-150: p < 0.001 compared with non-neuronal cultures of the same treatment.
F6-160: p < 0.001 compared with control within the same type of culture (two-way ANOVA with Tukey's multiple-comparisons test).
Discussion
During peripheral inflammation, increased activation of peripheral nociceptors is associated with increased release of tachykinins within the spinal cord (Hope et al., 1990; Schaible et al., 1990) and activation of NK1 receptors (Abbadie et al., 1997; Honore et al., 1999). Activation of spinal NK1 receptors results in increased excitability of spinal neurons (Dougherty et al., 1994; Neugebauer et al., 1995; Parsons et al., 1996) and hyperalgesia (Traub, 1996; Ma et al., 1998). Increased expression of NK1 receptors contributes to maintenance of tachykinin signaling in the spinal cord during peripheral inflammation (Schafer et al., 1993; McCarson and Krause, 1994). This is the first report linking an extracellular signaling molecule with increased expression of NK1 receptors in spinal neurons. Although this result remains to be confirmed in vivo in adult animals, the data add another dimension to understanding cotransmission of substance P and CGRP released from primary afferent neurons and, more broadly, the significance of peptide receptor signaling in the nervous system.
CGRP increased expression of NK1 receptors by a cAMP-dependent pathway
The occurrence of postsynaptic CGRP receptors in the spinal cord has been known for more than a decade (Tschopp et al., 1985; Garry et al., 1991), but the physiological significance of these receptors has not been well defined. The present results demonstrate that CGRP increases expression of NK1 receptors in spinal neurons by receptors that couple to activation of adenylyl cyclase and an intracellular pathway leading ultimately to activation of the transcription factor CREB. Effects of CGRP on production of cAMP, levels of NK1 receptor mRNA, and 125I-SP binding were concentration-dependent. Furthermore, the CGRP receptor antagonist CGRP8–37 attenuated CGRP-induced changes. Collectively, these data support receptor-mediated events in CGRP regulation of NK1 receptor expression. Evidence that CGRP increased cAMP levels and CRE-dependent gene expression, and that H89, an inhibitor of protein kinase A, blocked the effect of CGRP on CRE-dependent gene expression, implicates protein kinase A in a pathway from the CGRP receptor to phosphorylation of CREB. CGRP also increased levels of NK1 receptor mRNA in spinal neurons, although the increase in mRNA may reflect increased transcription or increased stability of the mRNA. Data that CGRP increased pCREB-immunoreactivity in neurons, that pCREB-immunoreactivity increases in NK1 receptor-immunoreactive neurons after a noxious chemical stimulus (Anderson and Seybold, 2000), and that the rat NK1 receptor gene has a CREB binding site (Gerard et al., 1991; Hershey et al., 1991) support the conclusion that increased gene transcription most likely contributed to the increase in expression of NK1 receptors. Extracellular signal-regulated kinase (ERK)–mitogen-activated protein kinase may also be an intermediate in the pathway for CGRP regulation of NK1 receptor expression. CGRP phosphorylates ERK by a protein kinase A-dependent pathway (Parameswaran et al., 2000), and ERK contributes to the increased expression of spinal NK1 receptors during peripheral inflammation (Ji et al., 2002).
Whereas CGRP8–37 always attenuated the effects of CGRP, the antagonist did not reduce the effect of CGRP to basal levels in some multiple-treatment paradigms. The long treatment periods in these experimental protocols may have contributed to the partial inhibition by CGRP8–37. Alternatively, it is possible that CGRP8–37 only partially blocked high concentrations of CGRP, because CGRP activated multiple types of receptors in spinal cord cultures. Blockade of CGRP effects by CGRP8–37 defines a CGRP1receptor-mediated response (Quirion et al., 1992), and CGRP1 receptors generally couple via G-proteins to stimulate adenylyl cyclase in neurons (Zona et al., 1991; Baidan et al., 1992; Parsons and Seybold, 1997). However, the biochemistry of CGRP receptors has recently been complicated by the discovery of accessory proteins that contribute to the functional receptor. Currently, CGRP receptors are believed to be a complex of a seven-transmembrane protein called a calcitonin receptor-like receptor (CRLR), a receptor activity-modifying protein (RAMP) that defines the relative potency of ligands for the receptor, and a receptor component protein (RCP) that defines the G-protein to which the receptor couples (McLatchie et al., 1998; Chakravarty et al., 2000; Evans et al., 2000). The CGRP1 receptor, which binds CGRP and CGRP8–37 with the highest affinity is made up of CRLR, RAMP1, and RCP and couples to production of cAMP (McLatchie et al., 1998). Although CGRP receptors in neurons generally couple to cAMP, CGRP receptors in other tissues couple to the generation of inositol phosphates and the release of calcium from intracellular stores (Laufer and Changeux, 1989; Aiyar et al., 1999). Therefore, other RCP proteins may exist. Although we found no evidence that CGRP receptors couple to production of inositol phosphates in primary cultures of neonatal rat spinal neurons (Parsons and Seybold, 1997), the occurrence of multiple CGRP receptor accessory proteins raises the possibility that some effects of CGRP may be mediated by intracellular signaling pathways activated by other CGRP receptor complexes.
H89 blocked the effect of CGRP at a concentration (10 μm) that was required to inhibit protein kinase A in another population of neurons (Huang et al., 2002). Although this concentration of H89 also inhibits protein kinase G in a cell-free system (Chijiwa et al., 1990), CGRP does not increase production of cGMP at concentrations of <1 μm in primary cultures of neonatal rat spinal cord (Parsons and Seybold, 1997), and cGMP does not increase expression of NK1 receptor mRNA in this preparation (Abrahams et al., 1999). Furthermore, another protein kinase A inhibitor, 8-Br-Rp-cAMPS, also inhibited the response to CGRP. Therefore, it is likely that the effect of H89 on CRE-dependent gene expression that occurred in response to CGRP was mediated by blocking protein kinase A.
Not all receptors that couple to production of cAMP increase SP binding
Multiple receptors can couple to activation of adenylyl cyclase. During peripheral inflammation, spinal levels of PGE2 increase and contribute to central mechanisms of hyperalgesia (Yang et al., 1996; Samad et al., 2001). Although PGE2 activates several subtypes of EP2 receptors that couple to production of cAMP (Narumiya et al., 1999), we found no evidence that PGE2 had effects on SP binding that were similar to those of CGRP. A concentration of PGE2 that increased cAMP in the spinal cord cultures decreased SP receptor binding over the same time course during which CGRP increased binding. The different effects of CGRP and PGE2 on SP binding could be caused by activation of receptors on different cells, compartmentalization of intracellular messengers, competing effects of other receptors activated by PGE2, or inhibition of transcriptional proteins by PGE2 (Riquet et al., 2000). Nonetheless, the difference in effects suggests that CGRP may be unique among ligands that couple to production of cAMP in spinal neurons in its regulation of NK1 receptors.
Significance for cotransmission of CGRP and substance P
Substance P and CGRP coexist to a large extent (70%) in terminals of primary afferent neurons in the dorsal horn of the spinal cord (Tuchscherer and Seybold, 1989). Consequently, they are released in response to the same stimuli (Duggan et al., 1988; Morton and Hutchison, 1989). Considerable evidence indicates that SP contributes directly to central mechanisms of hyperalgesia by increasing the excitability of spinal neurons and indirectly by facilitating the activation of NMDA receptors (Urban et al., 1994).
The role of CGRP in spinal cord physiology is less clear. Although CGRP causes no acute behavioral effect when injected by itself (Wiesenfeld-Hallin et al., 1984; Gamse and Saria, 1986; Welch et al., 1989), intrathecal administration of CGRP causes hyperalgesia to mechanical stimuli (Oku et al., 1987), and intrathecal injection of CGRP antiserum decreases thermal and mechanical hyperalgesia during peripheral inflammation (Kuraishi et al., 1988; Kawamura et al., 1989). These effects are most likely indirect, mediated in part by CGRP receptors on terminals of primary afferent neurons that facilitate the release of substance P and excitatory amino acids (Oku et al., 1987;Ryu et al., 1988; Kangrga and Randic, 1990) and CGRP receptors on spinal neurons that enhance voltage-gated calcium currents evoked by other agonists (Murase et al., 1989). However, the most compelling mechanism underlying this interaction is that CGRP competes with SP for catabolism by endopeptidases (LeGreves et al., 1985; Mao et al., 1992;Schaible et al., 1992; Saleh et al., 1996), resulting in prolonged extracellular concentrations of SP after release of the peptides from primary afferent neurons. Thus, concentrations of CGRP that have no overt effect by themselves on spinal neurons in vivofacilitate the actions of SP.
The present data provide evidence for interaction of CGRP and SP neurotransmission over a time course of many hours. We found no evidence of an acute interaction of CGRP receptors and NK1 receptors postsynaptically. CGRP did not increase SP binding to NK1 receptors either directly or after treatment with CGRP up to 4 hr. Furthermore, CGRP does not modulate NK1 receptor coupling to the production of inositol phosphates (Parsons and Seybold, 1997). However, CGRP receptor activation resulted in increased levels of mRNA for NK1 receptors and increased 125I-SP binding. The time course of increased receptor binding is consistent with the time required for synthesis of the receptor protein and parallels the time course for cAMP effects on NK1 receptor expression (Abrahams et al., 1999).
Conclusion
Data from a variety of experiments indicate that activation of NK1 receptors contributes to the hyperalgesia that accompanies peripheral inflammation and that expression of this receptor is increased in conjunction with peripheral inflammation. In addition to blocking NK1 receptors with drugs to decrease hyperalgesia, it may be important to block the increased synthesis of NK1 receptors that could overcome receptor blockade. Evidence that CGRP receptors activate an intracellular pathway that increased expression of NK1 receptors extends understanding of the significance of SP and CGRP cotransmission: they are cocontained in primary afferent neurons; CGRP enhances the bioavailability of SP; and CGRP increases the expression of receptors activated by SP. This interaction is likely to extend to other populations of neurons that express CGRP and NK1 receptors. CGRP regulation of gene expression may also extend to other proteins. For example, CGRP inhibits development of tolerance to morphine analgesia at the level of the spinal cord (Menard et al., 1996; Powell et al., 2000). CGRP-induced expression of proteins other than NK1 receptors may contribute to this effect.
Footnotes
This work was supported by National Institutes of Health Grants NS41302 (P.G.M.) and NS17702 (V.S.S.), National Institute on Drug Abuse Grant DA12505 (K.E.M.), United States Public Health Service Institutional Training Grant DA07234 (R.D.G.), and the University of Minnesota graduate school (V.S.S.). We thank David Linden and Michelle Winter for their technical expertise in isolation and measurement of mRNA.
Correspondence should be addressed to Dr. Virginia Seybold, Department of Neuroscience, University of Minnesota, 6–145 Jackson Hall, 321 Church Street, Southeast, Minneapolis, MN 55455. E-mail:ginger@med.umn.edu.
References
- 1.Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. doi: 10.1016/0306-4522(95)00343-h. [DOI] [PubMed] [Google Scholar]
- 2.Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17:8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abrahams LG, Reutter MA, McCarson KE, Seybold VS. Cyclic AMP regulates the expression of neurokinin1 receptors by neonatal rat spinal neurons in culture. J Neurochem. 1999;73:50–58. doi: 10.1046/j.1471-4159.1999.0730050.x. [DOI] [PubMed] [Google Scholar]
- 4.Aiyar N, Disa J, Stadel JM, Lysko PG. Calcitonin gene-related peptide receptor independently stimulates 3′, 5′-cyclic adenosine monophosphate and Ca2+ signaling pathways. Mol Cell Biochem. 1999;197:179–185. doi: 10.1023/a:1006962221332. [DOI] [PubMed] [Google Scholar]
- 5.Anderson LE, Seybold VS. Phosphorylated cAMP response element binding protein increases in neurokinin-1 receptor-immunoreactive neurons in rat spinal cord in response to formalin-induced nociception. Neurosci Lett. 2000;283:29–32. doi: 10.1016/s0304-3940(00)00908-3. [DOI] [PubMed] [Google Scholar]
- 6.Baidan LV, Fertel RH, Wood JD. Effects of brain-gut related peptides on cAMP levels in myenteric ganglia of guinea-pig small intestine. Eur J Pharmacol. 1992;255:21–27. doi: 10.1016/0922-4106(92)90034-s. [DOI] [PubMed] [Google Scholar]
- 7.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 8.Bray D. Isolated chick neurons for the study of axonal growth. In: Banker G, Goslin K, editors. Culturing nerve cells. MIT Press; Cambridge: 1991. pp. 119–135. [Google Scholar]
- 9.Chakravarty P, Suthar TP, Coppock HA, Nicholl CG, Bloom SR, Legon S, Smith DM. CGRP and adrenomedullin binding correlates with transcript levels for calcitonin receptor-like receptor (CRLR) and receptor activity modifying proteins (RAMPS) in rat tissues. Br J Pharmacol. 2000;130:189–195. doi: 10.1038/sj.bjp.0702975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chan CF, Sun WZ, Lin JK, Lin-Shiau SY. Activation of transcription factors of nuclear factor kappa B, activator protein-1 and octamer factors in hyperalgesia. Eur J Pharmacol. 2000;402:61–68. doi: 10.1016/s0014-2999(00)00431-3. [DOI] [PubMed] [Google Scholar]
- 11.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 12.Cho HJ, Kim JK, Zhou XF, Rush RA. Increased brain-derived neurotrophic factor immunoreactivity in rat dorsal root ganglia and spinal cord following peripheral inflammation. Brain Res. 1997;764:269–272. doi: 10.1016/s0006-8993(97)00597-0. [DOI] [PubMed] [Google Scholar]
- 13.Chomczynski P, Sacchi N. Single-step method of RNA isolation by guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 14.Donaldson LF, Harmar AJ, McQueen DS, Seckl JR. Increased expression of preprotachykinin, calcitonin gene-related peptide, but not vasoactive intestinal peptide messenger RNA in dorsal root ganglia during the development of adjuvant monoarthritis in the rat. Brain Res Mol Brain Res. 1992;16:143–149. doi: 10.1016/0169-328x(92)90204-o. [DOI] [PubMed] [Google Scholar]
- 15.Dougherty PM, Palecek J, Paleckova V, Willis WD. Neurokinin 1 and 2 antagonists attenuate the responses and NK1 antagonists prevent the sensitization of primate spinothalamic tract neurons after intradermal capsaicin. J Neurophysiol. 1994;72:1464–1475. doi: 10.1152/jn.1994.72.4.1464. [DOI] [PubMed] [Google Scholar]
- 16.Duggan AW, Hendry IA, Morton CR, Hutchison WD, Zhao ZQ. Cutaneous stimuli releasing immunoreactive substance P in the dorsal horn of the cat. Brain Res. 1988;451:261–273. doi: 10.1016/0006-8993(88)90771-8. [DOI] [PubMed] [Google Scholar]
- 17.Evans BN, Rosenblatt MI, Mnayers LO, Oliver KR, Dickerson IM. CGRP-RCP, a novel protein required for signal transduction at calcitonin gene-related peptide and adrenomedullin receptors. J Biol Chem. 2000;275:31438–31443. doi: 10.1074/jbc.M005604200. [DOI] [PubMed] [Google Scholar]
- 18.Galeazza MT, Garry MG, Yost HJ, Strait KA, Hargreaves KM, Seybold VS. Plasticity in the synthesis and storage of substance P and calcitonin gene-related peptide in primary afferent neurons during peripheral inflammation. Neuroscience. 1995;66:443–458. doi: 10.1016/0306-4522(94)00545-g. [DOI] [PubMed] [Google Scholar]
- 19.Gamse R, Saria A. Nociceptive behavior after intrathecal injections of substance P, neurokinin A and calcitonin gene-related peptide in mice. Neurosci Lett. 1986;70:143–147. doi: 10.1016/0304-3940(86)90453-2. [DOI] [PubMed] [Google Scholar]
- 20.Garry MG, Kajander KC, Bennett GJ, Seybold VS. Quantitative autoradiographic analysis of [125I]-human CGRP binding sites in the dorsal horn of the rat following chronic constriction injury or dorsal rhizotomy. Peptides. 1991;12:1365–1373. doi: 10.1016/0196-9781(91)90221-a. [DOI] [PubMed] [Google Scholar]
- 21.Gerard NP, Garraway LA, Eddy RL, Jr, Shows TB, Iijima H, Paquet JL, Gerard C. Human substance P receptor (NK-1): organization of the gene, chromosome localization, and functional expression of cDNA clones. Biochemistry. 1991;30:10640–10646. doi: 10.1021/bi00108a006. [DOI] [PubMed] [Google Scholar]
- 22.Groth RD, Aanonsen LM. Spinal brain-derived neurotrophic factor (BDNF) produces hyperalgesia in normal mice while antisense directed against either BDNF or trkB, prevent inflammation-induced hyperalgesia. Pain. 2002;100:171–181. doi: 10.1016/s0304-3959(02)00264-6. [DOI] [PubMed] [Google Scholar]
- 23.Hershey AD, Dykema PE, Krause JE. Organization, structure and expression of the gene encoding the rat substance P receptor. J Biol Chem. 1991;266:4366–4374. [PubMed] [Google Scholar]
- 24.Honore P, Menning PM, Rogers SD, Nichols ML, Basbaum AI, Besson JM, Mantyh PW. Spinal substance P receptor expression and internalization in acute, short-term, and long-term inflammatory pain states. J Neurosci. 1999;19:7670–7678. doi: 10.1523/JNEUROSCI.19-17-07670.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hope PJ, Jarrott B, Schaible HG, Clarke RW, Duggan AW. Release and spread of immunoreactive neurokinin A in the cat spinal cord in a model of acute arthritis. Brain Res. 1990;533:292–299. doi: 10.1016/0006-8993(90)91352-h. [DOI] [PubMed] [Google Scholar]
- 26.Huang CC, Chen YL, Lo SW, Hsu KS. Activation of cAMP-dependent protein kinase suppresses the presynaptic cannabinoid inhibition of glutamatergic transmission at corticostriatal synapses. Mol Pharmacol. 2002;61:578–585. doi: 10.1124/mol.61.3.578. [DOI] [PubMed] [Google Scholar]
- 27.Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- 28.Ji RR, Rupp F. Phosphorylation of transcription factor CREB in rat spinal cord after formalin-induced hyperalgesia: relationship to c-fos induction. J Neurosci. 1997;17:1776–1785. doi: 10.1523/JNEUROSCI.17-05-01776.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ji RR, Befort K, Brenner GJ, Woolf CJ. ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity. J Neurosci. 2002;22:478–485. doi: 10.1523/JNEUROSCI.22-02-00478.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kangrga I, Randic M. Tachykinins and calcitonin gene-related peptide enhance release of endogenous glutamate and aspartate from the rat spinal dorsal horn slice. J Neurosci. 1990;10:2026–2038. doi: 10.1523/JNEUROSCI.10-06-02026.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kawamura M, Kuraishi Y, Minami M, Satoh M. Antinociceptive effect of intrathecally administered antiserum against calcitonin gene-related peptide on thermal and mechanical noxious stimuli in experimental hyperalgesic rats. Brain Res. 1989;497:199–203. doi: 10.1016/0006-8993(89)90990-6. [DOI] [PubMed] [Google Scholar]
- 32.Kuraishi Y, Nanayama T, Ohno H, Minami M, Satoh M. Antinociception induced in rats by intrathecal administration of antiserum against calcitonin gene-related peptide. Neurosci Lett. 1988;92:325–329. doi: 10.1016/0304-3940(88)90611-8. [DOI] [PubMed] [Google Scholar]
- 33.Laufer R, Changeux JP. Calcitonin gene-related peptide and cyclic AMP stimulate phosphoinositide turnover in skeletal muscle cells. Interaction between two second messenger systems. J Biol Chem. 1989;264:2683–2689. [PubMed] [Google Scholar]
- 34.Laughlin TM, Vanderah TW, Lashbrook J, Nicholas ML, Ossipov M, Porreca F, Wilcox GL. Spinally administered dynorphin A produces long-lasting allodynia: involvement of NMDA but not opioid receptors. Pain. 1997;72:253–260. doi: 10.1016/s0304-3959(97)00046-8. [DOI] [PubMed] [Google Scholar]
- 35.Lee MK, Tuttle JB, Rebhun LL, Cleveland DW, Frankfurter A. The expression and posttranslational modification of a neuron-specific tubulin isotype during chick embryogenesis. Cell Motil Cytoskeleton. 1990;17:118–132. doi: 10.1002/cm.970170207. [DOI] [PubMed] [Google Scholar]
- 36.LeGreves P, Nyberg F, Terenius L, Hokfelt T. Calcitonin gene-related peptide is a potent inhibitor of substance P degradation. Eur J Pharmacol. 1985;115:309–311. doi: 10.1016/0014-2999(85)90706-x. [DOI] [PubMed] [Google Scholar]
- 37.Lin SK, Wang CC, Huang S, Lee JJ, Chiang CP, Lan WH, Hong CY. Induction of dental pulp fibroblast matrix metalloproteinase-1 and tissue inhibitor of metalloproteinase-1 gene expression by interleukin-1alpha and tumor necrosis factor-alpha through a prostaglandin-dependent pathway. J Endod. 2001;27:185–189. doi: 10.1097/00004770-200103000-00012. [DOI] [PubMed] [Google Scholar]
- 38.Ma QP, Allochorne AJ, Woolf CJ. Morphine, the NMDA receptor antagonist MK801 and tachykinin NK1 receptor antagonist RP67580 attenuate the development of inflammation-induced progressive tactile hypersensitivity. Pain. 1998;77:49–57. doi: 10.1016/S0304-3959(98)00081-5. [DOI] [PubMed] [Google Scholar]
- 39.Mannion RJ, Costigan M, Decosterd I, Amaya F, Ma QP, Holstege JC, Ji RR, Acheson A, Lindsay RM, Wilkinson GA, Woolf CJ. Neurotrophins: peripherally and centrally acting modulators of tactile stimulus-induced inflammatory pain hypersensitivity. Proc Natl Acad Sci USA. 1999;96:9385–9390. doi: 10.1073/pnas.96.16.9385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mao J, Coghill RC, Kellstein DE, Frenk H, Mayer DJ. Calcitonin gene-related peptide enhances substance P-induced behaviors via metabolic inhibition: in vivo evidence for a new mechanism of neuromodulation. Brain Res. 1992;574:157–163. doi: 10.1016/0006-8993(92)90812-n. [DOI] [PubMed] [Google Scholar]
- 41.Mapp PI, Terenghi G, Walsh DA, Chen ST, Cruwys SC, Garrett N, Kidd BL, Polak JM, Blake DR. Monoarthritis in the rat knee induces bilateral and time-dependent changes in substance P and calcitonin gene-related peptide immunoreactivity in the spinal cord. Neuroscience. 1993;57:1091–1096. doi: 10.1016/0306-4522(93)90051-g. [DOI] [PubMed] [Google Scholar]
- 42.McCarson KE, Krause JE. NK-1 and NK-3 type tachykinin receptor mRNA expression in the rat spinal cord dorsal horn is increased during adjuvant or formalin-induced nociception. J Neurosci. 1994;14:712–720. doi: 10.1523/JNEUROSCI.14-02-00712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McCarson KE, Krause JE. The formalin-induced expression of tachykinin peptide and neurokinin receptor messenger RNA in rat sensory ganglia and spinal cord is modulated by opiate preadministration. Neuroscience. 1995;64:729–739. doi: 10.1016/0306-4522(94)00442-8. [DOI] [PubMed] [Google Scholar]
- 44.McLatchie LM, Fraser NJ, Main MJ, Wise A, Brown J, Thompson N, Solari R, Lee MG, Foord SM. RAMPS regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature. 1998;393:333–339. doi: 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- 45.Menard DP, van Rossum D, Kar S, St Pierre S, Sutak M, Jhamandas K, Quirion R. A calcitonin gene-related peptide receptor antagonist prevents the development of tolerance to spinal morphine analgesia. J Neurosci. 1996;16:2342–2351. doi: 10.1523/JNEUROSCI.16-07-02342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Messersmith DJ, Kim DJ, Iadarola MJ. Transcription factor regulation of prodynorphin gene expression following rat hindpaw inflammation. Brain Res Mol Brain Res. 1998;53:260–269. doi: 10.1016/s0169-328x(97)00308-2. [DOI] [PubMed] [Google Scholar]
- 47.Morton CR, Hutchison WD. Release of sensory neuropeptides in the spinal cord: studies with calcitonin gene-related peptide and galanin. Neuroscience. 1989;31:807–815. doi: 10.1016/0306-4522(89)90443-0. [DOI] [PubMed] [Google Scholar]
- 48.Murase K, Ryu PD, Randic M. Excitatory and inhibitory amino acids and peptide-induced responses in acutely isolated rat spinal dorsal horn neurons. Neurosci Lett. 1989;103:56–63. doi: 10.1016/0304-3940(89)90485-0. [DOI] [PubMed] [Google Scholar]
- 49.Narumiya S, Sugimoto Y, Ushikubi F. Prostanoid receptors: structures, properties, and functions. Physiol Rev. 1999;79:1193–1226. doi: 10.1152/physrev.1999.79.4.1193. [DOI] [PubMed] [Google Scholar]
- 50.Neugebauer V, Weiretter F, Schaible HG. Involvement of substance P and neurokinin-1 receptors in the hyperexcitability of dorsal horn neurons during development of acute arthritis in rat's knee joint. J Neurophysiol. 1995;73:1574–1583. doi: 10.1152/jn.1995.73.4.1574. [DOI] [PubMed] [Google Scholar]
- 51.Ohnishi T, Suwa M, Oyama T, Arakaki N, Torii M, Daikuhara Y. Prostaglandin E2 predominantly induces production of hepatocyte growth factor/scatter factor in human dental pulp in acute inflammation. J Dent Res. 2000;79:748–755. doi: 10.1177/00220345000790020801. [DOI] [PubMed] [Google Scholar]
- 52.Oku R, Satoh M, Fujii N, Otaka A, Yajima H, Takagi H. Calcitonin gene-related peptide promotes mechanical nociception by potentiating release of substance P from the spinal dorsal horn in rats. Brain Res. 1987;403:350–354. doi: 10.1016/0006-8993(87)90074-6. [DOI] [PubMed] [Google Scholar]
- 53.Parameswaran N, Disa J, Spielman WS, Brooks DP, Nambi P, Aiyar N. Activation of multiple mitogen-activated protein kinases by recombinant calcitonin gene-related peptide receptor. Eur J Pharmacol. 2000;389:125–130. doi: 10.1016/s0014-2999(99)00874-2. [DOI] [PubMed] [Google Scholar]
- 54.Parsons AM, Seybold VS. Calcitonin gene-related peptide induces formation of second messengers in primary cultures of neonatal rat spinal cord. Synapse. 1997;26:235–242. doi: 10.1002/(SICI)1098-2396(199707)26:3<235::AID-SYN5>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 55.Parsons AM, El-Fakahany EE, Seybold VS. Tachykinins alter inositol phosphate formation, but not cAMP levels, in neonatal rat spinal neurons through activation of neurokinin receptors. Neuroscience. 1995;68:855–865. doi: 10.1016/0306-4522(95)00140-e. [DOI] [PubMed] [Google Scholar]
- 56.Parsons AM, Honda CN, Jia YP, Budai D, Xu XJ, Wiesenfeld-Hallin S, Seybold VS. Spinal neurokinin1 receptors contribute to the increased excitability of the nociceptive flexor reflex during persistent peripheral inflammation. Brain Res. 1996;739:263–275. doi: 10.1016/s0006-8993(96)00833-5. [DOI] [PubMed] [Google Scholar]
- 57.Powell KJ, Ma W, Sutak M, Doods H, Quirion R, Jhamandas K. Blockade and reversal of spinal morphine tolerance by peptide and non-peptide calcitonin gene-related peptide receptor antagonists. Br J Pharmacol. 2000;131:875–884. doi: 10.1038/sj.bjp.0703655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quirion R, van Rossum D, Dumont Y, St-Pierre S, Fournier A. Characterization of CGRP1 and CGRP2 receptor subtypes. Ann NY Acad Sci. 1992;657:88–105. doi: 10.1111/j.1749-6632.1992.tb22759.x. [DOI] [PubMed] [Google Scholar]
- 59.Riquet FB, Lai WF, Birkhead JR, Suen LF, Karsenty G, Goldring MB. Suppression of type I collagen gene expression by prostaglandins in fibroblasts is mediated at the transcriptional level. Mol Med. 2000;6:705–719. [PMC free article] [PubMed] [Google Scholar]
- 60.Ruda MA, Iadarola MJ, Cohen LV, Young WS. In situ hybridization histochemistry and immunohistochemistry reveal an increase in spinal dynorphin biosynthesis in rat model of peripheral inflammation and hyperalgesia. Proc Natl Acad Sci USA. 1988;85:622–626. doi: 10.1073/pnas.85.2.622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ryu PD, Gerber G, Murase K, Randic M. Calcitonin gene-related peptide enhances calcium current of rat dorsal root ganglion neurons and spinal excitatory synaptic transmission. Neurosci Lett. 1988;89:305–312. doi: 10.1016/0304-3940(88)90544-7. [DOI] [PubMed] [Google Scholar]
- 62.Saleh TM, Kombina SB, Zidichouski JA, Pittman QJ. Peptidergic modulation of synaptic transmission in the parabrachial nucleus in vitro: importance of degradative enzymes in regulating synaptic efficacy. J Neurosci. 1996;16:6046–6055. doi: 10.1523/JNEUROSCI.16-19-06046.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Samad A, Moore KA, Sapirstein A, Billet S, Allchorne A, Poole S, Bonventre JV, Woolf CJ. An interleukin-1b-mediated induction of Cox-2 in the central nervous system contributes to inflammatory pain hypersensitivity. Nature. 2001;22:471–475. doi: 10.1038/35068566. [DOI] [PubMed] [Google Scholar]
- 64.Schafer MK, Nohr D, Krause JE, Weihe E. Inflammation-induced upregulation of NK1 receptor mRNA in dorsal horn neurons. NeuroReport. 1993;4:1007–1010. doi: 10.1097/00001756-199308000-00003. [DOI] [PubMed] [Google Scholar]
- 65.Schaible HG, Jarrott B, Hope PJ, Duggan AW. Release of immunoreactive substance P in the spinal cord during development of acute arthritis in the knee joint of the cat: a study with antibody microprobes. Brain Res. 1990;529:214–223. doi: 10.1016/0006-8993(90)90830-5. [DOI] [PubMed] [Google Scholar]
- 66.Schaible HG, Hope PJ, Lang CW, Duggan AW. Calcitonin gene-related peptide causes intraspinal spreading of substance P released by peripheral stimulation. Eur J Neurosci. 1992;4:750–757. doi: 10.1111/j.1460-9568.1992.tb00184.x. [DOI] [PubMed] [Google Scholar]
- 67.Seybold VS, Abrahams LG. Characterization and regulation of neurokinin1 receptors in primary cultures of rat neonatal spinal neurons. Neuroscience. 1995;69:1263–1273. doi: 10.1016/0306-4522(95)00304-2. [DOI] [PubMed] [Google Scholar]
- 68.Sweeney MI, Sawynok J. Evidence that substance P may be a modulator rather than a transmitter of noxious mechanical stimulation. Can J Physiol Pharmacol. 1986;64:1324–1327. doi: 10.1139/y86-224. [DOI] [PubMed] [Google Scholar]
- 69.Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67:151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- 70.Tschopp FA, Henke H, Petermann JB, Tobler PH, Janzer R, Hökfelt T, Lundberg JM, Cuello C, Fischer JA. Calcitonin gene-related peptide and its binding sites in the human central nervous system and pituitary. Proc Natl Acad Sci USA. 1985;82:248–252. doi: 10.1073/pnas.82.1.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tuchscherer MM, Seybold VS. A quantitative study of the coexistence of peptides in varicosities within the superficial laminae of the dorsal horn of the rat spinal cord. J Neurosci. 1989;9:195–205. doi: 10.1523/JNEUROSCI.09-01-00195.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Urban L, Thompson SWN, Dray A. A modulation of spinal excitability: co-operation between neurokinin and excitatory amino acid neurotransmitters. Trends Neurosci. 1994;17:432–438. doi: 10.1016/0166-2236(94)90018-3. [DOI] [PubMed] [Google Scholar]
- 73.Welch SP, Singha AK, Dewey WL. The antinociception produced by intrathecal morphine, calcium, A23187, U50, 488H, [d-Ala2,N-Me-Phe4,Gly-ol]enkephalin and [d-Pen2,d-Pen5]enkephalin after intrathecal administration of calcitonin gene-related peptide in mice. J Pharmacol Exp Ther. 1989;251:1–8. [PubMed] [Google Scholar]
- 74.Wiesenfeld-Hallin Z, Hokfelt T, Lundberg JM, Forssmann WG, Reinecke M, Tschopp FA, Fischer JA. Immunoreactive calcitonin gene-related peptide and substance P coexist in sensory neurons to the spinal cord and interact in spinal behavioral responses of the rat. Neurosci Lett. 1984;52:199–204. doi: 10.1016/0304-3940(84)90374-4. [DOI] [PubMed] [Google Scholar]
- 75.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP Kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yang LC, Marsala M, Yaksh TL. Characterization of time course of spinal amino acids, citrulline and PGE2 release after carrageenan/kaolin-induced knee joint inflammation: a chronic microdialysis study. Pain. 1996;67:345–354. doi: 10.1016/0304-3959(96)03106-5. [DOI] [PubMed] [Google Scholar]
- 77.Yashpal K, Radhakrishnan V, Coderre TJ, Henry JL. CP-96, 345, but not its stereoisomer, CP-96, 344, blocks the nociceptive responses to intrathecally administered substance P and to noxious thermal and chemical stimuli in the rat. Neuroscience. 1993;52:1039–1047. doi: 10.1016/0306-4522(93)90550-y. [DOI] [PubMed] [Google Scholar]
- 78.Zona C, Farini D, Palma E, Eusebi F. Modulation of voltage-activated channels by calcitonin gene-related peptide in cultured rat neurones. J Physiol (Lond) 1991;433:631–643. doi: 10.1113/jphysiol.1991.sp018447. [DOI] [PMC free article] [PubMed] [Google Scholar]