Abstract
Previous studies have shown that D1receptor blockade disrupts and D2 receptor blockade enhances long-term potentiation. These data lead to the prediction that D1 antagonists will attenuate and D2 antagonists will potentiate at least some types of learning. The prediction is difficult to test, however, because disruptions in either D1 or D2 transmission lead to reduced locomotion, exploration, and response execution and are therefore likely to impair learning that requires behavioral responding (including exploration of an environment) during the learning episode. Under a paradigm that minimizes motor requirements, rats were trained to enter a food compartment during pellet presentation. Animals then received tone–food pairings under the influence of D1antagonist SCH23390 (0, 0.4, 0.8, and 0.16 mg/kg) or D2antagonist raclopride (0, 0.2, 0.4, and 0.8 mg/kg). An additional group received unpaired presentations of tone and food. On a drug-free test day 24 hr later, animals that had been under the influence of SCH23390 (like animals that had received unpaired presentations of tone and food) showed reduced head entries in response to the tone, whereas animals that had been under the influence of raclopride showed increased head entries in response to the tone compared with vehicle controls. These data demonstrate that, under a conditioned approach paradigm, D1 and D2 family receptor antagonists disrupt and promote learning, respectively, as predicted by the effects of D1 and D2 receptor blockade on neuronal plasticity.
Keywords: dopamine, learning motivation, plasticity, conditioning, D1, D2, rat
Introduction
Long-term potentiation (LTP) is blocked by D1 antagonists (Calabresi et al., 2000; Kerr and Wickens, 2001) and is enhanced by D2 antagonists and in D2receptor knock-out mice (Calabresi et al., 1997; Yamamoto et al., 1999). These opposite effects of D1 and D2 receptor blockade on LTP appear to result from their opposing effects on those intracellular cascades within striatal neurons that lead to dopamine and cAMP-regulated phosphoprotein of 32 kDa (DARPP-32) phosphorylation. D1receptor binding causes a G-protein-mediated increase in the activity of adenylyl cyclase, increased cAMP formation, stimulation of protein kinase A, and phosphorylation (i.e., activation) of DARPP-32 (Hemmings et al., 1984; Nishi et al., 1997). D2 binding produces the opposite effect on DARPP-32 activation, both by reducing adenylyl cyclase activity and through a mechanism that involves calcineurin activation (Nishi et al., 1997; Greengard et al., 1999). It is known that the activation and deactivation state of DARPP-32 critically mediates the facilitative and inhibitory effects of D1 and D2 binding on LTP induction, respectively (Calabresi et al., 2000; Centonze et al., 2001). Thus, via opposing effects on the activation state of DARPP-32, D1 activity promotes and D2activity restricts LTP.
As predicted on the basis of its role in striatal plasticity, D1 receptor activity plays an important role in learning (Beninger and Miller, 1998; Smith-Roe and Kelley, 2000; Azzara et al., 2001; Baldwin et al., 2002). Although a large number of studies demonstrate that D1 receptor blockade disrupts appetitive learning, the effects of D2 receptor blockade are more difficult to interpret because of the profound motor impairments typically produced by D2antagonists (Beninger and Miller, 1998).
A critical methodological consideration in such studies concerns the fact that dopamine (DA) (both D1 and D2) antagonists increase response latencies (Horvitz, 2001). Imagine that rats are presented a number of pairings of a conditioned stimulus (CS) that signals delivery of an unconditioned food stimulus (US) and that drugged animals fail to show normal increases in responsiveness to the CS with repeated trials. The reduced responsiveness to the CS could reflect motor suppression, disrupted learning, or a combination of the two effects. Although animals might be tested for appetitive responses to the CS on the following day, after the drug effects had subsided, it would remain critical to demonstrate that, on the acquisition day, drugged animals had received the food with normal latency. If drug-induced motor suppression increased the animal's latency to retrieve food after CS presentation on the acquisition day, drugged animals would have an artificially increased CS–US interval, which could itself impair the acquisition of the CS–US association. In this case, the reduced learning would not be attributable to a direct effect of the drug on the learning process but rather to a drug-induced motor deficit that changed the nature of the CS–US relationship. We report here that, using an appetitive head-entry paradigm that ensures normal CS–US exposure during learning trials, D1 and D2 antagonists produce opposite effects on learning and that these learning effects mirror those predicted by studies of D1 and D2antagonist effects on LTP.
Materials and Methods
Subjects
One hundred twenty-one Sprague Dawley rats (275–300 gm) were obtained from Charles River Laboratories (Kinsington, NY). Animals were housed in pairs within Plexiglas cages (22 × 22 × 46 cm) mounted on a rack within an animal colony, with food and water available ad libitum. The colony was maintained at ∼23°C, with a 12 hr light/dark cycle (lights on at 8:00 A.M.). Animals were handled and gentled during their first 2 weeks of arrival and were then placed on a 23 hr food deprivation schedule for 3 additional days before the experiment began. Animals were maintained on this food-restricted diet for the remainder of the experiment.
Apparatus
Behavioral test sessions were conducted in conditioning chambers (29 × 29 × 25 cm; Coulbourn Instruments, Allentown, PA), individually housed within sound- and light-attenuated enclosures. Two walls of the chamber were Plexiglas; the other two were metal. A house light was located at the top center of one of the metal walls, 2 cm below the ceiling of the chamber. Recessed within the bottom center of this wall, 2 cm above the chamber floor, was a food compartment (4 × 3 × 2.5 cm) into which food pellets (F0021, 45 mg; Bioserve, Frenchtown, NJ) were delivered. Activation of the food magazine produced a 400 msec 78 dB sound immediately before pellet delivery. A speaker (8 × 7.5 cm) was located adjacent to the food compartment (2 cm above the chamber floor). When activated, the speaker generated a 6 sec duration 1500 Hz tone (80 dB measured from the center of the chamber, 5.5 cm above the floor). An infrared photo emitter and detector, located on the sides of the food compartment, was interrupted by the animal's head entry and signaled to the computer the presence of the animal's head within the food compartment. Locomotor activity was recorded with an activity monitor that detected the movement of infrared body heat across small compartments of a ceiling-mounted lens (Pitts and Horvitz, 2000;Horvitz et al., 2001). The computer (a Dell Pentium running Coulbourn L2T2 data acquisition software) recorded the time of head entries, head withdrawals, pellet deliveries, locomotor counts, and tone presentations with 0.05 sec resolution.
Drug
Selective D1 receptor antagonist SCH23390 (SCH) (Iorio et al., 1983) and D2 receptor antagonist raclopride (RAC) (Hall et al., 1988) (obtained from Research Biochemicals, Natick, MA) were dissolved in isotonic saline. Intraperitoneal injections of SCH23390 or raclopride were delivered in a volume of 1 ml/kg body weight.
Procedure
Magazine training. Rats were acclimated to the conditioning chamber for a single 30 min session during which no food pellets were delivered. On the following day, animals began the first of 17 daily magazine training sessions. Seconds after placement in the chamber, the onset of a house light signaled the start of the session and remained illuminated until the session ended. During each session, rats received 28 pellets (trials) delivered individually into the food compartment on a variable time 70 sec (VT 70) schedule (with a minimum interpellet interval of 30 sec). Rats received 17 d of this magazine training. We found previously that this amount of pretraining protects animals from the otherwise disruptive effects of SCH23390 and raclopride on latencies to retrieve pellets (Choi et al., 2000; Horvitz and Eyny, 2000). The latency to enter the food compartment during pellet presentation was measured on each of these magazine training days.
Conditioning–drug treatment. On the following day (day 18), 28 pellets were delivered on a VT 116 sec schedule (with a 50 sec minimum interpellet interval), and a tone (6 sec duration) was presented 3 sec before each pellet delivery. Pilot studies showed that these parameters for CS duration, CS–US interval, and intertrial interval produced robust conditioning. On this day, animals were pretreated with vehicle (VEH/PAIRED; n = 24), 0.04 (n = 12), 0.08 (n = 10), or 0.16 (n = 10) mg/kg of the D1antagonist SCH23390 (SCH/PAIRED), or 0.2 (n = 9), 0.4 (n = 9), or 0.8 (n = 9) mg/kg of the D2 antagonist raclopride (RAC/PAIRED) 30 min before the tone–food conditioning session. In addition, an UNPAIRED group received vehicle injections 30 min before a session in which 28 pellets were presented on the same schedule as for the paired groups. For this group, the 28 tone presentations occurred randomly throughout the session, with the constraint that they never occurred within 13 sec before or after pellet delivery. For all groups, the time of each head entry, head withdrawal, pellet delivery, and tone presentation was recorded. In addition, locomotor counts were recorded throughout the session.
Test day. On the following day (day 19), all animals received a test session during which the 6 sec tone was presented on a schedule identical to that of the previous day, with the exceptions that animals were drug free and no food was delivered. The time of each head entry, head withdrawal, and tone presentation was recorded for each animal.
Results
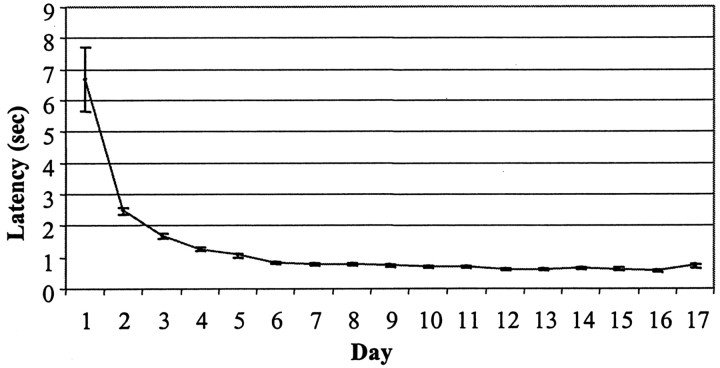
Individual ANOVAs were conducted on SCH (0, 0.04, 0.08, and 0.16 mg/kg) and RAC (0, 0.2, 0.4, and 0.8 mg/kg) data. Over the course of the 17 d magazine training sessions, the mean latency to retrieve food pellets decreased from ∼6.7 ± 1.0 on day 1 to 0.7 ± 0.1 sec on day 17 (Fig. 1). On day 18, neither SCH nor RAC disrupted latencies to enter the food compartment during pellet presentation (F(3,52) = 0.40, p = NS; F(3,47)= 0.51, p = NS, respectively), in accordance with previous findings that latencies to respond to an overtrained food cue are relatively invulnerable to neuroleptic challenge (Choi et al., 2000; Horvitz and Eyny, 2000). It is critical that latencies in neuroleptic-treated animals were normal during the conditioning session, because it ensured that all animals received the same number of CS–US pairings and that drug treatment groups experienced normal CS–US intervals. Figure 2 illustrates the head-entry behavior of three individual rats under vehicle, SCH, or RAC treatment during these conditioning trials. Note the intact latencies to enter the food compartment during pellet presentation in the neuroleptic-treated rats.
Fig. 1.
Latency to enter the food compartment during food presentation during 17 d of magazine training.
Fig. 2.
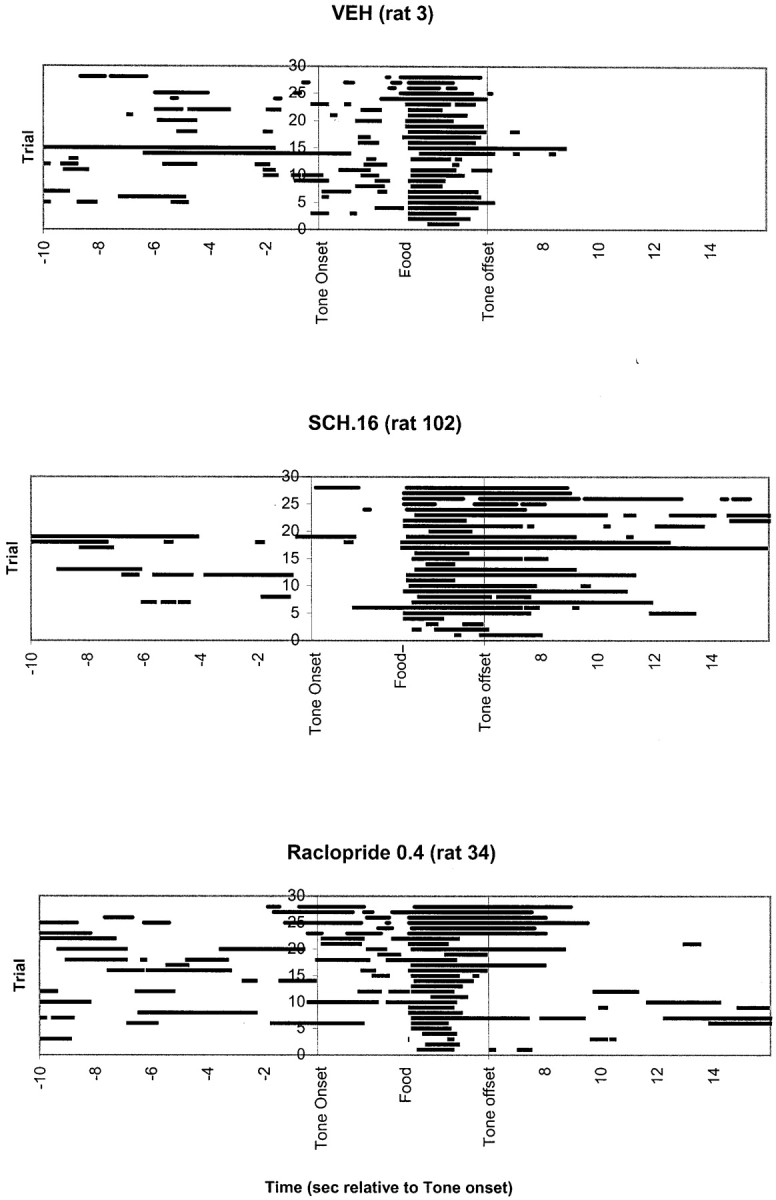
Head entries during the 10 sec before CS onset, the 6 sec CS, and the 16 sec after CS offset. Food was delivered 3 sec after CS onset. The figure shows head entries for three representative rats, one in the vehicle group (top), one in SCH at 0.16 mg/kg (middle), and one in raclopride at 0.4 mg/kg (bottom). Horizontal bars indicate the presence of the rat's head in the food compartment during each of the 28 conditioning trials (with successive trials represented frombottom to top). Note the normal latencies to respond to food presentation in SCH- and RAC-treated rats.
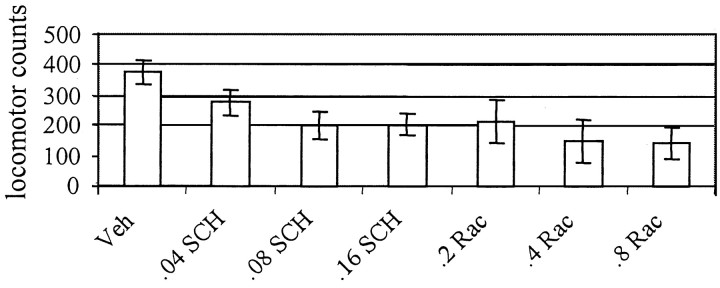
Although the neuroleptics did not increase latencies to enter the food compartment during pellet delivery, SCH and RAC reduced the duration of time for which the animal's head was in the food compartment from the onset of the CS period to the time of food delivery (F(3,52) = 4.02, p < 0.05; F(3,47) = 2.87,p = 0.05, respectively). However, because day 18 behavior is assessed in DA antagonist-treated animals, it is not possible to know whether the reduced behavioral responding reflects learning deficits, drug-induced motor suppression, or a combination of the two. Both D1 (Fowler and Liou, 1994; Aberman et al., 1998) and D2 (Fowler and Liou, 1994;Aberman et al., 1998; Horvitz and Eyny, 2000) antagonist drugs are known to suppress motor behavior. As can be seen in Figure3, SCH23390 and raclopride both reduced locomotor activity during the day 18 tone–food conditioning trials (F(3,52) = 4.29, p < 0.01; F(3,47) = 4.96,p < 0.005, respectively).
Fig. 3.
Both SCH23390 and raclopride reduced locomotor activity on tone conditioning–drug treatment day
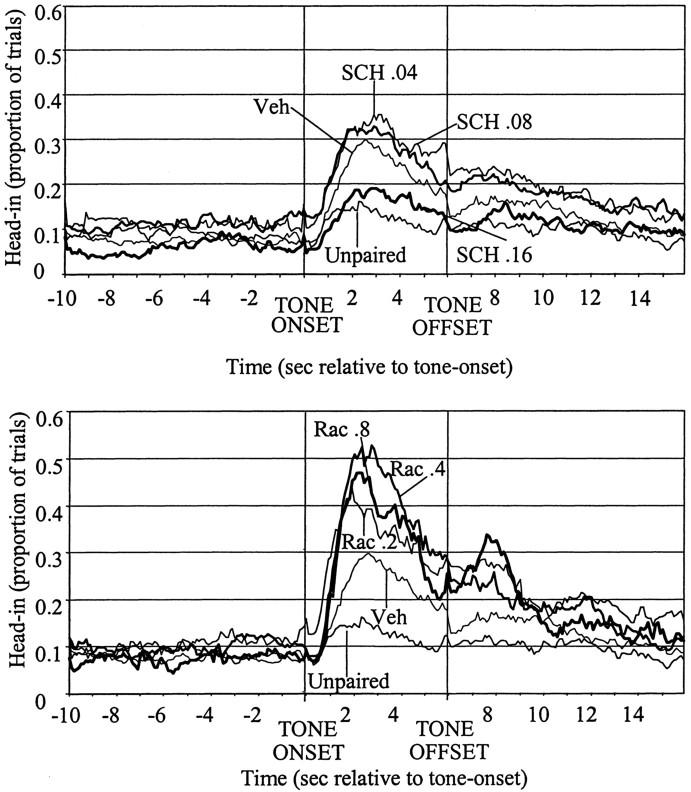
To examine whether DA receptor blockade affects learning, it was necessary to examine the learned behavior during a drug-free test day. Figure 4 shows the probability of an animal's head being inside the food compartment during consecutive 100 msec bins throughout the 10 sec pre-CS baseline, the 6 sec CS period, and a 16 sec post-CS period. Separate one-way ANOVAs examined the effects of (1) paired versus unpaired CS–US pairings, (2) SCH23390 dose, and (3) raclopride dose on mean head-in duration during baseline and CS periods. Animals that had received UNPAIRED presentations of CS and food showed reduced CS period head-in durations compared with PAIRED CS–food controls during the drug-free test session (F(1,35) = 7.90, p < 0.01). As can be seen in the top panel of Figure 4, CS-period head-in durations were also reduced in animals that had been under the influence of D1 antagonist SCH during the CS–food conditioning trials 24 hr earlier (F(3,52) = 3.25, p < 0.05). Post hoc LSD tests revealed that only the high dose (0.16 mg/kg SCH) group showed significant reductions in CS-period head duration compared with vehicle controls (p < 0.05). In contrast, animals that were under the influence of RAC during conditioning–drug treatment trials showed increased test day head-in durations during the CS period relative to vehicle controls (F(3,47) = 6.56, p < 0.01) (Fig. 4, bottom). All raclopride groups showed enhanced CS period head-in durations compared with vehicle controls (p < 0.05 for each of the RAC doses). These opposite effects of SCH and RAC on conditioned responding do not reflect general changes in behavioral responsiveness, because neither SCH nor RAC affected head-in durations during the baseline period 10 sec before CS presentation (F(3,52) = 2.35, p = NS; F(3,47)= 1.39, p = NS, for SCH and RAC, respectively), and neither did SCH or RAC treatment (administered on day 18) affect locomotor scores during the test session (F(3,52) = 0.731, p = NS; F(3,47) = 0.38, p= NS, for SCH and RAC, respectively).
Fig. 4.
Performance of each treatment group during day 19, the drug-free test session. The y-axis shows mean proportion of trials during which rats' heads were in the food compartment for each successive 100 msec bin during the 10 sec before tone onset (−10 to 0), the presentation of the tone (0–6), and the 10 sec after tone offset (6–16). As can be seen, rats that had received UNPAIRED presentations of CS and food showed reduced CS period head-in durations compared with paired CS–food controls. Animal that were under the influence of the highest SCH23390 dose during tone–food pairings also showed a reduced CS head-in probability. Animals that were under the influence of RAC during the tone–food pairings showed increased CS head-in probabilities compared with vehicle controls on test day.
Discussion
LTP is blocked by D1 receptor blockade and enhanced by D2 receptor blockade (for review, see Centonze et al., 2001). One might therefore expect D1 and D2 receptor blockade to produce opposite effects on learning, that is, for D1 blockade to disrupt and D2 blockade to facilitate learning. The present results confirm this prediction. Animals under the influence of D1 antagonist SCH23390 during the conditioning session showed reduced test day responding to the CS compared with vehicle controls. Animals that had been under the influence of D2 antagonist raclopride showed enhanced test day responding to the CS.
D1 antagonists have been shown previously to disrupt appetitive learning (Beninger and Miller, 1998; Azzara et al., 2001). However, for D2 antagonists, it has been difficult to dissociate drug-induced associative deficits from motor impairments (Hunt et al., 1994; Beninger and Miller, 1998). We believe that a critical feature of the current experimental design was that animals under the influence of varying doses of the D1 and D2 antagonists (1) received an identical number of CS–US pairings, (2) received normal CS–US intervals, and (3) were tested drug free. To ensure that drugged animals retrieved the food with normal latency, animals were overtrained to respond to the sound of the food magazine and then, in the presence or absence of the DA antagonists, were asked to transfer this response to an earlier CS. The paradigm may therefore be best described as a secondary conditioning paradigm.
Although the D2 antagonist promoted learning, it is unlikely to have done so by increasing the incentive motivational properties of the food; to the contrary, D2antagonists attenuate the incentive motivational or response-energizing properties of food (Ettenberg and Horvitz, 1990; Chausmer and Ettenberg, 1997). It is possible that D1 and D2 roles in neural plasticity and learning are distinct from their roles in incentive motivational processes. Consistent with this notion, intra-prefrontal D1antagonists disrupt the acquisition of an operant response at doses that do not affect free-feeding behavior (Baldwin et al., 2002).
A state-dependent learning hypothesis also fails to account for the present results, particularly the improved learning observed in D2 antagonist-treated animals. Given the opposing effects of the D1 and D2antagonist drugs on test day responses to the CS, any explanation that makes recourse to an altered pharmacological state on conditioning day is unlikely to account for the effects of both the D1 and D2 antagonists on test day performance.
Finally, differential effects of the drug on motor performance on conditioning day cannot easily account for these results, because both drugs had similar response-attenuating effects on head entries and locomotion during the conditioning day. Although D1 and D2 antagonists both exert suppressive effects on response expression (Fowler and Liou, 1994; Aberman et al., 1998; Horvitz and Eyny, 2000; Salamone and Correa, 2002), they may produce opposing effects on the acquisition of new learning.
D1 and D2 antagonists produce opposite effects on striatal plasticity, with D1 antagonists blocking (Calabresi et al., 2000;Kerr and Wickens, 2001) and D2 antagonists enhancing (Calabresi et al., 1997; Yamamoto et al., 1999) plasticity. The current results show that, under a learning paradigm that ensures normal CS–US exposure in drug-treated animals, D1 and D2 antagonists produce opposite effects on learning and that these learning effects mirror those predicted by D1 and D2 antagonist effects on neuronal plasticity.
Footnotes
This work was supported by National Institute on Drug Abuse Grant R29 DA11653 (J.C.H.). We gratefully acknowledge Melissa Donner, Joell Lerebours, Derek Narendra, Jennifer Adis, Michael Grody, Kimberly Fisher, and Gavin Williams for their invaluable assistance in the execution of this experiment.
Correspondence should be addressed to Dr. Jon C. Horvitz, Department of Psychology, Columbia University, 1190 Amsterdam Avenue, Room 406, New York, NY 10027. E-mail: jon@psych.columbia.edu.
References
- 1.Aberman JE, Ward SJ, Salamone JD. Effects of dopamine antagonists and accumbens dopamine depletions on time-constrained progressive-ratio performance. Pharmacol Biochem Behav. 1998;61:341–348. doi: 10.1016/s0091-3057(98)00112-9. [DOI] [PubMed] [Google Scholar]
- 2.Azzara AV, Bodnar RJ, Delamater AR, Sclafani A. D1 but not D2 dopamine receptor antagonism blocks the acquisition of a flavor preference conditioned by intragastric carbohydrate infusions. Pharmacol Biochem Behav. 2001;68:709–720. doi: 10.1016/s0091-3057(01)00484-1. [DOI] [PubMed] [Google Scholar]
- 3.Baldwin AE, Sadeghian K, Kelley AE. Appetitive instrumental learning requires coincident activation of NMDA and dopamine D1 receptors within the medial prefrontal cortex. J Neurosci. 2002;22:1063–1071. doi: 10.1523/JNEUROSCI.22-03-01063.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beninger RJ, Miller R. Dopamine D1-like receptors and reward-related incentive learning. Neurosci Biobehav Rev. 1998;22:335–345. doi: 10.1016/s0149-7634(97)00019-5. [DOI] [PubMed] [Google Scholar]
- 5.Calabresi P, Saiardi A, Pisani A, Baik JH, Centonze D, Mercuri NB, Bernardi G, Borrelli E. Abnormal synaptic plasticity in the striatum of mice lacking dopamine D2 receptors. J Neurosci. 1997;17:4536–4544. doi: 10.1523/JNEUROSCI.17-12-04536.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Calabresi P, Gubellini P, Centonze D, Picconi B, Bernardi G, Chergui K, Svenningsson P, Fienberg AA, Greengard P. Dopamine and cAMP-regulated phosphoprotein 32 kDa controls both striatal long-term depression and long-term potentiation, opposing forms of synaptic plasticity. J Neurosci. 2000;20:8443–8451. doi: 10.1523/JNEUROSCI.20-22-08443.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Centonze D, Picconi B, Gubellini P, Bernardi G, Calabresi P. Dopaminergic control of synaptic plasticity in the dorsal striatum. Eur J Neurosci. 2001;13:1071–1077. doi: 10.1046/j.0953-816x.2001.01485.x. [DOI] [PubMed] [Google Scholar]
- 8.Chausmer AL, Ettenberg A. A role for D2, but not D1, dopamine receptors in the response-reinstating effects of food reinforcement. Pharmacol Biochem Behav. 1997;57:681–685. doi: 10.1016/s0091-3057(96)00388-7. [DOI] [PubMed] [Google Scholar]
- 9.Choi W, Eyny YS, Matin L, Horvitz JC. Effects of D1 antagonist SCH 23390 on spontaneous versus stimulus-elicited behavioral responses in the rat. Soc Neurosci Abstr. 2000;26:2252. [Google Scholar]
- 10.Ettenberg A, Horvitz JC. Pimozide prevents the response-reinstating effects of water reinforcement in rats. Pharmacol Biochem Behav. 1990;37:465–469. doi: 10.1016/0091-3057(90)90014-9. [DOI] [PubMed] [Google Scholar]
- 11.Fowler SC, Liou JR. Microcatalepsy and disruption of forelimb usage during operant behavior: differences between dopamine D1 (SCH-23390) and D2 (raclopride) antagonists. Psychopharmacology (Berl) 1994;115:24–30. doi: 10.1007/BF02244747. [DOI] [PubMed] [Google Scholar]
- 12.Greengard P, Allen PB, Nairn AC. Beyond the dopamine receptor: the DARPP-32/protein phosphatase-1 cascade. Neuron. 1999;23:435–447. doi: 10.1016/s0896-6273(00)80798-9. [DOI] [PubMed] [Google Scholar]
- 13.Hall H, Kohler C, Gawell L, Farde L, Sedvall G. Raclopride, a new selective ligand for the dopamine-D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 1988;12:559–568. doi: 10.1016/0278-5846(88)90001-2. [DOI] [PubMed] [Google Scholar]
- 14.Hemmings HC, Jr, Nairn AC, Greengard P. DARPP-32, a dopamine- and adenosine 3′:5′-monophosphate-regulated neuronal phosphoprotein. II. Comparison of the kinetics of phosphorylation of DARPP-32 and phosphatase inhibitor 1. J Biol Chem. 1984;259:14491–14497. [PubMed] [Google Scholar]
- 15.Horvitz JC. The effects of D1 and D2 receptor blockade on the acquisition and expression of a conditioned appetitive response. Appetite. 2001;37:119–120. doi: 10.1006/appe.2001.0419. [DOI] [PubMed] [Google Scholar]
- 16.Horvitz JC, Eyny YS. Dopamine D2 receptor blockade reduces response likelihood but does not affect latency to emit a learned sensory-motor response: implications for Parkinson's disease. Behav Neurosci. 2000;114:934–939. [PubMed] [Google Scholar]
- 17.Horvitz JC, Williams G, Joy R. Time-dependent actions of D2 family agonist quinpirole on spontaneous behavior in the rat: dissociation between sniffing and locomotion. Psychopharmacology (Berl) 2001;154:350–355. doi: 10.1007/s002130000677. [DOI] [PubMed] [Google Scholar]
- 18.Hunt GE, Atrens DM, Jackson DM. Reward summation and the effects of dopamine D1 and D2 agonists and antagonists on fixed-interval responding for brain stimulation. Pharmacol Biochem Behav. 1994;48:853–862. doi: 10.1016/0091-3057(94)90192-9. [DOI] [PubMed] [Google Scholar]
- 19.Iorio LC, Barnett A, Leitz FH, Houser VP, Korduba CA. SCH 23390, a potential benzazepine antipsychotic with unique interactions on dopaminergic systems. J Pharmacol Exp Ther. 1983;226:462–468. [PubMed] [Google Scholar]
- 20.Kerr JN, Wickens JR. Dopamine D-1/D-5 receptor activation is required for long-term potentiation in the rat neostriatum in vitro. J Neurophysiol. 2001;85:117–124. doi: 10.1152/jn.2001.85.1.117. [DOI] [PubMed] [Google Scholar]
- 21.Nishi A, Snyder GL, Greengard P. Bidirectional regulation of DARPP-32 phosphorylation by dopamine. J Neurosci. 1997;17:8147–8155. doi: 10.1523/JNEUROSCI.17-21-08147.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pitts SM, Horvitz JC. Similar effects of D(1)/D(2) receptor blockade on feeding and locomotor behavior. Pharmacol Biochem Behav. 2000;65:433–438. doi: 10.1016/s0091-3057(99)00249-x. [DOI] [PubMed] [Google Scholar]
- 23.Salamone JD, Correa M. Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behav Brain Res. 2002;137:3–25. doi: 10.1016/s0166-4328(02)00282-6. [DOI] [PubMed] [Google Scholar]
- 24.Smith-Roe SL, Kelley AE. Coincident activation of NMDA and dopamine D1 receptors within the nucleus accumbens core is required for appetitive instrumental learning. J Neurosci. 2000;20:7737–7742. doi: 10.1523/JNEUROSCI.20-20-07737.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yamamoto Y, Nakanishi H, Takai N, Shimazoe T, Watanabe S, Kita H. Expression of N-methyl-d-aspartate receptor-dependent long-term potentiation in the neostriatal neurons in an in vitro slice after ethanol withdrawal of the rat. Neuroscience. 1999;91:59–68. doi: 10.1016/s0306-4522(98)00611-3. [DOI] [PubMed] [Google Scholar]