Abstract
A clear relationship exists between moment-to-moment behavioral elements and hippocampal rhythmical synchronous activity (RSA) (theta rhythm). However, behavioral elements are not isolated events but are part of behavioral sequences in a context of behavioral activity. By concurrently monitoring open field behavior and hippocampal EEG, EEG correlates of open field behavior in relation to preceding and following behavior were studied in Sprague Dawley rats to determine whether the behavioral context influences EEG correlates of behavior. Results show that preceding and subsequent behavioral patterns influenced the spectral power correlates of behavior. RSA power was increased when a “type 1 behavior” (voluntary movement) preceded the behavior compared with when a “type 2 behavior” (automatic movement, awake immobility) preceded it. The modulating effect of behavioral transitions was shown for several types of behaviors, and systematic modulation of hippocampal EEG correlates of behavior was demonstrated. The present report shows that the strong and systematic relationship between hippocampal RSA and behavior is modulated by the behavioral–sequential context. Thus, in addition to the well established relationship between RSA and motor activity, a second nonmotor process seems to contribute to hippocampal RSA. A likely candidate is a sensory process, which is in accordance with theories on the sensorimotor function of hippocampal RSA.
Keywords: EEG, behavior, open field, sequential analysis, transitions, rats, hippocampal RSA, sensorimotor integration
Introduction
Hippocampal rhythmical synchronous activity (RSA) [theta rhythm (6–10 Hz)] has been described previously by Jung and Kornmüller (1939). In the search of its behavioral correlates, RSA has been related to psychological concepts such as memory and learning (Elazar and Adey, 1967; Buzsáki, 1989), arousal and attention (Green and Arduini, 1954; Kemp and Kaada, 1975), the orienting response (Grastyán et al., 1959), “type I” motor movement (Vanderwolf, 1969; Coenen, 1975), and sensory (-motor) activity (Komisaruk, 1970; Sainsbury, 1998). On the basis of movement correlates with theta activity, behavior can be divided into “type 1 behavior” (or “voluntary movement”) and “type 2 behavior” (“automatic movement” and awake immobility) (Vanderwolf, 1969, 1992; Coenen, 1975). Type 1 behavior is correlated with theta and includes behavior such as walking, running, rearing, swimming, and changes in body posture; type 2 behavior is accompanied by large amplitude irregular activity and includes behavior such as body grooming, face washing, and awake immobility. For type 1 behavior, more vigorous movements are accompanied by higher-amplitude RSA (Whishaw and Vanderwolf, 1973). Active exploratory sniffing is also highly correlated with theta activity (Komisaruk, 1970; Forbes and Macrides, 1984; Chang, 1992). Different types of sniffing (sniffing air or sniffing an object) show differences in their electroencephalographic (EEG) power spectra (Coenen, 1975), indicating that sniffing includes a group of behaviors that are not homogenous with respect to amount of theta activity.
So far, the moment-to-moment relationship between behavior and EEG seems clear. However, behavioral elements are not isolated events but are part of behavioral sequences in a context of behavioral activity. Previous experiments (Van Lier et al., 2003) showed that a context of low or high exploratory activity could modulate hippocampal EEG correlates of behavior. The data suggested that, in addition to main motor components, sensory components contribute to hippocampal EEG correlates of exploratory behavior. To further investigate the relationship between behavior and the hippocampal EEG in its behavioral context, the question was addressed whether behavior such as sniffing is the same in terms of its physiological correlate in association with voluntary movements compared with automatic movements. In an open field, bouts of exploratory activity alternate with bouts of inactivity and grooming behaviors. In this environment, epochs of behavioral elements in association with either type 1 or 2 behavior can be collected when behavior is continuously scored. By concurrently monitoring open field behavior and EEG, RSA correlates of open field behavior in relation to preceding and following behavior were studied to determine whether the behavioral context influences RSA correlates of behavior. It was studied whether the RSA correlate of a behavioral element differs contingent on its association with either type 1 or type 2 behavior. Behavioral elements included sniffing behaviors, as well as type 1 and type 2 behaviors.
Materials and Methods
Animals. Thirteen male Sprague Dawley rats were obtained from Harlan (Bicester, UK). The animals were housed individually in macrolon cages with access to water and food ad libitum and maintained on a reversed 12 hr light/dark cycle, with lights on at 7:00 P.M. The animals were handled once per day for 5 min starting 1 week before testing. Experiments were performed at Organon (Newhouse, UK). Permission for all procedures was granted from the United Kingdom Home Office (Animals Scientific Procedures Act of 1986).
Surgical procedure. Surgery was performed under isoflurane anesthesia. The rat was placed in a stereotactic apparatus (David Kopf Instruments, Tujunga, CA) with bregma and lambda in the same horizontal plane. Local analgesic [xylocaine spray (Lidocaine)] was applied to the exposed tissue of the head. Animals were injected preoperatively with an antibiotic [Amfipen, 0.3 ml, s.c. (anhydrous ampicillin, 100 mg/ml)] and postoperatively with an analgesic [Carprofen, 1 ml/kg, s.c. (Rimadyl, nonopioid analgesic, 1:10)]. Rats were instrumented with bipolar recording electrode sets bilaterally in three cortical areas and the dorsal hippocampus (only hippocampal data used in this article). The two wires of the bipolar electrode sets were separated 1 mm vertically for hippocampal electrodes. The coordinates were as follows (in mm): −4.0 anteroposterior; ±2.0 lateral relative to bregma; and −3/−2 (depth from skull) (Paxinos and Watson, 1986). A screw was used as ground electrode. Electrodes [stainless steel wire; diameter, 0.004 mm (California Fine Wire, Grover Beach, CA)] connected to a pin (031-9540-000; ITT Cannon) with a small insert (track pins; 04.11.T1559; Display Elektronica, Amsterdam, The Netherlands) [typical resistance, 7.5 ± 0.1 (mean ± SE) kΩ] were inserted and fixated with superglue (Cyanolit) and dental cement. All electrode pins were fitted into an 18-hole connector (CTA3-IS-53; ITT Cannon). The electrodes and connector were embedded in dental cement, and the tissue was sutured.
Behavioral testing. Rats recovered for at least 2 weeks after surgery, before behavioral testing, at the age of 16–22 weeks. Animals were habituated to experimental conditions by connecting them to a dummy cable and swivel in their home cage the night before the experiment. Recording took place individually in an enriched open field, which was cleaned with ethanol (70%) between sessions to prevent rats using olfactory cues left by previously tested rats. Observations lasted 25 min per animal and were performed under dimmed red light conditions between the second and the fifth hour of the dark period to minimize circadian influences.
Open field. The open field (black plastic, 1 × 1 × 0.4 m) had one side made of clear acrylic glass to allow camera side view. The open field was supplied with a fixed amount of food, access to the spout of a drinking bottle, and enriched with an object that could not be displaced by the animal (pyramidal shaped, glass object, 6 × 6 × 11.5 cm).
Behavioral scoring. Behavior was recorded simultaneously on two cameras placed at side and top view of the animal, and behavior was scored off-line using the Observer Video-Pro (Noldus Information Technology, Wageningen, The Netherlands) with a time resolution of 0.04 sec. A detailed analysis of behavior included 21 behavioral elements (Van Lier et al., 2003), based on the work ofTimmermans (1978) and Vossen (1966) and our own observations. Each behavioral element was placed into one of the following categories: type 1 behavior, type 2 behavior (Coenen, 1975; Vanderwolf and Robinson, 1981), or sniffing behavior. Type 1 behavior included walking, running, hopping, exploratory walking, climbing, rearing, rearing supported, rearing object, and manipulating food. Type 2 behavior included eating, drinking, face washing, body grooming, genital grooming, scratching, and sitting. Sniffing behavior included sniffing object, sniffing up, sniffing down, sniffing wall, and sniffing food. To enable quantification of the intensity of motor movements during sniffing behavior, intensity scores were calculated on the basis of the distance (in centimeters) moved by the head and the paws of the animal for each 0.5 sec epoch.
Analysis of behavioral data. Using the Observer, a lag sequential analysis was obtained. Number of transitions between behaviors was calculated, and statistical significances were tested with one-way ANOVA (with post hoc Scheffé test) using SPSS software (SPSS, Chicago, IL). Because of structural zeros in the data, Pearson χ2 statistic and adjusted residuals were calculated according to procedures using log-linear approaches as recommended by Bakeman and Quera (1995). An iterative proportional fitting procedure was used (Fienberg, 1980); adjusted residuals were calculated using the Newton-Raphson algorithm (Haberman, 1979). All rats were included in the analysis.
EEG data acquisition. Bipolar recordings were obtained. Signals were filtered high pass at 1 Hz and low pass at 100 Hz and amplification at 300 μV/V. Digitization (sampling rate 1024 Hz) and data recording was done using Windaq (Dataq Instruments, Akron, OH).
Analysis of EEG data. A computer program segmented the EEG according to the files with behavioral scoring. EEG segments were not overlapped and averaged for each rat and behavior. Spectral power density (in square volts) was calculated, reflecting energy and thus electrical activity rather than amplitude (in volts). For the data in Figures 2 and 3, power spectral density was calculated for segments of 1 sec. For Figure 2, EEG of behavioral epochs <1 sec was discarded, and epochs >1 sec were divided into the largest number of integer 1 sec segments. For Figure 3, epochs <1 sec were discarded, whereas only the first second of epochs >1 sec was used. For the data of Figure 4, behavioral epochs of at least 2 sec were used and divided into four consecutive segments of 0.5 sec for power spectral density calculation. For analysis after transition, the beginning of the first segment of an EEG epoch was synchronous with the behavioral transition, and EEG was analyzed up to 2 sec after transition. For analysis before transition, the ending of the first segment was synchronous with the transition, the second segment ended at the beginning of the first segment, and so leading back to 2 sec before transition. Only animals showing the characteristic pattern of hippocampal electrical activity were selected for the analyses. From previous experiments, it was clear from histological verification that electrodes were then adequately placed in the hippocampus (van Luijtelaar and Coenen, 1984). Data from both the left and right brain hemisphere were used in the analysis. Student's paired t test statistics and one-way ANOVA (withpost hoc Scheffé test) were calculated when appropriate (SPSS 10). Regression analysis and covariance analysis were performed on movement intensity and 8 Hz EEG power, including normalized data from seven rats (SPSS).
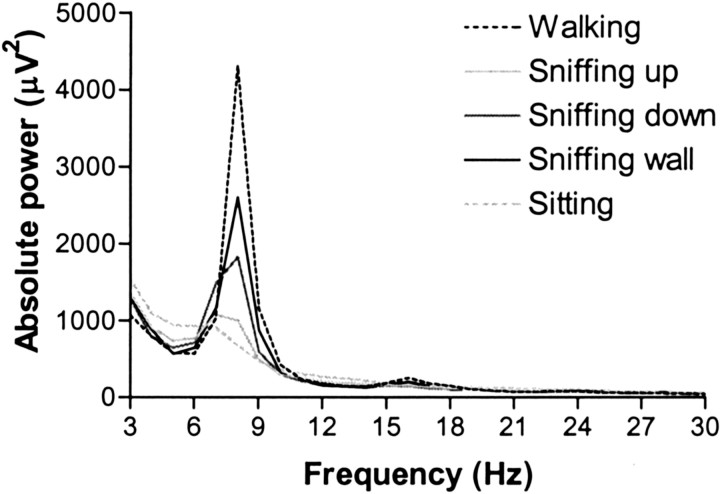
Fig. 2.
Hippocampal power spectra for visually distinguished types of sniffing (sniffing up, sniffing down, and sniffing wall). Also shown are power spectra for walking and sitting (n = 8).
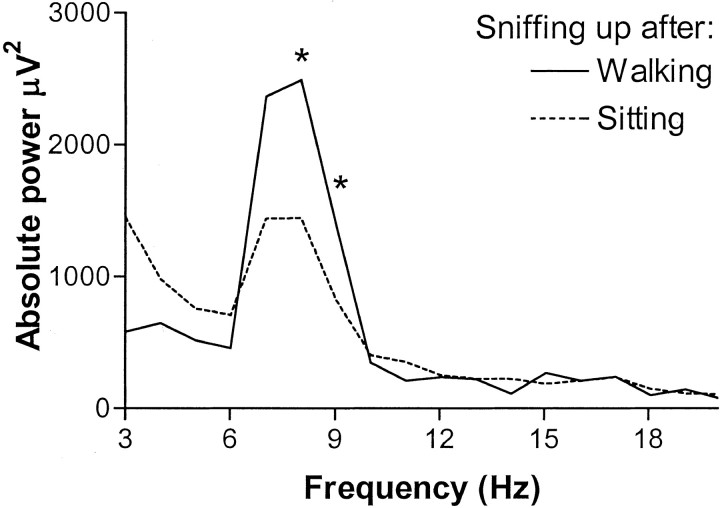
Fig. 3.
Power density spectrum for sniffing up after walking or after sitting. The first second of each sniffing up segment is used. * indicates that absolute power differs at 8 Hz (t(7) = 6.003; p < 0.01) and 9 Hz (t(7) = 2.558;p < 0.05).
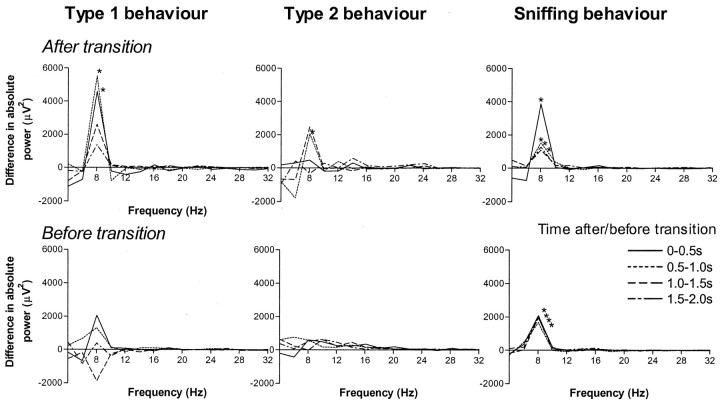
Fig. 4.
Transition effect. The transition effect is shown for type 1, type 2, and sniffing behavior, each linespecifying one of four consecutive time segments of 0.5 sec. On thetop, each line represents the difference in absolute power between following after type 1 and following after type 2 behavior (After transition). On thebottom, each line represents the difference in absolute power between preceding before type 1 and before type 2 behavior (Before transition). * indicates significant difference in absolute power at 8 Hz between transitions with type 1 and type 2 behavior (p < 0.05). Note that the difference between the two spectra is shown.
Results
Sequential analysis of open field behavior
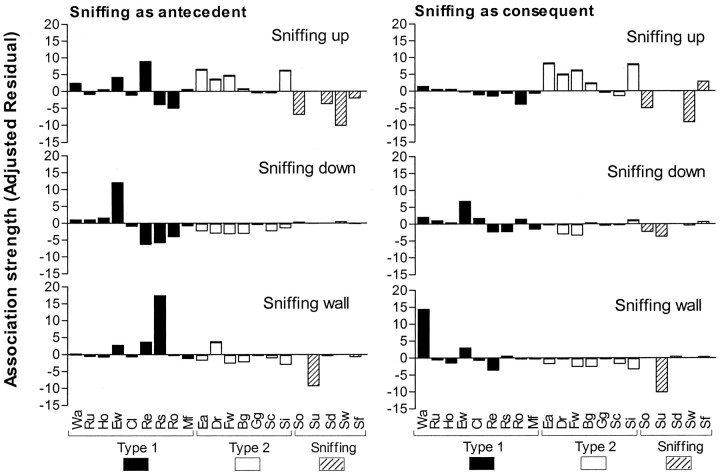
In Figure 1, results are given of sequential analysis of the open field behavior. Association strength between behavioral elements is depicted as adjusted residuals for the different types of sniffing. Behavior was not independent of the immediately preceding or following behavior [χ2(539) = 3356.54;p < 0.001]. A high adjusted residual shows a high association (a higher number of transitions than can be expected based on the occurrence of the behaviors) between behaviors. “Sniffing up” showed a high association with type 2 behaviors: sitting, face washing, and eating, as well as with rearing. “Sniffing down” was highly associated with “exploratory walking;” “sniffing wall” was associated with “rearing supported” and “walking.” The mean ± SE number of transitions with type 1 behavior as a percentage of number of transitions with type 1 and 2 behavior was as follows: sniffing up, 0.71 ± 0.17; sniffing down, 0.86 ± 0.12; and sniffing wall, 0.91 ± 0.06 (n = 13). Sniffing up had more transitions with type 2 behaviors compared with sniffing down and sniffing wall (F(2,36) = 9.277; p < 0.001; post hoc p < 0.05 andp < 0.01, respectively).
Fig. 1.
Behavioral transitions with sniffing behaviors. The figure shows the association strength (as adjusted residuals) of sniffing up, sniffing down, and sniffing wall with other types of behavior. A positive-adjusted residual reflects a higher number of transitions between two behaviors than can be expected on the basis of the occurrence of the two behaviors; a negative-adjusted residual means a lower number of transitions between two behaviors than can be expected on the basis of the occurrence of the two behaviors. The larger the adjusted residual, the stronger the association between the two behaviors is. Adjusted residuals are shown for transitions with sniffing behaviors as first act, with the behaviors on thex-axis representing following behaviors (Sniffing as antecedent, left), and for transitions with sniffing behaviors as second act, with the behaviors on thex-axis representing preceding behaviors (Sniffing as consequent, right). Wa, Walking; Ru, running; Ho, hopping;Ew, exploratory walking; Cl, climbing;Re, rearing; Rs, rearing supported;Ro, rearing object; Mf, manipulating food; Ea, eating; Dr, drinking;Fw, face washing; Bg, body grooming;Gg, genital grooming; Sc, scratching;Si, sitting; So, sniffing object;Su, sniffing up; SD, sniffing down;Sw, sniffing wall; Sf, sniffing food.
EEG power spectra of sniffing
The power spectra for visually distinguished types of sniffing fell between those for walking and sitting (Fig.2). Sniffing up most resembled the spectrum for sitting; sniffing wall was most similar to the spectrum of walking, and sniffing down fell between the spectra of sitting and walking. Sniffing up showed a significant lower RSA amplitude at 8 Hz compared with sniffing wall (F(2,36) = 3.954; p < 0.05; post hoc p < 0.05).
EEG effects of behavioral transitions
The power at theta frequencies of 8 Hz (t(7) = 6.003; p < 0.01) and 9 Hz (t(7) = 2.558;p < 0.05) was increased during sniffing up following after walking compared with during sniffing up following after sitting (Fig. 3). The effect of preceding behavior on RSA power was not specific for sniffing up behavior but was present during other types of sniffing as well. Also, during other type 1 behaviors and during other type 2 behaviors, RSA power was increased if a type 1 behavior preceded the behavior compared with if a type 2 behavior preceded it. In Figure 4, behavioral elements were grouped into the categories of sniffing, type 1 behavior, and type 2 behavior. Individual behavioral elements systematically showed the effects as shown for the grouped categories. Figure 4 (top) shows the difference in spectral power during a behavior between following after a type 1 compared with after a type 2 behavior. A marked difference occurred specifically for 8 Hz. In time, the effect of preceding behavior on 8 Hz power during sniffing was most prominent in the first half-second after transition (t(7) = 3.500; p < 0.05) and still present 0.5–1.0 sec (t(7) = 2.990; p < 0.05), 1.0–1.5 sec (t(7) = 3.134; p < 0.05), and 1.5–2.0 sec (t(7) = 2.412;p < 0.05) after the behavioral transition. During type 1 behavior, the effect was demonstrated for the first two half-second segments (0–1.0 sec) (t(7) = 3.662,p < 0.01; and t(7) = 2.418, p < 0.05, respectively); during type 2 behavior, the effect was shown for the second half-second after transition (0.5–1.5 sec) (t(7) = 3.093; p < 0.05). Not only did preceding behavior have an effect on 8 Hz power, but following behavior had an effect as well (Fig. 4, bottom). This effect of following behavior, however, was smaller than the effect of preceding behavior. It could only be demonstrated during sniffing and not during type 1 or type 2 behavior. It was present in all four half-second segments 0–2.0 sec before behavioral transition (t(7) = 2.663, p < 0.05;t(7) = 2.800, p < 0.05; t(7) = 3.250, p < 0.05; andt(7) = 3.344, p < 0.05, respectively).
Intensity of head and paw movements during sniffing at behavioral transitions
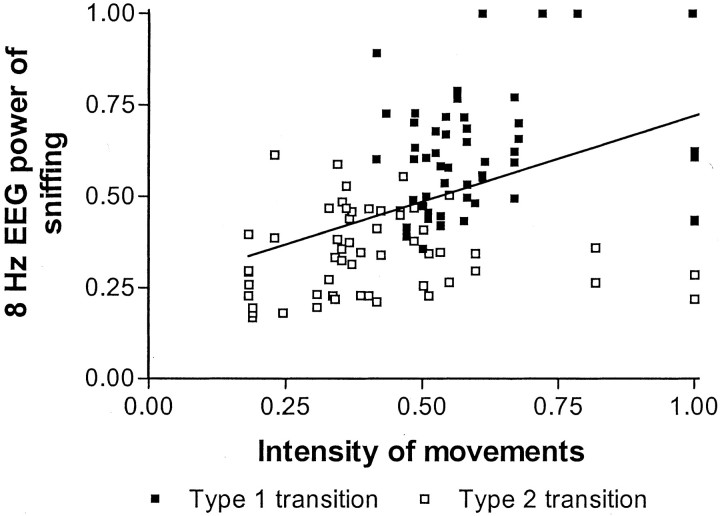
Intensity of motor movements of the head and limbs during sniffing behavior in the first 2 sec preceding or following transition differed between transitions with a type 1 or type 2 behavior (Table1). For all four time segments for both after and before transition, intensity of head and limb movements during sniffing behavior was higher for transitions with a type 1 behavior than for transitions with a type 2 behavior. Data from the movement intensity and 8 Hz EEG power were combined and further analyzed. EEG power at 8 Hz was higher for type 1 transitions compared with type 2 transitions (F(1,110) = 103.227; p < 0.001), as well as intensity of movement (F(1,110) = 40.357; p< 0.001). Data of both 8 Hz EEG power and intensity of head and limb movements during sniffing behavior were fitted in a regression analysis (Fig. 5). Intensity of movement and 8 Hz EEG power correlated significantly (p < 0.001) with r = 0.453, explaining only 20.5% of the total variance. On the basis of the correlation between intensity of movement and 8 Hz EEG power, a covariance analysis was performed to establish whether the difference in 8 Hz EEG power could be attributed to differences in intensity of movement. The analysis of covariance with intensity of movement as covariate showed that the difference in 8 Hz EEG power during sniffing remained significant between transitions with a type 1 and type 2 behavior (F(1,109)= 62.866; p < 0.001). Unstandardized residuals of the regression differed between type 1 and type 2 transitions (F(1,110) = 40.192; p< 0.0001), showing that data points of type 1 transitions lay on average above the regression line (0.1 ± 0.02, mean ± SEM) and data points of type 2 transitions on average under the line (−0.1 ± 0.02, mean ± SEM).
Table 1.
Intensity of motor movements of head and limbs during sniffing behavior
| Time segment | Intensity of movements (centimeters moved by head and/or limbs) | Paired t test | ||
|---|---|---|---|---|
| Type 1 | Type 2 | t value | p value | |
| After transition | ||||
| 0–0.5 sec | 4.17 (0.35) | 2.75 (0.31) | 5.463 | <.01* |
| 0.5–1.0 sec | 2.82 (0.35) | 1.96 (0.28) | 3.687 | <.01* |
| 1.0–1.5 sec | 2.74 (0.32) | 2.01 (0.17) | 3.283 | <.05* |
| 1.5–2.0 sec | 2.79 (0.20) | 2.07 (0.21) | 8.449 | <.001* |
| Before transition | ||||
| 0–0.5 sec | 5.23 (0.62) | 2.26 (0.39) | 7.161 | <.001* |
| 0.5–1.0 sec | 3.03 (0.30) | 1.73 (0.29) | 3.965 | <.007* |
| 1.0–1.5 sec | 2.77 (0.30) | 1.58 (0.16) | 2.984 | <.05* |
| 1.5–2.0 sec | 2.84 (0.28) | 1.88 (0.16) | 2.633 | <.05* |
Intensity was quantified as total centimeters moved by head and limbs during each half-second segment. Intensity of head and paw movements during sniffing was higher for transitions with type 1 behavior compared with type 2 behavior (n = 7) (paired samples t test).
Fig. 5.
Regression analysis of intensity of movements and 8 Hz power of sniffing. For each rat, each time segment, and for both after and before transition, data points of intensity of movements were plotted against 8 Hz EEG power of sniffing. Transitions between sniffing and type 1 behavior are represented by filled symbols; transitions with type 2 behavior are represented byopen symbols. Intensity of movements and 8 Hz EEG power were positively correlated; r = 0.453. Data points of type 1 transitions are on average above the regression line, and data points of type 2 transitions on average under the regression line.
Discussion
The type of behavior preceding and following a behavioral element influenced its spectral power. Transitions with either a type 1 or a type 2 behavior modulated the RSA component. This modulating effect was shown during several types of behavior. It was prominently present during sniffing and type 1 behavior and was less clearly found during type 2 behaviors. The duration of the effect made it evident that the effect could not solely be attributed to blurred starting or end points of behaviors. It has been reported that EEG appears to precede the EMG by 0.5 sec in the case of a spontaneous transition from standing to walking in dogs (Arnolds et al., 1979). Our results showed a prolonged effect on RSA power, demonstrating a systematic modulation of hippocampal EEG correlates of behavior by behavioral transitions.
This modulating effect on RSA power could contribute to the differences in EEG patterns between types of sniffing behavior. The power spectrum of sniffing up resembled spectra of type 2 behavior with regard to RSA peak power. In contrast, sniffing down and sniffing wall more closely resembled spectra of type 1 behavior. This corresponds with observations made by Coenen (1975). Sequential analysis of behavior showed that more transitions with type 2 behaviors relative to type 1 behaviors occurred in sniffing up compared with the other types of sniffing. Furthermore, a behavioral transition with a type 2 behavior resulted in a lower RSA peak power than a transition with a type 1 behavior. The combination of these two results would lead to the lower RSA power in the spectrum of sniffing up. It remains yet to be quantified to what extent the EEG effect of behavioral transitions could contribute to the differences in power spectra between types of sniffing relative to other putative influences. Such influences include differences in the level of automation or stereotypy between the sniffing behaviors (Coenen, 1975), differences in motor patterns, and differences in the vigor of execution of head or paw movements that can occur concurrent with the sniffing (Vanderwolf and Robinson, 1981).
A clear relationship between behavior and hippocampal RSA has been amply demonstrated over the last decades. Our results indicate that, in this relationship, previous and following behavior systematically modulate the RSA contents of the hippocampal EEG correlates of behavior. The strong and systematic relationship between hippocampal RSA and behavior appears to be further modulated systematically by the behavioral–sequential context. Studies on hippocampal lesions have demonstrated the role of the hippocampus in sequential behavior (Terlecki and Sainsbury, 1978; Cannon et al., 1992). The modulating effect of behavioral transitions could indicate that the link between behavioral output and RSA is not as direct. Our results show different hippocampal EEG patterns (namely RSA) for the same visually scored behavior in terms of movement patterns.
However, sniffing behavior was not the same in terms of the intensity of movements of the head and paws. Concurrent with increased RSA power during sniffing for transitions with type 1 behavior, increased intensity of head and paw movements during sniffing behavior was demonstrated. It has been described that more vigorous movements are accompanied by higher-amplitude RSA (Whishaw and Vanderwolf, 1973). Our data confirm a positive correlation between EEG power at 8 Hz and the intensity of head and paw movements during sniffing. Therefore, differences in intensity of movement will have contributed to the transition effect. However, the percentage of variance explained by differences in movement intensity was low, and covariance analysis showed that, even when corrected for intensity of movements, transitions with type 1 behavior still had a higher 8 Hz power. Thus, the higher 8 Hz power for transitions with type 1 behavior cannot be solely explained by the intensity of movements of head and paws during sniffing. A second factor seems to be involved, which is not dependent on the intensity of movements.
The estimate of only 20.5% of variance explained by intensity of motor movements is based on a linear association and assumes accurate measurement of actual intensity of motor movements. Movements were quantified by determining distances moved by the head and paws on a two-dimensional video screen, although movements are three dimensional. Thus, distance moved could be underestimated, increasingly so for larger movements. At equal two-dimensional movement scores, however, this underestimation should be equal. For the data points in the area of intensity scores between 0.47 and 0.60 (Fig. 5), intensity scores are equal for type 1 and type 2 transitions. In this area, 8 Hz power still differs between the two groups. Thus, the higher 8 Hz EEG power for type 1 transitions will still hold, even if actual movements were underestimated. Video analysis of motor intensity, however, could not measure muscle tension that did not result in movement. With regard to the assumption of linearity, several nonlinear curves have been fitted on the data. Nonlinear fits decreasedR2 or only marginally increasedR2. For example, an exponential relationship rendered an R2 of 0.26 (0.20 for a linear association). The highestR2 was 0.30 for a third-order polynomial fit. However, this kind of association between 8 Hz power and movement intensity is hard to interpret. Thus, nonlinear associations between 8 Hz EEG power and movement did not greatly influence the amount of variance explained by the association. Consequently, the suggested presence of a second factor that is independent of movement does not critically depend on the assumption of a linear association between 8 Hz EEG power and intensity of motor movements.
A likely possibility for a process that additionally contributes to the relationship between hippocampal theta and behavior is a sensory process, in accordance with recent theories that relate hippocampal theta to sensorimotor mechanisms (Oddie and Bland, 1998;Sainsbury, 1998; Vanderwolf, 2001). Bland and Oddie (2001) formulated a model that involves the hippocampal theta rhythm in mechanisms underlying sensorimotor integration. In this model, type 1 theta gives a direct indication of the level of activation of the motor systems involved in type 1 behavior, whereas type 2 theta indicates the processing of sensory information. This type 2 theta is always coincidental with type 1 theta and provides the motor systems with continually updated feedback information on changing sensory conditions (Bland and Oddie, 2001). The modulation of the hippocampal EEG correlates of behavior by the sequential context reported here can be fitted to this sensorimotor integration model. Higher RSA power after or before a transition with a voluntary movement could indicate the contribution of sensory information processing in the form of type 2 theta. This is based on the assumption that, in the open field situation in these experiments, more sensory information processing takes place in a sequence of voluntary movements (exploration) than in a sequence of type 2 behaviors.
The contribution of sensory processes to hippocampal theta in addition to motor activity could not only explain our data but seems more reasonable because complications with a simple motor movement hypothesis have been reported repeatedly. Many other species show obvious hippocampal theta during motionless vigilance states, as well as during movement. Rabbits, for example, display trains of theta during immobility (Klemm, 1971). Theta can be recorded from animals anesthetized with ether (MacLean, 1959), and theta appears in immobile rats just before a jump avoidance response (Vanderwolf, 1969). Thus, our data confirm problems with a simple motor movement hypothesis and offer experimental support for the recent theories on a sensorimotor function of hippocampal theta.
Nevertheless, other processes could explain the data: for example, a process that slowly changes the rat's brain state to increase both the probability of type 1 behavior activity and the tendency for the production of theta rhythm. Our data cannot distinguish between the two possibilities, but these possibilities could well be one and the same process. Such a process as slowly changing brain states could operate by modulating the processing of sensory information. Thus, at behavioral transitions, a second component of theta is revealed, reflecting the processing of sensory stimuli that are relevant to the initiation and maintenance of voluntary motor behaviors.
Another alternative is that higher-order functions could, in addition to motor activity, contribute to the relationship between RSA and behavior. However, it is not yet clear whether these functions could contribute to RSA and through which specific mechanism. Higher-order functions include attention (Wall and Messier, 2001), memory (Redish, 2001), spatial navigation and learning (Jarrard, 1995; Whishaw et al., 2001), and anxiety (File et al., 2000).
To conclude, a systematic modulating effect of behavioral transitions on hippocampal EEG correlates of open field behavior was shown. This indicates that the behavioral–sequential context modulates the relationship between behavior and hippocampal RSA. Results of the present studies suggest the presence of (at least) two superimposed processes in the relationship between hippocampal RSA and behavior. In addition to the well established relationship between RSA and motor activity, a second nonmotor process appears to contribute to hippocampal RSA. A likely candidate is a sensory process, which is in accordance with theories on the sensorimotor function of the hippocampus and hippocampal EEG.
Footnotes
This experiment was conducted by H.v.L. while she and W.H.I.M.D. were at Organon Laboratories (Newhouse, Scotland). We kindly acknowledge Organon Laboratories for their financial and practical support. Dr. Vijn is kindly acknowledged for his support in data acquisition and processing and Dr. van Luijtelaar for his advice.
Correspondence should be addressed to Hester van Lier, Nijmegen Institute for Cognition and Information, Department of Biological Psychology, University of Nijmegen, Montessorilaan 3, P.O. Box 9104, 6500 HE Nijmegen, The Netherlands. E-mail: h.vanlier@nici.kun.nl.
References
- 1.Arnolds DEAT, Lopes da Silva FH, Aitink JW, Kamp A. Hippocampal EEG and behaviour in dog. I. Hippocampal EEG correlates of gross motor behaviour. Electroencephalogr Clin Neurophysiol. 1979;46:552–570. doi: 10.1016/0013-4694(79)90009-9. [DOI] [PubMed] [Google Scholar]
- 2.Bakeman R, Quera V. Log-linear approaches to lag-sequential analysis when consecutive codes may and cannot repeat. Psychol Bull. 1995;118:272–284. [Google Scholar]
- 3.Bland BH, Oddie SD. Theta band oscillation and synchrony in the hippocampal formation and associated structures: the case for its role in sensorymotor integration. Behav Brain Res. 2001;127:119–136. doi: 10.1016/s0166-4328(01)00358-8. [DOI] [PubMed] [Google Scholar]
- 4.Buzsáki G. Two-stage model of memory trace formation: a role for “noisy” brain states. Neuroscience. 1989;31:551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- 5.Cannon RL, Paul DJ, Baisden RH, Woodruff ML. Alterations in self-grooming sequences in the rat as a consequence of hippocampal damage. Psychobiology. 1992;20:205–218. [Google Scholar]
- 6.Chang F.-C.T. Modification of medullary respiratory-related discharge patterns by behaviors and states of arousal. Brain Res. 1992;571:281–292. doi: 10.1016/0006-8993(92)90666-w. [DOI] [PubMed] [Google Scholar]
- 7.Coenen AML. Frequency analysis of rat hippocampal electrical activity. Physiol Behav. 1975;14:391–394. doi: 10.1016/0031-9384(75)90053-0. [DOI] [PubMed] [Google Scholar]
- 8.Elazar A, Adey WR. Spectral analysis of low frequency components in the electrical activity of the hippocampus during learning. Electroencephalogr Clin Neurophysiol. 1967;23:225–240. doi: 10.1016/0013-4694(67)90119-8. [DOI] [PubMed] [Google Scholar]
- 9.Fienberg SE. The analysis of cross-classified categorical data, Ed 2, pp 142–145. MIT; Cambridge, MA: 1980. [Google Scholar]
- 10.File SE, Kenny PJ, Cheeta S. The role of the dorsal hippocampal serotonergic and cholinergic systems in the modulation of anxiety. Pharmacol Biochem Behav. 2000;66:65–72. doi: 10.1016/s0091-3057(00)00198-2. [DOI] [PubMed] [Google Scholar]
- 11.Forbes WB, Macrides F. Temporal matching of sensory-motor behavior and limbic theta rhythm deteriorates in aging rats. Neurobiol Aging. 1984;5:7–17. doi: 10.1016/0197-4580(84)90080-0. [DOI] [PubMed] [Google Scholar]
- 12.Grastyán E, Lissák K, Madarász I, Donhoffer H. Hippocampal electrical activity during the development of conditional reflexes. Electroencephalogr Clin Neurophysiol. 1959;11:409–430. doi: 10.1016/0013-4694(59)90040-9. [DOI] [PubMed] [Google Scholar]
- 13.Green JD, Arduini AA. Hippocampal electrical activity in arousal. J Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- 14.Haberman SJ. Analysis of qualitative data, Vol 2, p 454. Academic; New York: 1979. [Google Scholar]
- 15.Jarrard LE. What does the hippocampus really do? Behav Brain Res. 1995;71:1–10. doi: 10.1016/0166-4328(95)00034-8. [DOI] [PubMed] [Google Scholar]
- 16.Jung R, Kornmüller AE. Eine Methodik der Ableitung lokalisierter Potentialschwankungen aus subcorticalen Hirngebieten. Arch Psychiatrische Nervenkrankheit. 1939;109:1–30. [Google Scholar]
- 17.Kemp IR, Kaada BR. The relation of hippocampal theta activity to arousal, attentive behaviour and somato-motor movements in unrestrained cats. Brain Res. 1975;95:323–342. doi: 10.1016/0006-8993(75)90110-9. [DOI] [PubMed] [Google Scholar]
- 18.Klemm WR. EEG and multiple-unit activity in limbic and motor systems during movement and immobility. Physiol Behav. 1971;7:337–343. doi: 10.1016/0031-9384(71)90311-8. [DOI] [PubMed] [Google Scholar]
- 19.Komisaruk BR. Synchrony between limbic system theta activity and rhythmical behavior in rats. J Comp Physiol Psychol. 1970;70:482–492. doi: 10.1037/h0028709. [DOI] [PubMed] [Google Scholar]
- 20.MacLean PD. The central nervous system and behavior (Brazier MAB, ed), Transactions of the Second Macy Conference. Josiah Macy Jr Foundation; New York: 1959. The limbic system with respect to two basic life principles. p. 358. [Google Scholar]
- 21.Oddie SD, Bland BH. Hippocampal formation theta activity and movement selection. Neurosci Biobehav Rev. 1998;22:221–231. doi: 10.1016/s0149-7634(97)00003-1. [DOI] [PubMed] [Google Scholar]
- 22.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic; New York: 1986. [DOI] [PubMed] [Google Scholar]
- 23.Redish AD. The hippocampal debate: are we asking the right questions? Behav Brain Res. 2001;127:81–98. doi: 10.1016/s0166-4328(01)00356-4. [DOI] [PubMed] [Google Scholar]
- 24.Sainsbury RS. Hippocampal theta: a sensory-inhibition theory of function. Neurosci Biobehav Rev. 1998;22:237–241. doi: 10.1016/s0149-7634(97)00011-0. [DOI] [PubMed] [Google Scholar]
- 25.Terlecki LJ, Sainsbury RS. Effects of fimbria lesions on maternal behavior in the rat. Physiol Behav. 1978;21:89–97. doi: 10.1016/0031-9384(78)90281-0. [DOI] [PubMed] [Google Scholar]
- 26.Timmermans PJA. PhD thesis. University of Nijmegen; 1978. Social behaviour in the rat. [Google Scholar]
- 27.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 28.Vanderwolf CH. The electrocorticogram in relation to physiology and behavior: a new analysis. Electroencephalogr Clin Neurophysiol. 1992;82:165–175. doi: 10.1016/0013-4694(92)90164-d. [DOI] [PubMed] [Google Scholar]
- 29.Vanderwolf CH. The hippocampus as an olfacto-motor mechanism: were the classical anatomists right after all? Behav Brain Res. 2001;127:25–47. doi: 10.1016/s0166-4328(01)00354-0. [DOI] [PubMed] [Google Scholar]
- 30.Vanderwolf CH, Robinson TE. Reticulo-cortical activity and behavior: A critique of the arousal theory and a new synthesis. Behav Brain Sci. 1981;4:459–514. [Google Scholar]
- 31.Van Lier H, Drinkenburg WHIM, Coenen AML. Strain differences in hippocampal EEG are related to strain differences in behaviour in rats. Physiol Behav. 2003;78:91–97. doi: 10.1016/s0031-9384(02)00893-4. [DOI] [PubMed] [Google Scholar]
- 32.van Luijtelaar ELJM, Coenen AML. An averaging technique for automated sleep-wake stage identification in the rat. Physiol Behav. 1984;33:837–841. doi: 10.1016/0031-9384(84)90056-8. [DOI] [PubMed] [Google Scholar]
- 33.Vossen JMH. PhD thesis. University of Nijmegen; 1966. Exploratief gedrag en leergedrag bij de rat. [Google Scholar]
- 34.Wall PM, Messier C. The hippocampal formation–orbitomedial prefrontal cortex circuit in the attentional control of active memory. Behav Brain Res. 2001;127:99–117. doi: 10.1016/s0166-4328(01)00355-2. [DOI] [PubMed] [Google Scholar]
- 35.Whishaw IQ, Vanderwolf CH. Hippocampal EEG and behavior: change in amplitude and frequency of RSA (theta rhythm) associated with spontaneous and learned movement patterns in rats and cats. Behav Biol. 1973;8:461–484. doi: 10.1016/s0091-6773(73)80041-0. [DOI] [PubMed] [Google Scholar]
- 36.Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127:49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]