Abstract
In the mammalian olfactory bulb, mitral cell dendrites release glutamate onto the dendritic spines of granule cells, which in turn release GABA back onto mitral dendrites. This local synaptic circuit forms the basis for reciprocal dendrodendritic inhibition mediated by ionotropic GABAA receptors in mitral cells. Surprisingly little is known about neurotransmitter modulation of dendrodendritic signaling in the olfactory bulb. In this study, we examine whether metabotropic GABAB receptors modulate dendrodendritic signaling between mitral and granule cells. We find that the selective GABAB agonist baclofen reduces mitral cell recurrent inhibition mediated by dendrodendritic synapses. GABABreceptor activation causes only a weak inhibition of field EPSCs in the external plexiform layer and only slightly reduces glutamate-mediated mitral cell self-excitation. Although GABAB receptors depress mitral cell glutamate release only weakly, baclofen causes a marked reduction in the amplitude of granule-cell-evoked, GABAA-mediated IPSCs in mitral cells. In addition to reducing the amplitude of granule-cell-evoked IPSCs, baclofen causes a change from paired-pulse depression to paired-pulse facilitation, suggesting that GABAB receptors modulate GABA release from granule cells. To explore the mechanism of action of GABAB receptors further, we show that baclofen inhibits high-voltage-activated calcium currents in granule cells. Together, these findings suggest that GABAB receptors modulate dendrodendritic inhibition primarily by inhibiting granule cell calcium channels and reducing the release of GABA. Furthermore, we show that endogenous GABA regulates the strength of dendrodendritic inhibition via the activation of GABAB autoreceptors.
Keywords: GABAB, baclofen, olfactory bulb, mitral cell, granule cell, dendrites, presynaptic, calcium channel
Introduction
The first stage of olfactory information processing in the brain occurs when olfactory nerve fibers release glutamate onto the distal primary dendrites of principal mitral cells in the olfactory bulb. Mitral cell axons convey olfactory input to higher brain centers such as the pyriform cortex. Within the olfactory bulb, local processing of olfactory input can occur at several levels. Transmitter release from olfactory nerve terminals in the glomerular layer of the olfactory bulb can be modulated by the activation of presynaptic dopamine receptors (Hsia et al., 1999; Ennis et al., 2001), and periglomerular interneurons have been suggested to inhibit olfactory nerve transmission via the activation of presynaptic GABAB receptors (Keller et al., 1998;Aroniadou-Anderjaska et al., 2000; Palouzier-Paulignan et al., 2002). Periglomerular cells are also believed to mediate local GABAAreceptor inhibition on the distal glomerular tufts of mitral cell primary dendrites (Shepherd and Greer, 1998).
An important local circuit in the bulb occurs at synaptic contacts formed between the lateral dendrites of mitral cells and the dendrites of GABAergic granule cells. Mitral cell dendrites release glutamate onto the dendritic spines of granule cells, which in turn release GABA back onto mitral cell dendrites. This reciprocal synaptic circuit underlies self and lateral dendrodendritic inhibition (Mori and Takagi, 1978; Jahr and Nicoll, 1980; Nowycky et al., 1981; Isaacson and Strowbridge, 1998; Schoppa et al., 1998; Urban and Sakmann, 2002); it is believed to play an important role in olfactory information processing (Yokoi et al., 1995; Mori et al., 1999).
Only a small number of neurotransmitter systems have been suggested to underlie the modulation of dendrodendritic inhibition in the olfactory bulb. Experiments in vivo indicate that noradrenergic (Wilson and Leon, 1988; Okutani et al., 1998) and cholinergic (Elaagouby et al., 1991) receptors can influence olfactory bulb excitability. Noradrenergic receptor activation inhibits the release of GABA from granule cells and reduces dendrodendritic inhibition (Jahr and Nicoll, 1982) in the turtle. Activation of metabotropic glutamate receptor 2 (mGluR2) inhibits spontaneous GABA release from granule cells in the mouse accessory olfactory bulb (Hayashi et al., 1993). In addition to glutamate, GABA is a major transmitter in the bulb that could mediate metabotropic receptor modulation of dendrodendritic transmission. However, a role for metabotropic GABA receptors in dendrodendritic signaling in the olfactory bulb has yet to be elucidated. In this study we address whether dendritic GABAB receptors modulate dendrodendritic transmission between mitral and granule cells in rat olfactory bulb slices.
Materials and Methods
Sprague Dawley rats (14–28 d of age) were anesthetized with a ketamine/zylazine mixture (200:14 mg/kg, i.m.) and decapitated. The olfactory bulbs were quickly removed and placed into ice-cold artificial CSF(aCSF) containing (in mm): 83 NaCl, 2.5 KCl, 0.5 CaCl2, 3.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, 22 glucose, and 72 sucrose equilibrated with 95% O2 and 5% CO2. Horizontal slices (350 μm) were cut using a vibratome and incubated at 36°C in sucrose aCSF for 30 min. Slices were then maintained at room temperature until they were transferred to a recording chamber on an upright microscope equipped with differential contrast optics (BX50;Olympus Optical, Tokyo, Japan). Slices were superfused (for at least 15 min before recording) with aCSF containing (in mm): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26.2 NaHCO3, and 22 glucose, which was equilibrated with 95% O2and 5% CO2. The mGluR antagonist (S)-α-methyl-4-carboxyphenylglycine(MCPG; 100 μm) was added to the aCSF in some experiments examining dendrodendritic inhibition. There were no obvious differences in experiments with or without MCPG, and results under the two conditions were combined. Calcium current recordings were performed at room temperature, and all other experiments were performed at 31–33°C.
For experiments examining recurrent inhibition, patch electrodes (3–5 MΩ) contained (in mm): 130 KCl, 10 HEPES, 10 phosphocreatine, 0.2 EGTA, 2.5 glutamate, 3 MgATP, and 0.5 NaGTP. For all other experiments, the internal solution contained (in mm): 110 CsCl, 10 TEA-Cl, 20 HEPES, 12 phosphocreatine, 0.2 EGTA, 2.5 glutamate, 3 MgATP, and 0.5 NaGTP. EGTA was increased to 10 mm in calcium current recordings. Series resistance, which was always <10 MΩ, was routinely compensated by >90%. Monosynaptic IPSCs were evoked via a bipolar stimulating electrode placed in the external plexiform layer (EPL) or granule cell layer and recorded in the presence of 2,3-dioxo-6-nitro-1,2,3,4 tetrahydrobenzo[f]quinoxaline-7-sulfonamide(NBQX; 10 μm) and (+)-5-methyl-10,11-dihydro-5H-dibenzo [a,d] cyclohepten-5,10-imine maleate(MK-801; 40 μm). Dendrodendritic field EPSPs (fEPSPs) were evoked via a bipolar electrode placed in the EPL and recorded with an aCSF-filled patch electrode. Voltage-clamp and field potential responses were recorded with an Axopatch-200B amplifier (Axon Instruments, Foster City, CA). All responses were filtered at 2 kHz and digitized at 5 kHz (ITC-18; Instrutech, Mineola, NY). Data were collected and analyzed using Axograph (Axon Instruments) and IGOR Pro (Wavemetrics, Lake Oswego, OR). Miniature IPSCs were identified using a template- and threshold-based detection method in Axograph. Miniature IPSCs were recorded over a 5 min period before and after drug application. At least 100 events were analyzed in each cell for the measurement of amplitude and frequency. The amplitudes of mitral cell dendrodendritic inhibition and self-excitation were quantified by integrating the response during the 500 msec after stimulation. Bicuculline methiodide (20–30 μm) was applied at the end of each experiment examining recurrent and dendrodendritic inhibition to allow subtraction of residual currents generated by the voltage steps. Similarly, APV (100 μm) was added at the end of self-excitation experiments. Baclofen was applied in all experiments at a concentration of 25–50 μm. Unless otherwise indicated, all traces represent the average of 5–10 trials. Summary data are expressed as means ±SEM. Statistical significance was assessed by paired Student'sttest.
Results
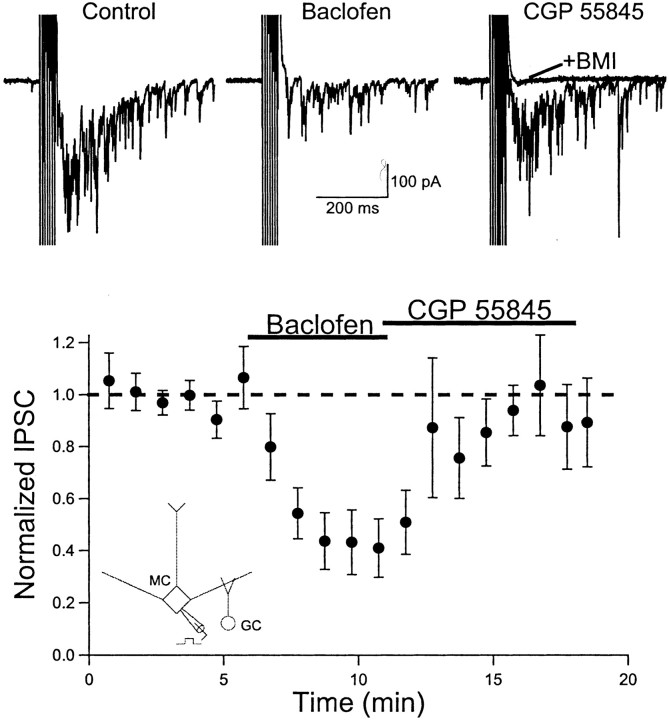
We first examined the effects of the specific GABAB agonist baclofen on recurrent inhibition in mitral cells. Mitral cells were voltage-clamped at −70 mV using a KCl-based internal solution. Under these recording conditions, the chloride driving force for GABAA-mediated responses results in depolarizing IPSCs. Trains of brief voltage steps to 0 mV (2 msec, 4 msec interval, 5–10 pulses) were used to evoke glutamate release from mitral cell axons and dendrites. Mitral cell stimulation evoked a long-lasting barrage of inward GABAA receptor-mediated IPSCs (Fig.1). Bath application of baclofen (25–50 μm) caused a marked reduction in the amplitude of recurrent inhibition (42.6 ± 11.5% of control;n = 11; p< 0.01). At the same time, baclofen did not alter the holding current or the input resistance (measured by a 5 mV voltage step) in mitral cells (data not shown). This lack of effect on membrane properties indicates that GABAB receptors are not coupled to G protein-coupled inwardly rectifying K+(GIRK) channels (Luscher et al., 1997) in mitral cells. Subsequent application of the GABAB antagonist (2S)-3-{[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinicacid(CGP 55845; 5 μm) rapidly reversed the reduction in recurrent inhibition produced by baclofen. These findings indicate that GABAB receptor activation reduces mitral cell recurrent inhibition.
Fig. 1.
GABAB receptor activation reduces mitral cell recurrent inhibition. Top, Individual records from one mitral cell (Vm = −70 mV, KCl internal solution). A brief train of depolarizing voltage steps (2 msec, +70 mV, 7 pulses) elicits action currents, followed by a long-lasting barrage of synaptic currents (Control). The synaptic response is reduced after bath application of the GABAB agonist baclofen (50 μm). Switching to a solution containing the GABAB antagonist CGP 55845 (5 μm) reverses the action of baclofen. Subsequent application of the GABAAantagonist bicuculline methiodide (20 μm) abolishes the synaptic response (+BMI). Bottom, Summary plot of the results for 11 cells. Points represent the average of two consecutive responses evoked every 30 sec. The dashed line represents unity. Inset, Recording configuration. MC, Mitral cell; GC, granule cell.
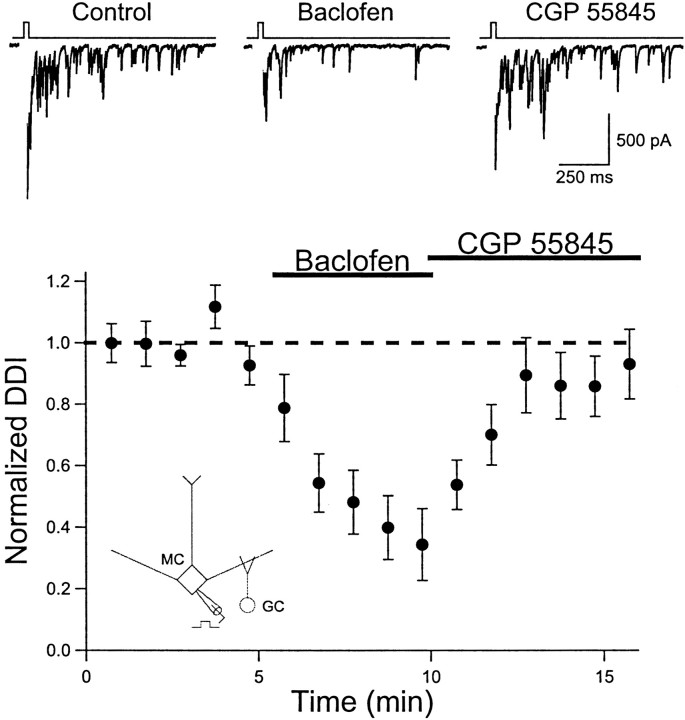
To isolate dendrodendritic synapses, we recorded from mitral cells using a CsCl-based internal solution in aCSF containing tetrodotoxin (TTX; 1 μm). TTX blocks Na+-dependent action potentials and prevents axonal transmitter release. We increased the duration of the depolarizing voltage step (10–50 msec) delivered to mitral cells to activate calcium channels to trigger dendritic glutamate release (Isaacson and Strowbridge, 1998). Baclofen caused a marked reduction in dendrodendritic inhibition (34.9 ± 11.6% of control; n = 10; p < 0.01) that was reversed by the subsequent application of the antagonist CGP 55845 (Fig.2). These results indicate that dendritic GABABreceptors modulate dendrodendritic signaling in the olfactory bulb.
Fig. 2.
GABAB receptor activation reduces mitral cell dendrodendritic inhibition. Top, Individual records from one cell (CsCl internal solution) in the presence of TTX (1 μm). A brief (25 msec) voltage step to 0 mV evokes a calcium current (blanked) followed by a long-lasting barrage of IPSCs (Control). Baclofen (50 μm) reduces dendrodendritic inhibition (DDI), and the response recovers after the application of CGP 55845 (5 μm). Bottom, Summary of the results for 10 cells. Points represent the average of two consecutive responses evoked every 30 sec. The dashed line represents unity.Inset, Recording configuration.
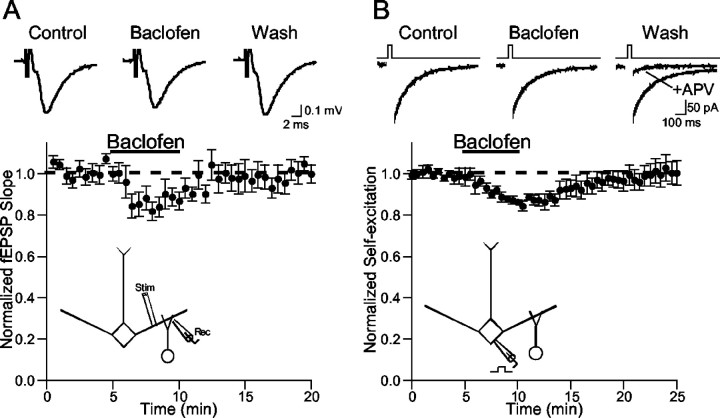
Where do GABAB receptors exert their action in this dendrodendritic circuit? Dendrodendritic inhibition relies on glutamate release from mitral cells followed by GABA release from granule cell dendrites; thus, one or both of these release sites could be modulated by GABAB receptors. We first studied the actions of baclofen on mitral dendrite glutamate release. fEPSPs were evoked and recorded in the EPL of bulb slices. Dendritic synapses are the major elements in this region of the slice, and fEPSPs in the EPL are generally believed to reflect mitral dendrite glutamate release onto granule cells (Aroniadou-Anderjaska et al., 1999;Isaacson, 2001). Bath application of baclofen caused a modest and reversible reduction in the slope of the dendritic fEPSP (84.4 ± 4.0% of control; n = 9; p < 0.01) (Fig.3A). This result suggests that GABAB receptors inhibit mitral dendrite glutamate release only weakly.
Fig. 3.
GABAB receptors weakly modulate mitral cell glutamatergic transmission. A, Baclofen (25–50 μm) application causes a small and reversible reduction in the slope of fEPSPs recorded in the external plexiform layer.Bottom, Results from nine slices. Top, fEPSPs from one representative experiment. B, Baclofen causes a small and reversible reduction in mitral cell self-excitation. Mitral cells (Vm= −50 mV) were recorded in the presence of TTX (1 μm) and picrotoxin (100 μm) in Mg2+-free aCSF. Self-excitation was evoked by brief (5–25 msec) voltage steps to 0 mV.Top, Representative traces from one experiment.Bottom, Summary of results (n= 7). Self-excitation was abolished at the end of each experiment by APV, confirming that the current was mediated by NMDARs. The dashed lines represent unity.
To confirm the modest inhibitory role of GABABreceptors on mitral cell dendritic glutamate release, we studied mitral cell self-excitation (Nicoll and Jahr, 1982; Isaacson, 1999; Friedman and Strowbridge, 2000; Salin et al., 2001). Mitral cells were voltage-clamped (Vm = −60 mV) using a CsCl internal solution and an aCSF containing no added Mg2+. The aCSF was supplemented with TTX (1 μm), and picrotoxin (100 μm) was added to block GABAA IPSCs. Under these conditions, brief depolarizing voltage steps (0 mV, 5–20 msec) reveal an EPSC because of the activation of dendritic NMDA receptors (NMDARs) by glutamate released from the same mitral cell (self-excitation). Bath application of baclofen (50 μm) caused only a slight and reversible reduction in the amplitude of the evoked EPSC (86.7 ± 1.8% of control; n = 7; p < 0.01) (Fig.3B). The self-excitatory response was blocked at the end of each experiment by bath application of APV (100 μm) (Fig. 3B), confirming that NMDARs mediate the EPSC. Application of the GABAB antagonists 3-[[(3,4-dichlorophenyl)-methyl]aminopropyl](diethoxymethyl)phosphinicacid(CGP 52432; 10–50 μm) or CGP 55845 (5–10 μm) alone did not enhance self-excitation (CGP 52432: 102 ± 6% of control, n = 8, p > 0.5; CGP 55845: 99 ± 7% of control, n = 4, p > 0.5), ruling out the possibility that endogenous GABA activated GABAB receptors on mitral cells. Together, the modest action of baclofen on the dendritic fEPSP and self-excitation suggest that GABAB receptors are not strong regulators of mitral dendrite glutamate release.
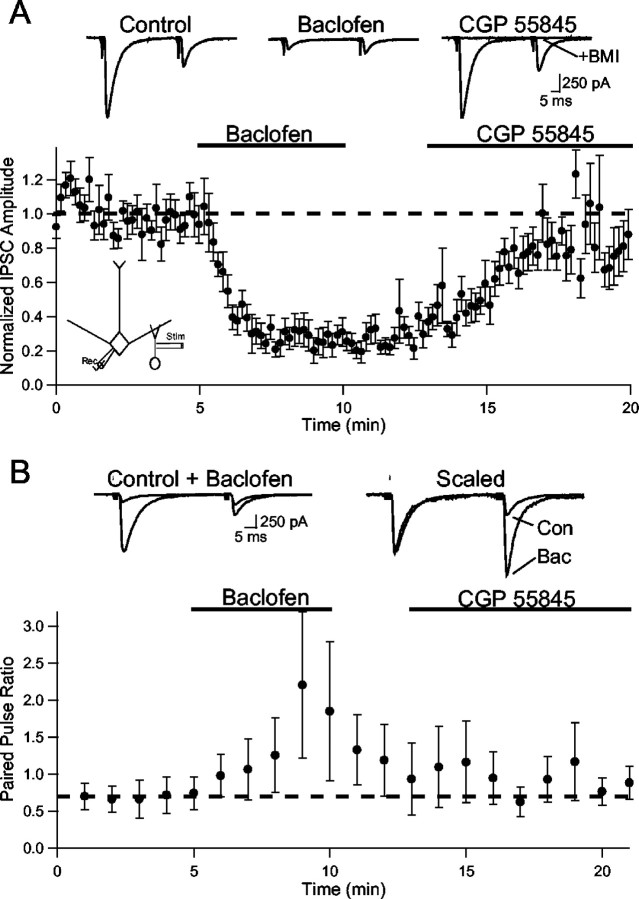
We next examined whether GABAB receptors modulated GABA release from granule cells. Mitral cells were voltage-clamped (Vm = −70 mV) with a CsCl internal solution, and monosynaptic IPSCs were evoked by stimulation in the EPL or granule cell layer. Paired pulse stimulation (50 msec interval) revealed paired-pulse depression of IPSCs (Fig.4). Bath application of baclofen caused a marked reduction in the amplitude of the evoked IPSC (27.3 ± 7.8% of control; n = 9; p < 0.01); this effect was reversed by CGP 55845 (5 μm) (Fig. 4). Baclofen also had a marked effect on the response to paired-pulse stimulation. On average, the paired-pulse ratio (PPR; pulse 2 amplitude/pulse 1 amplitude) changed from depression to facilitation in the presence of baclofen (control PPR, 0.68 ± 0.20; baclofen PPR, 1.77 ± 0.81; n = 4; p < 0.05) (Fig.4B). The GABAB antagonist CGP 55845 also reversed this action on the PPR.
Fig. 4.
GABAB receptor activation inhibits IPSCs evoked in mitral cells by granule cell stimulation.A, Summary plot (n = 9) of the action of baclofen (25–50 μm) on IPSC amplitude. After the washout of baclofen, CGP 55845 (5 μm) caused a recovery of IPSC amplitude. Top, Traces from a representative experiment using paired-pulse stimulation (50 msec interstimulus interval). At the end of the experiment, bicuculline methiodide (+BMI; 20 μm) abolished the IPSC, confirming that it was mediated by GABAA receptors. Thedashed line represents unity. B, Summary of results from cells in which paired-pulse stimulation was applied (n= 4, each point is the average of 6 consecutive IPSCs). Baclofen caused a change from paired-pulse depression to paired-pulse facilitation that was reversed after washout into CGP 55845. Top, Traces from one cell showing the IPSCs before (Control) and after baclofen and after the traces are scaled to the peak of the first IPSC (Scaled). The dashed line represents the average ratio during control.
Modulation of the PPR of synaptic responses is typically associated with presynaptic changes in the probability of transmitter release (Zucker and Regehr, 2002). We next considered the mechanism underlying the strong inhibition of granule-cell-evoked IPSCs by GABAB receptors. One possibility is that GABAB receptors may inhibit granule cells directly by the activation of GIRK channels. However, in current-clamp recordings of granule cells with a K+-based internal solution, baclofen had no effect on resting membrane potential or input resistance (n = 6, data not shown). Another possibility is that GABAB receptors may directly inhibit the machinery governing GABA exocytosis. This possibility reflects findings in other systems showing that GABAB and other G-protein-coupled receptors can reduce the frequency of miniature synaptic currents (Scanziani et al., 1992; Dittman and Regehr, 1996), which reflect the spontaneous fusion of individual vesicles of transmitter at the presynaptic release site. However, in the presence of TTX (1 μm) and cadmium (100 μm), baclofen did not affect either the frequency (control, 1.20 ± 0.28 Hz; baclofen, 1.21 ± 0.28 Hz) or amplitude (control, 101.7 ± 14.2 pA; baclofen, 101.6 ± 24.8 pA) of miniature IPSCs recorded in mitral cells (n = 4). This result indicates that GABAB receptors are unlikely to influence exocytosis directly in granule cell dendrites.
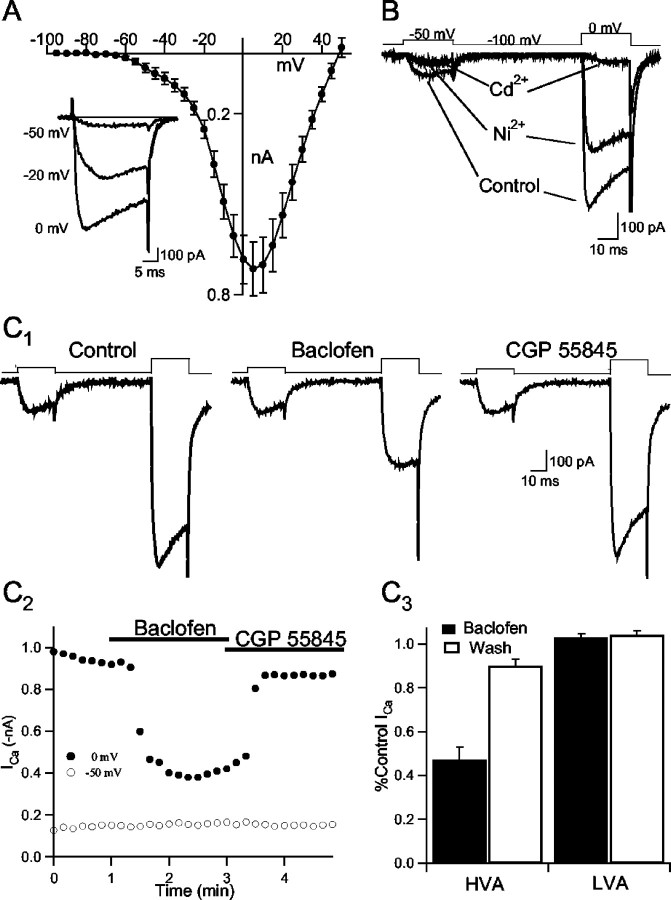
Presynaptic calcium channels represent an important target for the modulation of transmitter release in a variety of conventional nerve endings in the CNS (Miller, 1998). Previous findings suggest that high-voltage-activated (HVA) calcium channels govern dendritic transmitter release in the olfactory bulb (Isaacson and Strowbridge, 1998; Isaacson, 2001). We subsequently examined whether GABAB receptors modulate calcium channels in granule cells. To isolate calcium currents, voltage-clamp recordings (Vm = −100 mV) from granule cells were made in the presence of TTX (1 μm), TEA (5 mm), picrotoxin (100 μm), NBQX (10 μm), and MK-801 (40 μm). We first examined the current–voltage relationship of calcium currents in granule cells. Granule cells were held at −100 mV, and depolarizing voltage steps (20 msec) were applied in 5 mV increments. The peak amplitude of the evoked current over the first 5 msec of each test potential was averaged in eight cells (Fig.5A). The current–voltage (I–V) relationship revealed a marked “shoulder” at negative test potentials, indicating that channels activated with a threshold of approximately −60 mV. The maximal current amplitude was reached at +5 mV. The shoulder in the I–V plot at negative test potentials suggests that calcium currents in granule cells have both low-voltage-activated (LVA) and HVA components (Hille, 2001). LVA currents are typically associated with T-type calcium channels in neuronal cells and are generally found to be more sensitive to Ni2+than HVA calcium channels (Avery and Johnston, 1996). We next examined the actions of inorganic calcium channel blockers on LVA and HVA currents using voltage steps to −50 and 0 mV, respectively. Ni2+(100 μm) markedly reduced the LVA current, and the subsequent application of Cd2+(100 μm) had little additional effect (Fig.5B). In contrast, a considerable component of HVA current remained in the presence of Ni2+, and the Ni2+-insensitive current was almost completelyabolished in the presence of cadmium. Similar results were obtained in three other granule cells.
Fig. 5.
GABAB receptor activation causes a reduction in HVA calcium current in granule cells. A, Current–voltage relationship of calcium current in granule cells (Vm = −100 mV; n = 8).Inset, Currents in one cell in response to the voltage steps indicated to the leftof the sweeps.B, Voltage steps to −50 and 0 mV evoke LVA and HVA calcium currents, respectively, in a granule cell (Vm = −100 mV). Application of Ni2+(100 μm) almost completely abolishes LVA current and partially blocks HVA current. Subsequent application of Cd2+(100 μm) abolishes the remaining HVA current.C1, Response to voltage steps to −50 and 0 mV in one cell before (Control) and after the application of baclofen (50 μm). Baclofen reduced the HVA but not the LVA calcium current; the action was reversed after washout into CGP 55845.C2, Time course of the experiment shown in C1. Filled circles, HVA current; open circles, LVA current.C3, Summary of the actions of baclofen on HVA and LVA current in granule cells (n= 10).
We next examined whether granule cell calcium currents were modulated by GABAB receptors. Cells were held at −100 mV and voltage steps were applied to −50 and 0 mV to study LVA and HVA channels in the same sweeps. Baclofen caused a marked reduction in the peak amplitude of HVA currents in granule cells (47.1 ± 6.0% of control; n = 10; p < 0.01) (Fig.5B). The reduction in HVA current was rapidly reversed on washout of baclofen into aCSF containing CGP 55845 (90 ± 3.0% of control; p < 0.01). G-protein-mediated inhibition of calcium currents is typically accompanied by a slowing in channel activation kinetics (Bean, 1989; Isaacson, 1998). We measured the activation kinetics of granule cell calcium current by fitting an exponential curve to its rising phase. Consistent with G-protein-mediated inhibition, baclofen caused a marked slowing of HVA current activation (control τ = 1.5 ± 0.2 msec, baclofen τ = 4.8 ± 1.5 msec). In the same cells, LVA currents were unaffected by baclofen (103 ± 1.6% of control). Together, these results indicate that GABAB receptors can strongly inhibit HVA but not T-type calcium channels in olfactory bulb granule cells.
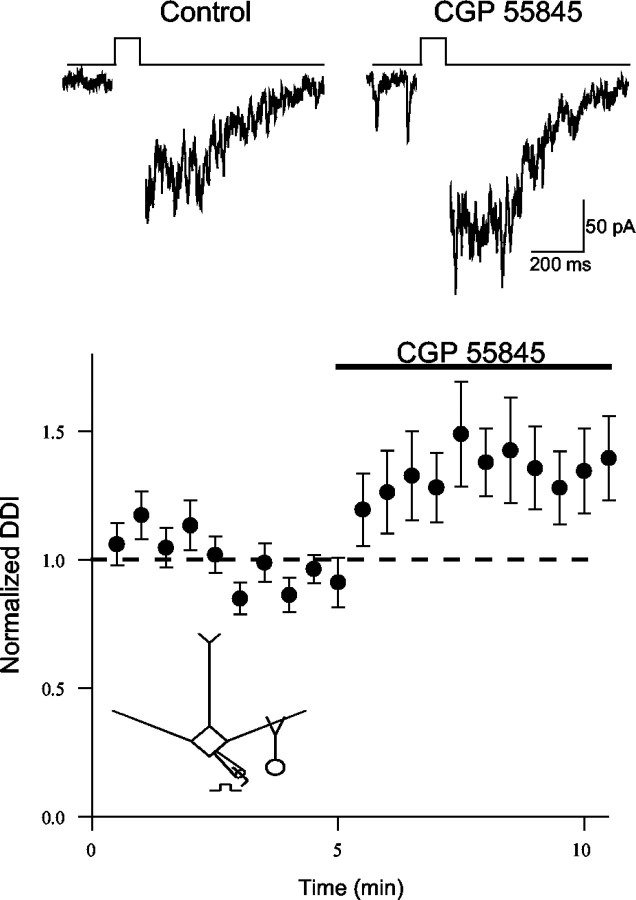
Thus far, our data indicate that the GABABagonist baclofen modulates dendritic signaling in the olfactory bulb. Does endogenously released GABA modulate dendrodendritic inhibition via activation of GABAB receptors? To address this question, we examined the action of the antagonist CGP 55845 alone on dendrodendritic inhibition. Bath application of the GABAB antagonist to naive slices caused a modest enhancement in dendrodendritic inhibition (134 ± 16% of control;n = 11; p < 0.05) (Fig.6A). This result suggests that endogenous GABA acting on GABABautoreceptors can modulate dendrodendritic signaling.
Fig. 6.
Endogenous activation of GABABreceptors limits the strength of mitral cell dendrodendritic inhibition (DDI). Summary plot of the actions of the GABAB antagonist CGP 55845 (5–10 μm) applied alone to slices (n = 11). The GABABantagonist enhances dendrodendritic inhibition in naive slices.Top traces are from one cell (50 msec step) before (Control) and after CGP 55845. The dashed line represents unity.
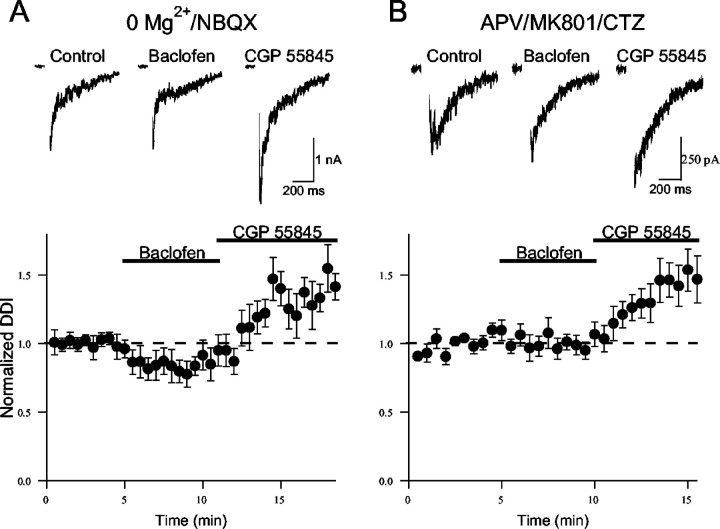
We next considered whether the role of GABABautoreceptors in dendrodendritic inhibition is activity dependent. NMDARs typically play a major role in driving granule cell GABA release during dendrodendritic inhibition (Isaacson and Strowbridge, 1998;Schoppa et al., 1998). Indeed, dendrodendritic inhibition is greatly enhanced by removing extracellular Mg2+to facilitate NMDAR-mediated responses in granule cells (Isaacson and Strowbridge, 1998; Schoppa et al., 1998). We next examined the role of GABAB receptors in dendrodendritic inhibition in Mg2+-free aCSF in the presence of the AMPA receptor (AMPAR) antagonist NBQX (10 μm). Under these conditions, baclofen application caused a smaller reduction in dendrodendritic inhibition (81 ± 9% of control; n = 10;p < 0.05) (Fig. 7) compared with its action in physiological aCSF (Fig. 2). However, the subsequent application of CGP 55845 markedly enhanced dendrodendritic inhibition (143 ± 12% of control; p < 0.01) in these same cells. The simplest interpretation of these results in Mg2+-free aCSF is that the action of baclofen was partially occluded by the activation of GABAB receptors by endogenously released GABA.
Fig. 7.
Enhancing excitatory drive to granule cells increases the activation of GABAB receptors by endogenous GABA. A, The action of baclofen is partially occluded when dendrodendritic inhibition (DDI) is evoked in Mg2+-free aCSF and NBQX (10 μm).Bottom, Summary of results (n = 10).Top, Representative results from one experiment.B, Baclofen has no effect when dendrodendritic inhibition is enhanced in the presence of cyclothiazide (CTZ, 100 μm). The aCSF was supplemented with APV (25 μm) and MK-801 (40 μm) to block NMDARs. Bottom, Summary of results (n = 8). Top, Representative responses from one experiment. The dashed lines represent unity.
Recent studies suggest that calcium influx through NMDARs can directly trigger GABA exocytosis from granule cell dendrites in Mg2+-free solution (Chen et al., 2000;Halabisky et al., 2000). This raises the possibility that baclofen is less effective in Mg2+-free aCSF because presynaptic NMDARs are an unlikely target for GABAB modulation. To address this issue, we examined the action of baclofen when dendrodendritic inhibition is enhanced in an NMDAR-independent manner. In the absence of NMDA receptors, dendrodendritic inhibition can be evoked by manipulations that slow the kinetics of AMPA receptors or increase granule cell excitability (Schoppa and Westbrook, 1999; Isaacson, 2001). Cyclothiazide (100 μm), a drug that slows AMPAR gating kinetics and greatly prolongs the time course of AMPAR-mediated EPSCs (Partin et al., 1995; Isaacson and Walmsley, 1996), was used to enhance dendrodendritic inhibition (Schoppa and Westbrook, 1999; Isaacson, 2001). Cyclothiazide was added to the aCSF in the presence of the NMDAR antagonists APV (25 μm) and MK-801 (40 μm). Application of cyclothiazide and the NMDAR blockers greatly enhanced dendrodendritic inhibition (503 ± 122% of control; n = 5;p < 0.01; data not shown). Because the AMPARs at granule spine synapses are believed to contain GluR2 subunits (Schoppa and Westbrook, 1999; Sassoe-Pognetto and Ottersen, 2000;Isaacson, 2001), the trigger for exocytosis under these conditions is likely to reflect calcium influx via HVA calcium channels rather than the AMPARs themselves. In the presence of cyclothiazide, baclofen had no effect on dendrodendritic inhibition (98 ± 7% of control;n = 8; p > 0.05) (Fig. 7B); however, the subsequent addition of CGP 55845 caused a marked increase in the response (148 ± 16% of control; n = 8;p < 0.05). Like the experiments in Mg2+-free solution, these results suggest that endogenously released GABA activates GABABreceptors more effectively when dendrodendritic inhibition is enhanced. Together, these findings suggest that although enhancing glutamatergic drive to granule cells augments dendrodendritic self-inhibition, it also increases the activation of GABABautoreceptors.
Discussion
In this study, we find that metabotropic GABAB receptors modulate dendrodendritic transmission between mitral and granule cells of the rat olfactory bulb. The selective GABAB agonist baclofen reduces dendrodendritic inhibition in mitral cells. We show that GABAB receptor activation weakly inhibits glutamate release from mitral cell dendrites and strongly reduces GABA release evoked from granule cells. In granule cells, GABAB receptor activation causes a marked inhibition of HVA calcium channels. Furthermore, we find that GABAB receptor activation by endogenously released GABAB governs the strength of dendrodendritic inhibition.
Dendrodendritic inhibition is generally thought to play an important role in olfactory processing. The reciprocal synaptic circuit between mitral dendrites and granule cell spines provides a mechanism for spatially localized self-inhibition. This local inhibition may be important for regulating temporal features of odor-evoked activity in mitral cells (Laurent, 1999) as well as dendritic spike propagation (Xiong and Chen, 2002). In addition, lateral inhibition between mitral cells can be mediated by dendrodendritic synapses (Isaacson and Strowbridge, 1998; Urban and Sakmann, 2002). Lateral inhibition is thought to improve the signal-to-noise ratio of olfactory bulb mitral cells by sharpening the responses of cells that are tuned to particular odorant qualities (Yokoi et al., 1995). Although dendrodendritic transmission is a major feature in olfaction, surprisingly little is known about the neurotransmitter modulation of dendrodendritic inhibition in the mammalian olfactory bulb.
GABA plays a central role as the transmitter underlying dendrodendritic inhibition: GABA release from granule cell dendritic spines activates GABAA receptors on mitral dendrites. In this study, we show that metabotropic GABAB receptors also play a role at dendrodendritic synapses. Baclofen reduced recurrent inhibition evoked by trains of Na+-dependent action currents in mitral cells voltage-clamped with a K+-based internal solution. The specific antagonist CGP 55845 reversed the action of baclofen, confirming the involvement of GABAB receptors. Although GABAB receptors can generate postsynaptic inhibition via the activation of GIRK channels in neurons (Dutar and Nicoll, 1988; Luscher et al., 1997), baclofen did not alter the membrane properties of mitral cells. This suggests that GABAB receptors do not modulate recurrent inhibition by altering mitral cell excitability.
Baclofen caused a similar reduction in dendrodendritic inhibition evoked in mitral cells recorded in the presence of TTX. This indicates that Na+-dependent action potentials are not required in mitral or granule cells for the GABAB-mediated modulation of mitral cell self-inhibition. Because dendrodendritic inhibition relies on both glutamate release from mitral dendrites and GABA release from granule cell dendritic spines, GABAB receptors could modulate transmission at either or both synaptic sites. We found that GABAB receptor activation causes only a small reduction in glutamatergic transmission in this circuit. Baclofen had a relatively weak inhibitory effect on dendritic fEPSPs in the external plexiform layer. This suggests that GABAB receptors do not have major effects on glutamatergic transmission between mitral and granule cells. Similarly, baclofen had only a small inhibitory effect on NMDAR-mediated mitral cell self-excitation. The weak action of baclofen on mitral cell self-excitation confirms that GABAB receptors do not cause a major reduction in glutamate release during dendrodendritic transmission.
Given the weak role of GABAB receptors in modulating glutamate release, we next focused on the role of GABAB receptors in granule cells. In contrast to the small inhibition of glutamatergic transmission, baclofen dramatically reduced monosynaptic IPSCs evoked in mitral cells by granule cell stimulation. The reduction in evoked IPSC amplitude was accompanied by a change in short-term synaptic plasticity induced by paired-pulse stimulation of granule cells. Under control conditions, paired-pulse stimulation caused a depression of the amplitude of the second evoked IPSC relative to the first; baclofen caused a shift in the paired-pulse ratio from synaptic depression to facilitation. Because baclofen did not affect the amplitude of miniature IPSCs in mitral cells, the simplest interpretation is that GABAB receptors mediate a reduction in GABA release from granule cells.
Baclofen did not alter the membrane properties of granule cells, ruling out the possibility that GABAB receptors couple to GIRK channels in granule cells. However, GABABreceptors also cause G-protein-mediated inhibition of voltage-gated calcium channels in many types of neurons. Calcium channel modulation can be a major site of action for GABAB-mediated presynaptic inhibition (Dittman and Regehr, 1996; Isaacson, 1998). Indeed, we found that baclofen caused a marked reduction in HVA calcium current in granule cells. In contrast, GABABreceptors did not mediate the inhibition of LVA T-type channels in the same cells. Previous findings suggest that HVA calcium channels in granule cell spines play an important role in triggering GABA exocytosis under physiological conditions (Isaacson, 2001). Together, our results are consistent with the fact that GABAB receptors reduce dendrodendritic inhibition primarily via inhibition of the calcium channels governing GABA exocytosis in granule cells.
Given the dramatic action of GABAB receptors on the amplitude of evoked IPSCs, it may at first seem surprising that baclofen reduced dendrodendritic inhibition by only ∼60%. However, several factors are likely to account for the somewhat modest action of baclofen on dendrodendritic inhibition. First, baclofen had less of an inhibitory effect on the second IPSC evoked by paired-pulse stimulation of granule cells. Indeed, baclofen shifted the paired-pulse ratio from depression to facilitation. This finding is consistent with previous results indicating that GABAB-mediated presynaptic inhibition is reduced during the repetitive activation of release sites (Isaacson and Hille, 1997; Brenowitz et al., 1998). A likely possibility is that many of the asynchronous IPSCs underlying dendrodendritic inhibition reflect multiple release events at the same granule cell spines. In this scenario, GABAB modulation of the barrage of events during dendrodendritic inhibition would be expected to be less dramatic than the inhibition of a synchronous, evoked IPSC.
We also find that the GABAB antagonist CGP 55845 causes an enhancement of dendrodendritic inhibition under normal conditions. This suggests that GABA released during dendrodendritic inhibition can partially occlude the action of baclofen by activating GABAB receptors. We think it is unlikely that ambient levels of GABA tonically activate GABABreceptors, because baclofen application exerts strong effects on evoked IPSCs in mitral cells. Rather, we believe that GABA released during dendrodendritic inhibition activates both GABAA receptors on mitral cells and GABAB receptors on granule cells. Although GABAB receptor-mediated responses activate relatively slowly (Sodickson and Bean, 1996), the slow time course of dendrodendritic inhibition (hundreds of milliseconds) provides ample time for GABAB receptors to influence GABA release. These findings are consistent with the idea that GABA regulates its own release during dendrodendritic inhibition by activating GABAB autoreceptors.
GABAB receptors have a high (∼1 μm) affinity for GABA (Sodickson and Bean, 1996), raising the question of why the receptors are not always saturated by GABA released during dendrodendritic inhibition at granule cell spines. However, recent immunolocalization studies suggest that presynaptic and postsynaptic GABAB receptors are typically located at perisynaptic regions (Fritschy et al., 1999; Lopez-Bendito et al., 2002). Indeed, in other brain regions, activation of GABAB autoreceptors at inhibitory synapses or mGluR autoreceptors at excitatory synapses requires trains of stimuli or the activation of many release sites (Lambert and Wilson, 1994;Misgeld et al., 1995; Scanziani et al., 1997). This activity dependence for autoreceptor activation is thought to reflect the fact that transmitter clearance from synaptic sites is extremely efficient and that high local concentrations of transmitter are required to reach perisynaptic receptors.
We believe that GABAB autoreceptors on granule cell dendrites are also likely to regulate GABA release in an activity-dependent manner. Consistent with this idea, we found that enhancing dendrodendritic inhibition by facilitating NMDARs or AMPARs almost completely occluded the action of baclofen. In the same cells, application of GABAB antagonist greatly augmented the response. These results were observed under conditions in which granule cell GABA release was driven exclusively by either NMDARs or AMPARs, suggesting that it was the increase in GABA release rather than the specific type of glutamate receptor governing release that was the critical factor. One simple explanation for these findings is that enhancing the excitatory drive to granule cell spines increases GABA release. In addition to potentiating GABAA input to mitral cells, the increase in GABA release also leads to near maximal activation of presynaptic granule cell GABAB receptors.
Although our results point to granule cell voltage-gated calcium channels as the major site of action for GABABreceptor modulation of dendrodendritic inhibition, we cannot rule out a contribution from other sites. The weak inhibition of mitral cell glutamate release may contribute to the baclofen-induced reduction in dendrodendritic inhibition. GABAB-mediated modulation of mitral dendrite calcium channels may also account for the small reduction in glutamatergic transmission we observed.
What role might dendrodendritic GABAB receptors play in the processing of olfactory information? One possibility is that GABAB autoreceptors serve to limit mitral cell self-inhibition during strong odor stimulation. This “disinhibition” of strongly activated mitral cells would promote lateral inhibition onto other, weakly activated mitral cells. In this scenario, GABAB autoreceptors on granule cells may facilitate the role of mitral cell lateral inhibition to increase the signal-to-noise ratio of olfactory principal cells (Yokoi et al., 1995; Mori et al., 1999).
Footnotes
This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC04682. J.S.I. was supported by a Burroughs Wellcome Fund Career Award and a McKnight Scholar Award. We thank Gabe Murphy for comments on this manuscript.
Correspondence should be addressed to Dr. Jeffry S. Isaacson, Department Neuroscience, MC 0608, Basic Science Building, Room 3065, University of California San Diego, School of Medicine, 9500 Gilman Drive, La Jolla, CA 92093-0608. E-mail: jisaacson@ucsd.edu.
References
- 1.Aroniadou-Anderjaska V, Ennis M, Shipley MT. Current-source density analysis in the rat olfactory bulb: laminar distribution of kainate/AMPA- and NMDA-receptor-mediated currents. J Neurophysiol. 1999;81:15–28. doi: 10.1152/jn.1999.81.1.15. [DOI] [PubMed] [Google Scholar]
- 2.Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. doi: 10.1152/jn.2000.84.3.1194. [DOI] [PubMed] [Google Scholar]
- 3.Avery RB, Johnston D. Multiple channel types contribute to the low-voltage-activated calcium current in hippocampal CA3 pyramidal neurons. J Neurosci. 1996;16:5567–5582. doi: 10.1523/JNEUROSCI.16-18-05567.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 5.Brenowitz S, David J, Trussell L. Enhancement of synaptic efficacy by presynaptic GABA(B) receptors. Neuron. 1998;20:135–141. doi: 10.1016/s0896-6273(00)80441-9. [DOI] [PubMed] [Google Scholar]
- 6.Chen WR, Xiong W, Shepherd GM. Analysis of relations between NMDA receptors and GABA release at olfactory bulb reciprocal synapses. Neuron. 2000;25:625–633. doi: 10.1016/s0896-6273(00)81065-x. [DOI] [PubMed] [Google Scholar]
- 7.Dittman JS, Regehr WG. Contributions of calcium-dependent and calcium-independent mechanisms to presynaptic inhibition at a cerebellar synapse. J Neurosci. 1996;16:1623–1633. doi: 10.1523/JNEUROSCI.16-05-01623.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dutar P, Nicoll RA. A physiological role for GABAB receptors in the central nervous system. Nature. 1988;332:156–158. doi: 10.1038/332156a0. [DOI] [PubMed] [Google Scholar]
- 9.Elaagouby A, Ravel N, Gervais R. Cholinergic modulation of excitability in the rat olfactory bulb: effect of local application of cholinergic agents on evoked field potentials. Neuroscience. 1991;45:653–662. doi: 10.1016/0306-4522(91)90278-v. [DOI] [PubMed] [Google Scholar]
- 10.Ennis M, Zhou FM, Ciombor KJ, Aroniadou-Anderjaska V, Hayar A, Borrelli E, Zimmer LA, Margolis F, Shipley MT. Dopamine D2 receptor-mediated presynaptic inhibition of olfactory nerve terminals. J Neurophysiol. 2001;86:2986–2997. doi: 10.1152/jn.2001.86.6.2986. [DOI] [PubMed] [Google Scholar]
- 11.Friedman D, Strowbridge BW. Functional role of NMDA autoreceptors in olfactory mitral cells. J Neurophysiol. 2000;84:39–50. doi: 10.1152/jn.2000.84.1.39. [DOI] [PubMed] [Google Scholar]
- 12.Fritschy JM, Meskenaite V, Weinmann O, Honer M, Benke D, Mohler H. GABAB-receptor splice variants GB1a and GB1b in rat brain: developmental regulation, cellular distribution and extrasynaptic localization. Eur J Neurosci. 1999;11:761–768. doi: 10.1046/j.1460-9568.1999.00481.x. [DOI] [PubMed] [Google Scholar]
- 13.Halabisky B, Friedman D, Radojicic M, Strowbridge BW. Calcium influx through NMDA receptors directly evokes GABA release in olfactory bulb granule cells. J Neurosci. 2000;20:5124–5134. doi: 10.1523/JNEUROSCI.20-13-05124.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hayashi Y, Momiyama A, Takahashi T, Ohishi H, Ogawa-Meguro R, Shigemoto R, Mizuno N, Nakanishi S. Role of a metabotropic glutamate receptor in synaptic modulation in the accessory olfactory bulb. Nature. 1993;366:687–690. doi: 10.1038/366687a0. [DOI] [PubMed] [Google Scholar]
- 15.Hille B. Ion channels of excitable membranes, Ed 3. Sinauer; Sunderland, MA: 2001. [Google Scholar]
- 16.Hsia AY, Vincent JD, Lledo PM. Dopamine depresses synaptic inputs into the olfactory bulb. J Neurophysiol. 1999;82:1082–1085. doi: 10.1152/jn.1999.82.2.1082. [DOI] [PubMed] [Google Scholar]
- 17.Isaacson JS. GABAB receptor-mediated modulation of presynaptic currents and excitatory transmission at a fast central synapse. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- 18.Isaacson JS. Glutamate spillover mediates excitatory transmission in the rat olfactory bulb. Neuron. 1999;23:377–384. doi: 10.1016/s0896-6273(00)80787-4. [DOI] [PubMed] [Google Scholar]
- 19.Isaacson JS. Mechanisms governing dendritic γ-aminobutyric acid (GABA) release in the rat olfactory bulb. Proc Natl Acad Sci USA. 2001;98:337–342. doi: 10.1073/pnas.021445798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Isaacson JS, Hille B. GABA(B)-mediated presynaptic inhibition of excitatory transmission and synaptic vesicle dynamics in cultured hippocampal neurons. Neuron. 1997;18:143–152. doi: 10.1016/s0896-6273(01)80053-2. [DOI] [PubMed] [Google Scholar]
- 21.Isaacson JS, Strowbridge BW. Olfactory reciprocal synapses: dendritic signaling in the CNS. Neuron. 1998;20:749–761. doi: 10.1016/s0896-6273(00)81013-2. [DOI] [PubMed] [Google Scholar]
- 22.Isaacson JS, Walmsley B. Amplitude and time course of spontaneous and evoked excitatory postsynaptic currents in bushy cells of the anteroventral cochlear nucleus. J Neurophysiol. 1996;76:1566–1571. doi: 10.1152/jn.1996.76.3.1566. [DOI] [PubMed] [Google Scholar]
- 23.Jahr CE, Nicoll RA. Dendrodendritic inhibition: demonstration with intracellular recording. Science. 1980;207:1473–1475. doi: 10.1126/science.7361098. [DOI] [PubMed] [Google Scholar]
- 24.Jahr CE, Nicoll RA. Noradrenergic modulation of dendrodendritic inhibition in the olfactory bulb. Nature. 1982;297:227–229. doi: 10.1038/297227a0. [DOI] [PubMed] [Google Scholar]
- 25.Keller A, Yagodin S, Aroniadou-Anderjaska V, Zimmer LA, Ennis M, Sheppard NF, Jr, Shipley MT. Functional organization of rat olfactory bulb glomeruli revealed by optical imaging. J Neurosci. 1998;18:2602–2612. doi: 10.1523/JNEUROSCI.18-07-02602.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lambert NA, Wilson WA. Temporally distinct mechanisms of use-dependent depression at inhibitory synapses in the rat hippocampus in vitro. J Neurophysiol. 1994;72:121–130. doi: 10.1152/jn.1994.72.1.121. [DOI] [PubMed] [Google Scholar]
- 27.Laurent G. A systems perspective on early olfactory coding. Science. 1999;286:723–728. doi: 10.1126/science.286.5440.723. [DOI] [PubMed] [Google Scholar]
- 28.Lopez-Bendito G, Shigemoto R, Kulik A, Paulsen O, Fairen A, Lujan R. Expression and distribution of metabotropic GABA receptor subtypes GABABR1 and GABABR2 during rat neocortical development. Eur J Neurosci. 2002;15:1766–1778. doi: 10.1046/j.1460-9568.2002.02032.x. [DOI] [PubMed] [Google Scholar]
- 29.Luscher C, Jan LY, Stoffel M, Malenka RC, Nicoll RA. G protein-coupled inwardly rectifying K+channels (GIRKs) mediate postsynaptic but not presynaptic transmitter actions in hippocampal neurons. Neuron. 1997;19:687–695. doi: 10.1016/s0896-6273(00)80381-5. [DOI] [PubMed] [Google Scholar]
- 30.Miller RJ. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 31.Misgeld U, Bijak M, Jarolimek W. A physiological role for GABAB receptors and the effects of baclofen in the mammalian central nervous system. Prog Neurobiol. 1995;46:423–462. doi: 10.1016/0301-0082(95)00012-k. [DOI] [PubMed] [Google Scholar]
- 32.Mori K, Takagi SF. An intracellular study of dendrodendritic inhibitory synapses on mitral cells in the rabbit olfactory bulb. J Physiol (Lond) 1978;279:569–588. doi: 10.1113/jphysiol.1978.sp012362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori K, Nagao H, Yoshihara Y. The olfactory bulb: coding and processing of odor molecule information. Science. 1999;286:711–715. doi: 10.1126/science.286.5440.711. [DOI] [PubMed] [Google Scholar]
- 34.Nicoll RA, Jahr CE. Self-excitation of olfactory bulb neurones. Nature. 1982;296:441–444. doi: 10.1038/296441a0. [DOI] [PubMed] [Google Scholar]
- 35.Nowycky MC, Mori K, Shepherd GM. GABAergic mechanisms of dendrodendritic synapses in isolated turtle olfactory bulb. J Neurophysiol. 1981;46:639–648. doi: 10.1152/jn.1981.46.3.639. [DOI] [PubMed] [Google Scholar]
- 36.Okutani F, Kaba H, Takahashi S, Seto K. The biphasic effects of locus coeruleus noradrenergic activation on dendrodendritic inhibition in the rat olfactory bulb. Brain Res. 1998;783:272–279. doi: 10.1016/s0006-8993(97)01371-1. [DOI] [PubMed] [Google Scholar]
- 37.Palouzier-Paulignan B, Duchamp-Viret P, Hardy AB, Duchamp A. GABA(B) receptor-mediated inhibition of mitral/tufted cell activity in the rat olfactory bulb: a whole-cell patch-clamp study in vitro. Neuroscience. 2002;111:241–250. doi: 10.1016/s0306-4522(02)00003-9. [DOI] [PubMed] [Google Scholar]
- 38.Partin KM, Bowie D, Mayer ML. Structural determinants of allosteric regulation in alternatively spliced AMPA receptors. Neuron. 1995;14:833–843. doi: 10.1016/0896-6273(95)90227-9. [DOI] [PubMed] [Google Scholar]
- 39.Salin PA, Lledo PM, Vincent JD, Charpak S. Dendritic glutamate autoreceptors modulate signal processing in rat mitral cells. J Neurophysiol. 2001;85:1275–1282. doi: 10.1152/jn.2001.85.3.1275. [DOI] [PubMed] [Google Scholar]
- 40.Sassoe-Pognetto M, Ottersen OP. Organization of ionotropic glutamate receptors at dendrodendritic synapses in the rat olfactory bulb. J Neurosci. 2000;20:2192–2201. doi: 10.1523/JNEUROSCI.20-06-02192.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Scanziani M, Capogna M, Gahwiler BH, Thompson SM. Presynaptic inhibition of miniature excitatory synaptic currents by baclofen and adenosine in the hippocampus. Neuron. 1992;9:919–927. doi: 10.1016/0896-6273(92)90244-8. [DOI] [PubMed] [Google Scholar]
- 42.Scanziani M, Salin PA, Vogt KE, Malenka RC, Nicoll RA. Use-dependent increases in glutamate concentration activate presynaptic metabotropic glutamate receptors. Nature. 1997;385:630–634. doi: 10.1038/385630a0. [DOI] [PubMed] [Google Scholar]
- 43.Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nat Neurosci. 1999;2:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- 44.Schoppa NE, Kinzie JM, Sahara Y, Segerson TP, Westbrook GL. Dendrodendritic inhibition in the olfactory bulb is driven by NMDA receptors. J Neurosci. 1998;18:6790–6802. doi: 10.1523/JNEUROSCI.18-17-06790.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shepherd GM, Greer CA. The olfactory bulb. In: Shepherd GM, editor. The synaptic organization of the brain. Oxford UP; New York: 1998. pp. 159–203. [Google Scholar]
- 46.Sodickson DL, Bean BP. GABAB receptor-activated inwardly rectifying potassium current in dissociated hippocampal CA3 neurons. J Neurosci. 1996;16:6374–6385. doi: 10.1523/JNEUROSCI.16-20-06374.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Urban NN, Sakmann B. Reciprocal intraglomerular excitation and intra- and interglomerular lateral inhibition between mouse olfactory bulb mitral cells. J Physiol (Lond) 2002;542:355–367. doi: 10.1113/jphysiol.2001.013491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wilson DA, Leon M. Noradrenergic modulation of olfactory bulb excitability in the postnatal rat. Brain Res. 1988;470:69–75. doi: 10.1016/0165-3806(88)90202-7. [DOI] [PubMed] [Google Scholar]
- 49.Xiong W, Chen WR. Dynamic gating of spike propagation in the mitral cell lateral dendrites. Neuron. 2002;34:115–126. doi: 10.1016/s0896-6273(02)00628-1. [DOI] [PubMed] [Google Scholar]
- 50.Yokoi M, Mori K, Nakanishi S. Refinement of odor molecule tuning by dendrodendritic synaptic inhibition in the olfactory bulb. Proc Natl Acad Sci USA. 1995;92:3371–3375. doi: 10.1073/pnas.92.8.3371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zucker RS, Regehr WG. Short-term synaptic plasticity. Annu Rev Physiol. 2002;64:355–405. doi: 10.1146/annurev.physiol.64.092501.114547. [DOI] [PubMed] [Google Scholar]