Abstract
Elevation of extracellular Ca2+(↑[Ca2+]e) stimulates the Ca2+ receptor (CaR) to induce secretion of 5-hydroxytryptamine (5-HT) from the calcium-sensing parafollicular (PF) cells. The CaR has been reported to couple to Gαq with subsequent activation of protein kinase C-γ (PKCγ). We have identified a parallel transduction pathway in primary cultures of sheep PF cells by using a combinatorial approach in which we expressed adenoviral-encoded dominant-negative signaling proteins and performed in vitro kinase assays. The role of the CaR was established by expression of a dominant-negative CaR that eliminated calcium-induced 5-HT secretion but not secretion in response to KCl or phorbol esters. The calcium-induced secretion was inhibited by a dominant-negative p85 regulatory subunit of phosphatidylinositol 3-kinase (PI3-K). PI3-K activity was also assayed using isoform-specific antibodies. The activity of p85/p110β (PI3-Kβ) immunocomplexes was elevated by ↑[Ca2+]e and activated by Gβγ subunits. In addition, secretion of 5-HT was antagonized by the expression of a minigene encoding a peptide scavenger of Gβγ subunits (C-terminal fragment peptide of bovine β-adrenergic receptor kinase). One target of PI3-K activity is phosphoinositide-dependent kinase-1 (PDK1), which in turn activated PKCζ. Expression of a dominant-negative PKCζ in PF cells reduced 5-HT secretion. Together, these observations establish that ↑[Ca2+]e evokes 5-HT secretion from PF cells by stimulating both Gαq- and Gβγ-signaling pathways downstream of the CaR. The βγ cascade subsequently activates PI3-Kβ-dependent signaling that is coupled to PDK1 and the downstream effector PKCζ, and results in an increase in 5-HT release.
Keywords: serotonin, parafollicular cells, gene transfer, atypical PKC, Gβγ subunits, secretion, calcitonin
Introduction
Parafollicular (PF) cells are neural crest-derived cells that neuralize when grown in a permissive environment (Barasch et al., 1987a; Clark et al., 1995). Although PF cells are endocrine, they share many properties of neurons and have thus been called paraneurons (Fujita, 1987; Lanigan et al., 1998). Like the adrenal chromaffin cell, the PF cell is useful as a neuronal model (Russo et al., 1996). PF cells costore 5-HT and calcitonin in a single population of secretory vesicles (Zabel, 1984; Barasch et al., 1987b). Both 5-HT and calcitonin are secreted in response to increased extracellular Ca2+(↑[Ca2+]e) (Nunez and Gershon, 1978). PF cells respond to ↑[Ca2+]e because they express a Gi- and Gq-coupled [Ca2+]e receptor (CaR) (Herbert and Brown, 1995; Ruat et al., 1996; Tamir et al., 1996;Brown and MacLeod, 2001). Events that follow CaR stimulation include depolarization (McGehee et al., 1997), acidification of the interiors of secretory vesicles (Tamir et al., 1994b), and secretion (Tamir et al., 1990, 1994b; McGehee et al., 1997; Liu et al., 2000). Signal transduction after CaR stimulation is complex because the receptor is coupled to multiple signaling cascades that in turn activate several effectors. The cascade that leads to the acidification of the interiors of secretory vesicles can hereby be distinguished experimentally from that which leads to secretion (Tamir et al., 1996).
CaR-induced 5-HT secretion by PF cells is resistant to pertussis toxin and is initiated by Gq (Liu et al., 2000). At least two isoforms of PKC are involved in mediating ↑[Ca2+]e-evoked 5-HT secretion. PKCγ-evoked 5-HT secretion is stimulated by exogenous phorbol esters and by endogenous diacylglycerol, which is liberated by phosphatidylinositol-specific phospholipase C. Downregulation of PKCγ abolishes phorbol ester-stimulated 5-HT secretion but only slightly inhibits secretion initiated by ↑[Ca2+]e (Tamir et al., 1990; McGehee et al., 1997; Liu et al., 2000). Our previous observations have suggested that activation of PKCζ, which is neither stimulated nor downregulated by phorbol esters, may account for the ability of the CaR to induce secretion even after downregulation of PKCγ. This PKCζ activation after exposure of PF cells to ↑[Ca2+]e is antagonized by inhibitors of phosphatidylinositol 3-kinase (PI3-K) (Liu et al., 2000).
The PI3-K holoenzyme is formed by association of one regulatory subunit (p50α, p55α/γ, p85α/β, or p101) with one of the following catalytic subunits: p110α, p110β, p110γ, p110δ, or p110θ (Fruman et al., 1998). The p85/p110β isoform (PI3-Kβ) and p101/p110γ isoform (PI3-Kγ) are thought to be activated primarily by G-protein-coupled receptors, whereas PI3-Kα and PI3-Kδ are coupled to signaling receptor tyrosine kinases (Clapham and Neer, 1997; Kurosu et al., 1997; Stephens et al., 1997; Vanhaesebroeck et al., 1997; Maier et al., 1999; Takasuga et al., 1999; Murga et al., 2000; Bony et al., 2001). Stimulation of PI3-K in cells generates phosphatidylinositol 3,4-bisphosphate [PtdIns(3,4)P2] and phosphatidylinositol 3,4,5-trisphosphate [PtdIns(3,4,5)P3]. These lipids bind and activate phosphoinositide-dependent kinase-1 (PDK1), which in turn phosphorylates the serine and threonine kinases PKCζ and Akt, also called protein kinase B (PKB), on conserved threonine residues within the catalytic loop. Phosphorylation of these residues is required for optimal kinase activation and necessary for inducing the kinase activities of these downstream kinases. In addition, PtdIns(3,4)P2 and PtdIns(3,4,5)P3 bind directly to the pleckstrin homology domains of Akt and induce its translocation to the plasma membrane (Franke et al., 1997). Many cellular responses are mediated by Akt and have been a focus of research (Vanhaesebroeck et al., 1997; Kandel and Hay, 1999; Scheid and Woodgett, 2001). Less is known about the function of the PI3-K target PKCζ in cells and in vivo. To date, PDK1 is the only known kinase that phosphorylates and activates PKCζ (Balendran et al., 2000). However, previous biochemical evidence has suggested that PKCζ and Akt coexist in signaling complexes in cells downstream of PI3-K and PDK1. As a consequence of this direct protein interaction, it has been suggested that regulation of PKCζ can affect Akt activity and vice versa, possibly by altering the efficiency of the interaction of PKCζ with PDK1 (Hodgkinson and Sale, 2002).
The current study was undertaken to confirm that ↑[Ca2+]e-evoked 5-HT secretion is attributable to CaR stimulation and to identify the molecular targets that mediate stimulation–secretion coupling. Hypotheses were tested by investigating the ↑[Ca2+]e-induced secretion of 5-HT by PF cells that express dominant-negative (DN) forms of the CaR and downstream-signaling molecules. Observations indicate that Ca2+ evokes 5-HT secretion by stimulating the CaR that is coupled via Gq to a set of PI3-K-dependent effectors. Downstream of PI-3K, PKCζ and Akt regulate different biological responses in which PKCζ induces 5-HT release, whereas Akt might be involved in other cell functions.
Materials and Methods
Isolation of PF cells. Fresh sheep thyroids were obtained from a slaughterhouse. PF cells were isolated by the phagocytic chromatography technique, as described previously (Bernd et al., 1981; Barasch et al., 1987b, 1988; Cidon et al., 1991). Essentially, thyroids were dissociated with trypsin, and the dissociated cells were incubated with thyrotropin (TSH) to stimulate the follicular cells to become phagocytic. The suspension of TSH-stimulated thyroid cells was passed through a Sepharose–thyroglobulin column, and follicular cells bind to the column, whereas the PF cells pass through it. Red blood cells were removed from the filtrate by centrifugation through a layer of Ficoll. Approximately 97% of cells in the final preparation were PF; the remaining cells were primarily fibroblasts, and there were no detectable follicular cells. The purified PF cells were cultured overnight to recover from the trauma of their isolation. Cultures were maintained at 37°C in Eagle's minimum essential medium, supplemented with 10% fetal bovine serum, and buffered by CO2.
Analysis of 5-HT secretion from PF cells.Ca2+ (0.5–10 mm) was added to isolated PF cells (1 × 106 cells per milliliter) to induce secretion (10 min; 37°C). Control cells were treated with 1 mm EGTA instead of ↑[Ca2+]e. When the effect of inhibitors was to be studied, cells were preincubated with the test compound for 30 min before challenging them with ↑[Ca2+]e. Stimulation was terminated by quickly cooling (on ice) and centrifuging the cells (800 × g). 5-HT and its metabolite, 5-hydroxyindole acetic acid, were extracted from both the pellets of the cells and the supernatant and measured by reverse-phase HPLC with electrochemical detection (Tamir et al., 1994a). The amounts of secreted 5-HT (defined as that present in the supernatant) were normalized to the cellular 5-HT content (pellet). To estimate the ↑[Ca2+]e-induced percentage of 5-HT secretion, the normalized 5-HT content of the supernatant of control cells was subtracted from that in the supernatant obtained from cells subjected to stimulation.
Gel electrophoresis and immunoblotting. Purified PF cells (107) were harvested, washed with PBS, and resuspended in Ca2+-free Earl's buffered salt solution containing 1 mmMgCl2. Cells were stimulated as described above. After stimulation, cells were washed in ice-cold PBS and lysed for 1 hr at 4°C in 300 μl of lysis buffer containing 1% Triton X-100, 20 mm Tris, pH 7.4, 150 mmNaCl, 1 mm sodium vanadate, 100 nm okadaic acid, 100 nmcalyculin A (Cell Signaling, Beverly, MA), and a mixture of protease inhibitors (1:500 dilution) (Sigma, St. Louis, MO). Lysates were microfuged for 10 min at 13,000 rpm to remove insoluble material, and an aliquot was removed from each sample to determine protein concentrations (detergent compatible protein assay; Bio-Rad, Hercules, CA). Samples were then adjusted so that each contained an equal amount of protein. Proteins present in the supernatant (100 μg) were separated by 8.5% SDS-PAGE under denaturation conditions and electroblotted onto nitrocellulose membranes. Proteins were visualized by staining for 2 min in Ponceau S solution (0.02% in 0.3% trichloroacetic acid) (Tamir et al., 1990). To detect PI3-K subunits, the membranes were washed and the subunits were detected with specific antibodies (1 μg/ml, overnight at 4°C) (Santa Cruz Biotechnology, Santa Cruz, CA) to the following PI3-K subunits: p85α, p110α, p110β, and p110γ. Reactive bands were visualized with a 1:1000 dilution of horseradish peroxidase-labeled goat anti-rabbit secondary antibodies (Santa Cruz Biotechnology). Phosphorylated products of PDK1 were detected by using polyclonal antibodies raised against a synthetic peptide resembling the phospho-(threonine) PDK1 substrate motif that is commonly found in PDK1 substrates (Cell Signaling). Bound antibodies were visualized on blots by enhanced chemiluminescence (Amersham Biosciences, Arlington Heights, IL).
Assay of PI3-K activity. PF cells were washed with PBS, exposed to ↑[Ca2+]e or EGTA (control, 1 mm), and lysed in buffer supplemented with 1% Triton X-100. PI3-K activity was measured as described previously (Liu et al., 2000), by using minor modifications of methods published previously (Ettinger et al., 1996; Herrera-Velit and Reiner, 1996). Briefly, aliquots of cell lysates (750 μg of protein) were immunoprecipitated with polyclonal antibodies to the p110β subunit of PI3-K (5 μg per sample; Santa Cruz Biotechnology) and incubated at 4°C for 60 min. Protein A/G-agarose (30 μl) was added, and the preparations were allowed to incubate with shaking for an additional 60 min at 4°C. The agarose–antigen–antibody complexes were collected by centrifugation, washed with Tris lysis buffer (see above), and resuspended in 35 μl of kinase assay buffer (30 mm HEPES, 30 mmMgCl2, and 200 μmadenosine) containing 20 μg of freshly sonicated soybean phosphatidylinositol. PI3-K assays were performed by adding 50 μm ATP and 10 μCi of [γ-32P]-ATP in 5 μl of kinase buffer and incubating for 10 min at room temperature. The reaction was stopped by adding 100 μl of 1N HCl. Lipids were extracted into 200 μl of chloroform:methanol (1:1), spotted onto silica gel thin-layer chromatography plates, and developed in a mobile phase consisting of chloroform, methanol, water, and NH4OH (18:14:3:1 v/v). Spots corresponding to phosphatidylinositol 3-phosphate (PI3-P) were detected after autoradiography and identified on the basis of their Rf values. Kinase activities were quantified by counting the spots corresponding to PI3-P in a liquid scintillation counter.
Infection of PF cells with adenoviral vectors containing dominant-negative and constitutively active constructs. Plasmids encoding dominant-negative forms of the CaR, the PI3-K regulatory subunit p85 and PKCζ, as well as a plasmid encoding a scavenger peptide of Gβγ subunits were packaged separately into adenoviral vectors. The plasmid encoding the dominant-negative form of the CaR (epitope tagged with Flag) (185Q) was obtained by mutation of arginine into glutamine at position 185 in the extracellular domain, resulting in a mutant protein with sevenfold decreased affinity for [Ca2+]e (Bai et al., 1996). This construct was provided by Dr. Mei Bai (The Brigham and Women's Hospital, Boston, MA). The plasmid encoding the dominant-negative form of the p85 regulatory subunit of PI3-K was a deletion of the inter-Src homology 2 domain of p85 that will prevent the mutant Δp85 protein from binding to the p110 catalytic subunit of PI3-K. As a result, p110 cannot be activated (Crowder and Freeman, 1998). The construct encoding the mutated p85 was obtained from Dr. Robert S. Freeman (Rochester University, Rochester, NY). The construct encoding the dominant-negative form of PKCζ (epitope tagged with influenza hemagglutinin) was obtained after mutating lysine to arginine in the ATP binding site of PKCζ (Soh et al., 1999). This construct was obtained from Dr. Bernard Weinstein (Columbia University, New York, NY). The minigene encoding the C-terminal fragment peptide of bovine β-adrenergic receptor kinase (βARK)-1 (βARKct), packaged in an adenoviral vector, was provided by Dr. Robert J. Lefkowitz (Duke University, Durham, NC). This peptide contains the Gβγ binding motif QXXER that is found in the βARK and several other effector proteins downstream of Gβγ subunits (Koch et al., 1994; Chen et al., 1995). Plasmids encoding dominant-negative mutants of CaR, p85, and PKCζ were cloned into a shuttle plasmid in preparation for packaging in replication-deficient adenoviral vectors using standard methods at the University of Iowa Gene Transfer Vector Core Facility (Iowa City, IA). Briefly, the coding sequences of the various mutants were cloned by blunt-end ligation into pAd5CMVK-NpA. The resultant plasmid and adenovirus backbone were transfected into human embryonic kidney 293 (HEK293) cells, and plaques were isolated and amplified for expression analysis of the mutant protein. A replication-deficient adenovirus encoding the various constructs was generated. Recombinant adenoviral vectors were triple plaque purified before use.
PF cells were infected with adenoviral vectors at 20–50 pfu per cell for 17 hr. The control vector was used at a multiplicity of infection (MOI) that was identical to that of the test vector. The percentage of cells infected by the viral vectors was measured for each experiment. Infected cells were identified by immunocytochemistry to demonstrate Flag or hemagglutinin markers encoded by the vectors. The total cell population was determined by counting with interference contrast optics, and the percentage of labeled (infected) cells was calculated. Experiments on the mechanism of secretion were considered valid if the adenovirus had infected ≥80% of the total population. In control experiments, cells were infected with an adenoviral vector that lacked an experimental construct. The viability of the cells after infection with an adenovirus was measured routinely and assessed by using a Trypan Blue exclusion assay. In all experiments, viability was >80%.
Determination of CaR cell-surface expression. Isolated PF cells (107 cells; 500 μg) were infected for 17 hr with an adenovirus expressing the dominant-negative form of PI3-K (MOI of 20), with an adenovirus expressing the dominant-negative form of CaR (MOI of 50), or with an adenoviral vector (as a control; MOI of 50) that lacked an experimental construct. Cells were washed twice with PBS, resuspended in 250 μl of PBS, and incubated overnight at 4°C with monoclonal antibodies (10 μg) directed against the extracellular domain of the CaR (a gift from Dr. E. Brown, The Brigham and Women's Hospital). Antibody-treated cells were washed twice with 1 ml of ice-cold PBS to remove unbound antibodies and resuspended in 250 μl of PBS containing 0.1 μCi of125I-labeled antibodies to mouse Ig (750–3000 Ci/mmol; Amersham Biosciences). Cells were incubated with the secondary antibodies for 5 hr at 4°C. The immunolabeled cells were then centrifuged at 3000 × gfor 5 min, washed three times with ice-cold PBS, and recentrifuged; the radioactivity of the pellets was subsequently measured using a scintillation counter.
Statistics. All experiments were repeated independently at least three times. Quantification of ECL immunoblots and autoradiographic data was performed using NIH Image software (version 1.62). All results are presented as mean ± SEM and, where applicable, p values were determined using an unpaired Student's t test.
Drugs, chemicals, and antibodies. Drugs and chemicals were obtained from Sigma unless otherwise specified. 2-(4-morpholinyl)-8-phenyl-4H-1-benzopyran-4-one (LY294002), a specific inhibitor of PI3-K, was purchased from Biomol Research Laboratories (Plymouth Meeting, PA). The wortmannin was purchased from Alexis Biochemicals (San Diego, CA). Protein A/G-agarose CL-4B was obtained from Amersham Biosciences. G-protein βγ-subunits, isolated from bovine brain, were purchased from Calbiochem (San Diego, CA). The QEHA27 peptide, a scavenger for G-protein βγ-subunits, was a generous gift from Dr. Ravi Iyangar (Mount Sinai Medical School, New York, NY). Antibodies to the p85α (z-8), 110α, 110β, and 110γ subunits of PI3-K were purchased from Santa Cruz Biotechnology. Antibodies to phosphorylated products of PDK1, phospho-PKCζ/λ (pT410/403), Akt, phospho-Akt (pS473), and phospho-Akt (pT308) were purchased from Cell Signaling. Earl's balanced salt solution was purchased from Invitrogen (Grand Island, NY). Goat anti-rabbit IgG coupled to rabbit horseradish peroxidase was purchased from Santa Cruz Biotechnology. Silica gel G 60 was purchased from Electron Microscopy Sciences (Gibbstown, NJ). Solvents used with all compounds were routinely tested for their possible effects on secretion, but none were found in the concentration ranges that we used.
Results
Expression of DN CaR inhibits ↑[Ca2+]e-elicited 5-HT secretion
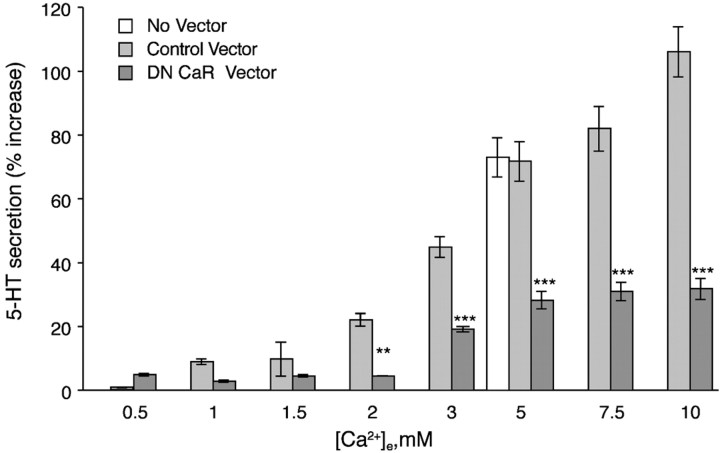
PF cells secrete 5-HT in response to ↑[Ca2+]e (Fig.1). After infection with a control adenovirus (empty vector), ↑[Ca2+]e evoked a concentration-dependent secretion of 5-HT. The secretion of 5-HT by PF cells infected with control virus was not significantly different from that of uninfected cells. These data indicate that PF cells can be infected with an adenoviral vector without affecting their ability to secrete 5-HT in response to ↑[Ca2+]e. In contrast to infection with the control vector, infection of PF cells with an adenovirus encoding a dominant-negative CaR significantly inhibited ↑[Ca2+]e-induced 5-HT secretion by up to 80% (Fig. 1). This observation is consistent with the idea that the CaR physiological function is to respond to elevated ↑[Ca2+]e (>1.5 mm). To test the specificity of the dominant-negative mutant of the CaR, we also evaluated the secretion of 5-HT evoked by depolarization with high K+ (50 mm) or exposure to phorbol 12-myristate 13-acetate (PMA; 0.1 μm). Infection of PF cells with the adenovirus encoding dominant-negative CaR affected neither the secretion of 5-HT evoked by high K+ nor that induced by PMA. ↑[Ca2+]e-induced 5-HT secretion was 3.9 ± 0.5, K+-induced secretion was 4.4 ± 0.3, and PMA-induced secretion was 3.8 ± 0.4 pmol/106 cells/min. Our results indicate that secretion of 5-HT is not affected by expression of the dominant-negative form of the CaR when it is induced by mechanisms that do not involve the CaR. In addition, we did not observe any cytotoxicity after infection. The effects of expressing the dominant-negative form of the CaR within PF cells are thus specific for inhibiting the cellular response to ↑[Ca2+]e.
Fig. 1.
Expression of dominant-negative CaR blocks ↑[Ca2+]e-induced secretion of 5-HT. PF cells were infected with an adenovirus containing a DN CaR. The ↑[Ca2+]e-induced 5-HT secretion is significantly antagonized in cells expressing the DN mutant at all concentrations of [Ca2+]e ≥2 mm. Infection of cells with a control adenoviral vector (lacking the DN construct) did not affect the secretion induced by 5 mm [Ca2+]e. The data show mean ± SE derived from at least four independent preparations of PF cells. **p < 0.005; ***p < 0.001 (vs cells infected with the control adenovirus at the same concentration of [Ca2+]e).
Our previous studies (Tamir et al., 1996), as well as those of others (Herbert and Brown, 1995; Ruat et al., 1996; Brown and Macleod, 2001), demonstrated that the CaR is coupled to Gi and Gq. Because pertussis toxin, which inactivates Gi, had no effect on ↑[Ca2+]e-induced secretion (Liu et al., 2000), it is likely that CaR-mediated secretion involves the coupling of the CaR to downstream effectors that are activated downstream of Gqα and/or Gqβγ.
p85/p110β participates in signal transduction from the CaR
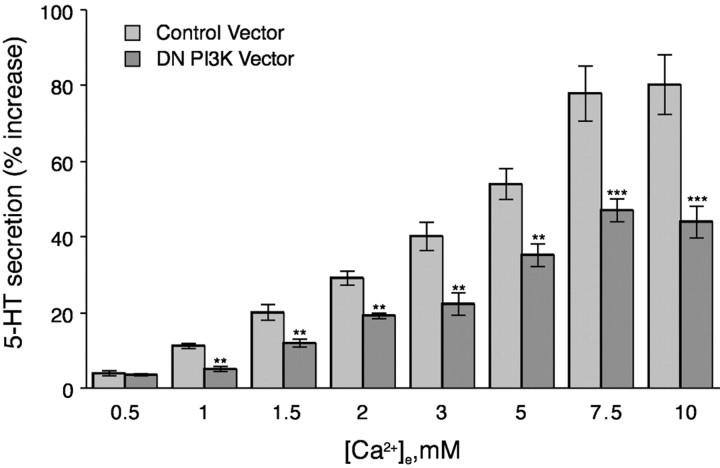
Our previous studies showed that the relatively selective PI3-K inhibitors wortmannin and LY294002 both antagonize ↑[Ca2+]e-mediated 5-HT secretion and suggested that stimulation of the CaR evokes secretion by activating PI3-K (Liu et al., 2000). We therefore sought to test the hypothesis that CaR-initiated secretion is mediated by PI3-K and, if so, to identify the PI3-K isoforms and the molecular mechanisms that link the stimulation of the CaR to PI3-K activation. To evaluate the role of PI3-K in ↑[Ca2+]e-evoked secretion of 5-HT, adenoviral infection was used to express a dominant-negative form of p85, one of the regulatory subunits of PI3-K. Expression of dominant-negative p85 significantly inhibited the secretion of 5-HT by PF cells in response to ↑[Ca2+]e (Fig.2). These observations confirm our pharmacological studies showing that PI3-K activation is necessary for ↑[Ca2+]e-evoked secretion of 5-HT by PF cells. Moreover, because p85 has the potential to regulate the catalytic activity only of the p110α, p110β, p110δ, or p110θ catalytic subunits of PI3-K, but not p110γ (Igarashi and Michel, 2001), our data suggest that only these isoforms of the catalytic subunit can be involved in 5-HT secretion. PF cells express both the p110α and p110β of the catalytic subunits of PI3-K (Liu et al., 2000). In this study, p110α and p110β were detected by Western blotting in PF cell extracts, but neither the p110γ nor the p110δ could be found (data not shown). Neither the expression of the dominant-negative form of the p85 regulatory subunit of PI3-K (Fig. 2) nor the application of the PI3-K inhibitors wortmannin and LY294002 (Liu et al., 2000) fully suppressed ↑[Ca2+]e-evoked secretion of 5-HT by PF cells. Separately, the dominant-negative mutant and the pharmacological inhibitors reduced the secretion of 5-HT to approximately one-half the level that was observed in control cells. The failure of PI3-K inhibition to fully abolish secretion might indicate that stimulation of the CaR results in the simultaneous activation of both PI3-K-dependent and PI3-K-independent signaling pathways.
Fig. 2.
Expression of dominant-negative PI3-kinase inhibits ↑[Ca2+]e-induced secretion of 5-HT. PF cells were infected with an adenovirus containing a DN form of p85 that consists of a regulatory subunit of PI3-K lacking the p110-interaction domain. The ↑[Ca2+]e-induced 5-HT secretion is significantly reduced (in comparison with cells infected with the control adenovirus) in cells expressing the DN mutant at all concentrations of [Ca2+]e ≥2 mm. Each data point represents the mean ± SE derived from at least three independent preparations of PF cells. **p < 0.005; ***p < 0.001 (vs cells infected with the control adenovirus at the same concentration of [Ca2+]e).
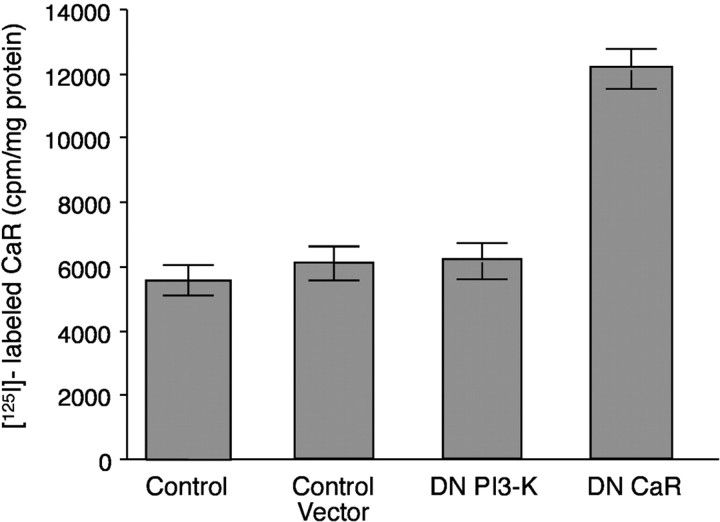
Cell-surface CaR expression was evaluated to determine whether expression of the dominant-negative form of PI3-K inhibits ↑[Ca2+]e-evoked secretion of 5-HT by primarily affecting the cell-surface expression of the CaR or by altering downstream signaling. Intact PF cells were exposed to antibodies that react with the extracellular domain of the CaR. The level of bound antibodies was measured with125I-labeled secondary antibodies. Antibody exposure was performed at 4°C to minimize internalization by endocytosis. The level of cell-surface anti-CaR binding to cells infected with the adenovirus expressing the dominant-negative form of PI3-K was comparable with that of uninfected cells and cells infected with control adenoviral vector (Fig. 3). As a positive control, cells were infected with the adenoviral vector expressing the dominant-negative CaR (which contains an immunoreactive extracellular domain), and these cells showed increased binding (Fig.3). These data indicate that neither adenoviral infection nor the expression of a dominant-negative form of PI3-K result in decreased CaR expression on the surface of PF cells. Because cell-surface expression was detectably increased after infection with adenovirus expressing a dominant-negative form of the CaR, the assay was valid as a measure of cell-surface CaR expression. The increase in expression also indicates that the dominant-negative form of the CaR expressed after adenoviral infection reached the plasma membrane.
Fig. 3.
Cell-surface expression of the CaR is not affected by adenoviral infection or expression of a dominant-negative form of PI3-K. Isolated PF cells were infected with an adenoviral vector that lacked a construct, with an adenoviral vector that expressed a dominant-negative form of PI3-K, or with adenovirus expressing a dominant-negative form of the CaR. The cell-surface CaR was detected with antibodies that recognize the extracellular domain of the CaR and the extracellular domain of the dominant-negative CaR and quantified with secondary antibodies labeled with 125I. Each data point represents the mean ± SE derived from three independent preparations of PF cells. An increase in CaR immunoreactivity at the cell surface was induced by expression of the dominant-negative CaR; however, neither the adenoviral vector nor the expression of the dominant-negative form of PI3-K affected cell-surface CaR immunoreactivity.
Total PI3-K activity in PF cells increases when cells are exposed to ↑[Ca2+]e (Liu et al., 2000). However, previous assays, which used antibodies to p85 to immunoprecipitate the holoenzyme, were not able to distinguish whether the increased PI3-K activity is mediated by the p110α or p110β isoforms of the catalytic subunit. The antibodies used in those studies will immunoprecipitate complexes of p85 that might contain both p110α and p110β. We now report that PI3-K activity is enhanced by exposure of PF cells to ↑[Ca2+]e, using isoform-specific antibodies to the p110β subunit rather than antibodies to p85 for the determination of PI3-K activity (Ettinger et al., 1996; Herrera-Velit and Reiner, 1996; Liu et al., 2000). The level of PI3-K activity found with antibodies to the p110β subunit (Fig.4A) is elevated (52 ± 5%) after exposure to ↑[Ca2+]e. A similar level of PI3-K activity has been seen when the holoenzyme is immunoprecipitated with antibodies to p85 (50 ± 5%). Thus, if both antibodies should precipitate the proteins under investigation equally well, the activity of the p110β isoform is likely to be sufficient to fully account for the activation of PI3-K activity after stimulation of the CaR.
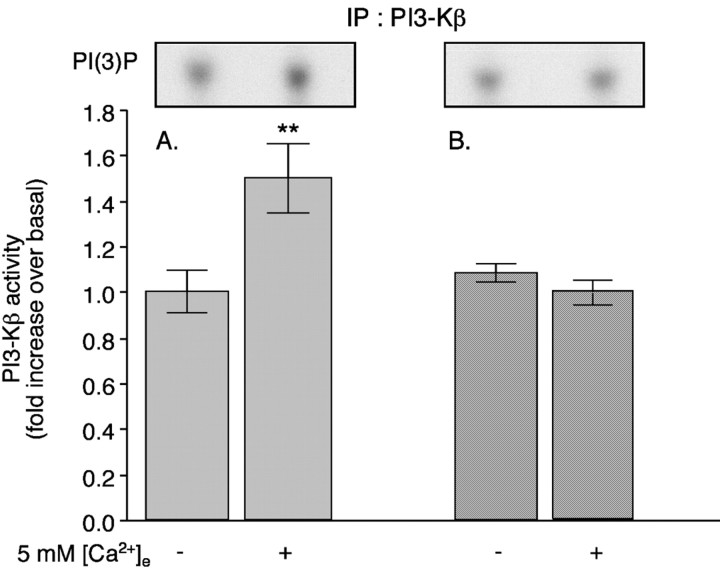
Fig. 4.
Expression of βARKct inhibits the ↑[Ca2+]e-evoked stimulation of PI3-Kβ. PF cells were infected with either control adenoviral vector or adenovirus expressing a minigene encoding the Gβγ scavenger (βARKct). ↑[Ca2+]e (5 mm) was used to stimulate the CaR in both sets of cells. Cells were lysed, and 750 μg of the resulting lysates were immunoprecipitated (IP) with antibodies to the p110β subunit of PI3-Kβ. The ↑[Ca2+]e-evoked activity of PI3-Kβ is significantly greater in cells infected with a control adenoviral vector (A) than in cells that express βARKct (B). Autoradiographs of typical spots of PI(3)32P obtained in the assays are illustrated in theinsets above the corresponding bar graphs. Each data point represents the mean ± SE derived from at least three independent preparations of PF cells. **p < 0.005 (vs 0 mm[Ca2+]e).
The CaR activates p85/p110β via Gβγ subunits
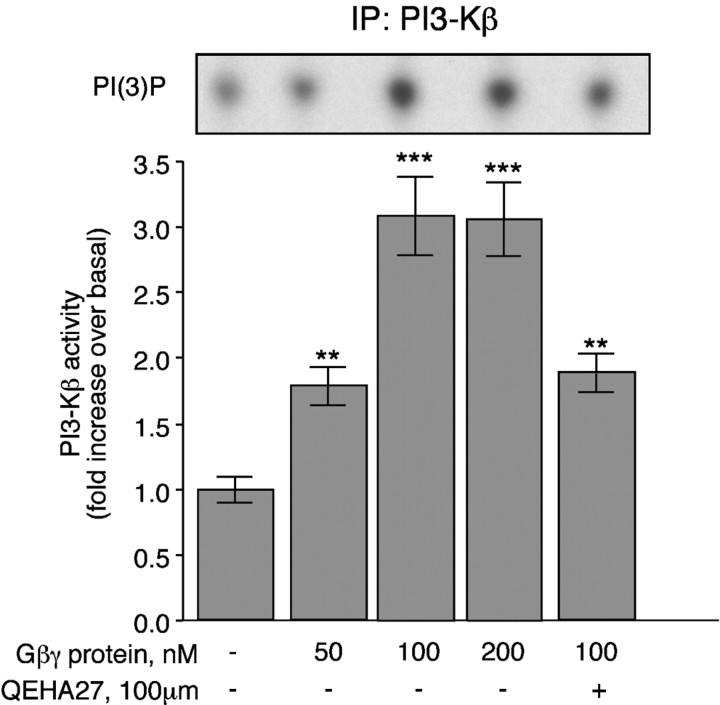
Stimulation of the CaR of PF cells activates two G-proteins (Gq and Gi) (Liu et al., 2000). Therefore, βγ subunits liberated from the CaR-activated G-proteins could be the potential messengers that couple the CaR to PI3-K. Because the p85/p110β PI3-K complex is activated by Gβγ subunits (Clapham and Neer, 1997), the role of Gβγ subunits in ↑[Ca2+]e-mediated PI3-K activation was evaluated. A minigene encoding a well characterized peptide scavenger of Gβγ subunits (βARKct; packaged in an adenoviral vector) (Koch et al., 1994) was expressed in PF cells. Expression of βARKct completely prevented activation of PI3-K after the exposure of PF cells to ↑[Ca2+]e (Fig.4B). To verify that the effect of βARKct was attributable to the scavenging of Gβγ subunits, we added purified Gβγ subunits to immunoprecipitated PI3-Kβ and measured the reconstituted kinase activity (Fig. 5). The Gβγ subunits increased the enzymatic activity of PI3-Kβ in a concentration-dependent manner. QEHA27, a peptide that scavenges Gβγ subunits (Chen et al., 1995), also reduced the Gβγ-dependent activation of PI3-K (Fig. 5). Denaturing the Gβγ subunits by boiling abolished the effect (data not shown). These observations indicate that Gβγ subunits activate the p85/p110β PI3-K complex. They are consistent with our conclusion that βARKct interferes with the Gβγ-mediated activation of the p85/p110β PI3-K complex in PF cells.
Fig. 5.
Gβγ subunits stimulate PI3-Kβ activity. PF cells were lysed, and 750 μg of the resulting lysates were immunoprecipitated (IP) with antibodies to the p110β subunit of PI3-Kβ. Gβγ subunits were added at the indicated concentrations. The measured activity of PI3-Kβ was stimulated by βγ subunits in a concentration-dependent manner. **p < 0.005; ***p < 0.001 (vs no addition of Gβγ). The effect of Gβγ subunits was inhibited by the addition of the scavenging peptide QEHA27. Autoradiographs of typical spots of PI(3)32P obtained in the assays are illustrated in the inset above the correspondingbar graphs. Each data point represents the mean ± SE derived from at least three independent preparations of PF cells.
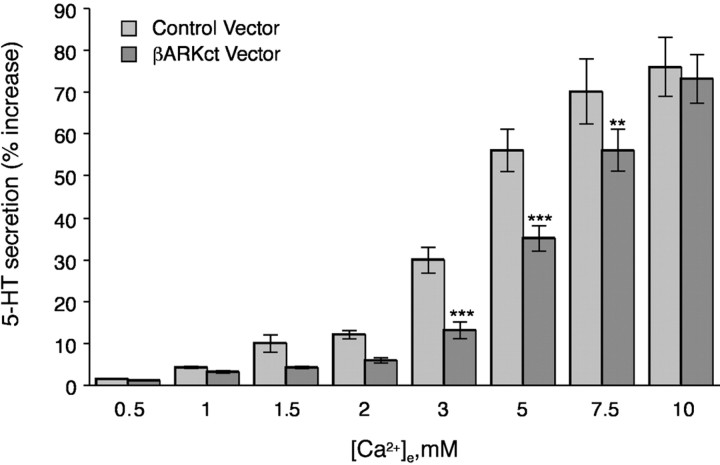
Because the expression of βARKct abolished PI3-K activity, βARKct expression would be expected to inhibit the ↑[Ca2+]e-evoked secretion of 5-HT in a manner similar to the expression of a dominant-negative form of p85. When PF cells were exposed to physiological levels of [Ca2+]e (>2 mm), expression of βARKct did indeed inhibit ↑[Ca2+]e-evoked 5-HT secretion (Fig. 6). Moreover, the extent of inhibition of the 5-HT secretion (50–60%) was comparable with that observed after overexpressing dominant-negative p85 or after treating cells with wortmannin or LY294002 inhibitors of PI3-K (Liu et al., 2000). However, when cells were exposed to higher physiological levels of [Ca2+]e(7.5–10 mm), the inhibition of 5-HT secretion by βARKct was lost. These observations suggest that Gβγ-dependent 5-HT secretion is probably most important at physiological concentrations of [Ca2+]e, and that Gβγ-independent 5-HT secretion becomes more relevant at supraphysiological concentrations of [Ca2+]e. The great stimulation of the CaR by supraphysiological concentrations of [Ca2+]e may also indicate that there are additional pathways to PI3-K and PKCζ that do not depend on Gβγ and are operational at high concentrations of [Ca2+]e.
Fig. 6.
Expression of βARKct inhibits the ↑[Ca2+]e-evoked secretion of 5-HT. PF cells were infected with an adenovirus containing a minigene encoding the Gβγ scavenger βARKct. At physiological concentrations of [Ca2+]e (3–5 mm), expression of βARKct inhibited the ↑[Ca2+]e-mediated secretion. The inhibition of 5-HT secretion by βARKct was reversed at a supraphysiological concentration of [Ca2+]e (10 mm). **p < 0.005; ***p < 0.001 (vs cells infected with the control adenovirus at the same concentration of [Ca2+]e).
Stimulation of the CaR leads to the phosphorylation of PDK1 substrates
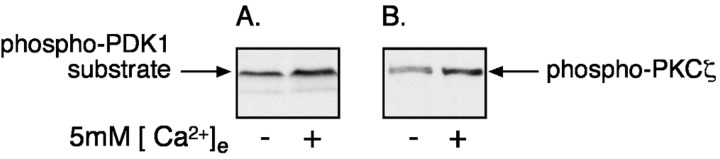
D3-phosphorylated phosphatidylinositol-phosphates are generated by PI3-K and activate PDK1 serine–threonine kinase activity (Le Good et al., 1998). PDK1 phosphorylates and activates downstream protein kinases, including PKC (Le Good et al., 1998), protein kinase A, and Akt (Chan et al., 1999; Balendran et al., 2000). We have demonstrated previously that stimulation of PF cells with ↑[Ca2+]e leads to the activation of PKCζ, an atypical isoform of PKC, and that PKCζ inhibitors antagonize the ↑[Ca2+]e-evoked secretion of 5-HT. These observations suggest that the PI3-K-dependent secretion of 5-HT may involve the activation of PDK1 and lead to the phosphorylation and stimulation of PKCζ. To test this hypothesis, the consequences of stimulating the CaR with ↑[Ca2+]e were determined by measuring the activities of PKCζ and PDK1. PDK1 activity was measured by Western blot analysis of whole-cell lysates and by using antibodies that specifically recognize phosphoproteins after they have been phosphorylated in the common PDK1 phosphorylation motif. Exposure of PF cells to ↑[Ca2+]e was found to increase the levels of phosphothreonine products of PDK1 (Fig.7A). To determine whether the phosphorylated form of PKCζ was among the products of PDK1 activity, the CaR of isolated PF cells was stimulated with ↑[Ca2+]e, and PKCζ was immunoprecipitated with specific antibodies that detect PKCζ independently of its phosphorylation state. The immunoprecipitated PKCζ was detected by immunoblotting with phosphospecific antibodies that recognize PKCζ only after phosphorylation in the PDK1-dependent phosphorylation site (threonine-410). Exposure of PF cells to ↑[Ca2+]eincreased the amount of phosphorylated PKCζ (66 ± 8%) (Fig.7B). These data are consistent with the idea that PI3-K stimulates PDK1 and promotes secretion through PDK1-dependent phosphorylation of PKCζ.
Fig. 7.
Exposure of PF cells to ↑[Ca2+]e stimulates PDK1-dependent phosphorylation. PF cells were incubated in the presence of 0 or 5 mm [Ca2+]e.A, The cells were lysed, and lysates (150 μg) were subjected to Western blot analysis with antibodies that detect substrates of PDK1 after phosphorylation in the PDK1 phosphorylation motif. B, In a separate experiment, PKCζ was immunoprecipitated with specific antibodies to PKCζ. The immune complex was blotted with antibodies against PDK1 substrates. Stimulation of PF cells with ↑[Ca2+]e increased the phosphorylation of PKCζ and that of other substrates of PDK1. Similar results were obtained in three independent experiments.
PKCζ mediates 5-HT secretion by PF cells
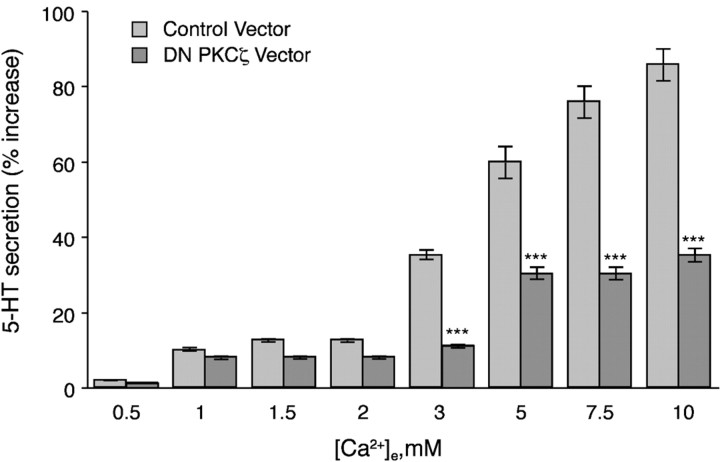
To test the hypothesis that the phosphorylated substrate of PDK1 mediating the ↑[Ca2+]e-evoked 5-HT secretion is PKCζ, a dominant-negative form of PKCζ was expressed in PF cells. Expression of the dominant-negative form of PKCζ significantly reduced the ↑[Ca2+]e-evoked secretion of 5-HT by PF cells, both physiological and by higher concentrations of [Ca2+]e (>2 mm) (Fig. 8). However, secretion of 5-HT was not fully abolished by expression of the dominant-negative form of PKCζ, suggesting that PKCζ activity alone is not sufficient for regulating 5-HT secretion.
Fig. 8.
Expression of dominant-negative PKCζ inhibits the ↑[Ca2+]e-evoked secretion of 5-HT. PF cells were infected with an adenovirus containing dominant-negative PKCζ. The ↑[Ca2+]e-induced 5-HT secretion is significantly inhibited in cells expressing the DN mutant at all concentrations of [Ca2+]e ≥3 mm. The data show means ± SE derived from at least three independent preparations of PF cells. ***p < 0.001 (vs cells infected with the control adenovirus at the same concentration of [Ca2+]e).
The serine–threonine kinase Akt is also a direct downstream target of PI3-K and, like PKCζ, is activated via PDK1 (Franke et al., 1997). PDK1 phosphorylates Thr308 within the activation loop of Akt (Stephens et al., 1998). Immunoblots revealed that PF cells contain Akt (data not shown). To examine the regulation of endogenous Akt after stimulation of the CaR with ↑[Ca2+]e, Western blot analysis was performed using phosphospecific antibodies that detect Akt protein only when it is phosphorylated on residues that are required for its activity (Alessi and Cohen, 1998). No phosphorylated Akt was detected in cells, regardless of whether these cells were stimulated with ↑[Ca2+]e. Thus, our data suggest that 5-HT secretion involves the stimulation of PI3-K and activation of PKCζ via PDK1. Our results do not establish whether the induction of endogenous Akt activity participates in CaR-induced secretion.
Discussion
The main goal of this study was to characterize the intracellular signaling cascades that couple the CaR of PF cells to the secretion of 5-HT. In particular, we wanted to test the hypothesis that a second pathway uses PKCζ as an effector in parallel to the PKCγ transduction cascade that we described previously (Liu et al., 2000). Secretion of 5-HT is evoked when PF cells are exposed to ↑[Ca2+]e, which is their natural secretogogue. It has been postulated that ↑[Ca2+]e evokes secretion by stimulating the CaR (Brown and Macleod, 2001). We have confirmed the involvement of the CaR by demonstrating that ↑[Ca2+]e-evoked secretion of 5-HT was abolished when a dominant-negative form of the CaR was expressed in PF cells. The involvement of PKCζ as an effector mediating the ↑[Ca2+]e-evoked secretion of 5-HT was consolidated by expressing a dominant-negative form of PKCζ in PF cells. In contrast to the dominant-negative form of the CaR, which, when expressed in PF cells, virtually eliminated ↑[Ca2+]e-evoked 5-HT secretion, expression of the dominant-negative form of PKCζ reduced 5-HT secretion by 50–60%. The partial but significant antagonism of ↑[Ca2+]e-evoked 5-HT secretion after using the dominant-negative form of PKCζ is consistent with the idea that PKCζ is only one of the major effectors of 5-HT secretion. Other effectors of 5-HT secretion include PKCγ.
CaR-mediated secretion involves Gq rather than Gi, because Gq is insensitive to pertussis toxin. G-protein-coupled receptors are often promiscuous with respect to the signaling cascades that they can activate (Hamm, 1998). The steps in the signaling cascade, which activates PKCζ, were identified by expressing dominant-negative forms of intermediate signal molecules and that of a scavenger of βγ subunits before demonstrating that the expressed proteins inhibited the ↑[Ca2+]e-evoked secretion of 5-HT. The secretion of 5-HT by PF cells in response to ↑[Ca2+]e was found to be antagonized by the expression of a dominant-negative form of the p85 regulatory subunit of PI3-K. QEHA27 and the expression of βARKct scavenge Gβγ subunits, and both prevented the activation of PI3-K and also ↑[Ca2+]e-evoked 5-HT secretion. These findings suggest that the coupling of the stimulated CaR to Gq liberates βγ subunits, which, in turn, activate PI3-K. The ability of Gβγ subunits to stimulate PI3-K activity was confirmed by direct measurement of PI3-K lipid kinase activity. The primary form of activated PI3-K in our experimental model was PI3-Kβ (p85/p110β), as demonstrated by immunocomplex assays using isoform-specific antibodies.
Although PI3-K is commonly thought of as a participant in signaling cascades initiated by the stimulation of receptor tyrosine kinases, PI3-K can also be activated by G-protein-coupled receptors (Clapham and Neer, 1997; Kurosu et al., 1997; Stephens et al., 1997; Vanhaesebroeck et al., 1997; Maier et al., 1999; Takasuga et al., 1999; Murga et al., 2000; Bony et al., 2001). In contrast to classical G-protein signaling, in our experiments, PI3-K appears to be activated by Gβγ rather than α subunits. The observation that PI3-Kβ is the isoform that is activated in PF cells by βγ subunits was surprising, because PI3-Kγ is the isoform that has primarily been associated with G-protein-coupled receptors (Stephens et al., 1997; Hamm, 1998). However, expression of PI3-Kγ is more restricted than that of PI3-Kβ (Vanhaesebroeck et al., 1997) and indeed, PI3-Kα and PI3-Kβ, but not PI3-Kγ, were detected in PF cells. The activation of PI3-Kβ by G-protein-coupled receptors is not unique to PF cells and has also been reported in other systems (Igarashi and Michel, 2001;Yart et al., 2002). The regulatory subunit of the PI3-K that is stimulated after CaR activation in PF cells is p85α, which can associate with p110β but not with p110γ (Stephens et al., 1997). This rules out the involvement of the p110γ catalytic isoform as an intermediate messenger in the secretion pathway. However, no phosphotyrosine peptides were detected after activation of CaR, and ↑[Ca2+]e-evoked 5-HT secretion was not inhibited by genistein (our unpublished data). Thus, it is not clear how dominant-negative p85 can inhibit the catalytic activity of p110β in the absence of phosphotyrosine signaling. Dominant-negative p85 is thought to compete with full-length p85 for activated phosphotyrosine residues on receptor tyrosine kinases or downstream adaptor molecules. However, a recent publication may offer an explanation for the inhibitory effect of dominant-negative p85 in our system (Ueki et al., 2002). Its authors have demonstrated that the stability of the catalytic subunit of p110 is diminished in the absence of p85. Thus, the inhibitory effect of dominant-negative p85 may primarily be downregulating the expression levels of the catalytic subunit. Finally, the existence of multiple species of Gβγ-sensitive PI3-kinase that are not affected by phosphotyrosyl peptides, including p85/p110β, has been reported previously (Kurosu et al., 1997).
One strength of this study is that we have examined CaR signaling in primary cultures of cells that respond to [Ca2+]e under physiological conditions. The involvement of PI3-K in CaR signaling was not observed in studies that examined the CaR in heterologous cell systems. When stably expressed in transfected HEK293 cells, the CaR was reported to couple to phosphatidylinositol 4-kinase (PI4-K) and not to PI3-K (Huang et al., 2002). In those studies, the coupling of the CaR to PI4-K is independent of G-proteins and relies on the activation of Rho. It is conceivable that ectopic overexpression of the CaR in HEK293 cells results in a coupling of the CaR to signaling pathways that would not be used under physiological conditions. It is also possible that the coupling of the CaR via Gβγ subunits to PI3-K was not detected in HEK293 cells, because these studies did not examine PI3-K activity directly but instead measured Akt activity. However, in PF cells, the activation of Akt is negligible when compared with that of PKCζ. As a logical consequence, any failure to detect the activation of Akt in HEK293 cells after CaR stimulation does not yet rule out the participation of PI3-K in CaR signaling.
We investigated the role of Gβγ subunits in regulating the activity of PI3-K. Their involvement was established by several techniques, including the expression of a minigene that sequesters Gβγ. This minigene, βARKct, only inhibited the secretion of 5-HT when PF cells were exposed to physiological concentrations of the agonist [Ca2+]e. If the concentration of [Ca2+]e to which cells were exposed was supraphysiological (10 mm), the βARKct-dependent blockade of secretion was overcome and cells that expressed βARKct secreted 5-HT comparably as well as control cells. This phenomenon is consistent with the assumption that the mechanisms of 5-HT secretion at supraphysiological concentrations of [Ca2+]e are different from those in the physiological range. The PF cell secretion of 5-HT depends on the depolarization of PF cells via nonselective cation channels and [Ca2+]e influx through L-type calcium channels (McGehee et al., 1997). The influx of [Ca2+]e in response to stimulation of the chemokine receptor CX3CR1 has been demonstrated recently to depend on PI3-K, which was activated by βγ subunits that open L-type calcium channels (Kansra et al., 2001). Even if it is not yet clear whether the PI3-K that is activated by βγ subunits in PF cells functions similarly in the gating of the L-type channels, the influx of [Ca2+]e at a supraphysiological concentration of [Ca2+]e may moot the gating of calcium channels and trigger secretion. An alternative mechanism, in which the supraphysiological concentration of [Ca2+]e could increase secretion, might involve Akt-dependent potentiation of L-type channels (Blair et al., 1999).
The coupling of PI3-kinase to PKCζ appeared to be accomplished via PDK1, because stimulation of PF cells with ↑ [Ca2+]e led to an increase in the phosphorylated products of PDK1. PDK1 is known to activate several members of the AGC family of kinases, such as PKCζ, by phosphorylation of conserved serine–threonine residues in the T-loop of the kinase domain (T410 in PKCζ) (Alessi and Cohen, 1998; Coffer and Woodgett, 1998). PKCζ also contains a hydrophobic motif that acts as a “docking site” and permits its recruitment to PDK1 so it can be a substrate for that enzyme (Balendran et al., 2000). Thus, it is very likely that PDK1 and PKCζ (and maybe even Akt) coexist in a signaling complex that is activated by the stimulated CaR as a result of an increased local concentration of PI3-K products. Pleckstrin homology interactions with phospholipid products of PI3-K might hereby enable the signaling molecules to translocate to the plasma membrane, where they can interact with one another (Lemmon et al., 1996). The subsequent downstream pathways remain obscured because protein substrates of PKCζ in this pathway have not been identified.
Polyphosphoinositides have been implicated in the recycling of synaptic vesicles in neurons (Miller, 1998; Cremona et al., 1999). They have been postulated to play a wider role at synapses in the control of signaling, membrane traffic, and the actin cytoskeleton. Heterotrimeric G-protein-coupled receptors are also known to modulate the secretion of neurotransmitters (Alford and Grillner, 1991; Harris et al., 2000). Gβγ subunits have been shown recently to mediate the effect of 5-HT on neurotransmission, where they are functioning downstream of Ca2+ entry. The action of Gβγ subunits is thought to target the exocytic fusion machinery to presynaptic terminals (Blackmer et al., 2001). These properties may reflect the neural crest-origin and the neuron-like properties of PF cells. However, the amount of 5-HT secreted by isolated PF cells is less than that secreted at synapses. The CaR is also expressed by neurons, including those of the enteric nervous system (Brown and Macleod, 2001), which originate from the same level of the neural crest as PF cells (Le Douarin and Kalcheim, 1999) and the CNS (Brown and Macleod, 2001). The involvement of Gβγ subunits and phosphoinositides in the secretion of 5-HT by PF cells in response to stimulation of the G-protein-coupled CaR thus appears to be analogous to mechanisms that operate in the modulation of the secretion of neurotransmitters at some synapses. As a consequence, our studies in PF cells are most likely very relevant to other neural systems in which 5-HT secretion is induced by Ca2+ entry and includes synaptic transmission.
Footnotes
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant DK2139 (H.T.), National Institute of Neurological Diseases Grant NS12969 (M.D.G.), Human Frontiers Science Program Organization Grant RGY0152/2001-8 (T.F.F.), and National Institute of Child Health and Human Development Grant HD25969 (A.F.R.).
Correspondence should be addressed to Dr. Hadassah Tamir, Department of Neuroscience, New York State Psychiatric Institute, 1051 Riverside Drive, New York, NY 10032. E-mail: ht3@columbia.edu.
References
- 1.Alessi D, Cohen P. Mechanism of activation and function of protein kinase B. Curr Opin Genet Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 2.Alford S, Grillner S. The involvement of GABAB receptors and coupled G-protein in spinal GABAergic presynaptic inhibition. J Neurosci. 1991;11:3718–3726. doi: 10.1523/JNEUROSCI.11-12-03718.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bai M, Quinn S, Trivedi S, Kifor O, Pearce S, Pollak M, Krapcho K, Herbert S, Brown E. Expression and characterization of inactivating and activating mutations in the human Ca2+o-sensing receptor. J Biol Chem. 1996;271:19537–19545. doi: 10.1074/jbc.271.32.19537. [DOI] [PubMed] [Google Scholar]
- 4.Balendran A, Biondi R, Cheung P, Casamayor A, Deak M, Alessi D. A 3-phosphoinositide-dependent protein kinase-1 (PDK1) docking site is required for the phosphorylation of protein kinase Cζ (PKCζ) and PKC-related kinase 2 by PDK1. J Biol Chem. 2000;275:20806–20813. doi: 10.1074/jbc.M000421200. [DOI] [PubMed] [Google Scholar]
- 5.Barasch JM, Mackey H, Tamir H, Nunez EA, Gershon MD. Induction of a neural phenotype in a serotonergic endocrine cell derived from the neural crest. J Neurosci. 1987a;7:2874–2883. doi: 10.1523/JNEUROSCI.07-09-02874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barasch JM, Tamir H, Nunez EA, Gershon MD. Serotonin-storing secretory granules from thyroid parafollicular cells. J Neurosci. 1987b;7:4017–4033. doi: 10.1523/JNEUROSCI.07-12-04017.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barasch JM, Gershon MD, Nunez EA, Tamir H, Al-Awqati Q. Thyrotropin induces the acidification of the secretory granules of parafollicular cells by increasing the chloride conductance of the granular membrane. J Cell Biol. 1988;107:2137–2148. doi: 10.1083/jcb.107.6.2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernd P, Gershon MD, Nunez EA, Tamir H. Separation of dissociated thyroid follicular and parafollicular cells: association of serotonin binding protein with parafollicular cells. J Cell Biol. 1981;88:499–508. doi: 10.1083/jcb.88.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackmer T, Larsen E, Takahashi M, Martin T, Alford S, Hamm H. G-protein βγ subunit-mediated presynaptic inhibition: regulation of exocytotic fusion downstream of Ca2+ entry. Science. 2001;292:293–297. doi: 10.1126/science.1058803. [DOI] [PubMed] [Google Scholar]
- 10.Blair L, Bence-Hanulec K, Mehta S, Franke T, Kaplan D, Marshal J. Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival. J Neurosci. 1999;19:1940–1951. doi: 10.1523/JNEUROSCI.19-06-01940.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bony C, Roche S, Shuichi U, Sasaki T, Crackower M, Penninger J, Mano H, Puceat M. A specific role of phosphatidylinositol 3-kinase gamma: a regulation of autonomic Ca2+ oscillation in cardiac cells. J Cell Biol. 2001;152:717–728. doi: 10.1083/jcb.152.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brown E, MacLeod R. Extracellular calcium sensing and extracellular calcium signaling. Physiol Rev. 2001;81:239–297. doi: 10.1152/physrev.2001.81.1.239. [DOI] [PubMed] [Google Scholar]
- 13.Chan T, Rittenhouse S, Tsichlis P. Akt/PKB and other D3 phosphoinositide-regulated kinases: kinase activation by phosphoinositide-dependent phosphorylation. Annu Rev Biochem. 1999;68:965–1014. doi: 10.1146/annurev.biochem.68.1.965. [DOI] [PubMed] [Google Scholar]
- 14.Chen J, DeVivo M, Dingus L, Harry A, Li L, Sui J, Carty D, Blank J, Exton J, Stoffel R, Inglese J, Logothetis D, Hilderbrandt J, Iyengar R. A region of adenylyl cyclase 2 critical for regulation by G-protein βγ subunits. Science. 1995;268:1166–1169. doi: 10.1126/science.7761832. [DOI] [PubMed] [Google Scholar]
- 15.Cidon S, Tamir H, Nunez EA, Gershon MD. ATP-dependent uptake of 5-hydroxytryptamine by secretory granules isolated from thyroid parafollicular cells. J Biol Chem. 1991;266:4392–4400. [PubMed] [Google Scholar]
- 16.Clapham D, Neer E. G-protein βγ subunits. Annu Rev Pharmacol Toxicol. 1997;37:167–203. doi: 10.1146/annurev.pharmtox.37.1.167. [DOI] [PubMed] [Google Scholar]
- 17.Clark M, Lanigan T, Page N, Russo A. Induction of a serotonergic and neuronal phenotype in thyroid C-cells. J Neurosci. 1995;15:6167–6178. doi: 10.1523/JNEUROSCI.15-09-06167.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coffer P, Woodgett JR. Protein kinase B (c-Akt): multifunctional mediator of phosphatidylinositol 3-kinase activation. Biochem J. 1998;335:1–13. doi: 10.1042/bj3350001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cremona O, Di Paolo G, Wenk M, Luthi A, Kim W, Takei A, Danielli L, Nemoto Y, Shears S, Flavell R, McCormick D, De Camilli P. Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell. 1999;99:179–188. doi: 10.1016/s0092-8674(00)81649-9. [DOI] [PubMed] [Google Scholar]
- 20.Crowder R, Freeman R. Phosphatidylinositol 3-kinase and Akt protein kinase are necessary and sufficient for the survival of nerve growth factor-dependent sympathetic neurons. J Neurosci. 1998;18:2933–2943. doi: 10.1523/JNEUROSCI.18-08-02933.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ettinger SL, Lauener RW, Duronio V. Protein kinase Cδ specifically associates with phosphatidylinositol 3-kinase following cytokine stimulation. J Biol Chem. 1996;271:14514–14518. doi: 10.1074/jbc.271.24.14514. [DOI] [PubMed] [Google Scholar]
- 22.Franke T, Kaplan D, Cantley L. PI3K: downstream AKTion blocks apoptosis. Cell. 1997;88:435–437. doi: 10.1016/s0092-8674(00)81883-8. [DOI] [PubMed] [Google Scholar]
- 23.Fruman DA, Meyers RE, Cantley LC. Phosphoinositide kinases. Annu Rev Biochem. 1998;67:481–507. doi: 10.1146/annurev.biochem.67.1.481. [DOI] [PubMed] [Google Scholar]
- 24.Fujita T. Paraneurons. In: Adelman G, editor. Encyclopedia of neuroscience, Vol II. Birkhaüser; Boston: 1987. pp. 921–923. [Google Scholar]
- 25.Hamm H. The many faces of G-protein signaling. J Biol Chem. 1998;273:669–672. doi: 10.1074/jbc.273.2.669. [DOI] [PubMed] [Google Scholar]
- 26.Harris T, Hartwieg E, Horvitz H, Jorgensen E. Mutations in synaptojanin disrupt synaptic vesicle recycling. J Cell Biol. 2000;150:589–600. doi: 10.1083/jcb.150.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Herbert SC, Brown EM. The extracellular calcium receptor. Curr Opin Cell Biol. 1995;7:484–492. doi: 10.1016/0955-0674(95)80004-2. [DOI] [PubMed] [Google Scholar]
- 28.Herrera-Velit P, Reiner N. Bacterial lipopolysaccharides induce the association and coordinate activation of p53/56lyn and phosphatidylinositol 3-kinase in human monocytes. J Immunol. 1996;156:1157–1165. [PubMed] [Google Scholar]
- 29.Hodgkinson C, Sale G. Regulation of both PDK1 and phosphorylation of PKC-ζ and -δ by a C-terminal PRK2 fragment. Biochemistry. 2002;41:561–569. doi: 10.1021/bi010719z. [DOI] [PubMed] [Google Scholar]
- 30.Huang C, Handlogten M, Miller R. Parallel activation of phosphoinositol 4-kinase and phospholipase C by the extracellular calcium-sensing receptor. J Biol Chem. 2002;277:20293–20300. doi: 10.1074/jbc.M200831200. [DOI] [PubMed] [Google Scholar]
- 31.Igarashi J, Michel T. Sphingosine 1-phosphate and isoform-specific activation of phosphoinositide 3-kinase β. J Biol Chem. 2001;276:36281–36288. doi: 10.1074/jbc.M105628200. [DOI] [PubMed] [Google Scholar]
- 32.Kandel ES, Hay N. The regulation and activities of the multifunctional serine/threonine kinase Akt/PKB. Exp Cell Res. 1999;253:210–229. doi: 10.1006/excr.1999.4690. [DOI] [PubMed] [Google Scholar]
- 33.Kansra VCG, Gutierrez-Ramos J, Polakiewicz R. Phosphatidylinositol 3-kinase-dependent extracellular calcium influx is essential for CX(3)CR1-mediated activation of the mitogen-activated protein kinase cascade. J Biol Chem. 2001;276:31831–31838. doi: 10.1074/jbc.M009374200. [DOI] [PubMed] [Google Scholar]
- 34.Koch W, Hawes B, Inglese J, Luttrell RJL. Cellular expression of the carboxyl terminus of a G-protein-coupled receptor kinase attenuates G βγ-mediating signaling. J Biol Chem. 1994;269:6193–6197. [PubMed] [Google Scholar]
- 35.Kurosu H, Maehama T, Okada T, Yamamoto T, Hoshino S-I, Fukui Y, Ui M, Hazeki O, Katada T. Heterodimeric phosphoinositide 3-kinase consisting of p85 and p110β is synergistically activated by the βγ subunits of G-proteins and phosphotyrosyl peptide. J Biol Chem. 1997;272:24252–24256. doi: 10.1074/jbc.272.39.24252. [DOI] [PubMed] [Google Scholar]
- 36.Lanigan T, DeRaad S, Russo A. Requirement of the MASH-1 transcription factor for neuroendocrine differentiation thyroid C cells. J Neurobiol. 1998;34:126–134. [PubMed] [Google Scholar]
- 37.Le Douarin NM, Kalcheim C. The neural crest, Ed 2. Cambridge UP; Cambridge, UK: 1999. [Google Scholar]
- 38.Le Good J, Ziegler W, Parekh D, Alessi D, Cohen P, Parker P. Protein kinase C isotypes controlled by phosphoinositide 3-kinase through the protein kinase PDK1. Science. 1998;281:2042–2045. doi: 10.1126/science.281.5385.2042. [DOI] [PubMed] [Google Scholar]
- 39.Lemmon M, Ferguson K, Schlessinger J. PH domain: diverse sequences with a common fold recruit signaling molecules to the cell surface. Cell. 1996;85:621–624. doi: 10.1016/s0092-8674(00)81022-3. [DOI] [PubMed] [Google Scholar]
- 40.Liu K, Hsiung S, Adlersberg M, Sacktor T, Gershon M, Tamir H. Ca2+-evoked serotonin secretion by parafollicular cells: roles in signal transduction of phosphatidylinositol 3′-kinase, and the γ and ζ isoforms of protein kinase C. J Neurosci. 2000;20:1365–1373. doi: 10.1523/JNEUROSCI.20-04-01365.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Maier U, Babich A, Nurenberg B. Roles of non-catalytic subunits in Gβγ-induced activation of class I phosphoinositide 3-kinase isoforms β and γ. J Biol Chem. 1999;274:29311–293117. doi: 10.1074/jbc.274.41.29311. [DOI] [PubMed] [Google Scholar]
- 42.McGehee D, Adlersberg M, Liu K, Hsiung SC, Heath M, Tamir H. Mechanism of extracellular Ca2+ receptor-stimulated hormone release from sheep thyroid parafollicular cells. J Physiol (Lond) 1997;502:31–44. doi: 10.1111/j.1469-7793.1997.031bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Miller R. Presynaptic receptors. Annu Rev Pharmacol Toxicol. 1998;38:201–227. doi: 10.1146/annurev.pharmtox.38.1.201. [DOI] [PubMed] [Google Scholar]
- 44.Murga C, Fukuhara S, Gutkind J. A novel role for phosphatidylinositol 3-kinaseβ in signaling from G-protein-coupled receptors to Akt. J Biol Chem. 2000;275:12069–12073. doi: 10.1074/jbc.275.16.12069. [DOI] [PubMed] [Google Scholar]
- 45.Nunez EA, Gershon MD. The cytophysiology of thyroid parafollicular cells. Int Rev Cytol. 1978;52:1–80. doi: 10.1016/s0074-7696(08)60753-6. [DOI] [PubMed] [Google Scholar]
- 46.Ruat M, Snowman A, Hester L, Snyder S. Cloned and expressed rat Ca2+-sensing receptor. J Biol Chem. 1996;271:5972–5975. doi: 10.1074/jbc.271.11.5972. [DOI] [PubMed] [Google Scholar]
- 47.Russo A, Clark M, Durham P. An accessible model for the study of serotonergic neurons. Mol Neurobiol. 1996;13:257–276. doi: 10.1007/BF02740626. [DOI] [PubMed] [Google Scholar]
- 48.Scheid M, Woodgett J. PKB/Akt: functional insights from genetic models. Nat Rev Cell Biol. 2001;2:760–768. doi: 10.1038/35096067. [DOI] [PubMed] [Google Scholar]
- 49.Soh J, Lee E, Frywes R, Weinstein B. Novel roles of specific isoforms of protein kinase C in activation of c-fos serum response element. Mol Cell Biol. 1999;19:1313–1324. doi: 10.1128/mcb.19.2.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stephens L, Eguinoa A, Erdjument-Bromage H, Lui M, Cooke F, Coadwell J, Smrcka A, Thelen M, Cadwallader K, Tempst P, Hawkins P. The G βγ sensitivity of PI3K is dependent upon tightly associated adaptor, p101. Cell. 1997;89:105–114. doi: 10.1016/s0092-8674(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 51.Stephens L, Anderson K, Stokoe D, Erdjument-Bromage H, Painter G, Holmes A, Gaffiney P, Reese C, McCormick F, Tempest P, Coadwell J, Hawkins P. Protein kinase B kinases that mediate the phosphatidylinositol 3,4,5-trisphosphate-dependent activation of protein kinase B. Science. 1998;279:710–714. doi: 10.1126/science.279.5351.710. [DOI] [PubMed] [Google Scholar]
- 52.Takasuga S, Katada T, Ui M, Hazeki O. Enhancement by adenosine of insulin-induced activation of phosphoinositide 3-kinase and protein kinase B in rat adipocytes. J Biol Chem. 1999;274:19545–19550. doi: 10.1074/jbc.274.28.19545. [DOI] [PubMed] [Google Scholar]
- 53.Tamir H, Liu KP, Hsiung SC, Adlersberg M, Nunez EA, Gershon MD. Multiple signal transduction mechanisms leading to the secretion of 5-hydroxytryptamine by MTC cells, a neurectodermally derived cell line. J Neurosci. 1990;10:3743–3753. doi: 10.1523/JNEUROSCI.10-11-03743.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tamir H, Liu KP, Heath M, Adlersberg M, Gershon MD. Stimulus-induced secretion and vesicle acidification in serotonergic paraneurons (parafollicular cells): the role of voltage-gated Ca2+ channels. Soc Neurosci Abstr. 1994a;20:1719. [Google Scholar]
- 55.Tamir H, Piscopo I, Liu KP, Hsiung SC, Adlersberg M, Nicolaides M, Al-Awqati Q, Gershon MD. Secretogogue-induced gating of chloride channels in the secretory vesicles of a paraneuron. Endocrinology. 1994b;135:2045–2057. doi: 10.1210/endo.135.5.7525261. [DOI] [PubMed] [Google Scholar]
- 56.Tamir H, Liu K-P, Adlersberg M, Hsiung SC, Gershon MD. Acidification of serotonin-containing secretory vesicles induced by a plasma membrane calcium receptor. J Biol Chem. 1996;271:6441–6450. doi: 10.1074/jbc.271.11.6441. [DOI] [PubMed] [Google Scholar]
- 57.Ueki K, Fruman D, Brachmann S, Tseng Y, Cantley L, Kahn C. Molecular balance between the regulatory and catalytic subunits of phosphoinositide 3-kinase regulates cell signaling and survival. Mol Cell Biol. 2002;22:965–977. doi: 10.1128/MCB.22.3.965-977.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vanhaesebroeck B, Leevers S, Panayotou G, Waterfield M. Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci. 1997;22:267–272. doi: 10.1016/s0968-0004(97)01061-x. [DOI] [PubMed] [Google Scholar]
- 59.Yart A, Roche S, Reinhard W, Laffargue M, Tonks N, Mayeux P, Chap H, Raynal P. A function for phosphoinositide 3-kinase β lipid products in coupling βγ to Ras activation in response to lysophosphatidic acid. J Biol Chem. 2002;277:21167–21178. doi: 10.1074/jbc.M110411200. [DOI] [PubMed] [Google Scholar]
- 60.Zabel M. Ultrastructural localization of calcitonin, somatostatin and serotonin in parafollicular cells of the rat thyroid. Histochem J. 1984;16:1265–1272. doi: 10.1007/BF01003725. [DOI] [PubMed] [Google Scholar]