● Cellular/Molecular
Chaperones Rescue a PolyQ Neurodegeneration
Heat Shock Protein 70 Chaperone Overexpression Ameliorates Phenotypes of the Spinal and Bulbar Muscular Atrophy Transgenic Mouse Model by Reducing Nuclear-Localized Mutant Androgen Receptor Protein
Hiroaki Adachi, Masahisa Katsuno, Makoto Minamiyama, Chen Sang, Gerassimos Pagoulatos, Charalampos Angelidis, Moriaki Kusakabe, Atsushi Yoshiki, Yasushi Kobayashi, Manabu Doyu, and Gen Sobue (see pages 2203–2211)
Spinal and bulbar muscular atrophy (SBMA), a so-called triple-repeat disease, is one of several inherited neurodegenerative diseases caused by expansion of the CAG codon for glutamine. In SBMA, the polyQ tract expansion is in the androgen receptor (AR) gene. Patients with SBMA, as well as the SBMA mouse, develop progressive muscle atrophy and weakness including the bulbar muscles (i.e. those innervated by cranial nerves), while bulbar and spinal motor neurons show nuclear inclusions of aggregated AR protein. Here, Adachi et al. use a hint from the pathology, the coprecipitation of mutant AR with chaperone and proteasomal proteins, to further investigate whether altered protein folding and/or degradation may underlie polyQ neurodegeneration. They cross-bred SBMA mice with mice that overexpress the chaperone protein heat shock protein 70 (HSP70). A 10-fold excess of chaperone proteins in the double-transgenic mice led to a reduction in the aggregated mutant AR protein as well as a reduction in muscle weakness. An HSP70-dependent decrease in both high-molecular-weight complexes and monomeric mutant AR proteins suggests an amplification of the ubiquitin degradation pathway, say the authors, although they point out that another possibility remains that the chaperone may aid in renaturation of mutant AR. Along with similar results in another mouse model of a triple-repeat disease (spinocerebellar ataxia type 1), these data suggest a possible strategy for therapy in these disorders.
▴ Development/Plasticity/Repair
A Homeobox Gene and Commissural Neuron Differentiation
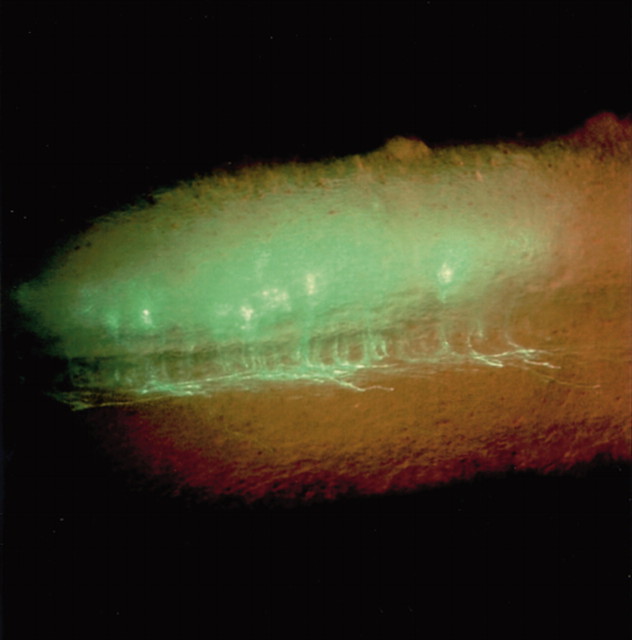
Mammalian BarH1 Confers Commissural Neuron Identity on Dorsal Cells in the Spinal Cord
Rie Saba, Norio Nakatsuji, and Tetsuichiro Saito (see pages 1987–1991)
The molecular cascade that determines the migration and axon projection of commissural neurons, which carry information from one side of the body to the other across the midline, has been well characterized, but the events leading to differentiation of these neurons remain more mysterious. In this brief report, Saba et al. use a transgenic reporter mouse to show selective expression of the mammalian homeobox gene BarH1 (MBH1) in commissural neurons in the dorsal horn of the developing spinal cord. They then used a gain-of-function strategy to further examine the role ofMBH1. Ectopic expression of MBH1 byin utero electroporation caused neurons to mimic the migration and projection patterns of endogenousMBH1-expressing commissural neurons [i.e. they migrated into the deep dorsal horn, expressed the commissural neuronal markers TAG-1 (tumor-associated glycoprotein-1) and DCC (Deleted in Colorectal Cancer), and developed contralateral axonal projections]. The authors conclude that MBH1 functions upstream of these migratory molecules to determine the fate of commissural neurons in the spinal cord.
Axon projection of embryonic dorsal horn neurons (embryonic day 13.5 spinal cord) 2 d after in utero spinal cord electroporation with the mammalian BarH1 gene. Ectopic expression resulted in a commissural neuronal phenotype.Right, Rostral; top, dorsal.
▪ Behavioral/Systems/Cognitive
Striatal ΔFosB Accumulation and Drug-Seeking Behavior
Striatal Cell Type-Specific Overexpression of ΔFosB Enhances Incentive for Cocaine
Christina R. Colby, Kim Whisler, Cathy Steffen, Eric J. Nestler, and David W. Self (see pages 2488–2493)
Multiple neuroadaptive mechanisms are thought to underlie the behavioral changes associated with drug addiction. In this issue, Colby et al. examine one potential mechanism. They overexpressed the transcription factor ΔFosB in striatal neurons, which led to sensitization to the incentive properties of cocaine. Normally, substance P–dynorphin-containing striatal neurons accumulate ΔFosB with repeated cocaine use, an effect that increases sensitivity to cocaine. The authors here used an inducible cell-specific transgenic system to overexpress ΔFosB only in these neurons. The mice that overexpressed ΔFosB learned to administer low-threshold doses of cocaine faster and took more injections than control mice, whereas there was no difference at high doses. The authors also measured motivation to continue taking the drug with “progressive ratio testing,” in which the amount of lever pressing required to receive low doses of cocaine steadily increased; the maximum number of presses reflects the effort an animal is willing to invest before giving up. The ΔFosB-expressing mice pressed the lever nearly twice as many times as did controls before quitting. Because the behavioral differences were seen only at low-threshold doses, the authors conclude that ΔFosB specifically sensitizes the reinforcing effects of cocaine, an effect that is perhaps relevant to the regulation of craving and relapse.