Abstract
Although many people drink alcohol regularly, only some become addicted. Several studies have shown that genetic and environmental factors contribute to individual differences in the vulnerability to the effects of alcohol (Nestler, 2000; Kreek, 2001; Crabbe, 2002). Among the environmental factors, stress is perhaps the most important trigger for relapse after a period of abstinence (Koob and Nestler, 1997; Piazza and Le Moal, 1998; Koob and Le Moal, 2001; Weiss et al., 2001). Here we show that ethanol withdrawal symptoms were completely absent in cannabinoid CB1 receptor-deficient mice, although acute effects of ethanol and ethanol tolerance and preference were basically normal. Furthermore, foot-shock stress had no affect on alcohol preference in Cnr1−/− mice, although it induced a dramatic increase in Cnr1+/+ animals. These results reveal a critical role for the CB1 receptor in clinically important aspects of alcohol dependence and provide a rationale for the use of CB1 receptor antagonists in the treatment of alcohol addiction.
Keywords: cannabinoid, ethanol, mice, mutation, withdrawal, addiction
Introduction
Three lines of evidence point to a possible involvement of the cannabinoid CB1 receptor in ethanol effects. First, although the mechanisms of action of ethanol and Δ9-tetrahydrocannabinol (THC), the natural CB1agonist found in Cannabis sativa preparations, are different, they produce a number of similar physiological and behavioral responses, including euphoria, motor incoordination, and hypothermia (Hungund and Basavarajappa, 2000a). Second, alcohol preference and self-administration can be modulated with CB1 receptor agonists and antagonists (Colombo et al., 1998; Gallate et al., 1999; Rodriguez de Fonseca et al., 1999;Hungund and Basavarajappa, 2000a; Lallemand et al., 2001). Finally,Buck et al. (1997) have identified a marker locus (D4Ncvs78) associated with alcohol withdrawal liability on chromosome 4 in close proximity to Cnr1.
We therefore asked whether a deletion of the CB1receptor would alter behavioral or physiological effects of alcohol. Chronic ethanol exposure selectively increased the synthesis of endocannabinoids in cell cultures (Basavarajappa and Hungund, 1999b;Basavarajappa et al., 2000) and in mouse brains (Hungund and Basavarajappa, 2000a). In addition, chronic ethanol treatment resulted in a reduction of CB1 receptor densities and a concomitant decrease in Bmax without any change in G-protein affinity Kd(Basavarajappa et al., 1998; Basavarajappa and Hungund, 1999a). The downregulation of CB1 receptors parallels in many aspects the changes in CB1 expression and signaling properties after chronic treatment with natural or synthetic CB1 agonists, including anandamide (Rodriguez de Fonseca et al., 1994; Basavarajappa and Hungund, 1999a). These homeostatic adaptations of the endocannabinoid system may therefore contribute to many of the physiological and behavioral effects of chronic ethanol exposure, including tolerance and dependence. Indeed, cross-tolerance between ethanol and THC have been reported from many studies in the literature (Hungund and Basavarajappa, 2000a). If this hypothesis were correct, one would expect to see alterations in the development of tolerance and dependence in CB1-deficient mice.
Materials and Methods
Acute ethanol effects, tolerance, and withdrawal.Six- to 8-week-old male Cnr1+/+ and Cnr1−/− mice, with a C57BL/6J genetic background, were used. Animals were housed individually under reversed light–dark conditions (lights on at 7:00 P.M. and lights off at 9:00 A.M.). To determine acute alcohol effects, animals received a single intraperitoneal injection of vehicle (PBS) and 1, 2, or 4 gm/kg ethanol. Body temperatures were measured with a rectal thermometer immediately before and 30 min after the treatment. Another group of animals was trained on a rotarod (4–25 rpm with an acceleration of 1 rpm/sec; Columbus Instruments, Columbus, OH) 10 times daily for 2 d. Animals that did not reach the training criterion (≥30 sec on the rotating rod without falling down, three times successively) were not included in the test. On the third day, the animals received a vehicle or ethanol (2 or 4 gm/kg) injection. Thirty minutes after treatment, we measured the time the animals remained on the rotating rod. To assess ethanol tolerance, animals were supplied with ethanol solutions as their only drinking source as follows: days 1–3, 4% ethanol; days 4–10, 8% ethanol; and days 11–21, 16% ethanol. Ethanol consumption (in grams per kilogram), food consumption, and the body weight were recorded twice per week. After 2 weeks of 16% ethanol drinking, the effect of acute ethanol treatment on the body temperature of the animals was determined again. The treatment procedure was the same as described above. For withdrawal studies, animals received the 16% ethanol solution for 3 more weeks (days 11–41) before it was replaced with water on day 42. Quantification of withdrawal symptoms was made by a person, who was blind to the experimental groups, using handling-induced convulsions performed as described previously (Watson et al., 1994) 3 hr after replacing ethanol with water. The behavioral ratings in response to gentle handling during ethanol withdrawal were as follows: 0, no tremor or convulsion; 1, mild tremor on lifting and turning; 2, continuous severe tremor on lifting and turning; and 3, clonic forelimb extensor spasm on lifting. For statistical analysis, the mean value and SE of the body temperature and of the time the mice spent on the rotarod were calculated. Groups were compared by two-way ANOVA (genotype × treatment), followed by Scheffe post hoctest. The differences between scores were calculated by nonparametric ANOVA, with the Kolmogorov–Smirnov test.
Open-field test. Mice were placed into the center of the open-field apparatus (44 × 44 × 30 cm; Med Associates, Georgia, VT) during the drinking procedure and 3 d after the withdrawal. Movements of the animals were tracked by an automatic monitoring system (Med Associates) for 10 min. Horizontal motor (distance traveled) and central activity (distance traveled in central area/total distance traveled) was evaluated. The experiment was performed under low-light conditions (∼5 lux). Mean value and SE was calculated in each group, which contained 10 animals. Groups were compared by two-way ANOVA (genotype × treatment), followed by the Fisher's test.
Elevated zero maze. Animals were treated with saline or ethanol (2 gm/kg) intraperitoneally in the volume of 10 ml/kg; 9–10 animals were tested in each group. Thirty minutes later, their activity on the zero maze was measured for 5 min. The maze consisted of an annular white platform (inner diameter of 46 cm, 5.6 cm width) elevated 40 cm above the ground level and equally divided into four quadrants. The two opposite quadrants were enclosed by white walls (24 cm high) on both edges of the platform. The behavior of mice was videotaped using a camera fixed above the maze and analyzed with a video-tracking system (Videomot; TSE Systems, Bad Homburg, Germany). The number of stretching postures was determined by an experienced observer unaware to strain or treatment. Time spent in the open area, distance traveled in the open and closed parts, and number of stretching postures were evaluated (Shepherd et al., 1994; Konig et al., 1996). Mean value and SE was calculated in each group, and groups were compared by one-way ANOVA, followed by the Fisher's test
Alcohol preference. Ethanol preference measurements were basically performed as described previously (Little et al., 1999). Briefly, two drinking bottles (with a metal ball in the sipper tubes to stop the dropping of fluids; Cascade 5; Hagen, Holm, Germany) were available to the animals during the experiment. One of these bottles contained 8% v/v alcohol, and the other contained drinking water. The positions of the bottles were changed daily. The ratio of alcohol to total fluid consumption, the amount of consumed ethanol (in grams per kilogram), the body weight, and the food consumption were determined twice per week.
Foot-shock procedure. This procedure was made with the animals that had access to an ethanol solution (8%) ad libitum for >5 weeks and maintained a stable ethanol intake. For the foot-shock stress, animals were kept in a dark chamber during the shock procedure, where a continuous background white noise (65 dB) was present. A few seconds before the shock, a warning signal (sound and light) was presented. Intermittent electric foot shocks (intensity, 0.5 mA; duration, 100 msec; interval between shocks, 55–60 sec) were then delivered five times through the grid floor by an isolated stimulator. The ratio of alcohol to total fluid consumption and the amount of consumed ethanol (in grams per kilogram) was determined 24 and 96 hr after the shock.
c-fos expression. Three brains from each experimental group were analyzed. Brain sections (10 μm) were cut using a cryostat and thaw mounted on Superfrost Plus glass slides (Fisher, Pittsburgh, PA). After drying, the slide-mounted sections were stored at −70°C. The template for c-fos was a murine cDNA (400 bp). Plasmid was linearized to generate either sense or antisense cRNA probes. In situ hybridization was performed as described previously (Campbell and Hess, 1999). Hybridized sections were covered with Kodak NTB emulsion (Eastman Kodak, Rochester, NY) and exposed for 4 weeks. After development, the background were stained in 0.5% Giemsa (Fluka, Neu-Ulm, Germany), and the sections were dried and covered with Cytoseal 60 (Richard-Allan Scientific, Kalamazoo, MI).
Results
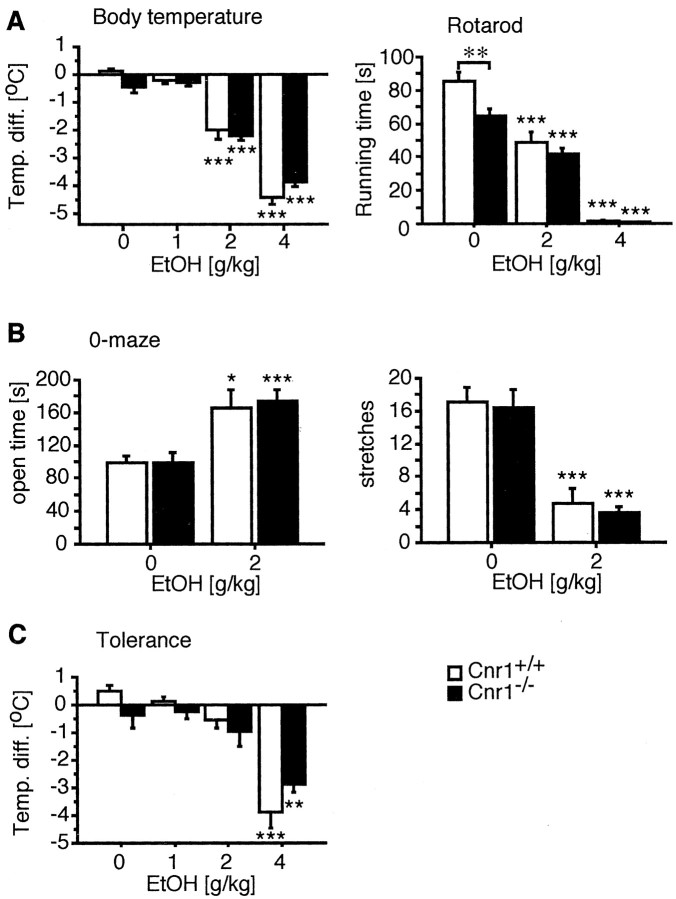
We first evaluated ethanol-induced hypothermia and motor-incoordination after a single intraperitoneal injection of ethanol solutions. As shown in Figure1A, ethanol produced a similar dose-dependent reduction in body temperature in mutant and wild-type animals (treatment effect,F(3,73) = 195.97, p < 0.0001; genotype effect, F(1,73) = 0.57, p = 0.45). In addition, motor coordination, as evaluated on the rotarod, was similarly affected by the ethanol treatment in both genotypes (treatment effect, Cnr1+/+,F(2,36) = 64.8, p ≤ 0.0001; Cnr1−/−,F(2,35) = 79.6, p ≤ 0.0001; treatment × strain,F(2,71) = 2.39, p = 0.0994), although Cnr1−/− mice did not perform as well in this test as Cnr1+/+animals (genotype effect: F(1,71) = 7.6, p ≤ 0.01). Anxiolytic effects of subchronic ethanol treatment (2 gm/kg, i.p.) were determined in the zero-maze test. Animals of both genotypes spent significantly more time in the open sectors after ethanol treatment and showed a reduced number of stretch-attend postures. Thus, the anxiolytic properties of ethanol were not affected by the mutation. (treatment × genotype,F = 0.09, p = 0.767). These results show that the CB1 receptor is not required for these acute ethanol effects.
Fig. 1.
Acute ethanol effects and tolerance are identical in wild-type and CB1 receptor-deficient mice.A, Cnr1+/+ and Cnr1−/− mice showed a similar dose-dependent reduction in body temperature and impairment of motor coordination on the rotarod after intraperitoneal injection of ethanol that was significant at 2 and 4 gm/kg. Interestingly, Cnr1+/+animals performed better in the rotarod test than Cnr1−/− mice (genotype effect,F(1,71) = 7.6, p = 0.0074). This is in contrast to our previous analysis of the mutant phenotype on a mixed (129 × C57BL/6J) genetic background, in which mutant animals showed a tendency toward a reduced running time but never performed significantly different from wild-type controls (Steiner et al., 1999). B, Anxiolytic effects of ethanol were similar in mice from both genotypes. *p≤ 0.05; **p < 0.001. When animals were forced to drink an ethanol solution for a period of 3 weeks (4–16%), before receiving an ethanol injection, only the highest dose tested (4 gm/kg) produced a significant reduction in body temperature.
We next wanted to examine the development of tolerance after chronic ethanol exposure. For this purpose, we restricted the animals to an ethanol solution (4–16%) as their only fluid source for a period of 3 weeks. The total liquid intake of Cnr1−/− and Cnr1+/+ animals was similar at ∼2–2.5 ml/d. Subsequently, we determined the physiological effects of an intraperitoneal ethanol injection by measuring the animals' body temperature. Only the highest dose of 4 gm/kg ethanol produced significant hypothermia in these mice (Fig. 1C), whereas 2 gm/kg were already effective in alcohol-naive animals. However, there was no difference between the two genotypes (treatment effect:,F(3,28) = 32.05, p < 0.0001; genotype effect, F(1,28) = 0.52, p = 0.48; treatment × genotype,F(3,1) = 0.747, p = 0.526) and, thus, ethanol tolerance was not affected by the CB1 deletion.
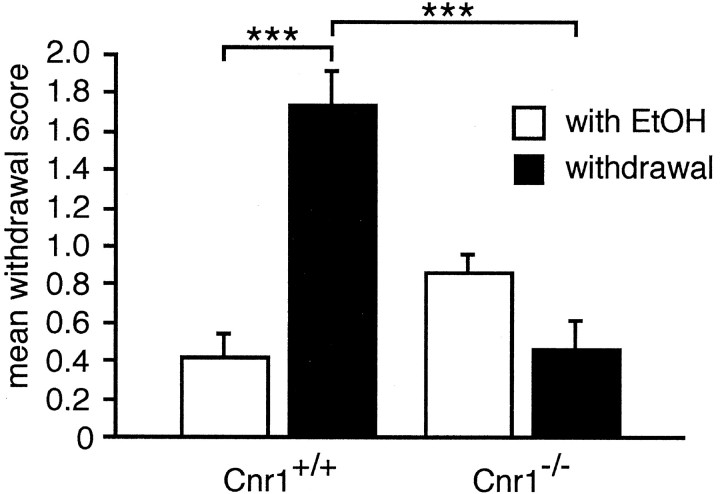
To study ethanol withdrawal symptoms, we restricted mice to a 16% ethanol solution as their only fluids source for 4 weeks and then replaced the ethanol solution with water. Withdrawal symptoms were evaluated 3 hr after replacing the ethanol solution. Although Cnr1+/+ animals displayed severe withdrawal symptoms (mean score, 1.74 ± 0.19; χ2 = 15.2; p = 0.001; Kolmogorov–Smirnov test), we could not detect any signs for ethanol withdrawal in Cnr1−/− animals (mean score, 0.46 ± 0.12; χ2 = 4.2;p = 0.24; Kolmogorov–Smirnov test) (Fig.2). We also measured withdrawal-induced hyperlocomotion in the open field, which is a different symptom for ethanol withdrawal in mice. As expected, Cnr1+/+ animals were significantly hyperactive 3 d after ethanol withdrawal (F(1,17) = 7.10; p = 0.016; ANOVA). However, there was no change in Cnr1−/− mice (F(1,11) = 1.00; p = 0.333; ANOVA). Thus, ethanol withdrawal symptoms were completely absent in Cnr1−/− mice.
Fig. 2.
Ethanol withdrawal symptoms are absent in Cnr1−/− mice. Animals had access to a 16% ethanol solution as their only drinking source for 4 weeks. Cnr1+/+ animals displayed severe withdrawal symptoms 3 hr after replacing the ethanol solution with water, whereas Cnr1−/− animals did not display any withdrawal symptoms. ***p ≤ 0.005; Kolmogorov–Smirnov test.
CB1 receptor agonists and antagonists are known modulators of appetite, food intake, and ethanol preference (Ameri, 1999). We therefore asked whether the preference for ethanol would be altered in the absence of CB1 receptors. When animals were given access ad libitum to either an ethanol solution (8%) or water, Cnr1−/− mice initially showed a significantly higher preference for the ethanol solution than Cnr1+/+ animals (Fig.3A). The absolute amount of ethanol consumed was also higher in Cnr1−/− mice (B). However, both genotypes established a similar stable level of ethanol intake within a few days. After the first week, the Cnr1−/− animals showed the same ethanol preference as Cnr1+/+ mice, and they consumed a similar amount of ethanol until the end of the experiment. There was no difference between the two genotypes in the amount of food consumed, nor was there any genotype difference in the body weight (data not shown).
Fig. 3.
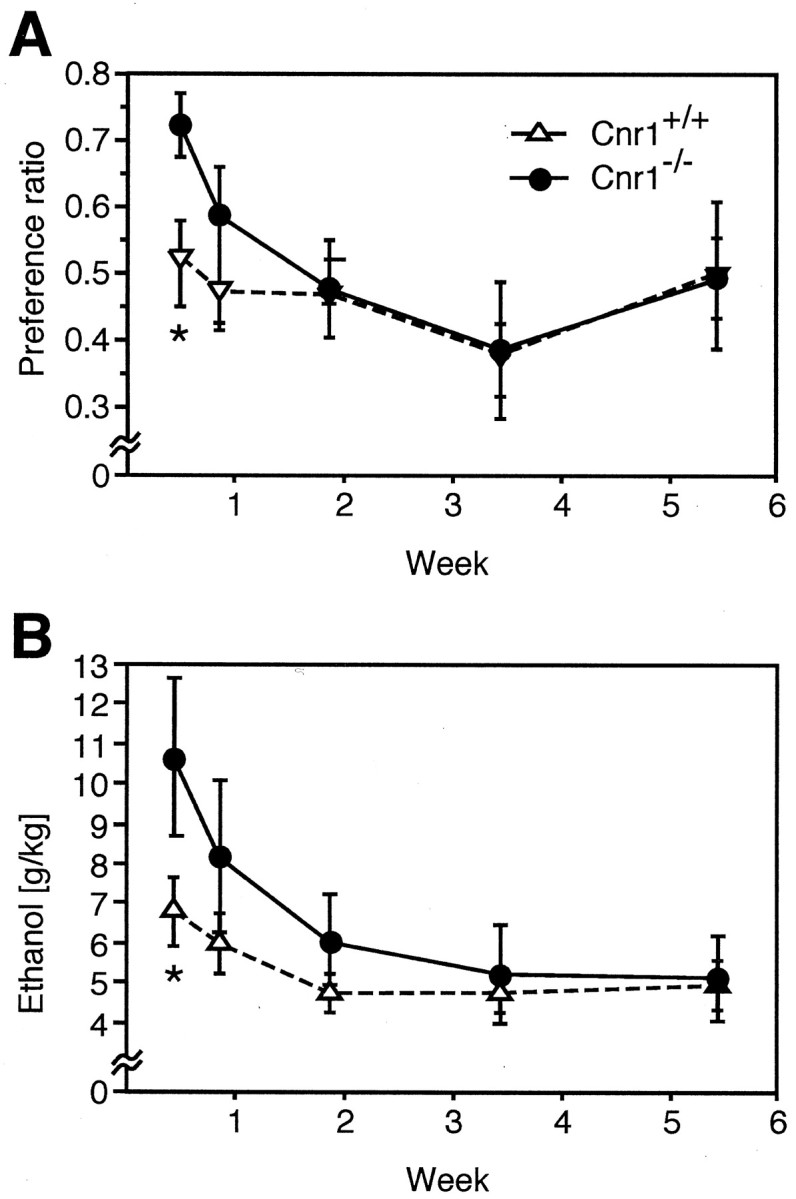
Ethanol drinking behavior in a two-bottle choice paradigm. A, Cnr1−/− mice initially showed a significantly higher preference for the ethanol (8%) solution than Cnr1+/+ animals. B, The absolute amount of ethanol consumed was also higher. Animals of both genotypes established a similar stable level of ethanol intake after the first week. *p ≤ 0.05; ANOVA.
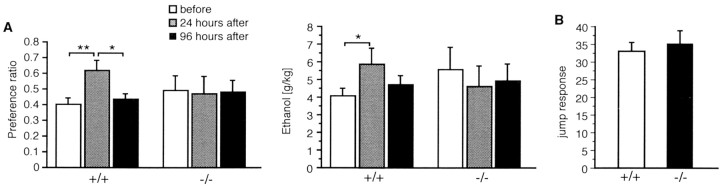
Stress is one of the most important factors known to trigger relapse after a period of abstinence in human patients and in animal models of drug addiction. In rodents, a brief exposure to a mild foot-shock stressor can reinstate drug-seeking behavior or increase ethanol preference. We therefore exposed mice that had access ad libitum to an ethanol solution (8%) for >5 weeks and maintained a stable ethanol intake to a mild 5 min foot shock. As expected, wild-type Cnr1+/+ mice drank more ethanol in the 24 hr period after receiving the foot shocks and also displayed a significant increase in their preference for ethanol during this period (Fig. 4). The stress-induced increase in ethanol preference was transient, because animals returned to prestress levels within 96 hr. In contrast, however, ethanol preference or absolute amount of ethanol consumed by Cnr1−/− mice were totally unaffected by the foot-shock stressor.
Fig. 4.
Absence of stress-induced increase of ethanol drinking in Cnr1−/− mice. Animals had accessad libitum to ethanol for a period of 5 weeks and reached a stable plateau of ethanol consumption. A,left and right, When exposed to a foot-shock stress, Cnr1+/+ control animals displayed a significant transient increase in ethanol preference and in the absolute amount of ethanol consumed. In contrast, Cnr1−/− mice were not affected by the foot-shock stressor. B, Jump responses elicited by the foot shocks were similar in both genotypes. *p ≤ 0.05; ANOVA; **p < 0.001.
We considered the possibility that the level of stress produced by the foot shock induced was different between the two genotypes. We therefore evaluated the amplitude of jump responses after the administration of foot shocks. As shown in Figure 4B, there was no difference in this parameter between Cnr1+/+ and Cnr1−/− mice, indicating that the immediate level of discomfort was similar. We also determined the level of c-fos induction after the foot-shock stressor in different brain regions (Fig. 5). Cnr1+/+ and Cnr1−/− mice showed a robust c-fos expression in the cortex, amygdala, hippocampus, and paraventricular-thalamic nuclei in stressed, but not in control animals. Together these results strongly indicate that the foot-shocks produced similar levels of stress in Cnr1−/− and Cnr1+/+ animals.
Fig. 5.
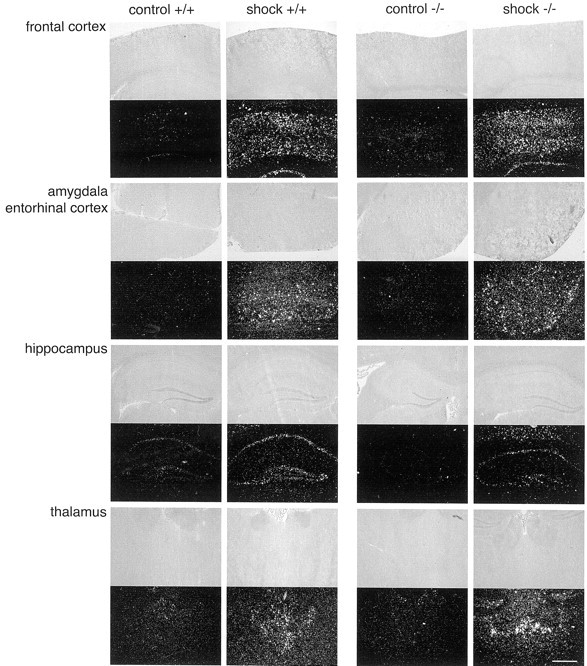
Stress-induced c-fos induction. Representative sections through different brain regions are shown. Cnr1+/+ and Cnr1−/− mice showed similar levels of c-fos induction in the cortex, amygdala, hippocampus, and thalamus.
Discussion
Mice with specific gene deletions have been used recently to investigate the role of the endocannabinoid system in drug reinforcement and addiction. In this manuscript, we analyzed ethanol responses in a mouse strain with a deletion in the cannabinoid CB1 receptor gene Cnr1. Our results demonstrate a crucial role of the CB1 receptor in the physiological manifestation of ethanol dependence and in stress-induced increase of ethanol preference.
The Cnr1 mutation was examined on a genetic C57BL/6J genetic background. Mice with this genetic background are known for their voluntary consumption of alcoholic solutions (McClearn and Rodgers, 1961; Crabbe and Belknap, 1980). Unlike mice from many other genetic backgrounds, C57BL/6J animals will readily drink ethanol solutions when these are presented together with regular tap water in a two-bottle choice paradigm. Surprisingly, we found that Cnr1−/− mice had initially an even higher preference for ethanol than C57BL/6J mice. This result was unexpected, because previous pharmacological studies suggested that blocking the CB1 receptor with the selective antagonist SR141716A reduced ethanol consumption. However, the interpretation of results obtained with this compound have been difficult, because SR141716A has a well known reverse agonist activity. In addition, [3H]GTPγS binding studies on Cnr1−/− brain tissues suggested that SR141716A may have activity on a still unidentified “CB3” receptor (Breivogel et al., 2001). Of course, it is also possible that the acute blockade of the receptor produces different effects than the continuous removal through the genetic ablation. In fact, a recent study has revealed differential effects of SR141716A treatment of ethanol preference, depending on the treatment regimen (Lallemand et al., 2001). The increased preference of Cnr1−/− mice for ethanol was only significant during the first days. After 1 week, the ethanol consumption was virtually identical between Cnr1+/+ and Cnr1−/− mice. Together, these results indicate that the endocannabinoid system is not a critical mediator of normal alcohol drinking behavior, although it may modulate behavioral responses to the first contact with ethanol.
A completely different picture emerged when mice, which had established a stable plateau of ethanol drinking, were exposed to a mild intermittent foot-shock stress. As expected, alcohol consumption increased significantly in Cnr1+/+ mice for a brief period after the stress, whereas Cnr1−/− mice showed no change in their ethanol preference. We can exclude the possibility that the foot shock was less stressful for Cnr1−/− animals, because (1) Cnr1−/− and Cnr1+/+ mice exhibited similar jumping responses and (2) c-fos induction through the foot-shock stress was similar in both genotypes. Furthermore, two Cnr1−/− animals, but none of the Cnr1+/+ mice, died shortly after the foot-shock stress. We often noticed an increased mortality in Cnr1−/− mice (Zimmer et al., 1999), especially when circumstances dictated higher stress levels (e.g., tape testing for pinworm infections and construction work in the animal facility). The cause of the deaths of these animals remains unclear but may involve epileptic seizures. Previous studies using an independently derived Cnr1−/− strain on an outbred genetic background have also indicated that Cnr1−/− mice are more emotional than Cnr1+/+ animals (Martin et al., 2002), although these animals exhibited reduced analgesia after a swim stress (Valverde et al., 2000). In addition, the acquisition and consolidation of conditioned auditory freezing behaviors after foot-shock stimulation was unaltered in a third independent Cnr1 knock-out strain (Marsicano et al., 2002).
Numerous clinical studies have revealed a general correlation between stress and drug relapse. Stressful life events in childhood (Simantov et al., 2000), daily job problems in adulthood (Delaney et al., 2002), and experiences like the September 11th terror attack (Vlahov et al., 2002) each may increase the risk for alcohol drinking and a concomitantly increased risk for alcoholism. Reinstatement of drug-seeking behaviors through intermittent foot-shock stress has been demonstrated for a number of drugs, including heroin (Shaham and Stewart, 1995), cocaine (Erb et al., 1996), nicotine (Buczek et al., 1999), and alcohol (Le et al., 1998), suggesting a drug-independent effect of the stressor. Although the molecular and cellular events underlying stress-induced reinstatement are primarily obscure, corticotropin-releasing factor, as well as dopamine-dependent and dopamine-independent mechanisms, have been implicated (Self and Nestler, 1998; Le and Shaham, 2002; Leri et al., 2002). There is almost no previous evidence for a genetic predisposition to stress-induced relapse, except for one study that demonstrated enhanced stress-induced ethanol drinking in corticotrophin-releasing hormone-1 receptor-deficient mice (Sillaber et al., 2002).
Our second important finding is the demonstration that withdrawal symptoms after the cessation of chronic ethanol administration were completely absent in CB1 knock-out mice. To our knowledge, this is the first direct evidence for an involvement of the endocannabinoid system in ethanol withdrawal. Importantly, however, the analysis of recombinant inbred strains for ethanol withdrawal severity lead to the identification of a quantitative trait locus (QTL) on chromosome 4 in close proximity to Cnr1 (Buck et al., 1997), which may be independent from another distal locus on the same chromosome (Fehr et al., 2002). It is well known that CB1 receptor densities and CB1 receptor agonist-stimulated [35S]GTPγS binding differ significantly between the parental alcohol-preferring C57BL/6 and alcohol-avoiding DBA/2J strains (Hungund and Basavarajappa, 2000b;Basavarajappa and Hungund, 2001). Therefore, it seems possible that the Cnr1 locus accounts for this QTL. Interestingly, a recent clinical study also associated a Cnr1 gene polymorphism with the severity of withdrawal symptoms in humans (Schmidt et al., 2002).
Ethanol withdrawal symptoms may involve homeostatic changes of the endocannabinoid system, including an increased synthesis of endocannabinoids and a concomitant downregulation of CB1 receptor binding sites (Basavarajappa and Hungund, 2002; Gonzalez et al., 2002). The endocannabinoid system may play a general role in the manifestation of physiological drug dependence, because a significant reduction of withdrawal symptoms was also observed in CB1-deficient mice after morphine withdrawal (Ledent et al., 1999).
In summary, the endocannabinoid system does not seem to be crucial for the rewarding effects of ethanol and the manifestation of normal ethanol drinking behaviors. However, it appears to play an important role in the manifestation of stress-induced alcohol drinking and ethanol withdrawal. These results support the notion that the neuronal mechanisms involved in drug reinforcement are dissociable from those involved in withdrawal and reinstatement (Shalev et al., 2002). Indeed, our results demonstrate that ethanol tolerance and physical dependence can be separated. Thus, CB1 receptor antagonists may be useful for the treatment of alcohol addiction.
Footnotes
This work was supported by grants from the Land Nordrhein Westphalen (Innovationsprogramm Forschung), the Deutsche Forschungsgemeinschaft (Sonderforschungsbereich 400), and the German National Genome Network. We thank members of the laboratory for suggestions on the experiments and this manuscript.
Correspondence should be addressed to Andreas Zimmer, Laboratory of Molecular Neurobiology, Clinic of Psychiatry, University of Bonn, Sigmund-Freud-Strasse 25, 53125 Bonn, Germany. E-mail:neuro@uni-bonn.de.
References
- 1.Ameri A. The effects of cannabinoids on the brain. Prog Neurobiol. 1999;58:315–348. doi: 10.1016/s0301-0082(98)00087-2. [DOI] [PubMed] [Google Scholar]
- 2.Basavarajappa BS, Hungund BL. Down-regulation of cannabinoid receptor agonist-stimulated [35S]GTP gamma S binding in synaptic plasma membrane from chronic ethanol exposed mouse. Brain Res. 1999a;815:89–97. doi: 10.1016/s0006-8993(98)01072-5. [DOI] [PubMed] [Google Scholar]
- 3.Basavarajappa BS, Hungund BL. Chronic ethanol increases the cannabinoid receptor agonist anandamide and its precursor N-arachidonoylphosphatidylethanolamine in SK-N-SH cells. J Neurochem. 1999b;72:522–528. doi: 10.1046/j.1471-4159.1999.0720522.x. [DOI] [PubMed] [Google Scholar]
- 4.Basavarajappa BS, Hungund BL. Cannabinoid receptor agonist-stimulated [35S]guanosine triphosphate gammaS binding in the brain of C57BL/6 and DBA/2 mice. J Neurosci Res. 2001;64:429–436. doi: 10.1002/jnr.1094. [DOI] [PubMed] [Google Scholar]
- 5.Basavarajappa BS, Hungund BL. Neuromodulatory role of the endocannabinoid signaling system in alcoholism: an overview. Prostaglandins Leukot Essent Fatty Acids. 2002;66:287–299. doi: 10.1054/plef.2001.0352. [DOI] [PubMed] [Google Scholar]
- 6.Basavarajappa BS, Cooper TB, Hungund BL. Chronic ethanol administration down-regulates cannabinoid receptors in mouse brain synaptic plasma membrane. Brain Res. 1998;793:212–218. doi: 10.1016/s0006-8993(98)00175-9. [DOI] [PubMed] [Google Scholar]
- 7.Basavarajappa BS, Saito M, Cooper TB, Hungund BL. Stimulation of cannabinoid receptor agonist 2-arachidonylglycerol by chronic ethanol and its modulation by specific neuromodulators in cerebellar granule neurons. Biochim Biophys Acta. 2000;1535:78–86. doi: 10.1016/s0925-4439(00)00085-5. [DOI] [PubMed] [Google Scholar]
- 8.Breivogel CS, Griffin G, Di Marzo V, Martin BR. Evidence for a new G protein-coupled cannabinoid receptor in mouse brain. Mol Pharmacol. 2001;60:155–163. [PubMed] [Google Scholar]
- 9.Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buczek Y, Le AD, Stewart J, Shaham Y. Stress reinstates nicotine seeking but not sucrose solution seeking in rats. Psychopharmacology (Berl) 1999;144:183–188. doi: 10.1007/s002130050992. [DOI] [PubMed] [Google Scholar]
- 11.Campbell DB, Hess EJ. L-type calcium channels contribute to the tottering mouse dystonic episodes. Mol Pharmacol. 1999;55:23–31. doi: 10.1124/mol.55.1.23. [DOI] [PubMed] [Google Scholar]
- 12.Colombo G, Agabio R, Fa M, Guano L, Lobina C, Loche A, Reali R, Gessa GL. Reduction of voluntary ethanol intake in ethanol-preferring sP rats by the cannabinoid antagonist SR-141716. Alcohol Alcohol. 1998;33:126–130. doi: 10.1093/oxfordjournals.alcalc.a008368. [DOI] [PubMed] [Google Scholar]
- 13.Crabbe JC. Genetic contributions to addiction. Annu Rev Psychol. 2002;53:435–462. doi: 10.1146/annurev.psych.53.100901.135142. [DOI] [PubMed] [Google Scholar]
- 14.Crabbe JC, Belknap JK. Pharmacogenetic tools in the study of drug tolerance and dependence. Subst Alcohol Actions Misuse. 1980;1:385–413. [PubMed] [Google Scholar]
- 15.Delaney WP, Grube JW, Greiner B, Fisher JM, Ragland DR. Job stress, unwinding and drinking in transit operators. J Stud Alcohol. 2002;63:420–429. doi: 10.15288/jsa.2002.63.420. [DOI] [PubMed] [Google Scholar]
- 16.Erb S, Shaham Y, Stewart J. Stress reinstates cocaine-seeking behavior after prolonged extinction and a drug-free period. Psychopharmacology (Berl) 1996;128:408–412. doi: 10.1007/s002130050150. [DOI] [PubMed] [Google Scholar]
- 17.Fehr C, Shirley RL, Belknap JK, Crabbe JC, Buck KJ. Congenic mapping of alcohol and pentobarbital withdrawal liability loci to a <1 cM interval of murine chromosome 4: identification of Mpdz as a candidate gene. J Neurosci. 2002;22:3730–3738. doi: 10.1523/JNEUROSCI.22-09-03730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gallate JE, Saharov T, Mallet PE, McGregor IS. Increased motivation for beer in rats following administration of a cannabinoid CB1 receptor agonist. Eur J Pharmacol. 1999;370:233–240. doi: 10.1016/s0014-2999(99)00170-3. [DOI] [PubMed] [Google Scholar]
- 19.Gonzalez S, Fernandez-Ruiz J, Sparpaglione V, Parolaro D, Ramos JA. Chronic exposure to morphine, cocaine or ethanol in rats produced different effects in brain cannabinoid CB(1) receptor binding and mRNA levels. Drug Alcohol Depend. 2002;66:77–84. doi: 10.1016/s0376-8716(01)00186-7. [DOI] [PubMed] [Google Scholar]
- 20.Hungund BL, Basavarajappa BS. Are anandamide and cannabinoid receptors involved in ethanol tolerance? A review of the evidence. Alcohol Alcohol. 2000a;35:126–133. doi: 10.1093/alcalc/35.2.126. [DOI] [PubMed] [Google Scholar]
- 21.Hungund BL, Basavarajappa BS. Distinct differences in the cannabinoid receptor binding in the brain of C57BL/6 and DBA/2 mice, selected for their differences in voluntary ethanol consumption. J Neurosci Res. 2000b;60:122–128. doi: 10.1002/(SICI)1097-4547(20000401)60:1<122::AID-JNR13>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 22.Konig M, Zimmer AM, Steiner H, Holmes PV, Crawley JN, Brownstein MJ, Zimmer A. Pain responses, anxiety and aggression in mice deficient in pre-proenkephalin. Nature. 1996;383:535–538. doi: 10.1038/383535a0. [DOI] [PubMed] [Google Scholar]
- 23.Koob GF, Le Moal M. Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology. 2001;24:97–129. doi: 10.1016/S0893-133X(00)00195-0. [DOI] [PubMed] [Google Scholar]
- 24.Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 25.Kreek MJ. Drug addictions. Molecular and cellular endpoints. Ann NY Acad Sci. 2001;937:27–49. [PubMed] [Google Scholar]
- 26.Lallemand F, Soubrie PH, De Witte PH. Effects of CB1 cannabinoid receptor blockade on ethanol preference after chronic ethanol administration. Alcohol Clin Exp Res. 2001;25:1317–1323. [PubMed] [Google Scholar]
- 27.Le A, Shaham Y. Neurobiology of relapse to alcohol in rats. Pharmacol Ther. 2002;94:137. doi: 10.1016/s0163-7258(02)00200-0. [DOI] [PubMed] [Google Scholar]
- 28.Le AD, Quan B, Juzytch W, Fletcher PJ, Joharchi N, Shaham Y. Reinstatement of alcohol-seeking by priming injections of alcohol and exposure to stress in rats. Psychopharmacology (Berl) 1998;135:169–174. doi: 10.1007/s002130050498. [DOI] [PubMed] [Google Scholar]
- 29.Ledent C, Valverde O, Cossu G, Petitet F, Aubert JF, Beslot F, Bohme GA, Imperato A, Pedrazzini T, Roques BP, Vassart G, Fratta W, Parmentier M. Unresponsiveness to cannabinoids and reduced addictive effects of opiates in CB1 receptor knockout mice. Science. 1999;283:401–404. doi: 10.1126/science.283.5400.401. [DOI] [PubMed] [Google Scholar]
- 30.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Little HJ, O'Callaghan MJ, Butterworth AR, Wilson J, Cole J, Watson WP. Low alcohol preference among the “high alcohol preference” C57 strain of mice; preference increased by saline injections. Psychopharmacology (Berl) 1999;147:182–189. doi: 10.1007/s002130051159. [DOI] [PubMed] [Google Scholar]
- 32.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgansberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 33.Martin M, Ledent C, Parmentier M, Maldonado R, Valverde O. Involvement of CB1 cannabinoid receptors in emotional behaviour. Psychopharmacology (Berl) 2002;159:379–387. doi: 10.1007/s00213-001-0946-5. [DOI] [PubMed] [Google Scholar]
- 34.McClearn G, Rodgers D. Genetic factors in alcohol preference of laboratory mice. J Comp Physiol Psychol. 1961;54:116–119. [Google Scholar]
- 35.Nestler EJ. Genes and addiction. Nat Genet. 2000;26:277–281. doi: 10.1038/81570. [DOI] [PubMed] [Google Scholar]
- 36.Piazza PV, Le Moal M. The role of stress in drug self-administration. Trends Pharmacol Sci. 1998;19:67–74. doi: 10.1016/s0165-6147(97)01115-2. [DOI] [PubMed] [Google Scholar]
- 37.Rodriguez de Fonseca F, Gorriti MA, Fernandez-Ruiz JJ, Palomo T, Ramos JA. Downregulation of rat brain cannabinoid binding sites after chronic delta 9-tetrahydrocannabinol treatment. Pharmacol Biochem Behav. 1994;47:33–40. doi: 10.1016/0091-3057(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 38.Rodriguez de Fonseca F, Roberts AJ, Bilbao A, Koob GF, Navarro M. Cannabinoid receptor antagonist SR141716A decreases operant ethanol self administration in rats exposed to ethanol-vapor chambers. Zhongguo Yao Li Xue Bao. 1999;20:1109–1114. [PubMed] [Google Scholar]
- 39.Schmidt LG, Samochowiec J, Finckh U, Fiszer-Piosik E, Horodnicki J, Wendel B, Rommelspacher H, Hoehe MR. Association of a CB1 cannabinoid receptor gene (CNR1) polymorphism with severe alcohol dependence. Drug Alcohol Depend. 2002;65:221–224. doi: 10.1016/s0376-8716(01)00164-8. [DOI] [PubMed] [Google Scholar]
- 40.Self DW, Nestler EJ. Relapse to drug-seeking: neural and molecular mechanisms. Drug Alcohol Depend. 1998;51:49–60. doi: 10.1016/s0376-8716(98)00065-9. [DOI] [PubMed] [Google Scholar]
- 41.Shaham Y, Stewart J. Stress reinstates heroin-seeking in drug-free animals: an effect mimicking heroin, not withdrawal. Psychopharmacology (Berl) 1995;119:334–341. doi: 10.1007/BF02246300. [DOI] [PubMed] [Google Scholar]
- 42.Shalev U, Grimm JW, Shaham Y. Neurobiology of relapse to heroin and cocaine seeking: a review. Pharmacol Rev. 2002;54:1–42. doi: 10.1124/pr.54.1.1. [DOI] [PubMed] [Google Scholar]
- 43.Shepherd JK, Grewal SS, Fletcher A, Bill DJ, Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl) 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 44.Sillaber I, Rammes G, Zimmermann S, Mahal B, Zieglgansberger W, Wurst W, Holsboer F, Spanagel R. Enhanced and delayed stress-induced alcohol drinking in mice lacking functional CRH1 receptors. Science. 2002;296:931–933. doi: 10.1126/science.1069836. [DOI] [PubMed] [Google Scholar]
- 45.Simantov E, Schoen C, Klein JD. Health-compromising behaviors: why do adolescents smoke or drink?: identifying underlying risk and protective factors. Arch Pediatr Adolesc Med. 2000;154:1025–1033. doi: 10.1001/archpedi.154.10.1025. [DOI] [PubMed] [Google Scholar]
- 46.Steiner H, Bonner TI, Zimmer AM, Kitai ST, Zimmer A. Altered gene expression in striatal projection neurons in CB1 cannabinoid receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5786–5790. doi: 10.1073/pnas.96.10.5786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Valverde O, Ledent C, Beslot F, Parmentier M, Roques BP. Reduction of stress-induced analgesia but not of exogenous opioid effects in mice lacking CB1 receptors. Eur J Neurosci. 2000;12:533–539. doi: 10.1046/j.1460-9568.2000.00929.x. [DOI] [PubMed] [Google Scholar]
- 48.Vlahov D, Galea S, Resnick H, Ahern J, Boscarino JA, Bucuvalas M, Gold J, Kilpatrick D. Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacks. Am J Epidemiol. 2002;155:988–996. doi: 10.1093/aje/155.11.988. [DOI] [PubMed] [Google Scholar]
- 49.Watson WP, Misra A, Cross AJ, Green AR, Little HJ. The differential effects of felodipine and nitrendipine on cerebral dihydropyridine binding ex vivo and the ethanol withdrawal syndrome in mice. Br J Pharmacol. 1994;112:1017–1024. doi: 10.1111/j.1476-5381.1994.tb13184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Weiss F, Ciccocioppo R, Parsons LH, Katner S, Liu X, Zorrilla EP, Valdez GR, Ben-Shahar O, Angeletti S, Richter RR. Compulsive drug-seeking behavior and relapse. Neuroadaptation, stress, and conditioning factors. Ann NY Acad Sci. 2001;937:1–26. doi: 10.1111/j.1749-6632.2001.tb03556.x. [DOI] [PubMed] [Google Scholar]
- 51.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]