Abstract
PTPζ/RPTPβ, a receptor-type protein tyrosine phosphatase synthesized as a chondroitin sulfate (CS) proteoglycan, uses a heparin-binding growth factor pleiotrophin (PTN) as a ligand, in which the CS portion plays an essential role in ligand binding. Using an organotypic slice culture system, we tested the hypothesis that PTN-PTPζ signaling is involved in the morphogenesis of Purkinje cell dendrites. An aberrant morphology of Purkinje cell dendrites such as multiple and disoriented primary dendrites was induced in slice cultures by (1) addition of a polyclonal antibody against the extracellular domain of PTPζ, (2) inhibition of protein tyrosine phosphatase activity, (3) enzymatic removal of the CS chains, (4) addition of exogenous CS chains, and (5) addition of exogenous PTN, all of which disturb PTN-PTPζ signaling. These treatments also reduced the immunoreactivity to GLAST, a glial glutamate transporter, on Bergmann glial processes. Furthermore, a glutamate transporter inhibitor also induced the abnormal morphogenesis of Purkinje cell dendrites. Altogether, these findings suggest that PTN-PTPζ signaling regulates the morphogenesis of Purkinje cell dendrites and that the mechanisms underlying that regulation involve the GLAST activity in Bergmann glial processes.
Keywords: PTPζ/RPTPβ, pleiotrophin, GLAST, Purkinje cell, dendritic morphogenesis, cerebellum, organotypic slice culture
Introduction
Neurons are characterized by the specific morphology of dendritic trees and axons, which are essential for information processing. Although the molecular mechanisms of directed axonal outgrowth are beginning to be elucidated, those underlying the morphogenesis of dendritic trees are poorly understood. Among the neurons of the CNS, the cerebellar Purkinje cells have the most elaborate dendritic trees. Mature Purkinje cells have a single primary dendrite, which extends toward the pial surface, branches extensively in the molecular layer (ML), and makes synaptic contacts with parallel fibers, the axons of granule cells. The presence and differentiation of granule cells are necessary for normal development of Purkinje cell dendrites, as shown in agranular cerebella of x-irradiated and mutant animals (Altman and Anderson, 1972; Rakic and Sidman, 1973; Sotelo, 1975; Berry et al., 1978). Coculture experiments using dissociated Purkinje cells and granule cells clearly indicated that the granule–Purkinje cell interaction plays a crucial role in the branching and thickening of the Purkinje cell dendrites (Baptista et al., 1994; Hirai and Launey, 2000). It has been suggested that granule cells exert trophic effects on Purkinje cells by providing neurotrophic substances and electrical activity (Schwartz et al., 1997; Hirai and Launey, 2000). Although Purkinje cells displayed stimulated growth of dendrites in such coculture systems, most of them had multiple primary dendrites extending in various directions in contrast to Purkinje cellsin vivo having a single primary dendrite extending in only one direction. This suggests that the polarity of Purkinje cells is determined by alternative mechanisms. Recently, Yamada et al. (2000)found that the lamellate processes of Bergmann glia surrounded the differentiating dendritic trees of Purkinje cells, and more importantly, the growing tips of Purkinje cell dendrites entered the external granular layer (EGL) by contacting the rod-like processes of Bergmann glia. These observations suggest that the Bergmann glia–Purkinje cell interaction is involved in the directed growth and determination of polarity of Purkinje cell dendrites.
Phosphacan/6B4 proteoglycan, a chondroitin sulfate (CS) proteoglycan expressed predominantly in the CNS, is distributed around the cell surface of Purkinje cells during dendritic outgrowth (Maeda et al., 1992). Phosphacan corresponds to the extracellular domain of PTPζ/RPTPβ, a receptor-type protein tyrosine phosphatase composed of an N-terminal carbonic anhydrase-like domain, a fibronectin type III domain, a serine-, glycine-rich domain, a transmembrane segment, and two intracellular tyrosine phosphatase domains (Maurel et al., 1994;Peles et al., 1998). Phosphacan and the transmembrane-type molecules are generated by alternative splicing, and all of the splice variants are synthesized as CS proteoglycans (Maurel et al., 1994; Nishiwaki et al., 1998; Peles et al., 1998). Pleiotrophin (PTN) and midkine (MK), closely related heparin-binding growth factors, bind to PTPζ/phosphacan with high affinity and trigger signal transduction of this receptor (Maeda et al., 1996, 1999). The CS portion of PTPζ/phosphacan plays an essential role in binding to PTN and MK, and the removal of CS chains from PTPζ/phosphacan resulted in a marked decrease of the binding affinity to PTN and MK and in the loss of signal transduction (Maeda et al., 1996, 1999; Qi et al., 2001). Although Purkinje cells and Bergmann glia express PTPζ/phosphacan (Canoll et al., 1993; Snyder et al., 1996), PTN and MK distribute along Bergmann glial fibers in postnatally developing cerebellum (Matsumoto et al., 1994; Wewetzer et al., 1995). These expression patterns suggest that PTN/MK secreted by Bergmann glia binds with PTPζ on Purkinje cells or Bergmann glia, or both. Thus, the signaling of PTPζ/phosphacan and PTN/MK could be involved in cell–cell interaction between Purkinje cells and Bergmann glia.
In this study, we hypothesized that PTN-PTPζ signaling is involved in the Bergmann glia–Purkinje cell interaction required for the morphogenesis of Purkinje cell dendrites. To test this hypothesis, we used organotypic slice cultures of postnatal rat cerebellum, which preserve the cytoarchitecture of the cerebellar cortex and reproduce the series of processes in cerebellar cortical development (Tanaka et al., 1994). Using this system, we found that the perturbation of PTN-PTPζ signaling resulted in a marked increase in the number of Purkinje cells with abnormal dendrites, such as multiple and disoriented primary dendrites, showing that PTN-PTPζ signaling is involved in the morphogenesis of Purkinje cell dendrites. Furthermore, we obtained evidence suggesting that Bergmann glia play important roles in these mechanisms.
Materials and Methods
Slice culture. The methods for slice culture have been described previously (Tanaka et al., 1994). In brief, cerebella were dissected from 9-d-old Wistar rats. The vermes of the cerebella were cut parasagittally into ∼600-μm-thick slices in calcium- and magnesium-free PBS (CMF-PBS). The slices were mounted on a collagen-coated, porous (2.0 μm) polycarbonate membrane (Nuclepore; Whatman, Clifton, NJ) that was floated at the interface between the air and culture medium in a Petri dish (“interface” culture technique) (Freshney, 1987; Yamamoto et al., 1989; Stoppini et al., 1991; Tanaka et al., 1994). The culture medium consisted of 15% heat-inactivated horse serum (Invitrogen, Grand Island, NY), 25% Earle's balanced salt solution, 60% Eagle's basal medium, 5.6 gm/l glucose, 3 mml-glutamine, 5 μg/ml bovine insulin, 5 μg/ml human transferrin, 30 nm sodium selenite, 20 nm progesterone, 1 mmsodium pyruvate, 50 U/ml penicillin G potassium, and 100 μg/ml streptomycin sulfate. The rabbit polyclonal antibody (α6B4PG) against the extracellular domain of PTPζ (PTPζ-ECD) has been described previously (Maeda et al., 1996). The other reagents added to the culture medium were purchased as follows: rabbit IgG, fromChemicon (Temecula, CA); sodium orthovanadate, from Wako Pure Chemicals (Osaka, Japan); chondroitinase ABC (Chase ABC; protease free), CS-C and CS-D from shark cartilage, CS-E from squid cartilage, and CS-A from whale cartilage, from Seikagaku (Tokyo, Japan); recombinant human PTN, from Sigma (St. Louis, MO); anddl-threo-β-benzyloxyaspartate (dl-TBOA), from Tocris (Bristol, UK). Chase ABC was dissolved (6 U/ml) in 60 mm sodium acetate, pH 7.5, 80 mm sodium chloride, and bovine serum albumin (1 mg/ml), and stored as frozen aliquots. These reagents were added at 1 d in vitro (DIV). The cultures were incubated at 33°C in 5% CO2/95% air. Because a large number of cells degenerated in the bottom part (medium side) of the slice cultures, we analyzed the top half (air side) to obtain data in the present study.
Analysis of the Purkinje cell morphology. For immunohistochemistry of inositol 1,4,5-trisphosphate receptor (IP3R), cerebellar slice cultures were fixed with 4% paraformaldehyde in CMF-PBS for 20 min at room temperature. For analysis of the cerebellum in vivo, 250-μm-thick slices were prepared from the vermes of the cerebella of 9- or 15-d-old rats and fixed as mentioned above. The slices were preincubated for 30 min in 10% normal goat serum and 0.3% Triton X-100 in CMF-PBS and then incubated overnight at 4°C in CMF-PBS containing a rat anti-mouse IP3R monoclonal antibody (4C11; 1:20) (Maeda et al., 1989). The immunoreactivity was visualized using a Cy3-conjugated goat anti-rat IgG antibody and examined under a confocal laser scanning microscope (LSM510; Zeiss, Oberkochen, Germany). For imaging of Purkinje cells, we used a 63× water-immersion objective (numerical aperture = 0.9; Achroplan Water;Zeiss) and projected five to eight optical sections of 3 μm thickness to make one stacked image. For analysis of the morphology of primary dendrites, we used three to four slices from three independent experiments in each experimental condition and randomly selected 222–300 (in vivo) and 32–63 (slice cultures) Purkinje cells per slice [total 707–729 (in vivo) and 126–205 (slice cultures) cells per condition]. For statistical analysis of the ratios of the three types of Purkinje cells (see Results), the repeated measures ANOVA was used.
Immunohistochemistry of cryosections. For immunohistochemical analysis of IP3R, PTPζ, GLAST, PTN, CS, neuronal nuclei (NeuN), vesicular glutamate transporter 1 (VGLUT1), and glial fibrillary acidic protein (GFAP), cerebellar slices before or after culture were fixed with 4% paraformaldehyde in CMF-PBS for 20 min at room temperature, frozen in liquid nitrogen, and sectioned at 12 μm on a cryostat. The sections were preincubated for 30 min at room temperature in 10% normal goat serum in CMF-PBS with or without 0.3% Triton X-100, and then incubated overnight at 4°C in CMF-PBS containing a rat anti-IP3R monoclonal antibody (4C11; 1:20), a rabbit anti-PTPζ-ECD antibody (α6B4PG; 20 μg/ml), a mouse monoclonal antibody against the intracellular domain of PTPζ (PTPζ-ICD) (Transduction Laboratories, Lexington, KY; 1:100), a rabbit anti-GLAST antibody (Abcam, Cambridge, MA; 1:600), a guinea pig anti-GLAST antibody (Chemicon; 1:8000), a goat anti-PTN antibody (N-15; Santa Cruz Biotechnology, Santa Cruz, CA; 1:400), a mouse anti-CS monoclonal antibody (CS-56; Sigma; 1:800), a mouse anti-NeuN monoclonal antibody (Chemicon; 1:600), a guinea pig anti-VGLUT1 antibody (Chemicon; 1:10,000), or a rabbit anti-GFAP antibody (Immunon, Shandon, Pittsburgh, PA; 1:1000). For pretreatment with Chase ABC before PTN immunohistochemistry, the sections were incubated for 40 min at 37°C in CMF-PBS containing 20 mU/ml Chase ABC. The immunoreactivities to IP3R, PTPζ-ECD, GLAST (rabbit antibody), NeuN, and GFAP were visualized using Cy3-, Cy2-, or Cy5-conjugated secondary antibodies. The immunoreactivities to PTPζ-ICD, GLAST (guinea pig antibody), and PTN were visualized with a tyramide signal amplification system (TSA-Indirect; NEN Life Science Products, Boston, MA) and Cy2-conjugated streptavidin. Images for fluorescent microscopy were acquired with a confocal laser scanning microscope (LSM510;Zeiss). In some cases of PTN immunohistochemistry, the signals were chromogenically visualized with 3-amino-9-ethylcarbazole (AEC). The immunoreactivities to CS and VGLUT1 were visualized by the streptavidin–biotin affinity method and development with AEC.
Quantitative analysis of Purkinje and granule cells and Bergmann fibers. For measurement of the length of the longest dendrite per cell and counting the number of dendritic branching points per cell, the stacked confocal microscopic images of Purkinje cells obtained as described above were analyzed using the LSM Ver. 2.8 software (Zeiss). For evaluation of the density of Purkinje and granule cells, cryosections of slice cultures were stained by immunohistochemistry using antibodies against IP3R and NeuN as described above. The IP3R- and NeuN-positive cells within 150 μm of the Purkinje cell layer (PL) and internal granular layer (IGL) were enumerated to determine the density of Purkinje and granule cells, respectively, in confocal microscopic images of 2-μm-thick optical sections. As another type of evaluation of granule cell density, the number of NeuN-positive cells within square areas of 100 × 100 μm2 of the IGL was counted. We did these two types of evaluation of granule cell density because migration of these cells from the EGL to the IGL might result in an increase of only one of the number of granule cells per unit length of the IGL and that per unit area of the IGL in slice cultures. When counting the NeuN-positive cells, the cells >10 μm in diameter (<0.1% of total NeuN-positive cells in the IGL) were not included because they might be Golgi cells. For evaluation of Bergmann fibers, GFAP-positive fibers >30 μm within 150 μm of the ML were enumerated in confocal microscopic images of 3-μm-thick optical sections. Statistical analysis was done using Student's t test.
Western blotting. Slice culture and treatment with Chase ABC or CS chains were done as described above. Three cultured slices at 3 DIV were combined and rapidly frozen on dry ice. The frozen slices were homogenized in 200 μl of 1% NP-40, 0.1% SDS, 2 mm phenylmethylsulfonyl fluoride, 1.5 μm aprotinin, 30 μmE64, 40 μm leupeptin, 100 μm bestatin, and 20 μmpepstatin A. After centrifugation at 15,000 × g for 15 min at 4°C, the supernatants (15 μg protein) were applied to 12.5% SDS-PAGE and Western blotting using a goat anti-PTN antibody (R&D systems, Minneapolis, MN; 1:1,000). The density of the bands was quantified by an Epson desktop scanner (GT-9700F) using NIH image software.
Results
Comparison of the morphogenesis of Purkinje cell dendritesin vivo and in slice cultures
In the present study, we examined the morphogenesis of Purkinje cell dendrites in postnatal cerebellar development using an organotypic slice culture system of cerebellum from 9-d-old rats (Tanaka et al., 1994). Purkinje cells were visualized by immunohistochemical staining using a monoclonal antibody against IP3R (Maeda et al., 1989). This antibody stains clearly the overall structure of Purkinje cells, including dendrites, dendritic spines, axons, and cell bodies (Fig. 1).
Fig. 1.
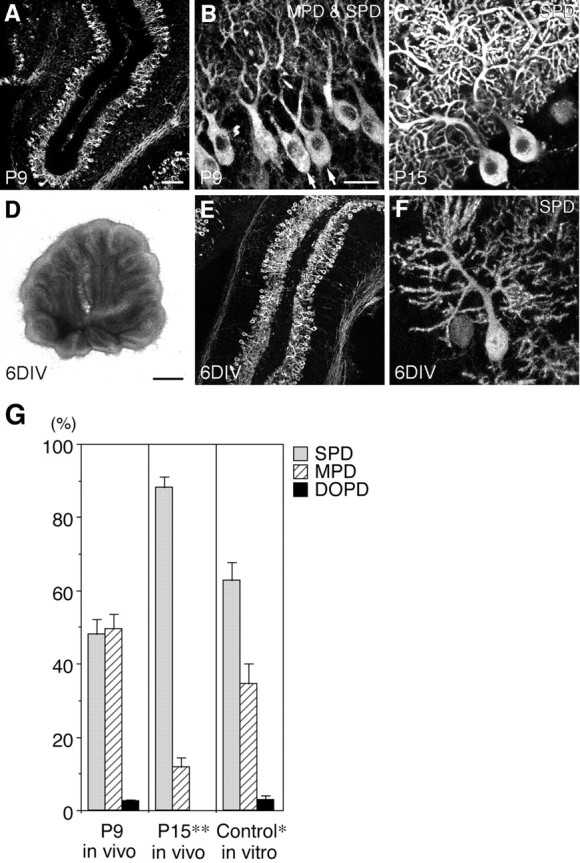
Morphogenesis of Purkinje cell dendrites in vivo and in slice cultures. A–C, Fluorescent immunohistochemistry using a monoclonal antibody against IP3R (4C11) showing the morphology of Purkinje cells in the cerebellum from P9 (A, B) and P15 (C) rats. A, Low-power view of an immunostained cryosection. B, C, Confocal microscopic images of immunostained slices. The multiple primary dendrite (MPD)-type (arrows) and single primary dendrite (SPD)-type Purkinje cells coexist on P9 (B), whereas most Purkinje cells are of the SPD type on P15 (C).D–F, Overview (D) and fluorescent immunohistochemistry using 4C11 (E, F) of cerebellar slices derived from P9 rats and cultured for 6 d under control conditions. E, Low-power view of an immunostained cryosection. F, A confocal microscopic image of an immunostained slice culture. Scale bars: (in A) A, E, 100 μm; (inB) B, C, F, 25 μm;D, 1 mm. G, Quantitative representation of the ratios of the three types of Purkinje cells in vivo (P9 and P15) and in slice cultures at 6 DIV under control conditions. The ratio of SPD-type Purkinje cells significantly increased in slice cultures at 6 DIV compared with that on P9, although the increase was not as marked as in vivo. Cell counts were made in slices, not in cryosections, which enabled us to reliably distinguish the morphology of Purkinje cell dendrites.DOPD, Disoriented primary dendrite.n = 4. Error bars represent SEM. *p < 0.05, **p < 0.001 versus P9.
The morphology of Purkinje cell dendrites changes dramatically during postnatal cerebellar development (Hendelman and Aggerwal, 1980;Armengol and Sotelo, 1991). In the first postnatal week in vivo, Purkinje cells have several primary dendrites. During the second postnatal week, most Purkinje cells lose all of their primary dendrites except one, which extends toward the pial surface, branches extensively in the ML, and forms numerous synapses with parallel fibers (Fig. 1A–C).
Our slice culture system preserves the overall structure of cerebellar slices (Fig. 1D) and the cytoarchitecture of the cerebellar cortex and reproduces the serial process of granule cell development, including proliferation, migration, and extension of parallel fibers within 6 DIV, as described previously (Tanaka et al., 1994). Purkinje cells also survive well, are arranged in a row at the PL as in vivo (Fig. 1E), extend arborized dendritic trees toward the pial surface (Fig. 1F), and form synapses with parallel fibers (Tanaka et al., 1994).
As the first step in the present study, we carefully observed the morphological changes of Purkinje cell dendrites in vivo and in slice cultures under control conditions. The immunohistochemical analysis was done using slices, not sections (cryosections or paraffin sections) of slices, which enabled us to reliably distinguish the morphology of Purkinje cell dendrites. The morphology of Purkinje cells was classified into three types: single primary dendrite (SPD), multiple primary dendrite (MPD), and disoriented primary dendrite (DOPD). The MPD type was defined as Purkinje cells with multiple primary dendrites, all of which extended toward the pial surface. The DOPD type was defined as Purkinje cells with multiple primary dendrites, at least some of which had an abnormal orientation, for example, extending horizontally in the PL or downward into the IGL. No Purkinje cells with a single primary dendrite extending abnormally were observed in the present study.
In the rat cerebellum on postnatal day (P) 9, approximately half (47.9%) of the Purkinje cells were of the SPD type and half (49.6%) were of the MPD type (Fig. 1B,G). Few (2.5%) Purkinje cells were of the DOPD type. On P15, most Purkinje cells showed the SPD-type morphology (SPD/MPD/DOPD = 88.3:11.7:0.0%) (Fig.1C,G). Also in our slice culture system, the ratio of SPD-type Purkinje cells significantly increased at 6 DIV compared with that on P9 (0 DIV), although somewhat fewer SPD-type and more MPD-type cells were observed under the culture conditions than in the P15 cerebellum (SPD/MPD/DOPD = 62.7:34.5:2.8%) (Fig.1F,G). These results indicated that nearly normal morphological changes of Purkinje cell dendrites occur in our slice cultures under control conditions, although these changes appeared to proceed insufficiently in vitro.
Expression patterns of PTPζ and PTN in postnatally developing cerebellum
The expression pattern of PTPζ in P9 rat cerebellum was examined by double-fluorescent immunohistochemistry using antibodies against PTPζ-ECD or -ICD and IP3R (Fig.2A,B). Signals obtained by a polyclonal antibody against PTPζ-ECD were abundant around Purkinje cells in the PL and ML (Fig.2A). On the other hand, the immunoreactivity to the monoclonal antibody against PTPζ-ICD was detected in the cytoplasm and surroundings of Purkinje cells (Fig. 2B). Western blotting showed that the amount of the transmembrane forms of PTPζ is very low compared with that of the secreted form (data not shown), which suggests that the signals of PTPζ-ECD mainly correspond to the presence of the secreted form, phosphacan. In addition, the signals of both PTPζ-ECD and -ICD were detected in the IGL and white matter.
Fig. 2.
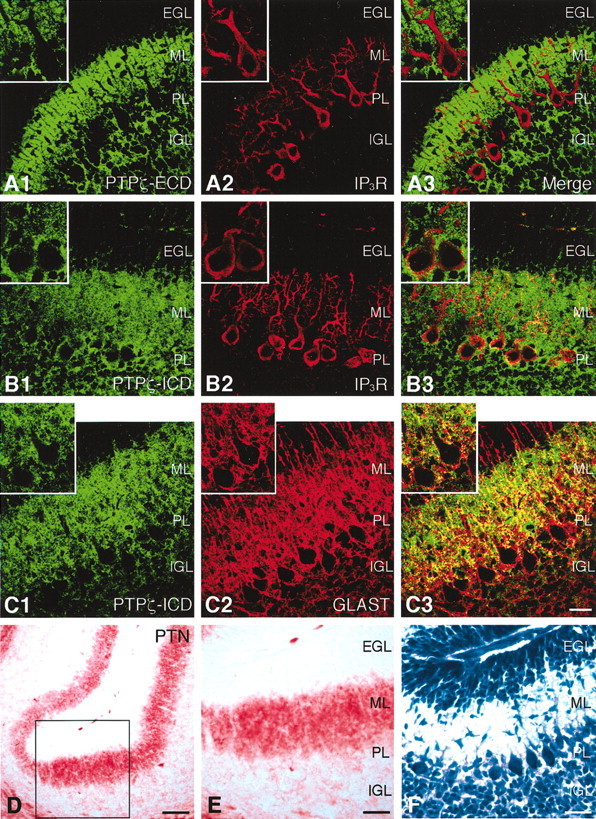
Expression patterns of PTPζ and PTN in postnatally developing cerebellum. A–C, Confocal microscopic images of cerebellar cryosections derived from P9 rats and double stained by fluorescent immunohistochemistry using antibodies against the extracellular domain (ECD) (A1) or the intracellular domain (ICD) (B1, C1) of PTPζ (Cy2) and IP3R (A2, B2) or GLAST (C2) (Cy3). A3, B3, andC3 are the merged images. The immunoreactivities to PTPζ-ICD and GLAST partially overlapped each other, especially around Purkinje cells. EGL, External granular layer;ML, molecular layer; PL, Purkinje cell layer; IGL, internal granular layer. Scale bar, 25 μm.D, E, A cerebellar cryosection derived from a P9 rat and stained by immunohistochemistry using an antibody against PTN. E is the high-magnification image of the enclosed area in D. PTN distributes abundantly in the ML. Scale bars: D, 50 μm; E, 25 μm.F, An adjacent section stained with toluidine blue. Scale bar, 25 μm.
During postnatal development of the cerebellum in vivo, differentiating dendrites of Purkinje cells are surrounded by the lamellate processes of Bergmann glia, which express GLAST, a glial glutamate transporter (Yamada et al., 2000). Double-fluorescent immunohistochemistry using antibodies against PTPζ-ICD and GLAST showed that the immunoreactivities to these proteins partially overlapped each other, especially around Purkinje cells. This suggests that the PTPζ-ICD-positive structures surrounding Purkinje cells were the GLAST-positive lamellate processes of Bergmann glia (Fig. 2C).
Immunohistochemical analysis using an antibody against PTN revealed that this growth factor shows a characteristic localization in the developing cerebellum (Fig. 2D–F). PTN was distributed abundantly in the ML and moderately in the IGL (Fig.2E). Thus, signal transduction of PTPζ by PTN could occur most strongly in the ML in the developing cerebellum. In addition, abundant signals of PTN were also observed in the white matter.
Involvement of PTPζ in the morphogenesis of Purkinje cell dendrites
From the abundant expression of PTPζ and PTN around developing Purkinje cells, we hypothesized that PTN-PTPζ signaling is involved in the morphogenesis of Purkinje cell dendrites. To test this possibility, we examined the effects of the polyclonal antibody against PTPζ-ECD, α6B4PG, on the morphogenesis of Purkinje cell dendrites in slice cultures. This antibody disturbs PTPζ signaling activated by PTN and MK (Maeda et al., 1996; Maeda and Noda, 1998; Qi et al., 2001). On addition of this antibody (200 μg/ml) into the medium of slice cultures, the MPD (Fig.3A) and DOPD (Fig.3B) types of Purkinje cells significantly increased at 6 DIV (SPD/MPD/DOPD = 32.4:48.4:19.2%) (Fig. 3F). Notably, the DOPD type of Purkinje cells increased remarkably. Addition of the control rabbit IgG (200 μg/ml) did not influence the morphogenesis of Purkinje cells (SPD/MPD/DOPD = 62.0:34.1:3.9%) (Fig. 3C,F). These findings suggest that PTPζ regulates the morphogenesis of Purkinje cell dendrites during cerebellar development. The length of the longest dendrites and the number of branching points per cell were not significantly different between the α6B4PG- and control IgG-added conditions, indicating that the growth of Purkinje cell dendrites itself was not influenced by this treatment (Table 1). Thus, PTPζ does not simply promote growth of Purkinje cell dendrites but is involved in the morphological change from the MPD- to the SPD-type Purkinje cells and the directed growth of Purkinje cell dendrites.
Fig. 3.
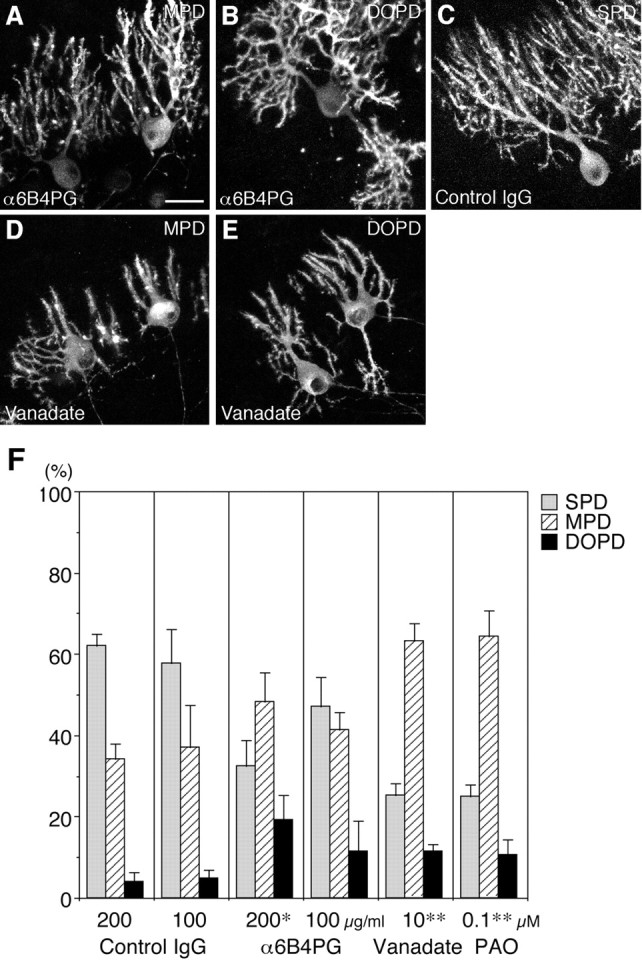
Involvement of PTPζ in the morphogenesis of Purkinje cell dendrites. A–E, Effects of the polyclonal antibody against PTPζ-ECD (α6B4PG) (A, B), control IgG (C), and sodium vanadate (D, E) on the morphogenesis of Purkinje cell dendrites. Shown are confocal microscopic images of cerebellar slices derived from P9 rats, cultured for 6 d with culture medium containing α6B4PG (200 μg/ml) (A, B), control IgG (200 μg/ml) (C), and sodium vanadate (10 μm) (D, E) and stained by fluorescent immunohistochemistry using 4C11. On addition of α6B4PG and sodium vanadate, the MPD (A, D) and DOPD (B,E) types of Purkinje cells markedly increased. Control IgG did not influence the morphogenesis of Purkinje cell dendrites (C). Scale bar, 25 μm. F, Quantitative representation of the effects of α6B4PG, control IgG, sodium vanadate, and phenylarsine oxide (PAO) on the morphogenesis of Purkinje cell dendrites in slice cultures at 6 DIV.n = 3 (Vanadate, PAO) or 4 (α6B4PG, Control IgG). Error bars represent SEM. *p < 0.05 versus control IgG (200 μg/ml); **p < 0.005 versus control (data in Fig.1G).
Table 1.
Quantitative evaluation of the growth of Purkinje cell dendrites and the density of Purkinje cells
| Culture conditions | Longest dendrite (μm) | Branching points (number/cell) | Cell density (cells/150 μm PL) |
|---|---|---|---|
| Control | 81.3 ± 3.1 (48) | 56.2 ± 5.1 (20) | 4.4 ± 0.3 (28) |
| CS-D | 75.1 ± 4.5 (22) | 54.2 ± 5.1 (13) | 4.4 ± 0.2 (17) |
| Control IgG | 82.1 ± 5.2 (24) | 56.3 ± 5.6 (17) | 4.2 ± 0.2 (27) |
| α6B4PG | 71.4 ± 3.0 (36) | 52.8 ± 7.1 (15) | 4.0 ± 0.3 (25) |
Cerebellar slices derived from P9 rats were cultured for 6 d with or without CS-D (50 μg/ml), control IgG (200 μg/ml), and α6B4PG (200 μg/ml). For measurement of the length of the longest dendrite per cell and counting the number of dendritic branching points per cell, confocal microscopic images of slice cultures stained by immunohistochemistry using an antibody against IP3R were analyzed. For analysis of the density of Purkinje cells, cryosections of slice cultures were stained by immunohistochemistry using an antibody against IP3R, and the IP3R-positive cells within 150 μm of the PL were enumerated in confocal microscopic images of 2-μm-thick optical sections. Values are expressed as the mean ± SEM (n). Statistical analysis using Student'st test detected no significant difference between any set of conditions.
Consistently, sodium vanadate and phenylarsine oxide, protein tyrosine phosphatase inhibitors, also increased the MPD and DOPD types of Purkinje cells at 6 DIV [SPD/MPD/DOPD = 25.4:63.3:11.4% (sodium vanadate; 10 μm); 25.1:64.3:10.7% (phenylarsine oxide; 0.1 μm)] (Fig. 3D–F). We cannot exclude the possibility that these treatments retarded Purkinje cell development, however, because they concomitantly resulted in reduced extension and branching of Purkinje cell dendrites [longest dendrite per cell = 64.8 ± 3.1 μm (n = 41), branching points per cell = 40.8 ± 6.0 (n = 16) under vanadate-added (compare Table 1)].
Involvement of CS and PTN in the morphogenesis of Purkinje cell dendrites
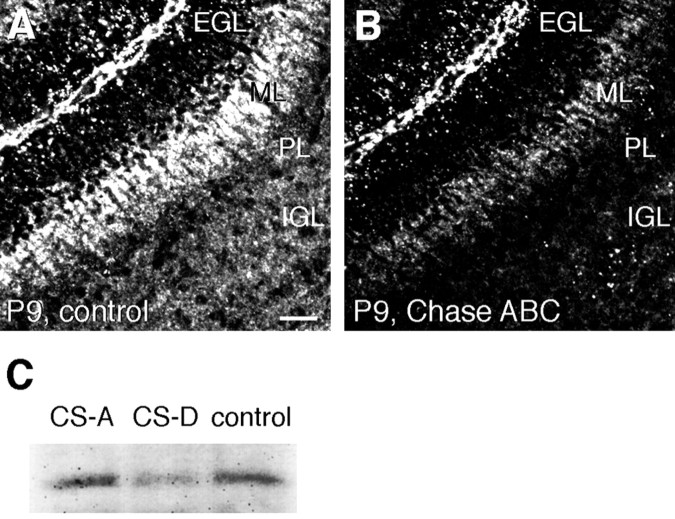
CS plays essential roles in the signal transduction of PTPζ, especially for the signaling of PTN and MK. As revealed by immunohistochemistry using a monoclonal antibody against CS, CS-56, this glycosaminoglycan is present in the ML, IGL, and white matterin vivo (data not shown) and in slice cultures (Fig.4A). Addition of Chase ABC (200 mU/ml), an enzyme that hydrolyzes CS chains, into the medium of slice cultures markedly decreased the immunoreactivity to CS-56 (Fig. 4B). This treatment also resulted in a significant increase in the MPD and DOPD types of Purkinje cells (SPD/MPD/DOPD = 36.5:50.2:13.3%; compare SPD/MPD/DOPD = 63.7:31.6:4.6% under control conditions) (Fig.4C,D,M), as in the case of α6B4PG. These results indicated that endogenous CS is involved in the morphogenesis of Purkinje cell dendrites.
Fig. 4.
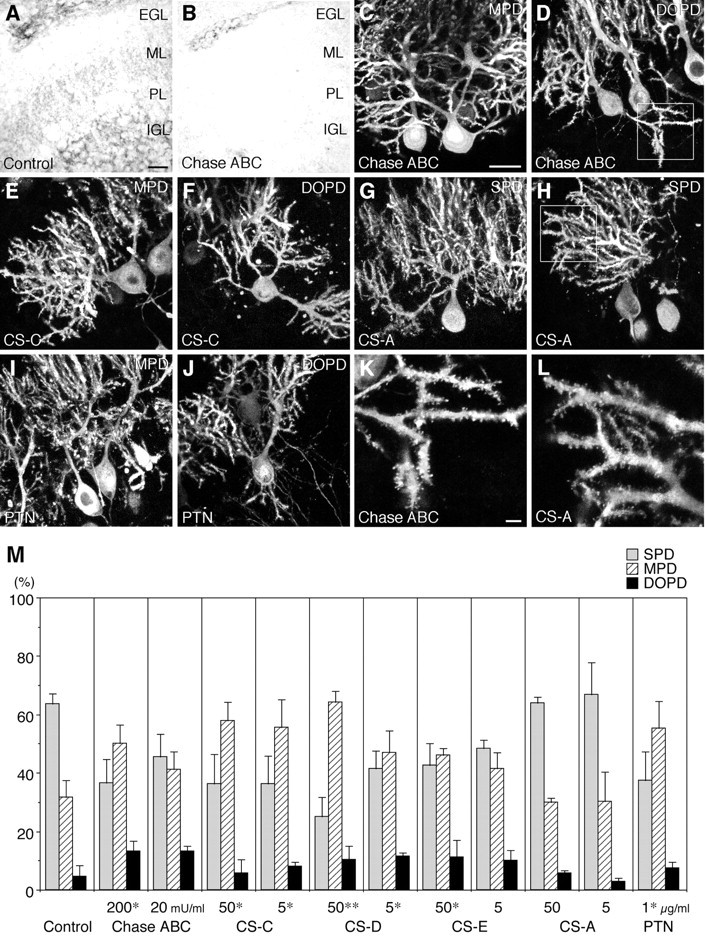
Involvement of chondroitin sulfate (CS) and PTN in the morphogenesis of Purkinje cell dendrites. A, B, Distribution of CS in the control (A) and chondroitinase ABC (Chase ABC)-treated (B) slice cultures. Cryosections of cerebellar slices derived from P9 rats, cultured for 6 d without (A) or with (B) Chase ABC (200 mU/ml), and stained by immunohistochemistry using a monoclonal antibody against CS (CS-56). Scale bar, 25 μm. C–J, Effects of Chase ABC, CS, and PTN on the morphogenesis of Purkinje cell dendrites. Shown are confocal microscopic images of cerebellar slices cultured for 6 d with culture medium containing Chase ABC (200 mU/ml) (C,D), CS-C (50 μg/ml) (E,F), CS-A (50 μg/ml) (G,H), and PTN (1 μg/ml) (I,J), and stained by fluorescent immunohistochemistry using 4C11. On addition of Chase ABC, CS-C, and PTN, the MPD (C, E,I) and DOPD (D, F,J) types of Purkinje cells increased. In contrast, many Purkinje cells had an SPD under the CS-A-added conditions (G, H). Scale bar, 25 μm. K, L, High-power views of the enclosed areas in D, H. Spine formation on Purkinje cell dendrites did not differ between the Chase ABC (K)- and CS-A (L)-added conditions. Scale bar, 3 μm. M, Quantitative representation of the effects of Chase ABC, CS, and PTN on the morphogenesis of Purkinje cell dendrites in slice cultures at 6 DIV.n = 3 (CS chains) or 4 (Control, Chase ABC,PTN). Error bars represent SEM. *p < 0.05, **p < 0.005 versus control.
Next we examined the effects of exogenously added CS chains. We indicated previously that various CS samples differentially inhibit the signaling of the PTN-PTPζ pathway (Maeda et al., 1996, 1999). Although CS-C, -D, and -E markedly inhibited the binding of PTN to PTPζ, CS-A scarcely influenced the binding. A similar selectivity was observed in the effects on the morphogenesis of Purkinje cell dendrites. CS-C, -D, and -E (50 μg/ml) increased the MPD and DOPD types of Purkinje cells when added to the medium of slice cultures [SPD/MPD/DOPD = 36.2:57.9:5.9% (CS-C); 25.1:64.4:10.5% (CS-D); 42.6:46.1:11.2% (CS-E)] (Fig.4E,F,M). In contrast, the morphology of Purkinje cell dendrites was not influenced by addition of 50 μg/ml CS-A (SPD/MPD/DOPD = 64.0:30.1:5.9%) (Fig. 4G,H,M). These observations suggested that PTN signaling is involved in the morphogenesis of Purkinje cell dendrites.
In our slice culture system, exogenously applied PTN also induced an abnormal morphology in Purkinje cell dendrites (SPD/MPD/DOPD = 37.4:55.2:7.4%) (Fig.4I,J,M). This may be caused by perturbation of normal distribution of the endogenous PTN by the exogenous PTN.
The morphological change of Purkinje cells from the MPD to the SPD type is accompanied by the formation of many dendritic spines in vivo. This later aspect of maturation appeared not to be influenced by treatment with the reagents affecting PTN-PTPζ signaling in slice cultures. Spine formation was apparently normal in the MPD and DOPD types of Purkinje cells in the slice cultures treated with Chase ABC and CS chains (Fig.4K,L).
In contrast to the dendrite formation of Purkinje cells, development of granule cells proceeded normally even in the presence of the reagents affecting PTN-PTPζ signaling. As under control conditions, migration of granule cells from the EGL to the IGL was nearly completed by 6 DIV in the treated cultures, as revealed by the almost complete disappearance of the EGL (Fig.5A,B). The density of granule cells in the IGL at 6 DIV was not significantly different between the treated and control conditions (Fig.5C,D, Table 2). No abnormalities such as increased pyknosis were observed in granule cells in sections of the treated cultures processed for Nissl staining (Fig.5A,B) and immunohistochemistry against NeuN (Fig. 5C,D). Furthermore, VGLUT1, a vesicular glutamate transporter that is localized to the synaptic vesicles (Bellocchio et al., 1998), was expressed in the ML under the treated as well as control conditions, suggesting that normal presynaptic structures of parallel fiber terminals were formed under both conditions (Fig.5E,F).
Fig. 5.
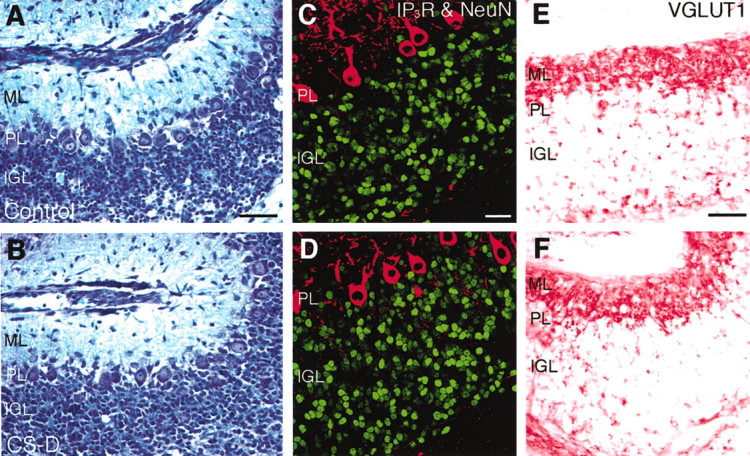
Development of granule cells in slice cultures under control and CS-D-added conditions. Cerebellar slices cultured for 6 d without (A,C,E) or with (B,D,F) CS-D were processed for several histological analyses.A, B, Nissl staining with toluidine blue. Migration of granule cells from the EGL to the IGL was nearly completed even in the CS-D-treated cultures. Scale bar, 50 μm.C, D, Confocal microscopic images of cryosections double stained by fluorescent immunohistochemistry using antibodies against NeuN (Cy2, green) and IP3R (Cy3, red). The density of granule cells in the IGL was not significantly different between these two conditions (Table 2). Scale bar, 25 μm. E,F, Cryosections stained by immunohistochemistry using an antibody against vesicular glutamate transporter 1 (VGLUT1). VGLUT1 was expressed in the ML under both conditions. Scale bar, 50 μm.
Table 2.
Evaluation of the density of granule cells
| Culture conditions | Density of granule cells | |
|---|---|---|
| (Cells/150 μm IGL) | (Cells/100 × 100 μm2) | |
| Control | 189.6 ± 7.1 (28) | 95.5 ± 1.9 (28) |
| CS-D | 189.3 ± 8.9 (17) | 97.8 ± 2.6 (17) |
| Control IgG | 186.6 ± 6.1 (27) | 97.7 ± 2.2 (27) |
| α6B4PG | 189.0 ± 5.5 (25) | 91.7 ± 2.1 (25) |
Cerebellar slices derived from P9 rats were cultured for 6 d with or without CS-D (50 μg/ml), control IgG (200 μg/ml), and α6B4PG (200 μg/ml). Cryosections of slice cultures were stained by immunohistochemistry using an antibody against NeuN, and the NeuN-positive cells within 150 μm or 100 × 100 μm2 of the IGL were enumerated in confocal microscopic images of 2-μm-thick optical sections. Values are expressed as the mean ± SEM (n). Statistical analysis using Student'st test detected no significant difference between any set of conditions.
Interestingly, Chase ABC digestion of the cerebellar sections markedly reduced the PTN immunoreactivity in the ML (Fig.6A,B), indicating that most of the endogenous PTN is present in a CS-bound form in this tissue. This also suggests that the effects of Chase ABC and CS chains on the morphogenesis of Purkinje cell dendrites were caused by the displacement of PTN from the ML. In fact, Western blotting showed that the addition of CS-D but not CS-A into the culture medium markedly decreased the PTN contents in the cerebellar slices [control/CS-D/CS-A = 100; 68 ± 8 (p< 0.01 vs control; t test); 100 ± 4% (n = 3)] (Fig. 6C).
Fig. 6.
Effects of the Chase ABC and CS treatments on PTN contents in the cerebellum. A, B, Confocal microscopic images of adjacent cryosections derived from the cerebellum of a P9 rat and stained by fluorescent immunohistochemistry using an antibody against PTN without (A) or with (B) pretreatment by Chase ABC (20 mU/ml; 37°C; 40 min). The pretreatment with Chase ABC reduced PTN immunoreactivity. Scale bar, 25 μm. C, Western blotting analysis showing effects of CS-D and -A on PTN contents in cerebellar slice cultures. Addition of CS-D but not CS-A decreased the PTN contents in the slices.
Involvement of GLAST on Bergmann glial processes
Because the GLAST-positive lamellate processes of Bergmann glia expressed PTPζ, we next examined whether the reagents affecting PTN-PTPζ signaling influence these processes of Bergmann glia. For this purpose, cryosections of cerebellar slice cultures were double stained by immunohistochemistry using the antibodies against IP3R and GLAST. Although the cell bodies and dendrites of Purkinje cells were surrounded by the GLAST-positive lamellate processes of Bergmann glia in slice cultures under control conditions (Fig.7A,B), the GLAST immunoreactivity was markedly reduced in the slice cultures treated with α6B4PG, Chase ABC, and CS-C, -D, and -E (Fig.7C,D). In contrast, the same treatments did not reduce the number of Bergmann fibers, the shaft processes of Bergmann glia that express GFAP, a glial intermediate filament protein (Fig.7E,F, Table3). This indicated that the reduction in GLAST immunoreactivity was not caused by the nonspecific degeneration of Bergmann glia. Western blotting also showed that the amount of GLAST but not GFAP was reduced by these treatments (data not shown). These observations strongly suggest that the morphogenesis of Purkinje cell dendrites involves their interaction with the GLAST-positive lamellate processes of Bergmann glia.
Fig. 7.
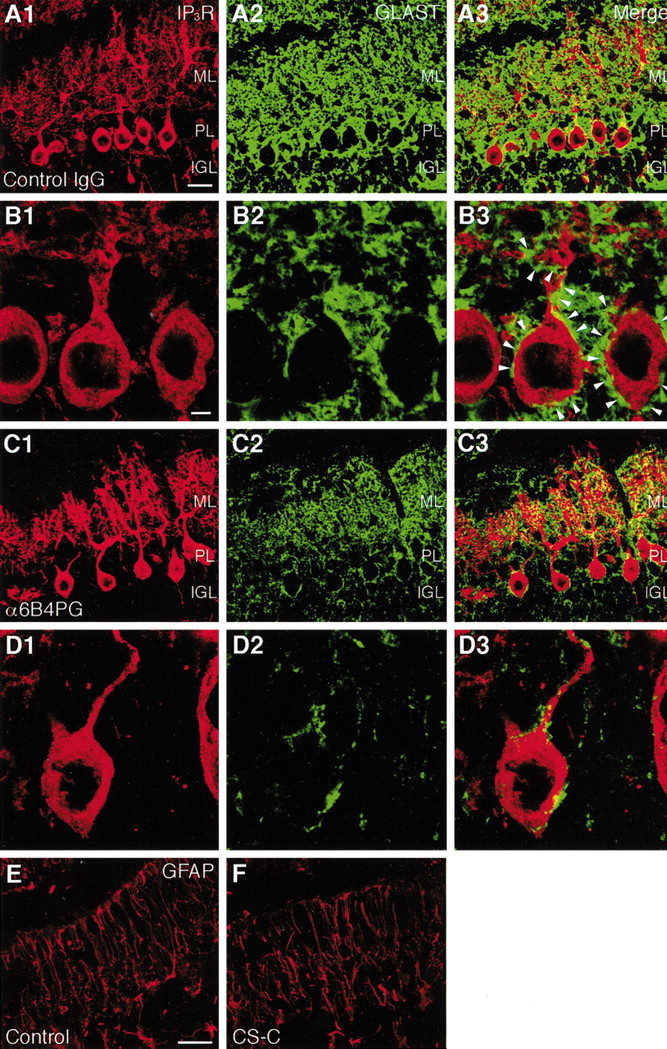
Reduction in GLAST immunoreactivity on Bergmann glial processes by the reagents affecting PTN-PTPζ signaling. Shown are confocal microscopic images of cryosections of cerebellar slices cultured for 6 d with culture medium containing control IgG (A, B) and α6B4PG (C, D) (200 μg/ml) and double stained by fluorescent immunohistochemistry using antibodies against IP3R (A1, B1, C1, D1) and GLAST (A2, B2, C2, D2). A3, B3, C3, and D3 are the merged images. Although the cell bodies and dendrites of Purkinje cells were surrounded by the GLAST-positive lamellate processes of Bergmann glia under the control IgG-treated conditions (B, arrowheads), the GLAST immunoreactivity was markedly reduced under the α6B4PG-treated conditions (C, D). Scale bars: (inA) A, C, 25 μm; (inB) B, D, 5 μm.E, F, Distribution of GFAP in cerebellar slice cultures. Confocal microscopic images of cryosections of slices cultured for 6 d without (E) or with (F) CS-C (50 μg/ml) and stained by fluorescent immunohistochemistry using an antibody against GFAP. The GFAP-positive shaft processes of Bergmann glia did not differ between the two culture conditions. Scale bar, 25 μm.
Table 3.
Evaluation of the number of Bergmann fibers
| Culture conditions | Bergmann fibers (cells/150 μm ML) |
|---|---|
| Control | 18.1 ± 1.5 (28) |
| CS-D | 18.8 ± 1.3 (27) |
| Control IgG | 16.6 ± 2.3 (20) |
| α6B4PG | 14.1 ± 3.1 (7) |
Cerebellar slices derived from P9 rats were cultured for 6 d with or without CS-D (50 μg/ml), control IgG (200 μg/ml), and α6B4PG (200 μg/ml). Cryosections of slice cultures were stained by immunohistochemistry using an antibody against GFAP, and GFAP-positive fibers >30 μm within 150 μm of the ML were enumerated in confocal microscopic images of 3-μm-thick optical sections. Values are expressed as the mean ± SEM (n). Statistical analysis using Student's t test detected no significant difference between any set of conditions.
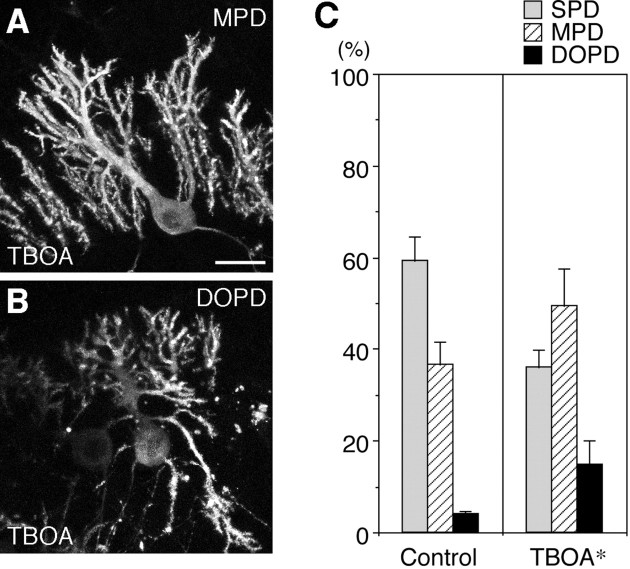
The reduction in GLAST immunoreactivity by the reagents affecting PTN-PTPζ signaling suggests that the glutamate-transporting activity of GLAST is involved in the morphogenesis of Purkinje cell dendrites presumably downstream of PTN-PTPζ signaling. To test this hypothesis, slice cultures were treated with dl-TBOA, an inhibitor of glutamate transporters (Shimamoto et al., 1998). dl-TBOA (10 μm) actually increased the MPD and DOPD types of Purkinje cells (SPD/MPD/DOPD = 36.0:49.3:14.7%; compare SPD/MPD/DOPD = 59.3:36.5:4.1% under control conditions) (Fig.8). This treatment reduced neither the survival of Purkinje cells [IP3R-positive cells per 150 μm of the PL under dl-TBOA-added conditions = 4.4 ± 0.3 (n = 28) (compare Table 1)] nor the extension or branching of Purkinje cell dendrites (Fig.8A,B). Thus, the morphogenesis of Purkinje cell dendrites may be regulated by the glutamate-transporting activity of GLAST on Bergmann glial processes.
Fig. 8.
Effects of a glutamate transporter inhibitor on the morphogenesis of Purkinje cell dendrites. A,B, Confocal microscopic images of cerebellar slices derived from P9 rats, cultured for 6 d with culture medium containing dl-threo-β-benzyloxyaspartate (TBOA) (10 μm), an inhibitor of glutamate transporters, and stained by fluorescent immunohistochemistry using 4C11. On addition of dl-TBOA, the MPD (A) and DOPD (B) types of Purkinje cells increased. Scale bar, 25 μm. C, Quantitative representation of the effects of dl-TBOA on the morphogenesis of Purkinje cell dendrites in slice cultures at 6 DIV. n = 4. Error bars represent SEM. *p < 0.05 versus control.
Discussion
The postnatal development of Purkinje cells is characterized by a specific pattern of dendritic morphogenesis. The change of Purkinje cells from the MPD or DOPD type to the SPD type strictly proceeds underin vivo conditions, and almost all of the Purkinje cells are of the SPD type in the matured cerebellum. In our organotypic slice culture system of postnatal rat cerebellum, the morphological changes of Purkinje cells basically proceeded as in vivo (Fig. 1). This suggested that some of the primary dendrites were withdrawn in slice cultures as in vivo. However, more MPD-type Purkinje cells were found in slice cultures at 6 DIV than in P15 cerebellum. In addition, a significant population of Purkinje cells was of the DOPD type at 6 DIV, whereas at the corresponding in vivo stage (P15), this type of Purkinje cell was not observed. We consider that this insufficient progress of Purkinje cell development in slice cultures is caused by a decrease in signal transduction levels required for the dendrite formation under the culture conditions. However, it seems that this property of the slice culture system makes it a highly sensitive assay system for Purkinje cell development. Using this culture system, we demonstrated that the perturbation of PTN-PTPζ signaling induced the aberrant morphology of Purkinje cell dendrites such as MPDs and DOPDs (Figs. 3, 4), clearly showing that PTN-PTPζ signaling is involved in the morphogenesis of Purkinje cell dendrites. Berry and Bradley (1976) reported that Purkinje cells already acquire their polarity on P9. However, our findings that the perturbation of PTN-PTPζ signaling induced the DOPD-type Purkinje cells suggest that this signaling needs to function continuously to maintain the polarity of Purkinje cells. If this signaling is lost, new primary dendrites could sprout and extend to an abnormal orientation even after P9.
CS proteoglycans are reported to contribute to the regulation of directed axonal outgrowth of various neurons, including retinal ganglion cells (Brittis and Silver, 1994; Chung et al., 2000) and spinal motor neurons (Bernhardt and Schachner, 2000). These findings suggest that the signal transductions mediated by CS proteoglycans are involved in the directed outgrowth of neurites, although it is not known what kinds of CS proteoglycans contribute to these phenomena. The present study identified PTPζ as a CS proteoglycan regulating the dendritic morphogenesis of cerebellar Purkinje cells.
The treatment with Chase ABC and CS chains induced an aberrant Purkinje cell morphogenesis in slice cultures (Fig. 4). The same treatments also markedly decreased PTN contents in the cerebellum (Fig. 6), indicating that most PTN is present in the CS proteoglycan-bound form in this tissue. It is notable that the effects of CS chains were highly dependent on the structure of CS chains. Although CS-C, -D, and -E influenced the morphogenesis of Purkinje cells, CS-A gave no effect on this type of cells. CS-C and -D contain ∼10–20% GlcUA(2-sulfate)β1–3GalNAc(6-sulfate) disaccharide units, and CS-E contains 60–65% GlcUAβ1–3GalNAc(4,6-disulfate) disaccharide units (Sakai et al., 2000). In contrast, the contents of these oversulfated disaccharide units are very low in CS-A, suggesting that the oversulfated portion of CS is functionally important for the binding to PTN. Exogenously applied PTN also induced an abnormal morphology in Purkinje cell dendrites (Fig. 4), suggesting that normal distribution of PTN is required for the action of PTN-PTPζ signaling in this phenomenon.
Although PTPζ is expressed by both Purkinje cells and Bergmann glia, PTN is expressed by the latter cells in the postnatal cerebellum (Matsumoto et al., 1994; Wewetzer et al., 1995). These expression patterns suggest two possibilities concerning the action of PTN-PTPζ signaling, although they are not mutually exclusive. One possibility is that PTN secreted by Bergmann glia binds with PTPζ on the same cells in an autocrine or paracrine manner (Fig.9, arrow 3). The other is that PTN secreted by Bergmann glia binds with PTPζ on Purkinje cells as one of the glia–neuron interactions (Fig. 9, arrow 3′). Before binding to the transmembrane form of PTPζ, PTN may be pooled by binding to the secreted form of PTPζ (phosphacan) in the extracellular matrix of the ML (Figs. 6, 9, arrow 2).
Fig. 9.
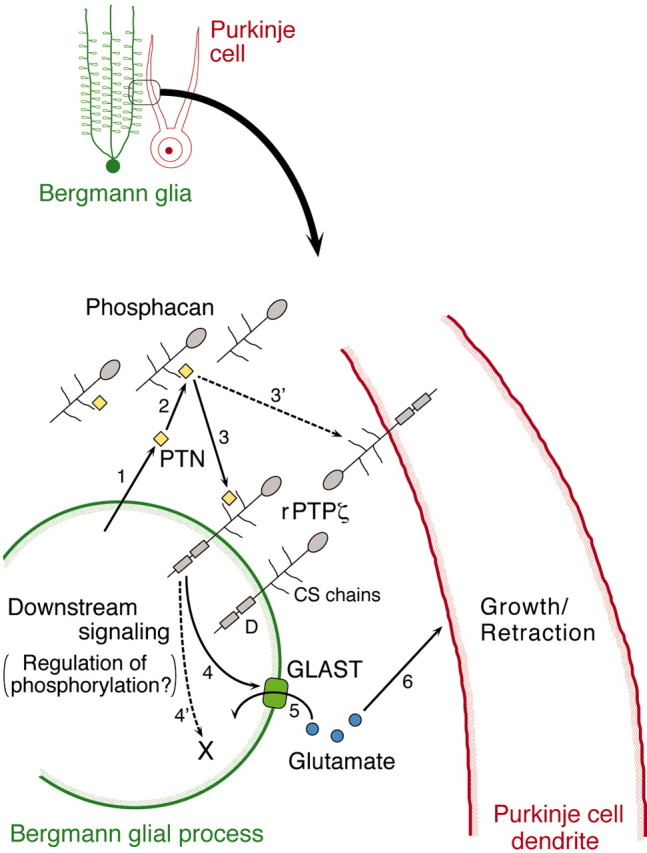
A model for regulation of the morphogenesis of Purkinje cell dendrites by PTN-PTPζ signaling. 1, PTN is produced by Bergmann glia. 2, Before binding to the transmembrane (receptor) form of PTPζ (rPTPζ), PTN may be pooled by binding to the secreted form of PTPζ (Phosphacan) in the extracellular matrix of the ML.3, PTN is suggested to bind with rPTPζ on Bergmann glia. 3′, The possibility that PTN directly binds with rPTPζ on Purkinje cells cannot be excluded. 4, PTPζ signaling controls the formation or maintenance, or both, of the GLAST-positive lamellate processes of Bergmann glia, the mechanism of which is not known at present. 4′, The existence of a GLAST-independent mechanism cannot be excluded. 5, 6, GLAST regulates the extracellular levels of glutamate, which can induce growth or retraction of Purkinje cell dendrites. D, Tyrosine phosphatase domain.
Our study showed that the immunoreactivity to the glutamate transporter GLAST on the lamellate processes of Bergmann glia was reduced in slice cultures treated with the reagents affecting PTN-PTPζ signaling (Fig.7). In contrast, the number of GFAP-positive Bergmann fibers was not reduced under the same conditions, indicating that the decrease in GLAST immunoreactivity was not caused by toxic effects of the reagents on Bergmann glia. Moreover, inhibition of glutamate transporter activity by dl-TBOA induced the aberrant morphogenesis of Purkinje cells just as in the case of the perturbation of PTN-PTPζ signaling (Fig. 8). These findings suggest that PTN-PTPζ signaling acts on the Bergmann glial processes and controls the formation and maintenance of the GLAST-positive lamellate processes of Bergmann glia, which regulate the morphogenesis of Purkinje cell dendrites (Fig. 9). Further studies are necessary to elucidate how PTN-PTPζ signaling controls GLAST expression (Fig. 9, arrow 4). On the other hand, we cannot exclude the possibility that PTN directly affects Purkinje cells (Fig. 9, arrow 3′).
In the developing cerebellum, glutamate is produced and released by granule cells (Levi et al., 1991; Miranda-Contreras et al., 1999). Studies using dissociated cell cultures have shown that glutamate stimulates the dendritic growth of Purkinje cells (Cohen-Cory et al., 1991; Hirai and Launey, 2000), suggesting that glutamate released in the extracellular space by granule cells influences the morphogenesis of Purkinje cell dendrites in vivo. However, it is not plausible that the aberrant morphogenesis of Purkinje cells observed in this study was caused by the abnormal development of granule cells, because the migration and differentiation of granule cells proceeded normally in our slice cultures even in the presence of the reagents affecting PTN-PTPζ signaling (Fig. 5, Table 2). Furthermore, although the growth of Purkinje cell dendrites was stimulated in the dissociated coculture system of Purkinje cells and granule cells, most of the Purkinje cells had multiple primary dendrites extending in various directions (Baptista et al., 1994; Hirai and Launey, 2000), suggesting that granule cells do not function in the determination of polarity in Purkinje cells.
In this context, Bergmann glia settle in a quite important position. The cell bodies of Bergmann glia closely associate with Purkinje cell bodies and extend polarized shaft processes with fine lamellate processes toward the pial surface. Of the two, the latter processes express GLAST and closely surround both the cell bodies and dendrites of Purkinje cells during postnatal development (Furuta et al., 1997;Ullensvang et al., 1997; Yamada et al., 2000) and in adults (Rothstein et al., 1994; Lehre et al., 1995), regulating the extracellular glutamate levels around this type of cell (Barbour et al., 1994). In the present study, we found that the GLAST immunoreactivity was reduced around the Purkinje cells of aberrant morphology and that the inhibition of the glutamate transporter activity induced the abnormal morphogenesis of Purkinje cell dendrites. On the basis of these findings, we suggest that at least one mechanism for Bergmann glia to regulate the morphogenesis of Purkinje cell dendrites is by modulating the extracellular glutamate levels through the glutamate-transporting activity of GLAST (Fig. 9, arrows 5,6).
Although the directed growth of Purkinje cell dendrites might be regulated simply by the interaction between the leading processes of the dendrites and the Bergmann glial processes (Yamada et al., 2000), the mechanism for the morphological change from the MPD- to the SPD-type Purkinje cells is a profound problem because both the growth and retraction of the primary dendrites have to occur in the same cell.Wilson and Keith (1998) found that glutamate can both facilitate and inhibit the dendritic growth of hippocampal neurons, depending on the exposure time to glutamate in dissociated cell cultures. Fine regulation of the extracellular glutamate levels by GLAST on Bergmann glial processes might produce a spatiotemporal pattern of the glutamate levels around MPDs, and such a pattern might influence the final selection of one primary dendrite. This mechanism may involve voltage-dependent calcium channels activated after glutamate stimulation, because a mutation in the gene encoding the α2δ-2 voltage-dependent calcium channel accessory subunit was found to increase the MPD-type Purkinje cells (Brodbeck et al., 2002). In addition, we can propose another mechanism for Bergmann glia to regulate Purkinje cell morphology. The Bergmann glial processes closely surrounding Purkinje cell bodies may prevent the sprouting of new primary dendrites and maintain the SPD-type morphology of Purkinje cells.
It was reported recently that mutant mice deficient in PTPζ (Shintani et al., 1998; Harroch et al., 2000) and PTN (Amet et al., 2001) showed no gross morphological abnormality, at least in adult animals. This suggests that the morphogenesis of Purkinje cell dendrites is regulated by multiple signaling mechanisms, including that of PTN-PTPζ, and that the loss of PTN or PTPζ alone is compensated for by the other molecules. For example, MK might compensate for PTN deficiency, and PTPζ deficiency might be compensated for by the other proteoglycans such as neurocan and syndecan-3, which bind with PTN (Bandtlow and Zimmermann, 2000). On the other hand, Purkinje cells in the GLAST-deficient mice were abnormal in that they were multiply innervated by the climbing fibers even at the adult stage (Watase et al., 1998). A detailed description of Purkinje cell development in these mutant mice has not been reported, and it will be interesting to analyze the development of the cerebellum in single- and double-mutant mice for these genes.
Footnotes
This study was supported in part by grants-in-aid for scientific research from Fujita Health University and from the Ministry of Education, Science, Sports and Culture of Japan.
Correspondence should be addressed to Masahiko Tanaka, Division of Cell Biology, Institute for Comprehensive Medical Science, Fujita Health University, Toyoake, Aichi 470-1192, Japan. E-mail:mtanaka@fujita-hu.ac.jp.
References
- 1.Altman J, Anderson WJ. Experimental reorganization of the cerebellar cortex. I. Morphological effects of eliminating all microneurons with prolonged X-irradiation started at birth. J Comp Neurol. 1972;146:355–406. doi: 10.1002/cne.901460305. [DOI] [PubMed] [Google Scholar]
- 2.Amet LEA, Lauri SE, Hienola A, Croll SD, Lu Y, Levorse JM, Prabhakaran B, Taira T, Rauvala H, Vogt TF. Enhanced hippocampal long-term potentiation in mice lacking heparin-binding growth-associated molecule. Mol Cell Neurosci. 2001;17:1014–1024. doi: 10.1006/mcne.2001.0998. [DOI] [PubMed] [Google Scholar]
- 3.Armengol J-A, Sotelo C. Early dendritic development of Purkinje cells in the rat cerebellum. A light and electron microscopic study using axonal tracing in “in vitro” slices. Dev Brain Res. 1991;64:95–114. doi: 10.1016/0165-3806(91)90213-3. [DOI] [PubMed] [Google Scholar]
- 4.Bandtlow CE, Zimmermann DR. Proteoglycans in the developing brain: new conceptual insights for old proteins. Physiol Rev. 2000;80:1267–1290. doi: 10.1152/physrev.2000.80.4.1267. [DOI] [PubMed] [Google Scholar]
- 5.Baptista CA, Hatten ME, Blazeski R, Mason CA. Cell-cell interactions influence survival and differentiation of purified Purkinje cells in vitro. Neuron. 1994;12:243–260. doi: 10.1016/0896-6273(94)90268-2. [DOI] [PubMed] [Google Scholar]
- 6.Barbour B, Keller BU, Llano I, Marty A. Prolonged presence of glutamate during excitatory synaptic transmission to cerebellar Purkinje cells. Neuron. 1994;12:1331–1343. doi: 10.1016/0896-6273(94)90448-0. [DOI] [PubMed] [Google Scholar]
- 7.Bellocchio EE, Hu H, Pohorille A, Chan J, Pickel VM, Edwards RH. The localization of the brain-specific inorganic phosphate transporter suggests a specific presynaptic role in glutamatergic transmission. J Neurosci. 1998;18:8648–8659. doi: 10.1523/JNEUROSCI.18-21-08648.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernhardt RR, Schachner M. Chondroitin sulfates affect the formation of the segmental motor nerve in zebrafish embryo. Dev Biol. 2000;221:206–219. doi: 10.1006/dbio.2000.9673. [DOI] [PubMed] [Google Scholar]
- 9.Berry M, Bradley P. The growth of the dendritic trees of Purkinje cells in the cerebellum of the rat. Brain Res. 1976;112:1–35. doi: 10.1016/0006-8993(76)90331-0. [DOI] [PubMed] [Google Scholar]
- 10.Berry M, Bradley P, Borges S. Environmental and genetic determinants of connectivity in the central nervous system—an approach through dendritic field analysis. Prog Brain Res. 1978;48:133–146. doi: 10.1016/S0079-6123(08)61020-1. [DOI] [PubMed] [Google Scholar]
- 11.Brittis PA, Silver J. Exogenous glycosaminoglycans induce complete inversion of retinal ganglion cell bodies and their axons within the retinal neuroepithelium. Proc Natl Acad Sci USA. 1994;91:7539–7542. doi: 10.1073/pnas.91.16.7539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brodbeck J, Davies A, Courtney J-M, Meir A, Balaguero N, Canti C, Moss FJ, Page KM, Pratt WS, Hunt SP, Barclay J, Rees M, Dolphin AC. The ducky mutation in Cacna2d2 results in altered Purkinje cell morphology and is associated with the expression of a truncated α2δ-2 protein with abnormal function. J Biol Chem. 2002;277:7684–7693. doi: 10.1074/jbc.M109404200. [DOI] [PubMed] [Google Scholar]
- 13.Canoll PD, Barnea G, Levy JB, Sap J, Ehrlich M, Silvennoinen O, Schlessinger J, Musacchio JM. The expression of a novel receptor-type tyrosine phosphatase suggests a role in morphogenesis and plasticity of the nervous system. Dev Brain Res. 1993;75:293–298. doi: 10.1016/0165-3806(93)90035-9. [DOI] [PubMed] [Google Scholar]
- 14.Chung KY, Taylor JSH, Shum DKY, Chan SO. Axon routing at the optic chiasm after enzymatic removal of chondroitin sulfate in mouse embryo. Development. 2000;127:2673–2683. doi: 10.1242/dev.127.12.2673. [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Cory S, Dreyfus CF, Black IB. NGF and excitatory neurotransmitters regulate survival and morphogenesis of cultured cerebellar Purkinje cells. J Neurosci. 1991;11:462–471. doi: 10.1523/JNEUROSCI.11-02-00462.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Freshney RI. Culture of animal cells: a manual of basic technique, Ed 2. Alan R. Liss; New York: 1987. Three-dimensional culture systems. pp. 297–307. [Google Scholar]
- 17.Furuta A, Rothstein JD, Martin LJ. Glutamate transporter protein subtypes are expressed differentially during rat CNS development. J Neurosci. 1997;17:8363–8375. doi: 10.1523/JNEUROSCI.17-21-08363.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harroch S, Palmeri M, Rosenbluth J, Custer A, Okigaki M, Shrager P, Blum M, Buxbaum JD, Schlessinger J. No obvious abnormality in mice deficient in receptor protein tyrosine phosphatase β. Mol Cell Biol. 2000;20:7706–7715. doi: 10.1128/mcb.20.20.7706-7715.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hendelman WJ, Aggerwal AS. The Purkinje neuron: I. A Golgi study of its development in the mouse and in culture. J Comp Neurol. 1980;193:1063–1079. doi: 10.1002/cne.901930417. [DOI] [PubMed] [Google Scholar]
- 20.Hirai H, Launey T. The regulatory connection between the activity of granule cell NMDA receptors and dendritic differentiation of cerebellar Purkinje cells. J Neurosci. 2000;20:5217–5224. doi: 10.1523/JNEUROSCI.20-14-05217.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lehre KP, Levy LM, Ottersen OP, Storm-Mathisen J, Danbolt NC. Differential expression of two glial glutamate transporters in the rat brain: quantitative and immunocytochemical observations. J Neurosci. 1995;15:1835–1853. doi: 10.1523/JNEUROSCI.15-03-01835.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Levi G, Patrizio M, Gallo V. Release of endogenous and newly synthesized glutamate and of other amino acids induced by non-N-methyl-d-aspartate receptor activation in cerebellar granule cell cultures. J Neurochem. 1991;56:199–206. doi: 10.1111/j.1471-4159.1991.tb02581.x. [DOI] [PubMed] [Google Scholar]
- 23.Maeda N, Noda M. Involvement of receptor-like protein tyrosine phosphatase ζ/RPTPβ and its ligand pleiotrophin/heparin-binding growth-associated molecule (HB-GAM) in neuronal migration. J Cell Biol. 1998;142:203–216. doi: 10.1083/jcb.142.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeda N, Niinobe M, Inoue Y, Mikoshiba K. Developmental expression and intracellular localization of P400 protein characteristic of Purkinje cells in the mouse cerebellum. Dev Biol. 1989;133:67–76. doi: 10.1016/0012-1606(89)90297-2. [DOI] [PubMed] [Google Scholar]
- 25.Maeda N, Matsui F, Oohira A. A chondroitin sulfate proteoglycan that is developmentally regulated in the cerebellar mossy fiber system. Dev Biol. 1992;151:564–574. doi: 10.1016/0012-1606(92)90194-l. [DOI] [PubMed] [Google Scholar]
- 26.Maeda N, Nishiwaki T, Shintani T, Hamanaka H, Noda M. 6B4 proteoglycan/phosphacan, an extracellular variant of receptor-like protein-tyrosine phosphatase ζ/RPTPβ, binds pleiotrophin/heparin-binding growth-associated molecule (HB-GAM). J Biol Chem. 1996;271:21446–21452. doi: 10.1074/jbc.271.35.21446. [DOI] [PubMed] [Google Scholar]
- 27.Maeda N, Ichihara-Tanaka K, Kimura T, Kadomatsu K, Muramatsu T, Noda M. A receptor-like protein-tyrosine phosphatase PTPζ/RPTPβ binds a heparin-binding growth factor midkine. J Biol Chem. 1999;274:12474–12479. doi: 10.1074/jbc.274.18.12474. [DOI] [PubMed] [Google Scholar]
- 28.Matsumoto K, Wanaka A, Mori T, Taguchi A, Ishi N, Muramatsu H, Muramatsu T, Tohyama M. Localization of pleiotrophin and midkine in the postnatal developing cerebellum. Neurosci Lett. 1994;178:216–220. doi: 10.1016/0304-3940(94)90762-5. [DOI] [PubMed] [Google Scholar]
- 29.Maurel P, Rauch U, Flad M, Margolis RK, Margolis RU. Phosphacan, a chondroitin sulfate proteoglycan of brain that interacts with neurons and neural cell-adhesion molecules, is an extracellular variant of a receptor-type protein tyrosine phosphatase. Proc Natl Acad Sci USA. 1994;91:2512–2516. doi: 10.1073/pnas.91.7.2512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miranda-Contreras L, Benítez-Diaz PR, Mendoza-Briceño RV, Delgado-Saez MC, Palacios-Prü EL. Levels of amino acid neurotransmitters during mouse cerebellar neurogenesis and in histotypic cerebellar cultures. Dev Neurosci. 1999;21:147–158. doi: 10.1159/000017377. [DOI] [PubMed] [Google Scholar]
- 31.Nishiwaki T, Maeda N, Noda M. Characterization and developmental regulation of proteoglycan-type protein tyrosine phosphatase ζ/RPTPβ isoforms. J Biochem. 1998;123:458–467. doi: 10.1093/oxfordjournals.jbchem.a021959. [DOI] [PubMed] [Google Scholar]
- 32.Peles E, Schlessinger J, Grumet M. Multi-ligand interactions with receptor-like protein tyrosine phosphatase β: implications for intercellular signaling. Trends Biochem Sci. 1998;23:121–124. doi: 10.1016/s0968-0004(98)01195-5. [DOI] [PubMed] [Google Scholar]
- 33.Qi M, Ikematsu S, Maeda N, Ichihara-Tanaka K, Sakuma S, Noda M, Muramatsu T, Kadomatsu K. Haptotactic migration induced by midkine. Involvement of protein-tyrosine phosphatase ζ, mitogen-activated protein kinase, and phosphatidylinositol 3-kinase. J Biol Chem. 2001;276:15868–15875. doi: 10.1074/jbc.m005911200. [DOI] [PubMed] [Google Scholar]
- 34.Rakic P, Sidman RL. Organization of cerebellar cortex secondary to deficit of granule cells in weaver mutant mice. J Comp Neurol. 1973;152:133–162. doi: 10.1002/cne.901520203. [DOI] [PubMed] [Google Scholar]
- 35.Rothstein JD, Martin L, Levey AI, Dykes-Hoberg M, Jin L, Wu D, Nash N, Kuncl RW. Localization of neuronal and glial glutamate transporters. Neuron. 1994;13:713–725. doi: 10.1016/0896-6273(94)90038-8. [DOI] [PubMed] [Google Scholar]
- 36.Sakai T, Kyogashima M, Kariya Y, Urano T, Takada Y, Takada A. Importance of GlucUAβ1–3GalNAc(4S, 6S) in chondroitin sulfate E for t-PA- and u-PA-mediated Glu-plasminogen activation. Thromb Res. 2000;100:557–565. doi: 10.1016/s0049-3848(00)00365-0. [DOI] [PubMed] [Google Scholar]
- 37.Schwartz PM, Borghesani PR, Levy RL, Pomeroy SL, Segal RA. Abnormal cerebellar development and foliation in BDNF−/− mice reveals a role for neurotrophins in CNS patterning. Neuron. 1997;19:269–281. doi: 10.1016/s0896-6273(00)80938-1. [DOI] [PubMed] [Google Scholar]
- 38.Shimamoto K, LeBrun B, Yasuda-Kamatani Y, Sakaitani M, Shigeri Y, Yumoto N, Nakajima T. dl-threo-β-benzyloxyaspartate, a potent blocker of excitatory amino acid transporters. Mol Pharmacol. 1998;53:195–201. doi: 10.1124/mol.53.2.195. [DOI] [PubMed] [Google Scholar]
- 39.Shintani T, Watanabe E, Maeda N, Noda M. Neurons as well as astrocytes express proteoglycan-type protein tyrosine phosphatase ζ/RPTPβ: analysis of mice in which the PTPζ/RPTPβ gene was replaced with the LacZ gene. Neurosci Lett. 1998;247:135–138. doi: 10.1016/s0304-3940(98)00295-x. [DOI] [PubMed] [Google Scholar]
- 40.Snyder SE, Li J, Schauwecker PE, McNeill TH, Salton SRJ. Comparison of RPTPζ/β, phosphacan, and trkB mRNA expression in the developing and adult rat nervous system and induction of RPTPζ/β and phosphacan mRNA following brain injury. Mol Brain Res. 1996;40:79–96. doi: 10.1016/0169-328x(96)00039-3. [DOI] [PubMed] [Google Scholar]
- 41.Sotelo C. Anatomical, physiological and biochemical studies of the cerebellum from mutant mice. II. Morphological study of cerebellar cortical neurons and circuits in the weaver mouse. Brain Res. 1975;94:19–44. doi: 10.1016/0006-8993(75)90874-4. [DOI] [PubMed] [Google Scholar]
- 42.Stoppini L, Buchs P, Muller D. A simple method for organotypic cultures of nervous tissue. J Neurosci Methods. 1991;37:173–182. doi: 10.1016/0165-0270(91)90128-m. [DOI] [PubMed] [Google Scholar]
- 43.Tanaka M, Tomita A, Yoshida S, Yano M, Shimizu H. Observation of the highly organized development of granule cells in rat cerebellar organotypic cultures. Brain Res. 1994;641:319–327. doi: 10.1016/0006-8993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 44.Ullensvang K, Lehre KP, Storm-Mathisen J, Danbolt NC. Differential developmental expression of the two rat brain glutamate transporter proteins GLAST and GLT. Eur J Neurosci. 1997;9:1646–1655. doi: 10.1111/j.1460-9568.1997.tb01522.x. [DOI] [PubMed] [Google Scholar]
- 45.Watase K, Hashimoto K, Kano M, Yamada K, Watanabe M, Inoue Y, Okuyama S, Sakagawa T, Ogawa S, Kawashima N, Hori S, Takimoto M, Wada K, Tanaka K. Motor discoordination and increased susceptibility to cerebellar injury in GLAST mutant mice. Eur J Neurosci. 1998;10:976–988. doi: 10.1046/j.1460-9568.1998.00108.x. [DOI] [PubMed] [Google Scholar]
- 46.Wewetzer K, Rauvala H, Unsicker K. Immunocytochemical localization of the heparin-binding growth-associated molecule (HB-GAM) in the developing and adult rat cerebellar cortex. Brain Res. 1995;693:31–38. doi: 10.1016/0006-8993(95)00683-h. [DOI] [PubMed] [Google Scholar]
- 47.Wilson MT, Keith CH. Glutamate modulation of dendrite outgrowth: alterations in the distribution of dendritic microtubles. J Neurosci Res. 1998;52:599–611. doi: 10.1002/(SICI)1097-4547(19980601)52:5<599::AID-JNR12>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 48.Yamada K, Fukaya M, Shibata T, Kurihara H, Tanaka K, Inoue Y, Watanabe M. Dynamic transformation of Bergmann glial fibers proceeds in correlation with dendritic outgrowth and synapse formation of cerebellar Purkinje cells. J Comp Neurol. 2000;418:106–120. [PubMed] [Google Scholar]
- 49.Yamamoto N, Kurotani T, Toyama K. Neural connections between the lateral geniculate nucleus and visual cortex in vitro. Science. 1989;245:192–194. doi: 10.1126/science.2749258. [DOI] [PubMed] [Google Scholar]