Abstract
Afferent transmission can be regulated (or gated) so that responses to peripheral stimuli are adjusted to make them appropriate for the ongoing phase of a motor program. Here, we characterize a gating mechanism that involves regulation of spike propagation inAplysia mechanoafferent B21. B21 is striking in that afferent transmission to the motor neuron B8 does not occur when B21 is at resting membrane potential. Our data suggest that this results from the fact that spikes are not actively propagated to the lateral process of B21 (the primary contact with B8). When B21 is peripherally activated at its resting potential, electrotonic potentials in the lateral process are on average 11 mV. In contrast, mechanoafferent activity is transmitted to B8 when B21 is centrally depolarized via current injection. Our data suggest that central depolarization relieves propagation failure. Full-size spikes are recorded in the lateral process when B21 is depolarized and then peripherally activated. Moreover, changes in membrane potential in the lateral process affect spike amplitude, even when the somatic membrane potential is virtually unchanged. During motor programs, both the lateral process and the soma of B21 are phasically depolarized via synaptic input. These depolarizations are sufficient to convert subthreshold potentials to full-size spikes in the lateral process. Thus, our data strongly suggest that afferent transmission from B21 to B8 is, at least in part, regulated via synaptic control of spike initiation in the lateral process. Consequences of this control for compartmentalization in B21 are discussed, as are specific consequences for feeding behavior.
Keywords: sensorimotor integration, sensory gating, central pattern generator, mollusc, feeding, motor program
Introduction
Many motor behaviors are mediated by networks, central pattern generators (CPGs) that can generate rhythmic output in the absence of afferent input (Delcomyn, 1980; Marder, 2001). However, physiologically, CPGs often receive sensory input so that output is adjusted to compensate for changes in the periphery (Rossignol et al., 1988; Pearson, 1993; Marder, 2001; McCrea, 2001;Suster and Bate, 2002). When this occurs, changes in motor output are not always solely determined by stimulus properties. Instead, peripherally generated and centrally generated activity can be integrated so that stimulus-induced changes in motor output depend on the state of the ongoing motor program (Pearson and Ramirez, l997;McCrea, 2001). Thus, afferent transmission can be regulated (i.e., gated) during an ongoing motor program.
Mechanisms that gate-out afferent activity have been characterized most extensively. For example, terminals of sensory neurons can be rhythmically depolarized during motor programs at least in part via a conductance increase. When this occurs, spike amplitude and/or transmitter release can be decreased and afferent transmission can be inhibited (Clarac and Cattaert, 1996; Rudomin, 1999; Cattaert et al., 2001). Although this form of control has been described in a number of contexts, it is becoming increasingly apparent that afferent transmission can be gated via a number of diverse mechanisms (Sillar, 1991; Pearson and Ramirez, l997; DiCaprio, 1999; Gosgnach et al., 2000). In this study, we characterize a mechanism that gates-in rather than gates-out afferent activity.
Studies of afferent gating are often limited by technical difficulties. Consequently, a number of studies of afferent transmission have been performed in experimentally advantageous invertebrate preparations. We use one such preparation and study sensory neurons activated during feeding in the mollusc Aplysia (Evans and Cropper, 1998;Evans et al., 1999). These neurons have features that facilitate studies of afferent transmission. For example, their somata are centrally rather than peripherally located, which makes them easily reidentifiable. Their major processes are also relatively large and can be impaled with standard microelectrodes.
The neuron in this study, B21, is a bipolar mechanoafferent that innervates a muscle, the subradula tissue (SRT) (Cropper et al., 1996;Rosen et al., 2000) (see Fig. 1B). The SRT underlies the radula, a structure used to move food into the buccal cavity ofAplysia. In this study, we concentrate on one of the output connections of B21, its excitatory chemical connection with a radula-closer motor neuron (B8) (Klein et al., 2000; Rosen et al., 2000). When B21 is peripherally activated at its resting membrane potential, postsynaptic potentials (PSPs) are not observed in B8 (Rosen et al., 2000). In contrast, if B21 is centrally depolarized and then peripherally activated, PSPs are observed in B8 (Rosen et al., 2000). Thus, B21 afferent transmission to B8 must be actively gated-in. Preliminary data have provided insights into how gating may occur (Borovikov et al., 2000; Rosen et al., 2000). In this study, we verify and extend previous hypotheses. Moreover, we present data that indicate that afferent transmission is regulated during physiologically characterized motor programs.
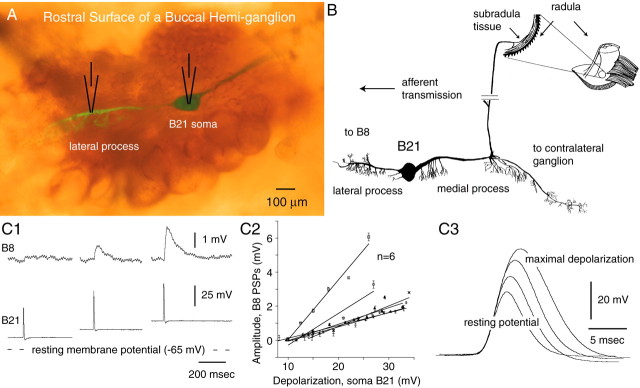
Fig. 1.
A, Verification of lateral process recordings. The rostral surface of a buccal hemi-ganglion viewed with both epifluorescence and epi-illumination is shown. B21 was injected with fast green dye before electrophysiological experiments, and carboxyfluorescein was injected after experiments.B, B21 morphology. The medial process of B21 bifurcates and innervates the contralateral buccal ganglion and both the ipsilateral and contralateral subradula tissue (only the contralateral innervation is shown). The lateral process is the primary point of contact with the radula-closer motor neuron B8. C1–C3, Somatic depolarization gates-in B21 afferent input to the radula-closer motor neuron B8 and increases the amplitude and half-width of somatic spikes. Experiments with a current passing and recording electrode in B21 and a single electrode in B8 are shown. C1,Bottom traces, Each mechanical stimulus triggered a single spike in B21 (as monitored in the soma). Top traces, PSPs (or lack thereof) in B8. As B21 was depolarized, PSPs became apparent and progressively increased in amplitude.C2, Group data. C3, Effects of somatic depolarization on somatic spikes. Increases in amplitude and half-width were both statistically significant (two-tailed paired ttest; p < 0.0001 for the half-width comparison;p = 0.0001 for the amplitude comparison).
Materials and Methods
Animals. Experiments were conducted with 23 200–300 gm Aplysia californica (Marinus, Long Beach, CA) that had been maintained in 14–16°C holding tanks. Animals were anesthetized by injection of isotonic MgCl2 and then dissected to create the reduced preparations described below. The nomenclature follows that of Gardner (1971). All experiments were conducted in artificial seawater composed of the following (in mm): 460 NaCl, 10 KCl, 11 CaCl2, 55 MgCl2, and 5 NaHCO3.
Identification of B21. B21 was identified as described previously (Rosen et al., 2000). The average resting potential of B21 (measured in its soma) was −63.8 ± 1.7 mV, and the average threshold for spike initiation when current was injected into the soma was −35.6 ± 1.8 mV (n = 5).
Preparations. Most experiments were conducted in preparations that consisted of the two buccal hemi-ganglia and the isolated SRT. The buccal mass was dissected so that the SRT could be removed from beneath the radula. Because the sensory innervation of the SRT passes through the radula nerve, this nerve was left intact (Borovikov et al., 2000). All other buccal nerves were severed. In motor-program experiments, preparations also included the cerebral ganglion and the cerebral buccal connectives.
Electrophysiology. Up to four simultaneous intracellular recordings were amplified and displayed using Getting Model 5A amplifiers (Getting Instruments, Iowa City, IA) modified for 100 nA current injection, Tektronix (Wilsonville, OR) AM 502 amplifiers, a four channel Tektronix storage oscilloscope (model 5111), and an eight channel Astro-Med chart recorder (model 9500; Grass Instruments, Quincy, MA). Some data were digitized using a Digidata (Axon Instruments, Foster City, CA) and were acquired using Axograph software (Axon Instruments) and a Macintosh G3 computer. Data were filtered electronically with a 1 kHz high-pass filter, and PSP recordings were filtered digitally with Axograph. To record from the somata of neurons, we used single-barrel electrodes fabricated from thin-walled glass capillary tubing filled with 2 m potassium acetate. Electrodes were beveled so that their impedances were generally 5–10 MΩ. To record from the lateral or medial process of B21, microelectrodes had a high resistance (generally ∼50 MΩ) and contained 3% 5(6)-carboxyfluorescein dye in 0.1 m potassium citrate (to verify recording sites as described below). Specifically, electrodes were backfilled by briefly touching the blunt end to the carboxyfluorescein solution. With this method, only the pulled tip of the electrode was filled with dye. To depolarize or hyperpolarize cells, DC was manually injected. When a current was injected via high-resistance carboxyfluorescein electrodes, the injection was continuously adjusted to compensate for progressive increases in electrode resistance.
In experiments in which we recorded from the lateral process of B21, we injected fast green dye into its soma. After ∼15–30 min, the lateral and medial processes could be visualized. To facilitate penetration of the lateral process, we often removed some of the overlying connective tissue and small cells using a glass micropipette. Physiological experiments were initiated by placing a microelectrode in the soma of B21. We then attempted to penetrate the lateral processes. We assumed that we were successful if we saw a simultaneous disturbance in the soma recording. In addition, we attempted to gate-in responses to peripheral stimulation and confirmed that lateral spikes were recorded after soma spikes. At the conclusion of experiments, we verified recording sites by injecting carboxyfluorescein dye (see Fig.1A).
Buccal motor programs. Buccal motor programs can be induced via stimulation of cerebral buccal interneurons (CBIs). Motor programs induced by CBI-2 activity have been characterized in detail previously (Rosen et al., 1991; Church and Lloyd, 1994; Morgan et al., 2000;Sanchez and Kirk, 2000; Jing and Weiss, 2001; Morgan et al., 2002). These programs can be clearly ingestive, egestive, or “intermediate” (Jing and Weiss, 2001; Morgan et al., 2002). Programs are ingestive if radula-closer motor neurons are predominately active during radula retraction and are egestive if radula-closer motor neurons are predominately active during radula protraction (Morton and Chiel, 1993a,b). In intermediate (or ambiguous) programs, radula-closer motor neurons are active during both protraction and retraction (Morgan et al., 2002).
In one set of experiments, we recorded intracellularly from CBI-2, one of the B4/5 neurons, the B21 soma, and the radula-closer motor neuron B8. We classified cycles of motor programs using B8 activity (Morton and Chiel, 1993a,b; Jing and Weiss, 2001; Morgan et al., 2002). If B8 fired at <1 Hz during the first phase of the motor program (protraction) and fired at a higher frequency during the second phase (retraction), we classified the cycle as ingestive. If B8 was active during protraction but fired at <1 Hz during retraction, we classified the cycle of the motor program as egestive. In other experiments, we recorded from CBI-2 the B21 soma and the B21 lateral process. In these experiments, cycles of motor programs were classified using B4/5 activity (Jing and Weiss, 2001), which correlates negatively with B8 activity. If the B4/5 firing frequency during retraction was low (i.e., <6 Hz), and if B4/5 was active for <50% of the duration of retraction, the cycle of the program was classified as ingestive. If B4/5 fired at >13 Hz and was active for >50% of the duration of retraction, the cycle of the motor program was classified as egestive.
Peripheral stimulation of the subradula tissue. The SRT was peripherally stimulated as described previously (Cropper et al., 1996). Briefly, mechanical stimuli were delivered by means of a mini-speaker (Quam) that had a wooden stick (tip diameter, 1 mm) perpendicularly attached to the speaker membrane. Reproducible movements of the speaker membrane were regularly elicited by driving the speaker with a stimulator at ∼0.5–2 Hz (Grass Instruments).
Fluorescence microscopy. Dye-filled cells were viewed with a Nikon (Tokyo, Japan) Labphot2 microscope with epifluorescence and both trans- and epi-illumination. The microscope was equipped with a filter set to visualize fluorescein (B-2A; EX 450–490/DM 505/BA 520). Digital images were captured using a Nikon CoolPix 990 camera and compiled into figures using Adobe PhotoShop and Adobe Illustrator (Adobe Systems, San Jose, CA).
Data analysis. Spike amplitude, half-width, and rise time were all measured with Axograph. Kaleidagraph (Synergy Software, Reading, PA) and StatView (SAS Institute, Cary, NC) were used to plot data and perform statistical analyses. All values are given as means ± SEM.
Results
When B21 is peripherally activated at its resting membrane potential, PSPs are not recorded in the follower neuron B8. However, if B21 is depolarized by injecting current into its soma, PSPs are observed (Rosen et al., 2000) (Fig.1C1). A goal of this study was to characterize a mechanism that could underlie this phenomenon. Depolarization does produce a progressive increase in spike amplitude and half-width (Fig. 1C3). For example, when B21 was at its resting potential, peripherally triggered spikes recorded in the soma were on average 35.7 ± 2.6 mV, and the half-width was 4.9 ± 0.1 msec. When B21 was depolarized so that it was just below threshold, spikes were 51.6 ± 3.2 mV with a half-width of 7.4 ± 0.1 msec (both differences are statistically significant). However, the primary point of contact between B21 and B8 appears to be the lateral process and not the soma (Borovikov et al., 2000). We therefore sought to determine whether changes in spike characteristics in the soma are correlated with an effect of membrane potential on spike characteristics in the part of B21 that contacts B8 (i.e., the lateral process).
Spike propagation at resting membrane potential
To study lateral-process spikes, we peripherally activated B21 and simultaneously recorded from the lateral process and soma. When B21 was at its resting potential, the mean amplitude of potentials in the lateral process was 10.6 ± 1.1 mV (Fig.2A1,B). When depolarizing current was injected into the soma, the amplitude of potentials increased to a maximum value (50.0 ± 2.8 mV) (a statistically significant difference) (Fig.2A2,B). Amplitude was not increased further with additional depolarization; therefore, we refer to these potentials as full-size spikes. Thus, when B21 is at its resting membrane potential, depolarizing potentials in the lateral process are attenuated to the point at which it would be expected that there would be an effect on transmitter release.
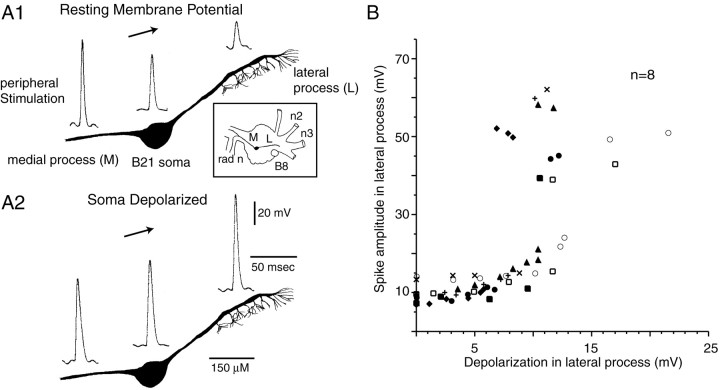
Fig. 2.
Spikes are attenuated in the lateral process of B21. A1, A2, B21 was peripherally activated, and intracellular recordings were obtained at approximately the positions indicated. Recording sites are indicated with respect to a camera lucida drawing of a typical cell. A1, When B21 was at its resting potential, spikes in the lateral process were attenuated. A2, When B21 was peripherally activated and depolarizing current was injected into the soma, spikes in the lateral process were no longer attenuated. Inset, Drawing that indicates the relative position of B8, the soma of B21, the medial process (M) of B21, and the lateral process (L) of B21. B, Relationship between the amount of depolarization in the lateral process and spike amplitude in the lateral process. A differentsymbol is used to plot data from each preparation. When B21 was centrally depolarized, there was a statistically significant increase in spike amplitude (two-tailed pairedt tests; p < 0.0001).
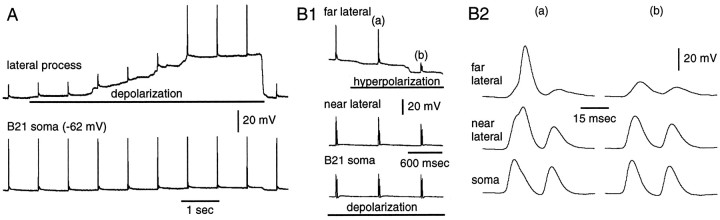
It is likely that potentials recorded from the lateral process are attenuated because they are passive reflections of spikes actively generated in medial parts of B21 (i.e., active spike propagation fails). Potentials recorded in the soma of B21 are generally smaller in amplitude than those recorded in the medial process, and potentials in the lateral process are generally smaller than those recorded in the soma (Fig. 2A1). To determine whether electrotonic decay occurs within the lateral process itself, we placed one electrode in the soma of B21 and a second electrode in the lateral process. We continuously activated B21 peripherally, and moved the lateral process electrode as far laterally as possible (n = 3). In other experiments, we recorded from the soma of B21 and simultaneously from two points in the lateral process (n = 2) (Fig.3). In both types of experiments, we found that when B21 was at its resting potential, the most laterally recorded potentials were the most attenuated (Fig.3B,C). We did not detect a reappearance of full-size spikes as we moved laterally. Thus, our data suggest that when B21 is at its resting potential, active spike propagation fails and afferent activity is not gated-in.
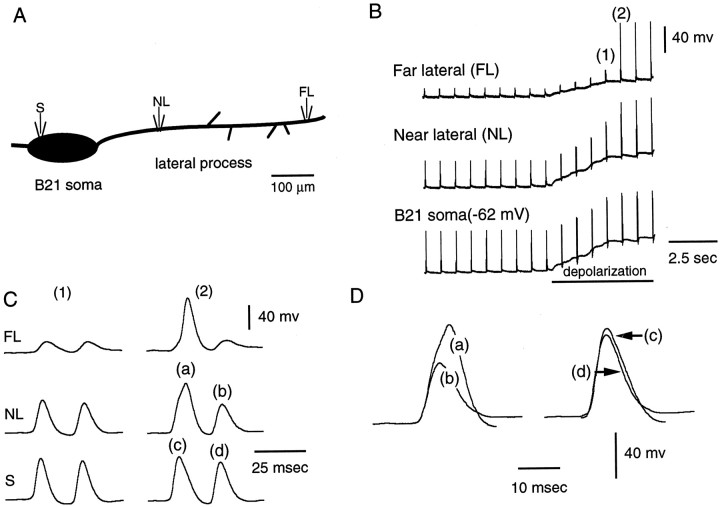
Fig. 3.
Spike attenuation and reflection occurs within the lateral process. A, Schematic representation of the neuron from which the data in B–D were obtained. One electrode was in the soma (S), and two electrodes were in the lateral process (NL, near lateral;FL, far lateral). B1, B2, B21 was peripherally activated at its resting potential (first part of record). Note that spikes were most attenuated at the far lateral site. Depolarizing current was injected into the soma (barunder bottom trace) and spikes became full-size at the lateral recording sites, indicating that the lateral process was not damaged. C1, C2, High-speed record of1 and 2 in B. Each stimulus triggered two spikes in B21. When full-size spikes were recorded at the far lateral site, spikes at the near lateral and soma sites were increased in amplitude and half-width (e.g., the left spike in 2). Da–Dd, Superimposition ofa–d from C. The effect of spike initiation at the far lateral site was more pronounced at the near lateral site then it was in the soma (i.e., the difference betweena and b is more than that betweenc and d).
Spike propagation with central depolarization: gating-in of mechanoafferent activity
As discussed above, when B21 is centrally depolarized and then activated peripherally, full-size spikes are recorded from the lateral process (Fig. 2A2). We hypothesize that this occurs because spike propagation no longer fails (i.e., spikes are actively generated in the lateral process). This model implies that the lateral process is capable of spike generation. To determine whether this is true, we injected current into the lateral process, and in 11 of 11 preparations found that spikes could be triggered (Fig.4A1). In most (8 of 11) cases, we found that spikes triggered by injecting current into the lateral process were significantly attenuated in the soma (suggesting that somatic parts of the cell were not contributing greatly to spike generation in these cases) (Fig. 4A1,left). Additionally, we isolated the lateral process from medial parts of B21 (i.e., the soma and medial process). Fast green dye was injected into B21, and the lateral process was visualized. We then severed the connection between the lateral process and the soma (Fig.4B1,B2). We impaled the isolated lateral process and found that spikes could be initiated by injecting depolarizing current (n = 7) (Fig.4B3). Thus, spikes can be initiated in the lateral process.
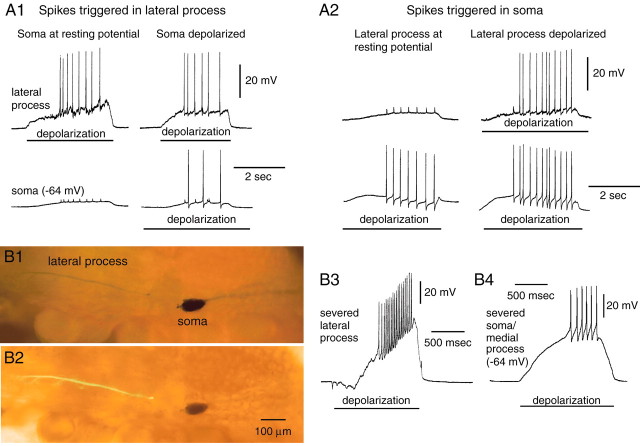
Fig. 4.
There are at least two sites for spike initiation in B21. A1, Spikes can be triggered by injecting current into the lateral process of B21 (bars under top traces). Left, In most preparations, spikes triggered in the lateral process were attenuated in the soma, suggesting that the soma was not making a major contribution to spike initiation. Right, To verify that the soma was not damaged, it was depolarized (bar under bottom trace), and current was injected into the lateral process. Full-size spikes were now observed in the soma. A2, Spikes can be triggered by injecting current into the soma of B21 (bars under bottom traces).Left, In 9 of 23 preparations, spikes triggered in this manner were attenuated in the lateral process, suggesting that the lateral process was not making a major contribution to spike initiation. Right, To verify that the lateral process was not damaged, it was depolarized (bar undertop trace), and current was injected again into the soma. B1–B4, Spikes can be initiated in both parts of B21 when the connection between the lateral process and soma is severed. B1, Preparation in which the data inB3 were obtained and viewed with epi-illumination alone (B1) or with both epifluorescence and epi-illumination (B2). B3, Spikes could be triggered in the isolated lateral process by injecting depolarizing current (bar). B4, Spikes could also be triggered by injecting current into the isolated soma/medial process (bar).
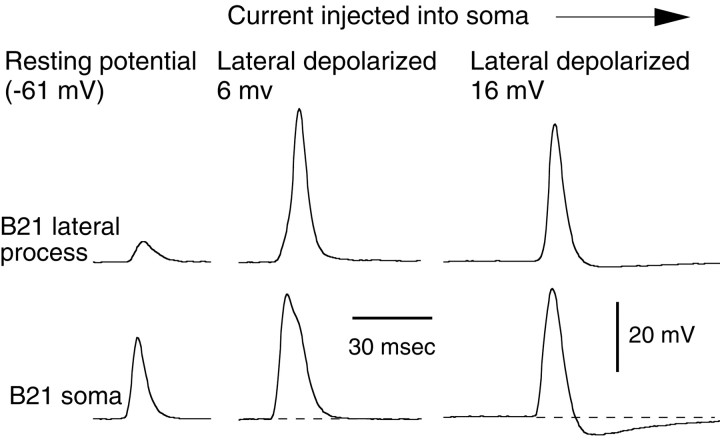
If active spike initiation in the lateral process is important for gating-in mechanoafferent input, changes in membrane potential in the lateral process itself (instead of the soma) should be able to affect afferent transmission. To determine whether this was the case, we induced afferent activity in B21 and injected depolarizing current in the lateral process (Fig. 5A). As expected, we did record full-size spikes in the lateral process. At resting membrane potential, depolarizing potentials were 10.4 ± 1.7 mV in amplitude, and with depolarization, potentials were 43.8 ± 4.4 mV (a statistically significant difference). We also found the converse to be true. If we depolarized the soma of B21 to the point at which spikes in the lateral process were full-size and then injected hyperpolarizing current into the lateral process, the amplitude of depolarizing potentials was reduced from 52.9 ± 1.9 to 16.5 ± 1.7 mV (also a statistically significant difference) (Fig. 5B1,B2).
Fig. 5.
Changes in membrane potential in the lateral process affect spike propagation. A, Depolarization of the lateral process increases spike amplitude. B21 was peripherally activated at its resting potential, and spikes in the lateral process were attenuated (first response). When depolarizing current was injected into the lateral process (bar under top trace), spike amplitude in the lateral process was increased despite the relatively small change in membrane potential in the soma. The increase was statistically significant (paired ttest; p = 0.0008; n = 5).B1, B2, Hyperpolarization decreases spike amplitude. An experiment with one electrode in the soma of B21 and two in the lateral process is shown. Peripheral stimulation triggered two spikes in B21. B1a, B1b, B21 was peripherally activated and depolarized (by injecting current into the soma) so that spikes in the lateral process were full-size (first response). When hyperpolarizing current was injected into the lateral process, spike amplitude was significantly decreased (e.g., third response) (paired t test; p < 0.0001; n = 3). B2a,B2b, Responses a and bfrom B1 at a faster sweep speed. Note that, when spikes were full-size at the far lateral recording site, spike amplitude and half-width were increased at both the near lateral and soma recording sites.
In the latter experiments, the lateral process and the soma are necessarily connected. Theoretically, changes in membrane potential in the lateral process could therefore produce changes in membrane potential and spike generation in the soma. However, note that in these experiments, changes in membrane potential that affected spike amplitude in the lateral process produced very little change in membrane potential in the soma. Specifically, the average change in somatic membrane potential was 3.0 ± 0.8 mV (range, 0–6.4 mV). In fact, in some (three of five) preparations, we were able to change spike initiation in the lateral process without any measurable change in the somatic membrane potential. In other preparations, changes in somatic membrane potential were relatively small. It is likely, therefore, that active processes in the soma are not necessary for spike initiation when afferent activity is gated-in. Together, our data support the idea that active spike propagation fails when mechanoafferent transmission does not occur, and that when mechanoafferent activity is gated-in, this propagation failure is relieved.
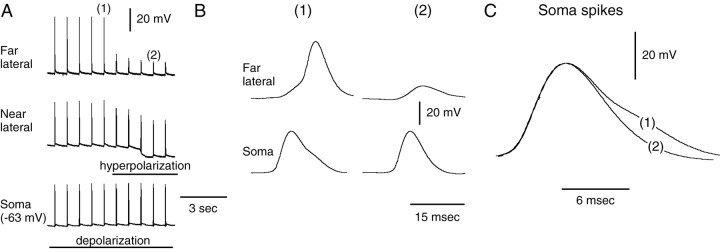
The above experiments emphasize the importance of active spike initiation in the lateral process for the gating-in of mechanoafferent activity. However, they do not indicate whether or not spikes are additionally actively triggered in the soma of B21. To verify that spike generation in the somatic region can occur, we triggered spikes by injecting current into the soma of B21 and simultaneously recorded from the lateral process. In 9 of 23 preparations, we found that spikes could be triggered in the soma/medial process spike-initiation zone when small attenuated potentials were recorded in the lateral process (suggesting that the lateral process was not contributing to spike generation) (Fig. 4A2). Additionally, when the connection between the lateral process and soma was severed in all preparations tested, we found that spikes could be triggered by injecting current into the isolated soma/medial process (n = 7) (Fig. 4B4). Thus, there is at least one spike-initiation zone in medial parts of B21 (i.e., parts of B21 relatively isopotential with the soma). Therefore, there are at least two possibilities for the gating-in of mechanoafferent input: (1) only the lateral spike-initiation site could be activated, or (2) the soma/medial process spike-initiation zone(s) and the lateral-process zone(s) could both be activated.
It is likely that activation of the soma/medial spike-initiation zone does not occur when afferent activity is gated-in via injection of current directly into the lateral process. Full-size spikes can be recorded in the lateral process when the lateral process is depolarized, but there is very little change in somatic membrane potential (Fig. 5A). Interestingly, however, it also appears that the somatic spike-initiation zone(s) is not always activated when afferent activity is gated-in via current injection into the soma. Spikes initiated by injecting current into the soma of B21 always have a clear afterhyperpolarization (Fig. 4B4). In contrast, when mechanoafferent activity is gated-in via somatic depolarization, depolarizing potentials recorded in the soma do not always have a clear afterhyperpolarization (they did not in 9 of 12 preparations) (Fig. 6). These depolarizing potentials are likely to be electrotonic potentials and not spikes. Electrotonic potentials such as axon spikes often do not have afterhyperpolarizations. Thus, even with somatic depolarization, it appears that activation of the somatic/medial process spike-initiation site may not occur.
Fig. 6.
Peripherally triggered spikes in the soma of B21 do not always have a clear afterhyperpolarization. Left, B21 was peripherally activated at its resting potential, and spikes in the lateral process were attenuated (as expected).Middle, When depolarizing current was injected into the soma, spikes in the lateral process increased in amplitude. Initially, soma spikes did not have a clear afterhyperpolarization.Right, With additional depolarization, an afterhyperpolarization became apparent in the soma spike. Thedashed line indicates the resting membrane potential.
To identify a variable that could determine whether activation of the somatic/medial process spike-initiation site occurs, we varied the somatic membrane potential as we gated-in afferent activity and determined whether somatic recordings were characterized by an afterhyperpolarization. We found that depolarization often changed a somatic recording without an afterhyperpolarization into a spike with a clear afterhyperpolarization (Fig. 6, middle vsright). This suggests that with relatively little somatic depolarization, the soma/medial process spike-initiation site is not always activated, possibly because it has a relatively high threshold. In fact, to initiate a spike in the soma of B21 on average, it is necessary to depolarize cells by 27.2 ± 1.1 mV. In contrast, the lateral process appears to have a relatively low threshold for spike initiation (at least under conditions in which subthreshold electrotonic depolarizations are converted to action potentials). On average, full-size spikes were recorded in the lateral process with peripheral activation when the lateral process was depolarized by 11.8 ± 0.9 mV. Thus, our data suggest that when mechanoafferent activity is gated-in, spikes must be actively generated in the lateral process. Additionally, they can be actively generated in the soma of B21, or the somatic spike-initiation site can be skipped. The type of transmission that will occur is likely to depend both on where B21 receives synaptic input and on how much it is depolarized.
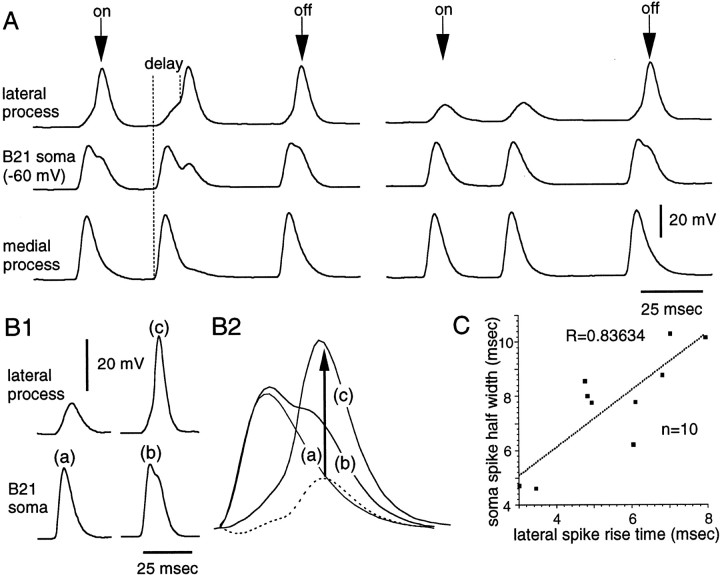
In final mechanistic experiments that examined the gating-in of mechanoafferent activity, we returned to an issue raised previously. Namely, in experiments in which we studied afferent transmission by recording PSPs from B8, we noted that as B21 was depolarized and PSPs appeared in B8, we observed changes in somatic spikes. Spike amplitude and half-width were increased (Fig. 1C3). Above, we emphasize the importance of active spike generation in the lateral process for the gating-in of afferent activity. We therefore sought to determine whether changes in soma spike characteristics could, at least in part, be caused by spike initiation in the lateral process. In these experiments, we took advantage of the fact that when somatic depolarizations are at a threshold value, the membrane potential of B21 can be held constant, and active spike initiation will occur in the lateral process when some peripheral stimuli are applied and will not occur when other stimuli are applied (as would be expected) (Segev and Schneidman, 1999) (Fig. 7A). When we compared somatic spikes that were recorded under these conditions, we found that soma spike amplitude was increased from 37.3 ± 2.3 to 39.9 ± 2 mV when lateral spikes were present. Soma spike half-width was increased from 6.1 ± 0.5 to 7.7 ± 0.6 msec. The effect on half-width was statistically significant, whereas the effect on amplitude was not (paired t test;p = 0.003 for the effect on half-width;p = 0.08 for the effect on amplitude). Thus, changes in somatic spikes are observed when full-size spikes are recorded in the lateral process, even when there is no change in the somatic membrane potential.
Fig. 7.
Spike initiation in the lateral process affects somatic spikes. A, Experiment in which B21 was peripherally activated so that two spikes were triggered as the probe contacted the SRT (on arrow above top trace) and one spike was triggered as an “off” response (off arrow). Depolarizing current was injected into the soma so that in some cases, full-size spikes were triggered in the lateral process (e.g., both on and off responses on theleft), and in other cases spikes were attenuated (e.g., the on response on the right). When a full-size spike was recorded in the lateral process, the half-width of the spike in the soma increased (e.g., on the right, compare themiddle traces during the on response with themiddle trace during the off response). Note that there can be a delay before spikes are initiated in the lateral process, (i.e., when medial parts of B21 spike, the lateral process is depolarized almost immediately) (left, dotted lines). Thus, the rise time of the spike in the lateral process varies. B1, B2, Changes in somatic spikes appear to be caused by spike initiation in the lateral process.B1a–B1c, Somatic spikes were generated without a lateral spike (action potential labeled a) and with a lateral spike (action potential labeled b).B2a–B2c, Superimposition of a–c fromB1. Also plotted is the difference betweena and b, which is indicated by adotted line. Note that the peak obtained by the subtraction reached its maximum value after c did.C, Data from 10 preparations showing that the rise time of the spike in the lateral process and the soma spike half-width are correlated (r = 0.84).
If changes in somatic spikes were at least in part a result of spike initiation in the lateral process, we would expect that they would satisfy several criteria. For example, we would expect them to be small. Spikes initiated in the lateral process produce relatively small depolarization in the soma (Fig. 4A1). To quantify changes in somatic spikes, we subtracted somatic recordings made when lateral spikes were not present from somatic recordings made when lateral spikes were present. In all cases, we obtained relatively small peaks of depolarization (Fig. 7B2, dotted line). Additionally, subtracted depolarizations should peak after or with action potentials in the lateral process. Presumably, if spiking in the lateral process is triggering the change in the soma spike, the change in the soma spike should not happen first. We found that subtracted depolarizations peaked after the lateral process spiked (Fig. 7B2). Thus, when spikes are initiated laterally, changes in spike characteristics are relatively small and peak after the lateral-process spike has peaked.
A second prediction stems from the observation that although spike initiation in medial parts of B21 is accompanied by a virtually simultaneous depolarization of the lateral process (Fig. 7A,left dotted line), in some cases the lateral process does not immediately spike (Fig. 7A, right dotted line, 3C, 5B2). In other systems, this pattern of depolarization has been referred to as a spike with a “foot” (Baccus, 1998). In other peripheral responses, there is less delay before spikes are initiated in the lateral process (Fig. 6,top middle trace). Thus, the rise time of the lateral-process spike varies. If lateral-process spike initiation does in fact affect somatic spike characteristics, it would be predicted that differences in lateral-spike rise time should be correlated with differences in somatic spike characteristics. We found a positive correlation between the lateral-spike rise time and the degree of broadening of the soma spike (Fig. 7C).
Finally, if spike initiation in the lateral process affects soma spikes, we would expect that if we inhibited spike initiation in the lateral process, we would see a change in the corresponding somatic spike. In three preparations, we were able to block spiking by injecting hyperpolarizing current into the lateral process with virtually no change in the somatic membrane potential (Fig.8A). In all cases, we found that spike half-width was decreased when the lateral process was hyperpolarized so that lateral spikes were not triggered (Fig.8B,C). In summary, our data indicate that when spikes are actively initiated in the lateral process of B21, the soma and medial parts of the cell are affected (i.e., a type of reflection occurs). This reflection is presumably more electrotonic then active (i.e., reflected depolarizations are small and appear to be graded) (Fig. 3D). If the lateral process generates action potentials relatively quickly, the amplitude of the soma spike is most likely to be affected. If the lateral process spikes with a delay, the half-width of the soma spike is most likely to be affected.
Fig. 8.
Inhibition of spiking in the lateral process alters spike half-width in the soma. An experiment with one electrode in the soma of B21 and two electrodes in the lateral process (similar to Fig. 3A) is shown. A1,A2, B21 was peripherally activated and depolarized (bar under bottom trace) so that full-size spikes were triggered in the lateral process. We then hyperpolarized the lateral process (bar undermiddle trace), which inhibited spiking in the lateral process. Lateral parts of the lateral process were presumably most affected, because potentials at the far lateral site were decreased in amplitude more than potentials at the near lateral site. [Presumably, this is a result of the fact that lateral parts of the lateral process were less depolarized (current was injected into the soma) and were therefore closer to threshold.] Note that the somatic membrane potential remained virtually unchanged. B,C, Peripheral responses 1 and2 from A at faster sweep speeds. Note that the half-width of the soma spike was decreased when spiking in the lateral process was prevented (most clearly seen in C, which is a superimposition of the soma spikes).
A possible significance of the potentiating effect of the lateral–medial-reflected depolarization is that it could decrease compartmentalization in B21 when afferent activity is gated-in. For example, it would be reasonable to predict that electrotonic potentials entering the somatic region of B21 would be decreased in size by the presence of the lateral process. [This would be predicted from cable theory (e.g., as a comparison of open-ended vs sealed-end cables). Additionally the input resistance of the cell is presumably decreased by the presence of the lateral process.] Experimentally, we verified this by comparing peripherally generated somatic spikes with and without the lateral process. We injected B21 neurons with fast green dye and then impaled somata with a current passing and recording electrode. We triggered activity peripherally and measured spike amplitude at different membrane potentials. We then removed electrodes and severed the connection between the soma and the lateral process. We reimpaled somata and found that in six of seven preparations, afferent spikes recorded at resting membrane potential were larger after the lateral process was severed (Fig. 9). Thus, the presence of the lateral process tends to decrease the size of entering electrotonic potentials. Interestingly, however, we found that as cells were depolarized, spike amplitude with the lateral process present increased more than spike amplitude without the lateral process, so that spikes generated with the lateral process were not statistically different from those without when depolarizations were >15 mV (Fig. 9). Thus, reflections of depolarizations from the lateral process could contribute to this “compensatory” response and reduce compartmentalization when afferent activity is gated-in.
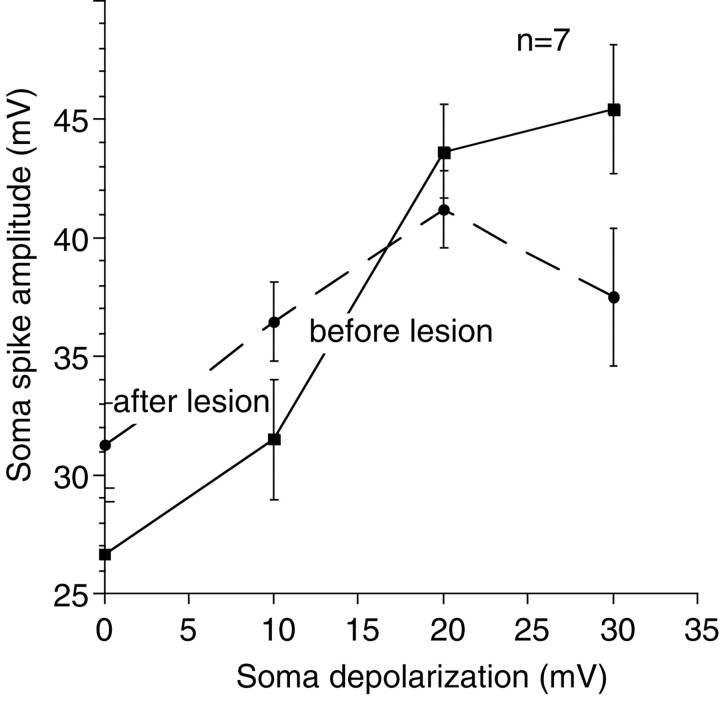
Fig. 9.
Afferent spikes in the soma before and after the lateral process lesions. When neurons were at their resting membrane potential, afferent spikes recorded after the lateral process had been lesioned were larger than spikes recorded before the lateral process was lesioned (two-tailed paired t test;p = 0.01) (a significant result even when a Bonferroni correction for the repeated measures is applied). When neurons were progressively depolarized, spike amplitude increased both with and without the lateral process (two-factor repeated-measures ANOVA; p < 0.001 for membrane potential;p = 0.0027 for membrane potential and lesion status). Note that, although spikes were initially smaller before the lesion, spike amplitude was increased at least as much as it was after the lesion (10, 20, and 30 mV spike amplitudes are not statistically different when a Bonferroni correction is applied). Overall, therefore, the effect of the lesion was not statistically significant (two-factor repeated-measures ANOVA; p = 0.87).
Afferent activity during ingestive motor programs
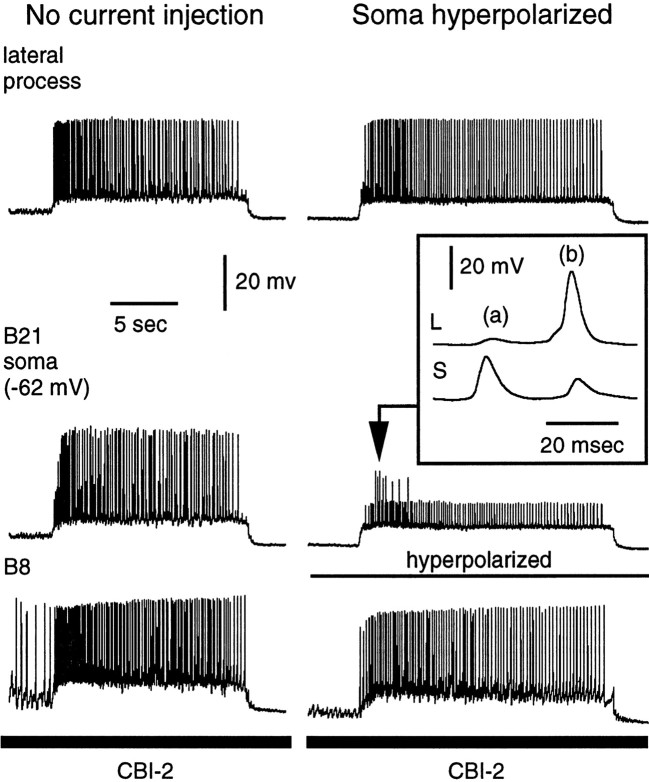
In a final set of experiments, we sought to determine whether changes in spike initiation could occur in the lateral process during physiologically characterized motor programs. Motor programs were induced via stimulation of the command-like neuron CBI-2, and in most (six of seven) preparations, B21 did not spike, or it only spiked occasionally before peripheral stimulation was initiated. Afferent transmission was not studied in the preparation in which there was a lot of centrally induced activity in B21. However, we did examine it to determine where B21 was receiving synaptic input (i.e., the lateral process vs the soma). We found that some central activity appeared to be initiated in somatic regions of the cell (i.e., spikes and/or excitatory synaptic potentials were larger in the soma than in the lateral process), and some activity appeared to be initiated in the lateral process (i.e., spikes and/or excitatory synaptic potentials were larger in the lateral process than the soma) (Fig.10). To verify that this was not unique to the one somewhat unusual preparation, we examined the central activity that we did observe in other preparations. Although there was much less of this activity, in all cases we were able to find examples of input to both the lateral process and soma.
Fig. 10.
Spikes can be initiated in the lateral process during motor programs. A motor program was induced by stimulation of CBI-2. In this preparation, an unusual amount of centrally induced activity was observed in B21. Only the retraction phase of the motor program is shown in its entirety. Left, Current was not injected into B21. Note that spikes are recorded from both the soma and lateral process. Right, To determine whether spikes originated in the soma or lateral process, hyperpolarizing current was injected into the soma. Many spikes were attenuated in the soma but remained full-size in the lateral process, suggesting that they were initiated in the lateral process. Inset, High-speed recording of the region indicated by the arrow.b, Note that, when spikes were full-size in the lateral process and attenuated in the soma, the peak of depolarization in the lateral process preceded the peak of depolarization in the soma.a, Note that the soma also appeared to be receiving synaptic input, and some depolarizing potentials were larger and peaked earlier than corresponding potentials in the lateral process.L, Lateral; S, soma.
Some cycles of motor programs were classified as ingestive, whereas others were classified as egestive (Jing and Weiss, 2001; Morgan et al., 2002). Some cycles of motor programs were not easily classified as either egestive or ingestive and have been referred to as intermediate (see Materials and Methods). During some cycles of motor programs, IPSPs were observed in B21 during retraction (i.e., during the time when B21 was centrally depolarized). These IPSPs were particularly apparent during egestive and intermediate cycles of motor programs. The effects of this inhibitory input on spike transmission will be considered in a separate study. Here, we concentrate on the effects of depolarization on afferent transmission and specifically study ingestive activity.
When B21 was peripherally activated (i.e., a mechanical stimulus was repeatedly applied to the SRT), action potentials were recorded in the B21 soma during both the protraction and retraction phases of ingestive motor programs (Fig. 11). To determine whether rhythmic depolarizations were sufficient to affect afferent transmission, we initially recorded from the soma of B21. We measured spike characteristics (amplitude and half-width) before motor programs were initiated and during the latter half of the retraction phase of the motor programs (i.e., when B21 was not receiving inhibitory input). In all cases (i.e., in six of six preparations), we observed an increase in both spike amplitude and half-width in soma recordings (Fig. 11A2). Both increases were statistically significant.
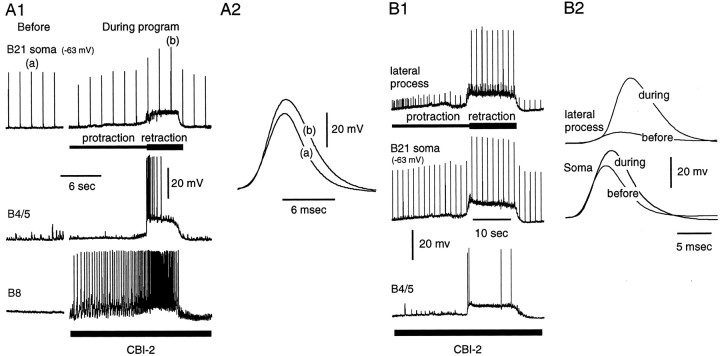
Fig. 11.
A1, A2, Somatic potentials are altered during the retraction phase of ingestive motor programs. A1, Left, Peripheral activation of B21 before the motor program. Right, A single cycle of a motor program induced by stimulation of CBI-2. B21 was centrally depolarized during the retraction phase of the motor program, but there was very little centrally induced spike activity in B21 (none is visible in the stretch of recording shown). The spikes apparent in the soma were peripherally triggered. A2, Superimposition ofA1a and A1b. Note that, when B21 was centrally depolarized, spikes were increased in amplitude and half-width. Both changes were statistically significant (two-tailed paired t test; p = 0.003 for the half-width comparison; p = 0.005 for the amplitude comparison). B1, B2, Increases in the amplitude and half-width of somatic potentials are correlated with changes in spike amplitude in the lateral process. A single cycle of a motor program induced by stimulation of CBI-2 is shown.B1, During the retraction phase of the motor program, B21 was centrally depolarized and spike amplitude increased in the lateral process. B2, Superimposition of soma (bottom traces) and lateral process (top traces) spikes before and during the retraction phase of the motor program. Soma spikes were increased in amplitude and half-width during retraction, and there was a corresponding change in the amplitude of the lateral process recording.
To determine whether changes in somatic potentials were indicative of changes in spike initiation in the lateral process, in three preparations we simultaneously recorded from the lateral process and the soma during motor programs. We found that changes in spike amplitude in the two regions were always correlated. As was expected, changes in spike amplitude in the lateral process were much more dramatic than changes in spike amplitude in the soma (Fig.11B2). On average, we observed a 222 ± 136% increase in spike amplitude in the lateral process and a 32 ± 8% increase in spike amplitude in the soma. The variability in the effect of spike amplitude in the lateral process was likely to be attributable to, at least in part, differences in the placement of the electrodes. For example, in one preparation, the increase in spike amplitude in the lateral process was only 25% and we were recording relatively close to the soma. In the other two preparations, we were farther from the soma, and the increases were 159 and 483%. In summary, we conclude that motor program-induced changes in membrane potential are sufficient to affect spike propagation to the lateral process of B21.
Discussion
Gating mechanism
The B21 to B8 connection is striking in that afferent activity is not relayed to B8 when B21 is at its resting potential (Rosen et al., 2000) (Fig. 1C1,C2). Our data suggest that this results from a failure of active spike propagation. Depolarizing potentials in the lateral process of B21, the primary contact with B8, are only ∼11 mV. Presumably, propagation failure results, at least in part, from impedance mismatch as activity is relayed from the medial process to the much larger soma (Yau, 1976; Haydon and Winlow, 1982;Altrup and Speckmann, 1984; Weiss et al., 1986; Luscher et al., 1994;Spruston et al., 1995; Antic et al., 2000). When spike propagation in bipolar and unipolar cells has been compared, it has become apparent that propagation is less reliable when cells are bipolar, particularly when somata are large (Luscher et al., 1994). Additionally, however, other factors may contribute. For example, B21 spikes are characterized by an afterhyperpolarization, which could inhibit conduction (Van Essen, 1973). In any case, active spike propagation fails, and potentials decrement as they spread throughout the lateral process. Potentials decrement to the point at which they are not likely to produce significant increases in intracellular calcium (Spruston et al., 1995). Consequently, transmitter release will be reduced.
In contrast, when B21 is centrally depolarized and then peripherally activated, EPSPs are recorded in B8 (Rosen et al., 2000) (Fig.1C1,C2). Thus, depolarization gates-in afferent input, presumably because spikes are now initiated in the lateral process. This gates-in afferent input in a graded manner (Fig.1C2), which may indicate that other mechanisms for plasticity are also present at this synapse (e.g., the release process itself may be voltage-sensitive). Under physiological conditions, spike initiation in the lateral process is presumably determined by chemical and/or electrical input to B21. During motor programs, we recorded excitatory potentials in the soma and lateral process of B21 that were of central origin. Moreover, when B21 was peripherally activated, motor program-induced depolarizations were sufficient to convert depolarizations in the lateral process to full-size action potentials. The regulation of afferent transmission from B21 to B8 could therefore be considered “presynaptic” in that afferent transmission is regulated in B21, which is presynaptic to B8. Presynaptic mechanisms for controlling afferent activity have been described in a number of contexts (Clarac and Cattaert, 1996; Mar and Drapeau, 1996; Apps, 1999;Rudomin, 1999; Wachowiak and Cohen, 1999; Cattaert et al., 2001).
If the proposed mechanism is considered presynaptic, it would be a form of presynaptic facilitation. However, presynaptic facilitation is often thought of as a process that alters spike-induced transmitter release (Klein, 1995). Spikes are propagated to terminals without facilitatory input, but facilitatory input enhances excitation–secretion coupling. With this arrangement, the input modifying afferent transmission is necessarily close to the site of transmitter release (as is also often the case in presynaptic inhibition) (Nusbaum et al., 1997). We have not yet identified the sites at which B21 receives facilitatory input, but depolarizations are recorded throughout the cell, indicating that input is not restricted to release sites. Thus, our results add to a growing body of work that indicates that spike propagation itself can be altered by synaptic input (Meyrand et al., 1992; Wall, 1995; Mar and Drapeau, 1996; Debanne et al., 1999; Johnston et al., 1999). This type of control has been referred to as pre-presynaptic (Wall, 1995). A distinction between presynaptic and pre-presynaptic control may be important, because different types of regulation can have different functional consequences (Segev, 1990).
Functional considerations of presynaptic phenomena often emphasize the resulting compartmentalization of a cell. For example, the output of one neuronal process can be altered, whereas the output of another process remains unchanged (Wall, 1995; Nusbaum et al., 1997; Clarac and Cattaert, 1999; Rudomin, 1999). Compartmentalization occurs when a neuron has output branches that are isolated from one another. Similarly, it might be predicted that the somatic region of B21 and its lateral process are different compartments, and that changes in spike initiation in the lateral process of B21 would be relatively inconsequential for somatic parts of the cell. A spike in the lateral process produces changes in membrane potential in the soma that are much more attenuated than the reflected spikes that exert potentiating actions in other contexts (Baccus, 1998; Baccus et al., 2000). During afferent transmission, however, depolarizations reflected “backward” (from the lateral process to the soma) summate with potentials traveling forward (from the medial process to the soma). Reflected potentials produce relatively small changes in somatic spikes, but these types of changes can produce dramatic effects on synaptic transmission in Aplysia in other contexts (Gingrich and Byrne, 1985; Gingrich et al., 1988). Thus, during afferent transmission, changes in spike initiation in the lateral process may exert important effects on the output of medial parts of B21, despite the unfavorable length constant. Therefore, our data suggest that in cases in which output regions of cells are not completely isolated, timing may be important in determining whether an electrical event in one part of the cell impacts another part. This type of consideration will obviously complicate the classification of one part of a cell as a separate compartment. A region may be a separate compartment under one set of circumstances but not another.
Consequences of gating for feeding in Aplysia
During ingestive behavior, B21 is presumably activated during the two antagonistic phases of the motor program. The tissue innervated by B21 is a muscle (Cropper et al., 1996) that contributes to radula opening (Borovikov et al., 2000). When the SRT contracts, B21 is activated (Borovikov et al., 2000). Thus, we have shown that B21 is activated during the radula-protraction phase of ingestive motor programs. Additionally, B21 is a low-threshold mechanoafferent that is activated when the radula is touched (Rosen et al., 2000). During ingestion, food will presumably activate B21, because the radula will close on food as it retracts. Thus, B21 will presumably be activated during both the radula-protraction and -retraction phases of ingestive motor programs.
If mechanoafferent input is transmitted to the radula-closer motor neuron B8 during radula protraction, the nature of the behavior will be altered. If the radula begins to close during protraction, food is pushed out of the buccal cavity. This is what occurs during egestive behaviors but not during ingestive behaviors (Morton and Chiel, 1993a,b). In contrast, if radula mechanoafferent input is transmitted to B8 during radula retraction, ingestive behavior will be enhanced. The radula will close more tightly as it pulls food into the buccal cavity. Thus, selective transmission of mechanoafferent input to B8 during radula retraction is likely to be functionally important for ingestive behaviors.
Although mechanoafferent input does not appear to be transmitted to B8 during the protraction phase of ingestive motor programs, it may be transmitted to another follower, B64. B64 differs from B8 in that the B21–B64 contact does not appear to be so exclusively via the lateral process (Borovikov et al., 2000). Spike attenuation in the lateral process is therefore likely to be of less relevance. B64 makes inhibitory connections with a number of protraction neurons and excitatory connections with retraction neurons (Hurwitz and Susswein, 1996; Hurwitz et al., 1997; Jing and Weiss, 2001, 2002). The activation of B64 is thought to be an important part of protraction–retraction phase transitions. Processes that accelerate B64 activation phase-advance the retraction phase of motor programs (Hurwitz and Susswein, 1996). B21 is particularly sensitive to the rate of contraction of the SRT (Borovikov et al., 2000). Thus, B21 will be strongly activated when radula opening (protraction) occurs quickly. Under these conditions, mechanoafferent input to B64 may be important for producing a corresponding phase advance of radula retraction. We have demonstrated that stimulation of B21 with brief current pulses decreases the duration of the protraction phase of CBI-2-induced motor programs (Borovikov et al., 2000).
In conclusion, during the protraction phase of ingestive activity, B21 is presumably functionally compartmentalized in that afferent activity is transmitted, at least to some degree, to the retraction interneuron B64, whereas it is not transmitted to the radula-closer motor neuron B8. At least in part, this is likely attributable to the fact that spike initiation in the lateral process fails, and electrotonic spikes are too small to induce transmitter release. Although spikes are also likely to be attenuated in medial parts of B21 (e.g., the soma), the attenuation is much less; in addition, the connection with B64 is electrical, and therefore presumably less dependent on the occurrence of full-size spikes.
During the retraction phase of ingestive motor programs, B21 is centrally depolarized, and spike initiation will occur in the lateral process. This will gate-in afferent input to B8 and contribute to increases in spike amplitude and half-width in somatic regions of B21. The changes in somatic spikes are likely to enhance mechanoafferent input to B64, which will, in turn, enhance retraction. Enhancements of both radula closing and retraction are likely to be important when food is ingested. Enhanced retraction will ensure that the radula moves deeply into the buccal cavity to deposit food in the esophagus. Enhanced closing will ensure that food is grasped tightly as it is internalized.
Footnotes
This work was supported by National Institutes of Health (NIH) K02 Award MH01267 and United States Public Health Service Grants MH51393 and MH35564. The National Resource for Aplysiaof the University of Miami provided some of the Aplysiaused in this study under National Center for Research Resources (NIH) Grant RR-10294. We thank K. R. Weiss and V. Brezina for valuable comments on a previous version of this manuscript.
Correspondence should be addressed to E. C. Cropper, Department of Physiology and Biophysics, P.O. Box 1218, Mount Sinai School of Medicine, 1 Gustave L. Levy Place, New York, NY 10029. E-mail:elizabeth.cropper@mssm.edu.
References
- 1.Altrup U, Speckmann EJ. Intrasomatically recorded action potentials in snail neurons (Helix pomatia): different shapes with different sites of origin in the neuronal arborization. A combined morphological and electrophysiological study. Comp Biochem Physiol [A] 1984;77:225–230. doi: 10.1016/0300-9629(84)90051-3. [DOI] [PubMed] [Google Scholar]
- 2.Antic S, Wuskell JP, Loew L, Zecevic D. Functional profile of the giant metacerebral neuron of Helix aspersa: temporal and spatial dynamics of electrical activity in situ. J Physiol (Lond) 2000;527:55–69. doi: 10.1111/j.1469-7793.2000.00055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Apps R. Movement-related gating of climbing fibre input to cerebellar cortical zones. Prog Neurobiol. 1999;57:537–562. doi: 10.1016/s0301-0082(98)00068-9. [DOI] [PubMed] [Google Scholar]
- 4.Baccus SA. Synaptic facilitation by reflected action potentials: enhancement of transmission when nerve impulses reverse direction at axon branch points. Proc Natl Acad Sci USA. 1998;95:8345–8350. doi: 10.1073/pnas.95.14.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baccus SA, Burrell BD, Sahley CL, Muller KJ. Action potential reflection and failure at axon branch points cause stepwise changes in EPSPs in a neuron essential for learning. J Neurophysiol. 2000;83:1693–1700. doi: 10.1152/jn.2000.83.3.1693. [DOI] [PubMed] [Google Scholar]
- 6.Borovikov D, Evans CG, Jing J, Rosen SC, Cropper EC. A proprioceptive role for an exteroceptive mechanoafferent neuron in Aplysia. J Neurosci. 2000;20:1990–2002. doi: 10.1523/JNEUROSCI.20-05-01990.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cattaert D, Libersat F, El Manira A. Presynaptic inhibition and antidromic spikes in primary afferents of the crayfish: a computational and experimental analysis. J Neurosci. 2001;21:1007–1021. doi: 10.1523/JNEUROSCI.21-03-01007.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Church PJ, Lloyd PE. Activity of multiple identified motor neurons recorded intracellularly during evoked feedinglike motor programs in Aplysia. J Neurophysiol. 1994;72:1794–1809. doi: 10.1152/jn.1994.72.4.1794. [DOI] [PubMed] [Google Scholar]
- 9.Clarac F, Cattaert D. Invertebrate presynaptic inhibition and motor control. Exp Brain Res. 1996;112:163–180. doi: 10.1007/BF00227635. [DOI] [PubMed] [Google Scholar]
- 10.Clarac F, Cattaert D. Functional multimodality of axonal tree in invertebrate neurons. J Physiol (Paris) 1999;93:319–327. doi: 10.1016/s0928-4257(00)80060-1. [DOI] [PubMed] [Google Scholar]
- 11.Cropper EC, Evans CG, Rosen SC. Multiple mechanisms for peripheral activation of the peptide-containing radula mechanoafferent neurons B21 and B22 of Aplysia. J Neurophysiol. 1996;76:1344–1351. doi: 10.1152/jn.1996.76.2.1344. [DOI] [PubMed] [Google Scholar]
- 12.Debanne D, Kopysova IL, Bras H, Ferrand N. Gating of action potential propagation by an axonal A-like potassium conductance in the hippocampus: a new type of non-synaptic plasticity. J Physiol (Paris) 1999;93:285–296. doi: 10.1016/s0928-4257(00)80057-1. [DOI] [PubMed] [Google Scholar]
- 13.Delcomyn F. Neural basis of rhythmic behavior in animals. Science. 1980;210:492–498. doi: 10.1126/science.7423199. [DOI] [PubMed] [Google Scholar]
- 14.DiCaprio RA. Gating of afferent input by a central pattern generator. J Neurophysiol. 1999;81:950–953. doi: 10.1152/jn.1999.81.2.950. [DOI] [PubMed] [Google Scholar]
- 15.Evans CG, Cropper EC. Proprioceptive input to feeding motor programs in Aplysia. J Neurosci. 1998;18:8016–8031. doi: 10.1523/JNEUROSCI.18-19-08016.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Evans CG, Alexeeva V, Rybak J, Karhunen T, Weiss KR, Cropper EC. A pair of reciprocally inhibitory histaminergic sensory neurons are activated within the same phase of ingestive motor programs in Aplysia. J Neurosci. 1999;19:845–858. doi: 10.1523/JNEUROSCI.19-02-00845.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971;173:550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- 18.Gingrich KJ, Byrne JH. Simulation of synaptic depression, posttetanic potentiation, and presynaptic facilitation of synaptic potentials from sensory neurons mediating gill-withdrawal reflex in Aplysia. J Neurophysiol. 1985;53:652–669. doi: 10.1152/jn.1985.53.3.652. [DOI] [PubMed] [Google Scholar]
- 19.Gingrich KJ, Baxter DA, Byrne JH. Mathematical model of cellular mechanisms contributing to presynaptic facilitation. Brain Res Bull. 1988;21:513–520. doi: 10.1016/0361-9230(88)90167-0. [DOI] [PubMed] [Google Scholar]
- 20.Gosgnach S, Quevedo J, Fedirchuk B, McCrea DA. Depression of group Ia monosynaptic EPSPs in cat hindlimb motoneurones during fictive locomotion. J Physiol (Lond) 2000;526:639–652. doi: 10.1111/j.1469-7793.2000.00639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Haydon PG, Winlow W. Multiple spike initiation sites and propagation failure allow neuronal compartmentalization. J Comp Physiol [A] 1982;147:503–510. [Google Scholar]
- 22.Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol. 1996;75:1327–1344. doi: 10.1152/jn.1996.75.4.1327. [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz I, Kupfermann I, Susswein AJ. Different roles of neurons B63 and B34 that are active during the protraction phase of buccal motor programs in Aplysia californica. J Neurophysiol. 1997;78:1305–1319. doi: 10.1152/jn.1997.78.3.1305. [DOI] [PubMed] [Google Scholar]
- 24.Jing J, Weiss KR. Neural mechanisms of motor program switching in Aplysia. J Neurosci. 2001;21:7349–7362. doi: 10.1523/JNEUROSCI.21-18-07349.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jing J, Weiss KR. Interneuronal basis of the generation of related but distinct motor programs in Aplysia: implications for current neuronal models of vertebrate intralimb coordination. J Neurosci. 2002;22:6228–6238. doi: 10.1523/JNEUROSCI.22-14-06228.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnston D, Hoffman DA, Colbert CM, Magee JC. Regulation of back-propagating action potentials in hippocampal neurons. Curr Opin Neurobiol. 1999;9:288–292. doi: 10.1016/s0959-4388(99)80042-7. [DOI] [PubMed] [Google Scholar]
- 27.Klein AN, Weiss KR, Cropper EC. Glutamate is the fast excitatory neurotransmitter of small cardioactive peptide-containing Aplysia radula mechanoafferent neuron B21. Neurosci Lett. 2000;289:37–40. doi: 10.1016/s0304-3940(00)01262-3. [DOI] [PubMed] [Google Scholar]
- 28.Klein M. Modulation of ion currents and regulation of transmitter release in short-term synaptic plasticity: the rise and fall of the action potential. Invert Neurosci. 1995;1:15–24. doi: 10.1007/BF02331828. [DOI] [PubMed] [Google Scholar]
- 29.Luscher C, Streit R, Quadroni R, Luscher HR. Action potential propagation through embryonic dorsal root ganglion cells in culture. I. Influence of the cell morphology on propagation properties. J Neurophysiol. 1994;72:622–633. doi: 10.1152/jn.1994.72.2.622. [DOI] [PubMed] [Google Scholar]
- 30.Mar A, Drapeau P. Modulation of conduction block in leech mechanosensory neurons. J Neurosci. 1996;16:4335–4343. doi: 10.1523/JNEUROSCI.16-14-04335.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marder E. Moving rhythms. Nature. 2001;410:755–756. doi: 10.1038/35071196. [DOI] [PubMed] [Google Scholar]
- 32.McCrea DA. Spinal circuitry of sensorimotor control of locomotion. J Physiol (Lond) 2001;533:41–50. doi: 10.1111/j.1469-7793.2001.0041b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meyrand P, Weimann JM, Marder E. Multiple axonal spike initiation zones in a motor neuron: serotonin activation. J Neurosci. 1992;12:2803–2812. doi: 10.1523/JNEUROSCI.12-07-02803.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morgan PT, Perrins R, Lloyd PE, Weiss KR. Intrinsic and extrinsic modulation of a single central pattern generating circuit. J Neurophysiol. 2000;84:1186–1193. doi: 10.1152/jn.2000.84.3.1186. [DOI] [PubMed] [Google Scholar]
- 35.Morgan PT, Jing J, Vilim FS, Weiss KR. Interneuronal and peptidergic control of motor pattern switching in Aplysia. J Neurophysiol. 2002;87:49–61. doi: 10.1152/jn.00438.2001. [DOI] [PubMed] [Google Scholar]
- 36.Morton DW, Chiel HJ. In vivo buccal nerve activity that distinguishes ingestion from rejection can be used to predict behavioral transitions in Aplysia. J Comp Physiol [A] 1993a;172:17–32. doi: 10.1007/BF00214712. [DOI] [PubMed] [Google Scholar]
- 37.Morton DW, Chiel HJ. The timing of activity in motor neurons that produce radula movements distinguishes ingestion from rejection in Aplysia. J Comp Physiol [A] 1993b;173:519–536. doi: 10.1007/BF00197761. [DOI] [PubMed] [Google Scholar]
- 38.Nusbaum MP, El Manira A, Gossard JP, Rossignol S. Presynaptic mechanisms during rhythmic activity in vertebrates and invertebrates. In: Stein PSG, Grillner S, Selverston A, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 237–253. [Google Scholar]
- 39.Pearson KG. Common principles of motor control in vertebrates and invertebrates. Annu Rev Neurosci. 1993;16:265–297. doi: 10.1146/annurev.ne.16.030193.001405. [DOI] [PubMed] [Google Scholar]
- 40.Pearson KG, Ramirez JM. Sensory modulation of pattern-generating circuits. In: Stein PSG, Grillner S, Selverston Al, Stuart DG, editors. Neurons, networks, and motor behavior. MIT; Cambridge, MA: 1997. pp. 225–236. [Google Scholar]
- 41.Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci. 1991;11:3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosen SC, Miller MW, Evans CG, Cropper EC, Kupfermann I. Diverse synaptic connections between peptidergic radula mechanoafferent neurons and neurons in the feeding system of Aplysia. J Neurophysiol. 2000;83:1605–1620. doi: 10.1152/jn.2000.83.3.1605. [DOI] [PubMed] [Google Scholar]
- 43.Rossignol S, Lund JP, Drew T. The role of sensory inputs in regulating patterns of rhythmical movements in higher vertebrates: a comparison between locomotion, respiration, and mastication. In: Cohen A, Rossignol S, Grillner S, editors. Neural control of rhythmic movements in vertebrates. Wiley; New York: 1988. pp. 201–283. [Google Scholar]
- 44.Rudomin P. Presynaptic selection of afferent inflow in the spinal cord. J Physiol (Paris) 1999;93:329–347. doi: 10.1016/s0928-4257(00)80061-3. [DOI] [PubMed] [Google Scholar]
- 45. Sanchez JA, Kirk MD. Short-term synaptic enhancement modulates ingestion motor programs of Aplysia. J Neurosci 20 2000. RC85(1–7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Segev I. Computer study of presynaptic inhibition controlling the spread of action potentials into axonal terminals. J Neurophysiol. 1990;63:987–998. doi: 10.1152/jn.1990.63.5.987. [DOI] [PubMed] [Google Scholar]
- 47.Segev I, Schneidman E. Axons as computing devices: basic insights gained from models. J Physiol (Paris) 1999;93:263–270. doi: 10.1016/s0928-4257(00)80055-8. [DOI] [PubMed] [Google Scholar]
- 48.Sillar KT. Spinal pattern generation and sensory gating mechanisms. Curr Opin Neurobiol. 1991;1:583–589. doi: 10.1016/s0959-4388(05)80032-7. [DOI] [PubMed] [Google Scholar]
- 49.Spruston N, Schiller Y, Stuart G, Sakmann B. Activity-dependent action potential invasion and calcium influx into hippocampal CA1 dendrites. Science. 1995;268:297–300. doi: 10.1126/science.7716524. [DOI] [PubMed] [Google Scholar]
- 50.Suster ML, Bate M. Embryonic assembly of a central pattern generator without sensory input. Nature. 2002;416:174–178. doi: 10.1038/416174a. [DOI] [PubMed] [Google Scholar]
- 51.Van Essen DC. The contribution of membrane hyperpolarization to adaptation and conduction block in sensory neurons of the leech. J Physiol (Lond) 1973;230:509–534. doi: 10.1113/jphysiol.1973.sp010201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wachowiak M, Cohen LB. Presynaptic inhibition of primary olfactory afferents mediated by different mechanisms in lobster and turtle. J Neurosci. 1999;19:8808–8817. doi: 10.1523/JNEUROSCI.19-20-08808.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wall PD. Do nerve impulses penetrate terminal arborizations? A pre-presynaptic control mechanism. Trends Neurosci. 1995;18:99–103. doi: 10.1016/0166-2236(95)93883-y. [DOI] [PubMed] [Google Scholar]
- 54.Weiss KR, Chiel HJ, Kupfermann I. Sensory function and gating of histaminergic neuron C2 in Aplysia. J Neurosci. 1986;6:2416–2426. doi: 10.1523/JNEUROSCI.06-08-02416.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yau KW. Receptive fields, geometry and conduction block of sensory neurons in the CNS of the leech. J Physiol (Lond) 1976;263:513–538. doi: 10.1113/jphysiol.1976.sp011643. [DOI] [PMC free article] [PubMed] [Google Scholar]