Abstract
Acetylcholine acts as a neurotransmitter in the retina. Although previous physiological studies have indicated that some retinal ganglion cells may be cholinergic, several immunohistochemical studies using antibodies to choline acetyltransferase (ChAT) have stained only amacrine cells but not ganglion cells. Recently, we identified a splice variant of ChAT mRNA, lacking exons 6–9, in rat peripheral nervous system. The encoded protein was designated as ChAT of a peripheral type (pChAT), against which an antiserum was raised. In the present study, we examined expression of pChAT in rat retina, both at the protein level by immunohistochemistry using the antiserum and at the mRNA level by RT-PCR. Immunohistochemistry revealed that although no positive neurons were found in untreated intact retinas, many neurons became immunoreactive for pChAT after intravitreal injection of colchicine. Damage of the optic nerve was also effective in disclosing positive cells. Such positive neurons were shown to be ganglion cells by double labeling with a retrograde tracer that had been injected into the contralateral superior colliculus. Western blot analysis and RT-PCR revealed a corresponding band to the pChAT protein and to the amplified pChAT gene fragment, respectively, in retinal samples. In addition, ChAT activity was definitely detected in retinofugal fibers of the optic nerve. These results indicate the presence of cholinergic ganglion cells in rat retina.
Keywords: retinal ganglion cells, immunohistochemistry, RT-PCR, neurotransmitter, alternative splicing, cholinergic transmission
Introduction
So far, neurotransmitters of retinal ganglion cells are generally unknown. A body of evidence indicates that glutamate is a major neurotransmitter of retinal ganglion cells. Glutamate immunoreactivity has been observed in these cells (Davanger et al., 1991; Crooks and Kolb, 1992; Kalloniatis and Fletcher, 1993;Jojich and Pourcho, 1996; Sun and Crossland, 2000) and their terminals (Montero, 1994; Ortega et al., 1995; Mize and Butler, 1996). In addition, glutamate receptors have been shown to be involved in the synaptic transmission in the retinogeniculate and retico-collicular pathways (Sillito et al., 1990; Roberts et al., 1991). Nevertheless, the role of glutamate as a major neurotransmitter of retinal ganglion cells remains to be confirmed. First of all, only 5–10% of the cells in the ganglion cell layer (GCL) have been reported to be labeled with [3H]-d-aspartate, the uptake of which is an indicator of neurons using glutamate as a transmitter (Beaudet et al., 1981; Ehinger, 1981). Second, glutamate has been proven not to be released from optic nerve terminals in a calcium-dependent manner (Sandberg and Corazzi, 1983; Tsai et al., 1990). The dipeptide N-acetylaspartylglutamate has also been suggested as a candidate transmitter, but this is still controversial (Anderson et al., 1987; Tsai et al., 1990; Tieman and Tieman, 1996). Some ganglion cells may have the synthetic capability for several neuropeptides (Kuljis et al., 1984; Cuenca and Kolb, 1989), which are not the major neurotransmitters of ganglion cells.
Acetylcholine (ACh) acts as a neurotransmitter in the retina (for review, see Puro, 1985; Ehinger and Dowling, 1987). The retina contains ACh and its biosynthetic enzyme, choline acetyltransferase (ChAT; E.C. 2.3.1.6). Although the cholinergic nature of retinal ganglion cells has once been suggested in the toad (Oswald and Freeman, 1980), many morphological studies have failed to support this possibility. Because there is no good histochemical technique for ACh itself, immunohistochemistry for ChAT has been regarded as the most reliable method for cholinergic neurons. Previous immunohistochemical studies using ChAT antibodies have constantly demonstrated ChAT immunoreactivity in amacrine cells but not in ganglion cells (Eckenstein and Thoenen, 1982; Tumosa et al., 1984; Schmidt et al., 1985; Pourcho and Osman, 1986a,b; Tumosa and Stell, 1986; Voigt, 1986). Even with such data, however, the possibility still remains that retinal ganglion cells use a different form of ChAT for synthesizing ACh.
Recently, Tooyama and Kimura (2000) cloned a different form of ChAT cDNA from rat pterygopalatine ganglion. This form of transcript lacks exons 6–9, indicating an alternative splicing event. An antiserum was raised against the peptide covering the splice joint of exons 5 and 10, which should be specific to the predicted protein. Immunohistochemistry using the antiserum clearly revealed peripheral cholinergic neurons, whereas it failed to reveal known cholinergic neurons in brain (Nakanishi et al., 1999; Nakajima et al., 2000; Tooyama and Kimura, 2000). Because of the preferential localization in peripheral neurons, the protein was designated pChAT (ChAT of a peripheral type), and the conventional or common form of the enzyme was termed cChAT (ChAT of the common type). The negative staining of peripheral cholinergic neurons by conventional cChAT antibodies suggests that epitopes of such cChAT antibodies preferentially lie within exons 6–9, which are absent in pChAT.
In the present study, we examined the possible existence of cholinergic ganglion cells in rat retina by immunohistochemistry and Western blot analysis using the pChAT antibody. In addition, expression of ChAT mRNA in the retina was analyzed by RT-PCR, and ChAT enzyme activity was examined in the optic nerve and retina of rats.
Finally, effects of light and darkness on the expression of pChAT were examined immunohistochemically in the retina, optic nerve, and optic tract. This is because concentrations of several putative neurotransmitters in the retina are known to be regulated by light/dark (Starr, 1973; Masland and Livingstone, 1976; Iuvone et al., 1978;Millar and Chubb, 1984). In addition, accumulating lines of evidence have shown that light stimuli induce the expression of Fos, an immediate early gene product, in the retina (Sagar and Sharp, 1990; Koistinaho and Sagar, 1995). Fos has been suggested to be among the transcription factors that regulate ACh synthesis (Koistinaho and Sagar, 1995). Thus, we were interested in studying the effect of light/dark adaptation on the expression of pChAT, possibly through the Fos-mediated mechanism.
Materials and Methods
Animals and surgical procedures. Male Wistar rats (Clea Japan, Tokyo, Japan), weighing 200–300 gm, were used. The animals were generally kept on a 12 hr light/dark cycle with ad libitum access to food and water. The rats were handled in compliance with the principles of the NIH Guide for the Care and Use of Laboratory Animals and the standards of animal experiments in our university.
All surgical procedures and intravitreal injections were performed under deep pentobarbital anesthesia (80 mg/kg). The first group of rats (n = 5) was used as normal animals without any treatment. In the second group of rats (n = 4), the right eye was enucleated. After unilateral enucleation, the animals were kept on a 12 hr light/dark cycle. They were perfused 14 or 28 d after the operation. In the third group of rats (n = 5), 10 μl of a solution of colchicine (Sigma, St. Louis, MO) (10 μg/μl dissolved in saline) was injected intravitreally into the right eye. After a survival period of 4–7 d, the animals were perfused. In the fourth group of rats (n = 6), the right optic nerve was damaged. Under deep anesthesia, the intraorbital portion of the optic nerve was approached by a skin incision on the cheek, and the Harderian gland and a part of the lacrimal gland were removed. The lateral and inferior rectus muscles were parted by blunt dissection, and the optic nerve was exposed. The nerve was then crushed twice for 30 sec each with a hemostatic forceps (n = 3) or pressure injected with 3 μl of 100% ethanol through a heat-pulled glass micropipette (n = 3). In either case, care was taken to avoid injury to the retinal blood supply. The rats were allowed to survive for 4–7 d.
Tissue preparations. Under pentobarbital anesthesia (80 mg/kg), the animals were perfused on crushed ice through the ascending aorta with 10 mm PBS, pH 7.4, followed by a fixative of 4% paraformaldehyde in 0.1 mphosphate buffer (PB), pH 7.4. After perfusion, bilateral eyes and the brain were removed. The brain tissues were immersed for 2 d in the same fixative at 4°C and then cryoprotected by immersion for 48 hr in 0.1 m PB containing 15% sucrose. They were frozen and cut into 20-μm-thick sections in a cryostat. The sections were collected and stored in 10 mm PBS containing 0.3% Triton X-100 (0.3% PBST) until stained.
The perfusion-fixed eye tissues were processed in two ways. First, gelatin-embedded transverse sections of the eye cup were prepared. In brief, the corneas and vitreous bodies were carefully removed from the eye tissues. The resultant eye cups were postfixed for 2 d with 4% paraformaldehyde in 0.1 m PB at 4°C. After rinsing with 0.1 m PBS, they were immersed for 4 hr in 0.1m PB containing 10% gelatin at 37°C and then placed for 1 hr in a cold chamber at 4°C. The gelatin-embedded specimens were cut into 5- to 7-mm-thick blocks that were postfixed overnight with 4% paraformaldehyde in 0.1 m PB at 4°C and then placed overnight in 0.1 m PB containing 15% sucrose at 4°C. Transverse sections of 30 μm thickness were cut in a cryostat and collected in 0.3% PBST.
Second, retinal whole mounts were prepared. The retinas were dissected out from the eye cups. After cutting radially, each retina was sandwiched between two sheets of filter paper and then fixed by immersion for 2 d in 4% paraformaldehyde in 0.1 m PB at 4°C. The fixed retinal tissues were then placed for 2 d in 0.1 m PB containing 15% sucrose at 4°C, before immunohistochemical staining. Bilateral eyes were simultaneously processed in all animals, and the eye tissue of the side with no surgical treatment or intraorbital injection always served as a control.
pChAT immunohistochemistry. Immunohistochemical staining was done in a free-floating state. The coronal sections of the brain and gelatin-embedded transverse sections of the eye cups were incubated for 3 d with the pChAT antiserum (diluted 1:80,000) or a goat anti-cChAT antibody (Chemicon, Temecula, CA) (AB144p, polyclonal; diluted 1:20,000) (Bruce et al., 1985) at 4°C, for 2 hr with biotinylated secondary antibody of appropriate species (Vector Laboratories, Burlingame, CA) (diluted 1:1000) at room temperature, and for 1 hr with the avidin–biotinylated peroxidase complex (Vector Laboratories; ABC Elite, diluted 1:2000) at room temperature. Dilution of the reagents and washing sections between each step were done with 0.3% PBST. Color was developed by reacting the sections for 20 min with a mixture containing 0.02% 3,3′-diaminobenzidine, 0.0045% H2O2, and 0.3% nickel ammonium sulfate in 50 mm Tris-HCl buffer, pH 7.6. The stained sections were air dried, washed in tap water, dried through a graded series of alcohol, cleared with xylene, and coverslipped with Entellan (Merck, Darmstadt, Germany).
To facilitate antibody penetration, the retinal whole mounts were freeze-thawed (Eldred et al., 1983) and then treated for 10 min with the protease papain (0.1 IU/ml in 0.1 m PBS) at room temperature, followed by fixation for 20 min with 4% paraformaldehyde at 4°C. After washing, they were immunostained for pChAT as described above, except that 0.6% PBST was used as a buffer system.
For immunohistochemical controls, the pChAT antiserum was replaced by the preimmune serum or by pChAT antiserum that had been preincubated overnight with the antigenic peptide of pChAT (41 amino acids) (Tooyama and Kimura, 2000). No positive staining was observed in these control studies. The number of cells labeled for pChAT was counted in 15 nonoverlapping areas (0.25 × 0.25 mm each) in the central and peripheral regions of the retina in three rats. The cell density in each region was calculated as the number of labeled cells per square millimeter. An estimate was also made for the total number of pChAT-positive cells in the retina, through the measurement of total area of each region and the mean pChAT-positive cell density per unit area.
Double staining for pChAT and a retrograde tracer, fluorescent latex microspheres. While the rats were under deep anesthesia with pentobarbital (80 mg/kg), 5 μl of a commercially available solution of red-fluorescent latex FluoSpheres (Molecular Probes, Eugene, OR) (mean diameter 40 nm; excitation 580 nm/emission 605 nm) (Persson and Gatzinsky, 1993; Balthazart and Absil, 1997) was stereotaxically injected into two sites in the left superior colliculus. In rat retina, the overwhelming majority of ganglion cells in an eyeball are known to project to the contralateral superior colliculus (Linden and Perry, 1983). Three days later, each rat received an intravitreal colchicine injection into the right eye as described above. They were allowed to survive for an additional 4 d and then perfused. The preparation and pretreatment of retinal whole mounts were performed as described. They were then incubated for 5 d with the pChAT antiserum (diluted 1:80,000) in 0.6% PBST at 4°C and for 2 hr with fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit IgG (ICN Pharmaceutical, Aurora, OH) (diluted 1:100) in 0.6% PBST at room temperature. After washing, all retinas were mounted as flat mounts on glass slides and coverslipped with glycerin. The retinal whole mounts were imaged on a laser scanning microscope (MRC- 600, Bio-Rad, Hercules, CA), and images were recorded and processed digitally. The numbers of double-labeled and single-labeled cells were counted on two representative composite confocal images of the mid-peripheral region covering a total area of 0.6 mm2, and the percentage of double-labeled as compared with single-labeled cells was calculated.
mRNA analysis by RT-PCR. Total RNA was isolated from rat striatum and retina using the acid guanidium thiocyanate-phenol method (Chomczynski and Sacchi, 1987). Before reverse transcription, the total RNA was incubated for 1 hr with 10 U of RNase-free DNase (Amersham Biosciences, Piscataway, NJ) and 20 U of recombinant RNase inhibitor (Wako Pure Chemicals, Osaka, Japan) at 37°C to eliminate any trace of DNA contamination. Five micrograms of each total RNA were then reverse transcribed for the first-strand cDNA synthesis using 80 U of SuperScript II (Invitrogen, Gaithersburg, MD) and 500 pmol of oligo dT12–18 (Amersham Biosciences) as primers.
Details of primers used for PCR in this study are shown in Table1. At the first step, PCR was performed using the pair of primers, P1 and P3, and the first-strand cDNA as a template. The reaction mixture consisted of 2 ng/μl of the template cDNA, 0.8 μm each of the primers, 0.2 mm of each of four deoxynucleotide triphosphates, 10 μmβ−mercaptoethanol (Wako Pure Chemicals), 16.6 mm ammonium sulfate, 2 mmMgCl2, and 2.0 U Taq polymerase (Toyobo, Osaka, Japan) in 67 mmTris-HCl, pH 8.8. Thirty-six cycles of PCR were performed with the profile of thermal cycles consisting of denaturation at 95°C for 1 min, annealing at 56°C for 1 min, and extension at 72°C for 2 min. The PCR products were diluted at 1:100 in autoclaved water.
Table 1.
Primers used in the present study
| Number | Sequence | Sense/antisense | Position1-a | Exon1-b |
|---|---|---|---|---|
| P1 | ATGCCCATCCTGGAAAAGGCTCCC | Sense | 202–225 | 2 |
| P2 | TGGGTCTCTGAATACTGGCTGAATG | Sense | 454–478 | 3–4 |
| P3 | AACATTTCAACCTCAACCTTCTGG | Antisense | 1317–1340 | 10 |
| P4 | CTCACTCACTGAGTCAGCCCTGAC | Antisense | 1282–1305 | 10 |
The number of nucleic acids is based on the rat ChAT cDNA sequence reported by Brice et al. (1989).
Exon organization of the rat ChAT gene is based on the report by Hahn et al. (1992).
The nested PCR of 24 cycles was then performed using another pair of primers, P2 and P4, and 0.5 μl of the diluted first PCR products as a template. The composition of the reaction mixture and the profile of thermal cycles were similar to the first-step PCR, except for the annealing temperature at 66°C. The optimal annealing temperature for each pair of primers was determined preliminarily using the RoboCycler (Stratagene, La Jolla, CA). The first-step and nested PCR products were electrophoresed on a 3% agarose gel and stained with ethidium bromide. After dissecting out the bands from the gel, the target DNA in each band was eluted and cloned using a TA cloning system (Invitrogen, San Diego, CA). The pCR 2.1 plasmid vector containing the target DNA insert was transfected into the hostEscherichia coli, IVFα′. After minipreparation of plasmid DNA, each PCR product was sequenced using the ThermoSequenase Cycle Sequencing kit (Amersham Biosciences Corp) and a Sequencer DDS-1 (Shimadzu, Kyoto, Japan).
Western blot analysis. Two rats were used. Each animal received a single intravitreal colchicine injection (100 ng) into the right eye. Four days later, they were perfused through the ascending aorta with 10 mm PBS to clear blood. Tissues of the retina of the injected and uninjected sides were collected separately and homogenized in 10 vol of ice-cold 50 mm Tris-HCl, pH 7.4, containing 0.5% Triton X-100 and protease inhibitor mixture tablets, Complete Mini (Roche Diagnostics, Mannheim, Germany) (one tablet per 10 ml). The fresh tissue of the striatum from each animal was also processed in parallel. The homogenates were centrifuged at 12,000 × g for 20 min at 4°C. The supernatants were collected as a crude protein fraction. Approximately 25 μg of the crude extracted protein and Prestained Precision Protein Standards (Bio-Rad) were electrophoresed on a 9% SDS-polyacrylamide gel containing 20 μm reduced cysteine under a reducing condition and then transferred to a polyvinylidene difluoride membrane (Immobilon-P, Millipore Japan, Tokyo, Japan). The membrane was incubated for 1 hr with 8% skim milk in 25 mm Tris-buffered saline (TBS, pH 7.4) at room temperature, and further incubated overnight with the cChAT antibody (Chemicon) (AB144p, polyclonal; diluted 1:500) or the pChAT antiserum (diluted 1:40,000) in 25 mm TBS containing 1% skim milk at room temperature. After washing with 25 mm TBS containing 0.1% Tween 20, the membrane was reacted for 2 hr with a peroxidase-coupled anti-rabbit IgG Fab′ fragment (Histofine; Nichirei Corporation, Tokyo, Japan) (diluted 1:50). The peroxidase labeling was detected by chemiluminescence using the ECL Western blotting analysis system (Amersham Biosciences).
Assay of ChAT activity. Rat retinas and optic nerves were processed for the assay of ChAT activity. Four rats received unilateral intracranial nerve crush to eliminate a possible contribution of retinopetal cholinergic fibers. For intracranial optic nerve crush, anesthesia was introduced in the rats with 2% halothane and maintained with 1% halothane in a mixture of 70% nitrogen and 30% oxygen to allow the animals to breathe spontaneously. Each animal was placed in the lateral position in a stereotaxic apparatus (Model SH-8; Narishige, Tokyo, Japan), and a linear skin and muscle incision was made between the left eyeball and external ear. The temporal muscle was retracted on either side of the middle of the muscle without removal of the muscle, zygomatic arch, or eyeball. Then a burr hole was opened in the basal surface of the temporal bone between the orbital fissure and the foramen ovale. The optic nerve was approached through the hole and then crushed twice for 30 sec each with a hemostatic forceps. At the end of the surgical operation, the incision was closed and anesthesia was discontinued. The animals were allowed to survive for 4 d.
The enzyme activity of ChAT was measured according to Fonnum's method (Fonnum, 1975) with a slight modification. Fresh optic nerves from intact and optic nerve-crushed rats as well as fresh retinas from intact rats were homogenized in 10 vol of 1 mm EDTA plus 0.5% Triton X-100, pH 7.4, at 4°C. After centrifugation at 10,000 × g for 30 min, the supernatant was collected. Protein concentration was assayed using Lowry's method (Lowry et al., 1951). Ten microliters of the supernatant (5–10 μg of protein) were added to 10 μl of a reaction mixture containing 50 mm sodium phosphate buffer, pH 7.4, 300 mm sodium chloride, 0.2 mmeserine salicylate (Sigma), 8 mm choline (Sigma), 300 μm acetyl-CoA (Sigma) and 2,220,000 dpm [3H] acetyl-CoA (20 Ci/mmol) (Amersham Biosciences Corp). After incubation for 25 min at 37°C, the reaction was terminated by cooling, and the resultant [3H] ACh was quickly extracted with 5 mg/ml sodium tetraphenylboron in acetonitrile/toluene. The radioactivity in the organic phase was counted. To determine the efficiency of ACh extraction, [14C] ACh was used as an internal standard. The enzyme activity was expressed as picomoles of [3H] ACh formed per minute per milligram of protein.
Animal manipulation for dark and light adaptation. For dark adaptation, rats were kept in a black box in a dark room for 1 or 4 d. They were anesthetized with pentobarbital (80 mg/kg) under infrared illumination and then perfused. For light exposure, the rats were placed in a cage and exposed to 300 lux of white light for 4 or 16 hr or to continuous fluorescent room light for 4 d. The retinas and brains from these dark-adapted and light-exposed rats were used for immunohistochemical investigations.
Results
pChAT immunohistochemistry in the retina and visual pathway in intact rats
In retinal whole mounts of control rats, pChAT-like immunoreactivity was present in thick fibers converging toward the central optic disc (Fig.1A). No cell bodies were found to be immunoreactive. In transverse sections of eye cups, positive fibers ran through the optic fiber layer of the retina and could be traced into the optic nerve head (Fig. 1B). At more caudal levels in the orbit, some optic nerve fibers were only weakly stained. Positive cell somata in the ciliary ganglia and positive fiber bundles in ciliary nerves were observed along the optic nerve. The localization of pChAT-positive nerve elements around the optic nerve will be described in detail elsewhere. In brain sections, the optic chiasm and the optic tract were devoid of positive fibers or contained a few weakly stained fibers for pChAT.
Fig. 1.
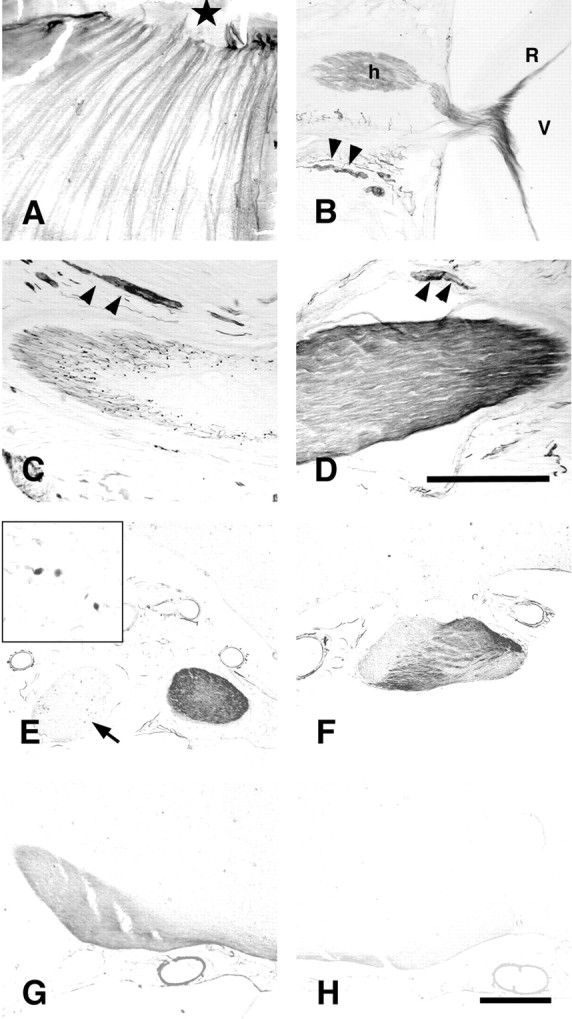
pChAT immunoreactive structures in the retina and primary visual pathway in an intact rat (A, B) and a rat with unilateral enucleation (C–H).A, B, A retinal whole mount (A) and a transverse section of the optic nerve head (B) in an intact rat. Black star in A indicates the central optic disk.Arrowheads in B indicate positive ciliary fibers. V, Vitreous body; R, retina;h, optic nerve head. C–H, A rat after unilateral enucleation of the right eye. C,D, Right (C) and left (D) optic nerve in the intraorbital portion.Arrowheads in C and Dindicate positive ciliary fibers. E, Optic nerves in the intracranial portion. Inset shows high-power photomicrograph of the right optic nerve, indicated by anarrow, showing fragmented positive fiber debris.F, Optic chiasm. G, H, Right (G) and left (H) optic tracts. Scale bars:D (for A–D),H (for E–H), 500 μm.
Enucleation
To verify the derivation of positive fibers in the optic nerve, particularly in the optic nerve head, the right eyeball was enucleated and the localization of the pChAT immunoreactivity was analyzed. Fourteen days after unilateral enucleation of the right eyeball, the pChAT immunostaining revealed positive staining in only a few fragmented nerve fibers in the optic nerve of the enucleated side (right) (Fig. 1C,E). In contrast, pChAT immunoreactivity was observed intensely in the contralateral left optic nerve (left) (Fig. 1D,E). In the optic chiasm, intensely stained fibers ran obliquely from the left side to the ventrolateral region of the right side, contrasting sharply with the remaining unstained region (Fig. 1F). However, the latter region contained a number of positive oval to round structures (data not shown). At more caudal levels, positive fibers could be traced to the right optic tract (Fig. 1G). Positive fibers were not observed in the left optic tract, although it was weakly and diffusely stained (Fig. 1H). Twenty-eight days after enucleation, the patterns of staining were similar to those seen at 14 d after surgery, except that the number of positive oval structures was greatly decreased in both optic nerve and optic chiasm of the enucleated side (data not shown). The positive oval to round structures were reasonably regarded as axonal debris of the optic nerve undergoing Wallerian degeneration after enucleation. Thus, most of the pChAT-positive fibers in the optic nerve were concluded to be retinofugal derived from certain population of retinal ganglion cells. In other words, some retinal ganglion cells were expected to express pChAT.
Visualization of pChAT-immunoreactive cells in the retina
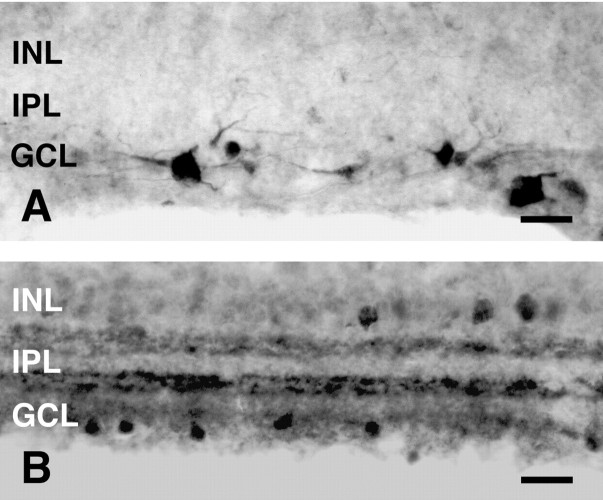
To verify the possible existence of retinal ganglion cells containing pChAT, colchicine, a blocker of axonal transport, was injected into the right vitreous body. As expected, 4 d after colchicine treatment, pChAT-positive cells became visible in the GCL. Figure 2A shows the positive cells in the GCL in transverse retinal sections after intravitreal colchicine injection. These cells frequently extended processes into the inner plexiform layer (IPL). Neither positive cell somata nor positive fibers were observed in other retinal layers. Most of such positive cells had small cell bodies, whereas a few large cells were also stained positively for pChAT. Occasionally, axon-like processes could be seen emanating from these cells.
Fig. 2.
Comparison of pChAT-positive cells after colchicine treatment (A) and cChAT-positive cells (B) in transverse sections of the retina.INL, Inner nuclear layer; IPL, inner plexiform layer; GCL, ganglion cell layer. Scale bars, 50 μm.
The pattern of staining with the pChAT antiserum was compared with those with the commercially obtained antibody to ChAT (cChAT) in the retina. cChAT immunoreactivity was present in two cell populations: one with cell bodies in the inner nuclear layer (INL) and the other with cell bodies in the GCL (Fig. 2B). The processes of these neurons ramified to form two strata in the IPL. The pattern was consistent with previous immunohistochemical studies in the retina of various species, in which cholinergic amacrine cells and displaced amacrine cells were described (Tumosa et al., 1984; Millar et al., 1985; Schmidt et al., 1985; Pourcho and Osman, 1986a; Tumosa and Stell, 1986; Voigt, 1986). As shown in Figure 2, A andB, the pattern of positive cell distribution for cChAT differed fundamentally from that for pChAT. Moreover, such cChAT-positive cells in the GCL were distinct in both shape and size from pChAT-positive ones. Therefore, pChAT- and cChAT-positive neurons appeared to represent separate populations in retinal layers.
In retinal whole mounts of rats pretreated with intravitreal colchicine, many pChAT-positive neurons were observed among intensely stained thick fiber trees converging toward the central optic disc (Fig. 3A). Apparently, there were at least two types of immunostained cells (Fig. 3B). The first group of positive cells had large cell bodies, ranging from 20 to 35 μm in diameter, with three to six thick fiber processes. The second group of positive cells had small cell bodies, fusiform or round, of diameters from 10 to 17 μm, with a few fine processes. In some sections, fiber processes emanating from such positive cells were observed to join fiber bundles, somewhat regularly arranged in groups to converge toward the central optic disc. Small cells outnumbered large ones. Cell counts were performed in an area of 0.25 × 0.25 mm at both the central and peripheral retinal regions. The density of positive cells was 292.7 ± 9.5 cells per square millimeter (means ± SEM; n = 3) in the central region and 370.3 ± 14.1 cells per square millimeter in the peripheral region, respectively. The total number of pChAT-positive cells in the whole retina was estimated to be ∼22,800.
Fig. 3.
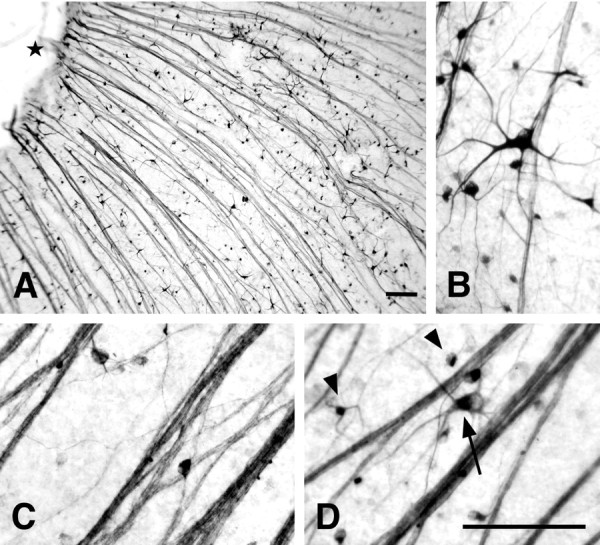
Visualization of pChAT-positive cells in the retinal whole mounts. A, B, Colchicine treatment. A, Low-power photomicrograph. Black star indicates the central optic disk. B, High-power photomicrograph showing configurations of positive cells.C, Optic nerve crush. D, Ethanol injection into the optic nerve. Note that two types of cells, large (arrow) and small (arrowheads), are positive for pChAT. Scale bars: A, B (forB-D), 100 μm.
Visualization of positive cells in the retina was also achieved by damaging the optic nerve. Either optic nerve crush (Fig. 3C) or alcohol injection into the optic nerve (Fig. 3D) caused many pChAT-immunoreactive cells to be visible in the retina, as was the case with intravitreal colchicine injection.
Retrograde tracer labeling
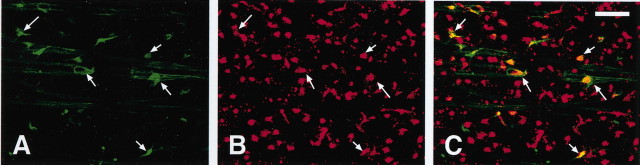
To confirm the identity of pChAT-immunoreactive cells with retinal ganglion cells, a double-labeling experiment was performed. Ganglion cells were labeled with a retrograde tracer, red latex FluoSpheres, which had been applied to the contralateral superior colliculus. After an intravitreal colchicine injection, pChAT-positive cells were labeled with FITC-conjugated secondary antibody. As shown in Figure4, virtually every pChAT-positive cell was also labeled with the retrograde tracer. In two composite images of the mid-peripheral region covering an area of 0.6 mm2, the numbers of double-labeled and single-labeled cells were counted. The density of FluoSphere-containing retinal ganglion cells was 1503 cells per square millimeter. Ninety-two percent of pChAT-positive cells contained FluoSphere, whereas 19% of FluoSphere-containing ganglion cells exhibited pChAT immunoreactivity. In contrast, our immunohistochemical examination for cChAT did not reveal any doubly labeled cells with the tracer experiment (data not shown).
Fig. 4.
The retinal whole mount doubly labeled for pChAT and a retrograde tracer, red latex FluoSperes, after injection into the contralateral superior colliculus. A, Confocal image of pChAT-immunoreactive cells in the retina (green).B, Retrogradely labeled cells with red latex FluoSperes (red). C, Superimposed image ofA and B showing double-labeled cells (yellow to orange).Arrows indicate corresponding cells. Scale bar, 50 μm.
Analysis of ChAT mRNA expression in the retina
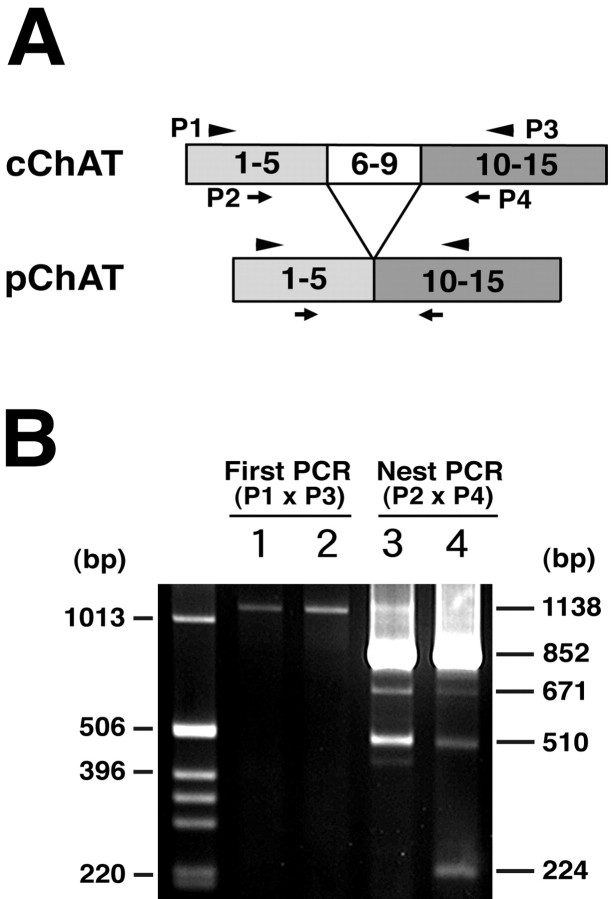
Expression of ChAT mRNA was analyzed by RT-PCR, using RNA preparations extracted from tissues of the striatum and retinas of control rats. As shown in Figure5, the first-step PCR using P1 and P3 primers revealed a single band of ∼1138 bp in both striatum and retina, which corresponded to the expected size of the PCR product from cChAT cDNA. To further characterize ChAT gene expression, nested PCR was done using P2 and P4 primers and the first PCR products as templates. In the nested PCR, multiple bands were detected in the striatum (three bands) and retina (four bands). The larger three bands were detected in both striatum and retina, whereas the smallest band was detected exclusively in the retina. Nucleotide sequence analysis revealed that the largest band represented the cChAT gene product [852 bp; molecular weight (MW) of the encoded protein, 72 kDa]. The second larger band corresponded to a ChAT gene product, which lacked exon 6 (671 bp). This type of ChAT mRNA encodes a truncated protein of 164 amino acid (MW 18 kDa). The nucleotide sequence of the third band (504 bp) lacked exons 7 and 8. This type of mRNA is expected to encode a full-length protein of 529 amino acids (MW 59 kDa). The smallest band, which was detected specifically in the retina, was identified to represent the PCR product of the pChAT cDNA (224 bp). The deduced molecular weight of pChAT is 49 kDa.
Fig. 5.
Expression of ChAT mRNA in the striatum and retina revealed by RT-PCR. A, Schematic drawing showing the exon organizations of cChAT and pChAT, as well as the positions of primers used. B, A 3% agarose gel showing the result of the first RT-PCR and the nested PCR for ChAT. Lane 1, Striatum (First PCR); lane 2, retina (First PCR); lane 3, striatum (Nest PCR); lane 4, retina (Nest PCR). The positions of DNA size markers (1 kb DNA ladder; Invitrogen) and those of positive bands are indicated to the left and right of the gel, respectively.
Western blot analysis
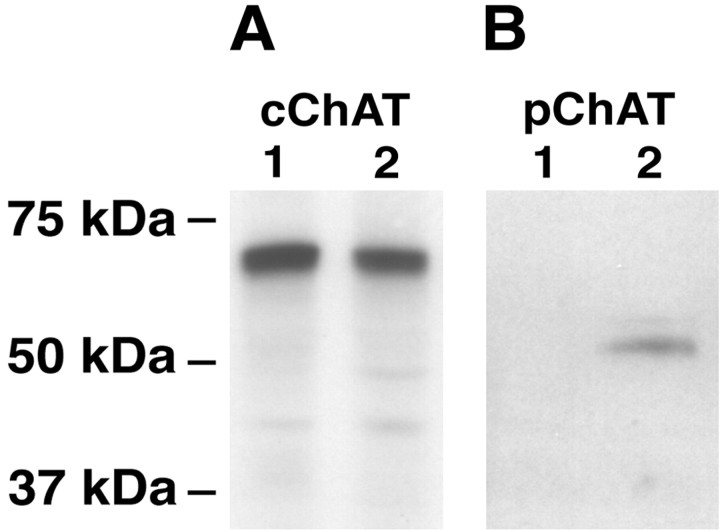
Tissue homogenates from the striatum and the retina were analyzed by Western blot using the cChAT and pChAT antibodies. As shown in Figure 6A, the cChAT antibody gave a positive band of ∼69 kDa in the striatum as well as the retina of both colchicine-injected and uninjected sides. In contrast, the pChAT antiserum detected a band at ∼55 kDa in the retina of the colchicine-treated side but not in the striatum (Fig.6B). The retina of the colchicine-untreated side showed no band or a weakly stained band at the same molecular weight as that in the colchicine-treated retina (data not shown). The size (55 kDa) was slightly larger than the deduced molecular weight (49 kDa) of pChAT.
Fig. 6.
Western blot analysis using tissues of the striatum and colchicine-treated retina. A, Probed with the cChAT antibody. B, Probed with the pChAT antiserum.Lane 1, Striatum; lane 2, colchicine-treated retina. Note that the cChAT antibody reveals a 69 kDa band in the striatum and retina, whereas the pChAT antiserum detects a 55 kDa band in the retina but not in the striatum. The positions of the protein size markers (Prestained Precision Standards; Bio-Rad) are indicated to theleft.
ChAT activities in the retina and optic nerve
To verify the cholinergic properties of retinal ganglion cells of rats, enzyme activities for ChAT were measured in the retina and optic nerve. The protein extracts from intact retinas and optic nerves gave ChAT activity of 1985.9 ± 107.8 and 30.9 ± 13.9 pmol · min−1 · mg−1protein (mean ± SEM; n = 6), respectively. Because the value in intact optic nerves showed significant individual variations, it was suggested that possible centrifugal cholinergic fibers might participate in the optic nerve. Hence we performed intracranial optic nerve crush unilaterally just anterior to the optic chasm. Four days after the nerve crush, the remaining optic nerve still exhibited significant ChAT activity with an acceptable variation (25.8 ± 2.4 pmol · min−1 · mg−1protein; n = 4). These data indicate that ChAT activity is indeed present in some retinofugal fibers in the optic nerve.
Effects of darkness and light exposure on pChAT staining
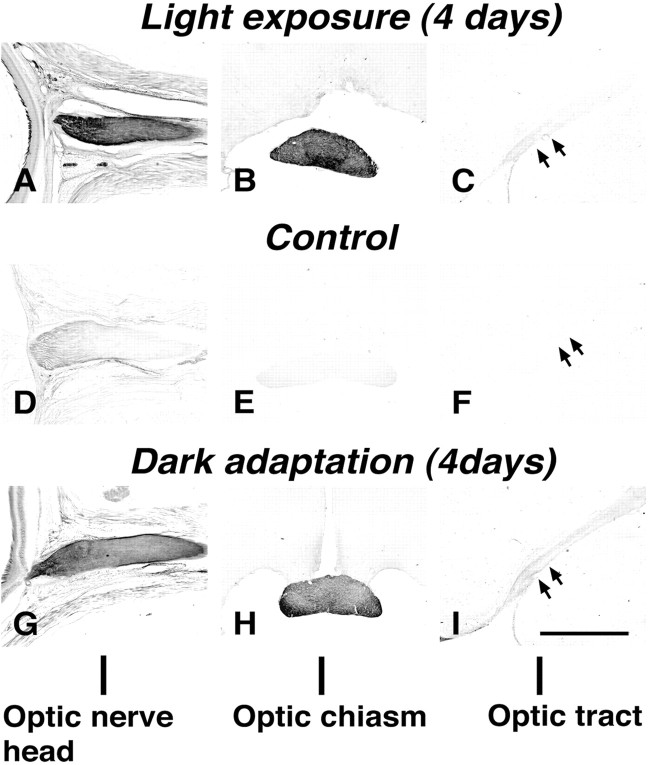
The effects of light and darkness on pChAT-positive structures in the pathway were examined immunohistochemically using the pChAT antiserum. The first group of rats were dark-adapted for 1 or 4 d. Although positive ganglion cells were not visible in any one of the rats that were dark-adapted for 1 d, an increase in pChAT immunoreactivity was observed in the optic nerve. This became more evident after prolonged adaptation for 4 d in darkness. Figure7A–C shows pChAT immunoreactivity in the primary visual pathway of the rat dark-adapted for 4 d. Intensely stained fibers ran through the optic nerve (Fig. 7A) and optic chiasm (Fig. 7B). Less intensely stained fibers were recognized in the optic tract (Fig.7C), and they could be traced up to the close vicinity of the superior colliculus. The expression of pChAT was apparently increased as compared with that in normal control rats, where staining was limited to the optic nerve head (Fig.7D–F). A similar effect was observed on exposure to light. A questionable increase in immunoreactivity was noted after exposure to light for 4 hr, but the increase was evident after overnight light exposure. Rats kept under continuous room light for 4 d showed a staining pattern similar to that seen after dark adaptation for 4 d (Fig. 7G–I). From these results, it is concluded that prolonged exposure to light and dark adaptation were both effective in increasing the expression of pChAT in the primary visual pathway.
Fig. 7.
Effects of darkness and light exposure on pChAT immunoreactivity in the primary visual pathway: the optic nerve head (A, D, G), optic chiasm (B, E, H), and optic tracts (C, F,I). A–C, Light exposure for 4 d. D–F, No treatment. G–I, Dark adaptation for 4 d. Arrows in C,F, and I indicate the optic tract. Note that both light exposure and dark adaptation are effective in increasing pChAT immunoreactivity in the visual pathway. Scale bar, 500 μm.
Discussion
The present study demonstrates pChAT-positive retinal ganglion cells in rats by immunohistochemistry. Biochemically, expression of pChAT in rat retina is confirmed at both levels of protein and mRNA. The enzyme activity of ChAT, the ability of ACh synthesis, is also verified in the optic nerve. These results indicate that a subpopulation of retinal ganglion cells are cholinergic. Our physiological experiments further suggest that these retinal cholinergic ganglion cells are regulated dynamically.
pChAT-positive cells in rat retina are ganglion cells
pChAT-positive cells became visible after pretreatment with colchicine. Damage given in two different ways to the optic nerve was also effective in visualizing pChAT-positive cells. Therefore, visualization should be the result of the blockage of axonal transport rather than the toxic stimulation of colchicine (Morgan and Mundy, 1982).
The double-labeling experiment in colchicine-treated retinas showed that 92% of pChAT-positive cells were retrogradely labeled after the injection of FluoSpheres into the contralateral superior colliculus. This percentage may be an underestimate because a small proportion of retinal ganglion cells might be unlabeled by our retrograde tracing technique. First, the density of retrogradely labeled ganglion cells in this study (1503 cells per square millimeter in the peripheral region) was slightly lower (approximately −6%) than that reported by Perry (1981) (1600 cells per square millimeter). Second, 5% of retinal ganglion cells of rats project to the ipsilateral superior colliculus (Linden and Perry, 1983). Thus, it is strongly assumed that every pChAT-positive cell in rat retina is a ganglion cell.
As mentioned above, the density of ganglion cells in rat retina was estimated to be 2500 cells per square millimeter in the central region and 1600 cells per square millimeter in the peripheral region (Perry, 1981). In this study, the densities of pChAT-positive cells in the colchicine-treated retina were 292.7 ± 9.5 cells per square millimeter (mean ± SEM) in the central region (12% of the total ganglion cells) and 370.3 ± 14.1 cells per square millimeter in the peripheral region (23% of ganglion cells). By double labeling, 19% of retrogradely labeled ganglion cells exhibited pChAT immunoreactivity in the peripheral region of the retina. It is uncertain whether the somewhat lower incidence of pChAT-positive ganglion cells in the central region may reflect some functional properties of pChAT.
In the cat, retinal ganglion cells have been classified into various types according to their sizes, dendritic patterns, and electrophysiological reactions to retinal illuminations. Although much less information is available in rats, three types of retinal ganglion cells have been described in rats: type I, II, and III cells (Perry, 1979, 1981) and RGA, RGB, and RGC cells (Huxlin and Goodchild, 1997). In the present study, two types of pChAT-positive cells, one with large somata characteristic of the type I cells (RGAcells) and another with small somata of type II or type III cells (RGB or RGC cells), respectively, were observed in the retinal whole mounts. Thus, the occurrence of pChAT immunoreactivity appears not to be cell type dependent. Future studies are needed for further morphological characterization of pChAT-positive retinal ganglion cells.
Multiple ChAT mRNAs in rat striatum and retina
Our RT-PCR technique has revealed expressions of multiple ChAT mRNAs in rat striatum and retina. They include mRNAs for cChAT and pChAT (ChAT5–10) and two additional splice variants of ChAT mRNA. The cChAT mRNA was expressed in both striatum and retina, whereas the pChAT mRNA was expressed exclusively in the retina. Two additional variants of ChAT mRNA that have never been reported before, one lacking exon 6 (ChAT5–7) and another lacking exons 7 and 8 (ChAT6–9), were unexpectedly identified in the striatum and retina. The splice pattern of ChAT5–7 mRNA generates a frame shift and, subsequently, a stop codon just downstream to the splice joint. Thus, it encodes a truncated protein (MW 18 kDa) lacking the catalytic domain in exon 10. In contrast, ChAT6–9 mRNA shows no frame shift and encodes a protein carrying domains essential for enzyme activity (MW 59 kDa).
Our pChAT antibody detected a single 55 kDa band only in the retina by Western blot analysis. Because our antibody does not recognize the recombinant ChAT6–9 protein (our unpublished observation), the 55 kDa band should represent pChAT but not other variant proteins. The difference between the molecular weight of the detected band (55 kDa) and the deductive size of pChAT (49 kDa) may indicate some post-translational modification. It remains to be clarified whether ChAT5–7 and ChAT6–9 proteins actually function in vivo.
ChAT activity in the retina and optic nerve
To address whether pChAT is capable of synthesizing ACh in the visual pathway, we measured ChAT activities in the retina and optic nerve. In accordance with previous studies (Ross et al., 1975; Mindel and Mittag, 1976), the retina exhibited high ChAT activity. To avoid the influence of enzyme activity from well known cholinergic amacrine cells that are all intrinsic within the retina, we measured the enzyme activity in the optic nerve. In intact optic nerves, ChAT activity was detectable with a large individual variation. Accordingly, ChAT activities in intact mouse optic nerves were shown to vary greatly among individuals (Ross and McDougal, 1976). Although the reason for such a large variation is unknown, one possible explanation may be that ChAT in the optic nerve is under rapid transportation. In addition, a possible presence of centrifugal cholinergic fibers in the optic nerve (Wenk et al., 1981) may make the analysis complicated. After intracranial optic nerve crush, however, a significant and consistent ChAT activity was still detected in the optic nerve peripheral to the lesioned site. The result indicates that the optic nerve contains retinofugal cholinergic fibers and thus provides evidence that some retinal ganglion cells are cholinergic.
Comparison with previous studies
To date, several methods have been used to demonstrate cholinergic cells, including (1) ChAT immunohistochemistry, (2) autoradiography for choline uptake and ACh synthesis, and (3) histochemistry for acetylcholinesterase (AChE), a metabolizing enzyme for ACh. All of the hitherto available antibodies against ChAT have failed to stain any retinal ganglion cell, but they consistently stained some amacrine cells in the retina (Eckenstein and Thoenen, 1982; Tumosa et al., 1984;Schmidt et al., 1985; Pourcho and Osman, 1986a,b; Tumosa and Stell, 1986; Voigt, 1986). This is compatible with the present results using the cChAT antibody. In contrast, the pChAT antibody did not stain amacrine cells but stained some ganglion cells after colchicine treatment. Therefore, the result suggests that some retinal ganglion cells contain pChAT, which is antigenically different from cChAT.
Several in vitro autoradiographic studies have also failed to reveal any cholinergic retinal ganglion cells. Freeze-dry autoradiographic studies have suggested that choline uptake (Baughman and Bader, 1977) as well as ACh synthesis (Masland and Mills, 1979;Hayden et al., 1980; Masland et al., 1984) are confined to amacrine cells in the retina. However, there are possible explanations for the negative labeling of ganglion cells in these studies. In our immunohistochemical study, retinal ganglion cell somata show no pChAT immunoreactivity under normal conditions. Therefore, the level of ACh synthesis may normally be too low to be detected by autoradiography. It is also possible that high-affinity choline uptake may occur at synaptic terminals in the target tissue as has been proposed (Suszkiw and Pilar, 1976). If this holds true, the negative labeling of ganglion cells may not indicate the noncholinergic nature of such cells but may only reflect the distance between the cell bodies in the retina and synaptic terminals in the superior colliculus. Moreover, autoradiographic data may vary according to methodological differences. For example, large retinal ganglion cells have been reported to contain positive ACh synthesis signals in tissues fixed with 5% phosophomolybdic acid (Pourcho and Osman, 1986a). It suffices to say, therefore, that there is room for argument regarding the results of previous autoradiographic studies.
The other technique for demonstrating cholinergic structures is AChE histochemistry. Although AChE is not a specific marker for cholinergic cells, the presence of AChE activity would be a necessity for such cells (Fibiger, 1982). Previous studies revealed that most ganglion cells, in addition to amacrine cells, showed AChE activity (Hebb, 1957;Reale et al., 1971; Millar et al., 1985; Pourcho and Osman, 1986b). In addition, it is noteworthy that some ganglion cells exhibited AChE activity even after diisopropyl-fluorophosphate pretreatment (Nichols and Koelle, 1968), the feature interpreted as one indicator of cholinergic cells (Butcher and Woolf, 1984).
In summary, previous morphological studies seem to provide no decisive evidence against the presence of cholinergic retinal ganglion cells. Rather, reappraisal of published information, in the light of the discovery of pChAT, indicates that some studies could favor their presence.
Significance of pChAT-positive retinal ganglion cells
The mechanism of the induction of pChAT mRNA and the functional significance of pChAT in the primary visual pathway are so far unclear. As mentioned, previous reports have demonstrated an increase in Fos-like immunoreactivity in the INL and GCL of the retina (Sagar and Sharp, 1990; Gudehithlu et al., 1993), occurring mostly in amacrine and ganglion cells (Koistinaho and Sagar, 1995). The expressions of c-fos, c-jun, and jun B mRNAs in the INL and GCL are induced transiently by light exposure (Gudehithlu et al., 1993; Yoshida et al., 1993; Imaki et al., 1995). Both Fos and Jun can bind to a DNA regulatory element known as AP-1, which is involved in gene expression (Morgan and Curran, 1989). AP-1 binding motifs are associated with regulation of various neuropeptides and neurotransmitter-synthesizing enzymes, including ChAT (Misawa et al., 1992). Indeed, the immunohistochemical localization of ChAT closely overlaps with that of Fos/Jun in rat spinal cord and lower brainstem (Herdegen et al., 1991) and in amacrine cells of rabbit retina (Koistinaho and Sagar, 1995). The stimulatory effect of light on ACh synthesis has also been reported in amacrine cells of rabbit retina (Masland and Livingstone, 1976). These facts may lead us to assume that light exposure may have directly elevated the expression of pChAT up to the detectable level through the Fos-mediated mechanism. However, similar consequences of continuous light exposure and constant darkness in pChAT staining in this study appear incompatible with the view that expression of pChAT is involved in the formation of circadian rhythm. In this context, it is noteworthy that after unilateral enucleation of an eyeball, pChAT immunoreactivity was increased in the contralateral intact optic nerve under a 12 hr light/dark cycle. Thus, it is possible that a factor regardless of the light/dark condition, such as attention, may augment its expression.
Another implication is that ACh produced by pChAT may not be acting merely as a neurotransmitter, but rather may play different roles in retinal ganglion cells, as has been suggested in non-neuronal ACh (Wessler et al., 1998). For example, ChAT, ACh, and ACh receptors have been detected in non-neuronal epithelial cells, in which ACh is suggested to participate in the regulation of important cell functions, such as trophic signaling, cell cycle, and cell–cell contact (Wessler et al., 1998). This notion may explain why the pChAT antibody failed to detect positive terminals in the superior colliculus of rats. Whatever is the case, the results suggest that expression of pChAT is physiologically regulated and that the cholinergic mechanism may play a role in the primary visual pathway. Future studies are needed to clarify the mechanism of pChAT upregulations as an important clue for understanding the functional significance of pChAT in retinal ganglion cells.
Footnotes
This work was supported in part by a grant-in-aid for Scientific Research from the Ministry of Education, Science, Sports, Culture and Technology of Japan. We thank M. Suzaki and T. Yamamoto (Shiga University of Medical Science) and Dr. Y. Aoki (Nippon Shinyaku Company, Kyoto, Japan) for technical assistance.
Correspondence should be addressed to Hiroshi Kimura, Molecular Neuroscience Research Center, Shiga University of Medical Science, Seta Tsukinowa-cho, Otsu 520-2192, Japan. E-mail:hkimura@belle.shiga-med.ac.jp.
References
- 1.Anderson KJ, Borja MA, Cotman CW, Moffett JR, Namboodiri MAA, Neale JH. N-acetylaspartylglutamate identified in the rat retinal ganglion cells and their projections in the brain. Brain Res. 1987;411:172–177. doi: 10.1016/0006-8993(87)90696-2. [DOI] [PubMed] [Google Scholar]
- 2.Balthazart J, Absil P. Identification of catecholaminergic inputs to and outputs from aromatase-containing brain areas of the Japanese quail by tract tracing combined with tyrosine hydroxylase immunocytochemistry. J Comp Neurol. 1997;382:401–428. [PubMed] [Google Scholar]
- 3.Baughman RW, Bader CR. Biochemical characterization and cellular localization of the cholinergic system in the chicken retina. Brain Res. 1977;138:469–485. doi: 10.1016/0006-8993(77)90684-9. [DOI] [PubMed] [Google Scholar]
- 4.Beaudet A, Burkhalter A, Reubi J-C, Cuénod M. Selective bidirectional transport of [3H]-D-aspartate in the pigeon retinotectal pathway. Neuroscience. 1981;6:2021–2034. doi: 10.1016/0306-4522(81)90040-3. [DOI] [PubMed] [Google Scholar]
- 5.Brice A, Berrard S, Raynaud B, Ansieau S, Coppola T, Weber MJ, Mallet J. Complete sequence of a cDNA encoding an active rat choline acetyltransferase: a tool to investigate the plasticity of cholinergic phenotype expression. J Neurosci Res. 1989;23:266–273. doi: 10.1002/jnr.490230304. [DOI] [PubMed] [Google Scholar]
- 6.Bruce G, Wainer BH, Hersh LB. Immunoaffinity purification of human choline acetyltransferase: comparison of the brain and placental enzymes. J Neurochem. 1985;45:611–620. doi: 10.1111/j.1471-4159.1985.tb04030.x. [DOI] [PubMed] [Google Scholar]
- 7.Butcher LL, Woolf NJ. Histochemical distribution of acetylcholinesterase in the central nervous system: clues to the localization of cholinergic neurons. In: Björklund A, Hökfelt T, Kuhar MJ, editors. Handbook of chemical neuroanatomy, Vol 3, Classical transmitters and transmitter receptors in the CNS, Part II. Elsevier; Amsterdam: 1984. pp. 1–50. [Google Scholar]
- 8.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 9.Crooks J, Kolb H. Localization of GABA, glycine, glutamate and tyrosine hydroxylase in the human retina. J Comp Neurol. 1992;315:287–302. doi: 10.1002/cne.903150305. [DOI] [PubMed] [Google Scholar]
- 10.Cuenca N, Kolb H. Morphology and distribution of neurons immunoreactive for substance P in the turtle retina. J Comp Neurol. 1989;290:391–411. doi: 10.1002/cne.902900308. [DOI] [PubMed] [Google Scholar]
- 11.Davanger S, Ottersen OP, Storm-Mathisen J. Glutamate, GABA, and glycine in the human retina: an immunocytochemical investigation. J Comp Neurol. 1991;311:483–494. doi: 10.1002/cne.903110404. [DOI] [PubMed] [Google Scholar]
- 12.Eckenstein F, Thoenen H. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J. 1982;1:363–368. doi: 10.1002/j.1460-2075.1982.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehinger B. [3H]-D-aspartate accumulation in the retina of pigeon, guinea-pig and rabbit. Exp Eye Res. 1981;33:381–391. doi: 10.1016/s0014-4835(81)80090-5. [DOI] [PubMed] [Google Scholar]
- 14.Ehinger B, Dowling JE. Retinal neurocircuitry and transmitters. In: Björklund A, Hökfelt T, Swanson LW, editors. Handbook of chemical neuroanatomy, Vol 5, Integrated systems of the CNS, Part I. Elsevier; Amsterdam: 1987. pp. 389–446. [Google Scholar]
- 15.Eldred WD, Zucker C, Karten HJ, Yazulla S. Comparison of fixation and penetration enhancement techniques for use in ultrastructural immunocytochemistry. J Histochem Cytochem. 1983;31:285–292. doi: 10.1177/31.2.6339606. [DOI] [PubMed] [Google Scholar]
- 16.Fibiger HC. The organization and some projections of cholinergic neurons of the mammalian forebrain. Brain Res. 1982;257:327–388. doi: 10.1016/0165-0173(82)90011-x. [DOI] [PubMed] [Google Scholar]
- 17.Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975;24:407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- 18.Gudehithlu KP, Neff NH, Hadjiconstantinou M. C-fos and NGFI-A mRNA of rat retina: evidence for light-induced augmentation and a role for cholinergic and glutamate receptors. Brain Res. 1993;631:77–82. doi: 10.1016/0006-8993(93)91189-y. [DOI] [PubMed] [Google Scholar]
- 19.Hahn M, Hahn SL, Stone DM, Joh TH. Cloning of the rat gene encoding choline acetyltransferase, a cholinergic neuron-specific marker. Proc Natl Acad Sci USA. 1992;89:4387–4391. doi: 10.1073/pnas.89.10.4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hayden SA, Mills JW, Masland EH. Acetylcholine synthesis by displaced amacrine cells. Science. 1980;210:435–436. doi: 10.1126/science.7433984. [DOI] [PubMed] [Google Scholar]
- 21.Hebb CO. The problem of identifying cholinergic neurons in the retina. Acta Physiol Pharmacol Neurol. 1957;6:621–631. [PubMed] [Google Scholar]
- 22.Herdegen T, Leah JD, Manisali A, Bravo R, Zimmermann M. C-jun-like immunoreactivity in the CNS of the adult rat: basal and transynaptically induced expression of an immediate-early gene. Neuroscience. 1991;41:643–654. doi: 10.1016/0306-4522(91)90356-s. [DOI] [PubMed] [Google Scholar]
- 23.Huxlin KR, Goodchild AK. Retinal ganglion cells in the albino rat: revised morphological classification. J Comp Neurol. 1997;385:309–323. [PubMed] [Google Scholar]
- 24.Imaki J, Yamashita K, Yamakawa A, Yoshida K. Expression of jun family genes in rat retinal cells: regulation by light/dark cycle. Mol Brain Res. 1995;30:48–52. doi: 10.1016/0169-328x(94)00270-o. [DOI] [PubMed] [Google Scholar]
- 25.Iuvone PM, Galli CL, Garrison-Grand CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 26.Jojich L, Pourcho RG. Glutamate immunoreactivity in the cat retina: a quantitative study. Vis Neurosci. 1996;13:117–133. doi: 10.1017/s0952523800007173. [DOI] [PubMed] [Google Scholar]
- 27.Kalloniatis M, Fletcher EL. Immunocytochemical localization of the amino acid neurotransmitters in the chicken retina. J Comp Neurol. 1993;336:174–193. doi: 10.1002/cne.903360203. [DOI] [PubMed] [Google Scholar]
- 28.Koistinaho J, Sagar SM. Light-induced c-fos expression in amacrine cells in the rabbit retina. Mol Brain Res. 1995;29:53–63. doi: 10.1016/0169-328x(94)00218-4. [DOI] [PubMed] [Google Scholar]
- 29.Kuljis RO, Krause JE, Karten HJ. Peptide-like immunoreactivity in anuran optic nerve fibers. J Comp Neurol. 1984;226:222–237. doi: 10.1002/cne.902260206. [DOI] [PubMed] [Google Scholar]
- 30.Linden R, Perry VH. Massive retinotectal projection in rats. Brain Res. 1983;272:145–149. doi: 10.1016/0006-8993(83)90371-2. [DOI] [PubMed] [Google Scholar]
- 31.Lowry OH, Rossbourgh NI, Farr AL, Randall RJ. Protein measurement with folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 32.Masland RH, Livingstone CJ. Effect of stimulation with light on synthesis and release of acetylcholine by an isolated mammalian retina. J Neurophysiol. 1976;39:1210–1219. doi: 10.1152/jn.1976.39.6.1210. [DOI] [PubMed] [Google Scholar]
- 33.Masland RH, Mills JW. Autoradiographic identification of acetylcholine in the rabbit retina. J Cell Biol. 1979;83:159–178. doi: 10.1083/jcb.83.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Masland RH, Mills JW, Hayden SA. Acetylcholine-synthesizing amacrine cells: identification and selective staining by using radioautography and fluorescent markers. Proc R Soc Lond B Biol Sci. 1984;223:79–100. doi: 10.1098/rspb.1984.0084. [DOI] [PubMed] [Google Scholar]
- 35.Millar T, Ishimoto I, Johnson CD, Epstein ML, Chubb IW, Morgan IG. Cholinergic and acetylcholinesterase-containing neurons of the chicken retina. Neurosci Lett. 1985;61:311–316. doi: 10.1016/0304-3940(85)90482-3. [DOI] [PubMed] [Google Scholar]
- 36.Millar TJ, Chubb IW. Substance P in the chick retina: effects of light and dark. Brain Res. 1984;307:303–309. doi: 10.1016/0006-8993(84)90484-0. [DOI] [PubMed] [Google Scholar]
- 37.Mindel JS, Mittag TW. Choline acetyltransferase in ocular tissues of rabbits, cats, cattle and humans. Invest Ophthalmol Vis Sci. 1976;15:808–814. [PubMed] [Google Scholar]
- 38.Misawa H, Ishii K, Deguchi T. Gene expression of mouse choline acetyltransferase. Alternative splicing and identification of a highly active promoter region. J Biol Chem. 1992;267:20392–20399. [PubMed] [Google Scholar]
- 39.Mize RR, Butler GD. Postembedding immunocytochemistry demonstrates directly that both retinal and cortical terminals in the cat superior colliculus are glutamate immunoreactive. J Comp Neurol. 1996;371:633–648. doi: 10.1002/(SICI)1096-9861(19960805)371:4<633::AID-CNE11>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 40.Montero VM. Quantitative immunogold evidence for enrichment of glutamate but not aspartate in synaptic terminals of retino-geniculate, geniculo-cortical, and cortico-geniculate axons in the cat. Vis Neurosci. 1994;11:675–681. doi: 10.1017/s0952523800002984. [DOI] [PubMed] [Google Scholar]
- 41.Morgan IG, Mundy PG. Ganglion cells of chicken retina possess nicotinic rather than muscarinic acetylcholine receptors. Neurochem Res. 1982;7:267–274. doi: 10.1007/BF00965639. [DOI] [PubMed] [Google Scholar]
- 42.Morgan JI, Curran T. Stimulus-transcription coupling in neurons: role of cellular immediate-early genes. Trends Neurosci. 1989;11:459–462. doi: 10.1016/0166-2236(89)90096-9. [DOI] [PubMed] [Google Scholar]
- 43.Nakajima K, Tooyama I, Yasuhara O, Aimi Y, Kimura H. Immunohistochemical demonstration of choline acetyltransferase of a peripheral type (pChAT) in the enteric nervous system of rats. J Chem Neuroanat. 2000;18:31–40. doi: 10.1016/s0891-0618(99)00058-7. [DOI] [PubMed] [Google Scholar]
- 44.Nakanishi Y, Tooyama I, Yasuhara O, Aimi Y, Kitajima K, Kimura H. Immunohistochemical localization of choline acetyltransferase of a peripheral type in rat larynx. J Chem Neuroanat. 1999;17:21–32. doi: 10.1016/s0891-0618(99)00020-4. [DOI] [PubMed] [Google Scholar]
- 45.Nichols CW, Koelle GB. Comparison of the localization of acetylcholinesterase and non-specific cholinesterase in mammalian and avian retinas. J Comp Neurol. 1968;133:1–16. doi: 10.1002/cne.901330102. [DOI] [PubMed] [Google Scholar]
- 46.Ortega F, Hennequet L, Sarria R, Streit P, Grandes P. Changes in the pattern of glutamate-like immunoreactivity in rat superior colliculus following retinal and visual cortical lesions. Neuroscience. 1995;67:125–134. doi: 10.1016/0306-4522(95)00057-p. [DOI] [PubMed] [Google Scholar]
- 47.Oswald RE, Freeman JA. Optic nerve transmitters in lower vertebrate species. Life Sci. 1980;27:527–533. doi: 10.1016/0024-3205(80)90301-x. [DOI] [PubMed] [Google Scholar]
- 48.Perry VH. The ganglion cell layer of the retina of the rat: a Golgi study. Proc R Soc Lond B Biol Sci. 1979;204:363–375. doi: 10.1098/rspb.1979.0033. [DOI] [PubMed] [Google Scholar]
- 49.Perry VH. Evidence for an amacrine cell system in the ganglion cell layer of the rat retina. Neuroscience. 1981;6:931–944. doi: 10.1016/0306-4522(81)90174-3. [DOI] [PubMed] [Google Scholar]
- 50.Persson HG, Gatzinsky KP. Distribution of retrogradely transported fluorescent latex microspheres in rat lumbosacral ventral root axons following peripheral crush injury: a light and electron microscopic study. Brain Res. 1993;630:115–124. doi: 10.1016/0006-8993(93)90649-8. [DOI] [PubMed] [Google Scholar]
- 51.Pourcho RG, Osman K. Cytochemical identification of cholinergic amacrine cells in the cat retina. J Comp Neurol. 1986a;247:497–504. doi: 10.1002/cne.902470409. [DOI] [PubMed] [Google Scholar]
- 52.Pourcho RG, Osman K. Acetylcholinesterase localization in cat retina: a comparison with choline acetyltransferase. Exp Eye Res. 1986b;43:585–594. doi: 10.1016/s0014-4835(86)80025-2. [DOI] [PubMed] [Google Scholar]
- 53.Puro DG. Cholinergic systems. In: Morgan WH, editor. Retinal transmitters and modulators: models for the brain, Vol I. CRC; Boca Raton, FL: 1985. pp. 63–91. [Google Scholar]
- 54.Reale E, Lucinano L, Spitznas M. The fine structural localization of acetylcholinesterase activity in the retina and optic nerve of rabbit. J Histochem Cytochem. 1971;19:85–96. doi: 10.1177/19.2.85. [DOI] [PubMed] [Google Scholar]
- 55.Roberts WA, Eaton SA, Salt TE. Excitatory amino acid receptors mediate synaptic responses to visual stimuli in superior colliculus neurones of the rat. Neurosci Lett. 1991;129:161–164. doi: 10.1016/0304-3940(91)90451-x. [DOI] [PubMed] [Google Scholar]
- 56.Ross CD, McDougal DB., Jr The distribution of choline acetyltransferase activity in vertebrate retina. J Neurochem. 1976;26:521–526. doi: 10.1111/j.1471-4159.1976.tb01505.x. [DOI] [PubMed] [Google Scholar]
- 57.Ross CD, Cohen AI, McDougal DB., Jr Choline acetyltransferase and acetylcholinesterase activities in normal and biologically fractionated mouse retinas. Invest Ophthalmol Vis Sci. 1975;14:756–761. [PubMed] [Google Scholar]
- 58.Sagar SM, Sharp FR. Light induces a Fos-like nuclear antigen in retinal neurons. Mol Brain Res. 1990;7:17–21. doi: 10.1016/0169-328x(90)90068-o. [DOI] [PubMed] [Google Scholar]
- 59.Sandberg M, Corazzi L. Release of endogenous amino acids from superior colliculus of the rabbit: in vitro studies after retinal ablation. J Neurochem. 1983;40:917–921. doi: 10.1111/j.1471-4159.1983.tb08074.x. [DOI] [PubMed] [Google Scholar]
- 60.Schmidt M, Wässle H, Humphrey M. Number and distribution of putative cholinergic neurons in the cat retina. Neurosci Lett. 1985;59:235–240. doi: 10.1016/0304-3940(85)90137-5. [DOI] [PubMed] [Google Scholar]
- 61.Sillito AM, Murphy PC, Salt TE. The contribution of the non-N-methyl-d-aspartate group of excitatory amino acid receptors to retinogeniculate transmission in the cat. Neuroscience. 1990;34:273–280. doi: 10.1016/0306-4522(90)90137-s. [DOI] [PubMed] [Google Scholar]
- 62.Starr MS. Effect of dark adaptation on the GABA system in the retina. Brain Res. 1973;59:331–338. doi: 10.1016/0006-8993(73)90271-0. [DOI] [PubMed] [Google Scholar]
- 63.Sun H, Crossland WJ. Quantitative assessment of localization and colocalization of glutamate, aspartate, glycine, and GABA immunoreactivity in the chick retina. Anat Rec. 2000;260:158–179. doi: 10.1002/1097-0185(20001001)260:2<158::AID-AR60>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 64.Suszkiw JB, Pilar GR. Selective localization of a high affinity choline uptake system and its role in ACh formation in cholinergic nerve terminals. J Neurochem. 1976;26:1133–1138. doi: 10.1111/j.1471-4159.1976.tb06996.x. [DOI] [PubMed] [Google Scholar]
- 65.Tieman SB, Tieman DG. N-acetylaspartylglutamate immunoreactivity in human retina. Vision Res. 1996;36:941–947. doi: 10.1016/0042-6989(95)00185-9. [DOI] [PubMed] [Google Scholar]
- 66.Tooyama I, Kimura H. An alternative splice variant of choline acetyltransferase is localized preferentially in peripheral nerve cells and fibers: implication for a peripheral cholinergic marker. J Chem Neuroanat. 2000;17:217–226. doi: 10.1016/s0891-0618(99)00043-5. [DOI] [PubMed] [Google Scholar]
- 67.Tsai G, Stauch BL, Vornov JJ, Deshpande JK, Coyle JT. Selective release of N-acetylaspartylglutamate from rat optic nerve terminals in vivo. Brain Res. 1990;518:313–316. doi: 10.1016/0006-8993(90)90989-o. [DOI] [PubMed] [Google Scholar]
- 68.Tumosa N, Stell WK. Choline acetyltransferase immunoreactivity suggests that ganglion cells in the goldfish retina are not cholinergic. J Comp Neurol. 1986;244:267–275. doi: 10.1002/cne.902440212. [DOI] [PubMed] [Google Scholar]
- 69.Tumosa N, Eckenstein F, Stell WK. Immunocytochemical localization of putative cholinergic neurons in the goldfish retina. Neurosci Lett. 1984;48:255–259. doi: 10.1016/0304-3940(84)90047-8. [DOI] [PubMed] [Google Scholar]
- 70.Voigt T. Cholinergic amacrine cells in the rat retina. J Comp Neurol. 1986;248:19–35. doi: 10.1002/cne.902480103. [DOI] [PubMed] [Google Scholar]
- 71.Wenk H, Bigl V, Meyer U. Cholinergic mechanisms in the retina of rats. J Hirnforsch. 1981;22:481–490. [PubMed] [Google Scholar]
- 72.Wessler I, Kirkpatrick CJ, Racké K. Non-neuronal acetylcholine, a locally acting molecule, widely distributed in biological systems: expression and function in humans. Pharmacol Ther. 1998;77:59–79. doi: 10.1016/s0163-7258(97)00085-5. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida K, Kawamura K, Imaki J. Differential expression of c-fos mRNA in rat retinal cells: regulation by light/dark cycle. Neuron. 1993;10:1049–1054. doi: 10.1016/0896-6273(93)90053-t. [DOI] [PubMed] [Google Scholar]