Abstract
Theiler's virus infection of the CNS induces an immune-mediated demyelinating disease in susceptible mouse strains and serves as a relevant infection model for human multiple sclerosis (MS). Cannabinoids may act as immunosuppressive compounds that have shown therapeutic potential in chronic inflammatory disorders. Using the Theiler's murine encephalomyelitis virus model, we report here that treatment with the synthetic cannabinoids WIN 55,212–2, ACEA, and JWH-015 during established disease significantly improved the neurological deficits in a long-lasting way. At a histological level, cannabinoids reduced microglial activation, abrogated major histocompatibility complex class II antigen expression, and decreased the number of CD4+ infiltrating T cells in the spinal cord. Both recovery of motor function and diminution of inflammation paralleled extensive remyelination. Overall, the data presented may have potential therapeutic implications in demyelinating pathologies such as MS; in particular, the possible involvement of cannabinoid receptor CB2 would enable nonpsychoactive therapy suitable for long-term use.
Keywords: Theiler's virus, CB1 agonists, CB2 agonists, demyelination, neuroinflammation, remyelination, spinal cord, rotarod
Introduction
Multiple sclerosis (MS) is the most common chronic inflammatory, demyelinating disease of the CNS in humans and is characterized by neurological deficits including sensory deficits, motor weakness, tremor, and ataxia. Demyelination is considered to occur as a consequence of a chronic inflammation in which circulating T cells and macrophages infiltrated into the CNS get involved in the development of the demyelinated plaques that characterize the disease (Noseworthy et al., 2000). The mechanisms of the immune-mediated injury of myelin sheaths are thought to involve an indirect bystander injury and an autoimmune response against myelin epitopes. The etiology of MS is still unknown, but epidemiological studies suggest a role for viruses in triggering the disease (Johnson, 1994). Several viruses have been shown to induce CNS demyelination in animals, and Theiler's virus provides the best studied model. Theiler's murine encephalomyelitis virus (TMEV) is a picornavirus natural pathogen in mice that, when injected intracerebrally in susceptible strains, induces a chronic, progressive, demyelinating disease that resembles progressive MS (Dal Canto and Lipton, 1977). An immune response directed against viral and myelin epitopes takes place in the CNS of TMEV-infected mice (Miller et al., 1997), and this event is considered to be the cause of the TMEV-induced demyelinating disease (TMEV-IDD). Mice exhibit several clinical deficits, including progressive impaired motor coordination, incontinence, and paralysis associated with axonal loss and electrophysiological abnormalities (McGavern et al., 2000).
Cannabinoids, the bioactive components of Cannabis sativa, are immunosuppressors by affecting cell function (Zhu et al., 1998; Mc Coy et al., 1999) and by inhibiting proinflammatory soluble mediators (Klein et al., 2000). On this basis, cannabidiol, the nonpsychoactive cannabinoid, ameliorated chronic inflammation in a mouse model of rheumatoid arthritis (Malfait et al., 2000). In experimental autoimmune encephalomyelitis (EAE), Δ9-tetrahydrocannabinol (THC), the major psychotropic constituent of marijuana, was effective in delaying the onset of disease when administered before induction (Lyman et al., 1989). Synthetic cannabinoids also reduce spasticity and tremor in mice with chronic relapsing EAE (Baker et al., 2000), accordingly to their actions on pain and motor pathways in the CNS. Limited clinical studies have suggested beneficial effects of cannabinoids (for review, seePertwee, 2002), and the possible role of the endocannabinoid system in MS symptomatology has also been discussed (Di Marzo et al., 2000).
Cannabinoids are hydrophobic compounds that exert most of their actions via the activation of specific G-protein-coupled receptors. To date, two cannabinoid receptors have been cloned and characterized, cannabinoid type 1 receptor (CB1) and cannabinoid type 2 receptor (CB2), although additional receptors may exist (Matsuda et al., 1990; Di Marzo et al., 2001). The CB1 receptor is expressed primarily in the CNS (Herkenham et al., 1990) and is responsible for the psychotropic effects of cannabinoids, whereas the CB2 receptor is expressed predominantly in immune cells (Bouaboula et al., 1993;Munro et al., 1993). Nevertheless, CB1 receptor expression is also reported in immune cells (Galiegue et al., 1995). Several endogenous lipids have been isolated and characterized as natural ligands for both receptors (Devane et al., 1992; Mechoulam et al., 1995), and an endocannabinoid system is known to operate, but its regulation and functions are not yet well understood (for review, seePiomelli et al., 2000).
Our study reports a therapeutic effect of synthetic cannabinoids on TMEV-IDD. Cannabinoid treatment during established clinical disease restores motor coordination, diminishes inflammation, and promotes remyelination in TMEV-infected mice.
Materials and Methods
Animals and Theiler's virus inoculation. We used female SJL/J mice, susceptible to TMEV-IDD development, from our in-house colony (Cajal Institute, Madrid, Spain), maintained on food and water ad libitum in a 12 hr light/dark cycle. Four-week-old mice were inoculated intracerebrally in the right cerebral hemisphere with 106 pfu of BeAn TMEV strain in 30 μl of DMEM supplemented with 10% of FCS as previously described (Lledó et al., 1999). Handling of animals was performed in compliance with the guidelines of animal care set by the European Union (86/609/EEC).
Evaluation of motor coordination. To evaluate neurological deficits of mice, we used the rotarod test, which measures balance, coordination, and motor control. The rotarod apparatus (Ugo Basile, Comerio, Italy) consists of a suspended rod able to run at constant or at accelerating speed. All mice were exposed to a training period at constant speed to familiarize them with the apparatus before cannabinoid treatment and at accelerating speed while the test was being performed. Data were collected from mouse rotarod performance 1 d before the beginning of treatment, 1 d after the end of the treatment, and 25 d later. The trial was terminated when mice fell from the apparatus or after a maximum of 5 min. We analyzed data with a two-way ANOVA and Student's ttest.
Experimental procedure. At 60 d after TMEV infection, neurological dysfunction was tested by the rotarod assay, and mice were assigned to four groups, with initially no significant differences between them in their ability to perform the rotarod test. Mice from these groups were injected intraperitoneally once a day for 10 d with WIN 55,212–2 (nonselective CB1/CB2 agonist), ACEA (>1400-fold CB1 selectivity over CB2), JWH-015 (23-fold CB2 selectivity over CB1), or vehicle. Doses were calculated based on their receptor binding affinities, but this estimate does not take account of pharmacokinetics of the individual compounds (Pertwee, 1999). We evaluated effects of WIN 55,212–2 and ACEA on motor function by testing spontaneous locomotor activity (Activity Monitor System; Omnitech Electronics, Columbus, OH). Naive SJL/J mice were injected intraperitoneally either with 5 mg/kg of WIN 55,212–2, 2.5 mg/kg of ACEA, or the appropriate vehicle and immediately introduced into the activity cage for a 60 min session. Mice injected with WIN-55,212–2 displayed lower locomotor response than controls, but no typical signs of catalepsy, as frozen postures or immobility were observed (our unpublished results). To avoid potential habituation, cannabinoid agonist doses were increased through time as treatment advanced as follows: 2.5 mg/kg of WIN 55,212–2 for 3 d, 3.75 mg/kg on days 4–6, and 5 mg/kg on days 7–10. WIN 55,212–2 doses were based on a previous report (Baker et al., 2000). ACEA doses were 1.25 mg/kg on days 1–3, 1.9 mg/kg on days 4–6, and 2.5 mg/kg on the last 4 d of treatment. JWH-015 was injected at 0.6 mg/kg for 3 d, 0.9 mg/kg on days 4–6, and 1.2 mg/kg on the last 4 d. Vehicle-injected mice received a solution of 5% BSA and 0.2% DMSO in PBS. The number of mice for each treatment was 12; half were killed to process the spinal cord the day after treatment termination, and the remainder were maintained for 25 d to reach 5 weeks from the beginning of treatment; this period is considered optimal to evaluate remyelination induced by any event (Warrington et al., 2000).
Tissue processing. Mice were anesthetized by intraperitoneal pentobarbital administration and perfused transcardially with 4% paraformaldehyde in 0.1 m phosphate buffer (PB). Spinal cords were collected and divided into five segments. From each segment, ∼1 mm was postfixed in 2.5% glutaraldehyde and 2% paraformaldehyde in 0.1 m PB for 1 hr, stained with 1% osmium tetraoxide in water for 1 hr, and embedded in Araldite epoxy resin (TAAB, Aldermaston, UK). Tissue was then cut with an ultramicrotome to obtain 1 μm semithin sections and stained with toluidine blue. The remaining tissue was postfixed in 4% paraformaldehyde in 0.1 PB, cryoprotected with a 30% solution of sucrose in 0.1 PB, and frozen in dry ice. We then obtained 35-μm-thick coronal cryostat sections and processed them to visualize microglia/macrophages using Mac-1 anti-CD11b antibody (Serotec, Oxford, UK), CD4+ T cells (BD PharMingen, San Diego, CA) and major histocompatibility complex (MHC) class II antigen expression using OX-6 anti H-2A class II antibody (Serotec). Immunostaining was visualized with Alexa-conjugated secondary anti-mouse and anti-rat antibodies (Molecular Probes, Eugene, OR). Negative controls for MHC class II detection were performed, and immunostaining was absent in microglial cells.
Determination of reactive microglial cell number. To determine the number of microglia with reactive morphology, five randomly selected ventral spinal cord images per mouse at different levels were obtained by confocal microscopy, with constant laser beam intensity and photodetector sensitivity. Images were analyzed with NIH Image software fixed to detect Mac-1+cells larger than 25 μm2, a size we consider as reactive microglia. To confirm that this was a correct size threshold, we analyzed spinal cords from sham mice, and the software detected no cells in spinal cord, in which all microglial cells were resting. Moreover, reactive microglia detected by this method showed the same labeling intensity in vehicle-treated compared with cannabinoid-treated mice. Data were analyzed with one-way ANOVA followed by Tukey's multiple comparison test.
Determination of CD4+. To determine CD4+ cell number, we counted positive cells in four random microscopic fields using a 40× objective. Five coronal sections were quantified per mouse at different spinal cord levels. The four fields counted per section were always two from the ventral and two from the dorsal region; two fields were from one hemisphere and two from the other. We performed one-way ANOVA followed by Tukey's multiple comparison test with data obtained.
Quantitation of spinal cord demyelination. Toluidine blue-stained semithin cross sections (1-μm-thick) of cervical, thoracic, and lumbar spinal cord were used for quantification. We counted both remyelinating fibers (a thin myelin sheath compared with normally myelinated axon fibers) and demyelinated axons (naked axons devoid of myelin) using a 100× objective. Counts were made in random fields within affected demyelinating areas in lateral, anterolateral, and anterior columns (the effect was minimal in the posterior column). We analyzed six animals per treatment, five sections per mouse, and five fields per area in each hemisphere. To determine statistical differences, we performed one-way ANOVA and Tukey's post-test.
Results
Cannabinoid agonist treatment improves motor function on established neurological symptomatology
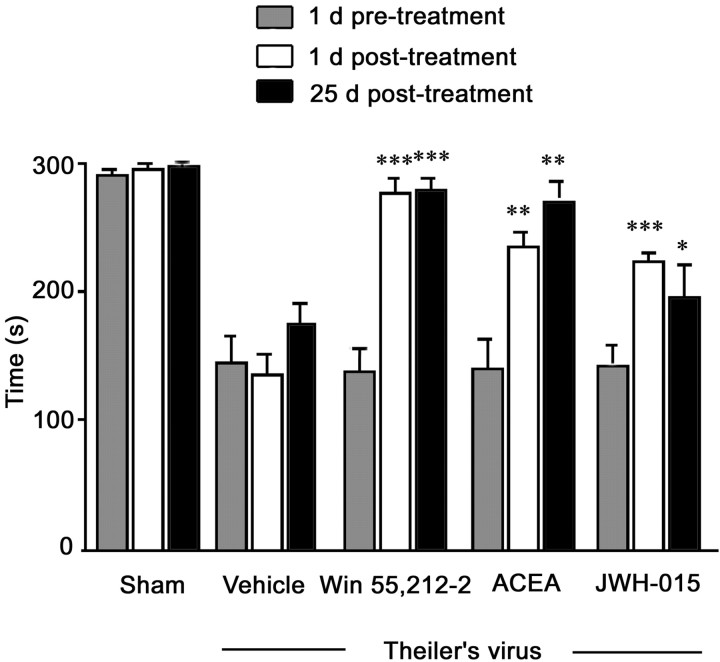
Several studies correlate poor ability to perform rotarod test with demyelination induced by TMEV infection (McGavern et al., 1999). TMEV-infected mice exhibited reduced ability in rotarod performance before cannabinoid treatment compared with sham mice (Fig.1). A two-way ANOVA revealed a significant effect of treatment (p < 0.001), time (p < 0.001), and treatment × time interaction (p < 0.001). To evaluate the effect of cannabinoids compared with the pretreatment situation, we performed Student's t test within each cannabinoid-treated group. One day after a 10 d treatment protocol with the CB1/CB2 nonselective cannabinoid agonist WIN 55,212–2, mice showed increased ability to perform the test correctly (p < 0.001); this effect was also observed 25 d after cessation of treatment (p < 0.001). The selective CB1 agonist ACEA also increased the ability of TMEV-infected mice to perform the rotarod assay 1 d after the end of the treatment (p < 0.01) as well as at 25 d (p < 0.01). The CB2 selective agonist JWH-015, although it did not improve the motor ability of TMEV-infected mice to values similar to those of WIN 55,212–2 and ACEA, also improved rotarod performance at 1 d (p < 0.001) and 25 d after treatment (p < 0.05). Thus, all cannabinoids used induce functional recovery of TMEV-infected mice that is maintained for at least 25 d after the termination of treatment.
Fig. 1.
Cannabinoids induce long-term improvement of motor function. Data from the rotarod assay show a significant increase in motor function of WIN 55,212–2-treated (dose schedule: 2.5 mg/kg for 3 d, 3.75 mg/kg on days 4–6, and 5 mg/kg on days 7–10), ACEA-treated (dose schedule: 1.25 mg/kg on days 1–3, 1.9 mg/kg on days 4–6, and 2.5 mg/kg on the last 4 d of treatment), and JWH-015-treated (dose schedule: 0.6 mg/kg for 3 d, 0.9 mg/kg on days 4–6, and 1.2 mg/kg on the last 4 d) mice 1 d after the end of the 10 d treatment protocol, which is maintained for at least 25 d after cessation of treatment. (***p< 0.001 vs vehicle; **p < 0.01 vs vehicle; *p < 0.05 vs vehicle).
Cannabinoid agonists reduce microglial activation in TMEV-infected mice
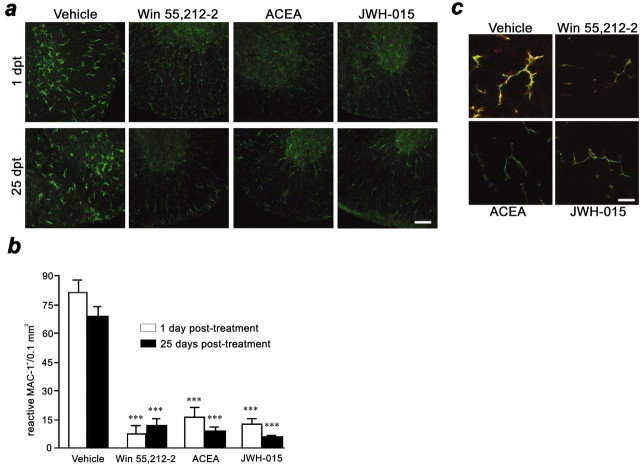
Microglial cells in the spinal cord of TMEV-infected mice showed a reactive morphology in white and gray matter; 10 d treatment with WIN 55,212–2, ACEA, or JWH-015 markedly switched their morphology toward a resting one (Fig.2a). This effect was observed 1 d after treatment and was maintained for at least 25 d after treatment (Fig. 2a). Quantification of reactive microglia within the spinal cord and statistical analysis showed that the three cannabinoid agonists induced a significant reduction (p < 0.001 for all agonists) in the number of reactive microglial cells, both 1 and 25 d after the end of treatment (Fig. 2b).
Fig. 2.
Cannabinoids inhibit microglial activation.a, Confocal images with constant laser beam and photodetector sensitivity of microglia/macrophages (CD11b+ cells) in ventral spinal cord sections. Microglial cells in vehicle-treated mice show a reactive morphology at 1 and 25 d after treatment. In contrast, WIN 55,512–2, ACEA, and JWH-015 treatments markedly inhibit reactive morphology of microglia at 1 and 25 d after treatment. Scale bar, 50 μm. b,Treatment with cannabinoid agonists reduce the number of reactive microglial cells in the spinal cord at 1 and 25 d after treatment (***p < 0.001 vs vehicle). c,Confocal images with constant laser beam and photodetector sensitivity of microglial MHC class II antigen expression. CD11b is shown ingreen, and the MHC class II complex is shown inred. Note antibody colocalization (yellow) in vehicle-treated mice and the presence of cells other than microglia expressing MHC class II molecules. Cannabinoids abrogate microglial MHC class II expression. Images are representative of 1 and 25 d after 10 d cannabinoid treatment. Scale bar, 20 μm.
Macrophage/microglial cells can process and present myelin epitopes, in association with MHC class II molecules, to CD4+ T cells within the CNS of TMEV-infected mice (Katz-Levy et al., 1999). Double immunostaining for Mac-1 and H-2A (SJL/J MHC class II haplotype) revealed a predominant colocalization of MHC class II antigen expression on Mac-1+ cells in the white and gray matter of TMEV-infected mice spinal cords (Fig. 2c). One day after cannabinoid treatment, positive immunostaining for MHC class II almost disappeared in microglial cells with all three agonists. Importantly, this effect did not diminish by 25 d after the end of treatment (Fig. 2c).
Cannabinoid treatment reduces the number of CD4+infiltrated T cells in the spinal cord of TMEV-infected mice
TMEV-infected mice exhibited CD4+infiltrated T cells within spinal cord white and gray matter (Fig.3a). Cannabinoid treatment caused a significant reduction in the number of CD4+ T cells in the spinal cord 1 d after treatment (p < 0.05 for WIN 55,212–2; p < 0.001 for ACEA and JWH-015), as well as at 25 d after treatment (p < 0.05 for WIN 55,212–2 and JWH-015; p < 0.01 for ACEA) (Fig.3b). Therefore, treatment of TMEV-infected mice with cannabinoids for 10 d reduced CD4+ T cell infiltration in spinal cord for at least 25 d after treatment cessation.
Fig. 3.
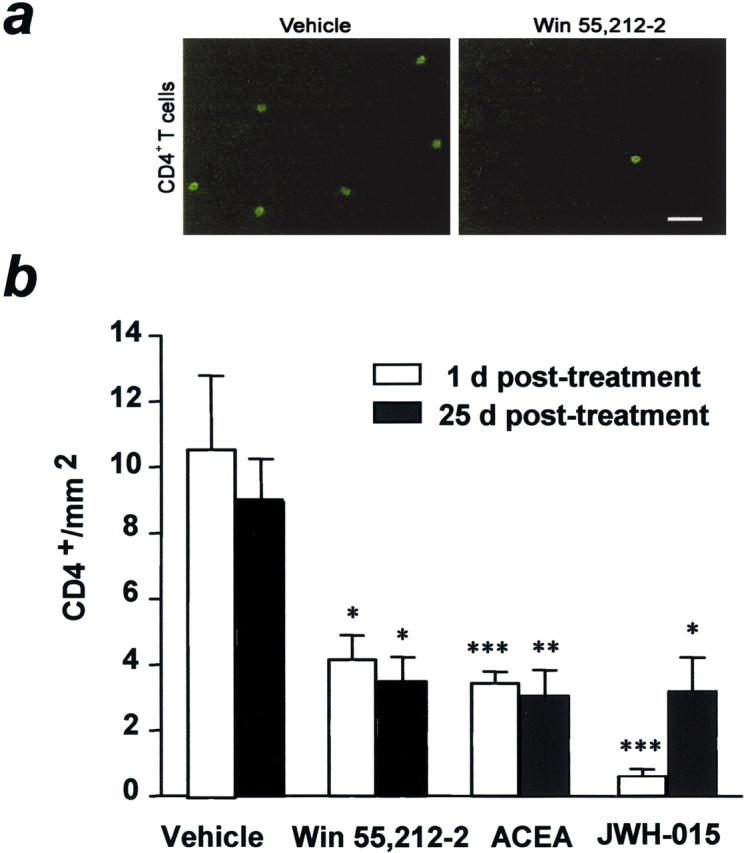
Cannabinoids reduce the number of CD4+-infiltrated T cells. a,Fluorescence microscopy images show CD4+ T cells infiltrated in the spinal cord of TMEV-infected mice. Note the clear reduction in CD4+-infiltrated T cells in spinal cord from cannabinoid-treated mice. Scale bar, 8 μm. b,Cannabinoid agonist-treated mice show a reduction in the number of infiltrated CD4+ T cells to less than half that of the vehicle-treated group at 1 d after treatment; this effect is maintained for 25 d (*p < 0.05 vs vehicle; **p < 0.01 vs vehicle; ***p < 0.001 vs vehicle).
Cannabinoid agonists promote remyelination in TMEV-infected mice
Spinal cords of TMEV-infected mice showed disperse demyelination (Fig. 4a), preferentially distributed in anterior, anterolateral, and lateral columns. The number of axons affected (demyelinated plus remyelinated) in vehicle-treated and cannabinoid-treated mice was not significantly different, indicating that myelin lesions were similar in the different groups (data not shown). In spinal cords from cannabinoid-treated mice, however, there was a reduction in the number of demyelinated axons and a significant increase in the number of remyelinating axons (p < 0.001 vs vehicle for the three agonists) (Fig. 4b). In fact, the percentage of remyelinating axons in cannabinoid-treated mice was more than twofold higher than in vehicle-treated mice.
Fig. 4.
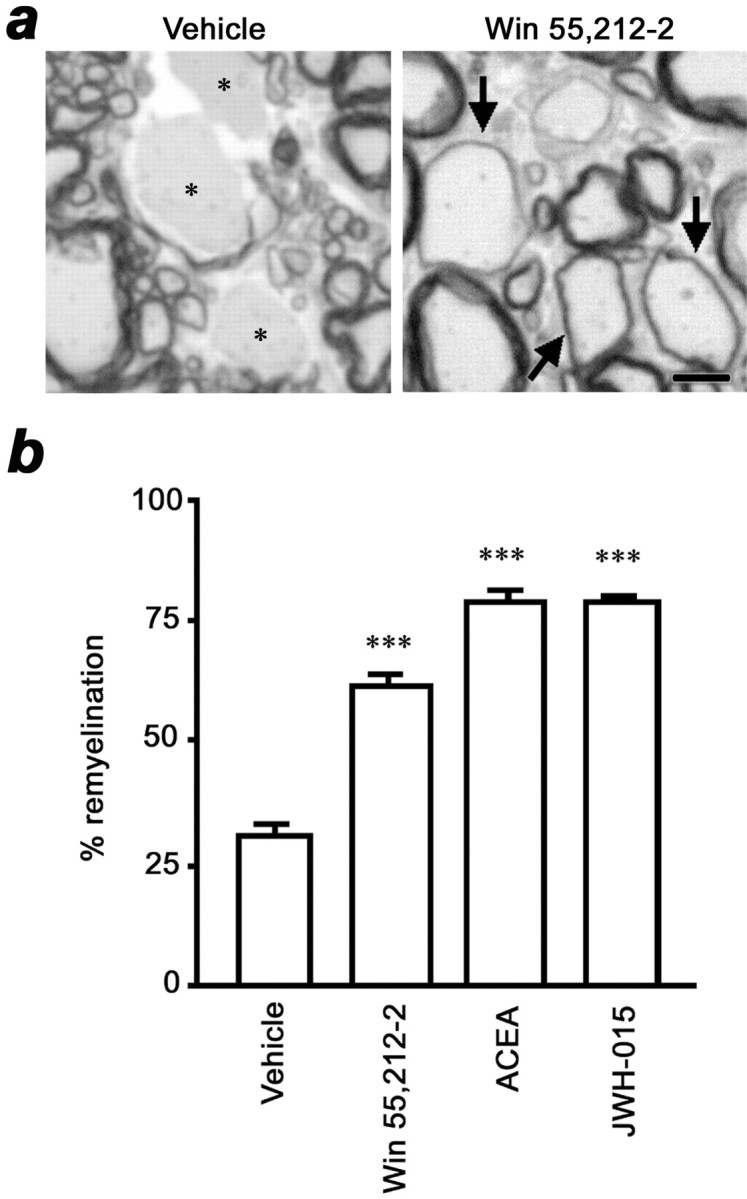
Cannabinoids promote spinal cord remyelination.a, Toluidine blue-stained semithin sections of spinal cord show demyelinated axons (asterisk) in a vehicle-treated mouse and thin compact myelin sheets in large axons, indicative of remyelination (arrows), in a WIN55,212–2-treated mouse. Scale bar, 5 μm. b, The percentage of remyelinated axons in cannabinoid-treated animals was more than twofold higher than in vehicle-treated mice (***p < 0.001 vs vehicle).
Discussion
The present study reports that functional recovery of TMEV-infected mice induced by cannabinoid treatment parallels reduction in CNS inflammation and extensive remyelination.
As evaluated by rotarod performance, treatment with the cannabinoids, WIN 55,212–2, ACEA and JWH-015, improve motor coordination of TMEV-infected mice, suggesting the involvement of both CB receptors on such effect. However, because JWH-015 has also a low affinity for CB1 receptors, it cannot be excluded as a putative CB1-mediated effect. In the chronic relapsing EAE model of MS,Baker et al. (2000) found reductions on limb spasticity and tremor in mice treated with CB1 or CB2 selective agonists. Besides, the level of endocannabinoids increased in the spinal cord of diseased mice (Baker et al., 2001) and changes in the number of CB1 receptors occurred in motor pathways (Berrendero et al., 2001). Together, these observations suggest that endocannabinoids tonically control spasticity in animal models of MS. Therefore, a direct action of cannabinoids on the motor system may contribute to the amelioration observed in our study. However, the maintenance of recovery for at least 25 d after the end of treatment suggests that effects other than single modulation of motor pathways are involved.
Cannabinoids may promote long-lasting functional recovery by interfering with the inflammatory demyelinating process and by favoring myelin repair. All three agonists reduced the number of reactive microglia, almost suppressed microglial MHC class II antigen expression, and diminished CD4+ T cell infiltration within the spinal cord of TMEV-infected mice. Therefore, cannabinoid treatment diminished components of the MHC class II-restricted CD4+ T cell response. In addition, we show, to our knowledge for the first time, that cannabinoid treatment favors remyelination in the spinal cord of TMEV-infected mice. One possibility is that cannabinoids enhance myelin repair indirectly by inhibiting the immune response that contributes to demyelination or that hampers remyelination. Our results showing decreased number of CD4+ T cells in cannabinoid-treated mice support an immunomodulatory mechanism. In accordance with this, depletion of CD4+ T cells enhanced myelin repair (Fiette et al., 1993), and inhibition of the MHC class II-mediated response using anti-H-2A antibodies ameliorates TMEV-IDD (Friedmann et al., 1987). Further support derives from the observation that interferon-β, a cytokine used in MS treatment, inhibits MHC class II expression (Barna et al., 1989). Therefore, the reduction in the MHC class II-restricted CD4+ T cell response may be a key event leading to an increased capacity to remyelinate naked axons in cannabinoid-treated mice. Moreover, cannabinoids suppress the production of inflammatory molecules by astrocytes (F. Molina-Holgado et al., 1997, 2002) and microglial cells (Puffenbarger et al., 2000) perhaps, contributing to the effects observed in the present study.
The percentage of remyelinating axons in cannabinoid-treated mice reached values more than twofold higher than in vehicle-treated mice. In the progressive form of MS, the remyelination process remains abortive, and although multiple factors may be involved, the inflammatory environment has been considered a reliable candidate for such impediment (Noseworthy et al., 2000). The failure of remyelination in TMEV-IDD, which resembles progressive MS, may also be related to the inflammatory environment, because the decrease inflammation observed in cannabinoid-treated mice parallels an increase in remyelination. The administration of high doses of Δ9-THC decreased clinical signs in EAE and also reduced CNS inflammation (Lyman et al., 1989). Recent observations indicate that remyelination progress more efficiently when is associated to the inflammatory process (for review, see Franklin, 2002). However, this occurs mainly in models of toxic demyelination in which the inflammatory response is a consequence of demyelination and not its cause, as occurs in immune-mediated demyelination. Under our experimental conditions, downregulation of the inflammatory response seems to establish a more favorable CNS environment for oligodendroglial cells to resume the myelination program. Oligodendroglial cells are damaged by inflammatory stimuli, whereas reduction of inflammation markedly rescues cell survival (Merril and Benveniste, 1996; Molina-Holgado et al., 2001). Thus, anti-inflammatory actions of cannabinoids may be important not only to restrain the demyelination process, but also to enhance the endogenous reparative remyelination. In addition, cannabinoids may favor myelin repair directly by acting on oligodendrocytes. Such hypothesis is supported by the expression of cannabinoid receptors in oligodendroglial cells and the protection of progenitors from apoptosis induced by deprivation of trophic support through a PI3K/Akt-dependent mechanism (E. Molina-Holgado et al., 2002). Therefore, we suggest that the effects of cannabinoids on remyelination result from both anti-inflammatory actions and direct effects on oligodendrocyte survival and differentiation.
In conclusion, our data show a protective role of cannabinoids in experimental progressive, inflammatory demyelination. Such action of cannabinoids is probably exerted at several levels, as discussed above. The ability of cannabinoids to ameliorate established disease may have potential therapeutic implications in demyelinating pathologies such as MS, because treatment is started after the onset of clinical symptomatology. The involvement of CB2 receptors would enable nonpsychoactive therapy suitable for long-term use. Moreover, the pharmacological modulation of endocannabinoid system (Di Marzo et al., 2001) would be a future research target for the development of cannabinoid-based therapy in animal models of MS. Studies in progress are aimed to evaluate whether cannabinoids are actually involved in oligodendrocyte precursor recruitment and differentiation after demyelination.
Footnotes
This work was supported by grants from the Ministerio de Ciencia y Tecnología (Spain, SAF-2001/1246). We gratefully appreciate Dr. R. P. Roos (University of Chicago) for delivery of Theiler's virus. We express our gratitude to E. Baides, C. Bailón, and C. Hernández for their excellent technical assistance.
Correspondence should be addressed to Carmen Guaza, Cajal Institute, Consejo Superior de Investigaciones Científicas, Avenida Doctor Arce 37, 28002 Madrid, Spain. E-mail: cgjb@cajal.csic.es.
References
- 1.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Huffman JW, Layward L. Cannabinoids control spasticity and tremor in a multiple sclerosis model. Nature. 2000;404:84–87. doi: 10.1038/35003583. [DOI] [PubMed] [Google Scholar]
- 2.Baker D, Pryce G, Croxford JL, Brown P, Pertwee RG, Makriyannis A, Khanolkar A, Layward L, Fezza F, Bisogno T, Di Marzo V. Endocannabinoids control spasticity in a multiple sclerosis model. FASEB J. 2001;15:300–302. doi: 10.1096/fj.00-0399fje. [DOI] [PubMed] [Google Scholar]
- 3.Barna BP, Chou SM, Jacobs B, Yen-Lieberman B, Ransohoff RM. Interferon-β impairs induction of HLA-DR antigen expression in cultured human astrocytes. J Neuroimmunol. 1989;23:45–53. doi: 10.1016/0165-5728(89)90072-6. [DOI] [PubMed] [Google Scholar]
- 4.Berrendero F, Sanchez A, Cabranes A, Puerta C, Ramos JA, García-Merino A, Fernández Ruiz JJ. Changes in cannabinoid CB1 receptors in striatal and cortical regions of rat with experimental allergic encephalomyelitis, an animal model of multiple sclerosis. Synapse. 2001;41:195–202. doi: 10.1002/syn.1075. [DOI] [PubMed] [Google Scholar]
- 5.Bouaboula M, Rinaldi M, Carayon P, Carillon C, Delpech B, Shire D, Le Fur G, Casellas P. Cannabinoid-receptor expression in human leukocytes. Eur J Biochem. 1993;214:173–180. doi: 10.1111/j.1432-1033.1993.tb17910.x. [DOI] [PubMed] [Google Scholar]
- 6.Dal Canto MC, Lipton HL. Multiple sclerosis-animal model: Theiler's virus infection in mice. Am J Pathol. 1977;88:497–500. [PMC free article] [PubMed] [Google Scholar]
- 7.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etingen A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 8.Di Marzo V, Bifulco M, De Petrocellis L. Endocannabinoids and multiple sclerosis: a blessing from the inner bliss? Trends Pharmacol Sci. 2000;21:195–197. doi: 10.1016/s0165-6147(00)01487-5. [DOI] [PubMed] [Google Scholar]
- 9.Di Marzo V, Bisogno T, De Petrocellis L. Anandamide: some like it hot. Trends Pharmacol Sci. 2001;22:346–349. doi: 10.1016/s0165-6147(00)01712-0. [DOI] [PubMed] [Google Scholar]
- 10.Fiette L, Aubert C, Brahic M, Peña Rossi C. Theiler's virus infection of β2-microglobulin-deficient mice. J Virol. 1993;67:589–592. doi: 10.1128/jvi.67.1.589-592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Franklin RJ. Why does remyelination fail in multiple sclerosis? Nat Neurosci. 2002;3:705–714. doi: 10.1038/nrn917. [DOI] [PubMed] [Google Scholar]
- 12.Friedmann A, Frankel G, Lorch Y, Steinman L. Monoclonal anti-I-A antibody reverses chronic paralysis and demyelination in Theiler's virus-infected mice: critical importance of timing of treatment. J Virol. 1987;61:898–903. doi: 10.1128/jvi.61.3.898-903.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P. Expression of central and cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 14.Herkenham M, Lynn AB, Little MD, Johson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johnson RT. The virology of demyelinating diseases. Ann Neurol. 1994;36:54–60. doi: 10.1002/ana.410360715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katz-Levy Y, Neville KL, Girvin AM, Vanderlugt CL, Pope JG, Tan LJ, Miller SD. Endogenous presentation of self myelin epitopes by CNS-resident APCs in Theiler's virus-infected mice. J Clin Invest. 1999;104:599–610. doi: 10.1172/JCI7292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein TW, Lane B, Newton C, Friedman H. Cannabinoid receptors and immunity. Proc Soc Exp Biol Med. 2000;225:1–8. doi: 10.1177/153537020022500101. [DOI] [PubMed] [Google Scholar]
- 18.Lledó A, Borrell J, Guaza C. Dexamethasone regulation of interleukin-1-receptors in the hippocampus of Theiler's virus-infected mice: effects on virus-mediated demyelination. Eur J Pharmacol. 1999;372:75–83. doi: 10.1016/s0014-2999(99)00187-9. [DOI] [PubMed] [Google Scholar]
- 19.Lyman WD, Sonett JR, Brosnan CF, Elkin R, Bornstein MB. Δ9-tetrahydrocannabinol: a novel treatment for experimental autoimmune encephalomyelitis. J Neuroimmunol. 1989;23:73–81. doi: 10.1016/0165-5728(89)90075-1. [DOI] [PubMed] [Google Scholar]
- 20.Malfait AM, Gallily R, Sumariwalla PF, Malik AS, Andreakos E, Mechoulam R, Feldmann M. The nonpsychoactive cannabis constituent cannabidiol is an oral anti-arthritic therapeutic in murine collagen-induced arthritis. Proc Natl Acad Sci USA. 2000;97:9561–9566. doi: 10.1073/pnas.160105897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 22.McCoy KL, Matmeyeva M, Carlisle SJ, Cabral GA. Cannabinoid inhibition of the processing of intact lysozyme by macrophages: evidence for CB2 receptor participation. J Pharmacol Exp Ther. 1999;289:1620–1625. [PubMed] [Google Scholar]
- 23.McGavern DB, Zoecklein L, Drescher KM, Rodriguez M. Quantitative assessment of neurologic deficits in a chronic progressive murine model of CNS demyelination. Exp Neurol. 1999;158:171–181. doi: 10.1006/exnr.1999.7082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGavern DB, Murray PD, Rivera-Quiñones C, Schmelzer JD, Low PA, Rodriguez M. Axonal loss results in spinal cord atrophy, electrophysiological abnormalities and neurological deficits following demyelination in a chronic inflammatory model of multiple sclerosis. Brain. 2000;123:519–531. doi: 10.1093/brain/123.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mechoulam R, Ben Shabat S, Hanus L, Ligumsky M, Kaminski NE, Shatz AR, Gopher A, Almog S, Martin BR, Compton DR, Pertwee RG, Griffin G, Bayewitch M, Barg J, Vogel Z. Identification of an endogenous 2-monoglyceride, present in canine gut, that binds to cannabinoid receptors. Biochem Pharmacol. 1995;50:83–90. doi: 10.1016/0006-2952(95)00109-d. [DOI] [PubMed] [Google Scholar]
- 26.Merril JE, Benveniste EN. Cytokines in inflammatory brain lesions: helpful and harmful. Trends Neurosci. 1996;19:331–338. doi: 10.1016/0166-2236(96)10047-3. [DOI] [PubMed] [Google Scholar]
- 27.Miller SD, Vanderlugt CL, Begolka WS, Pao W, Yauch RL, Neville KL, Katz-Levy Y, Carrizosa A, Kim BS. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat Med. 1997;3:1133–1136. doi: 10.1038/nm1097-1133. [DOI] [PubMed] [Google Scholar]
- 28.Molina-Holgado E, Vela JM, Arévalo-Martín A, Guaza C. LPS/IFN-γ cytotoxicity in oligodendroglial cells: role of nitric oxide and protection by the anti-inflammatory cytokine IL-10. Eur J Neurosci. 2001;13:493–502. doi: 10.1046/j.0953-816x.2000.01412.x. [DOI] [PubMed] [Google Scholar]
- 29.Molina-Holgado E, Vela JM, Arévalo-Martín A, Molina-Holgado F, Almazán G, Borrell J, Guaza C. Expression of cannabinoid CB1 receptors in oligodendroglial cells: activation of PI-3K/Akt signaling pathway promotes cell survival. J Neurosci. 2002;22:9742–9753. doi: 10.1523/JNEUROSCI.22-22-09742.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Molina-Holgado F, Lledo A, Guaza C. Anandamide suppresses nitric oxide and TNF-α responses to Theiler's virus or endotoxin in astrocytes. NeuroReport. 1997;8:1929–1933. doi: 10.1097/00001756-199705260-00027. [DOI] [PubMed] [Google Scholar]
- 31.Molina-Holgado F, Molina-Holgado E, Guaza C, Rothwell N. Role of CB1 and CB2 receptors in the inhibitory effects of cannabinoids on lipopolysaccharide induced nitric oxide release in astrocyte cultures. J Neurosci Res. 2002;67:829–836. doi: 10.1002/jnr.10165. [DOI] [PubMed] [Google Scholar]
- 32.Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- 33.Noseworthy JH, Lucchinetti C, Rodriguez M, Weinshenker BG. Multiple sclerosis. N Engl J Med. 2000;343:938–952. doi: 10.1056/NEJM200009283431307. [DOI] [PubMed] [Google Scholar]
- 34.Pertwee RG. Pharmacology of cannabinoid receptor ligands. Curr Med Chem. 1999;6:635–664. [PubMed] [Google Scholar]
- 35.Pertwee RG. Cannabinoids and multiple sclerosis. Pharmacol Ther. 2002;95:165–174. doi: 10.1016/s0163-7258(02)00255-3. [DOI] [PubMed] [Google Scholar]
- 36.Piomelli D, Giuffrida A, Calignano A, Rodriguez de Fonseca F. The endocannabinoid system as a target for therapeutic drugs. Trends Pharmacol Sci. 2000;21:218–224. doi: 10.1016/s0165-6147(00)01482-6. [DOI] [PubMed] [Google Scholar]
- 37.Puffenbarger RA, Boothe AC, Cabral GA. Cannabinoids inhibit LPS-inducible cytokine mRNA expression in rat microglial cells. Glia. 2000;29:58–69. [PubMed] [Google Scholar]
- 38.Warrington AE, Asakura K, Bieber AJ, Ciric B, Van Keulen V, Kaveri SV, Kyle RA, Pease LR, Rodríguez M. Human monoclonal antibodies reactive to oligodendrocytes promote remyelination in a model of multiple sclerosis. Proc Natl Acad Sci USA. 2000;97:6820–6825. doi: 10.1073/pnas.97.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu W, Friedman H, Klein TW. Δ9-Tetrahydrocannabinol induces apoptosis in macrophages and lymphocytes: involvement of bcl-2 and caspase-1. J Pharmacol Exp Ther. 1998;286:1103–1109. [PubMed] [Google Scholar]