Abstract
The bed nucleus of the stria terminalis (BNST) is in a key position to influence the integration of motivational and visceral functions, receiving inputs from limbic regions, including the amygdala, and sending projections to areas central to reward processing, including the ventral tegmental area and nucleus accumbens. The BNST also possesses a high density of noradrenergic fibers. The purpose of the present studies was to characterize the effects of cocaine self-administration on the regulation of norepinephrine transporter (NET) distribution and functional activity in the BNST of rhesus monkeys in the initial (5 d) or chronic (100 d) phases of cocaine self-administration. NET binding site densities in the BNST were assessed with quantitative in vitro receptor autoradiography using [3H]nisoxetine, and rates of local cerebral glucose utilization in the BNST were measured in the same monkeys using the 2-[14C]deoxyglucose method. Chronic exposure to cocaine self-administration resulted in significantly higher NET binding site densities (up to 52% relative to controls) throughout the BNST. Furthermore, cerebral metabolism was depressed significantly in a time-dependent manner with larger decreases after 100 d of cocaine self-administration. These data represent the first report of significant changes in the regulation of the NET resulting from cocaine exposure in primates. Furthermore, given the role of the BNST in cocaine withdrawal and stress-related reinstatement of self-administration, the changes reported here may provide a substrate for these phenomena.
Keywords: cocaine, self-administration, bed nucleus of the stria terminalis, norepinephrine, receptor autoradiography, local cerebral glucose utilization
Introduction
Cocaine is a powerful psychomotor stimulant known to be one of the most strongly reinforcing drugs of abuse. Cocaine binds to dopamine, serotonin, and norepinephrine (NE) transporters (NET) (Ritz et al., 1990; Florin et al., 1994), blocking the reuptake of these monoamines into presynaptic terminals, thereby increasing neurotransmitter concentrations. The interaction of cocaine with dopamine systems has been considered critical to its reinforcing effects (Roberts et al., 1980; Goeders and Smith, 1983; Ritz et al., 1987; Giros et al., 1996); therefore, much attention has been paid to alterations in the regulation of dopamine (DA) transporters and receptors as a result of long-term exposure to cocaine (Farfel et al., 1992; Volkow et al., 1993, 1999; Letchworth et al., 2001; Nader et al., 2002; Porrino et al., 2002a).
Far less attention has been paid, however, to the role of noradrenergic systems as mediators of the acute or chronic actions of cocaine, although cocaine accumulates in high concentrations in NE-rich brain regions (Madras and Kaufman, 1994). Several reports, however, have documented altered noradrenergic tone during cocaine withdrawal in human cocaine abusers (McDougle et al., 1994;Kampman et al., 2001). The administration of the α2-adrenergic receptor antagonist yohimbine immediately after the cessation of cocaine use produced an increased susceptibility to panic anxiety (McDougle et al., 1994), suggesting that dysregulation of the noradrenergic system may accompany long-term cocaine exposure.
The bed nucleus of the stria terminalis (BNST), a key component of the extended amygdala, contains one of the highest regional densities of noradrenergic fiber inputs (Brownstein and Palkovits, 1984) and NET binding sites (Tejani-Butt, 1992), receiving projections primarily from the noradrenergic cell-containing regions of the caudal medulla (Roder and Ciriello, 1994). Recent reports have suggested that the BNST plays a role in drug reinforcement processes. The dopamine D1 receptor antagonist SCH 23390 administered bilaterally into the rat BNST effectively reduces the reinforcing effects of cocaine (Epping-Jordan et al., 1998), and the BNST has been shown to be critical in mediating stress-induced reinstatement of cocaine self-administration in rodent models (Shaham et al., 2000; Leri et al., 2002).
Given the role of the BNST in mediating the effects of cocaine, in conjunction with dense noradrenergic innervation of the BNST, the purpose of the present study was to investigate in monkeys the effects of chronic cocaine self-administration in the BNST on both functional activity, as measured with the 2-[14C]deoxyglucose (2-DG) method, and the concentration of the NET, measured autoradiographically in the same animals. To this end, a nonhuman primate model of self-administration was used in which the effects of increasing durations of cocaine exposure on both glucose utilization and NET density were measured within the BNST.
Materials and Methods
Subjects. Fourteen experimentally naive adult male rhesus monkeys (Macaca mulatta), 6–13 years old and weighing 7.5–13.0 kg under free-feeding conditions at the inception of the study, were housed individually in stainless steel cages and maintained in temperature- and humidity-controlled colony rooms on a 14/10 hr light/dark cycle (lights on at 6:00 A.M.). Monkeys were maintained at 90–95% of their free-feeding weights, and water was available ad libitum. All procedures were performed in accordance with established practices as described in the NIH Guide for Care and Use of Laboratory Animals. Protocols were reviewed and approved by the Animal Care and Use Committee of Wake Forest University.
Cocaine self-administration. Training and self-administration procedures have been described previously (Nader et al., 2002). Monkeys were trained initially to respond under a fixed-interval 3 min schedule of food presentation. When food-maintained responding was stable, responding was extinguished for five consecutive sessions. After food-maintained responding was reestablished, indwelling intravenous catheters and vascular access ports for drug delivery were implanted. Control monkeys (5 d,n = 4; 100 d, n = 2) continued to respond under the same food presentation schedule for the duration of testing.
Cocaine self-administration under the fixed-interval 3 min schedule was established in two groups of monkeys (n = 4 per group) by substituting cocaine (0.3 mg/kg per injection) for food. The cocaine groups differed by length of cocaine exposure: 5 d was chosen to represent a model of the earliest stages of cocaine exposure, and 100 d represented chronic cocaine exposure. All sessions ended after 30 reinforcer presentations. Experimental sessions were conducted 7 d/week at approximately the same time each day. Total lifetime cocaine intake was 45 and 900 mg/kg for the 5 and 100 d groups, respectively. The average weekly cocaine intake of the chronic monkeys (63 mg/kg) was within the range of reported weekly intakes of human cocaine addicts (43–329 mg/kg on the basis of the average 70 kg man) (Volkow et al., 1993, 1999). A detailed analysis of the behavioral data has been reported previously (Nader et al., 2002).
Measurement of local cerebral glucose utilization. The animals' catheters exited through an opening in the rear of the chamber, allowing all infusions and sampling to be accomplished remotely with minimal disruption to the animal. The 2-DG procedure has been described in detail previously (Porrino et al., 2002b). Briefly, a pulse of 75 μCi/kg 2-deoxy-d-[14C]glucose (specific activity, 50–55 mCi/mmol; DuPont NEN, Boston, MA) was infused intravenously, followed by the collection of timed arterial blood samples drawn at a schedule sufficient to define the time course of the arterial 2-[14C]deoxyglucose and glucose concentrations. At ∼45 min after tracer injection, the animals were killed by an intravenous overdose of sodium pentobarbital (100 mg/kg). Brains were removed rapidly, blocked, frozen in isopentane, and stored at −70°C until processed. Coronal sections (20 μm) were cut in a cryostat, thaw mounted on glass coverslips, and apposed to Kodak MR-1 film (Eastman Kodak, Rochester, NY) for 15–30 d, along with a set of [14C]methylmethacrylate standards (Amersham Biosciences, Arlington Heights, IL) calibrated previously for their equivalent 14C concentration in 20 μm brain sections.
[3H]nisoxetine autoradiography. Coronal sections (20 μm) adjacent to sections used for 2-DG autoradiography were thaw mounted onto chrome-alum–gelatin-subbed slides or electrostatically charged slides, desiccated, and stored at −80°C until processed.
The distribution of [3H]nisoxetine binding sites was assessed with quantitative in vitroreceptor autoradiography according to procedures adapted from previous research (Tejani-Butt, 1992). Tissue sections were preincubated at room temperature in buffer (in mm: 50 Tris, 300 NaCl, and 5 KCl, pH 7.4) for 20 min to remove any residual cocaine and [14C]2-DG. Sections were then incubated for 4 hr at 4°C in buffer containing 3.0 nm[3H]nisoxetine (80 Ci/mmol) (PerkinElmer Life Sciences, Boston, MA) in the presence (nonspecific binding) or absence (total binding) of 1 μm mazindol. Sections were rinsed three times (5 min each) in buffer at 4°C, with a final 10 sec rinse in ice-cold water. Sections were dried immediately under a stream of cold air and placed on Hyperfilm-3H in the presence of [3H] standards (Amersham Biosciences). After exposure times of 2–6 weeks, films were developed with Kodak GBX developer, fixed, and rinsed.
Measurement of rates of glucose utilization and [3H]nisoxetine binding was conducted by quantitative densitometry with a computerized image processing system (MCID; Imaging Research, St. Catharines, Ontario) as described previously (Porrino et al., 2002b). Nomenclature and identification of the BNST subdivisions measured for optical densities were according to the work of Martin et al. (1991) in thionin-stained sections. Tissue equivalent values (2-DG, μmol/100 gm per minute; NET, femtomoles per milligram of wet weight tissue) were determined from the optical densities and from a calibration curve obtained by densitometric analysis of 14C or tritium standards, respectively. Specific binding was determined by subtracting nonspecific binding values from total binding values measured in adjacent sections.
Statistical analysis. Statistical analyses (SPSS, Chicago, IL) of rates of glucose utilization and specific binding values were performed for each BNST subdivision by means of a one-way ANOVA, followed by least-significant difference multiple comparisons of cocaine self-administration groups to controls.
Results
No significant differences in metabolism or NET density were observed between the 5 and 100 d control subjects (n = 4 and n = 2, respectively); therefore, their results were combined throughout.
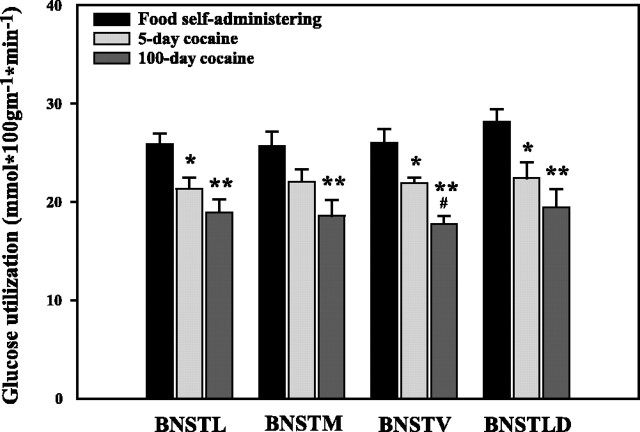
Cocaine self-administration produced large duration-dependent reductions in cerebral glucose utilization throughout all segments of the BNST (Fig. 1). After 5 d of self-administration, glucose utilization was altered, compared with controls, in the lateral subdivision of the BNST (BNSTL) (−17.5%), ventral subdivision of the BNST (BNSTV) (−15.6%), and lateral dorsal BNST (BNSTLD) (−20.3%). After chronic exposure, alterations in cerebral metabolism were of a greater magnitude and included the entire BNST–BNSTL (−26.8%), medial subdivision of the BNST (BNSTM) (−27.7%), BNSTV (−31.6%), and the BNSTLD (−30.8%).
Fig. 1.
The effect of cocaine self-administration on cerebral metabolism in the BNST. Monkeys with 100 d of cocaine self-administration experience exhibited significantly reduced glucose utilization in the BNST compared with food controls (*p < 0.05; **p < 0.01) and monkeys with 5 d of exposure to cocaine (#p < 0.05).
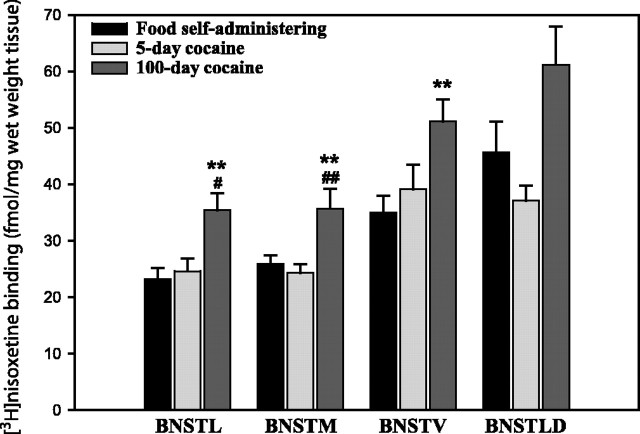
The binding pattern of [3H]nisoxetine to NET in control animals was highly heterogeneous (Fig.2). The highest NET binding densities were observed in the BNSTLD (45.6 fmol/mg wet weight tissue) and the BNSTV (35.0 fmol/mg). Concentrations of [3H]nisoxetine binding were lower in the BNSTM (25.9 fmol/mg) and the BNSTL (23.2 fmol/mg).
Fig. 2.
The effect of cocaine self-administration on NET distribution in the BNST. In most of the subregions of the BNST, monkeys with 100 d of cocaine self-administration experience exhibited significantly greater [3H]nisoxetine binding densities than food controls (*p < 0.05; **p ≤ 0.01) and monkeys with 5 d of exposure to cocaine (#p < 0.05; ##p < 0.01).
Cocaine self-administration induced duration-dependent increases in [3H]nisoxetine binding site densities in all BNST subregions examined (Figs. 2,3). Although 5 d of cocaine self-administration did not significantly alter the density of [3H]nisoxetine binding sites, 100 d of cocaine exposure resulted in significantly higher [3H]nisoxetine binding site densities compared with controls. After chronic self-administration, the increases in NET density were significant in the BNSTL (+52%), the BNSTM (+38%), and the BNSTV (+46%).
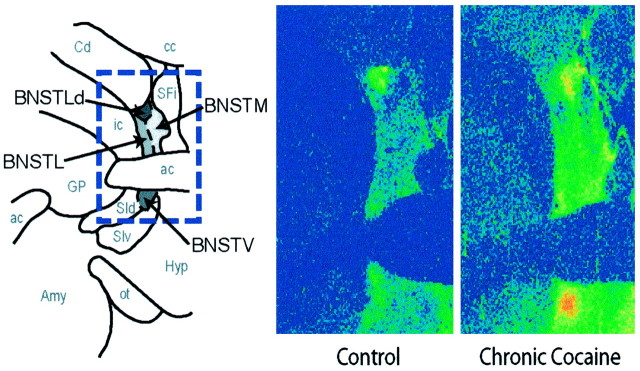
Fig. 3.
Comparison of [3H]nisoxetine binding in the BNST of representative control and chronic cocaine self-administration monkeys. Shown are coronal sections through the BNST. At left is a schematic diagram depicting the spatial organization of subregions within the BNST in which the densities of [3H]nisoxetine binding sites were quantified. At right are color-coded transformations of autoradiograms of [3H]nisoxetine binding sites in the BNST of a food control (left) and chronic (100 d) cocaine monkey (right). Each colorrepresents a range of values expressed as femtomoles per milligram of wet weight tissue. Higher levels of [3H]nisoxetine binding are visible throughout the BNST of the chronic cocaine monkey.ac, Anterior commissure; amy, amygdala;cc, central commissure; Cd, caudate nucleus; GP, globus pallidus; Hyp, hypothalamus; ic, internal capsule; ot, optic tract; SFi, septofimbrial nucleus;SId, dorsal substantia innominata; SIv, ventral substantia innominata.
Discussion
The present data demonstrate that profound structural and functional alterations develop throughout all portions of the BNST as a result of chronic cocaine self-administration. Cerebral metabolism in the BNST was changed even in the earliest stages of cocaine exposure. These changes intensified over time, suggesting that information processing in this brain region, critical to stress and autonomic function, is disrupted increasingly over the course of cocaine self-administration experience. In addition to these functional alterations, changes in the regulation of the NET within the BNST developed in response to continued cocaine self-administration, with apparent upregulation evident in all of the subdivisions of the BNST. The changes in NET density reported here were particularly profound, larger in percentage than any changes in DA transporter or the density of DA D1 or D2 receptors reported within the striatum of the same monkeys (Letchworth et al., 2001; Nader et al., 2002; Porrino et al., 2002a) or in studies of the DA system in human cocaine abusers (Volkow et al., 1993, 1999; Staley et al., 1994; Staley and Mash, 1996; Little et al., 1999). Although many of the behavioral effects of cocaine have generally been attributed to its actions on the DA system, the robust changes in the distribution of the NET reported here, along with the increasing magnitude of the functional alterations that accompany them, suggest that the noradrenergic system of the BNST may be an important component in the development of the neuroadaptations that accompany chronic cocaine use. The BNST in humans can be difficult to distinguish with most neuroimaging techniques but was readily visualized autoradiographically in the present study of nonhuman primates. These data, then, represent the first report of significant changes in either functional activity or the regulation of the noradrenergic system in a primate species.
Several reports have demonstrated an altered responsiveness to acute administration of cocaine in NET (Xu et al., 2000) and α1b-adrenergic receptor (Drouin et al., 2002) knock-out mice, supporting the potential importance of central NE systems in the effects of cocaine. It is, however, with chronic cocaine treatment that the role of NE systems in the BNST is most apparent. Antagonism of NE function by the activation of α2-adrenergic receptors in the amygdala and prefrontal cortex and by the blockade of β-adrenergic receptors in the ventrolateral BNST, for example, has been reported to prevent the stress-mediated reinstatement of cocaine seeking in abstinent rats while not altering reinstatement induced by cocaine itself (Shaham et al., 2000; Leri et al., 2002). Moreover, noradrenergic function during cocaine withdrawal in rats has been implicated pharmacologically in brain stimulation reward threshold elevations, changes indicative of “anhedonia” (Markou et al., 1992). The role for the noradrenergic system in cocaine withdrawal is consistent with findings of similar noradrenergic involvement in opiate withdrawal (Aston-Jones et al., 1999). The upregulation of the NET reported here may provide a substrate for the involvement of NE systems in the BNST after long-term exposure to cocaine.
The BNST is an important substrate of autonomic and visceral functions, including autonomic responses to stressors (Roder and Ciriello, 1994; Koob, 1999). In addition to possessing one of the highest densities of cerebral noradrenergic fiber inputs, the BNST exhibits immunolabeling for corticotropin-releasing factor (CRF) and contains a dense population of CRF receptors (Brownstein and Palkovits, 1984; Shaham et al., 2000; Van Bockstaele et al., 2001). CRF injected into the BNST in the absence of external stressors can induce the reinstatement of cocaine seeking in rats, whereas administration of a CRF receptor antagonist into the BNST attenuates stress-induced reinstatement (Shaham et al., 2000; Erb et al., 2001). In turn, the BNST in both nonhuman primates and rodents has been shown to have strong inputs to dopaminergic populations in the ventral tegmental area (VTA) and substantia nigra (Krettek and Price, 1978; Fudge and Haber, 2001), and its stimulation can activate DA neurons in the VTA (Aston-Jones et al., 1999; Georges and Aston-Jones, 2001, 2002). The increasing reductions in functional activity in the BNST as reported here, then, may inhibit activation of DA neurons throughout the reward circuitry. Such suppression may lead to the perpetuation of drug taking as a means to compensate for this deficit. Furthermore, because NE release in the BNST stimulates release of the stress hormone CRF in this region (Koob, 1999) and because anhedonia, as measured by elevated intra-VTA self-stimulation reward thresholds, is accompanied by profound increases in CRF concentrations in the BNST (Stout et al., 2000), it is conceivable that elevated CRF levels in the BNST associated with increased noradrenergic tone may also dysregulate brain reward function. Behaviorally and anatomically, then, the BNST acts as an interface between stress and reward systems.
In human cocaine abusers, high levels of anxiety are a frequent symptom of the early phases of drug abstinence. During this time, panic attacks can be elicited by the α2-adrenergic receptor antagonist yohimbine in cocaine-dependent subjects, and the β-adrenergic antagonist propranolol was shown recently to both ameliorate cocaine withdrawal symptom severity effectively in early abstinence and improve treatment outcome (McDougle et al., 1994; Kampman et al., 2001). The present findings may provide a mechanism for the heightened reactivity of the noradrenergic system that accompanies cocaine withdrawal.
However, the finding that the NET removes both NE and DA from the synaptic space makes it difficult to determine with certainty the specificity of the cause and function of NET upregulation during chronic cocaine exposure. In fact, in this regard, the NET possesses an even higher affinity for DA than for NE (Pacholczyk et al., 1991). Therefore, DA binding to the NET may also contribute to the chronic effects of cocaine. In vivo microdialysis studies have shown that cocaine dose dependently elevates extracellular DA levels in the BNST (Carboni et al., 2000). It is not clear, therefore, whether NET upregulation in the BNST results from altered dopaminergic or noradrenergic function, or both, and which neurotransmitter system is most influenced by this upregulation. Additional experimentation is necessary to address this question.
A number of studies have demonstrated that cocaine dose dependently increases extracellular NE in the rat hippocampus, prefrontal cortex, and nucleus accumbens (Florin et al., 1994; Li et al., 1996). In turn, NE appears to suppress neuronal firing in the BNST (Casada and Dafny, 1993), thereby inhibiting the release of the excitatory transmitter glutamate in the BNST (Forray et al., 1999). This cocaine-induced tonic inhibition may translate into depressed cerebral metabolic rates (Porrino et al., 2002b). Consequently, prolonged presence of cocaine could account for both increased NET binding densities (neuroadaptive upregulation caused by increased NE concentrations) and reduced cerebral metabolism (caused by noradrenergic inhibition of glutamatergic activity) in the BNST.
The BNST and central nucleus of the amygdala are both components of the “extended amygdala” and share similar morphology, connectivity, and function (including involvement in stress-induced reinstatement of cocaine-seeking behavior) (Alheid and Heimer, 1988; Leri et al., 2002). Although rates of glucose utilization were reduced similarly in both regions after chronic cocaine self-administration (27–32 vs 26%, respectively), the levels of NET binding in the central nucleus of the amygdala were unaltered at this time point (our unpublished observations). Changes in the density of the NET consequent to chronic cocaine are therefore highly selective within the extended amygdala.
In conclusion, the results of this study suggest that chronic cocaine exposure leads to significant functional neuroadaptations in the BNST, accompanied by a profound upregulation in NET binding site density. Given the stress- and reward-related functions of the BNST in the CNS and the recent findings that the noradrenergic system can possess reinforcing properties, the presence of long-term neuroadaptations in this region may contribute to the manifestation of cocaine withdrawal-induced stress and reduced reward function.
Footnotes
This work was supported by United States Public Health Service Grants DA-09085 and DA-06634 from the National Institute on Drug Abuse. We thank Susan Nader, Clifford Hubbard, and Tonya Moore for assistance in the conduct of these experiments.
Correspondence should be addressed to Dr. Linda Porrino, Department of Physiology and Pharmacology, Wake Forest University School of Medicine, Medical Center Boulevard, Winston-Salem, NC 27157. E-mail:lporrino@wfubmc.edu.
References
- 1.Alheid GF, Heimer L. New perspectives in basal forebrain organization of special relevance for neuropsychiatric disorders: the striatopallidal, amygdaloid, and corticopetal components of substantia innominata. Neuroscience. 1988;27:1–39. doi: 10.1016/0306-4522(88)90217-5. [DOI] [PubMed] [Google Scholar]
- 2.Aston-Jones G, Delfs JM, Druhan J, Zhu Y. The bed nucleus of the stria terminalis: a target site for noradrenergic actions in opiate withdrawal. Ann NY Acad Sci. 1999;877:486–498. doi: 10.1111/j.1749-6632.1999.tb09284.x. [DOI] [PubMed] [Google Scholar]
- 3.Brownstein MJ, Palkovits M. Classical transmitters in the CNS, Pt 1. In: Bjorklund A, Hokfelt T, editors. Handbook of chemical neuroanatomy, Vol 2. Elsevier; Amsterdam: 1984. pp. 23–54. [Google Scholar]
- 4. Carboni E, Silvagni A, Rolando MT, Di Chiara G. Stimulation of in vivo dopamine transmission in the bed nucleus of stria terminalis by reinforcing drugs. J Neurosci 20 2000. RC102(1–5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casada JH, Dafny N. Responses of neurons in bed nucleus of the stria terminalis to microiontophoretically applied morphine, norepinephrine and acetylcholine. Neuropharmacology. 1993;32:279–284. doi: 10.1016/0028-3908(93)90112-g. [DOI] [PubMed] [Google Scholar]
- 6.Drouin C, Darracq L, Trovero F, Blanc G, Glowinski J, Cotecchia S, Tassin JP. α1b-Adrenergic receptors control locomotor and rewarding effects of psychostimulants and opiates. J Neurosci. 2002;22:2873–2884. doi: 10.1523/JNEUROSCI.22-07-02873.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Epping-Jordan MP, Markou A, Koob GF. The dopamine D-1 receptor antagonist SCH 23390 injected into the dorsolateral bed nucleus of the stria terminalis decreased cocaine reinforcement in the rat. Brain Res. 1998;784:105–115. doi: 10.1016/s0006-8993(97)01190-6. [DOI] [PubMed] [Google Scholar]
- 8.Erb S, Salmaso N, Rodaros D, Stewart J. A role for the CRF-containing pathway from central nucleus of the amygdala to bed nucleus of the stria terminalis in the stress-induced reinstatement of cocaine seeking in rats. Psychopharmacology (Berl) 2001;158:360–365. doi: 10.1007/s002130000642. [DOI] [PubMed] [Google Scholar]
- 9.Farfel GM, Kleven MS, Woolverton WL, Seiden LS, Perry BD. Effects of repeated injections of cocaine on catecholamine receptor binding sites, dopamine transporter binding sites and behavior in rhesus monkey. Brain Res. 1992;578:235–243. doi: 10.1016/0006-8993(92)90252-5. [DOI] [PubMed] [Google Scholar]
- 10.Florin SM, Kuczenski R, Segal DS. Regional extracellular norepinephrine responses to amphetamine and cocaine and effects of clonidine pretreatment. Brain Res. 1994;654:53–62. doi: 10.1016/0006-8993(94)91570-9. [DOI] [PubMed] [Google Scholar]
- 11.Forray MI, Bustos G, Gysling K. Noradrenaline inhibits glutamate release in the rat bed nucleus of the stria terminalis: in vivo microdialysis studies. J Neurosci Res. 1999;55:311–320. doi: 10.1002/(SICI)1097-4547(19990201)55:3<311::AID-JNR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 12.Fudge JL, Haber SN. Bed nucleus of the stria terminalis and extended amygdala inputs to dopamine subpopulations in primates. Neuroscience. 2001;104:807–827. doi: 10.1016/s0306-4522(01)00112-9. [DOI] [PubMed] [Google Scholar]
- 13. Georges F, Aston-Jones G. Potent regulation of midbrain dopamine neurons by the bed nucleus of the stria terminalis. J Neurosci 21 2001. RC160(1–6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Georges F, Aston-Jones G. Activation of ventral tegmental area cells by the bed nucleus of the stria terminalis: a novel excitatory amino acid input to midbrain dopamine neurons. J Neurosci. 2002;22:5173–5187. doi: 10.1523/JNEUROSCI.22-12-05173.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 16.Goeders NE, Smith JE. Cortical dopaminergic involvement in cocaine reinforcement. Science. 1983;221:773–775. doi: 10.1126/science.6879176. [DOI] [PubMed] [Google Scholar]
- 17.Kampman KM, Volpicelli JR, Mulvaney F, Alterman AI, Cornish J, Gariti P, Cnaan A, Poole S, Muller E, Acosta T, Luce D, O'Brien C. Effectiveness of propranolol for cocaine dependence treatment may depend on cocaine withdrawal symptom severity. Drug Alcohol Depend. 2001;63:69–78. doi: 10.1016/s0376-8716(00)00193-9. [DOI] [PubMed] [Google Scholar]
- 18.Koob GF. Corticotropin-releasing factor, norepinephrine, and stress. Biol Psychiatry. 1999;46:1167–1180. doi: 10.1016/s0006-3223(99)00164-x. [DOI] [PubMed] [Google Scholar]
- 19.Krettek JE, Price JL. Amygdaloid projections to subcortical structures within the basal forebrain and brainstem in the rat and cat. J Comp Neurol. 1978;178:225–254. doi: 10.1002/cne.901780204. [DOI] [PubMed] [Google Scholar]
- 20.Leri F, Flores J, Rodaros D, Stewart J. Blockade of stress-induced but not cocaine-induced reinstatement by infusion of noradrenergic antagonists into the bed nucleus of the stria terminalis or the central nucleus of the amygdala. J Neurosci. 2002;22:5713–5718. doi: 10.1523/JNEUROSCI.22-13-05713.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21:2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li MY, Yan QS, Coffey LL, Reith ME. Extracellular dopamine, norepinephrine, and serotonin in the nucleus accumbens of freely moving rats during intracerebral dialysis with cocaine and other monoamine uptake blockers. J Neurochem. 1996;66:559–568. doi: 10.1046/j.1471-4159.1996.66020559.x. [DOI] [PubMed] [Google Scholar]
- 23.Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry. 1999;156:238–245. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- 24.Madras BK, Kaufman MJ. Cocaine accumulates in dopamine-rich regions of primate brain after i.v. administration: comparison with mazindol distribution. Synapse. 1994;18:261–275. doi: 10.1002/syn.890180311. [DOI] [PubMed] [Google Scholar]
- 25.Markou A, Hauger RL, Koob GF. Desmethylimipramine attenuates cocaine withdrawal in rats. Psychopharmacology (Berl) 1992;109:305–314. doi: 10.1007/BF02245878. [DOI] [PubMed] [Google Scholar]
- 26.Martin LJ, Powers RE, Dellovade TL, Price DL. The bed nucleus-amygdala continuum in human and monkey. J Comp Neurol. 1991;309:445–485. doi: 10.1002/cne.903090404. [DOI] [PubMed] [Google Scholar]
- 27.McDougle CJ, Black JE, Malison RT, Zimmermann RC, Kosten TR, Heninger GR, Price LH. Noradrenergic dysregulation during discontinuation of cocaine use in addicts. Arch Gen Psychiatry. 1994;51:713–719. doi: 10.1001/archpsyc.1994.03950090045007. [DOI] [PubMed] [Google Scholar]
- 28.Nader MA, Daunais JB, Moore T, Nader SH, Moore RJ, Smith HR, Friedman DP, Porrino LJ. Effects of cocaine self-administration on striatal dopamine systems in rhesus monkeys: initial and chronic exposure. Neuropsychopharmacology. 2002;27:35–46. doi: 10.1016/S0893-133X(01)00427-4. [DOI] [PubMed] [Google Scholar]
- 29.Pacholczyk T, Blakely RD, Amara SG. Expression cloning of a cocaine- and antidepressant-sensitive human noradrenaline transporter. Nature. 1991;350:350–354. doi: 10.1038/350350a0. [DOI] [PubMed] [Google Scholar]
- 30.Porrino LJ, Lyons DJ, Letchworth SR, Freedland CS, Nader MA. Structural and functional neuroimaging of the effects of cocaine in human and nonhuman primates. In: Massaro EJ, editor. Handbook of neurotoxicology, Vol 2. Humana; Totowa, NJ: 2002a. pp. 413–435. [Google Scholar]
- 31.Porrino LJ, Lyons D, Miller MD, Smith HR, Friedman DP, Daunais JB, Nader MA. Metabolic mapping of the effects of cocaine during the initial phases of self-administration in the nonhuman primate. J Neurosci. 2002b;22:7687–7694. doi: 10.1523/JNEUROSCI.22-17-07687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 33.Ritz MC, Cone EJ, Kuhar MJ. Cocaine inhibition of ligand binding at dopamine, norepinephrine and serotonin transporters: a structure-activity study. Life Sci. 1990;46:635–645. doi: 10.1016/0024-3205(90)90132-b. [DOI] [PubMed] [Google Scholar]
- 34.Roberts DC, Koob GF, Klonoff P, Fibiger HC. Extinction and recovery of cocaine self-administration following 6-hydroxydopamine lesions of the nucleus accumbens. Pharmacol Biochem Behav. 1980;12:781–787. doi: 10.1016/0091-3057(80)90166-5. [DOI] [PubMed] [Google Scholar]
- 35.Roder S, Ciriello J. Collateral axonal projections to limbic structures from ventrolateral medullary A1 noradrenergic neurons. Brain Res. 1994;638:182–188. doi: 10.1016/0006-8993(94)90648-3. [DOI] [PubMed] [Google Scholar]
- 36.Shaham Y, Erb S, Stewart J. Stress-induced relapse to heroin and cocaine seeking in rats: a review. Brain Res Brain Res Rev. 2000;33:13–33. doi: 10.1016/s0165-0173(00)00024-2. [DOI] [PubMed] [Google Scholar]
- 37.Staley JK, Mash DC. Adaptive increase in D3 dopamine receptors in the brain reward circuits of human cocaine fatalities. J Neurosci. 1996;16:6100–6106. doi: 10.1523/JNEUROSCI.16-19-06100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. J Pharmacol Exp Ther. 1994;271:1678–1685. [PubMed] [Google Scholar]
- 39.Stout SC, Mortas P, Owens MJ, Nemeroff CB, Moreau J. Increased corticotropin-releasing factor concentrations in the bed nucleus of the stria terminalis of anhedonic rats. Eur J Pharmacol. 2000;401:39–46. doi: 10.1016/s0014-2999(00)00412-x. [DOI] [PubMed] [Google Scholar]
- 40.Tejani-Butt SM. [3H]nisoxetine: a radioligand for quantitation of norepinephrine uptake sites by autoradiography or by homogenate binding. J Pharmacol Exp Ther. 1992;260:427–436. [PubMed] [Google Scholar]
- 41.Van Bockstaele EJ, Bajic D, Proudfit H, Valentino RJ. Topographic architecture of stress-related pathways targeting the noradrenergic locus coeruleus. Physiol Behav. 2001;73:273–283. doi: 10.1016/s0031-9384(01)00448-6. [DOI] [PubMed] [Google Scholar]
- 42.Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- 43.Volkow ND, Fowler JS, Wang GJ. Imaging studies on the role of dopamine in cocaine reinforcement and addiction in humans. J Psychopharmacol. 1999;13:337–345. doi: 10.1177/026988119901300406. [DOI] [PubMed] [Google Scholar]
- 44.Xu F, Gainetdinov RR, Wetsel WC, Jones SR, Bohn LM, Miller GW, Wang YM, Caron MG. Mice lacking the norepinephrine transporter are supersensitive to psychostimulants. Nat Neurosci. 2000;3:465–471. doi: 10.1038/74839. [DOI] [PubMed] [Google Scholar]