Abstract
In rodents, cyclically fluctuating levels of gonadal steroid hormones modulate neural plasticity by altering synaptic transmission and synaptogenesis. Alterations of mood and cognition observed during the menstrual cycle suggest that steroid-related plasticity also occurs in humans. Cycle phase-dependent differences in cognitive performance have almost exclusively been found in tasks probing lateralized neuronal domains, i.e., cognitive domains such as language, which are predominantly executed by one hemisphere. To search for neural correlates of hormonally mediated neural plasticity in humans, we thus conducted a functional magnetic resonance imaging study measuring brain activity related to a semantic decision task in the language domain. This was contrasted with a letter-matching task in the perceptual domain, in which we expected no steroid hormone-mediated effect. We investigated 12 young healthy women in a counterbalanced repeated-measure design during low-steroid menstruation and high-steroid midluteal phase. Steroid serum levels correlated with the volume and lateralization of particular brain activations related to the semantic task but not with brain activity related to the perceptual task. More specifically, bilateral superior temporal recruitment correlated positively with progesterone and medial superior frontal recruitment with both progesterone and estradiol serum levels, whereas activations in inferior and middle frontal cortex were unaffected by steroid levels. In contrast to these specific interactions, testosterone levels correlated nonselectively with overall activation levels by neural and/or vascular factor(s). In conclusion, our data demonstrate steroid hormone responsivity in the adult human brain by revealing neural plasticity in the language domain, which appears hormone, task, and region specific.
Keywords: language, steroid, sex hormones, progesterone, estrogen, fMRI, menstrual cycle, neural plasticity, language dominance
Introduction
Plasma concentrations of gonadal steroid hormones such as estradiol and progesterone vary systematically during the menstrual cycle, with low plasma concentrations during menstruation, high preovulatory estradiol levels at the end of the follicular phase, and high levels of both progesterone and estradiol during the luteal phase in the second half of the cycle after ovulation. This changing hormonal milieu seems to be responsible for cyclic modulations of mood (Sherwin and Gelfand, 1985) and certain cognitive abilities (Hampson and Kimura, 1992). Behavioral studies assessing cyclic fluctuations in cortical plasticity suggested, for instance, that language operations are more lateralized to the left hemisphere during menstruation than the midluteal phase, indicating that progesterone alone or in combination with estradiol can alter cortical representations of language (Altemus et al., 1989; Hausmann and Güntürkün, 2000; Alexander et al., 2002; Hausmann et al., 2002). However, behavioral techniques such as dichotic listening or tachistoscopic visual half-field stimulation assess only the relative contribution of each hemisphere to a given task. They cannot answer the crucial question of whether the midluteal reduction in asymmetry is based on a specific corecruitment of the contralateral, subdominant hemisphere or on a more general increase in neural recruitment, including brain areas in the subdominant hemisphere. Moreover, behavioral approaches cannot determine which particular areas within a hemisphere may selectively be modulated by gonadal steroid hormones.
In contrast, functional imaging techniques such as functional magnetic resonance imaging (fMRI) should be ideally suited to track hormone-dependent neural plasticity. However, the results of initial fMRI studies did not concur with the behavioral observations (Altemus et al., 1989; Hausmann and Güntürkün, 2000; Alexander et al., 2002; Hausmann et al., 2002). Whereas one study (Veltman et al., 2000) found no interaction between cycle phase and language-related activations, another (Dietrich et al., 2001) found weaker or smaller, but similarly lateralized, activations during menstruation than the preovulatory estradiol surge. These fMRI examinations were performed during either the low-progesterone follicular phase or a presumed luteal phase, but without confirmation by progesterone measurement, thereby potentially missing the brief progesterone peak.
To explore hormone-dependent cortical plasticity in adulthood, we used a counterbalanced repeated-measure design testing whether naturally occurring differences in serum levels of gonadal steroid hormones between menstruation (days 2–4 of the cycle) and the midluteal phase (confirmed by hormone assessment) (see Table 1) affect fMRI activations associated with a semantic–perceptual contrast (Fernández et al., 2001) with high test–retest reliability (Fernández et al., 2003). To explore the interaction between steroid hormones and brain activity in greater detail, we related serum concentrations of estradiol, progesterone, and testosterone directly to fMRI data and to individual fMRI measures such as functional cerebral asymmetry and the level and volume of task-related activations.
Table 1.
Results of serum hormone assessment
| Hormone | Midluteal phase (Mean ± SD serum level) | Menstruation (Mean ± SD serum level) | t value (Paired-sample t test) | p value |
|---|---|---|---|---|
| Oestradiol (pg/ml) | 131.0 ± 35.6 | 46.6 ± 10.3 | 8.5 | p< 0.0001 |
| Progesterone (ng/ml) | 11.5 ± 6.2 | 0.4 ± 0.1 | 6.1 | p < 0.0001 |
| FAI | 3.4 ± 3.1 | 2.7 ± 2.7 | 2.2 | p < 0.05 |
| DHEAS (μg/dl) | 230.8 ± 181.2 | 208.1 ± 124.8 | 0.8 | NS |
| LH (IU/l) | 2.96 ± 1.4 | 4.70 ± 2.4 | −2.1 | NS |
| FSH (IU/l) | 1.98 ± 0.69 | 5.85 ± 1.91 | −8.4 | p < 0.0001 |
Materials and Methods
This study was approved by the Medical Ethics Committee of the University of Bonn, and all women gave their written informed consent. Each woman was tested once during menstruation (between days 2 and 4 after onset of menstruation) and once during the midluteal phase [7 d after the luteinizing hormone (LH) peak as estimated by ClearPlan Ovulation Predictor Tests (Unipath, Bedford, UK)]. The order of investigations was counterbalanced across the women. To reduce the effect of circadian fluctuations in hormone levels (e.g., corticosteroids), all investigations were performed between 8:00 and 11:00 P.M. Four women initially enrolled were excluded because of a failure of serum progesterone levels to rise above 4 ng/ml (two women), hyperandrogenism (one woman), or pregnancy (one woman). Twelve healthy women completed the study after meeting all inclusion criteria: no hormonal medication within the last 6 months, regular cycle duration (±2 d), right-handedness [Edinburgh Handedness Index (Oldfield, 1971) of ≥90], German as the first language, normal vision, no use of CNS active medication or illicit drugs, and no regular consumption of nicotine or alcohol. The mean age was 27.5 years (range, 22–34). Mean length of menstrual cycle was 28.7 d (range, 26–31).
To make our study sample as homogeneous as possible, left-handed women were excluded. Because the incidence of typical left-hemispheric language dominance in left-handed subjects is ∼80% (Szaflarski et al., 2002), we would have needed a very large study sample (∼75 women) to explore the interactions between steroid hormones, left-handedness, and atypical language dominance.
Hormone assessment. A venous blood sample was drawn before each MRI investigation for determination of LH, follicle-stimulating hormone (FSH), estradiol, progesterone, dehydroepiandrosterone, sex hormone binding globulin (SHBG), and testosterone. Parameters were measured by commercially available immunometric assays with an automated chemiluminescent immunoassay system (ImmuliteTM; Diagnostic Products, Los Angeles, CA). To assess the biological active testosterone fraction (Carter et al., 1983), the free androgen index (FAI) was calculated: FAI = 100 × testosterone (nmol/l)/SHBG (nmol/l).
Behavioral procedure. In the scanner, a series of item pairs, either word- or consonant string pairs, were presented back-projected onto a translucent screen, which women viewed by way of a mirror. Both constituents of each pair were simultaneously presented for 4 sec, above and below a central fixation cross. A semantic condition (synonym-judgment task) alternated with a perceptual condition (letter-matching task) every 25 sec, so that six item pairs were presented for each of 30 half-cycles. Hence, the behavioral experiment took 12.5 min in total.
We created two stimuli sets with identical characteristics. The verbal stimuli were 360 common German nouns (5–11 letters), forming 90 pairs of words with identical or highly similar meanings (synonyms) and 90 pairs of semantically unrelated words. The consonant strings were developed pseudorandomly to represent 90 pairs of two identical strings and 90 pairs in which one letter was different between the two constituents. Strings were matched with words with regard to the number of letters. Across women, the order of stimuli sets and stimuli pairs within each condition was counterbalanced. Women were required to push a button with the index finger of their right hand whenever they identified a pair of two synonyms or identical letter strings.
MRI data acquisition. For each investigation, 16 axial slices were collected at 1.5 T (Symphony; Siemens, Erlangen, Germany): 248 T2*-weighted, gradient echo planar imaging (EPI) scans, including eight initial scans that were discarded to achieve steady-state magnetization (slice thickness, 6 mm; interslice gap, 0.6 mm; matrix size, 64 × 64; field of view, 220 mm; echo time, 50 msec; repetition time, 3.125 sec). Thereafter, we acquired a sagittal T1-weighted three-dimensional FLASH sequence for each woman during the first investigation for anatomical localization (number of slices, 120; slice thickness, 1.5 mm (no interslice gap); matrix size, 256 × 256; field of view, 230 mm; echo time, 4 msec; repetition time, 11 msec).
MRI data analysis. MR images were processed using SPM99 (www.fil. ion.ucl.ac.uk/spm) and the following steps: (1) registration of motion correction parameters aligning each image of the time series to the first image for head movement correction; (2) calculation of parameters for the normalization onto the Montreal Neurological Institute (MNI) atlas based on the first EPI scan using the EPI template (default values for nonlinear corrections); (3) normalization, i.e., transformation of all images into the standard space defined by the MNI atlas using a sinc-interpolation algorithm to allow intersubject averaging; (4) smoothing of the normalized images with a 7 mm full-width at half-maximum isotropic Gaussian kernel to account for intersubject variance of functional anatomy; (5) modeling the expected hemodynamic responses to each task as an increase of signal intensity during task-related stimulation (boxcar regressor in a general linear model); this regressor was convolved with a canonical hemodynamic response function (hrf) to represent brain physiology; (6) temporal filtering of the acquired time series to reduce high- and low-frequency noise attributable to scanner drifts and physiological noise (i.e., with the hrf as a low-pass filter and 106 sec for the cutoff period of a high-pass filter); (7) calculation of parameter estimates for each trial type covariate from the least mean squares fit of the model to the data; parameter estimates stored as separate images for each subject; (8) definition of the preexperimentally planed effects of interest (synonym-judgment > letter-matching and letter-matching > synonym-judgment) by the relevant contrast of these parameter estimates and generation of contrast images for each woman and each effect of interest; (9) entering contrast images into repeated-measures one-sample or two-sample t test respectively, to test whether, across subjects, the mean of the parameter estimates of the contrast differed from zero (random effects model); and (10) entering contrast images into regression analyses, correlating serum hormone concentrations with levels of activations (parameter estimates).
Three fMRI measures [weighted lateralization index (LI), level of activation, and volume of activation] were derived from individualt maps after masking the supratentorial brain and excluding three sagittal midline planes to minimize errors attributable to normalization. To estimate these measures, we had to objectively adjust individual statistical thresholds as described previously (Fernández et al., 2001), because of intersubject variability in general activation levels. This was achieved by first calculating a mean maximum t value defined as the mean of those 5% of voxels showing the highest level of activation. Those voxels with at value exceeding 50% of this mean maximum tvalue were included into the calculation of LI, level of activation, and volume of activation. The level of activation was defined as the mean t value of all voxels exceeding the individually defined threshold and the volume of activation as the number of these voxels. The LI was calculated by the following formula:
where V is the set of activated voxels,XL is the t value of left hemispheric voxels, and XR is the t value of right hemispheric voxels.
Results
Hormone assessment
As expected, estradiol and progesterone levels were much higher, and testosterone levels were slightly higher during midluteal phase than menses. In contrast, FSH levels were lower during midluteal phase than menses (Table 1).
Behavioral performance
The rate of correctly identified synonyms was 96.4 ± 3.7% (mean ± SD), with 0.2 ± 0.5% false positive responses. The mean rate of correctly recognized identical consonant strings was 94.4 ± 6.6%, with 10.3 ± 5.2% false positive responses. Mean reaction times for correct responses to synonyms was 1705 ± 349 msec and to consonant strings was 2529 ± 428 msec. Both cycle phases were associated with similar performance and reaction times, and no pairwise comparison revealed a reliable difference (eachp > 0.05). Hence, differences in imaging results between midluteal phase and menstruation cannot be attributed to differences in performance.
MRI data
The synonym-judgment compared with the letter-matching task produced blood-oxygen level-dependent activations in multiple, predominantly left hemispheric regions (mean LI, 0.66 ± 0.25). These regions comprised a temporoparietal area, the dorsal part of the inferior and middle frontal gyrus, and the ventral as well as medial aspect of the superior frontal gyrus. The opposite contrast (letter-matching > synonym-judgment) revealed bilateral posterior activations, extending from occipital to inferior temporal and parietal regions following the dorsal and ventral visual processing stream. In addition, primary and secondary motor areas were activated within the frontal lobe (Fig.1a,b).
Fig. 1.

Brain regions significantly activated in all women (random effects model) in response to the synonym-judgment task contrasted with the letter-matching task (left columns) and to the letter-matching task contrasted with the synonym-judgment task (right columns) were overlaid onto an individual, randomly selected brain.t maps are thresholded at p = 0.01, and only clusters with an extent of >15 voxels are shown. Results are depicted separately for menstruation (a) and the midluteal phase (b) and their second level comparison (c). The three bottom rows depict activity in cerebral areas significantly correlated with hormone serum levels: progesterone (d), estradiol (e), and FAI (f).
Compared with menstruation, measurements during the midluteal phase revealed larger temporal and medial superior frontal activations for the synonym-judgment > letter-matching contrast. More voxels in both hemispheres (Figs. 1, 2) reached our statistical threshold during the midluteal phase than during menstruation (mean menses midluteal difference of the number of significantly activated voxels: left hemisphere, 101.8 ± 122.8; right hemisphere, 120.6 ± 144.2;t(11) = −0.48; NS), indicating a bilateral increase of neural recruitment. This increase was, however, correlated with reduced asymmetry (r = 0.798;p < 0.002; mean LI menses, 0.80 ± 0.20; mean LI midluteal phase, 0.57 ± 0.28;t(11) = −2.89; p < 0.02). Stronger left lateralization during menstruation than midluteal phase was found in 10 women. The two remaining women had strongly lateralized activations and only small differences between both cycle phases (LI, 0.95–1.00 and 0.94–1.00).
Fig. 2.
Mean ± SEM number of significantly activated voxels in response to the synonym-judgment task in contrast to the letter-matching task shown separately for the left hemisphere, right hemisphere, menstruation, and the midluteal phase.
The correlation of serum hormone levels with fMRI group data revealed that, for the semantic decision task, brain activity in the superior temporal gyrus correlated bilaterally with progesterone serum levels, and activity in the medial superior frontal gyrus correlated with both progesterone and estradiol serum levels (Fig.1d,e). However, activity in inferior and middle frontal cortex (Fig. 1a,b) did not correlate with any of these hormone levels (Fig. 1d,e), although these areas exhibited the strongest activation related to the semantic decision task (Fig. 1a,b). Moreover, brain activity related to the letter-matching task correlated with neither progesterone nor estradiol levels. Unlike progesterone and estradiol, FAI correlated with a nonspecific increase of disseminated activations in all brain areas activated by both tasks (Fig.1f).
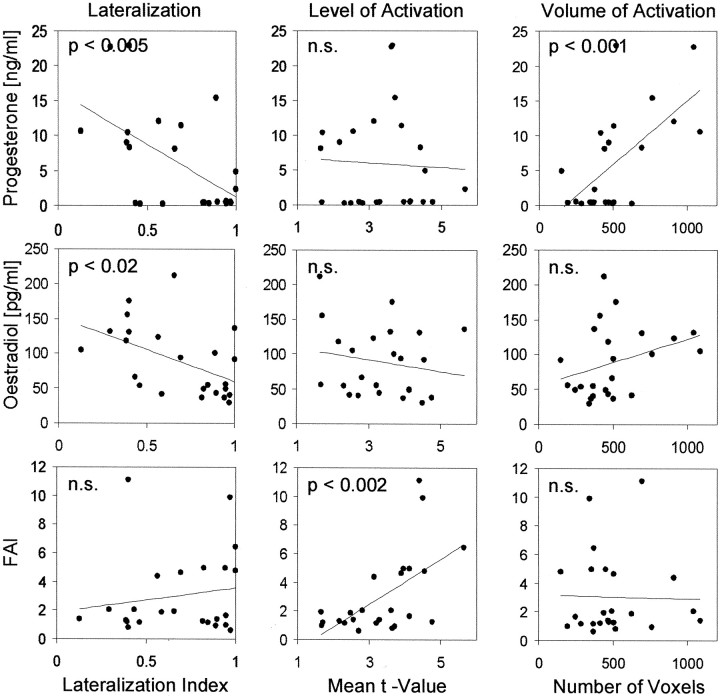
This dissociation between the effect of different hormones and task-related activations was examined further by correlations between individual hormone serum levels and measures of functional asymmetry, activation level, and activation volume based on individualt maps. No reliable correlations were revealed for the letter-matching > synonym-judgment contrast (maxr < 0.4; NS), confirming that brain activity related to the letter-matching task was not systematically influenced by the hormonal state. In contrast, language-related activations revealed specific correlations (Fig. 3) with progesterone (r = −0.57; p < 0.005) and, although to a lesser degree, estradiol levels (r = −0.49; p < 0.02), with lower serum levels associated with stronger left lateralization. FAI did not correlate with lateralization indices. The volume of brain activations (which is not independent of the level of activation because a statistical threshold is used) also showed a reliable correlation with progesterone levels (r = 0.63; p < 0.001), with higher serum levels of progesterone associated with larger areas of activation. Conversely, FAI did not correlate with the volume but with the level of activation (r = 0.59; p < 0.002), whereas estradiol and progesterone levels did not correlate with the level of activation (max r < 0.25; NS). This dissociation between specific hormone effects on activation level and activation volume is an additional indication that testosterone nonspecifically increases the contrast-to-noise ratio by neural and/or vascular factors, whereas progesterone and estradiol have specific effects on the volume of cortical recruitment.
Fig. 3.
Scatterplots with least-squares linear regression lines contrasting individual fMRI measures derived fromt maps based on the synonym-judgment > letter-matching contrast for lateralization (LI), the level of activation (mean t values), and the volume of activation (number of significantly activated voxels) with individual serum hormone levels of progesterone, estradiol, and FAI.
Finally, three stepwise regressions with the independent variables FAI, estradiol, and progesterone serum levels confirmed this pattern of interactions: the variance in the volume of activation was explained best (39%) if only progesterone was included in the model (F(1,22) = 14.07; p < 0.001). A similar result was obtained for lateralization. Again only progesterone was included, and the model accounted for 32% of the variance (F(1,22) = 10.38, p < 0.005). The model for the level of activation included only FAI and accounted for 18% of the variance (F(1,22) = 4.87; p < 0.05).
Discussion
Our results reveal that transient elevations of progesterone and estradiol serum levels, as they naturally occur during the menstrual cycle, are accompanied by spatially more extended recruitment of specific, symmetric brain areas involved in a semantic decision task. The additional recruitment is located in the superior temporal gyrus and the medial wall of the superior frontal gyrus. Because no general effect (like that of testosterone with nonspecific differences in contrast-to-noise ratio) caused the modulation of recruitment, the relationship between neural recruitment and gonadal steroids appears specific.
The two contrasts used (synonym-judgment > letter-matching and letter-matching > synonym-judgment) are not orthogonally opposed to each other. However, in brain regions that exclusively or predominantly process language (i.e., Broca's area or the superior temporal gyrus) or that exclusively or predominantly support higher-order visual processing (i.e., dorsal visual stream), the alternative condition can be regarded as a control condition inducing no or almost no hemodynamic response. Because our central findings are almost exclusively limited to brain regions that either predominately process words or letter strings, our conclusions can be attributed specifically to either language or visuo-perceptual processing. The design chosen excludes the possible confound introduced by simple control conditions such as a “resting” state, in which conceptual processing is known to be accompanied by activations of language-related brain areas (Binder et al., 1999).
It is well recognized that gonadal steroids have many effects on the brain beyond their role in reproduction (Rupprecht and Holsboer, 1999;McEwen, 2001; Smith et al., 2002). They act directly after passing the blood–brain barrier (Bixo et al., 1997) and after local metabolization or synthesis of so called neurosteroids (Le Goascogne et al., 1987). Progesterone, for instance, is metabolized to tetrahydroprogesterone, inducing rapid effects on neuronal excitability by allosteric interaction with a putative steroid recognition site on the GABAA receptor–ion channel complex, increasing the frequency and duration of chloride channel openings (Majewska et al., 1986). Moreover, there is ample evidence for cycle-dependent synaptogenesis in rodents (Woolley et al., 1990; Yankova et al., 2001). Estradiol causes an increase in the excitatory drive on pyramidal neurons leading to new dendritic spines with higher density of glutamatergic receptors and increased synaptic activity among neurons (Segal and Murphy, 2001). Such modulatory effects on neurotransmission, synaptogenesis, or both may cause the cycle-dependent plasticity observed here in the adult human brain. However, gonadal steroid effects are so complex that the question of how plasticity is mediated must remain open to speculation.
The cycle-dependent changes in language lateralization found here as a corollary of a symmetric increase of neural recruitment during the midluteal phase are in full accord with behavioral studies estimating the relative contribution of each hemisphere to a given language task in different cycle phases (Altemus et al., 1989; Hausmann and Güntürkün, 2000; Hausmann et al., 2002). Moreover, in line with those studies, which have analyzed the relationship between functional hemispheric asymmetries and hormone levels more closely (Hausmann and Güntürkün, 2000; Hausmann et al., 2002), we show that progesterone exerts a larger impact on functional hemispheric lateralization than estradiol. It has been hypothesized that high levels of progesterone lead to an interhemispheric decoupling based on a decrease of commissural activity (Hausmann and Güntürkün, 2000). Although the superior temporal region is connected with the opposite cerebral hemisphere by way of the anterior commissure and the corpus callosum (Cipolloni and Pandya, 1985), neither this temporal region nor the medial aspect of the superior frontal gyrus have a disproportional large number of commissural fibers (Pandya et al., 1971). Hence, the region-specific results obtained here (no steroid hormone effect on inferior and middle frontal activations but strong hormone effects on superior temporal and frontal activations) cannot simply be explained by a steroid effect on commissural transmission. Additional local- and regional-specific effects are necessary to explain our results of a symmetrical midluteal extension of regions engaged in the semantic decision task causing reduced functional lateralization and correlating with high progesterone and estradiol levels during this phase of the cycle.
Our finding that language lateralization is modulated by gonadal hormone levels could explain why imaging studies investigating interactions of gender and language lateralization have produced variable results. Whereas some studies found stronger left lateralization in men than women (Shaywitz et al., 1995; Kansaku et al., 2000), others did not (Buckner et al., 1995; Frost et al., 1999). Because we found a mean intrasubject difference of ∼30% in left lateralization between menstruation and midluteal phase, contrasting outcomes could be attributable to systematic differences between studypopulations in terms of their hormonal states (e.g., cycle phase or use of hormonal contraception). Moreover, differences in task demands could cause different degrees of language lateralization in men and women. Kansaku and Kitazawa (2001), for instance, suggested on the basis of a meta analysis that studies using a sublexical task or passive story listening found clear gender differences in language lateralization, whereas studies using tasks involving semantic processing of individual words failed to do so. Following this line of argument, the semantic task used here may have revealed cycle-dependent variations, which tasks like story listening or rhyming might not have detected. In any case, the hormonal state of female subjects should be considered as an important biological factor influencing activation pattern and limiting within-subject reproducibility in future imaging studies.
In conclusion, our data show that neural recruitment in a cognitive task is highly responsive to the kind of fluctuations of gonadal steroid hormones (McEwen, 1999; Breedlove and Jordan, 2001), which naturally occur during the menstrual cycle. Regardless of the underlying mechanism(s), our findings demonstrate that cortical representations of language are variable in the fully developed brain and that progesterone and estradiol may modulate neuronal plasticity in a task- and region-specific manner. For the time being, the evidence for a link between these gonadal steroids and cortical representation is limited to the two tasks implemented here. Additional investigations are necessary to assess whether gonadal steroid levels are related to neuronal plasticity in other cognitive tasks and domains.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft. We thank P. Hagoort, P. Indefrey, and M. van Turennout for helpful comments on previous drafts and H. Elfadil and S. Schür for technical support.
Correspondence should be addressed to Dr. Guillén Fernández, F. C. Donders Centre, P.O. Box 9101, 6500 HB Nijmegen, The Netherlands. E-mail:guillen.fernandez@fcdonders.kun.nl.
References
- 1.Alexander GM, Altemus M, Peterson BS, Wexler BE. Replication of a premenstrual decrease in right-ear advantage on language-related dichotic listening tests of cerebral laterality. Neuropsychologia. 2002;40:1293–1299. doi: 10.1016/s0028-3932(01)00220-2. [DOI] [PubMed] [Google Scholar]
- 2.Altemus M, Wexler BE, Boulis N. Changes in perceptual asymmetry with the menstrual cycle. Neuropsychologia. 1989;27:233–240. doi: 10.1016/0028-3932(89)90174-7. [DOI] [PubMed] [Google Scholar]
- 3.Binder JR, Frost JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Conceptual processing during the conscious resting state. A functional MRI study. J Cognit Neurosci. 1999;11:80–95. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- 4.Bixo M, Andersson A, Winblad B, Purdy RH, Backstrom T. Progesterone 5alpha-pregnane-320-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997;764:173–178. doi: 10.1016/s0006-8993(97)00455-1. [DOI] [PubMed] [Google Scholar]
- 5.Breedlove SM, Jordan CL. The increasingly plastic, hormone-responsive adult brain. Proc Natl Acad Sci USA. 2001;98:2956–2957. doi: 10.1073/pnas.071054098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buckner RL, Raichle ME, Petersen SE. Dissociation of human prefrontal cortical areas across different speech production tasks and gender groups. J Neurophysiol. 1995;74:2163–2173. doi: 10.1152/jn.1995.74.5.2163. [DOI] [PubMed] [Google Scholar]
- 7.Carter GD, Holland SM, Alaghband-Zadeh J, Rayman G, Dorrington-Ward P, Wise PH. Investigation of hirsutism: testosterone is not enough. Ann Clin Biochem. 1983;20:262–263. doi: 10.1177/000456328302000502. [DOI] [PubMed] [Google Scholar]
- 8.Cipolloni PB, Pandya DN. Topography and trajectories of commissural fibers of the superior temporal region in the rhesus monkey. Exp Brain Res. 1985;57:381–389. doi: 10.1007/BF00236544. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich T, Krings T, Neulen J, Willmes K, Erberich S, Thron A, Sturm W. Effects of blood estrogen level on cortical activation patterns during cognitive activation as measured by functional MRI. NeuroImage. 2001;13:425–432. doi: 10.1006/nimg.2001.0703. [DOI] [PubMed] [Google Scholar]
- 10.Fernández G, de Greiff A, von Oertzen J, Reuber M, Lun S, Klaver P, Ruhlmann J, Reul J, Elger CE. Language mapping in less than 15 minutes: real-time functional MRI during routine clinical investigation. NeuroImage. 2001;14:585–594. doi: 10.1006/nimg.2001.0854. [DOI] [PubMed] [Google Scholar]
- 11.Fernández G, Specht K, Weis S, Tendolkar I, Reuber M, Fell J, Klaver P, Ruhlmann J, Reul J, Elger CE. Intrasubject reproducibility of presurgical language lateralization and mapping using fMRI. Neurology. 2003;60:969–975. doi: 10.1212/01.wnl.0000049934.34209.2e. [DOI] [PubMed] [Google Scholar]
- 12.Frost JA, Binder JR, Springer JA, Hammeke TA, Bellgowan PS, Rao SM, Cox RW. Language processing is strongly left lateralized in both sexes. Evidence from functional MRI. Brain. 1999;122:199–208. doi: 10.1093/brain/122.2.199. [DOI] [PubMed] [Google Scholar]
- 13.Hampson E, Kimura D. Sex differences and hormonal influences on cognitive function in humans. In: Becker JB, Breedlove SM, Crews D, editors. Behavioral endocrinology. MIT; Cambridge, MA: 1992. pp. 357–398. [Google Scholar]
- 14.Hausmann M, Güntürkün O. Steroid fluctuations modify functional cerebral asymmetries: the hypothesis of progesterone-mediated interhemispheric decoupling. Neuropsychologia. 2000;38:1362–1374. doi: 10.1016/s0028-3932(00)00045-2. [DOI] [PubMed] [Google Scholar]
- 15.Hausmann M, Becker C, Gather U, Güntürkün O. Functional cerebral asymmetries during the menstrual cycle: a cross-sectional and longitudinal analysis. Neuropsychologia. 2002;40:808–816. doi: 10.1016/s0028-3932(01)00179-8. [DOI] [PubMed] [Google Scholar]
- 16.Kansaku K, Kitazawa S. Imaging studies on sex differences in the lateralization of language. Neurosci Res. 2001;41:333–337. doi: 10.1016/s0168-0102(01)00292-9. [DOI] [PubMed] [Google Scholar]
- 17.Kansaku K, Yamaura A, Kitazawa S. Sex differences in lateralization revealed in the posterior language areas. Cereb Cortex. 2000;10:866–872. doi: 10.1093/cercor/10.9.866. [DOI] [PubMed] [Google Scholar]
- 18.Le Goascogne C, Robel P, Gouezou M, Sananes N, Baulieu EE. Neurosteroids: cytochrome P-450scc in rat brain. Science. 1987;237:1212–1215. doi: 10.1126/science.3306919. [DOI] [PubMed] [Google Scholar]
- 19.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 20.McEwen BS. Permanence of brain sex differences and structural plasticity of the adult brain. Proc Natl Acad Sci USA. 1999;96:7128–7130. doi: 10.1073/pnas.96.13.7128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McEwen BS. Estrogens effects on the brain: multiple sites and molecular mechanisms. J Appl Physiol. 2001;91:2785–2801. doi: 10.1152/jappl.2001.91.6.2785. [DOI] [PubMed] [Google Scholar]
- 22.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 23.Pandya DN, Karol EA, Heilbronn D. The topographical distribution of interhemispheric projections in the corpus callosum of the rhesus monkey. Brain Res. 1971;32:31–43. doi: 10.1016/0006-8993(71)90153-3. [DOI] [PubMed] [Google Scholar]
- 24.Rupprecht R, Holsboer F. Neuroactive steroids: mechanisms of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 25.Segal M, Murphy D. Oestradiol induces formation of dendritic spines in hippocampal neurons: functional correlates. Horm Behav. 2001;40:156–159. doi: 10.1006/hbeh.2001.1688. [DOI] [PubMed] [Google Scholar]
- 26.Shaywitz BA, Shaywitz SE, Pugh KR, Constable RT, Skudlarski P, Fulbright RK, Bronen RA, Fletcher JM, Shankweiler DP, Katz L. Sex differences in the functional organization of the brain for language. Nature. 1995;373:607–609. doi: 10.1038/373607a0. [DOI] [PubMed] [Google Scholar]
- 27.Sherwin BB, Gelfand MM. Sex steroids and affect in the surgical menopause: a double-blind cross-over study. Psychoneuroendocrinology. 1985;10:325–335. doi: 10.1016/0306-4530(85)90009-5. [DOI] [PubMed] [Google Scholar]
- 28.Smith MJ, Adams LF, Schmidt PJ, Rubinow DR, Wassermann EM. Effects of ovarian hormones on human cortical excitability. Ann Neurol. 2002;51:599–603. doi: 10.1002/ana.10180. [DOI] [PubMed] [Google Scholar]
- 29.Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 2002;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- 30.Veltman DJ, Friston KJ, Sanders G, Price CJ. Regionally specific sensitivity differences in fMRI and PET: where do they come from? NeuroImage. 2000;11:575–588. doi: 10.1006/nimg.2000.0581. [DOI] [PubMed] [Google Scholar]
- 31.Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10:4035–4039. doi: 10.1523/JNEUROSCI.10-12-04035.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yankova M, Hart SA, Woolley CS. Estrogen increases synaptic connectivity between single presynaptic inputs and multiple postsynaptic CA1 pyramidal cells: a serial electron-microscopic study. Proc Natl Acad Sci USA. 2001;98:3525–3530. doi: 10.1073/pnas.051624598. [DOI] [PMC free article] [PubMed] [Google Scholar]