Abstract
Networks of interconnected inhibitory neurons, such as the thalamic reticular nucleus (TRN), often regulate neural oscillations. Thalamic circuits generate sleep spindles and may contribute to some forms of generalized absence epilepsy, yet the exact role of inhibitory connections within the TRN remains controversial. Here, by using mutant mice in which the thalamic effects of the anti-absence drug clonazepam (CZP) are restricted to either relay or reticular nuclei, we show that the enhancement of intra-TRN inhibition is both necessary and sufficient for CZP to suppress evoked oscillations in thalamic slices. Extracellular and intracellular recordings show that CZP specifically suppresses spikes that occur during bursts of synchronous firing, and this suppression grows over the course of an oscillation, ultimately shortening that oscillation. These results not only identify a particular anatomical and molecular target for anti-absence drug design, but also elucidate a specific dynamic mechanism by which inhibitory networks control neural oscillations.
Keywords: thalamus, generalized absence epilepsy, spike wave discharge, benzodiazepines, interneuronal network, inhibition
Introduction
Interconnected networks of inhibitory neurons regulate oscillations throughout the CNS. One such network, the thalamic reticular nucleus (TRN), participates in many thalamocortical oscillations, including 7–14 Hz sleep spindles and spike-wave seizures characteristic of generalized absence epilepsy. Spindles result from a well studied cycle of events, in which TRN neurons inhibit thalamocortical (TC) relay neurons, eliciting rebound bursts mediated by T-type calcium currents, and resulting in re-excitation of TRN (von Krosigk et al., 1993). Similar mechanisms may contribute to absence epilepsy. Knocking out the T-type calcium channel gene α1g eliminates rebound bursts in TC neurons, and the resulting mice are resistant to drug-induced spike-wave discharges (Kim et al., 2001). Because TRN is the source of the hyperpolarizing input that deinactivates T-current in TC neurons, this suggests that TRN may be necessary for absence seizures. Indeed, TRN lesions abolish spontaneous seizures in a genetic model (Avanzini et al., 1993) and suppress drug-induced absence seizures (Banerjee and Snead, 1994). Note however that cortex alone supports some forms of spike-wave discharge (Steriade and Contreras, 1998).
Thus, intra-TRN inhibition may regulate different thalamic oscillations, and one hypothesis is that it suppresses epileptiform activity. GABAA receptor antagonists essentially eliminate intra-TRN inhibition and transform spindles into slower, more synchronized epileptiform discharges in vitro (von Krosigk et al., 1993; Huguenard and Prince, 1994). A similar transformation follows knockout of the β3 subunit of the GABAA receptor, which selectively disrupts intra-TRN inhibition (Huntsman et al., 1999). Alternatively, intra-TRN inhibition may spread (Bazhenov et al., 1999) or sustain thalamic oscillations (Steriade et al., 1987).
The anti-absence drug clonazepam (CZP) suppresses rhythmic activity in thalamic and thalamocortical slices (Huguenard and Prince, 1994; Zhang et al., 1996), but the locus for this suppression is unclear, because CZP modulates IPSCs in TRN and TC neurons (Browne et al., 2001), and effects in thalamocortical slices may reflect cortical actions (Oh et al., 1995). Here we use genetic manipulations to locate the site of action of CZP and elucidate the function of intra-TRN inhibition. Mutating one residue renders α subunits of the GABAA receptor insensitive to classical benzodiazepines, including CZP, without affecting their sensitivity to GABA (Wieland et al., 1992; Benson et al., 1998). Within the thalamus, α1 and α3 are selectively expressed in the relay and reticular nuclei, respectively (Wisden et al., 1992; Pirker et al., 2000). As a result, in slices from mice with the α1(H101R) mutation, 100 nm CZP selectively modulates IPSCs in TRN neurons without affecting TC neurons (Huntsman et al., 2000), whereas in α3(H126R) mice, CZP selectively modulates IPSCs in TC neurons and has no effect in the TRN (Porcello et al., 2001).
By comparing effects of CZP on oscillations in wild-type (WT), α1(H101R), and α3(H126R) mice (Rudolph et al., 1999; Low et al., 2000), we demonstrate that CZP suppresses thalamic oscillations by enhancing intra-TRN inhibition. CZP specifically suppresses synchronous spikes during rhythmic bursts of population activity and ultimately shortens the duration of oscillations. This identifies a specific anatomical and molecular target for anti-absence drug design and suggests a mechanism for oscillatory control by inhibitory networks.
Materials and Methods
Slice preparation. All procedures involving animals were performed in accordance with protocols approved by the Stanford Institutional Animal Care and Use Committee, and investigators adhered to the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals. Slices were made as described inHuguenard and Prince (1994). Slices were made from 11–13 d old Sprague Dawley (Simonsen) rat pups and 12–21 d old mouse pups. Animals were deeply anesthetized with pentobarbital (50 mg/kg) and decapitated. Their brains were then rapidly removed and placed in chilled (4°C) slicing solution consisting of (in mm): 234 sucrose, 11 glucose, 24 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 10 MgSO4, and 0.5 CaCl, equilibrated with a 95% O2, 5% CO2 mixture. Horizontal slices (400 μm) were obtained using a Vibratome (TPI, St. Louis, MO). The slices were incubated in 32°C oxygenated saline for at least 1 hr before recording.
Recording procedures. All recordings were made in an interface chamber at 34 ± 1°C. The superfusion solution consisted of (in mm): 126 NaCl, 26 NaHCO3, 2.5 KCl, 1.25 NaH2PO4, 1 or 1.2 MgCl2, 2 CaCl, and 10 glucose. Electrical stimuli were delivered to the internal capsule through a pair of 50–100 KΩ tungsten electrodes (FHC, Bowdoinham, ME), with a separation of ∼100 μm. Clonazepam (obtained from Sigma, St. Louis, MO) was dissolved in DMSO before being added to the final solution, such that the final concentration of DMSO in the superperfusion solution was ≤0.03%. The concentration of clonazepam used was always 100 nm, unless noted otherwise. Extracellular multiunit recordings, which also used 50–100 KΩ tungsten electrodes, were normally bandpass filtered between 100 Hz and 3 kHz.
Intracellular recordings were made with sharp microelectrodes, which were pulled from borosilicate glass (1B100F-4, outer diameter 1.0 mm, inner diameter 0.58 mm; WPI Inc., Sarasota, FL) using a Flaming Brown Micropipette Puller (P-80, Sutter Instruments, Novato, CA). Sharp electrodes had resistances between 80 and 120 MΩ when filled with 4 m KAc and 100 mm KCl. Recordings were amplified using the AxoClamp 2A (Axon Instruments, Union City, CA). We report results from TRN neurons that had stable membrane potentials more negative than −60 mV.
Note that in this and all subsequent sections, we use the terms “oscillation” and “sweep” to refer to the response, recorded by one electrode, to a single stimulus, e.g., a set of spikes occurring over 1–5 sec. In contrast, a “recording” is the entire set of oscillations recorded from one electrode in different conditions (control, drug application, and drug washout) over tens of minutes.
Data analysis. To find spikes in extracellular multiunit recordings, we wrote software that detected sufficiently steep negative deflections followed by sufficiently steep positive deflections within a sufficiently short time window. The threshold for spike detection was scaled by the root-mean-square of the background noise in each recording, and the other parameters were set in accordance with the known properties of action potentials in TRN and TC neurons. Finally, in several cases, output from this spike detection algorithm was cross-checked against manual inspection of extracellular recordings to verify that the sensitivity and specificity of the algorithm were both within reasonable bounds.
To ensure that our observations reflect experimental manipulations rather than nonspecific shifts in excitability, we excluded recordings in which changes observed during drug application, e.g., shifts of >10% in the total number of spikes per evoked oscillation, did not reverse during the subsequent wash.
The period of an evoked oscillation, recorded from one electrode, was computed from the autocorrelogram of the multiunit spike train as follows. First, we counted spikes in 10-msec-wide bins to obtain the spike rate as a function of time (referred to as the “ratemeter”). The first bin always began 50–100 msec after the stimulus to exclude stimulus artifacts and firing induced directly by the stimulus (rather than indirectly via intrathalamic circuitry). Second, we computed the autocorrelogram, A(τ), of the ratemeter,r(t): A(τ) = Σ0≤t≤Nr(t+τ) r(t), where N is the total number of bins. We excluded autocorrelograms in which the second tallest peak (referred to as the “satellite peak” to distinguish it from the tallest peak, which is located at τ = 0) was not clearly distinguishable or was located at a value of τ > 250 msec. Then, we computed the period, T, as: T = Στ[A(τ) −A0]/ΣA(τ) −A0, where the sums are taken over values of τ between the troughs on either side of the satellite peak, and A0 is the mean of the levels of these two troughs. In this formula, the period is determined from a weighted average taken over the entire area between the troughs on either side of the satellite peak, rather than from just a single point at the satellite peak.
We defined the duration of an oscillation as the time at which the last rhythmic burst occurred. A burst was defined as at least five spikes in two adjoining, 10-msec-wide bins, and to be classified as “rhythmic,” a burst had to occur <400 msec after the preceding burst. A sample of durations determined by this algorithm were compared with those obtained via manual inspection and found to be similar.
We also compared the sizes and shapes of bursts during different conditions: control, CZP application, and CZP washout. To do this, we first detected bursts during control oscillations. A burst was defined as a local maximum in the ratemeter that was at least 80 msec after the preceding burst (before detecting maxima, we smoothed the ratemeter so that each point was an average of three consecutive 10-msec-wide bins). If we simply wanted to compare the nth burst in control conditions with the nth burst during CZP application, we could have detected bursts in this manner for each sweep in each different condition. However, on a given sweep, small bursts may or may not occur between larger bursts, making the exact timing of thenth burst highly variable. Such variability means that the fourth burst on one particular control sweep might not really be comparable with the fourth burst on a particular CZP sweep. Therefore, to compare bursts in control conditions with bursts that occurred at approximately the same time during oscillations in CZP, we used the following approach. After detecting the nth burst in several consecutive control sweeps, we computedtn, the average time of occurrence for thenth burst. Then, for each sweep in each condition, we found the local maximum in a 120-msec-wide window centered ontn and then output the ratemeter centered around the time of this local maximum. We then averaged together the recentered ratemeters for all of the control sweeps to obtain a profile of a “control burst” and did the same thing for CZP sweeps to obtain the “CZP burst,” etc.
Results
CZP suppresses evoked thalamic oscillations
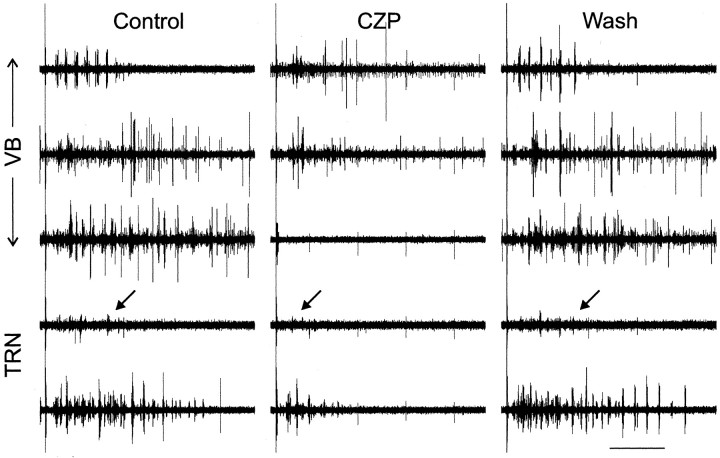
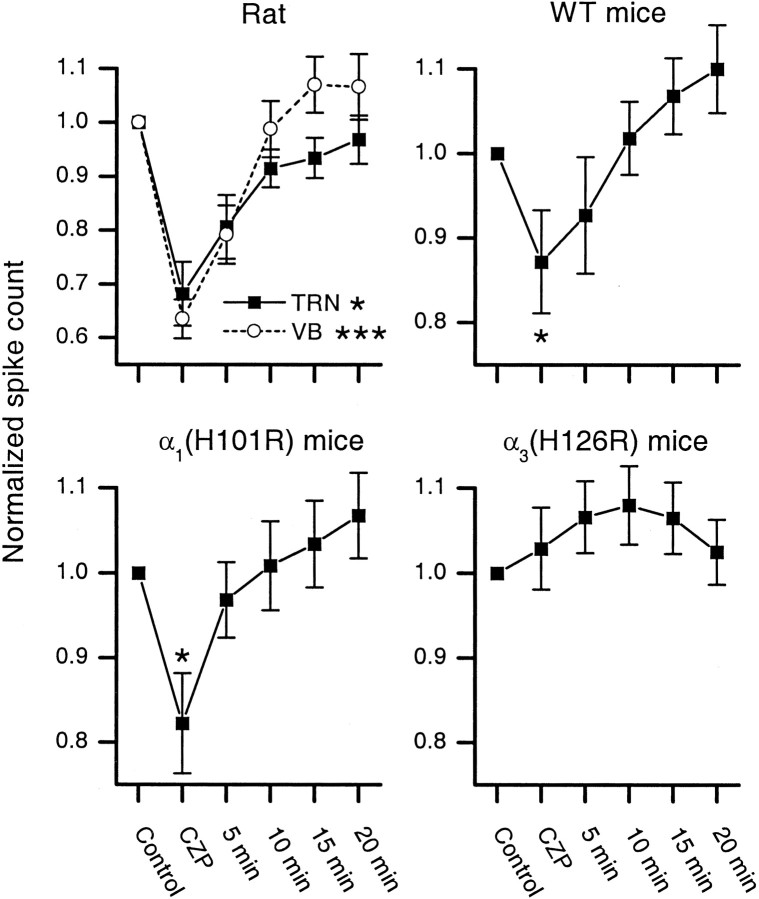
In rat thalamic slices, we recorded from TC neurons in the ventrobasal complex (VB) and TRN neurons. A single electrical shock to the internal capsule elicited spindle-like oscillations. These oscillations, which could extend across several electrodes in both VB (22 recordings from nine slices) and TRN (16 recordings from six slices), had frequencies between 4.7 and 8.2 Hz (mean = 6.0 Hz) and lasted 1–5 sec. Figure 1 shows evoked multiunit activity in one slice (top three recordings from VB; bottom two recordings from TRN). The evoked oscillation is shown in control conditions (left), during the application of CZP (middle), and after drug washout (right). CZP caused suppression of the oscillations, including a shortening of their duration, and this suppression reversed after washing out CZP. Figure 3 (left) shows the average amount of CZP-induced suppression in all recordings from rat slices, along with the degree of suppression at various time points during the drug washout. The number of spikes during each oscillation was reduced by 32 ± 6% (mean ± SEM) in TRN (n = 16; p < 0.05) and 36 ± 4% in VB (n = 22; p < 0.001) (Table 1).
Fig. 1.
Clonazepam (CZP) reversibly suppresses evoked oscillations in rat thalamic slices. Five simultaneous multiunit recordings from a rat thalamic slice in control conditions (left), during CZP application (middle), and after CZP washout (right). In each condition, the top three recordings are from thalamocortical neurons in the ventrobasal complex, and the bottom two recordings are from thalamic reticular neurons. In the top TRN trace, unit amplitude is small, so arrows point to the location of the last detected burst in each oscillation. The stimulus artifact is visible at the left of each recording. Calibration: 1 sec.
Fig. 3.
Comparison of the magnitude and washout of the clonazepam-mediated suppression of spikes during evoked oscillations in slices from rats, wild-type (WT) mice, α1(H101R) mice, and α3(H126R) mice. For each case, the total number of spikes in each evoked oscillation, relative to control, is plotted for various conditions (control, CZP application, and at 5 min intervals during drug washout). For rat slices, data from recordings in the ventrobasal complex (VB, ○) and the thalamic reticular nucleus (TRN, ▪) are plotted separately. In mouse slices, all recordings were made in VB. CZP significantly reduces the number of spikes during evoked oscillations in rat TRN, rat VB, WT mice, and α1 mutant mice but has no effect in α3 mutant mice (*p < 0.05; ***p < 0.001). Error bars indicate ± 1 SEM.
Table 1.
CZP effects in rats, WT mice, α1(H101R) mice, and α3(H126R) mice
| Spike count in CZP (% of control) | Change in duration after CZP application (msec) | Period (msec) | ||
|---|---|---|---|---|
| Control | CZP | |||
| Rat TRN | 68 ± 6*** | −359 ± 109** | 170 ± 9 | 177 ± 10 |
| Rat VB | 64 ± 4* | −417 ± 137** | 165 ± 5 | 169 ± 9 |
| WT mouse (VB) | 87 ± 6* | −279 ± 95* | 139 ± 6 | 141 ± 7 |
| α1(H101R) mouse (VB) | 82 ± 6* | −275 ± 102* | 152 ± 4 | 146 ± 5 |
| α3(H126R) mouse (VB) | 103 ± 5 | 117 ± 99 | 141 ± 5 | 141 ± 7 |
* p < 0.05; ** p < 0.01; *** p < 0.001.
α1 mutants but not α3 mutants retain CZP-mediated suppression
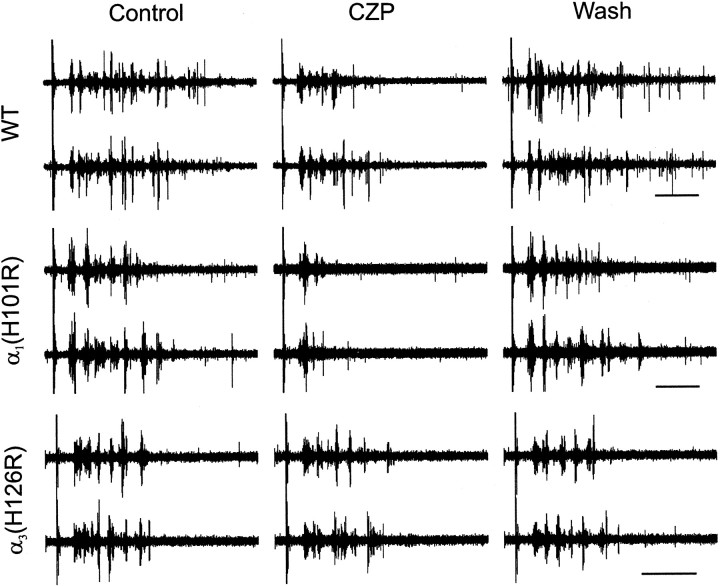
Having found that CZP suppresses evoked thalamic oscillations in rats, we looked for similar effects in WT and mutant mice. Because the CZP-mediated suppression is hypothesized to result from the enhancement of inhibitory synapses between TRN neurons, we expect the CZP-mediated suppression of oscillations to be intact in α1(H101R) mice, in which the effects of CZP are restricted to TRN neurons. By the same argument, CZP should not suppress thalamic oscillations in α3(H126R) mice, in which CZP modulates IPSCs in relay neurons but not in TRN neurons. Indeed, CZP produced a reversible suppression of evoked oscillations in recordings from the VB of WT and α1(H101R) mice. Figure2 shows multiunit activity during two consecutive evoked oscillations in WT (top) and α1(H101R) (middle) slices in control conditions (left), during the application of CZP (middle), and after CZP washout (right). In contrast, CZP had no noticeable effect on oscillations in recordings from α3(H126R) slices (Fig. 2, bottom). Figure 3 summarizes how the average number of spikes during each oscillation changed during CZP application and at various time points during the wash, for slices from rats, WT mice, α1(H101R) mice, and α3(H126R) mice.
Fig. 2.
Clonazepam (CZP) reversibly suppresses evoked oscillations in thalamic slices from wild-type (WT, top) mice and mice with mutations in the α1 subunit of the GABAA receptor (α1(H101R), middle), but not in slices from mice with mutant α3 subunits (α3(H126R), bottom). For each condition, control (left), CZP (middle), and washout (right), multiunit recordings from the same electrode during two consecutive evoked oscillations are shown. The stimulus artifact is visible at the left of each recording. Calibration: 500 msec.
In the following sections, we further characterize this CZP-mediated suppression. Because this suppression occurs in rats as well as WT and α1(H101R) mice, we will present data from rats alongside those from mice. In most respects, the rat and mouse data are very similar; the differences are addressed in Discussion.
CZP reduces the duration of thalamic oscillations
There are several independent mechanisms through which CZP might reduce the number of spikes per oscillation. These include reductions in the duration of each oscillation, the number of spikes on each oscillatory cycle, and the oscillation frequency. For rats, we did not observe changes in the oscillation period in either TRN (mean period in control = 170 ± 9 msec; mean period in CZP = 177 ± 10 msec; n = 13; p = 0.23) or VB (mean period in control = 165 ± 5 msec; mean period in CZP = 169 ± 9 msec; n = 12;p = 0.55). Similarly, CZP had no effect on the period in either WT (mean period in control = 139 ± 6 msec; mean period in CZP = 141 ± 7 msec; n = 13;p = 0.42) or α1(H101R) mice (mean period in control = 152 ± 4 msec; mean period in CZP = 146 ± 5 msec; n = 18;p = 0.09). However, as described below, the decrease in the number of spikes per oscillation did result from both a loss of spikes during the early part of the oscillation and a reduced duration of that oscillation.
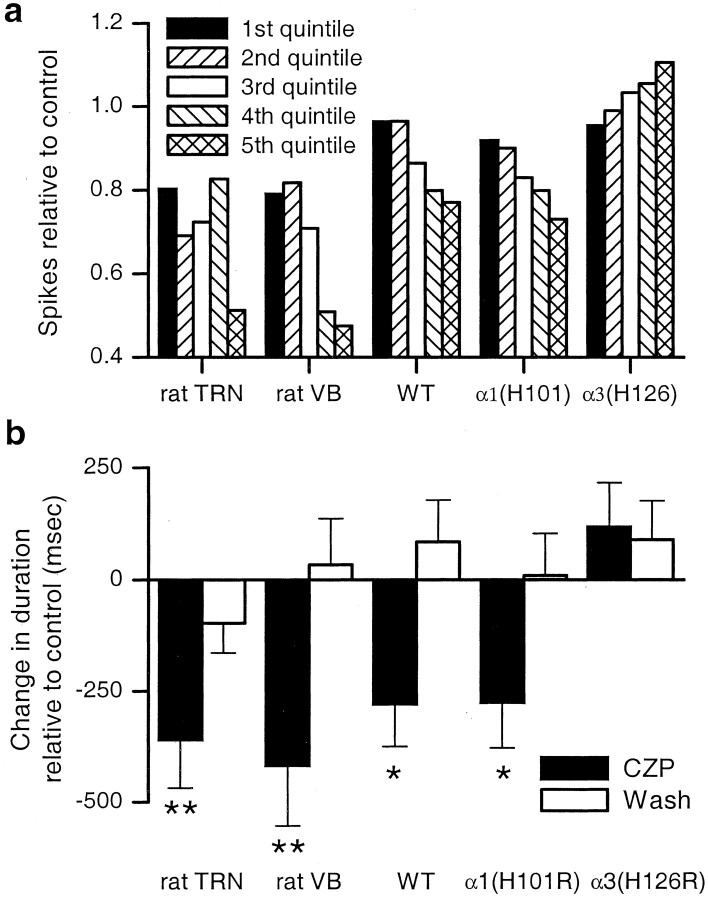
For each set of oscillations recorded from one electrode, we divided the time span over which one oscillation occurred into five domains. Each domain contained exactly one-fifth of the spikes in control conditions, e.g., if for one recording, an average of 100 spikes occurred during each control oscillation, then the “first spike quintile” is the time interval from the occurrence of the 1st spike until the occurrence of the 20th spike. We then computed the number of spikes that occurred within the same time interval after CZP application. We plotted the number of spikes that occurred in each quintile in CZP, relative to the number that had occurred in the same quintile in control conditions. If CZP suppressed equal numbers of spikes during the early and late portions of an oscillation, then the relative spike count should be the same for each quintile. However, if the relative spike count was near one for early spike quintiles, but small for late spike quintiles, it would indicate that CZP only suppressed spikes late in the oscillation, i.e., CZP simply shortened the duration of the oscillation. In fact Figure 4a shows that, for recordings from either rat TRN or rat VB, CZP suppresses spikes both early and late in the oscillation, with a much larger suppression (∼50%) occurring near the end of the oscillation (population data from n= 22 recordings in VB and n = 16 recordings in TRN). Figure 4a also shows the relative spike count for WT mouse VB (n = 13) and α1(H101R) VB (n = 21). In these cases, there is almost no suppression (< 10%) in the early quintiles, but the amount of suppression increases steadily over the course of an oscillation.
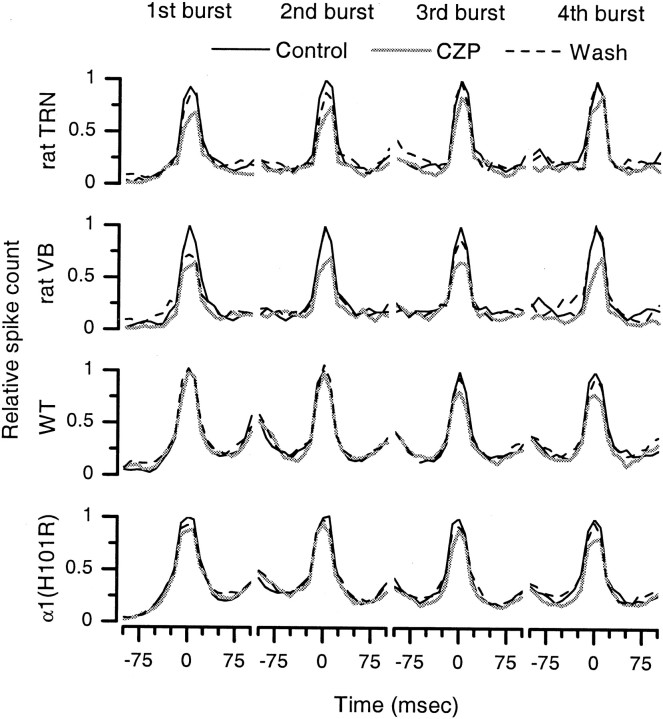
Fig. 4.
Clonazepam (CZP) both suppresses spikes throughout oscillations and shortens the duration of oscillations.a, The number of spikes suppressed by CZP increases over the course of an oscillation. Each oscillation is divided into five time intervals (quintiles), each of which represents a successively later portion of the oscillation and contains one-fifth of the spikes in control conditions. For each quintile, the number of spikes in CZP relative to that in control is shown. In the VB and TRN of rats, CZP suppresses some spikes (20–30%) early in the oscillation and a much greater fraction (∼50%) late in the oscillation. In the VB of WT and α1(H101R) mice, the CZP-mediated suppression is initially small but grows progressively over the course of the oscillation. b, CZP significantly shortens the duration of evoked oscillations in rat TRN, rat VB, WT mouse VB, and α1(H101R) mouse VB (*p < 0.05; **p < 0.01), and this shortening reverses after CZP washout. In the VB of α3(H126R) mice, CZP prolongs the duration, but this is not statistically significant.
Figure 4a shows that CZP suppresses some spikes during the early and intermediate parts of the oscillation and a much larger number late in the oscillation. This suggests that the CZP-mediated suppression of total spike count derives in part from a shorter duration of oscillations in CZP. Indeed, as shown in Figure 4b, in rat VB and TRN, and in the VB of WT and α1(H101R) mice, CZP significantly reduces the duration of oscillations (rat TRN: 353 ± 109 msec reduction, n = 16, p < 0.01; rat VB: 417 ± 137 msec reduction, n = 22,p < 0.01; WT VB: 279 ± 95 msec reduction,n = 13, p < 0.05; α1(H101R) VB: 276 ± 102 msec reduction,n = 22, p < 0.05). In contrast, CZP produces a nonsignificant prolongation of oscillations in the VB of α3(H126R) mice (117 ± 99 msec prolongation; n = 18; p = 0.25). The lack of an effect on, or possible prolongation of, duration in α3(H126R) slices is consistent with the fact that in these slices, CZP actually increases the number of spikes late in the oscillation (Fig. 4a, right) (n = 18). Note, as illustrated by Figure 2, the duration varied considerably from recording to recording. Despite this variability, however, there were no systematic differences in duration among WT, α1(H101R), and α3(H126R) mice, and, as shown by Figure 4b, CZP consistently decreased the duration of oscillations in slices from WT and α1(H101R) mice.
CZP suppresses synchronous spikes
Figure 3 shows that CZP suppresses spikes during thalamic oscillations in rats, WT mice, and α1(H101R) mice, and Figure 4a shows which spikes are suppressed, on the time scale of an oscillation. On the finer time scale of individual bursts, however, it is still not clear which spikes are affected. As shown in Figures 1 and 2, each cycle of an evoked oscillation consists of a population burst, in which many neurons burst, followed by a period of relative quiescence. CZP may suppress spikes at the peaks of these bursts, at the beginning or end of the bursts, and/or in the intervals between bursts. To elucidate exactly which spikes are suppressed on the time scale of bursts, we found the first, second, etc. bursts during oscillations in control conditions and compared each of these with bursts that occurred at approximately the same time during oscillations in CZP or after CZP washout (see Materials and Methods for details). Figure 5 shows the first through fourth bursts in control conditions in comparison with corresponding bursts in CZP and after CZP washout. It is clear that the major effect of CZP is to suppress synchronous spikes that occur at the peaks of the population bursts. Although some spikes at other times are suppressed, this effect both is smaller and appears later than the suppression of the peaks. For example, in rat VB, the peak is strongly suppressed on all four bursts, whereas spikes during the interburst intervals are only modestly suppressed, and this suppression is evident only on the fourth burst. In wild-type and α1(H101R) mice, during the fourth burst, spikes within 25 msec of the peak are significantly suppressed (WT: 25% suppression, n = 10,p < 0.001; α1(H101R): 24% suppression, n = 19; p < 0.01), whereas spikes >60 msec from the peak are not affected (WT: 3% enhancement, n = 10, p = 0.77; α1(H101R): 5% suppression, n = 19; p = 0.64).
Fig. 5.
Clonazepam (CZP) suppresses synchronous firing during evoked oscillations. We compared the bursts of synchronous population activity during oscillations in control conditions (solid black) with bursts at similar times during oscillations in CZP (gray) and after CZP washout (dotted black). Each burst shown here is an average computed from several sweeps from several experiments. In recordings from the TRN and VB of rats and from the VB of WT and α1(H101R) mice, the main effect of CZP is to suppress spikes at the peaks of the bursts.
Figure 5 also reinforces the time course for the CZP-mediated suppression suggested by Figure 4. In rats, the CZP-mediated suppression is present from the very first burst. In contrast, in WT and α1(H101R) mice, CZP has relatively small effects on the first two bursts and larger effects on the third and fourth bursts. A straightforward interpretation of this observation is that in mice, CZP does not appreciably suppress initial bursts but does produce a suppression that grows as the oscillation progresses. However, because the bursts in Figure 5 are averages taken over several sweeps and several experiments, there is an alternative to this “progressive suppression” interpretation. In particular, it is possible that the only effect of CZP is to accelerate the end of each oscillation in an abrupt, all-or-nothing manner. If the degree of premature termination was variable, this effect would manifest as a progressive decline in the amplitude of the averaged bursts, although bursts in individual sweeps were suppressed either completely or not at all. To verify the former progressive suppression interpretation, we compared ratemeters on individual control sweeps with those on individual CZP sweeps from the same recording. Figure6 shows binned spike counts (ratemeters) from four consecutive control sweeps alongside those obtained later, during CZP application, for a recording from a WT mouse slice. Comparing the two sets of ratemeters confirms that CZP progressively suppresses the bursts. Early in the oscillation, bursts are similar in control and CZP; however, soon thereafter, the bursts become markedly smaller in CZP, and this leads to the premature end of the oscillation.
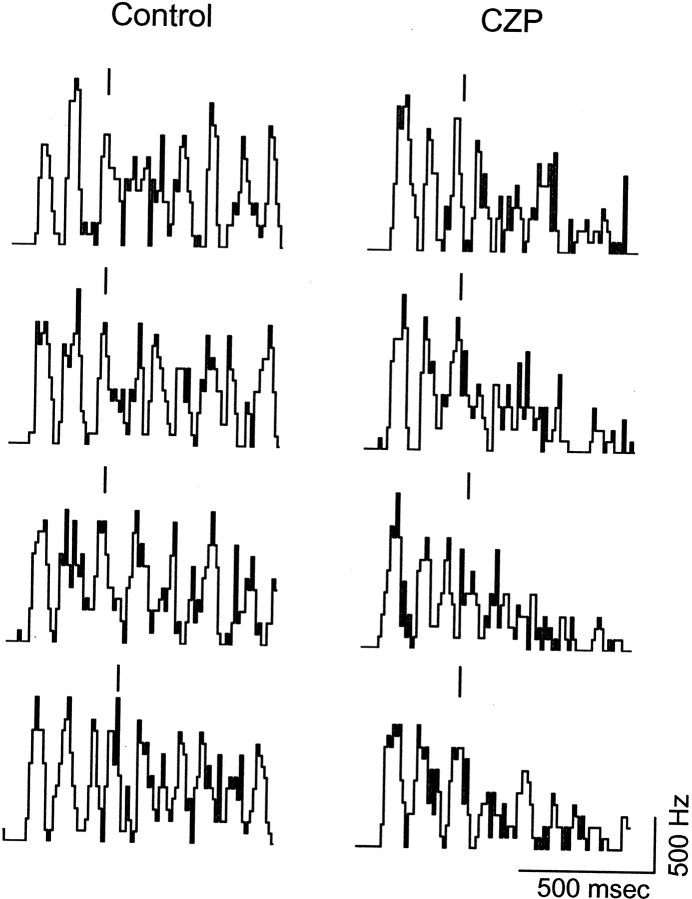
Fig. 6.
Binned spike counts (ratemeters) during four consecutive evoked oscillations in control (left) and CZP (right) during a recording from WT mouse VB. A single shock to internal capsule elicits a sustained oscillation, consisting of a series of bursts of population firing. The amplitude of early bursts is similar in control and CZP, but the later bursts are strongly suppressed in CZP, so that in the latter condition, oscillations come to a premature end. Each bar (‖) marks the center of a burst near the average time at which the third burst would occur during control oscillations in this recording. Calibration: horizontal, 500 msec; vertical, 5 spikes; bin width, 10 msec.
Figure 6 also demonstrates how our algorithm identifies “temporally corresponding” bursts for comparison. Above each ratemeter, a bar marks the burst that the algorithm selected as being close to the time at which the third control burst usually occurred. In most cases (six of eight), the bar is clearly aligned with the peak of the third burst. In the other two cases, however, the algorithm selected the fourth burst, because it searches for the local maximum of the ratemeter (after it has been smoothed) in a time window centered on the average time at which the third burst occurred. Thus, the algorithm compares bursts that occur close in time, rather than simply comparing the nth burst on different sweeps (because these might occur at very different times).
CZP reduces TRN neuron bursting in mice
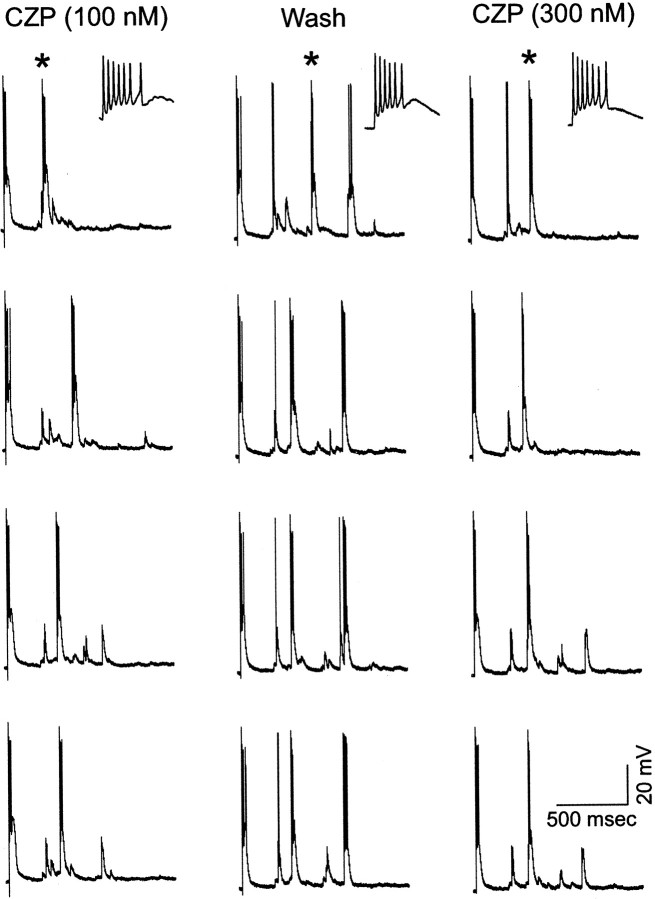
The action of CZP to suppress evoked oscillations, shown in Figures 1-3, presumably results from effects on TRN neurons because it is present in α1(H101R) but not α3(H126R) mice. These effects may include a reduction in the number of TRN neuron bursts and/or a reduction in the number of spikes per TRN neuron burst. To clarify which of these mechanisms might be at work, we recorded intracellularly from TRN neurons during evoked oscillations in WT and α1(H101R) mice. In these recordings (two recordings from WT slices and one recording from an α1(H101R) slice), CZP always produced a significant reduction in the number of bursts, but we never observed a reduction in the number of spikes per burst, even for bursts immediately after internal capsule stimulation (Table 2). Figure7 shows the activity of an α1(H101R) TRN neuron in three different conditions: during application of 100 nm CZP (left), after washout with control ACSF (middle), and during the subsequent application of 300 nm CZP (right). For each condition, the activity during four consecutive evoked oscillations is shown. Excluding the initial, stimulus-evoked burst (at left of each trace), this neuron consistently bursts three times in control ACSF, but only once or, rarely, twice in CZP. Each burst contains an average of 4.3 spikes in control conditions and 5.6 spikes in 100 nm CZP. As in all of our recordings, the CZP-induced reduction in bursting was not accompanied by a tonic hyperpolarization of the neuron. These observations are similar to those in a more thorough quantification of the effects of CZP on rat TRN neurons during evoked oscillations (Sohal and Huguenard, 2001b). They also accord with multiunit recordings from TRN, which clearly contain fewer TRN cell bursts after CZP application. As shown in Figure 8, to the extent that bursts from individual neurons could be discerned in multiunit recordings, they contained similar numbers of spikes in control conditions and after the application of CZP.
Table 2.
CZP effects on intracellularly recorded RE cell bursts
| α1(H101R) | Wild-type 1 | Wild-type 2 | ||||
|---|---|---|---|---|---|---|
| Control | 100 nmCZP | Control | 100 nm CZP | Control | 100 nm CZP | |
| Bursts/sweep | 2.9 ± 0.2 | 1.3 ± 0.2*** | 1.3 ± 0.4 | 0.3 ± 0.3* | 6.0 ± 0 | 4.3 ± 0.6* |
| Spikes/burst | 4.3 ± 0.5 | 5.6 ± 0.7 | 2.7 ± 0.4 | 3 ± 0 | 1.4 ± 0.1 | 1.4 ± 0.2 |
| Spikes immediately after intracellular stimulation | 7.2 ± 0.2 | 7.3 ± 0.2 | 7.7 ± 0.2 | 8.0 ± 0 | 5 ± 0 | 4.8 ± 0.3 |
CZP reduces the number of bursts, but not spikes per burst, in intracellular recordings from RE cells. The number of bursts per evoked oscillation, the number of spikes per burst, and the number of spikes in the 50–100 msec immediately after internal capsule stimulation are shown for each of three intracellularly recorded RE cells. In every case, the number of bursts per oscillation is significantly reduced, whereas the number of spikes per burst and the number of spikes immediately after internal capsule stimulation remain unchanged. *p < 0.05; ***p < 0.001.
Fig. 7.
Clonazepam (CZP) reduces the number of bursts in reticular neurons. Intracellular recordings from a reticular neuron in an α1 receptor mutant slice during evoked oscillations in three different conditions: application of 100 nm CZP (left), washout with control ACSF (middle), and subsequent application of 300 nm CZP (right). For each condition, the activity during four consecutive evoked oscillations is shown. Excluding the initial, stimulus-evoked burst (at left of each trace), this neuron consistently bursts three times in control ACSF, but only once or, rarely, twice in CZP. In the top three panels, bursts indicated by asterisks are shown on an expanded time scale in the insets to confirm that bursts consist of similar numbers of spikes in the three conditions: 100 nm CZP, wash, and 300 nmCZP.
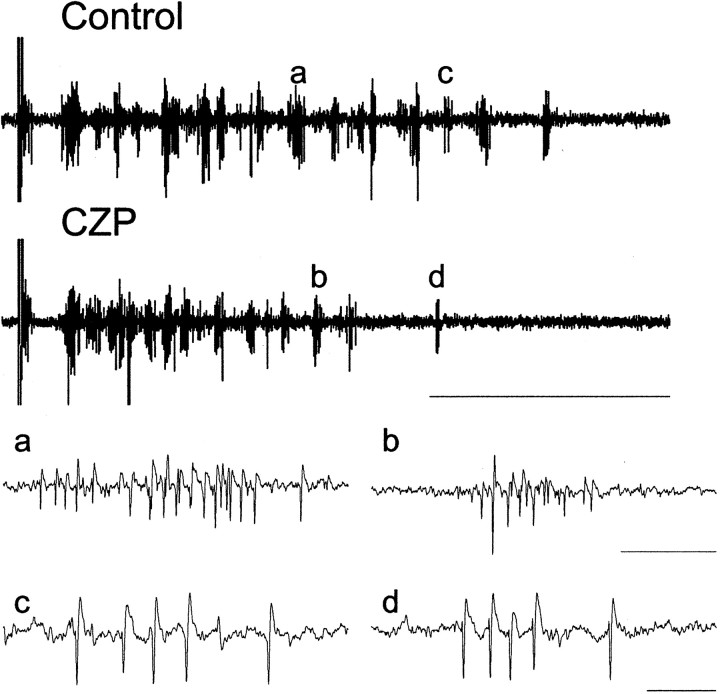
Fig. 8.
Clonazepam reduces the number of bursts, but not burst morphology, in multiunit activity recorded from TRN. Top, Multiunit recording from TRN in an α1(H101R) mouse in control conditions and after the application of 100 nm CZP (calibration: 1 sec). a and b show a population burst in each of the two conditions on an expanded time scale (calibration: 25 msec). Note the spikes of varying amplitudes and high interspike frequency, suggesting that the population burst contains spikes from multiple bursting RE cells. The top traces together with a and b show that fewer RE cell bursts occur in CZP than in control conditions. cand d show a burst, likely from a single RE cell, in the two conditions (calibration: 10 msec). In this experiment, one to three bursts that had similar spike morphologies and overall burst pattern were discernable in each control sweep compared with at most one burst from this presumed unit that was visible in each CZP sweep (data not shown). In both conditions, these bursts contained four to five spikes, consistent with the idea that although CZP may decrease the number of RE cell bursts, the number of spikes in each burst by this RE cell does not change dramatically.
Discussion
CZP suppresses thalamic oscillations by enhancing intra-TRN inhibition
We found that CZP suppresses evoked spindle-like oscillations in thalamic slices from rats, WT mice, and α1(H101R) mice, but not in slices from α3(H126R) mice. In α1(H101R) and α3(H126R) mice, the effects of CZP are restricted to TRN or VB neurons, respectively (Huntsman et al., 2000; Porcello et al., 2001). Thus, enhancing inhibition between TRN neurons is both necessary and sufficient for CZP to suppress evoked thalamic oscillations.
The effects of CZP are dynamic over the course of an oscillation
How does enhancing intra-TRN inhibition suppress thalamic oscillations? First, the suppression is progressive: a relatively small reduction in spikes early on grows over the course of an oscillation, ultimately shortening the duration of that oscillation. Second, CZP does not suppress spikes indiscriminately. One large burst of spikes occurs during each cycle of an oscillation, and CZP preferentially suppresses the “synchronous” spikes that occur during the peaks of these population bursts. Third, in a limited number of intracellular recordings from TRN neurons in WT and α1(H101R) slices, and in more extensive recordings in rat slices (Sohal and Huguenard, 2001b), CZP reduces the number of times that TRN neurons burst during an oscillation.
These observations suggest a possible mechanism by which CZP could suppress thalamic oscillations. CZP enhances intra-TRN inhibition, and as a result, fewer TRN neurons burst synchronously during population bursts, reducing inhibitory output from TRN. Reduced TRN output elicits fewer or less intense rebound bursts in VB, attenuating synchronized bursts in TC neurons. Reduced TC activity would then recruit less TRN activity on the next cycle of the oscillation; this could explain how the effects of CZP grow over the course of an oscillation (Fig.4a).
Of course, the preceding mechanism may not be valid, because the effects of CZP, reduced TRN bursting, a progressive loss of synchronous spikes, and a reduction in the duration of the oscillation, may not be causally linked. Instead, a single factor might produce all three of these effects. For example, GABAA receptor activation could summate in TRN neurons over the course of an oscillation, so that the total GABAAreceptor-mediated conductance (gGABA-A,TRN) grows. Early in the oscillation, a relatively smallgGABA-A,TRN might have little effect. As the oscillation progresses and gGABA-A,TRNgrows, proportionately fewer spikes might occur. Ultimately,gGABA-A,TRN might be large enough to bring the oscillation to a premature end. In such a scheme, changes in the early part of the oscillation do not affect later parts of the oscillation. However, thalamic oscillations are dynamic phenomenon in which the initial pattern of activity controls the form that the oscillation ultimately takes [cf. colliding waves in vitroin Kim et al. (1995) and responses to differently sized stimuli in the simulations of Sohal and Huguenard (2000)]. This dynamism supports a mechanism in which CZP produces effects early in the oscillation that lead to later effects, e.g., shortening of the duration.
The progressive nature of CZP effects (Figs. 4a, 5) suggests that ongoing intrathalamic activity is sufficient to recruit meaningful levels of intra-TRN inhibition. An alternative would be that only broad activation of TRN, which follows the stimulation of corticothalamic fibers to initiate an oscillation, is enough to produce significant intra-TRN inhibition. In WT and α1(H101R) mice, however, CZP has little effect on the early portion of the oscillation, which immediately follows stimulation of corticothalamic fibers, but later in the oscillation, after intrathalamic activity has proceeded in the absence of corticothalamic stimulation, the CZP effect is much larger.
Role of intra-TRN inhibition
There are several hypothesized functions for intra-TRN inhibition. Our findings are consistent with studies suggesting that inhibitory synapses between TRN neurons suppress thalamic oscillations (von Krosigk et al., 1993; Huguenard and Prince, 1994; Huntsman et al., 1999; Sohal et al., 2000). One difference between our studies and those done in ferret slices (von Krosigk et al., 1993) is that the latter emphasized that blocking inhibition between neurons of perigeniculate nucleus (the visual analog of TRN) prolonged bursts. By contrast, data presented here (Fig. 7) and elsewhere (Sohal and Huguenard, 2001b) suggest that CZP may act by changing the number of bursts per oscillation.
Bazhenov et al. (1999) have proposed that rather than suppressing oscillations, GABAA receptor-mediated currents depolarize TRN neurons, spreading activity through TRN and initiating spindles. It is difficult to predict, on the basis of this model, how CZP should affect evoked spindles. One possibility is that by enhancing intra-TRN inhibition, CZP should spread activity. Alternatively, CZP might increase the amplitude or duration of IPSCs in TRN neurons beyond some critical value, so that these currents shunt, rather than elicit, bursts in TRN. We found that during evoked oscillations, CZP suppressed population bursts in multiunit recordings from TRN and reduced the number of bursts in intracellular recordings from TRN neurons. This suggests that if a regime exists within which intra-TRN inhibition depolarizes TRN neurons and elicits bursts, then during evoked oscillations, moderate augmentation of intra-TRN inhibition is sufficient to shift intra-TRN inhibition to a primarily shunting and anti-oscillatory role.
A third hypothesis is that TRN is the “pacemaker” for spindle oscillations (Steriade et al., 1987; Destexhe et al., 1994), i.e., oscillations occur when TRN neurons burst and inhibit each other, resulting in T-current deinactivation that elicits another cycle of rebound bursts. In this scheme, intra-TRN inhibition sustains and paces thalamic oscillations, so augmenting intra-TRN inhibition should enhance oscillations and alter their period. In contrast to this prediction, we not only observed marked suppression of oscillations by CZP, but we also observed that CZP did not affect the period of thalamic oscillations.
Synchrony in networks of inhibitory neurons
Weakening inhibition in interconnected inhibitory networks of the hippocampus disrupts population rhythmicity (Whittington et al., 1998). Here we studied an interconnected inhibitory network in the thalamus and found that selectively strengthening of intra-TRN inhibition suppressed synchronous spikes that occur at the peaks of population bursts. Thus, networks of inhibitory neurons can desynchronize network oscillations, and as described above, the suppression of synchronous activity might invoke dynamic mechanisms that ultimately reshape an oscillation, e.g., reducing its duration.
Why are synchronous spikes particularly affected by the strengthening of intra-TRN inhibition? Although modeling studies may yield further insights, we offer the following possible explanation. In TRN, synchronous spikes occur, by definition, during times of peak intra-TRN inhibition and thus are subject to increased shunting in CZP. Reduced TRN output, resulting from CZP-mediated enhancement of intra-TRN inhibition, may in turn elicit less intense rebound bursts from TC neurons. This translates into less activity at the peaks of population bursts. As long as many TC neurons rebound burst at varying times, however, spikes will still occur outside the peaks of the population bursts. Perhaps only later in the oscillation, when the inhibitory output from TRN decreases sufficiently, does the number of bursting TC neurons decrease so that spikes beyond the peaks of population bursts are affected.
Differences between the effects of CZP in rats and mice
CZP suppressed evoked oscillations in thalamic slices from rats, WT mice, and a1(H101R) mice. This suppression was much larger in rats than in mice, however, reflecting in large part the fact that CZP suppressed spikes throughout oscillations in rats, whereas in mice, CZP had a very small effect on early bursts. This difference may reflect the different contributions made by GABAB receptors during evoked thalamic oscillations in rats and mice. In intracellular recordings from TC neurons during evoked oscillations in mice, blocking GABAB receptors has no observable effect (Warren et al., 1994). By contrast, in rat TC neurons, the IPSP that immediately follows internal capsule stimulation has a large GABAB receptor-mediated component that is selectively suppressed by CZP (Huguenard and Prince, 1994). Thus, the strong suppression of the early part of an oscillation, which CZP produced in rats but not in mice, may reflect the effect of CZP to reduce GABAB receptor activation in TC neurons by altering the excitability of TRN neurons.
Larger contributions from GABAB receptors, which slow evoked thalamic oscillations (Jacobsen et al., 2001), may also explain why the period of oscillations was longer in rats than in mice.
Implications for anti-absence drug design
The same thalamic circuitry that generates spindles in vivo and spindle-like oscillations in vitro is hypothesized to contribute to the spike-wave discharges in absence epilepsy (Huguenard and Prince, 1997; Sohal and Huguenard, 2001a), and the effectiveness of CZP in treating absence epilepsy may derive from its ability to dampen thalamic oscillations (Huguenard and Prince, 1994; Zhang et al., 1996). Our results suggest that the suppression of thalamic oscillations by CZP results exclusively from its action on α3-containing GABAAreceptors in TRN. This suggests that drugs which selectively augment currents mediated by α3-containing GABAA receptors, or are in other ways specific for GABAA receptors in TRN, will share the therapeutic efficacy of CZP and may have fewer undesirable interactions with other GABAA receptors. Indeed, one major side effect of CZP is drowsiness, but when benzodiazepines and related drugs do not bind to α1-containing GABAA receptors, they are free of sedative effects (Rudolph et al., 1999).
Footnotes
This work was supported by National Institutes of Health Grants GM07365 from the National Institute of General Medical Sciences and NS34774 from the National Institute of Neurological Disorders and Stroke, the Pimley Research Fund, and the Swiss National Science Foundation.
Correspondence should be addressed to J. R. Huguenard, Department of Neurology and Neurological Sciences, Stanford University Medical Center, Stanford, CA 94305-5122. E-mail:john.huguenard@stanford.edu.
References
- 1.Avanzini G, Vergnes M, Spreafico R, Marescaux C. Calcium-dependent regulation of genetically determined spike and waves by the reticular thalamic nucleus of rats. Epilepsia. 1993;34:1–7. doi: 10.1111/j.1528-1157.1993.tb02369.x. [DOI] [PubMed] [Google Scholar]
- 2.Banerjee PK, Snead OC. Thalamic mediodorsal and intralaminar nuclear lesions disrupt the generation of experimentally induced generalized absence-like seizures in rats. Epilepsy Res. 1994;17:193–205. doi: 10.1016/0920-1211(94)90050-7. [DOI] [PubMed] [Google Scholar]
- 3.Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2:168–174. doi: 10.1038/5729. [DOI] [PubMed] [Google Scholar]
- 4.Benson JA, Löw K, Keist R, Mohler H, Rudolph U. Pharmacology of recombinant gamma-aminobutyric acid receptors rendered diazepam-insensitive by point-mutated α-subunits. FEBS Lett. 1998;431:400–404. doi: 10.1016/s0014-5793(98)00803-5. [DOI] [PubMed] [Google Scholar]
- 5.Browne SH, Kang J, Akk G, Chiang LW, Schulman H, Huguenard JR, Prince DA. Kinetic and pharmacological properties of GABA(A) receptors in single thalamic neurons and GABA(A) subunit expression. J Neurophysiol. 2001;86:2312–2322. doi: 10.1152/jn.2001.86.5.2312. [DOI] [PubMed] [Google Scholar]
- 6.Destexhe A, Contreras D, Sejnowski TJ, Steriade M. A model of spindle rhythmicity in the isolated thalamic reticular nucleus. J Neurophysiol. 1994;72:803–818. doi: 10.1152/jn.1994.72.2.803. [DOI] [PubMed] [Google Scholar]
- 7.Huguenard JR, Prince DA. Clonazepam suppresses GABAB-mediated inhibition in thalamic relay neurons through effects in nucleus reticularis. J Neurophysiol. 1994;71:2576–2581. doi: 10.1152/jn.1994.71.6.2576. [DOI] [PubMed] [Google Scholar]
- 8.Huguenard JR, Prince DA. Basic mechanisms of epileptic discharges in the thalamus. In: Steriade M, Jones EG, McCormick DA, editors. Thalamus, Vol II, Experimental and clinical aspects. Elsevier Science; Oxford: 1997. pp. 295–330. [Google Scholar]
- 9.Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR. Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science. 1999;283:541–543. doi: 10.1126/science.283.5401.541. [DOI] [PubMed] [Google Scholar]
- 10.Huntsman MM, Porcello DM, Rudolph U, Huguenard JR. GABA-A receptor knock-in mice reveal subtype-specific benzodiazepine modulation of inhibitory synaptic responses in thalamic neurons. Soc Neurosci Abstr. 2000;26:51.23. [Google Scholar]
- 11.Jacobsen RB, Ulrich D, Huguenard JR. GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol. 2001;86:1365–1375. doi: 10.1152/jn.2001.86.3.1365. [DOI] [PubMed] [Google Scholar]
- 12.Kim D, Song I, Keum S, Lee T, Jeong MJ, Kim SS, McEnery MW, Shin HS. Lack of the burst firing of thalamocortical relay neurons and resistance to absence seizures in mice lacking alpha(1G) T-type Ca(2+) channels. Neuron. 2001;31:35–45. doi: 10.1016/s0896-6273(01)00343-9. [DOI] [PubMed] [Google Scholar]
- 13.Kim U, Bal T, McCormick DA. Spindle waves are propagating synchronized oscillations in the ferret LGNd in vitro. J Neurophysiol. 1995;74:1301–1323. doi: 10.1152/jn.1995.74.3.1301. [DOI] [PubMed] [Google Scholar]
- 14.Low K, Crestani F, Keist R, Benke D, Brunig I, Benson JA, Fritschy JM, Rulicke T, Bluethmann H, Mohler H, Rudolph U. Molecular and neuronal substrate for the selective attenuation of anxiety. Science. 2000;290:131–134. doi: 10.1126/science.290.5489.131. [DOI] [PubMed] [Google Scholar]
- 15.Oh KS, Lee CJ, Gibbs JW, Coulter DA. Postnatal development of GABAA receptor function in somatosensory thalamus and cortex: whole-cell voltage-clamp recordings in acutely isolated rat neurons. J Neurosci. 1995;15:1341–1351. doi: 10.1523/JNEUROSCI.15-02-01341.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pirker S, Schwarzer C, Wieselthaler A, Sieghart W, Sperk G. GABA(A) receptors: immunocytochemical distribution of 13 subunits in the adult rat brain. Neuroscience. 2000;101:815–850. doi: 10.1016/s0306-4522(00)00442-5. [DOI] [PubMed] [Google Scholar]
- 17.Porcello DM, Huntsman MM, Rudolph U, Huguenard JR. GABA-A receptor mutant mice reveal subtype-selective benzodiazepine modulation of intra-inhibitory connections in reticular thalamus. Soc Neurosci Abstr. 2001;27:491.23. [Google Scholar]
- 18.Rudolph U, Crestani F, Benke D, Brünig I, Benson JA, Fritschy JM, Martin JR, Bluethmann H, Möhler H. Benzodiazepine actions mediated by specific gamma-aminobutyric acidA receptor subtypes. Nature. 1999;401:796–800. doi: 10.1038/44579. [DOI] [PubMed] [Google Scholar]
- 19.Sohal VS, Huguenard JR. It takes T to tango. Neuron. 2001a;31:3–4. doi: 10.1016/s0896-6273(01)00349-x. [DOI] [PubMed] [Google Scholar]
- 20.Sohal VS, Huguenard JR. Thalamic reticular cell activity during different oscillatory modes in vitro. Soc Neurosci Abstr. 2001b;27:559.6. [Google Scholar]
- 21.Sohal VS, Huntsman MM, Huguenard JR. Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J Neurosci. 2000;20:1735–1745. doi: 10.1523/JNEUROSCI.20-05-01735.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steriade M, Contreras D. Spike-wave complexes and fast components of cortically generated seizures. I. Role of neocortex and thalamus. J Neurophysiol. 1998;80:1439–1455. doi: 10.1152/jn.1998.80.3.1439. [DOI] [PubMed] [Google Scholar]
- 23.Steriade M, Domich L, Oakson G, Deschênes M. The deafferented reticular thalamic nucleus generates spindle rhythmicity. J Neurophysiol. 1987;57:260–273. doi: 10.1152/jn.1987.57.1.260. [DOI] [PubMed] [Google Scholar]
- 24.von Krosigk M, Bal T, McCormick DA. Cellular mechanisms of a synchronized oscillation in the thalamus. Science. 1993;261:361–364. doi: 10.1126/science.8392750. [DOI] [PubMed] [Google Scholar]
- 25.Warren RA, Agmon A, Jones EG. Oscillatory synaptic interactions between ventroposterior and reticular neurons in mouse thalamus in vitro. J Neurophysiol. 1994;72:1993–2003. doi: 10.1152/jn.1994.72.4.1993. [DOI] [PubMed] [Google Scholar]
- 26.Whittington MA, Traub RD, Faulkner HJ, Jefferys JG, Chettiar K. Morphine disrupts long-range synchrony of gamma oscillations in hippocampal slices. Proc Natl Acad Sci USA. 1998;95:5807–5811. doi: 10.1073/pnas.95.10.5807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wieland HA, Luddens H, Seeburg PH. A single histidine in GABAA receptors is essential for benzodiazepine agonist binding. J Biol Chem. 1992;267:1426–1429. [PubMed] [Google Scholar]
- 28.Wisden W, Laurie DJ, Monyer H, Seeburg PH. The distribution of 13 GABAA receptor subunit mRNAs in the rat brain. I. Telencephalon, diencephalon, mesencephalon. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang YF, Gibbs JW, III, Coulter DA. Anticonvulsant drug effects on spontaneous thalamocortical rhythms in vitro: ethosuximide, trimethadione, and dimethadione. Epilepsy Res. 1996;23:15–36. doi: 10.1016/0920-1211(95)00079-8. [DOI] [PubMed] [Google Scholar]