Abstract
The σ1 receptor is critically involved in the rewarding effect of cocaine, as measured using the conditioned place preference (CPP) procedure in mice. Neuroactive steroids exert rapid neuromodulatory effects in the brain by interacting with GABAA, NMDA, and σ1 receptors. At the σ1 receptor level, 3β-hydroxy-5-androsten-17-one [dehydroepiandrosterone (DHEA)] and 3β-hydroxy-5-pregnen-20-one (pregnenolone) act as agonists, whereas 4-pregnene-3,20-dione (progesterone) is an efficient antagonist. The present study sought to investigate the action of neuroactive steroids in acquisition of cocaine-induced CPP in C57BL/6 mice. None of these steroids induced CPP alone. However, pretreatment with DHEA or pregnenolone (5–20 mg/kg, s.c.) during conditioning with cocaine (10 mg/kg, i.p.) increased the conditioned score. On the contrary, pretreatment with either progesterone (10 or 20 mg/kg, s.c.) or finasteride (25 mg/kg, twice a day), a 5α-reductase inhibitor, blocked acquisition of cocaine (20 mg/kg)-induced CPP. A crossed pharmacology was observed between steroids and σ1 ligands. The σ1 antagonistN-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine blocked cocaine-induced CPP and its potentiation by DHEA or pregnenolone. Progesterone blocked cocaine-induced CPP and its potentiation by the σ1 agonist igmesine. These results showed that neuroactive steroids play a role in cocaine-induced appetence, through their interaction with the σ1receptor. Therefore, neuroendocrine control of cocaine addiction may not involve solely glucocorticoids. The importance of neuroactive steroids as factors of individual vulnerability to drug addiction should, thus, be considered.
Keywords: cocaine, neuroactive steroids, locomotor activity, reward, convulsions, σ1 receptor
Introduction
Cocaine is a major drug of abuse worldwide. It is a potent stimulant, exerting its effects primarily by its dopaminergic agonist activity on the mesocorticolimbic pathways. It is widely accepted that the addictive and reinforcing actions of cocaine are the results of its ability to block the reuptake of dopamine (DA) and thereby to increase DA neurotransmission (Ritz et al., 1987). Acute administration of cocaine induces hyperlocomotion and stereotypies, whereas repeated administrations provoke sensitization to the drug effects, with reinforcement and dependence appearing within a short time after the first administration (Koob and Nestler, 1997). Besides its action on DA, cocaine is known to affect the hypothalamopituitary adrenal axis, leading to increased levels of corticotropin-releasing hormone (Sarnyai et al., 1992), adrenocorticotropin hormone, and glucocorticoids (Moldow and Fischman, 1987). In turn, glucocorticoids play a particular role in vulnerability to cocaine intake and drug effects in terms of stress-induced regulation of dopaminergic neurons (Marinelli and Piazza, 2002).
However, with the exception of the anticonvulsant potency of 4-pregnene-3,20-dione (progesterone) metabolites against cocaine-induced seizures (Gasior et al., 1997), little is known regarding the influence on cocaine addiction of steroids synthesized upstream to glucocorticoids in adrenals, gonads, and the brain. Among them are neurosteroids (i.e., steroids synthesized in the brain and acting locally to modulate neuronal cell activity) (Baulieu, 1981). Neurosteroids are known to exert their modulatory effects primarily via nongenomic actions and are known to affect learning and memory processes, mood, and depression (Rupprecht and Holsboer, 1999). 3β-Hydroxy-5-pregnen-20-one (pregnenolone), 3β-hydroxy-5-androsten-17-one [dehydroepiandrosterone (DHEA)], and their sulfate esters are considered as excitatory steroids because they act as negative modulators of the GABAA receptor (Majewska et al., 1988, 1990) and positive modulators of NMDA receptors (Wu et al., 1991). Progesterone and its reduced metabolite 3α-hydroxy-5α-pregnan-20-one (allopregnanolone) act as positive modulators of the GABAA receptor (Schumacher and McEwen, 1989) and negative modulators of NMDA receptors (Smith, 1991). In addition, neuroactive steroids interact with the σ1 receptor, with in vitroaffinities for brain [3H](+)-SKF-10,047 (N-[3H]allyl-normetazocine)-labeled sites of 300 nm, 1 μm, and 3 μm for progesterone, DHEA, and pregnenolone, respectively (Su et al., 1988; Maurice et al., 1996). Moreover, pregnenolone, DHEA, and their sulfate esters also act as σ1 receptor agonists, whereas progesterone is a potent σ1 antagonist (Monnet et al., 1995;Bergeron et al., 1996; Maurice et al., 1999, 2001). The σ1 receptor is an intracellular neuronal protein associated with endoplasmic reticular, plasma, nuclear, and mitochondrial membranes (Alonso et al., 2000). The σ1 receptor ligands potently modulate intracellular Ca2+ mobilizations and extracellular Ca2+ influx (Hayashi et al., 2000) in addition to numerous responses to neurotransmitters (Monnet et al., 1992; Gonzalez-Alvear and Werling, 1994). This nonselective but efficient neuromodulatory system plays a major role in memory processes and response to stress or depression (for review, see Maurice et al., 1999, 2001). Moreover, the σ1 receptor is involved in several aspects of cocaine action (for review, see Maurice et al., 2002), including hyperlocomotion (Menkel et al., 1991), sensitization (Ujike et al., 1996), rewarding effects (Romieu et al., 2000, 2002), convulsions, and lethality (Matsumoto et al., 2001).
The present study examined the effects of neuroactive steroids, namely pregnenolone, DHEA, and progesterone, on cocaine-induced appetitive properties, using the conditioned place preference (CPP) paradigm in C57BL/6 mice. The results show that pregnenolone and DHEA, devoid of effect by themselves, markedly increased cocaine-induced CPP, whereas progesterone blocked it. A crossed pharmacology was observed between steroids and σ1 ligands, because the potentiating effects of pregnenolone and DHEA were blocked by preadministration of the σ1 antagonistN-[2-(3,4-dichlorophenyl)ethyl]-N-methyl-2-(dimethylamino)ethylamine (BD1047), whereas the potentiating effect of the σ1 agonist igmesine was blocked by progesterone. Neuroactive steroids may, thus, act through the σ1 receptor to modulate cocaine-induced reward.
Materials and Methods
Animals. Male C57BL/6 mice, 1 month of age and weighing 24–26 gm, were purchased from Iffa-Credo (Saint-Germain-sur-l'Arbresle, France). The animals were housed in groups with ad libitum access to food and water in a temperature- and humidity-controlled animal facility on a 12 hr light/dark cycle (lights off at 8:00 P.M.). Experiments were performed between 10:00 A.M. and 6:00 P.M. in a sound-attenuated and air-regulated experimental room, to which mice were habituated at least 30 min before each experiment. All animal procedures were conducted in strict adherence to the European Communities Council (EEC) Directive of November 24, 1986 (86-609/EEC).
Drugs. Cocaine was purchased from Cooper (Brive, France). Progesterone, pregnenolone, and DHEA were obtained from Sigma-Aldrich (Saint-Quentin Fallavier, France). (+)-N-cyclopropylmethyl-N-methyl-1,4-diphenyl-1-ethyl-but-3-en-1-ylamine (igmesine) was provided by Dr. F. J. Roman (Pfizer GRD, Fresnes, France), and BD1047 was synthesized in the Laboratory of Medicinal Chemistry as described previously (De Costa et al., 1992). Cocaine was dissolved in physiological saline, igmesine and BD1047 were dissolved in distilled water, and steroids were suspended in pure sesame oil (Sigma-Aldrich). They were injected intraperitoneally or subcutaneously in a volume of 100 μl per 20 gm of body weight.
CPP procedure. The apparatus consisted of a polyvinylchloride box divided into two compartments of equal size (15 × 15 × 35 cm high) separated by a sliding door, the first one with black walls and floor and the second one with white walls and floor. Each compartment presented different floor textures (smooth for the black one and covered by a wire mesh grid for the white one). A 60 W lamp lit the white compartment during all experiments.
The procedure consisted of three phases (Romieu et al., 2000, 2002). For preconditioning (day 1), each mouse was placed in the white compartment and doors were raised after 5 sec. The mouse freely explored the apparatus for 10 min. Preconditioning was repeated after 6 hr. The exploration was videotaped, and the amount of time spent in each compartment was recorded to determine the unconditioned preference. Animals showing a strong unconditioned preference (>570 sec) were discarded. Conditioning (days 2–5) was conducted using an unbiased procedure. In each group, one-half of the animals received drugs in the spontaneously preferred compartment and the other half in the nonpreferred compartment. Each mouse was confined to the drug-paired compartment for 30 min. In coadministration experiments, mice received steroids and/or σ1 ligands 10 min before cocaine. After a 6 hr washout period, vehicle solutions were injected and mice were confined in the other compartment for 30 min. During the postconditioning test (day 6), each mouse freely explored the apparatus for 10 min, and the time spent in each compartment was determined. The conditioned score (mean ± SEM) was calculated as the difference of time spent in the drug-paired compartment between the postconditioning and preconditioning. Data were analyzed using a parametric ANOVA, followed by a Newman–Keuls' post hoctest. The criterion for statistical significance was p< 0.05.
Results
Effects of neuroactive steroids on cocaine-induced CPP
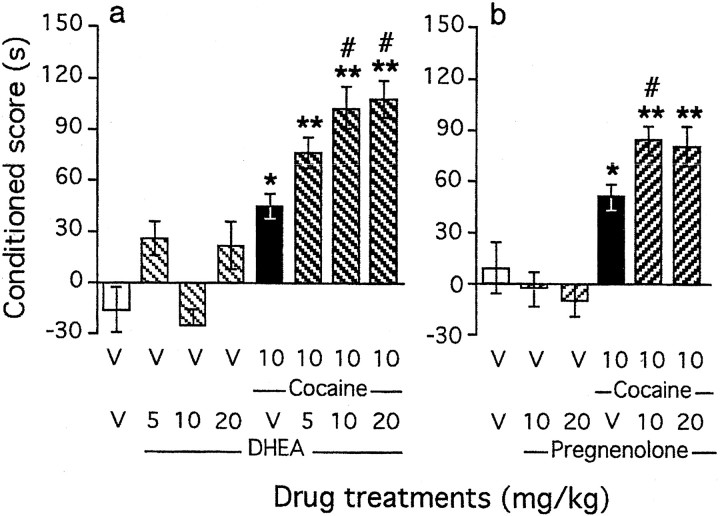
Cocaine conditioning during 4 d resulted in dose-dependent acquisition of CPP. A dose of 10 mg/kg led to a conditioned score of +60 sec (black columns; p < 0.05) (Fig.1a,b), and a dose of 20 mg/kg led to a conditioned score of +120 sec (black column;p < 0.01) (Fig.2a,b). The steroids DHEA (Fig. 1a) and pregnenolone (Fig. 1b), 5–20 mg/kg subcutaneously, failed to induce CPP alone. However, pretreatment before a low dose of cocaine (10 mg/kg) resulted in the potentiation of cocaine-induced CPP (F(7,107) = 7.41;p < 0.0001 for DHEA) (Fig. 1a) (F(5,61) = 3.70; p < 0.01 for pregnenolone) (Fig. 1b). In particular, DHEA at 10 and 20 mg/kg and pregnenolone at 10 mg/kg provoked significant increases in the conditioned scores compared with cocaine-treated animals (p < 0.05 each).
Fig. 1.
Effects of neuroactive steroids on acquisition of cocaine-induced CPP: coadministration of DHEA (a) or pregnenolone (b). Steroids were administered subcutaneously 10 min before cocaine, which was given immediately before placement in the compartment during conditioning. Conditioned scores represent the difference in time spent in the drug-paired compartment between the postconditioning and preconditioning sessions. Mice per group: n = 12–16 (a) and n = 8–12 (b). V, Vehicle. *p < 0.05 and **p < 0.01 versus the V+V-treated group;#p < 0.05 versus the cocaine+V-treated group.
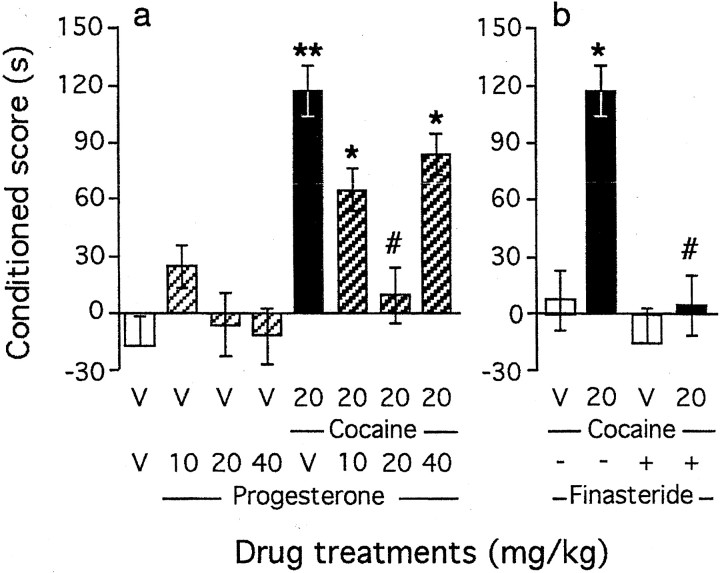
Fig. 2.
Effects of neuroactive steroids on acquisition of cocaine-induced CPP: coadministration of progesterone (a) or effect of the 5α-reductase inhibitor finasteride (b). Progesterone was administered subcutaneously 10 min before cocaine, given immediately before placement in the compartment during conditioning. Finasteride (25 mg/kg) was administered subcutaneously, every 12 hr during 6 d, starting 1 d before preconditioning. Conditioned scores represent the difference in time spent in the drug-paired compartment between the postconditioning and preconditioning sessions. Mice per group:n = 12–19 (a) andn = 10–19 (b). V, Vehicle. *p < 0.05 and **p < 0.01 versus the V+V-treated group; #p < 0.05 versus the cocaine+V-treated group.
Progesterone (10–40 mg/kg, s.c.) administration failed to induce CPP alone (Fig. 2a). However, preadministration of progesterone before cocaine (20 mg/kg) led to a U-shaped decrease in the conditioned score (F(7,115) = 3.56;p < 0.01) (Fig. 2a). Progesterone (20 mg/kg) pretreatment prevented acquisition of cocaine-induced CPP, whereas a higher dose (40 mg/kg) failed to affect the conditioned score. Finasteride is a selective inhibitor of the 5α-reductase enzyme that converts progesterone to 5α-pregnane-3,20-dione, the precursor of allopregnanolone (Trapani et al., 2002). When mice were repeatedly treated with finasteride (25 mg/kg, s.c., twice a day during 6 d), they failed to acquire cocaine-induced CPP (F(3,50) = 4.43; p < 0.01) (Fig. 2b). Finasteride treatment was, however, devoid of effect alone. Finasteride administration resulted in a moderate accumulation of progesterone and in depletion of both 5α-pregnane-3,20-dione and allopregnanolone levels (Trapani et al., 2002). The blockade of cocaine-induced CPP by finasteride suggested that not only low levels of progesterone but also sustained levels of allopregnanolone are important for the establishment of cocaine-induced CPP. Indeed, at the highest dose tested, the progesterone treatment led to increased allopregnanolone content, and consequently a U-shaped effect of progesterone was observed.
Involvement of the σ1 receptor in neuroactive steroid effects
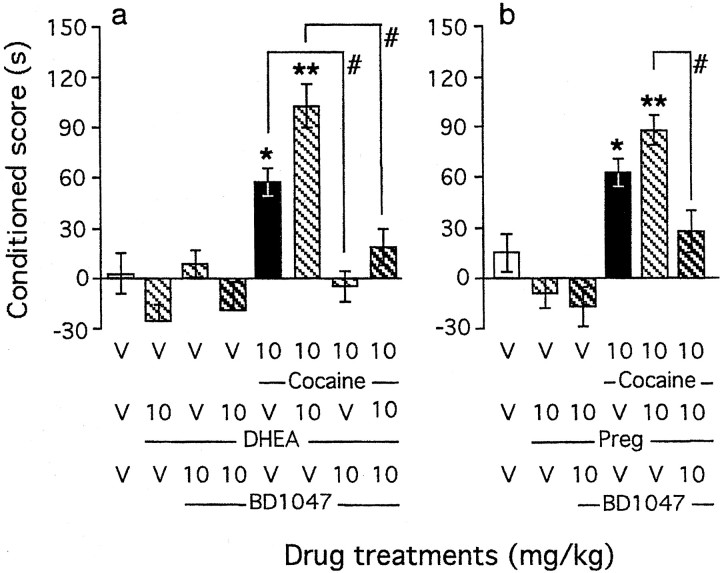
The σ1 receptor has been shown to be involved in the acquisition and expression of cocaine-induced CPP in previous studies, although selective σ1 ligands failed to induce CPP by themselves (Romieu et al., 2000, 2002). Because the σ1 receptor is a critical target for neuroactive steroids in the brain, crossed pharmacology studies were performed between steroids and σ1 ligands. First, the effect of the selective σ1antagonist BD1047 (10 mg/kg, s.c.) was examined. BD1047 pretreatment resulted not only in the significant prevention of the cocaine-induced CPP but also in blockade of the potentiation by DHEA (F(7,133) = 3.97; p < 0.001) (Fig. 3a). Similarly, BD1047 prevented the potentiating effect of pregnenolone (F(5,97) = 3.62; p < 0.01) (Fig. 3b). However, BD1047 administration failed to induce CPP alone and affected only the conditioned scores observed in cocaine-, DHEA-, or pregnenolone-treated groups (Fig.3a,b).
Fig. 3.
Involvement of the σ1 receptor in the modulation of cocaine-induced CPP by neuroactive steroids: blockade by the σ1 antagonist BD1047 of the potentiating effects of DHEA (a) or pregnenolone (b). BD1047 was administered intraperitoneally simultaneously with the steroid, given subcutaneously 10 min before cocaine, injected immediately before placement in the compartment during conditioning. Mice per group: n = 12–25 (a) and n = 12–26 (b). V, Vehicle. *p < 0.05 and **p < 0.01 versus the V+V-treated group;#p < 0.05 versus the cocaine+V-treated group.
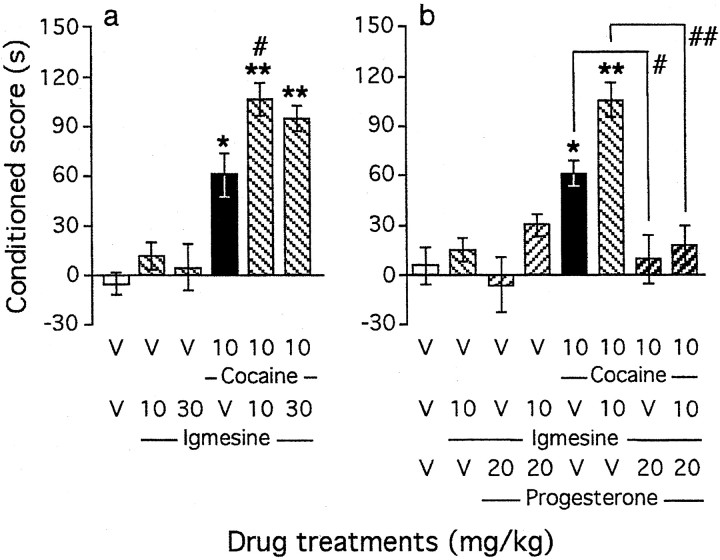
The selective σ1 agonist igmesine (10–30 mg/kg, i.p.) (Fig. 4a) failed to induce CPP alone in the 10–30 mg/kg dose range. However, pretreatment with the compound before cocaine (10 mg/kg, i.p.) significantly potentiated cocaine-induced CPP (F(5,92) = 5.48; p < 0.001) (Fig. 4a). At 10 mg/kg, igmesine significantly increased the cocaine-induced conditioned score (p < 0.05). We thus tested the antagonizing effect of progesterone (20 mg/kg, s.c.) on the facilitation of cocaine-induced CPP induced by igmesine (Fig. 4b). Progesterone not only significantly prevented cocaine-induced CPP but also blocked the potentiation by igmesine (F(7,125) = 2.83; p < 0.001) (Fig. 4b).
Fig. 4.
Involvement of the σ1 receptor in the modulation of cocaine-induced CPP by neuroactive steroids: coadministration of the σ1 agonist igmesine before cocaine (a) and blockade of its effect by progesterone (b). Progesterone was administered subcutaneously simultaneously with igmesine, given intraperitoneally 10 min before cocaine, injected immediately before placement in the compartment during conditioning. Mice per group: n= 11–19 (a) and n = 10–20 (b). V, Vehicle. *p < 0.05 and **p < 0.01 versus the V+V-treated group;#p < 0.05 and##p < 0.01 versus the cocaine+V-treated group.
Discussion
The present study provides evidence showing that neuroactive steroids, namely DHEA, pregnenolone, and progesterone, interfere with cocaine-induced reward in mice. None of the steroids tested alone induced CPP in mice, a behavioral response considered as a reliable index of drug-induced reward. However, pretreatment before cocaine significantly modulated the acquisition of cocaine-induced CPP. On the one hand, DHEA and its precursor pregnenenolone facilitated acquisition of cocaine-induced CPP. On the other hand, progesterone or its endogenous accumulation by finasteride inhibition of 5α-reductase activity blocked the cocaine-induced response.
These steroids, originating endogenously from either the adrenal or gonadal glands or the brain, are known to exert rapid neuromodulatory actions on nerve cells (Rupprecht and Holsboer, 1999). In particular, the sulfate ester of pregnenolone has been demonstrated to act as a positive allosteric modulator of the NMDA receptor and a negative allosteric modulator of the GABAA receptor (Majewska et al., 1988; Wu et al., 1991). DHEA sulfate also acts as a negative allosteric modulator of the GABAAreceptor (Majewska et al., 1990). On the contrary, progesterone potentiates GABA-induced chloride currents and attenuates some neuronal responses to NMDA (Wu et al., 1990; Smith, 1991). These effects through either GABAA or NMDA receptor modulation may contribute to the present observations. Indeed, mesolimbic DA neurons in the nucleus accumbens are under the control of inhibitory GABA neurons and excitatory glutamatergic neurons originating from the frontal cortex and hippocampus. For example, diazepam, a benzodiazepine acting as a positive GABAA receptor modulator, reduced DA release in the nucleus accumbens measured byin vivo microdialysis (Invernizzi et al., 1991) and as a consequence attenuated cocaine-induced CPP (Meririnne et al., 1999). In addition, presynaptic NMDA receptors facilitate central DA release (Ault et al., 1998). In turn, competitive or noncompetitive NMDA receptor antagonists decreased cocaine-induced locomotor stimulation and sensitization (Karler et al., 1989) as well as CPP (Cervo and Samanin, 1995). Because the respective pharmacological profile of DHEA, pregnenolone, and progesterone on GABAA or NMDA receptors is highly consistent, these interactions could be involved in their effects on cocaine-induced CPP. However, most of the GABAA or NMDA receptor modulators produced CPP or conditioned place aversion (CPA) by themselves. For instance, CPP was observed with the direct GABAA agonist meprobamate, the GABA metabolite γ-hydroxybutyric acid, and several benzodiazepine compounds, whereas benzodiazepine antagonists or inverse agonists produced CPA (for review, see Tzschentke, 1998). Notably, allopregnanolone, a neuroactive steroid devoid of affinity for the σ1 receptor but acting as a highly efficient GABAA receptor-positive modulator, induced CPP after exogenous administration (Finn et al., 1996). Whereas NMDA receptor competitive antagonists have also been reported to induce CPP, inconsistent results were reported with noncompetitive antagonists. As a particular example, dizocilpine has been described to induce either CPP or CPA (Tzschentke, 1998). In the present study, the neuroactive steroids failed to induce CPP or CPA alone, which seemed to indicate that at the dose range tested they did not efficiently or selectively interact with either GABAA or NMDA receptors.
The findings of the present study bring evidence that the σ1 receptor, another molecular target mediating some of the nongenomic effects of neuroactive steroids, is involved in steroidal effects on cocaine-induced reward. First, the involvement of the σ1 receptor in several cocaine-induced behavioral effects has been described previously (for review, seeMaurice et al., 2002). Selective σ1 antagonists blocked the locomotor stimulant effect of cocaine after acute administration (Menkel et al., 1991; Witkin et al., 1993; McCracken et al., 1999) and the development of behavioral sensitization after repeated cocaine injections and withdrawal (Ujike et al., 1996). Selective σ1 antagonists or antisense oligodeoxynucleotide probes targeting the σ1receptor also blocked acquisition or expression of cocaine-induced CPP in mice (Romieu et al., 2000, 2002). Second, a crossed pharmacology could be observed in the present study between neuroactive steroids and σ1 ligands on this behavioral response. Cocaine-induced CPP, and its stimulation by pretreatment with either DHEA or pregnenolone, could be fully blocked using the σ1 antagonist BD1047. Reciprocally, progesterone blocked not only cocaine-induced CPP but also its stimulation by the selective σ1 agonist igmesine. Such crossed pharmacology was previously observed on physiological responses involving the σ1receptor, such as the potentiation of several NMDA-evoked responsesin vitro or in vivo (Monnet et al., 1995;Bergeron et al., 1996). At the behavioral level, the antiamnesic effects induced by DHEA, pregnenolone, and their sulfate esters on several pharmacological models of amnesia involved an interaction with the σ1 receptor (Urani et al., 1998; Zou et al., 2000; Maurice et al., 2001). The antistress effects of neuroactive steroids in the conditioned fear stress or forced swim tests similarly involved the σ1 receptor (Noda et al., 2000;Urani et al., 2001). In these different behavioral consequences of the rapid nongenomic neuromodulation exerted by neuroactive steroids, a clear crossed pharmacology with σ1 receptors was described, suggesting a common mode of action and direct interaction (Maurice et al., 1999, 2001). Therefore, the present observations suggest that neuroactive steroids may influence cocaine-induced reward through their interaction with the σ1 receptor. Notably, both the σ1 antagonist BD1047 and progesterone completely blocked the potentiation of the cocaine-induced CPP by either igmesine or neuroactive steroids. As mentioned previously, neuroactive steroids may modulate dopaminergic neurons through their effects on glutamatergic or GABAergic systems, either at the presynaptic or postsynaptic level. However, activation of the σ1 receptor may be a common pathway, putatively downstream to these excitatory or inhibitory regulations, because its inhibition results in a complete blockade of the behavioral response.
At the physiological level, the role of peripheral steroid hormones, notably glucocorticoids, as endogenous modulators of the physiological response to natural reinforcers and drugs of abuse has been documented (Marinelli and Piazza, 2002). Other steroids and particularly centrally synthesized steroids (i.e., neurosteroids) were known to be important modulators of mood, depression, and affective disorders. Interestingly, a recent report by Barrot et al. (1999) showed that intracerebroventricular injection of pregnenolone sulfate increased DA efflux measured in the rat nucleus accumbens by in vivomicrodialysis and potentiated the morphine-induced release. Consequently, the authors suggested that this potentiation of mesolimbic DA concentration may suggest an involvement of neuroactive steroids in mood and motivation. The present observations brought behavioral evidence for such an effect by showing that neuroactive steroids could modulate responses to drug reward. DHEA, its precursor pregnenolone, and their sulfate esters may indeed serve as endogenous amplifiers of the rewarding effects of cocaine, whereas progesterone may act as an inhibitor/stabilizer. It should be noted that although no direct measure of brain steroid levels after cocaine has yet been performed, circulating levels of both DHEA and progesterone are affected by cocaine administration (Buydens-Branchey et al., 2002). Therefore, individual levels of these steroids and evolution of the DHEA/progesterone ratio after cocaine administration may putatively constitute a reliable marker of vulnerability. Additional studies must now be conducted to examine the involvement of DHEA and progesterone in other manifestations of cocaine addiction, notably self-administration, to validate their value as individual vulnerability markers.
Footnotes
This work was supported by Centre National de la Recherche Scientifique. We thank Dr. F. J. Roman for the gift of igmesine and Dr. R. R. Matsumoto (Oklahoma University, Oklahoma City, OK) for helpful discussions.
Correspondence should be addressed to Dr. Tangui Maurice, Centre National de la Recherche Scientifique Unité Mixte de Recherche 5102, University of Montpellier II, c.c. 090, place Eugène Bataillon, 34095 Montpellier cedex 5, France. E-mail:maurice@univ-montp2.fr.
References
- 1.Alonso G, Phan VL, Guillemain I, Saunier M, Legrand A, Anoal M, Maurice T. Immunocytochemical localization of the σ1 receptor in the adult rat central nervous system. Neuroscience. 2000;97:155–170. doi: 10.1016/s0306-4522(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Ault DT, Radeff JM, Werling LL. Modulation of [3H] dopamine release from rat nucleus accumbens by neuropeptide Y may involve a sigma1-like receptor. J Pharmacol Exp Ther. 1998;284:553–560. [PubMed] [Google Scholar]
- 3.Barrot M, Vallée M, Gingras MA, Le Moal M, Mayo W, Piazza PV. The neurosteroid pregnenolone sulphate increases dopamine release and the dopaminergic response to morphine in the rat nucleus accumbens. Eur J Neurosci. 1999;11:3757–3760. doi: 10.1046/j.1460-9568.1999.00816.x. [DOI] [PubMed] [Google Scholar]
- 4.Baulieu EE. Steroid hormones in the brain: several mechanisms? In: Fuxe K, Gustafson JA, Wettenberg L, editors. Steroid hormone regulation of the brain. Pergamon; Oxford: 1981. pp. 3–14. [Google Scholar]
- 5.Bergeron R, de Montigny C, Debonnel G. Potentiation of neuronal NMDA response induced by dehydroepiandrosterone and its suppression by progesterone: effects mediated via σ receptors. J Neurosci. 1996;16:1193–1202. doi: 10.1523/JNEUROSCI.16-03-01193.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Buydens-Branchey L, Branchey M, Hudson J, Majewska MD. Perturbations of plasma cortisol and DHEA-S following discontinuation of cocaine use in cocaine addict. Psychoneuroendocrinology. 2002;27:83–97. doi: 10.1016/s0306-4530(01)00037-3. [DOI] [PubMed] [Google Scholar]
- 7.Cervo L, Samanin R. Effects of dopaminergic and glutamatergic antagonists on the acquisition and expression of cocaine conditioning place preference. Brain Res. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- 8.De Costa BR, Radesca L, Di Paolo L, Bowen WD. Synthesis, characterization and biological evaluation of a novel class of N-(arylethyl)-N-alkyl-2-(1-pyrrolidinyl)ethylamines: structural requirements and binding affinity at the sigma receptor. J Med Chem. 1992;35:38–47. doi: 10.1021/jm00079a004. [DOI] [PubMed] [Google Scholar]
- 9.Finn DA, Phillips TJ, Okorn DM, Chester JA, Cunningham CL. Rewarding effect of the neuroactive steroid 3α-hydroxy-5α-pregnan-20-one in mice. Pharmacol Biochem Behav. 1996;56:261–264. doi: 10.1016/s0091-3057(96)00218-3. [DOI] [PubMed] [Google Scholar]
- 10.Gasior M, Carter RB, Goldberg SR, Witkin JM. Anticonvulsant and behavioral effects of neuroactive steroids alone and in conjunction with diazepam. J Pharmacol Exp Ther. 1997;282:543–553. [PubMed] [Google Scholar]
- 11.Gonzalez-Alvear GM, Werling LL. Regulation of [3H]dopamine release from rat striatal slices by sigma ligands. J Pharmacol Exp Ther. 1994;271:212–219. [PubMed] [Google Scholar]
- 12.Hayashi T, Maurice T, Su TP. Ca2+ signaling via sigma1-receptors: novel regulatory mechanism affecting intracellular Ca2+ concentration. J Pharmacol Exp Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- 13.Invernizzi R, Pozzi L, Samanin R. Release of dopamine is reduced more in the nucleus accumbens than in the caudate nucleus in conscious rats. Neuropharmacology. 1991;30:575–578. doi: 10.1016/0028-3908(91)90075-m. [DOI] [PubMed] [Google Scholar]
- 14.Karler R, Calder LD, Chaudhry IA, Turkanis SA. Blockade of “reverse tolerance” to cocaine and amphetamine by MK-801. Life Sci. 1989;45:599–606. doi: 10.1016/0024-3205(89)90045-3. [DOI] [PubMed] [Google Scholar]
- 15.Koob GF, Nestler EJ. The neurobiology of drug addiction. J Neuropsychiatry Clin Neurosci. 1997;9:482–497. doi: 10.1176/jnp.9.3.482. [DOI] [PubMed] [Google Scholar]
- 16.Majewska MD, Mienville JM, Vicini S. Neurosteroid pregnenolone sulfate antagonizes electrophysiological responses to GABA in neurons. Neurosci Lett. 1988;90:279–284. doi: 10.1016/0304-3940(88)90202-9. [DOI] [PubMed] [Google Scholar]
- 17.Majewska MD, Demirgören S, Spivak CE, London ED. The neurosteroid dehydroepiandrosterone sulfate is an allosteric antagonist of the GABAA receptor. Brain Res. 1990;526:143–146. doi: 10.1016/0006-8993(90)90261-9. [DOI] [PubMed] [Google Scholar]
- 18.Marinelli M, Piazza PV. Interaction between glucocorticoid hormones, stress and psychostimulant drugs. Eur J Neurosci. 2002;16:387–394. doi: 10.1046/j.1460-9568.2002.02089.x. [DOI] [PubMed] [Google Scholar]
- 19.Matsumoto RR, McCracken KA, Pouw B, Miller J, Bowen WD, Williams W, De Costa BR. N-alkyl substituted analogs of the σ receptor ligand BD1008 and traditional sigma receptor ligands affect cocaine-induced convulsions and lethality in mice. Eur J Pharmacol. 2001;411:261–273. doi: 10.1016/s0014-2999(00)00917-1. [DOI] [PubMed] [Google Scholar]
- 20.Maurice T, Roman FJ, Privat A. Modulation by neurosteroids of the in vivo (+)-[3H]SKF-10,047 binding to σ1 receptors in the mouse forebrain. J Neurosci Res. 1996;46:734–743. doi: 10.1002/(SICI)1097-4547(19961215)46:6<734::AID-JNR10>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 21.Maurice T, Phan VL, Urani A, Kamei H, Noda Y, Nabeshima T. Neuroactive neurosteroids as endogenous effector for the sigma1 (σ1) receptor: pharmacological evidences and therapeutic opportunities. Jpn J Pharmacol. 1999;81:125–155. doi: 10.1254/jjp.81.125. [DOI] [PubMed] [Google Scholar]
- 22.Maurice T, Urani A, Phan VL, Romieu P. The interaction between neuroactive steroids and the sigma1 (σ1) receptor function: behavioral consequences and therapeutic opportunities. Brain Res Brain Res Rev. 2001;37:116–132. doi: 10.1016/s0165-0173(01)00112-6. [DOI] [PubMed] [Google Scholar]
- 23.Maurice T, Martin-Fardon R, Romieu P, Matsumoto RR. Selective sigma1 (σ1) receptor antagonists as a new promising strategy to prevent cocaine-induced behaviors and toxicity. Neurosci Biobehav Res. 2002;26:499–527. doi: 10.1016/s0149-7634(02)00017-9. [DOI] [PubMed] [Google Scholar]
- 24.McCracken KA, Bowen WD, Matsumoto RR. Novel σ receptor ligands attenuate the locomotor stimulatory effects of cocaine. Eur J Pharmacol. 1999;365:35–38. doi: 10.1016/s0014-2999(98)00876-0. [DOI] [PubMed] [Google Scholar]
- 25.Menkel M, Terry M, Pontecorvo M, Katz JL, Witkin JM. Selective σ ligands block stimulant effects of cocaine. Eur J Pharmacol. 1991;201:251–252. doi: 10.1016/0014-2999(91)90355-t. [DOI] [PubMed] [Google Scholar]
- 26.Meririnne E, Kankaanpää A, Lillsunde P, Seppälä T. The effect of diazepam and zolpidem on cocaine- and amphetamine-induced place preference. Pharmacol Biochem Behav. 1999;62:159–164. doi: 10.1016/s0091-3057(98)00139-7. [DOI] [PubMed] [Google Scholar]
- 27.Moldow RM, Fischman AJ. Cocaine induced secretion of ACTH, β-endorphin, and corticosterone. Peptides. 1987;8:819–822. doi: 10.1016/0196-9781(87)90065-9. [DOI] [PubMed] [Google Scholar]
- 28.Monnet FP, Blier P, Debonnel G, de Montigny C. Modulation by sigma ligands of N-methyl-d-aspartate-induced [3H]noradrenaline release in the rat hippocampus: G-protein dependency. Naunyn Schmiedebergs Arch Pharmacol. 1992;346:32–39. doi: 10.1007/BF00167567. [DOI] [PubMed] [Google Scholar]
- 29.Monnet FP, Mahé V, Robel P, Baulieu EE. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-d-aspartate in the rat hippocampus. Proc Natl Acad Sci USA. 1995;92:3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Noda Y, Kamei H, Kamei Y, Nagai T, Nishida M, Nabeshima T. Neurosteroids ameliorate conditioned fear stress: an association with sigma receptors. Neuropsychopharmacology. 2000;23:276–284. doi: 10.1016/S0893-133X(00)00103-2. [DOI] [PubMed] [Google Scholar]
- 31.Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237:1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- 32.Romieu P, Martin-Fardon R, Maurice T. Involvement of the σ1 receptor in the cocaine-induced conditioned place preference. NeuroReport. 2000;11:2885–2888. doi: 10.1097/00001756-200009110-00011. [DOI] [PubMed] [Google Scholar]
- 33.Romieu P, Phan VL, Martin-Fardon R, Maurice T. The sigma1 receptor involvement in cocaine-induced conditioned place preference is consequent upon dopamine uptake blockade. Neuropsychopharmacology. 2002;26:444–455. doi: 10.1016/S0893-133X(01)00391-8. [DOI] [PubMed] [Google Scholar]
- 34.Rupprecht R, Holsboer F. Neuroactive steroids: mechanism of action and neuropsychopharmacological perspectives. Trends Neurosci. 1999;22:410–416. doi: 10.1016/s0166-2236(99)01399-5. [DOI] [PubMed] [Google Scholar]
- 35.Sarnyai Z, Biro E, Penke B, Telegdy G. The cocaine-induced elevation of plasma corticosterone is mediated by endogenous corticotropin-releasing factor (CRF) in rats. Brain Res. 1992;589:154–156. doi: 10.1016/0006-8993(92)91176-f. [DOI] [PubMed] [Google Scholar]
- 36.Schumacher M, McEwen BS. Steroid and barbiturate modulation of the GABAA receptor. Mol Neurobiol. 1989;3:275–280. doi: 10.1007/BF02740608. [DOI] [PubMed] [Google Scholar]
- 37.Smith SS. Progesterone administration attenuates excitatory amino acid responses of cerebellar Purkinje cells. Neuroscience. 1991;42:309–320. doi: 10.1016/0306-4522(91)90377-z. [DOI] [PubMed] [Google Scholar]
- 38.Su TP, London ED, Jaffe JH. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988;240:219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- 39.Trapani G, Dazzi L, Pisu MG, Reho A, Seu E, Biggio G. A rapid method for obtaining finasteride, a 5α-reductase inhibitor, from commercial tablets. Brain Res Brain Res Protoc. 2002;9:130–134. doi: 10.1016/s1385-299x(02)00146-0. [DOI] [PubMed] [Google Scholar]
- 40.Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drugs effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- 41.Ujike H, Kuroda S, Otsuki S. σ Receptor antagonists block the development of sensitization to cocaine. Eur J Pharmacol. 1996;296:123–128. doi: 10.1016/0014-2999(95)00693-1. [DOI] [PubMed] [Google Scholar]
- 42.Urani A, Privat A, Maurice T. The modulation by neurosteroids of the scopolamine-induced learning impairment in mice involves an interaction with sigma1 (σ1) receptors. Brain Res. 1998;799:64–77. doi: 10.1016/s0006-8993(98)00469-7. [DOI] [PubMed] [Google Scholar]
- 43.Urani A, Roman FJ, Phan VLP, Su TP, Maurice T. The antidepressant-like effect induced by sigma1 (σ1) receptor agonists and neuroactive steroids in mice submitted to the forced swimming test. J Pharmacol Exp Ther. 2001;298:1269–1279. [PubMed] [Google Scholar]
- 44.Witkin JM, Terry M, Menkel M, Hickey P, Pontecorvo M, Ferkany J, Katz JL. Effects of the selective sigma receptor ligand 6-[6-(4-hydroxypiperidinyl)hexyloxy]-3-methylflavone (NPC 16377), on behavioral and toxic effects of cocaine. J Pharmacol Exp Ther. 1993;266:473–482. [PubMed] [Google Scholar]
- 45.Wu FS, Gibbs TT, Farb DH. Inverse modulation of γ-aminobutyric acid- and glycine-induced currents by progesterone. Mol Pharmacol. 1990;37:597–602. [PubMed] [Google Scholar]
- 46.Wu FS, Gibbs TT, Farb DH. Pregnenolone sulfate: a positive allosteric modulator at the N-methyl-d-aspartate receptor. Mol Pharmacol. 1991;40:333–336. [PubMed] [Google Scholar]
- 47.Zou LB, Yamada K, Sasa M, Nakata Y, Nabeshima T. Effect of sigma1 receptor agonist SA4503 and neuroactive steroids on performance in a radial arm maze task in rats. Neuropharmacology. 2000;39:1617–1627. doi: 10.1016/s0028-3908(99)00228-2. [DOI] [PubMed] [Google Scholar]