Abstract
Subjects were trained on a pursuit task in which the target trajectory was predictable only on the horizontal axis. Half of them were sleep deprived on the first post-training night (n = 13). Three days later, functional magnetic resonance imaging revealed task-related increases in brain responses to the learned trajectory, as compared with a new trajectory. In the sleeping group (n = 12) as compared with the sleep-deprived group, subjects' performance was improved, and their brain activity was greater in the superior temporal sulcus (STS). Increased functional connectivity was observed between the STS and the cerebellum and between the supplementary eye field and the frontal eye field. These differences indicate sleep-related plastic changes during motor skill learning in areas involved in smooth pursuit eye movements.
Keywords: functional neuroimaging, functional magnetic resonance imaging, statistical parametric mapping, functional connectivity, procedural memory, memory consolidation, sleep, sleep deprivation, smooth pursuit eye movements
Introduction
Several lines of evidence indicate that sleep is involved in memory trace consolidation. First, sleep organization can be modified by recent learning both in animals (Hennevin et al., 1995) and in humans (Maquet, 2001). Second, neurons involved in recent waking experience are reactivated during post-training sleep in rodent hippocampus (Pavlides and Winson, 1989;Wilson and McNaughton, 1994; Kudrimoti et al., 1999; Nadasdy et al., 1999; Louie and Wilson, 2001) and in human cortex (Maquet et al., 2000). Third, sleep deprivation alters subsequent performance on the learned task in animals (Hennevin et al., 1995; Smith, 1995) and in humans (Maquet, 2001). Sleep deprivation studies suggest that sleep occurring during the first hours after training sessions in animals (Hennevin et al., 1995; Smith, 1995) or during the first post-training night in man (Stickgold et al., 2000) plays a critical role in memory trace consolidation, as measured by behavioral performance at a later date.
In several perceptual and motor skill learning tasks, performance continues to improve hours after the training session has ended (Karni and Sagi, 1993; Karni and Bertini, 1997; Karni et al., 1998). This so-called “slow learning” is believed to lead to the consolidation of the memory trace and to be sleep dependent (Maquet, 2001). Accordingly, the learning of the pursuit rotor task, a visuomotor procedural learning task, is known to be sensitive to sleep deprivation on the first post-training night (Smith and MacNeill, 1994).
The effects of sleep on the cerebral correlates of skill learning has not yet been characterized in humans. The aim of the present study was to compare learning-dependent changes in regional brain activity after sleep or sleep deprivation using a pursuit task (PT). We trained the participants on a particular version of the PT (Frith, 1973) in which the target trajectory was predictable on the horizontal but not on the vertical axis (see Fig. 1A). Half of the subjects were totally sleep deprived during the first post-training night (see Fig. 1B). Three days later, during a functional magnetic resonance imaging (fMRI) scanning session, they were exposed to the previously learned trajectory and also to a new one in which the predictable axis was vertical. This experimental design allowed for the assessment of the effects of learning on brain activity, using within-subject comparisons between learned and new conditions.
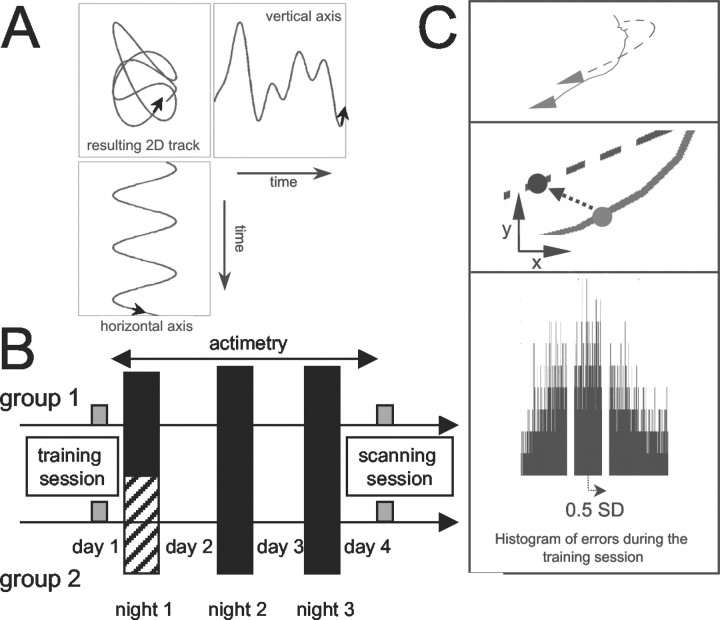
Fig. 1.
Experimental design. A, The bi-dimensional trajectory followed by the target during the training session combined a regular movement on the horizontal axis and an irregular movement on the vertical axis. B, Two experimental groups were compared: half of the subjects were totally sleep deprived during the first night after training on the PT and half were allowed to sleep normally. All subjects continuously wore an actimeter and were scanned while doing the PT on the third day after training (see Materials and Methods). C, Computation of the behavioral performance at the PT. Continuous line indicates the joystick trajectory; dotted line indicates the target trajectory. At each time point (40 msec), the distance between the target and the subject's trajectory was computed. The subject was considered on target if this distance (arrow) was smaller than half the SD of the joystick-to-target distances observed for the subject during the training session. The bottom display shows a typical distribution of the joystick-to-target distances for one subject.
Our objective was to provide evidence that sleep deprivation disrupts the slow processes that lead to memory consolidation. In contrast to others (Drummond et al., 2000), we were not aiming to characterize the immediate effect of sleep deprivation on human performance or cognition. This is the reason why we adopted an experimental protocol in which both sleeping and sleep-deprived subjects were retested after at least two complete nights of sleep, i.e., in a state of arousal that was similar across the two groups and between the training and retest sessions (Stickgold et al., 2000).
Materials and Methods
Subjects. Normal subjects (13 females, 12 males; age range: 19–24 years) were recruited by advertisement. They had no history of medical, neurological, or psychiatric disease. None of them was on medication. The quality of their usual sleep was assessed by the Pittsburgh Sleep Quality Index questionnaire (Buysse et al., 1989) to check for the absence of obvious disturbances of sleep/wakefulness cycles. The subjects were right-handed as indicated by the Edinburgh Inventory (Oldfield, 1971). The subjects gave their written informed consent to the study, which was approved by the Joint Ethics Committee of National Hospitals and Institute of Neurology.
Experimental protocol. Subjects performed the PT while lying in the scanner (see Fig. 1). A mirror box allowed them to view the display (18 × 23°) generated by a PC (480 × 640 resolution; refresh rate 60 Hz) and projected by liquid crystal display projector. Subjects were simultaneously shown the positions of a moving target (red circle, 1°) and of a joystick (yellow dot, 0.5°; refresh rate 25 Hz). By manipulating a custom-made joystick with their left hand, the subjects could move the position of the joystick on the screen. The instruction was to maintain the joystick position as close as possible to the moving target at all times. The left hand was chosen to ensure that performance on the PT would not rely on preexisting motor skills such as writing or drawing and to minimize interference with normal daytime activity during the post-training period (because the subjects were all right-handed). The subjects did not know that the trajectory followed by the target (Fig.1A) was manipulated in a similar way as in Frith (1973). The coordinates of the target were described by a single sine wave (frequency: 0.423 Hz) along the horizontal axis, and by the sum of four nonharmonic sine waves (frequency: 0.267, 0.341, 0.413, and 0.673 Hz) on the vertical axis. As a result, the trajectory followed by the target was easily predictable along the horizontal axis but very difficult to predict along the vertical axis. This trajectory was used to train the subjects and will be referred to as the “learned trajectory.”
The subjects were trained on the task in the scanner on the afternoon of day 1, during a period of 5 min, between 2 and 6 P.M. (Fig.1B). The subjects were not scanned during the training session. They were trained in the MR scanner to ensure that the task would be performed under the same conditions during the training and retest sessions, i.e., with the same physical characteristics for the presentation of visual inputs and same position for motor performance. The training session was deliberately kept short. The subjects developed only an imperfect skill on the task. In such a situation, learning and memory are more likely to depend on sleep processes (Hennevin et al., 1995).
The subjects were only scanned on day 4, at the same time of day as during the training session. During this scanning session, 30 18-sec-long blocks of PT were performed. Half of the blocks used the learned trajectory. In the remaining blocks, the trajectory was rotated by 90°, in such a way that the predictable axis became the vertical one. Because the subjects had never been exposed to it, this trajectory is referred to as the “new trajectory. The order of the learned and new trajectories was randomized over subjects. Periods of fixation, also 18 sec long, were interleaved between the PT blocks. The coordinates of the target and the joystick were recorded every 40 msec, during both the training and the scanning sessions (see below). Functional MRI time-series were acquired at 2 Tesla using a Magnetom VISION (Siemens, Erlangen, Germany) whole-body MRI system, equipped with a head volume coil. Multislice T2*-weighted fMRI images were obtained with a gradient echo-planar sequence using an axial slice orientation (echo time = 40 msec; repetition time = 3.65 sec; 64 × 64 × 48 voxels; voxel size: 3 × 3 × 3 mm3). After the six initial scans were discarded (to allow for magnetic saturation effects), each time-series comprised 300 volume images. A structural T1-weighted sequence scan was also obtained. The eye position was monitored on-line using an eye-trajectory system (ASL, Model 504; Applied Science Group, Bedford, MA).
The subjects were prospectively pseudorandomized into two groups (Fig.1B). In the first group (sleeping group), the subjects went home after the training session and slept as usual during the three post-training nights. In the second group (sleep-deprived group), the subjects stayed awake in the laboratory and were monitored during the first post-training night (until 7.00 A.M.). During this night, the ambient light and the subjects' physical activity were maintained as low as possible, and the subjects remained under the constant supervision of the experimenters. They pursued their usual activities on the following days and slept at home during the two remaining nights. After a single night of total sleep deprivation, individual performance on several tasks and subjective sleepiness are completely restored after two nights of recovery sleep (Bonnet, 2000).
The physical activity of all the subjects was monitored continuously by actimetry, from the end of the training session to the beginning of the scanning session (sampling rate: 1/30 Hz) (Actiwatch, Cambridge Technology). Subjects wore the actimeter on their right wrist and were also asked to fill in a sleep log during the entire experimental period.
All of the subjects were randomly assigned to each experimental group and exposed to the same task characteristics during the training session. Thus, no difference in the improvement in performance along time was expected between the two groups unless the sleep deprivation had a significant and deleterious effect on the acquisition of this visuomotor skill.
Analysis of behavioral data. First, the subject's error was computed as the Euclidean distance between the target and the joystick location for each time point of the training session, and the SD was computed (Fig. 1C). For the scanning session, the same measures were computed at each time point during the PT blocks. The time on target was used as the metric of subjects' performance. For each subject, it was computed as the number of time points (each 40 msec long) during which the distance was smaller than half the SD computed during the training session. This method ensured that the same metric was used to compute the performance in the training and scanning session on an individual basis. Summing these points within each PT block provided a measure of the time on target achieved during this block.
For the training session, the 5 min performance data were divided into 19 blocks of equal duration. These behavioral data were modeled by a general linear model with repeated measures, using the repetition of consecutive blocks as within-subject factor and the group (sleep vs sleep deprived) as between-subject factor. For the scanning session, the data were modeled by a general linear model with repeated measures, using the repetition of consecutive blocks and the trajectory (learned vs new) as within-subject factors and the group (sleep vs sleep deprived) as between-subject factor. Post hoc ttests were computed for differences between the groups or between trajectories.
The actimetry data were integrated over post-training periods of day (D2, D3) and night (N1, N2, N3), defined by the time-to-bed and wakeup times indicated in the individual sleep logs. The D4 data were not considered in the analyses because they usually spanned only a few hours (from wake up to the scanning session). Data were modeled by a general linear model with repeated measures, using consecutive night and day periods as within subject factor and the group (sleep versus sleep deprived) as between subject factor. Post hoc t tests checked for differences between the group for each relevant time period.
Analysis of fMRI data. Functional volumes were analyzed using Statistical Parametric Mapping http://www.fil.ion.ucl.ac.uk/spm/. They were corrected for head motion, spatially normalized to an echo planar imaging template of 3 × 3 × 3 mm3 voxels conforming to the Montreal Neurological Institute space, spatially smoothed with a Gaussian kernel of 8 mm full-width at half-maximum (FWHM), and high-pass filtered (1/140 Hz).
For each subject, changes in brain regional responses were estimated by a general linear model in which the activity evoked in the PT blocks with learned or new trajectory was modeled by boxcar waveforms convolved with a canonical hemodynamic response function. Movement parameters derived from realignment of the functional volumes were included as covariates of no interest. The effects of interest were then tested by linear contrasts, generating statistical parametric maps [SPM(T)]. The images resulting from the comparison between learned and new conditions were then further spatially smoothed (6 mm FWHM Gaussian kernel) and entered in a second-level analysis, corresponding to a random effects model, to account for intersubject variance in the main effect of learning. Two analyses were performed. First, parameter estimates for the learned and new conditions were compared in a one-sample t test across all subjects to describe the main effect of learning regardless of the group. Second, a two-samplet test was used to evaluate the trajectory-by-group interaction.
On the basis of published work on motion perception, smooth eye pursuit, eye–hand coordination, and motor learning, we expected that changes in brain responses would occur in areas that participate in performing the task: motion-related areas in the occipital and temporal cortices, intraparietal sulcus, premotor cortex [including frontal eye field (FEF)], supplementary motor area [including supplementary eye field (SEF)], primary motor cortex, and cerebellum. Small-volume correction of our fMRI results (Worsley, 1996) was computed on a 10 mm sphere around the average coordinates published for the corresponding relevant a priori location (Table 1, last column).
Table 1.
Functional MRI results
To examine whether sleep deprivation alters long-term functional connectivity, analyses of psychophysiological interactions were performed. These analyses searched for a modulation by the training condition of correlations between the learning-related areas (see below) [right dentate nucleus (DN); left supplementary motor area (SMA)] and other distant areas (Friston et al., 1997). A new linear model was constructed for each subject, using three regressors (plus the covariates of no interest as in the initial model). One regressor was the difference between the two main regressors of interest (learned minus new). The second regressor was the activity in the reference area. The third regressor represented the interaction of interest between the first (psychological) and second (physiological) regressors. Significant contrasts for this psychophysiological regressor indicated a learning-related change in the regression coefficients between any reported brain area and the reference region. After smoothing (6 mm FWHM Gaussian kernel), these contrast images were then entered into a second-level (random effects) analysis. A two-sample t test was performed to assess the between-group differences in learning-dependent changes in functional connectivity (voxelwise threshold, p < 0.001 uncorrected; small-volume correction at p < 0.05).
Results
Behavioral data
Two subjects were discarded: one in the sleeping group because of task-related movement artifacts in the fMRI time-series and another in the sleep-deprived group because the subject's sleep was compromised on nights 2 and 3 for professional reasons. The final number of subjects in the sleeping and sleep-deprived groups was 11 and 12, respectively. At debriefing, none of them was aware of the different spatial properties of the learned and new trajectories.
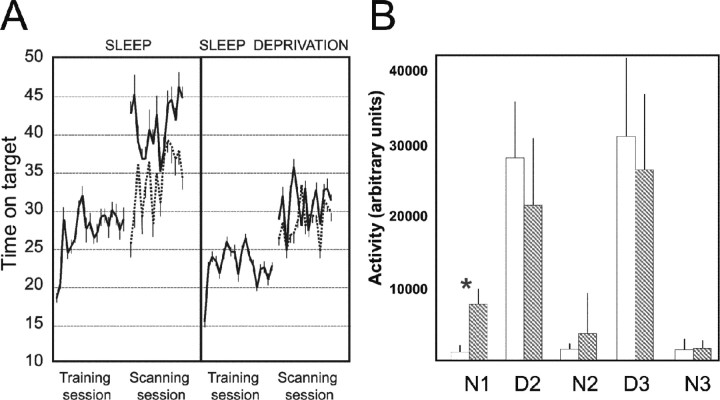
The behavioral results appear in Figure2A. The statistical analyses were run separately for the training and the scanning sessions. This is because the learning effect could be assessed within the scanning session, by comparison of the subjects' performance on the learned versus new trajectory. This analysis of behavioral data shadows the analysis of fMRI data, essentially based on the within-session learning effect during the scanning session (see below). For the training session, there was a significant effect of the repetition of training blocks (F(18) = 1.659; p = 0.044), reflecting the improvement of subjects' performance with time. There was also a significant effect of the group (sleep vs sleep deprived) (F(1) = 4.669; p = 0.041). Post hoc t tests confirmed that the performance of the sleep-deprived subjects was lower than that of the subjects in the sleeping group (p < 0.001). The repetition by group interaction was not significant (F(18,1) = 0.518; p = 0.950), suggesting that the rate of learning during the training session was not different between the two groups. For the post-training session during fMRI, the effect of the trajectory (learned vs new) was significant (F(1) = 18.603;p < 0.001). Post hoc paired ttests showed that the effect of trajectory (learned vs new) was significant in both the sleep group (p < 0.001) and the sleep-deprived group (p = 0.015). Most importantly, the trajectory-by-group interaction was also significant (F(1,21) = 4.862; p = 0.038), indicating that the sleeping subjects were significantly better on the trained than the new trajectory in comparison with the sleep-deprived group. The repetition (of the blocks) by group interaction showed a nonsignificant trend (F(14,9) = 2.778; p = 0.055). No other interactions were significant. The group effect was not significant (F(1) = 2.21;p = 0.151), suggesting that the sleep-deprived subjects were as good as the sleeping subjects (regardless of the status of the trajectory).
Fig. 2.
Behavioral data. A, Time on target (arbitrary units) during the training and scanning sessions, in the sleeping and sleep-deprived group, for the learned (continuous line) and new (dotted line) trajectories. Mean time on targets is shown for successive 15 sec blocks; error bars represent SEM. Units are the number of 40 msec intervals spent on the target. B, Average movement activity measured by actimetry in the sleep (white bars) and sleep deprived (hatched bars) during the post-training period (N1–N3). The activity was significantly higher during the first post-training night (N1) in the sleep-deprived group (*p < 0.01). No difference was noted on the following days (D2, D3) and nights (N2, N3). Error bars represent SEM.
The difference in performance during the training session is unlikely to confound our results. First, because of the pseudorandomization of the subjects and because the trajectories had the same features in all subjects, differences in performance during the training session could not be attributable to either a systematic population bias or a variation in task difficulty. Second, the learning of the pursuit task is a robust and replicable phenomenon (Eysenck and Frith, 1977). Consequently, no ceiling effect is expected with the pursuit task, even in the sleep-deprived subjects. Third and most importantly, the study was designed in such a way that the learning effect could be assessed by within-session effect. The critical contrast is the difference in performance between the learned and the new trajectory during the scanning session itself, regardless of the average value of performance. In our case, performances during the scanning session were matched between groups.
Actimetric data are shown on Figure 2B. The analysis showed a significant overall variation of activity across days and nights (F(4) = 125; p< 0.001) and a significant activity by group interaction (F(4,18) = 5.143; p = 0.001). Post hoc t tests comparing the two groups showed a significant increase in activity during the first night in the sleep-deprived subjects (p < 0.001), confirming the efficacy of the experimental treatment. The activity during the second day tended to be lower in the sleep-deprived group, although the difference was not significant (p = 0.071). No other comparison approached significance.
Functional MRI data
The results are summarized in Table 1.
Main effect of learning
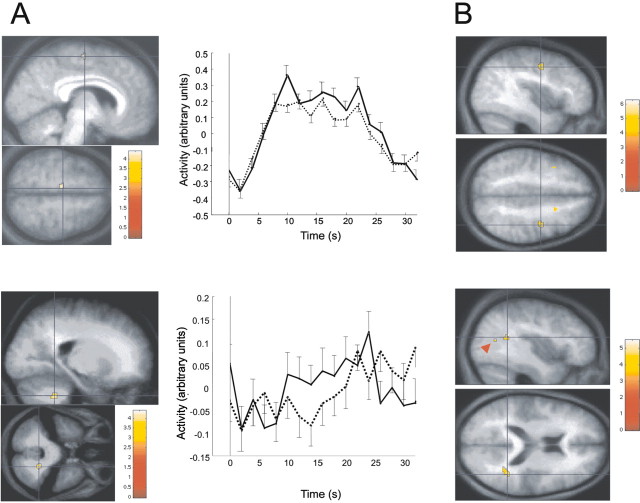
The responses to the learned trajectory were significantly larger than to the new trajectory in three regions, regardless of the group: the lateral nuclei of the cerebellum (hereafter referred to as DN), a left medial frontal area, and the right cuneus (Fig.3A). The latter did not survive small-volume correction, using the coordinates of the nearest motion-related area described in the literature (V3a; see references in Table 1). It will not be discussed further.
Fig. 3.
fMRI data. A, Main effect of learning. The first column shows the activation foci (SEF/SMA on the top panel; DN on the bottom panel), superimposed on the average normalized structural MR image of the group. The second columnshows the peristimulus time course of the response in the corresponding area (continuous line, responses to the learned trajectory; dotted line, for the new trajectory). Error bars represent SEM across subjects. B, Results of the second-level analysis based on psychophysiological interactions. On thetop and bottom panels, brain areas are connected with the SEF/SMA and DN, respectively, more tightly for learned than new trajectories, and more so in sleeping subjects than in the sleep-deprived group. The red arrowhead shows the second area detected in the STS. Displays are thresholded atp < 0.001 and coded according to the corresponding color scale.
The location of the DN activation was confirmed in reference to the cerebellar atlas of Schmahmann et al. (2000). The effect of learning on DN was contralateral to the moving hand. Contralateral cerebellar activations have already been reported in other learning situations, for instance in eyeblink conditioning (Logan and Grafton, 1995;Blaxton et al., 1996; Ramnani et al., 2000) and rhythm learning (Ramnani et al., 2000).
The left medial frontal area lies within the SMA (see references in Table 1) at a level identified as the supplementary eye field (SEF) (see references in Table 1). A medial prefrontal response ipsilateral to the used hand is not unexpected. There are extensive interhemispheric connections between homologous supplementary motor areas (McGuire et al., 1991). Moreover, it could also be the case, as for the left premotor cortex (Schluter et al., 1998, 2001), that the left SMA controls both hands and is dominant for action.
Trajectory-by-group interaction
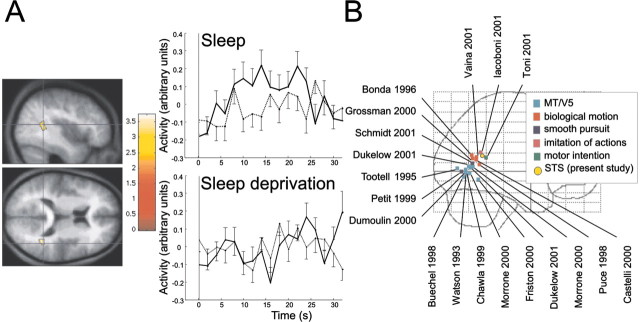
The responses in the depth of the posterior superior temporal sulcus (STS) to the learned trajectory were significantly larger in the sleep group than in the sleep-deprived group (Fig.4A). In other words, the posterior STS responded more to the learned trajectory than to the new one if the subjects were allowed to sleep on the first post-training night.
Fig. 4.
Trajectory by group interaction. A,Left panel, The superior temporal sulcus is significantly more active in the learned condition in sleeping subjects. The statistical results, displayed at p< 0.001, are superimposed on the average normalized structural MR image of the group. Right panel, Peristimulus time courses of STS response (continuous line, responses to the learned trajectory; dotted line, for the new trajectory; top row, sleep group; bottom row, sleep deprivation group). Error bars represent SEM across subjects. B, Lateral view of a glass brain in the MNI space, showing the projections of the reported STS, as well as MT/V5, biological motion, and related areas discussed in relation to STS. Sources of the data displayed are indicated by first author and year of publication.
Psychophysiological interactions
A psychophysiological analysis using the DN as reference region identified two areas in the STS area, a few millimeters away from the area detected in the trajectory-by-group interaction. This result indicates that these two areas are connected more tightly with the DN in the context of the learned than the new trajectory, and more so in sleeping subjects than in the sleep-deprived group. By applying a small-volume correction, both were included in the same sphere around the reference coordinates (Fig. 3B, bottom panel), but only one peak survived thep < 0.05 threshold.
The left SEF/SMA was more tightly correlated with the right premotor cortex in the sleeping than in the sleep-deprived subjects in response to the learned trajectory (Fig. 3B, top panel). The area within the premotor cortex corresponds to the frontal eye field (FEF) (see references in Table 1). The activation lay in the depth of the precentral sulcus, in keeping with the location of the pursuit area reported by Rosano et al. (2002).
Discussion
The present data reveal two important aspects of the cerebral correlates of PT learning. First, they extend previous positron emission tomography (PET) results obtained on the standard version of the task (pursuit rotor task using a circular trajectory). In our particular case, because of intrinsic properties of the target path, an optimal performance could only be achieved by developing implicitly some model of the motion characteristics of the learned trajectory. Furthermore, the pattern of brain responses observed here suggests that in learning the task, the acquisition of appropriate ocular responses is probably more critical than the development of new motor sequences for the hand or to the improvement of eye–hand coordination. Indeed, interactions between temporal cortex and the cerebellum as well as between the FEF and the SEF are both implicated in conventional pursuit eye movement pathways (Krauzlis and Stone, 1999).
Second, our results suggest that lack of sleep may hamper the consolidation of recent memory traces, with detrimental effects on later performance. In contrast, in the subjects allowed to sleep, further processing of the memory traces is permitted during the first post-training night. Consequently, their performance improves and significant changes in patterns of regional brain activity are revealed by functional neuroimaging.
Effect of learning
The performance on the learned trajectory was significantly better than on the new trajectory in both the sleeping and the sleep-deprived groups. The cerebral hemodynamic responses to the learned trajectory were significantly larger than to the new trajectory in a medial prefrontal area and the right DN.
The medial prefrontal area is probably the SEF. This would be consistent with the psychophysiological interaction showing that this area is functionally connected with the right FEF, a region involved in controlling eye movements. In nonhuman primates, neuronal activity in the SEF is related to smooth pursuit eye movements, especially when the target motion is predictable (Heinen and Liu, 1997). Electrical stimulation of this region modulates smooth pursuit eye movements (Tian and Lynch, 1995, 1996; Missal and Heinen, 2001). Alternatively, the medial prefrontal region could correspond to the part of SMA that is involved in hand action. In humans, an early PET study has shown that SMA activity correlates with the time on target during a pursuit rotor task (Grafton et al., 1994).
The increase in cerebellar signal is located in the DN. The DN has been involved in tracking tasks (Brooks et al., 1973; Vercher and Gauthier, 1988) and in the control of visually guided movements (Mushiake and Strick, 1993). Functional neuroimaging studies have described both decreases and increases in cerebellar activity in response to learning processes (Jenkins et al., 1994; Flament et al., 1996; Imamizu et al., 2000). Recent evidence shows that the cerebellar hemispheres tend to respond more with high movement errors, whereas a larger dentate activation is observed when tracking performance is good (Miall et al., 2001). The same observation is reported for other visually guided motor tasks (Nezafat et al., 2001; Doyon et al., 2002).
Effect of sleep on experience-dependent brain activation
The posterior STS was found to be the only region differentially more active for the learned trajectory in sleeping subjects than in the context of sleep deprivation. The posterior STS (Fig.4B) lies anterior to other motion-responsive areas, especially the middle temporal area (MT/V5) (Watson et al., 1993;Tootell et al., 1995; Dumoulin et al., 2000). Its functional role is not yet characterized precisely. It responds to biological motion (Bonda et al., 1996; Puce et al., 1998; Grossman et al., 2000; Grezes et al., 2001; Vaina et al., 2001) and to movement patterns of interacting geometrical shapes (Castelli et al., 2000).
The observed posterior STS is also close to regions of the temporal lobe that are active during smooth pursuit eye movements in humans (Petit and Haxby, 1999; Schmid et al., 2001) (Fig.4B). In nonhuman primates, stimulations (Komatsu and Wurtz, 1989) and lesions (Newsome et al., 1985; Dursteler and Wurtz, 1988) of the superior temporal sulcus [area MT and medial superior temporal (MST) area] affect saccades and pursuit eye movements.
The observed posterior superior temporal cortex is slightly anterior to the areas reported for biological motion or smooth eye movements (Fig.4B). It is even closer to the STS activation reported during imitation of action (Iacoboni et al., 2001). Imitation would require matching an observed action to an internal motor representation and using it to organize future behavior (Rizzolatti et al., 2001). Similarly, recent studies on motor preparation suggest that STS is involved in extracting contextual and intentional cues during goal-oriented behavior (Toni et al., 2001). We suggest that an internal model of motion characteristics of the learned trajectory is built up during the training session and consolidated during the post-training night. At retest, to minimize the error, the information provided by the current motion of the target has to be integrated with the stored representation. The STS would be involved in this on-line integration, which could not occur during the pursuit of the new trajectory. In consequence, our results support the view that STS is not specialized in the perception of social cues but is involved more generally in the evaluation of complex motion patterns. Future research will have to test these hypotheses.
Effect of sleep on experience-dependent changes in brain functional connectivity
Psychophysiological interactions showed that the responses of the DN were correlated with the activity in the posterior part of the STS more tightly when the trajectory was learned than when it was new and more so in sleeping subjects than in the context of sleep deprivation. The STS area is the same as the one detected by the trajectory-by-group interaction. This observation is consistent with a role of temporo–ponto–cerebellar circuits in ocular following tasks. First, projections from the superior temporal cortex to pontine nuclei are identified in nonhuman primates and contribute to cortico–ponto–cerebellar circuits (Ungerleider et al., 1984;Glickstein et al., 1985; Schmahmann and Pandya, 1991). These projections are thought to be involved in smooth pursuit eye movements in monkeys (Tusa and Ungerleider, 1988). Second, neurophysiological studies in primates show that ocular responses during trajectory tasks are mediated by a pathway involving temporal areas, pontine nuclei, and the cerebellum (Kawano et al., 1994; Takemura et al., 2001). Third, theoretical models hypothesize that temporal cortices are involved in building up an internal inverse model for eye movements during ocular following responses (Wolpert et al., 1998). These proposals refer to the STS in monkeys (areas MT and MST). The posterior STS area detected here is more anterior than the human MT/V5 complex. We suggest that the increased functional coupling between the DN and the STS might indicate that STS provides the (ponto–)cerebellar circuits with information on the eye trajectory appropriate for matching the learned trajectory. These interactions occurred only in the subjects who slept during the first post-training night.
Psychophysiological interactions also showed that the responses of SEF were correlated with the activity in the FEF more tightly when the trajectory was learned than when it was new and more so in sleeping subjects than in the context of sleep deprivation. In nonhuman primates, SEF and FEF are mutually connected (Huerta et al., 1987), and neural responses in the FEF are related to smooth pursuit eye movements (Gottlieb et al., 1994; Tanaka and Fukushima, 1998). Inactivation of FEF impairs smooth eye movements (Shi et al., 1998), whereas electrical stimulation of the FEF can generate pursuit eye movements (MacAvoy et al., 1991; Gottlieb et al., 1994) and modulate their gain (Tanaka and Fukushima, 1998; Tanaka and Lisberger, 2001, 2002). We suggest that the increased functional connectivity between SEF and FEF reflects a closer control of the eye movement parameters such as their direction, speed, and gain. This is possible because prediction of the target trajectory by the internal model becomes more accurate.
Effect of sleep on learning
It should be noted that this experiment was not designed to evaluate whether consolidation occurs exclusively during sleep. Even in the sleep-deprived subjects, the performance tended to be better for the learned trajectory during the scanning session than during the training session. This suggests that some consolidation does take place during wakefulness. Indeed, it has already been reported that consolidation of basic motor skills can occur within 5 hr of wakefulness (Shadmehr and Holcomb, 1997).
Our results are consistent with the hypothesis of a favorable influence of sleep processes on recent memory traces. The behavioral data confirmed the observation by Smith and MacNeill (1994). Total sleep deprivation during the first post-training night disrupts subsequent performance of the learned trajectory. The functional MRI data further show that when sleep is allowed during the first post-training night, regional responses are increased in critical regions usually activated by performing learned motor sequences. Furthermore, the functional connectivity is augmented between these regions and other areas known to participate in the conventional smooth pursuit eye movement network. These changes in connectivity might reflect better inverse modeling of the ocular following response and better control over the oculomotor output.
Footnotes
This research was supported by the Wellcome Trust. P.M. is Senior Research Assistant at the Fonds National de la Recherche Scientifique (Belgique). P.M. was also supported by the Queen Elisabeth Medical Foundation and the Royal Society. S.S. was supported by the Swiss National Science Foundation (Grant 8210-061240). We thank Karl Friston for his comments on a previous version of this manuscript.
Correspondence should be addressed to Pierre Maquet, Cyclotron Research Centre B30, University of Liège–Sart Tilman, 4000 Liège, Belgium. E-mail: pmaquet@ulg.ac.be.
References
- 1.Berman RA, Colby CL, Genovese CR, Voyvodic JT, Luna B, Thulborn KR, Sweeney JA. Cortical networks subserving pursuit and saccadic eye movements in humans: an FMRI study. Hum Brain Mapp. 1999;8:209–225. doi: 10.1002/(SICI)1097-0193(1999)8:4<209::AID-HBM5>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blaxton TA, Zeffiro TA, Gabrieli JD, Bookheimer SY, Carrillo MC, Theodore WH, Disterhoft JF. Functional mapping of human learning: a positron emission tomography activation study of eyeblink conditioning. J Neurosci. 1996;16:4032–4040. doi: 10.1523/JNEUROSCI.16-12-04032.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boecker H, Dagher A, Ceballos-Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J, Dettmers C, Conrad B, Brooks DJ. Role of the human rostral supplementary motor area and the basal ganglia in motor sequence control: investigations with H2 15O PET. J Neurophysiol. 1998;79:1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- 4.Bonda E, Petrides M, Ostry D, Evans A. Specific involvement of human parietal systems and the amygdala in the perception of biological motion. J Neurosci. 1996;16:3737–3744. doi: 10.1523/JNEUROSCI.16-11-03737.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bonnet M. Sleep deprivation. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine, Ed 3. W. B. Saunders; Philadelphia: 2000. pp. 53–71. [Google Scholar]
- 6.Brooks VB, Kozlovskaya IB, Atkin A, Horvath FE, Uno M. Effects of cooling dentate nucleus on tracking-task performance in monkeys. J Neurophysiol. 1973;36:974–995. doi: 10.1152/jn.1973.36.6.974. [DOI] [PubMed] [Google Scholar]
- 7.Buechel C, Josephs O, Rees G, Turner R, Frith CD, Friston KJ. The functional anatomy of attention to visual motion. A functional MRI study. Brain. 1998;121:1281–1294. doi: 10.1093/brain/121.7.1281. [DOI] [PubMed] [Google Scholar]
- 8.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 9.Castelli F, Happe F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. NeuroImage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 10.Chawla D, Buechel C, Edwards R, Howseman A, Josephs O, Ashburner J, Friston KJ. Speed-dependent responses in V5: a replication study. NeuroImage. 1999;9:508–515. doi: 10.1006/nimg.1999.0432. [DOI] [PubMed] [Google Scholar]
- 11.Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG. Experience-dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA. 2002;99:1017–1022. doi: 10.1073/pnas.022615199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Drummond SP, Brown GG, Gillin JC, Stricker JL, Wong EC, Buxton RB. Altered brain response to verbal learning following sleep deprivation. Nature. 2000;403:655–657. doi: 10.1038/35001068. [DOI] [PubMed] [Google Scholar]
- 13.Dukelow SP, DeSouza JF, Culham JC, van den Berg AV, Menon RS, Vilis T. Distinguishing subregions of the human MT+ complex using visual fields and pursuit eye movements. J Neurophysiol. 2001;86:1991–2000. doi: 10.1152/jn.2001.86.4.1991. [DOI] [PubMed] [Google Scholar]
- 14.Dumoulin SO, Bittar RG, Kabani NJ, Baker CL, Jr, Le Goualher G, Bruce Pike G, Evans AC. A new anatomical landmark for reliable identification of human area V5/MT: a quantitative analysis of sulcal patterning. Cereb Cortex. 2000;10:454–463. doi: 10.1093/cercor/10.5.454. [DOI] [PubMed] [Google Scholar]
- 15.Dursteler MR, Wurtz RH. Pursuit and optokinetic deficits following chemical lesions of cortical areas MT and MST. J Neurophysiol. 1988;60:940–965. doi: 10.1152/jn.1988.60.3.940. [DOI] [PubMed] [Google Scholar]
- 16.Eysenck HJ, Frith CD. Reminiscence, motivation, and personality. A case study in experimental psychology. Plenum; New York: 1977. [Google Scholar]
- 17.Fink GR, Frackowiak RS, Pietrzyk U, Passingham RE. Multiple nonprimary motor areas in the human cortex. J Neurophysiol. 1997;77:2164–2174. doi: 10.1152/jn.1997.77.4.2164. [DOI] [PubMed] [Google Scholar]
- 18.Flament D, Ellerman J, Kim S, Ugurbil K, Ebner T. Functional resonance magnetic imaging of cerebellar activation during the learning of a visuo-motor dissociation task. Hum Brain Mapp. 1996;4:210–226. doi: 10.1002/hbm.460040302. [DOI] [PubMed] [Google Scholar]
- 19.Friston KJ, Buechel C. Attentional modulation of effective connectivity from V2 to V5/MT in humans. Proc Natl Acad Sci USA. 2000;97:7591–7596. doi: 10.1073/pnas.97.13.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 21.Frith C. Learning rhythmic hand movements. Q J Exp Psychol. 1973;25:253–259. doi: 10.1080/14640747308400345. [DOI] [PubMed] [Google Scholar]
- 22.Glickstein M, May JG, III, Mercier BE. Corticopontine projection in the macaque: the distribution of labelled cortical cells after large injections of horseradish peroxidase in the pontine nuclei. J Comp Neurol. 1985;235:343–359. doi: 10.1002/cne.902350306. [DOI] [PubMed] [Google Scholar]
- 23.Gottlieb JP, MacAvoy MG, Bruce CJ. Neural responses related to smooth-pursuit eye movements and their correspondence with electrically elicited smooth eye movements in the primate frontal eye field. J Neurophysiol. 1994;72:1634–1653. doi: 10.1152/jn.1994.72.4.1634. [DOI] [PubMed] [Google Scholar]
- 24.Grafton S, Woods R, Tyszka M. Functional imaging of procedural motor learning: relating cerebral blood flow with individual subject performance. Hum Brain Mapp. 1994;1:221–234. doi: 10.1002/hbm.460010307. [DOI] [PubMed] [Google Scholar]
- 25.Grafton ST, Mazziotta JC, Presty S, Friston KJ, Frackowiak RS, Phelps ME. Functional anatomy of human procedural learning determined with regional cerebral blood flow and PET. J Neurosci. 1992;12:2542–2548. doi: 10.1523/JNEUROSCI.12-07-02542.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Grezes J, Fonlupt P, Bertenthal B, Delon-Martin C, Segebarth C, Decety J. Does perception of biological motion rely on specific brain regions? NeuroImage. 2001;13:775–785. doi: 10.1006/nimg.2000.0740. [DOI] [PubMed] [Google Scholar]
- 27.Grosbras MH, Lobel E, Van de Moortele PF, LeBihan D, Berthoz A. An anatomical landmark for the supplementary eye fields in human revealed with functional magnetic resonance imaging. Cereb Cortex. 1999;9:705–711. doi: 10.1093/cercor/9.7.705. [DOI] [PubMed] [Google Scholar]
- 28.Grossman E, Donnelly M, Price R, Pickens D, Morgan V, Neighbor G, Blake R. Brain areas involved in perception of biological motion. J Cognit Neurosci. 2000;12:711–720. doi: 10.1162/089892900562417. [DOI] [PubMed] [Google Scholar]
- 29.Heide W, Binkofski F, Seitz RJ, Posse S, Nitschke MF, Freund HJ, Kompf D. Activation of frontoparietal cortices during memorized triple-step sequences of saccadic eye movements: an fMRI study. Eur J Neurosci. 2001;13:1177–1189. doi: 10.1046/j.0953-816x.2001.01472.x. [DOI] [PubMed] [Google Scholar]
- 30.Heinen SJ, Liu M. Single-neuron activity in the dorsomedial frontal cortex during smooth-pursuit eye movements to predictable target motion. Vis Neurosci. 1997;14:853–865. doi: 10.1017/s0952523800011597. [DOI] [PubMed] [Google Scholar]
- 31.Hennevin E, Hars B, Maho C, Bloch V. Processing of learned information in paradoxical sleep: relevance for memory. Behav Brain Res. 1995;69:125–135. doi: 10.1016/0166-4328(95)00013-j. [DOI] [PubMed] [Google Scholar]
- 32.Huerta MF, Krubitzer LA, Kaas JH. Frontal eye field as defined by intracortical microstimulation in squirrel monkeys, owl monkeys, and macaque monkeys. II. Cortical connections. J Comp Neurol. 1987;265:332–361. doi: 10.1002/cne.902650304. [DOI] [PubMed] [Google Scholar]
- 33.Iacoboni M, Koski LM, Brass M, Bekkering H, Woods RP, Dubeau MC, Mazziotta JC, Rizzolatti G. Reafferent copies of imitated actions in the right superior temporal cortex. Proc Natl Acad Sci USA. 2001;98:13995–13999. doi: 10.1073/pnas.241474598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Imamizu H, Miyauchi S, Tamada T, Sasaki Y, Takino R, Putz B, Yoshioka T, Kawato M. Human cerebellar activity reflecting an acquired internal model of a new tool. Nature. 2000;403:192–195. doi: 10.1038/35003194. [DOI] [PubMed] [Google Scholar]
- 35.Jenkins IH, Brooks DJ, Nixon PD, Frackowiak RS, Passingham RE. Motor sequence learning: a study with positron emission tomography. J Neurosci. 1994;14:3775–3790. doi: 10.1523/JNEUROSCI.14-06-03775.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- 37.Jueptner M, Stephan KM, Frith CD, Brooks DJ, Frackowiak RS, Passingham RE. Anatomy of motor learning. I. Frontal cortex and attention to action. J Neurophysiol. 1997;77:1313–1324. doi: 10.1152/jn.1997.77.3.1313. [DOI] [PubMed] [Google Scholar]
- 38.Karni A, Bertini G. Learning perceptual skills: behavioral probes into adult cortical plasticity. Curr Opin Neurobiol. 1997;7:530–535. doi: 10.1016/s0959-4388(97)80033-5. [DOI] [PubMed] [Google Scholar]
- 39.Karni A, Sagi D. The time course of learning a visual skill. Nature. 1993;365:250–252. doi: 10.1038/365250a0. [DOI] [PubMed] [Google Scholar]
- 40.Karni A, Meyer G, Rey-Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG. The acquisition of skilled motor performance: fast and slow experience-driven changes in primary motor cortex. Proc Natl Acad Sci USA. 1998;95:861–868. doi: 10.1073/pnas.95.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kawano K, Shidara M, Watanabe Y, Yamane S. Neural activity in cortical area MST of alert monkey during ocular following responses. J Neurophysiol. 1994;71:2305–2324. doi: 10.1152/jn.1994.71.6.2305. [DOI] [PubMed] [Google Scholar]
- 42.Komatsu H, Wurtz RH. Modulation of pursuit eye movements by stimulation of cortical areas MT and MST. J Neurophysiol. 1989;62:31–47. doi: 10.1152/jn.1989.62.1.31. [DOI] [PubMed] [Google Scholar]
- 43.Krauzlis RJ, Stone LS. Tracking with the mind's eye. Trends Neurosci. 1999;22:544–550. doi: 10.1016/s0166-2236(99)01464-2. [DOI] [PubMed] [Google Scholar]
- 44.Kudrimoti HS, Barnes CA, McNaughton BL. Reactivation of hippocampal cell assemblies: effects of behavioral state, experience, and EEG dynamics. J Neurosci. 1999;19:4090–4101. doi: 10.1523/JNEUROSCI.19-10-04090.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Logan CG, Grafton ST. Functional anatomy of human eyeblink conditioning determined with regional cerebral glucose metabolism and positron-emission tomography. Proc Natl Acad Sci USA. 1995;92:7500–7504. doi: 10.1073/pnas.92.16.7500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Louie K, Wilson MA. Temporally structured replay of awake hippocampal ensemble activity during rapid eye movement sleep. Neuron. 2001;29:145–156. doi: 10.1016/s0896-6273(01)00186-6. [DOI] [PubMed] [Google Scholar]
- 47.Luna B, Thulborn KR, Strojwas MH, McCurtain BJ, Berman RA, Genovese CR, Sweeney JA. Dorsal cortical regions subserving visually guided saccades in humans: an fMRI study. Cereb Cortex. 1998;8:40–47. doi: 10.1093/cercor/8.1.40. [DOI] [PubMed] [Google Scholar]
- 48.MacAvoy MG, Gottlieb JP, Bruce CJ. Smooth-pursuit eye movement representation in the primate frontal eye field. Cereb Cortex. 1991;1:95–102. doi: 10.1093/cercor/1.1.95. [DOI] [PubMed] [Google Scholar]
- 49.Maquet P. The role of sleep in learning and memory. Science. 2001;294:1048–1052. doi: 10.1126/science.1062856. [DOI] [PubMed] [Google Scholar]
- 50.Maquet P, Laureys S, Peigneux P, Fuchs S, Petiau C, Phillips C, Aerts J, Del Fiore G, Degueldre C, Meulemans T, Luxen A, Franck G, Van Der Linden M, Smith C, Cleeremans A. Experience-dependent changes in cerebral activation during human REM sleep. Nat Neurosci. 2000;3:831–836. doi: 10.1038/77744. [DOI] [PubMed] [Google Scholar]
- 51.McGuire PK, Bates JF, Goldman-Rakic PS. Interhemispheric integration: I. Symmetry and convergence of the corticocortical connections of the left and the right principal sulcus (PS) and the left and the right supplementary motor area (SMA) in the rhesus monkey. Cereb Cortex. 1991;1:390–407. doi: 10.1093/cercor/1.5.390. [DOI] [PubMed] [Google Scholar]
- 52.Miall RC, Imamizu H, Miyauchi S. Activation of the cerebellum in coordinated eye and hand tracking movements: an fMRI study. Exp Brain Res. 2000;135:22–33. doi: 10.1007/s002210000491. [DOI] [PubMed] [Google Scholar]
- 53.Miall RC, Reckess GZ, Imamizu H. The cerebellum coordinates eye and hand tracking movements. Nat Neurosci. 2001;4:638–644. doi: 10.1038/88465. [DOI] [PubMed] [Google Scholar]
- 54.Missal M, Heinen SJ. Facilitation of smooth pursuit initiation by electrical stimulation in the supplementary eye fields. J Neurophysiol. 2001;86:2413–2425. doi: 10.1152/jn.2001.86.5.2413. [DOI] [PubMed] [Google Scholar]
- 55.Morrone MC, Tosetti M, Montanaro D, Fiorentini A, Cioni G, Burr DC. A cortical area that responds specifically to optic flow, revealed by fMRI. Nat Neurosci. 2000;3:1322–1328. doi: 10.1038/81860. [DOI] [PubMed] [Google Scholar]
- 56.Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol. 1993;70:2660–2664. doi: 10.1152/jn.1993.70.6.2660. [DOI] [PubMed] [Google Scholar]
- 57.Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsaki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Newsome WT, Wurtz RH, Dursteler MR, Mikami A. Deficits in visual motion processing following ibotenic acid lesions of the middle temporal visual area of the macaque monkey. J Neurosci. 1985;5:825–840. doi: 10.1523/JNEUROSCI.05-03-00825.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nezafat R, Shadmehr R, Holcomb HH. Long-term adaptation to dynamics of reaching movements: a PET study. Exp Brain Res. 2001;140:66–76. doi: 10.1007/s002210100787. [DOI] [PubMed] [Google Scholar]
- 60.O'Driscoll GA, Wolff AL, Benkelfat C, Florencio PS, Lal S, Evans AC. Functional neuroanatomy of smooth pursuit and predictive saccades. NeuroReport. 2000;11:1335–1340. doi: 10.1097/00001756-200004270-00037. [DOI] [PubMed] [Google Scholar]
- 61.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 62.Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Petit L, Haxby JV. Functional anatomy of pursuit eye movements in humans as revealed by fMRI. J Neurophysiol. 1999;82:463–471. doi: 10.1152/jn.1999.82.1.463. [DOI] [PubMed] [Google Scholar]
- 64.Petit L, Clark VP, Ingeholm J, Haxby JV. Dissociation of saccade-related and pursuit-related activation in human frontal eye fields as revealed by fMRI. J Neurophysiol. 1997;77:3386–3390. doi: 10.1152/jn.1997.77.6.3386. [DOI] [PubMed] [Google Scholar]
- 65.Puce A, Allison T, Bentin S, Gore JC, McCarthy G. Temporal cortex activation in humans viewing eye and mouth movements. J Neurosci. 1998;18:2188–2199. doi: 10.1523/JNEUROSCI.18-06-02188.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramnani N, Toni I, Josephs O, Ashburner J, Passingham RE. Learning- and expectation-related changes in the human brain during motor learning. J Neurophysiol. 2000;84:3026–3035. doi: 10.1152/jn.2000.84.6.3026. [DOI] [PubMed] [Google Scholar]
- 67.Rizzolatti G, Fogassi L, Gallese V. Neurophysiological mechanisms underlying the understanding and imitation of action. Nat Rev Neurosci. 2001;2:661–670. doi: 10.1038/35090060. [DOI] [PubMed] [Google Scholar]
- 68.Rosano C, Krisky CM, Welling JS, Eddy WF, Luna B, Thulborn KR, Sweeney JA. Pursuit and saccadic eye movement subregions in human frontal eye field: a high-resolution fMRI investigation. Cereb Cortex. 2002;12:107–115. doi: 10.1093/cercor/12.2.107. [DOI] [PubMed] [Google Scholar]
- 69.Schluter ND, Rushworth MF, Passingham RE, Mills KR. Temporary interference in human lateral premotor cortex suggests dominance for the selection of movements. A study using transcranial magnetic stimulation. Brain. 1998;121:785–799. doi: 10.1093/brain/121.5.785. [DOI] [PubMed] [Google Scholar]
- 70.Schluter ND, Krams M, Rushworth MF, Passingham RE. Cerebral dominance for action in the human brain: the selection of actions. Neuropsychologia. 2001;39:105–113. doi: 10.1016/s0028-3932(00)00105-6. [DOI] [PubMed] [Google Scholar]
- 71.Schmahmann J, Doyon J, Toga A, Petrides M, Evans A. MRI atlas of the human cerebellum. Academic; San Diego: 2000. [DOI] [PubMed] [Google Scholar]
- 72.Schmahmann JD, Pandya DN. Projections to the basis pontis from the superior temporal sulcus and superior temporal region in the rhesus monkey. J Comp Neurol. 1991;308:224–248. doi: 10.1002/cne.903080209. [DOI] [PubMed] [Google Scholar]
- 73.Schmid A, Rees G, Frith C, Barnes G. An fMRI study of anticipation and learning of smooth pursuit eye movements in humans. NeuroReport. 2001;12:1409–1414. doi: 10.1097/00001756-200105250-00023. [DOI] [PubMed] [Google Scholar]
- 74.Shadmehr R, Holcomb HH. Neural correlates of motor memory consolidation. Science. 1997;277:821–825. doi: 10.1126/science.277.5327.821. [DOI] [PubMed] [Google Scholar]
- 75.Shi D, Friedman HR, Bruce CJ. Deficits in smooth-pursuit eye movements after muscimol inactivation within the primate's frontal eye field. J Neurophysiol. 1998;80:458–464. doi: 10.1152/jn.1998.80.1.458. [DOI] [PubMed] [Google Scholar]
- 76.Smith C. Sleep states and memory processes. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 77.Smith C, MacNeill C. Impaired motor memory for a pursuit rotor task following stage 2 sleep loss in college students. J Sleep Res. 1994;3:206–213. doi: 10.1111/j.1365-2869.1994.tb00133.x. [DOI] [PubMed] [Google Scholar]
- 78.Stickgold R, James L, Hobson JA. Visual discrimination learning requires sleep after training. Nat Neurosci. 2000;3:1237–1238. doi: 10.1038/81756. [DOI] [PubMed] [Google Scholar]
- 79.Sunaert S, Van Hecke P, Marchal G, Orban GA. Motion-responsive regions of the human brain. Exp Brain Res. 1999;127:355–370. doi: 10.1007/s002210050804. [DOI] [PubMed] [Google Scholar]
- 80.Takemura A, Inoue Y, Gomi H, Kawato M, Kawano K. Change in neuronal firing patterns in the process of motor command generation for the ocular following response. J Neurophysiol. 2001;86:1750–1763. doi: 10.1152/jn.2001.86.4.1750. [DOI] [PubMed] [Google Scholar]
- 81.Tanaka M, Fukushima K. Neuronal responses related to smooth pursuit eye movements in the periarcuate cortical area of monkeys. J Neurophysiol. 1998;80:28–47. doi: 10.1152/jn.1998.80.1.28. [DOI] [PubMed] [Google Scholar]
- 82.Tanaka M, Lisberger SG. Regulation of the gain of visually guided smooth-pursuit eye movements by frontal cortex. Nature. 2001;409:191–194. doi: 10.1038/35051582. [DOI] [PubMed] [Google Scholar]
- 83.Tanaka M, Lisberger SG. Enhancement of multiple components of pursuit eye movement by microstimulation in the arcuate frontal pursuit area in monkeys. J Neurophysiol. 2002;87:802–818. doi: 10.1152/jn.00409.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tian JR, Lynch JC. Slow and saccadic eye movements evoked by microstimulation in the supplementary eye field of the cebus monkey. J Neurophysiol. 1995;74:2204–2210. doi: 10.1152/jn.1995.74.5.2204. [DOI] [PubMed] [Google Scholar]
- 85.Tian JR, Lynch JC. Functionally defined smooth and saccadic eye movement subregions in the frontal eye field of cebus monkeys. J Neurophysiol. 1996;76:2740–2753. doi: 10.1152/jn.1996.76.4.2740. [DOI] [PubMed] [Google Scholar]
- 86.Toni I, Krams M, Turner R, Passingham RE. The time course of changes during motor sequence learning: a whole-brain fMRI study. NeuroImage. 1998;8:50–61. doi: 10.1006/nimg.1998.0349. [DOI] [PubMed] [Google Scholar]
- 87.Toni I, Thoenissen D, Zilles K. Movement preparation and motor intention. NeuroImage. 2001;14:S110–117. doi: 10.1006/nimg.2001.0841. [DOI] [PubMed] [Google Scholar]
- 88.Tootell RB, Reppas JB, Kwong KK, Malach R, Born RT, Brady TJ, Rosen BR, Belliveau JW. Functional analysis of human MT and related visual cortical areas using magnetic resonance imaging. J Neurosci. 1995;15:3215–3230. doi: 10.1523/JNEUROSCI.15-04-03215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tusa RJ, Ungerleider LG. Fiber pathways of cortical areas mediating smooth pursuit eye movements in monkeys. Ann Neurol. 1988;23:174–183. doi: 10.1002/ana.410230211. [DOI] [PubMed] [Google Scholar]
- 90.Ungerleider LG, Desimone R, Galkin TW, Mishkin M. Subcortical projections of area MT in the macaque. J Comp Neurol. 1984;223:368–386. doi: 10.1002/cne.902230304. [DOI] [PubMed] [Google Scholar]
- 91.Vaina LM, Solomon J, Chowdhury S, Sinha P, Belliveau JW. Functional neuroanatomy of biological motion perception in humans. Proc Natl Acad Sci USA. 2001;98:11656–11661. doi: 10.1073/pnas.191374198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.van Mier H, Tempel LW, Perlmutter JS, Raichle ME, Petersen SE. Changes in brain activity during motor learning measured with PET: effects of hand of performance and practice. J Neurophysiol. 1998;80:2177–2199. doi: 10.1152/jn.1998.80.4.2177. [DOI] [PubMed] [Google Scholar]
- 93.Vercher JL, Gauthier GM. Cerebellar involvement in the coordination control of the oculo-manual tracking system: effects of cerebellar dentate nucleus lesion. Exp Brain Res. 1988;73:155–166. doi: 10.1007/BF00279669. [DOI] [PubMed] [Google Scholar]
- 94.Watson JD, Myers R, Frackowiak RS, Hajnal JV, Woods RP, Mazziotta JC, Shipp S, Zeki S. Area V5 of the human brain: evidence from a combined study using positron emission tomography and magnetic resonance imaging. Cereb Cortex. 1993;3:79–94. doi: 10.1093/cercor/3.2.79. [DOI] [PubMed] [Google Scholar]
- 95.Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- 96.Wolpert D, Miall C, Kawato M. Internal models in the cerebellum. Trends Cogn Neurosci. 1998;2:338–347. doi: 10.1016/s1364-6613(98)01221-2. [DOI] [PubMed] [Google Scholar]
- 97.Worsley K. A unified statistical approach for determining significant signals in images of cerebral activation. Hum Brain Mapp. 1996;4:58–73. doi: 10.1002/(SICI)1097-0193(1996)4:1<58::AID-HBM4>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]