● Cellular/Molecular
Exploring Neurodegeneration with Drosophila Mutants
blue cheese Mutations Define a Novel, Conserved Gene Involved in Progressive Neural Degeneration
Kim D. Finley, Philip T. Edeen, Robert C. Cumming, Michelle D. Mardahl-Dumesnill, Barbara J. Taylor, Maria H. Rodriguez, Calvin E. Hwang, Michael Benedetti, and Michael McKeown (see pages 1254–1264)
Neural Dysfunction and Neurodegeneration inDrosophila Na+/K+ ATPase Alpha Subunit Mutants
Michael J. Palladino, Jill E. Bower, Robert Kreber, and Barry Ganetzky (see pages 1276–1286)
Model organisms such as Drosophila are receiving increased attention for studies of human neurodegenerative disease. Although the merit of such systems for genetic studies is apparent, the relevance of a neurodegenerative phenotype in flies, whose lifespan is measured in weeks, to the long duration of human neurodegenerative diseases would seem less clear. However, two articles in this issue of the Journal provide additional evidence for the potential value of such studies. Finley et al. describe a novel loss-of-function mutant, blue cheese, that has a reduced life span, protein aggregates in the neuropil of “aged” animals, and neuronal apoptosis. The blue cheese protein, whose function is unknown, is expressed in the cytoplasm of CNS neurons and has a human homolog. In contrast, Palladino et al. report a neurodegenerative phenotype in Drosophila with mutations in a known protein, the α subunit of the Na/K ATPase. The sodium pump is fundamental to the maintenance of ion gradients in normal neurons. These mutants, identified in a screen for temperature-sensitive paralytic mutants, showed age-dependent, spongioform neurodegeneration but did not show protein aggregates. The mutations caused a reduced life span but could also cause hyperexcitability and seizures.
▴ Development/Plasticity/Repair
Micromodular Organization in Cerebral Cortex
Honeycomb-Like Mosaic at the Border of Layers 1 and 2 in the Cerebral Cortex
Noritaka Ichinohe, Fumino Fujiyama, Takeshi Kaneko, and Kathleen S. Rockland (see pages 1372–1382)
It is unusual these days for research studies to report new neuroanatomical features in brain. However, Ichinohe et al. used parvalbumin staining to reveal small (<100 μm) honeycomb-like structures, restricted to the border of layers 1 and 2 of rat visual cortex. The honeycombs were composed of intermingled dendritic bundles of GABAergic interneurons and pyramidal cells, zinc-rich corticocortical (CC) afferents, and thalamocortical (TC) nerve terminals. The honeycomb walls stained with parvalbumin as well as NMDA receptor 1 and glutamate receptor 1 receptor subunits. The laminar-specific substructures were also present in cat and monkey cortex and exhibited a high degree of regularity. The honeycomb organization appears to segregate CC and TC inputs into distinct dendritic compartments. Although the function of honeycomb organization remains unknown, the authors suggest that CC and TC represent two parallel systems in layer 2 that target different populations of pyramidal cell dendrites.
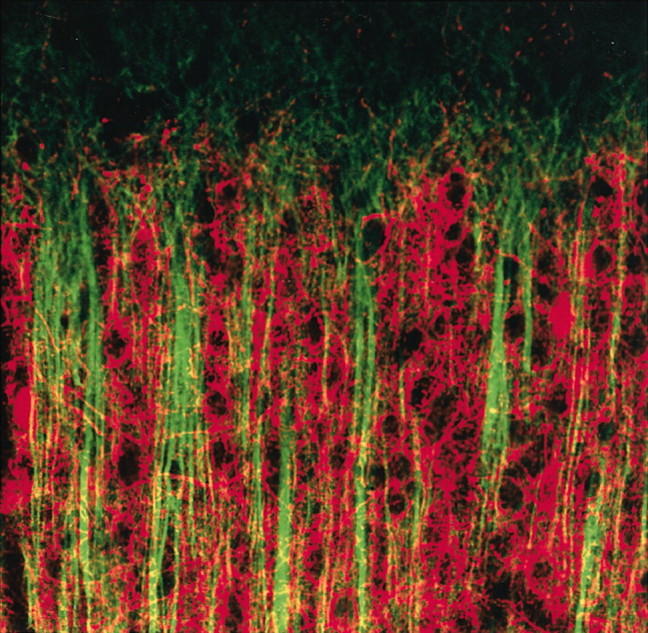
Labeling for microtubule-associated protein 2 (MAP2) (green) shows apical dendrites of pyramidal cells that end near the border of layers 1 and 2. The dendrites are surrounded by the parvalbumin-immunoreactive fibers (red) in the walls of each honeycomb.
▪ Behavioral/Systems/Cognitive
Energy Conservation, Locust-Style
Central Modulatory Neurons Control Fuel Selection in Flight Muscle of Migratory Locust
Tim Mentel, Carsten Duch, Heike Stypa, Gerhard Wegener, Uli Müller, and Hans-Joachim Pflüger (see pages1109–1113)
Although frequent-fliers may lament the declining quality of in-flight meals, the selection of fuel is even more vital to the success of long-distance flight for migratory insects such as the locust. During flight, locust muscle uses ATP at 100-fold the rest rate. To conserve energy, fuel consumption switches from carbohydrates during take-off to lipids during sustained flight, although carbohydrates are still present in muscle after 30 min of flight. What controls this active fuel selection? There is evidence for neuroendocrine control by adipokinetic hormones. However, Mentel et al. report that preflight activity of the dorsal unpaired median (DUM) neurons can drive carbohydrate consumption by flight muscles. The DUM neurons do not directly trigger contraction, but rather release the neuromodulator octopamine. The bioamine acts synergistically with AMP (generated by ATP hydrolysis) to increase carbohydrate consumption. The authors suggest that both octopamine-stimulated cAMP and IP3 may be required for this neural control of metabolism. During flight, DUM neurons are inhibited, thus allowing a switch to lipid metabolism. These studies provide a direct role for central modulatory neurons in the control of muscle glycolysis during exercise.


