Abstract
Hippocampal theta activity, a high-amplitude, slow (4–12 Hz) oscillation that occurs in a variety of behavioral contexts, is thought to emerge in infant rats only after 1 week of age. However, we report here that unanesthetized 2- and 4-d-old rats with electrodes implanted in the CA1 field of the hippocampus and tested in thermoneutral conditions exhibit theta activity. Moreover, this infant theta is characterized by the same neuronal bursting pattern and power spectrum that characterize theta in adults. Simultaneous measures of behavior and neck muscle tone indicated that bouts of theta occurred predominantly during periods of muscle atonia (with or without concurrent myoclonic twitching), indicative of REM sleep. In contrast, sharp waves were accompanied by startles (i.e., simultaneous and vigorous movement of all four limbs). These findings underscore the need for comprehensive in vivo investigations of the pharmacology, neural substrates, and behavioral correlates of hippocampal field activity in neonates.
Keywords: REM sleep, hippocampus, sharp waves, muscle tone, myoclonic twitching, behavior, temperature, startle
Introduction
The hippocampus is a forebrain structure long considered to play a role in learning, memory, and attentional processes (O'Keefe and Nadel, 1978). Interest in developmental aspects of the anatomy and function of this structure is increasing. Most recently, in vitroinvestigations of neonatal rat hippocampal activity have demonstrated the presence of synchronous giant depolarizing potentials (GDPs) (Leinekugel et al., 1997; Garaschuk et al., 1998). Based on in vivo evidence that rats younger than 8 d of age do not exhibit hippocampal theta activity (Leblanc and Bland, 1979; Leinekugel et al., 2002) but do exhibit hippocampal sharp waves (SPWs) that resemble the GDPs found in vitro (Leinekugel et al., 2002), it has been suggested that SPWs “represent the major source of correlated neuronal activity for the neonatal hippocampus” (Leinekugel et al., 2002).
The theta rhythm, defined in adults as a high-amplitude 4–12 Hz oscillation, occurs in modified form in a diverse array of species and in a variety of behavioral contexts, including walking, sniffing, chewing, and REM sleep (Robinson, 1980; Bland, 1986). Because of its hypothesized role in the modulation of long-term potentiation, the occurrence of the theta rhythm during REM sleep has helped fuel the still controversial notion that this stage of sleep represents a period of memory consolidation and neuronal plasticity (Winson, 1993;Vertes and Eastman, 2000; Graves et al., 2001; Stickgold et al., 2001). But despite the fact that REM sleep (or, more commonly, active sleep) is the predominant behavioral state of altricial infants (Jouvet-Mounier et al., 1970; Blumberg and Lucas, 1996), theta activity has been considered a missing component of this early REM sleep. The prevailing view that hippocampal theta does not exist in the neonate can be traced to one study in unanesthetized infant rats (Leblanc and Bland, 1979). In that study, recordings from CA1 and the dentate gyrus failed to indicate theta in pups younger than 7 d of age. Subsequent in vitro neurophysiological studies using hippocampal slices have provided direct and indirect support for the view that theta does not emerge until pups are at least 1 week of age (Konopacki et al., 1988; Strata, 1998). More recently, a secondin vivo experiment on three subjects reported the absence of theta in pups between 4 and 7 d of age (Leinekugel et al., 2002). In total, these studies indicate that the neonatal rat does not, and perhaps cannot, produce hippocampal theta. However, in the two previousin vivo experiments, pups were tested in conditions that were not thermally controlled and thus were not ideal for the expression of REM sleep (Blumberg, 2001), thus raising the question of whether theta might be expressed under thermoneutral conditions (Andersen and Moser, 1995).
Hippocampal electroencephalogram (EEG), nuchal muscle electromyogram (EMG), and sleep–wake behavior were recorded in unanesthetized 2-d-old (P2) and P4 rats. Based on previous experience with postsurgery infant rat behavior (Blumberg, 2001), particular care was taken to ensure that pups recovered in a warm (35°C), humidified incubator. In addition, to maximize the occurrence of REM sleep, pups were fed intragastrically before testing to ensure adequate nutrition (Lorenz et al., 1998) and were tested under thermoneutral conditions (Kreider and Blumberg, 2000; Karlsson and Blumberg, 2002). Data were acquired only when pups had fully recovered from the effects of surgery and anesthesia, as evidenced by the expression of normal nuchal EMG activity and sleep/wake behaviors (Robinson et al., 2000; Karlsson and Blumberg, 2002).
Materials and Methods
All experiments were performed under National Institutes of Health guidelines for the care and use of animals in research and were approved by the Institutional Animal Care and Use Committee of the University of Iowa.
Subjects. P2 (n = 11) and P4 (n = 4) male and female Sprague Dawley rats from nine litters were used (day of birth, P0). Body weights were 6.7–11.7 gm at the time of surgery. Mothers and their litters were housed in standard laboratory cages (48 × 20 × 26 cm) in the animal colony at the University of Iowa, where food and water were available ad libitum. Animals were maintained on a 12 hr light/dark cycle, with lights on at 7:00 A.M. All tests were conducted during the light phase of the cycle.
Surgery. On the day of testing, a pup with a visible milk band was removed from the litter, weighed, and anesthetized with isoflurane. For the measurement of hippocampal extracellular activity in the CA1 field, two stainless-steel single-ended electrodes (50 μm diameter; California Fine Wire, Grover Beach, CA) were implanted under stereotaxic and electrophysiological guidance (1.0–1.5 mm caudal to bregma; 1.8–2.0 mm from midline; depth, ∼2 mm); a silver indifferent electrode was implanted in the cerebral cortex anterior to bregma. All electrodes were secured to the skull using a light-curable epoxy adhesive (DSM Desotech, Elgin, IL). For measurement of nuchal EMG, two bipolar 50 μm stainless-steel hook electrodes were inserted bilaterally into the muscle, and a ground wire was attached to the skin of the back.
Procedure and data acquisition. After surgery, pups were placed on a soft felt platform and lightly secured in the supine position with the electrode wires running through an opening in the base of the platform. [Testing pups in the supine position allows for easy observation of myoclonic twitching of individual limbs and helps to minimize artifact in the electrophysiological recordings (Robinson et al., 2000; Karlsson and Blumberg, 2002).] Pups recovered from surgery for ∼2 hr inside a humidified incubator maintained at a temperature of 34–35°C. At the end of this period, pups were intubated with commercial half-and-half and then transferred to an electrically shielded, double-walled glass chamber (height, 17 cm; inner diameter, 12.5 cm) through which temperature-controlled water was circulated. The air temperature inside the chamber was 35°C, which is within the thermoneutral range for newborn pups (Blumberg et al., 1997). Air flow through the chamber was 300 ml/min. The electrodes were connected to differential amplifiers (A-M Systems, Carlsborg, WA) and a microcamera was placed above the chamber lid to record sleep–wake behaviors. After acclimation to the chamber for 20–30 min, electrophysiological and behavioral data were recorded uninterrupted for 60 min. The signals from the hippocampal and nuchal muscle electrodes were amplified (×10,000) with filter settings of 1–3000 and 300–5000 Hz, respectively. Amplified signals and video were recorded using a digital recording system (model DV8; Wintron Technologies, Rebersburg, PA). After the test, pups were perfused through the heart with formalin, and brain sections were stained with cresyl violet for the identification of electrode placement.
Statistical analysis. Electrophysiological signals were digitized at 6250–10,000 samples per second using a data acquisition system (BioPac Systems Inc., Santa Barbara, CA); behavior was simultaneously viewed on a monitor. The signal from one of the two hippocampal electrodes was used for analysis. The wide-band hippocampal EEG signal (1–3000 Hz) was digitally filtered to reveal slow-wave (1–35 Hz) and multiunit (300–3000 Hz) activity.
The two nuchal EMG signals were added together and full-wave rectified. For each subject's 1 hr of recording, bouts of theta activity were identified and analyzed. Data from subjects were discarded because of a poor electrophysiological signal (n = 1), severely disrupted sleep behavior (n = 1), and inability to identify electrode placement (n = 2).
After all theta bouts were identified, sleep–wake behavior was scored for a 3 sec period before and after the bout as well as during the bout. To do this, a trained observer, using an event recorder, pressed one of two keys when myoclonic twitching or awake behaviors were detected, as described previously (Karlsson and Blumberg, 2002). In this way, a digital record of a pup's electrophysiological activity and corresponding behavior was produced.
Two methods were used to measure theta frequency. First, for each theta bout, bout length (i.e., the number of complete cycles) was divided by bout duration to give theta frequency; the mean theta frequency across all 229 bouts was then determined. Second, Fourier analysis was performed on a subset of data by splicing together the five longest theta bouts from each of the seven subjects that exhibited theta, with no bout being <8 cycles long. This yielded a continuous 47 sec record of theta. The power spectrum was detrended and smoothed using a window size of 200 samples.
Results
Theta in a representative P2 rat
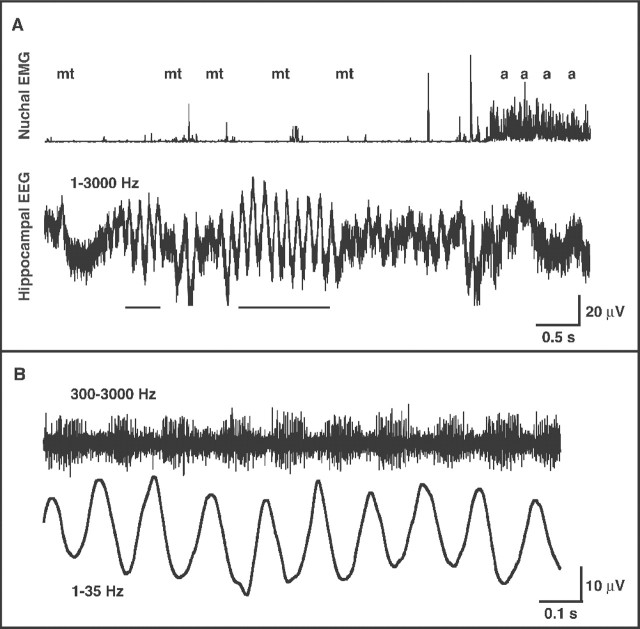
A 13 sec segment of data from a P2 subject is depicted in Figure1A, in which one short and one long bout of theta can be seen (Fig. 1A,bottom). During theta activity, the pup is exhibiting REM sleep, as characterized by low neck-muscle tone and the occurrence of sporadic myoclonic twitches. Toward the end of this segment, muscle tone abruptly increases, awake behaviors are displayed, and the hippocampus exhibits irregular activity. Selective filtering of the wide-band signal during a bout of theta (Fig. 1B) indicates that the neuronal burst activity occurs during the negative phase of the theta wave, as is typically seen in adults (Bland, 1986;Buzsáki, 2002).
Fig. 1.
Hippocampal theta activity in a P2 rat.A, Nuchal EMG (top) and wide-band (1–3000 Hz) hippocampal activity (bottom) in a P2 rat with an electrode implanted in the CA1 field. Two bouts of theta can be seen (identified by the horizontal bars at thebottom), both of them occurring against a background of low muscle tone. Myoclonic twitches (mt) and awake behaviors (a) are indicated at thetop. Mean ± SD theta amplitude is 55 ± 2 μV. B, The second bout of theta inA is filtered to highlight multiunit (300–3000 Hz) and theta (1–35 Hz) activity.
Similarity in the dominant frequency of infant and adult theta
Theta activity was detected in five P2 and two P4 subjects. For these subjects, electrode placement was within the CA1 field in five pups, at the CA1–subiculum boundary in one pup, and just dorsal to the lateral ventricle in one pup (Fig.2A); placements for subjects that did not exhibit theta were far dorsal to CA1 (n = 2), close to the CA1–subiculum boundary (n = 1), and close to CA3 (n = 1). For the seven subjects that exhibited theta, a total of 229 bouts of theta were documented (range, 4–92 bouts per subject); a bout of theta was defined as a slow wave containing at least three uninterrupted cycles. Using this definition, the mean number of cycles per bout was 9.6 ± 0.4, with a mode of 4 (Fig. 2B). The mean theta frequency over all 229 bouts was 8.4 ± 0.1 Hz (Fig.2C, left). In addition, Fourier analysis of a subset of theta bouts revealed a power spectrum with a peak frequency (7.9 Hz) and form characteristic of similar analyses performed in older infants and adults (Bronzino et al., 1987; Jarosiewicz et al., 2002) (Fig. 2C).
Fig. 2.
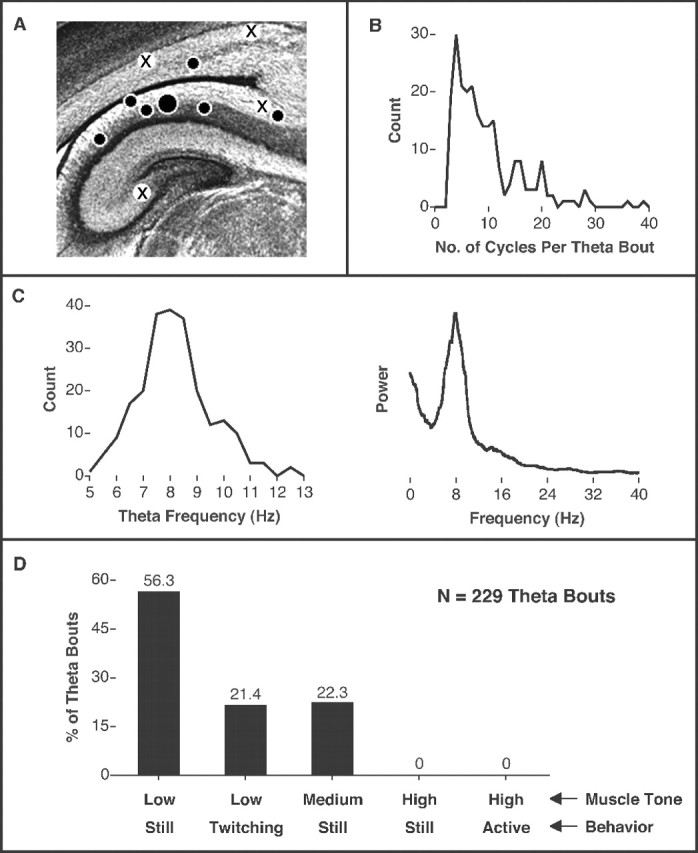
Characteristics of theta activity across all subjects. A, Coronal section from the brain of an infant rat showing the hippocampal formation and relative electrode placements for pups that exhibited bouts of theta activity. Filled circles indicate placements for subjects in which theta was detected (large circle denotes subject in Fig. 1);crosses indicate placements in which theta was not detected. B, Frequency distribution of the number of cycles per bout of hippocampal theta for 229 theta bouts.C,Left, Frequency distribution of theta frequency as determined from each of the 229 theta bouts, indicating a peak frequency of 8.4 Hz. C, Right, Fourier analysis of a subset of theta bouts across subjects, indicating a peak frequency of 7.9 Hz. For this analysis, a 47 sec continuous record of theta was constructed by splicing together the five longest theta bouts from each subject. D, Characterizations of nuchal muscle tone (Low, Medium, High) and behavior (Still, Twitching,Active) for all 229 theta bouts (expressed as percentage of total number of bouts). Only the five possible tone–behavior combinations are shown.
Infant theta occurs predominantly against a background of muscle atonia
Pups tested under the conditions used here cycle rapidly (approximately every 10–30 sec) between periods of REM sleep and awake behavior (Karlsson and Blumberg, 2002), thus providing an opportunity to assess the relationship between behavioral state and hippocampal activity. To do this, behavioral state was assessed immediately (i.e., 3 sec) before, during, and immediately (i.e., 3 sec) after each of the 229 theta bouts. During theta bouts, subjects were always either still (78.6% of theta bouts) or exhibiting sporadic twitching (21.4%) against a background of low (77.7%) or medium (22.3%) muscle tone (Fig. 2D). Similar results were found for the 3 sec periods before and after the bouts of theta. It should be stressed that the failure to detect even a single bout of theta in awake pups is not attributable to the infrequency of the waking state in pups at these ages; on the contrary, pups at this age exhibit awake behaviors (e.g., kicking, stretching) or high muscle tone ∼20–40% of the time (Karlsson and Blumberg, 2002).
Sharp waves are accompanied by startles
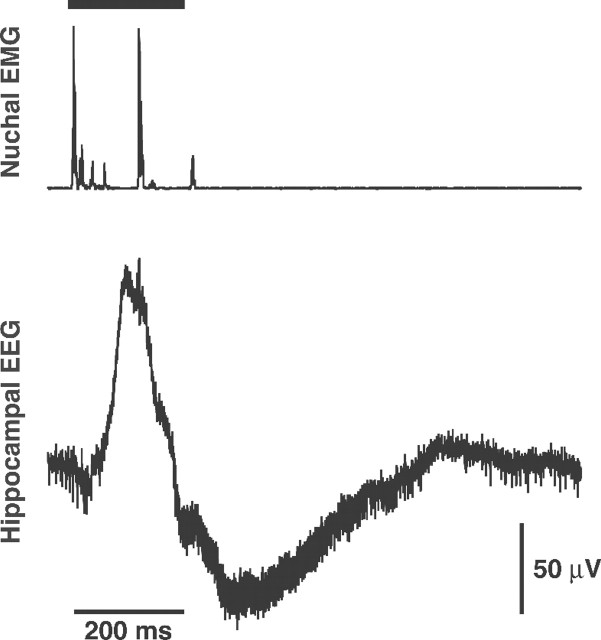
Because SPWs are considered to be the predominant neonatal hippocampal field pattern in the neonate (Leinekugel et al., 2002), hippocampal recordings were reviewed for evidence of SPWs. From three theta-producing subjects, the first five SPWs from each subject were selected for in-depth analysis. In all cases an identical pattern was observed: SPW activity was accompanied by concurrent spiking in the nuchal EMG (Fig. 3); frame-by-frame video analysis indicated near-simultaneous head and limb movements that were much more vigorous than the more common twitches of individual limbs. These movements resemble the startles that have been described previously and that are prevalent during the first postnatal week but become rare thereafter (Gramsbergen et al., 1970); interestingly, this developmental pattern mirrors that seen with SPWs and GDPs (Ben-Ari, 2001; Leinekugel et al., 2002). If the SPWs recorded in anesthetized (and therefore immobile) and unanesthetized pups are produced by identical mechanisms, as has been claimed (Leinekugel et al., 2002), then it is possible that SPWs play some role in the production of infant startles.
Fig. 3.
Representative SPW from a P2 rat. Nuchal muscle EMG (top) and wide-band (1–3000 Hz) SPW (bottom) in the same subject from Figure 1 are shown. The SPW bears a striking resemblance to those reported previously in freely moving infant rats (Leinekugel et al., 2002). Nuchal muscle activity, reflecting head movement, occurs before and during the SPW. The horizontal bar at the top indicates startle-like motor activity throughout the body during the SPW. This motor activation was confirmed by frame-by-frame video analysis. Sampling rate, 10 kHz.
Discussion
Based on a number of in vivo (Leblanc and Bland, 1979;Leinekugel et al., 2002) and in vitro (Konopacki et al., 1988) studies, it is generally believed that rats do not produce hippocampal theta until they are at least 7 d of age. However, the present experiment provides evidence to the contrary. Perhaps the most significant and unique feature of the present experiment was the provisioning of a thermoneutral test environment in which body temperatures of infants at the ages used here are maintained at ∼37°C (Blumberg et al., 1997). This feature of the experiment may be important for two reasons: First, hippocampal electrophysiological activity is influenced by local brain temperature (Andersen and Moser, 1995) and hippocampal slices from adult rats can be induced to exhibit theta-like activity only within a narrow thermal window of 33–37°C (Kowalczyk et al., 2001). Second, testing in an appropriate thermal environment permits the expression of many prolonged periods of REM (or active) sleep (Sokoloff and Blumberg, 1998), thereby maximizing the number and duration of artifact-free periods of hippocampal recording. In addition, we ensured that our pups had received milk just before testing, so as to maximize the expression of REM sleep (Lorenz et al., 1998). Thus, the procedures used here overcome many of the intrinsic difficulties that have hampered electrophysiological investigations of hippocampal activity in unanesthetized neonates (Leinekugel et al., 1998), difficulties that have led investigators interested in hippocampal development to focus predominantly on in vitropreparations.
As described above, theta activity in infants is similar to theta in adults with respect to its dominant frequency and the phase relations of multiunit activity. In contrast, whereas bouts of infant theta are very short, occurring for periods of ≤4 sec, the adult theta that occurs during REM sleep is expressed continuously for many minutes (Vanderwolf, 1969). Therefore, the brevity of REM sleep periods in infant rats (which last only 20–30 sec) was not a limiting factor in the production of theta. Interestingly, bouts of theta lasting only seconds have been observed during REM sleep in adult rats after atropine administration (Robinson et al., 1977).
The behavioral quiescence of pups during theta activity rules out the possibility that these signals resulted from gross movement artifact. Small movements of muscles in the head are also an unlikely source of artifact; for example, we examined our records for evidence of an association between jaw movements and theta activity and found no relationship between the two. In contrast to theta, frame-by-frame video analysis revealed that SPWs were associated with startle-like simultaneous activation of all four limbs, an association that was not reported in a recent study of SPWs in unanesthetized pups (Leinekugel et al., 2002); differences in observational methods may account for this discrepancy.
Oscillatory networks and synchronized bursting throughout the nervous system have been implicated in a variety of activity-dependent developmental processes (Shatz, 1990; Purves, 1994; Ben-Ari, 2001). Accordingly, it has been suggested that SPWs (and their putativein vitro counterparts, GDPs) are a primitive form of hippocampal activity that play a role in the development of hippocampal structure and function at a time at which mature activity, most notably in the form of theta, is not yet possible (Ben-Ari, 2001). However, the present results indicate that theta occurs in pups at ages much younger than previously suspected. It may be that the theta oscillations seen here are produced by intrinsic circuits comprising pyramidal cells within the CA1 field, a possibility that is not inconsistent with the prenatal origin of these cells (Bayer and Altman, 1974). Based on the linkage between theta and behavioral state, it is also possible that theta was driven by mesopontine nuclei that modulate REM sleep (Vertes and Kocsis, 1997; Siegel, 2000).
The present results add to a rapidly growing body of evidence indicating unexpected complexity in hippocampal activity in unanesthetized infant rats (Lahtinen et al., 2001; Leinekugel et al., 2002). Additional characterization of this activity must await simultaneous recordings from multiple regions of the hippocampus and related structures under a variety of testing conditions with concurrent assessment of behavior.
Footnotes
This work was supported by National Institutes of Health Grants MH50701, MH66424, and HD38708 (M.S.B.). We thank John Freeman, Amy Poremba, and Dan Nicholson for helpful comments on a previous draft of this manuscript and Dan Nicholson, Joseph Rathner, and Joy Kreider for technical assistance.
Correspondence should be addressed to Dr. Mark S. Blumberg, Department of Psychology, E11 Seashore Hall, University of Iowa, Iowa City, IA 52242. E-mail: mark-blumberg@uiowa.edu.
References
- 1.Andersen P, Moser EI. Brain temperature and hippocampal function. Hippocampus. 1995;5:491–498. doi: 10.1002/hipo.450050602. [DOI] [PubMed] [Google Scholar]
- 2.Bayer SA, Altman J. Hippocampal development in the rat: cytogenesis and morphogenesis examined with autoradiography and low-level X-irradiation. J Comp Neurol. 1974;158:55–80. doi: 10.1002/cne.901580105. [DOI] [PubMed] [Google Scholar]
- 3.Ben-Ari Y. Developing networks play a similar melody. Trends Neurosci. 2001;24:353–360. doi: 10.1016/s0166-2236(00)01813-0. [DOI] [PubMed] [Google Scholar]
- 4.Bland BH. The physiology and pharmacology of hippocampal formation theta rhythms. Prog Neurobiol. 1986;26:1–54. doi: 10.1016/0301-0082(86)90019-5. [DOI] [PubMed] [Google Scholar]
- 5.Blumberg MS. The developmental context of thermal homeostasis. In: Blass EM, editor. Handbook of behavioral neurobiology. Plenum; New York: 2001. pp. 199–228. [Google Scholar]
- 6.Blumberg MS, Lucas DE. A developmental and component analysis of active sleep. Dev Psychobiol. 1996;29:1–22. doi: 10.1002/(SICI)1098-2302(199601)29:1<1::AID-DEV1>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 7.Blumberg MS, Sokoloff G, Kirby RF. Brown fat thermogenesis and cardiac rate regulation during cold challenge in infant rats. Am J Physiol. 1997;272:R1308–R1313. doi: 10.1152/ajpregu.1997.272.4.R1308. [DOI] [PubMed] [Google Scholar]
- 8.Bronzino JD, Siok CJ, Austin K, Austin-Lafrance RJ, Morgane PJ. Spectral analysis of the electroencephalogram in the developing rat. Brain Res Dev Brain Res. 1987;35:257–267. doi: 10.1016/0165-3806(87)90050-2. [DOI] [PubMed] [Google Scholar]
- 9.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 10.Garaschuk O, Hanse E, Konnerth A. Developmental profile and synaptic origin of early network oscillations in the CA1 region of rat neonatal hippocampus. J Physiol (Lond) 1998;507:219–236. doi: 10.1111/j.1469-7793.1998.219bu.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gramsbergen A, Schwartze P, Prechtl HFR. The postnatal development of behavioral states in the rat. Dev Psychobiol. 1970;3:267–280. doi: 10.1002/dev.420030407. [DOI] [PubMed] [Google Scholar]
- 12.Graves L, Pack A, Abel T. Sleep and memory: a molecular perspective. Trends Neurosci. 2001;24:237–243. doi: 10.1016/s0166-2236(00)01744-6. [DOI] [PubMed] [Google Scholar]
- 13.Jarosiewicz B, McNaughton BL, Skaggs WE. Hippocampal population activity during the small-amplitude irregular activity state in the rat. J Neurosci. 2002;22:1373–1384. doi: 10.1523/JNEUROSCI.22-04-01373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jouvet-Mounier D, Astic L, Lacote D. Ontogenesis of the states of sleep in rat, cat, and guinea pig during the first postnatal month. Dev Psychobiol. 1970;2:216–239. doi: 10.1002/dev.420020407. [DOI] [PubMed] [Google Scholar]
- 15.Karlsson KÆ, Blumberg MS. The union of the state: myoclonic twitching is coupled with nuchal muscle atonia in infant rats. Behav Neurosci. 2002;116:912–917. doi: 10.1037//0735-7044.116.5.912. [DOI] [PubMed] [Google Scholar]
- 16.Konopacki J, Bland BH, Roth SH. The development of carbachol-induced EEG “theta” examined in hippocampal formation slices. Brain Res Dev Brain Res. 1988;38:229–232. doi: 10.1016/0165-3806(88)90048-x. [DOI] [PubMed] [Google Scholar]
- 17.Kowalczyk T, Golebiewski H, Eckersdorf B, Konopacki J. Window effect of temperature on carbachol-induced theta-like activity recorded in hippocampal formation in vitro. Brain Res. 2001;901:184–194. doi: 10.1016/s0006-8993(01)02355-1. [DOI] [PubMed] [Google Scholar]
- 18.Kreider JC, Blumberg MS. Mesopontine contribution to the expression of active “twitch” sleep in decerebrate week-old rats. Brain Res. 2000;872:149–159. doi: 10.1016/s0006-8993(00)02518-x. [DOI] [PubMed] [Google Scholar]
- 19.Lahtinen H, Palva JM, Sumanen S, Voipio J, Kaila K, Taira T. Postnatal development of rat hippocampal gamma rhythm in vivo. J Neurophysiol. 2001;88:1469–1474. doi: 10.1152/jn.2002.88.3.1469. [DOI] [PubMed] [Google Scholar]
- 20.Leblanc MO, Bland BH. Developmental aspects of hippocampal electrical activity and motor behavior in the rat. Exp Neurol. 1979;66:220–237. doi: 10.1016/0014-4886(79)90076-1. [DOI] [PubMed] [Google Scholar]
- 21.Leinekugel X, Medina I, Khalilov I, Ben-Ari Y, Khazipov R. Ca2+ oscillations mediated by the synergistic excitatory action GABAA and NMDA receptor in the neonatal hippocampus. Neuron. 1997;18:243–255. doi: 10.1016/s0896-6273(00)80265-2. [DOI] [PubMed] [Google Scholar]
- 22.Leinekugel X, Khalilov I, Ben-Ari Y, Khazipov R. Giant depolarizing potentials: the septal pole of the hippocampus paces the activity of the developing intact septohippocampal complex in vitro. J Neurosci. 1998;18:6349–6357. doi: 10.1523/JNEUROSCI.18-16-06349.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Leinekugel X, Khazipov R, Cannon R, Hirase H, Ben-Ari Y, Buzsáki G. Correlated bursts of activity in neonatal hippocampus in vivo. Science. 2002;296:2049–2052. doi: 10.1126/science.1071111. [DOI] [PubMed] [Google Scholar]
- 24.Lorenz DN, Poppe CJ, Quail C, Seipel K, Stordeur SA, Johnson E. Filling the gut activates paradoxical sleep in suckling rats. Dev Psychobiol. 1998;32:1–12. doi: 10.1002/(sici)1098-2302(199801)32:1<1::aid-dev1>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 25.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Clarendon; Oxford: 1978. [Google Scholar]
- 26.Purves D. Neural activity and the growth of the brain. Cambridge UP; Cambridge, UK: 1994. [Google Scholar]
- 27.Robinson SR, Blumberg MS, Lane MS, Kreber LA. Spontaneous motor activity in fetal and infant rats is organized into discrete multilimb bouts. Behav Neurosci. 2000;14:328–336. doi: 10.1037//0735-7044.114.2.328. [DOI] [PubMed] [Google Scholar]
- 28.Robinson TE. Hippocampal rhythmic slow activity (RSA; theta): a critical analysis of selected studies and discussion of possible species-differences. Brain Res Brain Res Rev. 1980;2:69–101. doi: 10.1016/0165-0173(80)90004-1. [DOI] [PubMed] [Google Scholar]
- 29.Robinson TE, Kramis RC, Vanderwolf CH. Two types of cerebral activation during active sleep: relations to behavior. Brain Res. 1977;124:544–549. doi: 10.1016/0006-8993(77)90954-4. [DOI] [PubMed] [Google Scholar]
- 30.Shatz CJ. Impulse activity and the patterning of connections during CNS development. Neuron. 1990;5:745–756. doi: 10.1016/0896-6273(90)90333-b. [DOI] [PubMed] [Google Scholar]
- 31.Siegel JM. Brainstem mechanisms generating REM sleep. In: Kryger MH, Roth T, Dement WC, editors. Principles and practice of sleep medicine. Saunders; Philadelphia: 2000. pp. 112–133. [Google Scholar]
- 32.Sokoloff G, Blumberg MS. Active sleep in cold-exposed infant Norway rats and Syrian golden hamsters: the role of brown adipose tissue thermogenesis. Behav Neurosci. 1998;112:695–706. doi: 10.1037//0735-7044.112.3.695. [DOI] [PubMed] [Google Scholar]
- 33.Stickgold R, Hobson JA, Fosse R, Fosse M. Sleep, learning, and dreams: off-line memory processing. Science. 2001;294:1052–1057. doi: 10.1126/science.1063530. [DOI] [PubMed] [Google Scholar]
- 34.Strata F. Intrinsic oscillations in CA3 hippocampal pyramids: physiological relevance to theta rhythm generation. Hippocampus. 1998;8:666–679. doi: 10.1002/(SICI)1098-1063(1998)8:6<666::AID-HIPO9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 35.Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr Clin Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- 36.Vertes RP, Eastman KE. The case against memory consolidation in REM sleep. Behav Brain Sci. 2000;23:867–876. doi: 10.1017/s0140525x00004003. [DOI] [PubMed] [Google Scholar]
- 37.Vertes RP, Kocsis B. Brainstem-diencephalo-septohippocampal systems controlling the theta rhythm of the hippocampus. Neuroscience. 1997;81:893–926. doi: 10.1016/s0306-4522(97)00239-x. [DOI] [PubMed] [Google Scholar]
- 38.Winson J. The biology and function of rapid eye movement sleep. Curr Opin Neurobiol. 1993;3:243–248. doi: 10.1016/0959-4388(93)90217-m. [DOI] [PubMed] [Google Scholar]