Abstract
This study sought to determine whether CA1 hippocampal neurons encode the duration of the trace interval during trace fear conditioning. Single neurons were recorded extracellularly in the CA1 of rabbits during and after a single trace fear classical conditioning session. Trace fear conditioning trials consisted of an auditory conditioned stimulus (CS; 3 sec) and a fear-producing shock unconditioned stimulus (US; 0.5 sec) separated by a silent trace interval. One group of rabbits was trained using a 10 sec trace interval (n = 5), and another group was trained using a 20 sec trace interval (n = 4). These groups were compared with pseudoconditioning control rabbits (n = 5 and n = 4, respectively) that received unpaired CSs and USs. One day after trace and pseudo fear conditioning rabbits received a CS-alone retention session in which no USs were presented. The trace conditioned groups showed larger bradycardiac-fear responses on CS-alone trials compared with the pseudoconditioning groups. A significant percentage of CA1 neurons from the 10 and 20 sec trace groups (24 and 28%, respectively) showed maximal firing on CS-alone retention trials timed to 10 sec (±1.5 sec) and 20 sec (±2.0 sec) after CS offset, respectively. These latencies were similar to the duration of the trace interval used on previous CS–trace–US trials. Timed CA1 firing was not seen in pseudoconditioning control animals, suggesting that a subset of CA1 neurons encoded the trace interval duration. The percentage of neurons encoding trace duration was largest when rabbits exhibited significant fear responses to the CS, suggesting that trace encoding was related to the strength of the CS and US association.
Keywords: learning, trace, temporal, conditioning, heart rate, CA1, hippocampus, single neuron
Introduction
One of the most important goals of neuroscience is to understand how neuronal activity represents or encodes learned information. Some of the most successful models of neuronal coding have exploited behavioral situations in which neuronal activity categorically represents a distinct behavior; for example, spatial information can be represented in a categorical manner by hippocampal place cells that fire when an animal is in a specific location in the spatial environment (O'Keefe and Nadel, 1978; Wilson and McNaughton, 1993). It is possible that the dimension of time also can be represented categorically by neuronal networks, such that firing patterns represent a specific temporal interval. Classical conditioning paradigms that incorporate a fixed temporal interval, or “trace” interval, between the conditioned stimulus (CS) and the unconditioned stimulus (US) could provide a useful scientific tool for examining how neuronal networks represent the duration of a specific temporal interval. One of these trace paradigms, called trace fear classical conditioning, requires animals to associate an auditory CS and a fear-producing shock US, which are separated by a silent trace interval of a specific duration. The trace fear classical conditioning paradigm could prove particularly useful for neurophysiological investigations, because the duration of the trace interval in this paradigm can be as long as 20 or even 30 sec (Moye and Rudy, 1987; McEchron et al., 1998), allowing the neuronal encoding of clearly distinct temporal intervals to be anchored to specific behavioral conditions.
The hippocampus is a brain structure that is critical for acquiring and remembering new information, and as a consequence, this structure has been studied extensively to try and understand how neurons encode information. Several studies have shown that neurons in the dorsal hippocampus of rats and rabbits are essential for trace fear classical conditioning (McEchron et al., 1998, 2000; Quinn et al., 2002). These studies demonstrated that the hippocampus was necessary for the association of the CS and US only when a trace interval separated these stimuli, but the hippocampus was not necessary for this association when no trace interval separated the stimuli. Thus, it seems likely that neurons in the dorsal hippocampus may play a role in the coding of temporal relationships between stimuli during trace fear classical conditioning.
The present study sought to address this question by recording the electrophysiological activity of CA1 single neurons in the hippocampus of rabbits during trace fear classical conditioning. Our previous work has shown that rabbits exhibit reliable decelerative-heart rate (HR) fear responses on CS-alone retention trials presented after trace fear conditioning with a 10 sec trace interval separating the CS and US (McEchron et al., 2000). We examined the CS-alone retention trials after trace fear conditioning with either a 10 sec trace interval or a 20 sec trace interval to determine whether CA1 single neurons encode the duration of specific trace intervals.
Materials and Methods
Subjects
A total of 14 New Zealand albino rabbits (4–7 months of age) were used in this study. Rabbits were housed individually and received food and water ad libitum. Before the fear conditioning experiments in this study, three of the rabbits received 10 d of classical eyeblink conditioning in a separate study (McEchron et al., 2001). The eyeblink conditioning was performed with a tone stimulus (3 kHz; 90 dB) and a corneal airpuff stimulus (150 msec; 3.0 psi), both of which were different from the stimuli used in the present investigation. These three rabbits were implanted with single neuron recording electrodes 1 week before the eyeblink training. One of these rabbits received unpaired pseudo eyeblink conditioning, and the other two received paired trace eyeblink conditioning using a 500 msec trace interval. After the eyeblink training, these three rabbits were allowed at least 7 d of rest in their home cage before the fear conditioning experiments used in this study. Analyses compared these animals with the naive animals in this study and showed that the previous eyeblink training had no effect on the measures of fear conditioning and single neuron encoding used in the present study.
Surgical implantation of electrodes
All rabbits were allowed to remain undisturbed in their cages for at least 1 week before any handling or surgery. Surgery was performed following procedures approved by the National Institutes of Health and Northwestern University Animal Care and Use Committee. Animals were anesthetized with ketamine (60 mg/kg, i.m.) and xylazine (10 mg/kg, i.m.), and the eyes were kept moist with a thin coat of antibacterial ophthalmic ointment. The skull was positioned in a stereotaxic frame with lambda 1.5 mm below bregma. The skull was then exposed, and a 3-mm-diameter hole was drilled above the left CA1 area of the hippocampus. Five self-tapping screws (2 × 1/4 inches) were inserted ∼2 mm into the skull to anchor the final dental cement-head assembly. In each animal, one or two nonmovable stereotrode recording bundles were stereotaxically lowered into the left CA1 area of the hippocampus (∼3 mm ventral to dura) until action potentials with pyramidal cell firing characteristics were recorded (Ranck, 1973). This procedure ensured that the electrode tip was located within the pyramidal cell layer of CA1. The coordinates for electrode placement were 5.0–5.2 mm caudal to bregma and 5.2–5.4 mm lateral to midline. Dental cement was then used to secure the electrodes to the skull and close the remaining wound area. Rabbits were given Buprenex (0.3 mg/kg, i.m.) to minimize discomfort after recovery from anesthesia.
Heart rate fear conditioning
One day before HR conditioning, animals were prepared for electrocardiographic (EKG) recording and acclimated to the conditioning chamber. Preparation for testing began by shaving and applying hair-removal cream to the chest and back for daily EKG recordings. Hair was also removed around the paraorbital region around the left eye. Topical lidocaine (5%) was applied to the paraorbital area followed by two Autoclip wound clips (Clay Adams, Parsippany, NJ), which served to deliver the shock US (0.5 sec; 3 mA alternating current shock). Immediately after preparation for EKG recording, rabbits were acclimated to the restrainer and the HR-conditioning chamber for 30 min. The aim of the acclimation was to reduce fear or arousal attributable to handling or restraint. No stimuli were presented during the acclimation session. During acclimation and all other testing, rabbits were restrained in a cloth bag and Plexiglas restrainer located within a sound-attenuating chamber. The ends of rubber tubes (1 cm diameter) were placed comfortably in each ear and served to deliver the auditory tone CS [3 sec; 6000 Hz; 80 dB; intertrial interval (ITI) of 60 ± 10 sec] from headphones. The EKG recording electrodes were sterilized stainless-steel safety pins inserted subcutaneously in the chest and back. The EKG activity was amplified 10,000×, filtered between 10 and 1000 Hz, sampled at 2224 Hz, and stored on a separate behavioral computer. The computer collected EKG activity continuously from 5 sec before CS onset to 5 sec after the scheduled US offset.
One day after the acclimation session, each rabbit received one habituation session followed immediately by either a trace fear conditioning session or a pseudoconditioning session. Rabbits then received one session of CS-alone retention trials 24 hr later.
Habituation. One day after acclimation, rabbits received one habituation session that consisted of 30 tone–CS-alone trials presented at an ITI of 60 ± 10 sec. This session was used to habituate the orienting HR response to the CS and reduce any sensitization HR responses to the CS.
Trace and pseudo fear conditioning. Immediately after habituation, rabbits received one of three behavioral conditions: (1) pseudo fear conditioning, (2) trace fear conditioning with a 10 sec trace interval, or (3) trace fear conditioning with a 20 sec trace interval. The trace fear conditioning sessions consisted of 35 paired trace trials (ITI of 90 ± 10 sec). For each trial of 10 sec trace fear conditioning, the CS was presented followed by a 10 sec empty trace interval, and then the US. For each trial of 20 sec trace fear conditioning, the CS was presented followed by a 20 sec empty trace interval, and then the US. A tone–CS-alone test trial was presented after every seven trace trials. The pseudoconditioning session consisted of CS-alone and US-alone trials (ITI of 45 ± 10 sec). During the pseudoconditioning session, the same stimulus was never presented more than two consecutive times. Both the trace and pseudo fear conditioning sessions were approximately the same duration (80 min) and contained the same number of CSs and USs. Three of the rabbits that received pseudoconditioning were trained 1 week later in 20 sec trace fear conditioning.
CS-alone retention. One day after trace and pseudo fear conditioning, animals were administered one retention session consisting of 30 CS-alone trials (ITI of 90 ± 10 sec). It is important to mention that immediately preceding the CS-alone retention session, three of the four rabbits in the 20 sec trace fear conditioning group were administered two reminder CS–trace–US trials that included a 20 sec trace interval, identical to the trials on the previous day of trace conditioning. These reminder trials were used to increase the likelihood of detecting timed neuronal responses on CS-alone retention trials.
Single neuron recording
Single neurons were recorded from rabbits during trace and pseudo fear conditioning using surgically implanted nonmoveable electrodes that were cemented in place. Each implanted recording electrode consisted of a bundle of six channels with a total diameter of ∼80 μm. Each channel was a Teflon-coated tungsten microwire (18 μm diameter when bare; 25 μm diameter when coated). The channels were bonded tightly together in parallel with epoxylite to form a 25 μm center-to-center spacing. During recording, two-wire stereotrode combinations were selected from the implanted probe that provided the largest and most heterogeneous ensembles of single neurons (2–10 neurons). This is an enhanced version of the stereotrode technique, which has been shown to allow large numbers of single neurons to be recorded and separated with much greater accuracy than single electrodes (McNaughton et al., 1983). Similar ensemble techniques have been used for recording tightly clustered groups of single neurons from a single probe (Apkarian et al., 2000).
Single neuron analog signals were amplified (10,000×), filtered (bandpass, 300 Hz to10 kHz), and collected with a DT 2821 Data Translation board (Data Translation, Marlboro, MA) attached to a 200 MHz Pentium computer that sampled each channel at 30 kHz. Single neuron data were collected continuously from 5 sec before CS onset to 10 sec after US onset using software from DataWave Technologies (Longmont, CO). The software recorded 1.5 msec epochs of data whenever a single neuron discharged a definable action potential. The action potentials of each of the different single neurons recorded on an electrode were separated off-line using a template-matching program developed by M. D. McEchron. This software allowed template windows to be defined for the characteristic waveform of each single neuron. All action potential waveforms that fell within the boundaries of a single template window belonged to an individual single neuron. The template window could account for any unique segment along the single neuron waveform, and the window could be minimized anywhere along the waveform to exclude other electrophysiological data that did not fit the exact shape of an individual single neuron. All action potential waveforms on a probe were also compared visually to ensure that the characteristic waveform of each individually defined single neuron was different from the waveforms of all other defined single neurons on the probe. This conservative approach ensured that the ensembles recorded from each probe were made up of unique single neurons that could be accurately followed throughout a single training session. Individual hippocampal pyramidal cells have been reported to exhibit complex spikes within a burst of activity where the action potentials of a single neuron decrease in height (Ranck, 1973). Based on parameters described by Quirk and Wilson (1999), the software was able to track patterns of activity that might represent complex spike activity. This prevented the complex spike activity of a single neuron from overlapping with more than one individually defined single neuron.
Single neuron activity was analyzed from a single day of training only if the single neurons on a stereotrode remained consistent throughout the entire training session. This ensured that the electrode did not drift during the recording session, which might produce an overlap of activity from more than one single neuron. However, it is important to note that the configuration of single neurons on a stereotrode changed from one day of recording to the next in almost all cases. Neurons were treated as the same neuron on consecutive days only if the same template parameters yielded the same configuration of single neurons on one probe. This does not rule out the possibility that small drifts in the electrode between recording sessions may have allowed new configurations of single neurons to form that included one or two of the neurons from the previous recording day. This conservative protocol was based on methods developed in our previous hippocampal single neuron recording work (McEchron et al., 2001).
After spike separation, an average waveform was computed for each single neuron to determine whether the neuron was a pyramidal or theta cell. Action potential widths were calculated from each average waveform as the peak time minus the valley time. Pyramidal cells were separated from theta cells using measurements of action potential width and background firing rate. Using criteria similar to those described by Fox and Ranck (1981), cells with a spike duration of ≥0.3 msec and a background firing rate of <5 Hz were classified as pyramidal cells, and cells with a spike duration of <0.3 msec and a background firing rate of ≥5 Hz were classified as interneurons.
Analyses
Single neuron analyses. All statistical analyses were performed with the aid of Microsoft (Redmond, WA) Visual Basic routines developed by M. D. McEchron and Minitab statistical software version 10.0 (State College, PA). Analyses of background single neuron firing rate were performed by calculating the mean discharge rate of each neuron before the delivery of each trial used in training. Changes in single neuron action potential firing rate during the trial were measured using standard t test scores. For each neuron, standard test scores were computed for time periods from 1000 to 5000 msec in duration after either the CS or US to capture discrete short latency increases or decreases in activity. The standard test scores were computed by subtracting the number of action potentials in the period preceding CS onset from the number of action potentials in the period after CS or US onset. The difference calculated for each period was divided by the sample SD during baseline. Group comparison of standard scores was accomplished using an independent t test. Each analysis used only one mean from an individual single neuron. An α of 0.05 was required for all significant analyses.
CS-alone retention trials were used to determine whether a single neuron fired timed action potentials that encoded the duration of the trace interval. Perievent time histograms were generated for each neuron using five trial blocks. The peak firing latency was determined for an individual neuron using the 1 sec bin with the most action potentials. A distribution was then plotted for the percentage of neurons that fired maximally during each 1 sec bin during the CS-alone trials. A binomial exact probability test described by Lee et al. (1989) was used to determine the probability and significance of detecting a specific percentage of neurons firing maximally at a specific latency. A two-group statistical comparison of proportions of neurons showing maximal firing at a specific latency was accomplished using the z-ratio–two-sample test for binomial proportions described by Rosner (1990). An α level of 0.05 was required for significance (two tailed).
Heart rate conditioning analyses. Conditioned HR fear responses were indicated as bradycardiac decreases in HR on CS-alone trials. The HR was quantified by measuring the duration of the interval between the R-peaks of the EKG waveform. The HR was calculated using the average rate in each 1 sec bin. Change scores were then computed for each bin after CS onset by subtracting the average HR in the 5 sec baseline period before CS onset. Individual trials were not included in any analyses if HR fluctuations exceeded 15 beats per min during the baseline period before CS onset. Trials with excessive tachycardiac–arrhythmic HR or movement artifact were also not included in any analyses. Using these criteria, only 1% (17 trials) of the HR trials were excluded from the final analyses.
Statistical analyses were performed by obtaining the mean HR change from baseline for each CS-alone test trial from each animal. Independent t tests were used to compare the mean HR change for two groups. Repeated-measures ANOVAs were used to examine behavioral data across five trial blocks. One mean was obtained for each animal per block of trials. The F ratio for repeated-measures ANOVAs that included between- and within-subjects factors was derived according to Erlebacher (1977). An α level of 0.05 was required for significance.
Histology. Marking lesions were placed at the tips of all electrodes by passing direct current (25 μA) for 20 sec. Animals were overdosed with sodium pentobarbital and perfused transcardially with saline (0.9% NaCl) followed by 10% formaldehyde in saline. Brains were then frozen, sectioned coronally (50 μm thick), mounted on albumin–gelatin-coated slides, and stained with neutral red. A light microscope (25× and 50× magnification) was used to locate electrode tips.
Results
A total of 14 rabbits were used for the experimental conditions in this study. Five of these rabbits received trace fear conditioning with a 10 sec trace interval. The behavioral and neural data from this 10 sec trace group were compared statistically with a separate group of five rabbits that received only pseudoconditioning. This formed a balanced comparison of 10 sec trace conditioning (n = 5) versus pseudoconditioning (n = 5).
A balanced comparison of 20 sec trace conditioning (n = 4) versus pseudoconditioning (n = 4) was accomplished using the following group assignment. Three rabbits received pseudo fear conditioning, and after a 1 week rest period, the same three rabbits received trace fear conditioning with a 20 sec trace interval. In addition to these three rabbits, one naive rabbit received only trace fear conditioning with a 20 sec trace interval. The fourth pseudoconditioning rabbit used for this balanced n = 4 analysis was randomly selected from one of the five pseudoconditioning rabbits used for the 10 sec trace conditioning analysis. Separate pseudoconditioning control groups were used for the 10 and 20 sec trace conditioning analyses, because the behavioral HR conditioning data for the 20 sec trace group required a longer sampling duration for each trial.
Heart rate fear conditioning
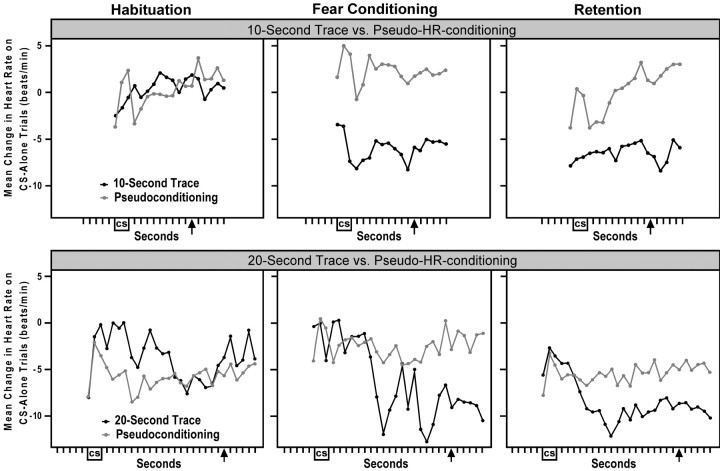
Each rabbit received one habituation session followed immediately by either a trace fear conditioning session or a pseudoconditioning session. Rabbits then received one session of CS-alone retention trials 24 hr later. The top panels of Figure1 show that the rabbits that received 10 sec trace conditioning (n = 5) exhibited larger bradycardiac responses compared with pseudoconditioned rabbits (n = 5) on CS-alone test trials during fear conditioning and on the initial CS-alone retention trials 24 hr later. Analyses confirmed that there was a group difference in HR responding on the five CS-alone test trials during 10 sec trace fear conditioning (t(8) = −2.54; p = 0.027). An analysis of the first five CS-alone retention trials approached but did not reach significance (t(8) = −1.83; p = 0.080). However, a similar analysis that used only the 5 sec period at the end of the 10 sec trace interval reached significance (t(8) = −2.47; p = 0.030). As shown in this figure, there was no significant group difference in HR responding during the last five CS-alone trials of habituation (t(8) = 1.00;p = 0.228). This figure also indicates that rabbits may have timed their HR responses to the duration of the trace interval. Moreover, analyses showed that on the CS-alone trials during fear conditioning and retention, three of the five rabbits in the 10 sec trace group showed average maximal bradycardiac responses ≥10 sec after the offset of the CS.
Fig. 1.
Conditioned HR fear responses. Top panels shows the HR change from baseline on CS-alone trials comparing 10 sec trace conditioned rabbits (n = 5) and the matched pseudoconditioning rabbits (n = 5).Bottom panels shows the HR change from baseline on CS-alone trials comparing the 20 sec trace group (n= 4) and the matched pseudoconditioning group (n = 4). Changes in HR were averaged across the last five CS-alone trials of habituation (left) and the five CS-alone test trials during fear conditioning and pseudoconditioning (middle). Right panels show changes in HR measured across the first five CS-alone trials of retention for the 10 sec trace group and the first 10 CS-alone trials of retention for the 20 sec trace group. The 10 and 20 sec trace groups showed larger bradycardiac-HR fear responses during trace fear conditioning and during the initial trials of retention compared with the matched pseudoconditioning control groups. Although no USs were presented on any of these CS-alone trials, the arrow marks the time of US delivery on CS–trace–US trials during the fear conditioning sessions.
The bottom panels of Figure 1 show that the rabbits that received 20 sec trace conditioning (n = 4) exhibited larger bradycardiac responses compared with the pseudoconditioning group (n = 4) on CS-alone trials during fear conditioning and on the initial CS-alone retention trials 24 hr later. Analyses confirmed that there was a significant group difference in HR responding on the CS-alone test trials during 20 sec trace fear conditioning (t(6) = −3.02;p = 0.015). An analysis of the first five CS-alone retention trials did not reach significance (t(6) = −0.84; p = 0.259); however, a similar analysis that used the first 10 CS-alone retention trials did show a group difference (t(6) = −3.25; p = 0.011). As shown in this figure, these groups showed no difference in HR responding on the final five CS-alone trials of the habituation session (t(6) = 0.95;p = 0.234). Unlike the 10 sec trace group, the 20 sec trace group did not show many HR responses timed to the duration of the 20 sec trace interval on CS-alone trials. In fact, only one rabbit during retention and two rabbits during fear conditioning exhibited maximal bradycardiac responses near the 20 sec time point after the offset of the CS.
CA1-single neuron recording
Habituation and fear conditioning
All of the animals used in this study had electrode tips placed directly in the pyramidal cell body layer of the CA1 area of the dorsal hippocampus. A total of 51 cells and 40 cells were recorded from the 10 sec trace group and the corresponding pseudoconditioning group, respectively, on the day of training that included habituation and fear conditioning. According to the pyramidal–theta cell criteria outlined by Fox and Ranck (1981), five cells had firing characteristics similar to theta cells and the remaining cells were similar to pyramidal cells. Pyramidal cells were used for all analyses in this study. The background-firing rate of the remaining cells with pyramidal cell firing characteristics was 0.57 Hz (SD of 0.77) and 0.28 Hz (SD of 0.36) for the 10 sec trace group and the corresponding pseudoconditioning group, respectively.
A total of 40 and 34 cells were recorded from the 20 sec trace group and the corresponding pseudoconditioning group, respectively, on the day of training that included habituation and fear conditioning. Only two cells had firing characteristics similar to theta cells, and the remaining cells were similar to pyramidal cells. The background firing rate of the remaining cells with pyramidal cell firing characteristics was 0.53 Hz (SD 1.15) and 0.33 Hz (SD 0.54) for the 20 sec trace group and the corresponding pseudoconditioning group, respectively.
CS-alone retention session
One day after the trace and pseudoconditioning session, all animals received one session of CS-alone retention trials. A total of 18 cells from all groups in this study could be reliably tracked from the first day of training to the next retention session. In addition, in four rabbits in this study, the single neuron recording yield improved dramatically between these 2 d of training. A total of 63 and 59 cells were recorded from the 10 sec trace group and the corresponding pseudoconditioning group, respectively, during the CS-alone retention session. All of the cells from these groups exhibited pyramidal cell characteristics and showed a background firing rate of <2 Hz, except for two cells that showed firing between 2 and 3 Hz. The background firing rate during retention was 0.51 Hz (SD of 0.61) and 0.36 Hz (SD of 0.40) for all cells from the 10 sec trace group and the corresponding pseudoconditioning group, respectively.
A total of 65 and 66 cells were recorded from the 20 sec trace group and the corresponding pseudoconditioning group, respectively, during the CS-alone retention session. All of the cells from these groups exhibited pyramidal cell characteristics, and the background firing rate of these cells was 0.34 Hz (SD of 0.39) and 0.45 Hz (SD of 0.46) for the 20 sec trace group and the corresponding pseudoconditioning group, respectively. Immediately preceding the CS-alone retention session, three of the four rabbits in the 20 sec trace fear conditioning group were administered two reminder CS–trace–US trials that included a 20 sec trace interval identical to the trials administered on the previous day of trace conditioning. These reminder trials were used to increase the likelihood of detecting timed neuronal responses on CS-alone retention trials. A comparison of the animal that did not receive the reminder trials with the other three animals revealed that the HR responses and the single neuron encoding of the trace interval on the subsequent CS-alone retention trials were similar for all four rabbits in this group. Thus, it appears that these reminder trials had little or no effect on HR conditioning or single neuron encoding and were therefore not necessary to obtain timed single neuron responses on CS-alone trials.
Encoding of trace interval duration
CA1 pyramidal neuron activity was analyzed during fear conditioning and retention to determine whether CA1 neurons encode information about the trace interval. Examination of the response profiles of individual single neurons recorded on the CS-alone retention trials revealed that a subset of neurons fired action potentials timed to the duration of the trace interval used on previous CS–trace–US trials. Analyses showed that a significant proportion of the neurons from the 10 sec trace conditioning group and the 20 sec trace conditioning group fired action potentials at 10 and 20 sec after the offset of the CS, respectively. This suggests that a subset of CA1 neurons was encoding the duration of the trace interval.
Figure 2 shows representative example histograms and raster plots from three CA1 single neurons recorded on the CS-alone retention trials from the 10 sec trace group and three CA1 neurons recorded from the 20 sec trace group. Although the US was not presented on any of these CS-alone retention trials, these neurons fired the most action potentials at the time point that the US was delivered on previous CS–trace–US trials. Furthermore, this timed firing at 10 and 20 sec after the offset of the CS was fairly consistent across the first 5 or 10 CS-alone retention trials, as shown by the raster plots. There is some variability in timing across trials in the rasters, and this is probably because each CS-alone retention trial also served as an extinction trial. It is likely that this extinction process affected the timed encoding from one trial to the next. Although other individual single neuron response profiles were encountered in CA1 during the retention session, each of the example neurons in Figure 2 was selected from a different rabbit to demonstrate that this encoding effect was consistent across animals.
Fig. 2.

Histograms and raster plots of exemplar CA1 single neurons recorded on CS-alone retention trials that showed encoding of trace interval duration. Each histogram shows the sum of action potentials in 500 msec bins across the initial 7–10 CS-alone retention trials after trace conditioning with a 10 sec trace interval (left) or a 20 sec trace interval (right). These neurons showed increases in firing on CS-alone trials ∼10 or 20 sec after CS offset for each of these groups, respectively. The rasters above each histogram plot the occurrence of each action potential (dots) on each of the first 10 CS-alone retention trials. The plots show that the timed responses for these example neurons were fairly consistent across the first 5 or 10 CS-alone retention trials. No USs were presented on any of the trials in this figure. The arrows mark the latency of the US onset used on previous trace fear conditioning trials. The number of trials used for each histogram is shown at thetop left of each panel. Although only a subset of all neurons recorded from each animal showed trace encoding, each of the example neurons in this figure was selected from a different rabbit, to demonstrate that this encoding effect was consistent across animals.
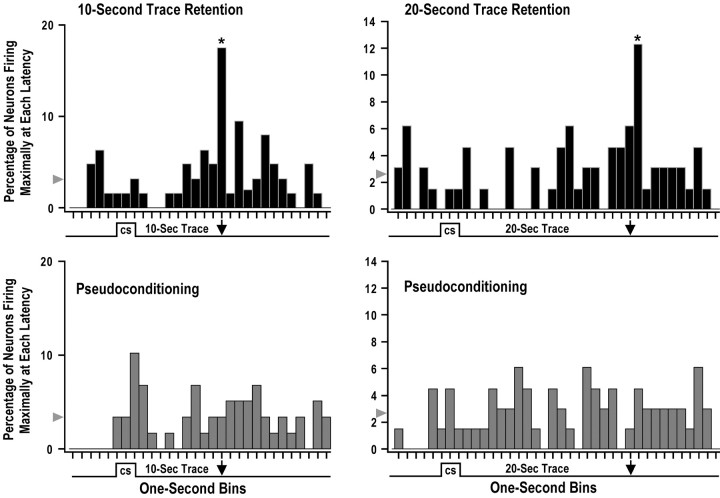
A series of analyses demonstrated that the timed CA1 firing at 10 and 20 sec after the offset of the CS on the CS-alone retention trials was learning related and specific to the duration of the trace interval used during the previous 10 and 20 sec trace fear conditioning session, respectively. Analyses used the latency of maximal firing for each individual single neuron on CS-alone retention trials to show that a significant percentage of CA1 neurons from the 10 and 20 sec trace groups showed maximal firing at 10 and 20 sec after the offset of the CS, respectively. Figure 3 shows the percentage of CA1 neurons firing maximally at each latency during the first five CS-alone retention trials from trace conditioned animals and the matched pseudoconditioning control animals. The left panels of Figure 3 show that 17.5% of the 63 CA1 neurons from the 10 sec trace conditioning group fired maximally 10 sec after CS offset (asterisk) on the initial CS-alone retention trials. The probability of ≥17.5% of the 63 neurons from this group firing maximally during any single 1 sec bin was p = 0.000011. The chance probability of a single neuron firing maximally in any one of the 30 1 sec bins in this analysis was 0.033 (3.3%). The bin with 17.5% of the 63 neurons from the 10 sec trace conditioning group (Fig.3, top left panel, asterisk at 10 sec after CS offset) was the only bin from this and the matched pseudoconditioning group (Fig. 3, bottom left panel) that was significantly different from chance (z = 2.895;p = 0.0038). The bottom left panel of Figure3 shows that there did not appear to be any distinct maximal firing latency for the 59 matched pseudoconditioning neurons, except perhaps immediately after the offset of the CS (10.2%; not significant).
Fig. 3.
Distribution of maximal firing latencies for CA1 neurons recorded on the initial CS-alone retention trials. The maximal firing latency was calculated for each single neuron using the first five CS-alone retention trials, and each 1 sec bin in this figure shows the percentage of single neurons that fired maximally at a specific latency during these CS-alone trials. Although the US was not presented on the CS-alone trials used in this analysis, the arrowshows when the US was presented on previous trace fear conditioning trials. The arrowhead indicates the chance percentage of maximal firing for each bin. The top left panel shows that 17.5% of the 63 CA1 neurons from the 10 sec trace conditioning group fired maximally 10 sec after CS offset (asterisk) on the initial CS-alone retention trials. This bin was significantly greater than chance, and the bottom left panel shows that there did not appear to be any distinct maximal firing latency for the 59 matched pseudoconditioning neurons. The top right panel shows that 12.3% of the 65 CA1 neurons from the 20 sec trace conditioning group fired maximally 21 sec after CS offset (asterisk) on the initial CS-alone retention trials. This bin was significantly greater than chance, and there did not appear to be any distinct maximal firing latency for the 66 matched pseudoconditioning neurons in the bottom right panel. These data show that a significant percentage of CA1 single neurons encode the duration of the trace interval during trace fear conditioning.
The CA1 neurons recorded from the 20 sec trace group also showed timed maximal firing on CS-alone retention trials at a latency that was similar to the duration of the trace interval. The right panels of Figure 3 show that 12.3% of the 65 CA1 neurons from the 20 sec trace conditioning group fired maximally 21 sec after CS offset (asterisk) on the initial CS-alone retention trials. The probability that ≥12.3% of the 65 neurons from this group fired maximally during any single 1 sec bin was p = 0.0003. The chance probability of a single neuron firing maximally in any one of the 38 1 sec bins in this analysis was 0.026 (2.6%). The bin with 12.3% of the 65 neurons from the 20 sec trace conditioning group (Fig.3, top right panel, asterisk at 21 sec after CS offset) was the only bin from this and the matched pseudoconditioning group (Fig. 3, bottom right panel) that was significantly different from chance (z = 2.342;p = 0.019). There did not appear to be any distinct maximal firing latency for the 66 matched pseudoconditioning neurons as shown in the bottom right panel of Figure 3. Note that no USs were presented on any of the CS-alone retention trials used for the analyses in Figure 3, suggesting that a significant percentage of CA1 neurons encoded the duration of the trace interval used on previous CS–trace–US trials. It should be noted that several additional control analyses were performed on the data shown in Figure 3. These control analyses moved the cutoff boundaries of each 1 sec bin earlier or later in 50 msec increments. Analyses were also performed using larger 2 sec bins. All of these control analyses revealed group effects similar to those shown in Figure 3, suggesting that the trace encoding at 10 and 20 sec after CS offset was not attributable to the selection of bin width or the placement of bin boundaries.
Similar latency distribution analyses of maximal reductions in firing (i.e., inhibition) were also attempted on the 10 and 20 sec trace conditioning groups. These approaches used 1 sec bins with the least firing, minima of linear averages of firing, and widest nonspiking intervals; however, none of these analyses revealed distinct latencies of inhibitory responding in either the 10 or 20 sec trace group.
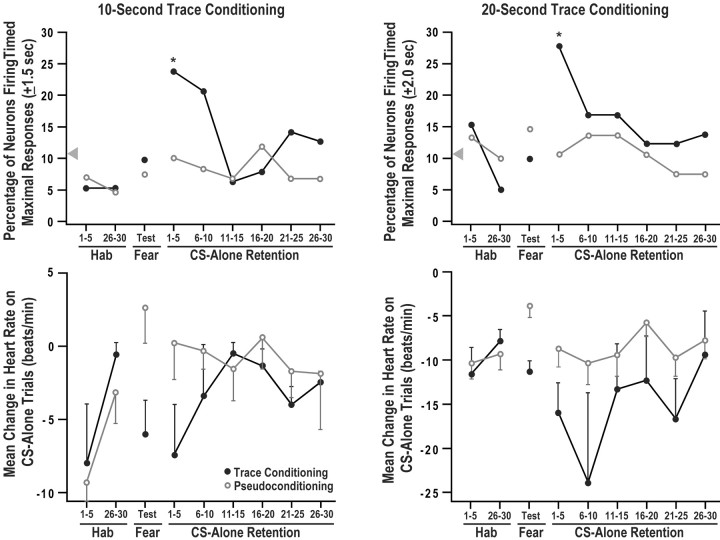
The CA1 encoding of the duration of the trace interval during the first five CS-alone retention trials shown in Figure 3 extinguished rapidly across subsequent CS-alone retention trials. This can be seen in thetop left panel of Figure 4, which shows the percentage of neurons firing maximally at 10 sec after the CS offset (±1.5 sec) for five trial blocks of CS-alone trials during habituation, fear conditioning, and retention for the 10 sec trace conditioning group compared with the matched pseudoconditioning group. This panel shows that there was a significant group difference in the percentage of neurons showing maximal firing timed to the trace interval duration on the first five trial block of CS-alone retention trials (z = 1.994; p = 0.0462; two-tailed). This group difference was nearly significant on the second five trial block of CS-alone retention trials (z = 1.893; p = 0.0584).
Fig. 4.
Conditioned HR responses and percentage of CA1 neurons firing timed maximal responses across five trial blocks for the 10 sec trace group (left) and the 20 sec trace group (right). The top panels show the percentage of neurons exhibiting maximal firing timed to the duration of the trace interval on the first and last five CS-alone trials of habituation (Hab), the CS-alone test trials of pseudo and fear conditioning (Fear), and each of the five trial blocks of the CS-alone retention session. Timed maximal firing occurred at 10 ± 1.5 and 20 ± 2.0 sec after the offset of the CS for the 10 and 20 sec trace groups, respectively. This produced a 3 and 4 sec duration response window for these groups, which is longer in duration than the 1 sec bins used in Figure 3; therefore, the percentage of neurons firing maximally will be larger in this figure. The asterisks indicate that during the first five CS-alone retention trials, the trace groups exhibited a significantly greater percentage of neurons with timed maximal responses within these response windows compared with the matched pseudoconditioning controls. The arrowhead shows the chance percentage of neurons showing maximal firing in the response window. The bottom panels show the mean change in HR for the same groups on the same blocks of CS-alone trials. Note that the y-axes are scaled differently in the bottom panels to highlight group differences in HR conditioning. Significant HR conditioning occurred during the fear conditioning session and during the initial blocks of retention. These data indicate that the time course for CA1 encoding of trace interval duration was parallel to the time course for the expression of the HR fear response. Bars indicate SEM.
Similar to the 10 sec trace group, CA1 encoding of trace interval duration by the 20 sec trace group extinguished rapidly across CS-alone retention trials. This can be seen in the top right panel of Figure 4, which shows the percentage of neurons firing maximally at 20 sec after the CS offset (±2.0 sec) for five trial blocks of CS-alone trials during habituation, fear conditioning, and retention for the 20 sec trace conditioning group versus the matched pseudoconditioning group. The duration of this window of timing (±2.0 sec) had a probability of 0.105 for detecting random maximal firing. This window duration was selected because it was nearly identical to the probability of detecting random maximal firing for the ±1.5 sec window used for the 10 sec trace group (0.107). The window of timed firing at 10 (±1.5) and 20 (±2.0) sec after CS offset produced a 3- and 4-sec-long response window. This window is longer in duration than the 1 sec bins used in Figure 3; therefore, the percentage of neurons firing maximally will be larger in Figure 4 compared with Figure 3. Thetop right panel of Figure 4 shows that on the first five trial block, there was a significant difference between the 20 sec trace group and the matched pseudoconditioning group for the percentage of neurons showing maximal firing timed to 20 sec after CS offset on CS-alone retention trials (z = 2.488; p= 0.0128). This group difference was not present during any other block of CS-alone trials. Analyses of maximal firing latency in Figure 4 used five trial blocks, because in most cases the low firing rate of individual hippocampal neurons required at least five trials to detect a maximal firing latency. The dramatic change in trace encoding from the first to the second five trial block shows that the CS-alone retention trials also served as extinction trials. This rapid extinction process prevented an examination of how the latency of maximal firing changed across CS-alone retention trials.
The bottom left panel of Figure 4 shows the mean change in HR for the 10 sec trace conditioning group and the matched pseudoconditioning group. The HR fear responses on the CS-alone trials in this bottom panel suggest that rabbits associated the CS and US during 10 sec trace fear conditioning and during the initial five trial block of CS-alone retention trials, but this fear response extinguished rapidly across subsequent CS-alone retention trials. A repeated-measures ANOVA was conducted on the HR data shown in thebottom left panel of Figure 4 with a blocks, group, and subject factor. This analysis revealed no significant effects; however, when the habituation blocks were removed, the analysis revealed a significant group effect (F(1,9) = 3.57; p = 0.0386). Surprisingly, the group × blocks interaction from this analysis was not significant. A Pearson correlation applied to the behavioral and encoding data in theleft panels of Figure 4 revealed a moderate inverse correlation (r = −0.29) that approached but did not reach significance. Nevertheless, the time course for CA1 encoding of trace interval duration appears to parallel the time course for the expression of the HR fear response.
The bottom right panel of Figure 4 shows the mean change in HR for the 20 sec trace conditioning group and the matched pseudoconditioning group. The HR fear responses on the CS-alone trials in this bottom panel suggest that rabbits associated the CS and US during 20 sec trace fear conditioning and during the initial 10 CS-alone retention trials, but this fear response appears to extinguish across subsequent CS-alone retention trials. A repeated-measures ANOVA was conducted on the HR data shown in the bottom right panelof Figure 4 with a blocks, group, and subject factor. Because three of the four rabbits in the 20 sec trace group were also used in the matched pseudoconditioning group, the group factor in this analysis was treated as a within-subjects factor according to Erlebacher (1977). This analysis revealed no significant effects; however, when the habituation blocks were removed, the analysis revealed a significant group effect (F(1,7) = 4.37;p = 0.0265). Surprisingly, the group × blocks interaction from this analysis was not significant. A Pearson correlation applied to the behavioral and encoding data in theright panels of Figure 4 revealed a significant (p < 0.05; two tailed) inverse correlation (r = −0.50). This suggests that the time course for CA1 encoding of trace interval duration was parallel to the time course for the expression of the HR fear response.
The data in Figure 4 provide additional evidence that the encoding of the trace interval duration is learning related and specific to the trace interval duration. Furthermore, the time course of trace encoding parallels the expression of HR fear responses, suggesting that the encoding of the trace interval duration may be related to the strength of the association of the CS and US. It is important to mention that the group differences in timed maximal firing at the beginning of the CS-alone retention session were not caused by an arbitrary selection of a response window duration. The group differences in timed maximal firing shown in the top left panel of Figure 4 were nearly identical when the response window was narrowed to ±1.0 sec or widened to ±3.5 sec. Similarly, the group differences in CA1 timing for 20 sec trace fear conditioning in the top right panel of this figure were identical if the response window was narrowed to ±1.0 sec or widened to ±3.0 sec. Furthermore, moving the original response window for the 10 and 20 sec trace groups 2.0 sec back or ahead of the original trace interval duration completely eliminated all group differences, providing additional evidence that the group differences were specific to the encoding of trace duration.
Average CA1-single neuron responses
Figure 5 shows histograms averaged across the CA1 single neuron activity from the last three CS-alone trials of habituation, the first three CS-alone test trials of fear conditioning, and the first three CS-alone trials of retention for the 10 sec trace conditioning group and the matched pseudoconditioning group. Figure 6 shows analogous CS-alone histograms from the 20 sec trace group and the matched pseudoconditioning group. During trace fear conditioning, one CS-alone trial was presented after every seven paired trials; therefore, the CS-alone test trials sampled changes in activity spread across the initial 24 fear conditioning and test trials. The average single neuron response topography revealed no group-specific pattern during the CS-alone trials of habituation or fear conditioning. Standard score measurements of activity during habituation and trace conditioning trials examined average changes in firing during the CS and trace period but revealed no systematic group differences. Individual single neuron responses to the tone CS were heterogeneous for all groups, consisting of various increases and decreases in firing to the CS. As a result, no group-specific pattern of responding to the CS could be isolated either during habituation or during fear conditioning. Furthermore, no individual response profile was revealed that encoded trace duration during the trace fear conditioning training session. Activity was averaged across only three trials in Figures 5 and 6, because three animals in the 10 sec trace group and two animals in the 20 sec trace group showed significant movement artifacts in the neural record in the latter half of the trace fear conditioning sessions. This was presumably attributable to the repeated presentation of the shock stimulus. Nevertheless, a trial-by-trial analysis was used to examine the development of single neuron encoding of trace duration across the first three CS-alone test trials during 10 and 20 sec trace fear conditioning, but no systematic pattern or trend could be isolated from any group.
Fig. 5.
Mean CA1 single neuron responses for the 10 sec trace conditioning group (top) and the matched pseudoconditioning group (bottom) on CS-alone trials. Histograms show single neuron activity in 500 msec bins averaged across the last three CS-alone trials during habituation (left), the first three CS-alone test trials of trace fear conditioning or pseudoconditioning (middle), and the first three CS-alone trials of retention (right). No significant group differences were revealed by average change score analyses, but the 10 sec trace group appears to show an increase in activity ∼10 sec after the offset of the CS on the initial CS-alone retention trials. Although no USs were presented on any of these CS-alone trials, the arrow marks 10 sec after CS offset, the time of US delivery during 10 sec trace fear conditioning (top middle). The duration of the CS was 3 sec.
Fig. 6.
Mean CA1 single neuron responses for the 20 sec trace conditioning group (top) and the matched pseudoconditioning group (bottom) on CS-alone trials. Histograms show single neuron activity in 500 msec bins averaged across the last three CS-alone trials during habituation (left), the first three CS-alone test trials of trace fear conditioning or pseudoconditioning (middle), and the first three CS-alone trials of retention (right). No significant group differences were revealed by average change score analyses, but the 20 sec trace group appears to show an increase in activity ∼20 sec after the offset of the CS on the initial CS-alone retention trials. Although no USs were presented on any of these CS-alone trials, the arrow marks 20 sec after CS offset, the time of US delivery during 20 sec trace fear conditioning (top middle). The duration of the CS was 3 sec.
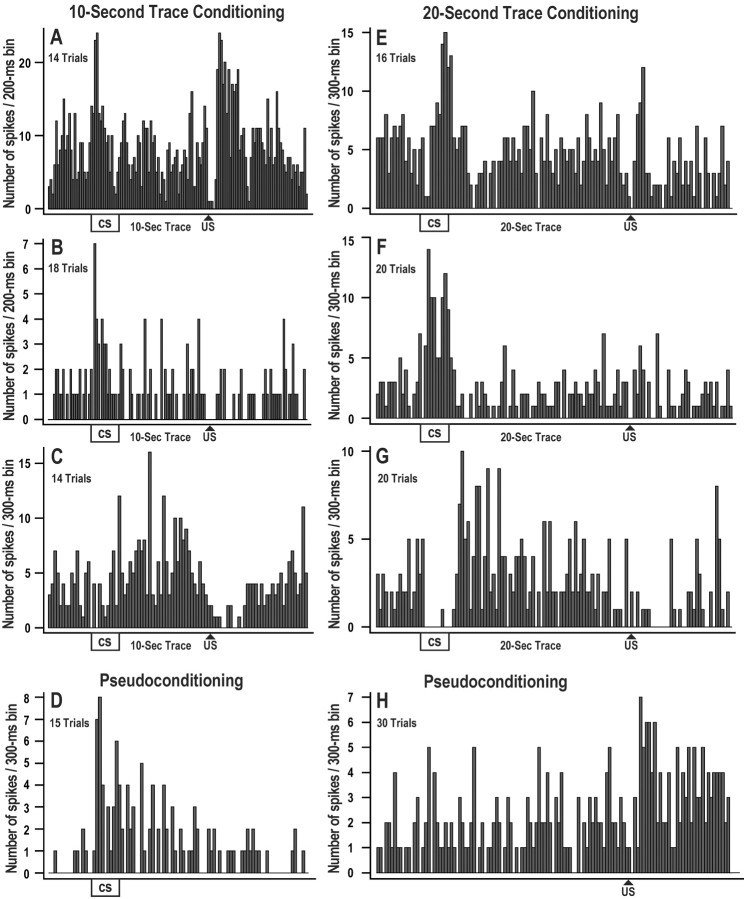
Although average standard score analyses revealed no consistent group differences during fear conditioning, individual single neuron response topographies suggest that a subset of CA1 neurons may play a role in processing CS, trace, or US information during fear conditioning trials. Figure 7 shows example neurons that exhibited excitatory and/or inhibitory responses during 10 and 20 sec trace fear conditioning. The example neurons in this figure were selected because they showed distinct responses to the CS, trace, or US. The response patterns in this figure were not necessarily the most common profiles; for example, neurons A and E showed excitatory responses to both the CS and US, and this pattern of responding was seen in only 5.8 and 15.0% of the neurons recorded from the 10 and 20 sec trace group, respectively. In fact, numerous combinations of excitatory and inhibitory responses to the CS or US were revealed, and very few if any response patterns were exhibited consistently across neurons using individual or average standard score analyses. This suggests that CA1 neurons do not process CS, trace, and US information by a narrow set of unimodal responses; rather, it is likely that numerous heterogeneous response profiles interact in an ensemble manner to encode information. Standard score responses were computed to the CS, trace interval, and US for each individual neuron using 5000 msec windows and 15 trial blocks during trace and pseudoconditioning. Table1 shows the percentage of neurons in each group exhibiting significant increases or decreases in response to the CS, trace, or US. This table shows that excitatory and inhibitory responses were comparable between the trace and pseudo groups. The only exception was that the pseudo group exhibited more inhibitory decreases in activity in response to the CS compared with the 10 sec trace group (z = −2.70;p = 0.007). It was unclear whether this was a true learning-related effect, because the same effect was not revealed for the 20 sec trace group. Analyses also used a trial-by-trial approach to determine how single neuron changes in activity in response to the CS and US developed across trials during the 10 and 20 sec trace fear conditioning sessions. Because of significant movement artifacts from some rabbits, these analyses were limited to the first half of trace and pseudoconditioning trials, and no systematic pattern or trend could be isolated from any group.
Fig. 7.
Histograms of exemplar CA1 single neurons recorded during trace fear conditioning or pseudoconditioning. Each histogram shows the sum of action potentials in 200 or 300 msec bins across 14–30 fear conditioning trials with a 10 sec trace interval (A–C) or a 20 sec trace interval (E–G). Neurons A andB show excitatory responses to the CS and US during trace fear conditioning. Neurons B and Fshow excitation only to the CS. Inhibitory responses are shown inC and G, with some excitation during the trace interval. Neurons D and H show excitatory responses during pseudoconditioning. Overall, excitatory and inhibitory responses to the CS and US were heterogeneous during pseudo and trace fear conditioning, and these histograms represent only a few of the response profiles observed. Analyses revealed no group differences or consistent timing effects during the fear conditioning session.
Table 1.
Percentage of significantly responsive CA1 neurons during fear conditioning (p < 0.05)
| Excitatory increases | Inhibitory decreases | |||||
|---|---|---|---|---|---|---|
| CS | Trace | US | CS | Trace | US | |
| 10 sec trace group (n = 51) | 7.8 | 11.8 | 17.6 | 9.8* | 0 | 11.8 |
| Pseudoconditioning group (n = 40) | 17.5 | 27.5 | 30.0 | 32.5 | 7.5 | 27.5 |
| 20 sec trace group (n = 40) | 20.0 | 22.5 | 20.0 | 22.5 | 2.5 | 25.0 |
| Pseudoconditioning group (n = 34) | 17.6 | 26.5 | 29.4 | 20.5 | 2.9 | 23.5 |
*p = 0.007.
Standard score measurements were also used to examine average changes in firing during the CS and trace period from the CS-alone retention session. Mean standard scores were compared between the 10 sec trace group and the corresponding pseudoconditioning group and between the 20 sec trace group and the corresponding pseudoconditioning group. These analyses used various trial–block combinations but revealed no significant group differences. However, the initial CS-alone retention trials revealed a modest yet noticeable increase in mean firing near the time point at which the US was delivered on the previous day of training. This can be seen in the retention portion of Figures 5 and 6, which shows several larger bins of activity ∼10 and 20 sec after the offset of the CS, respectively. Although this small number of bins did not produce statistical significance in the overall average analyses, this trend in timed maximal firing is consistent with the analyses depicted in Figures 2-4, which demonstrate that a significant proportion of CA1 neurons encode the duration of the trace interval.
Discussion
This study demonstrates that a significant percentage of CA1 hippocampal single neurons fired timed action potentials on CS-alone retention trials at a latency similar to the duration of the trace interval used on previous CS–trace–US trials. This timed CA1 firing on CS-alone retention trials specific to the duration of either a 10 sec or a 20 sec trace interval, and was not seen in matched pseudoconditioning control animals. This suggests that a significant percentage of CA1 single neurons encode the duration of the trace interval during auditory trace fear conditioning. The percentage of neurons encoding trace duration followed closely the time points at which rabbits exhibited significant fear responses to the CS. This is consistent with the notion that the encoding of trace interval duration was linked to the strength of the association of the CS and US.
A significant percentage (24–28%) of neurons encoded the duration of the trace interval; however, the remaining majority of the neurons in CA1 did not exhibit trace encoding. The subset of neurons exhibiting trace encoding was based on a very conservative definition of timed firing with an accuracy of ±1.5 or ±2.0 sec in the 10 and 20 sec trace groups, respectively. This analysis for trace encoding required a minimum of five trials per block, in part because of the very low firing rate for the CA1 hippocampal neurons. Therefore, a trial-by-trial analysis of encoding was not possible for most of the neurons recorded in this study. It is possible that other unrevealed subsets of neurons in this study showed encoding, but with poorer accuracy or for only one or two trials. Alternatively, the larger proportion of non-trace encoding neurons may have other roles in the encoding process in addition to timing the trace duration. Regardless of this issue, the accurate, categorical, and robust nature of CA1 trace encoding provides support for other studies that suggest that the hippocampus is involved in temporal processing (Rawlins et al., 1983;Meck et al., 1984; Jackson et al., 1998). A previous investigation byYoung and McNaughton (2000) demonstrated that subsets of hippocampal neurons exhibit appropriately timed responses to a temporal interval in an operant task in which reinforcement of lever pressing was contingent on a 15 sec interval. Although they admit that the timed responses in their study could be correlated with behavioral approach or inhibition, the network mechanisms responsible for the timed hippocampal responses in their study are probably similar to the mechanisms that control the CA1 encoding of trace duration reported in the present study.
The CA1 encoding of trace duration was short lasting, persisting approximately as long as the expression of the conditioned fear response. Trace interval encoding was seen only on CS-alone retention trials, which were also extinction trials. This extinction process quickly disrupted trace encoding, which was significantly diminished after approximately five CS-alone retention trials. It is possible that increasing the number of days of trace fear conditioning will increase the persistence of the CA1 trace encoding. Trace encoding in CA1 and HR responding were only moderately correlated. This may suggest that HR responses are a better indicator of CS–US associative strength rather than timing of the trace interval. Nevertheless, the encoding of trace duration shared a similar time course with the expression of the conditioned HR response, because both persisted for approximately five CS-alone retention trials. It is impossible to resolve whether the encoding of trace duration was a neurophysiological precursor event required for the expression of the conditioned fear response, or whether it was a more complex extended form of the neurophysiological representation of the CS–US association. A greater number of trials and training sessions in future studies may provide the number of time points necessary for examining the dynamics of this relationship between neural coding and behavior.
Earlier hippocampal research was dominated by the view that the hippocampus is strictly involved in processing spatial information during learning (Black et al., 1977; O'Keefe and Nadel, 1978; Morris et al., 1982; Nadel, 1991). In the present trace HR conditioning paradigm only nonspatial stimuli were used, and rabbits were restrained in a single fixed position, so there was no spatially encoded information required for learning. The present study adds to the numerous lines of converging evidence that suggest that the hippocampus is involved in processing nonspatial stimuli as well as spatial stimuli during learning. Many nonspatial hippocampus-dependent learning paradigms require an association of stimuli that are separated by a significant temporal interval (Solomon, 1977; Moore, 1979; Rawlins and Tsaltas, 1983; McEchron et al., 1999). One of these paradigms, trace eyeblink conditioning, has been used extensively to study hippocampal cellular mechanisms of plasticity related to learning. Although the trace interval in eyeblink conditioning (500 msec) is much shorter than in trace fear conditioning (10–30 sec), eyeblink and fear conditioning are similar, because hippocampal lesions disrupt learning when the trace interval separates the CS and US, but not when the CS overlaps with the US (Solomon et al., 1986; Moyer et al., 1990; Kim and Fanselow, 1992; Kim et al., 1995; McEchron et al., 1998, 2000). Studies have shown that hippocampal single neuron activity during the trace eyeblink trial is related to the acquisition of eyeblink conditioned responses (McEchron and Disterhoft, 1997), and trace eyeblink conditioning has been shown to produce learning-specific changes in synaptic plasticity and membrane excitability in the hippocampus (Moyer et al., 1996; Thompson et al., 1996; Power et al., 1997). Future studies may show that similar changes in plasticity occur in the hippocampus after trace fear conditioning.
Clearly the hippocampus is involved in processing spatial and nonspatial information during learning. The spatial and nonspatial roles of the hippocampus in learning are explained particularly well with the discontiguity theory outlined by Wallenstein et al. (1998). They argue that the hippocampus is critically involved in learning tasks in which discontiguous items must be associated, and these items can be temporally or spatially discontiguous, or both. A number of other researchers have described similar hippocampal theories of discontiguity (Rawlins, 1985; Rolls, 1990; Cohen and Eichenbaum, 1991;Gluck and Myers, 1993; Rudy and Sutherland, 1995; Levy, 1996). The notion of discontiguity applies well to trace fear conditioning, because learning requires an association between two stimuli, the tone CS and shock US, which do not overlap and are separated by a long temporal interval. As with other trace conditioning paradigms, the hippocampus is required for the association of the CS and US when a trace interval separates these stimuli, but not when these stimuli are contiguous or overlapping (Moyer et al., 1990; McEchron et al., 1998;Beylin et al., 2001).
Data from the 10 sec trace group demonstrate that the encoding of trace duration is retained for at least 24 hr after the trace fear conditioning session. This retention probably also occurs after 20 sec trace conditioning, but this was not directly tested in this study, because three of the four animals in this group received two reminder CS–trace–US trials immediately before the CS-alone retention trials. Future studies should address the maximal retention period of CA1-trace encoding. Other studies have shown that learning-related biophysical alterations in hippocampal membrane excitability persist for as long as 5 d after trace eyeblink conditioning (Moyer et al., 1996;Thompson et al., 1996).
Average standard score analyses did not reveal any consistent learning-related changes in responding to the CS, US, or trace interval during any of the fear conditioning sessions. However, individual CA1 neurons from both the trace and pseudoconditioning groups did show heterogeneous patterns of excitatory and inhibitory responses to the CS, trace, and US during training. These heterogeneous response patterns may suggest that more single neuron recordings are needed to see a consistent learning-related response pattern. Alternatively, it is possible that CA1 neurons do not process CS, trace, and US information by a narrow set of unimodal responses, but rather the heterogeneous response profiles may interact in an ensemble manner to encode information. Our previous work has shown that heterogeneous response profiles interact to encode information during trace eyeblink conditioning (McEchron et al., 2001). Another possibility is that the role of CA1 neurons is not to respond to and process CS, trace, and US information, but rather to process and store the duration of the trace interval. This would suggest that other areas of the hippocampal network may be responsible for processing and retaining CS, trace, and US information and passing this information to CA1. Lesioning work byGilbert et al. (2001) supports this notion of region-specific functions within the hippocampus. Their work shows that the function of temporal pattern separation is specific to the CA1 area rather than the dentate gyrus area of the hippocampus, and that spatial pattern separation is specific to the dentate gyrus rather than CA1 (Gilbert et al., 2001). However, the results of the present study do not rule out the possibility that the encoding of trace duration was transmitted directly from another area of the network such as the entorhinal cortex.
The CA1 encoding of trace duration in this study describes a learning-related categorical neuronal representation of a temporal component of the trace fear conditioning paradigm. Although CA1 neurons encoded the duration of the trace interval, no learning-specific CA1 single neuron response patterns were revealed that consistently sustained or bridged the duration of the empty trace interval. This suggests that other areas outside of CA1 may play a role in holding information across the trace interval. The categorical and reliable nature of trace encoding in CA1 should provide an excellent experimental tool for addressing the role of other areas of the hippocampal network in relation to CA1 during trace fear conditioning.
Footnotes
Correspondence should be addressed to Matthew D. McEchron, Department of Behavioral Science, Pennsylvania State College of Medicine, 500 University Drive., H181, Hershey, PA 17033. E-mail: mdm27@psu.edu.
References
- 1.Apkarian AV, Shi T, Bruggemann J, Airapetian LR. Segregation of nociceptive and non-nociceptive networks in the squirrel monkey somatosensory thalamus. J Neurophysiol. 2000;84:484–494. doi: 10.1152/jn.2000.84.1.484. [DOI] [PubMed] [Google Scholar]
- 2.Beylin AV, Gandhi CC, Wood GE, Talk AC, Matzel LD, Shors TJ. The role of the hippocampus in trace conditioning: temporal discontinuity or task difficulty? Neurobiol Learn Mem. 2001;76:447–461. doi: 10.1006/nlme.2001.4039. [DOI] [PubMed] [Google Scholar]
- 3.Black AH, Nadel L, O'Keefe J. Hippocampal function in avoidance learning and punishment. Psychol Bull. 1977;84:1107–1129. [PubMed] [Google Scholar]
- 4.Cohen NJ, Eichenbaum H. The theory that wouldn't die: a critical look at the spatial mapping theory of hippocampal function. Hippocampus. 1991;1:265–268. doi: 10.1002/hipo.450010312. [DOI] [PubMed] [Google Scholar]
- 5.Erlebacher A. Design and analysis of experiments contrasting the within- and between-subjects manipulation of the independent variable. Psychol Bull. 1977;84:212–219. [Google Scholar]
- 6.Fox SE, Ranck JB., Jr Electrophysiological characteristics of hippocampal complex-spike cells and theta cells. Exp Brain Res. 1981;41:399–410. doi: 10.1007/BF00238898. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert PE, Kesner RP, Lee I. Dissociating hippocampal subregions: double dissociation between dentate gyrus and CA1. Hippocampus. 2001;11:626–636. doi: 10.1002/hipo.1077. [DOI] [PubMed] [Google Scholar]
- 8.Gluck MA, Myers CE. Hippocampal mediation of stimulus representation: a computational theory. Hippocampus. 1993;3:491–516. doi: 10.1002/hipo.450030410. [DOI] [PubMed] [Google Scholar]
- 9.Jackson PA, Kesner RP, Amann K. Memory for duration: role of hippocampus and medial prefrontal cortex. Neurobiol Learn Mem. 1998;70:328–348. doi: 10.1006/nlme.1998.3859. [DOI] [PubMed] [Google Scholar]
- 10.Kim JJ, Fanselow MS. Modality-specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- 11.Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behav Neurosci. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- 12.Lee J, Lee HP, Fong NP. A SAS procedure for exact probability testing of difference between sample and population proportion. Comput Biol Med. 1989;19:137–143. doi: 10.1016/0010-4825(89)90006-1. [DOI] [PubMed] [Google Scholar]
- 13.Levy WB. A sequence predicting CA3 is a flexible associator that learns and uses context to solve hippocampal-like tasks. Hippocampus. 1996;6:579–590. doi: 10.1002/(SICI)1098-1063(1996)6:6<579::AID-HIPO3>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 14.McEchron MD, Disterhoft JF. Sequence of single neuron changes in CA1 hippocampus of rabbits during acquisition of trace eyeblink conditioned responses. J Neurophysiol. 1997;78:1030–1044. doi: 10.1152/jn.1997.78.2.1030. [DOI] [PubMed] [Google Scholar]
- 15.McEchron MD, Bouwmeester H, Tseng W, Weiss C, Disterhoft JF. Hippocampectomy disrupts trace auditory cued fear conditioning and contextual fear conditioning in the rat. Hippocampus. 1998;8:638–646. doi: 10.1002/(SICI)1098-1063(1998)8:6<638::AID-HIPO6>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 16.McEchron MD, Tseng W, Disterhoft JF. Neurotoxic lesions of the dorsal hippocampus disrupt auditory-cued trace heart rate (fear) conditioning in rabbits. Hippocampus. 2000;10:739–751. doi: 10.1002/1098-1063(2000)10:6<739::AID-HIPO1011>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 17.McEchron MD, Weible AP, Disterhoft JF. Aging and learning-specific changes in single-neuron activity in CA1 hippocampus during rabbit trace eyeblink conditioning. J Neurophysiol. 2001;86:1839–1857. doi: 10.1152/jn.2001.86.4.1839. [DOI] [PubMed] [Google Scholar]
- 18.McNaughton BL, O'Keefe J, Barnes CA. The stereotrode: a new technique for simultaneous isolation of several single units in the central nervous system from multiple unit records. J Neurosci Methods. 1983;8:391–397. doi: 10.1016/0165-0270(83)90097-3. [DOI] [PubMed] [Google Scholar]
- 19.Meck WH, Church RM, Olton DS. Hippocampus, time, and memory. Behav Neurosci. 1984;98:3–22. doi: 10.1037//0735-7044.98.1.3. [DOI] [PubMed] [Google Scholar]
- 20.Moore JW. Information processing in space-time by the hippocampus. Physiol Psychol. 1979;7:224–232. [Google Scholar]
- 21.Morris RGM, Garrud P, Rawlins JNP, O'Keefe JO. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 22.Moye TB, Rudy JW. Ontogenesis of trace conditioning in young rats: dissociation of associative and memory processes. Dev Psychobiol. 1987;20:405–414. doi: 10.1002/dev.420200405. [DOI] [PubMed] [Google Scholar]
- 23.Moyer JR, Deyo RA, Disterhoft JF. Hippocampectomy disrupts trace eye-blink conditioning in rabbits. Behav Neurosci. 1990;104:243–252. doi: 10.1037//0735-7044.104.2.243. [DOI] [PubMed] [Google Scholar]
- 24.Moyer JR, Thompson LT, Disterhoft JF. Trace eyeblink conditioning increases CA1 excitability in a transient and learning-specific manner. J Neurosci. 1996;16:5536–5546. doi: 10.1523/JNEUROSCI.16-17-05536.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nadel L. The hippocampus and space revisited. Hippocampus. 1991;1:221–229. doi: 10.1002/hipo.450010302. [DOI] [PubMed] [Google Scholar]
- 26.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford UP; London: 1978. [Google Scholar]
- 27.Power JM, Thompson LT, Moyer JR, Jr, Disterhoft JF. Enhanced synaptic transmission in CA1 hippocampus after eyeblink conditioning. J Neurophysiol. 1997;78:1184–1187. doi: 10.1152/jn.1997.78.2.1184. [DOI] [PubMed] [Google Scholar]
- 28.Quinn JJ, Oommen SS, Morrison GE, Fanselow MS. Post-training excitotoxic lesions of the dorsal hippocampus attenuate forward trace, backward trace, and delay fear conditioning in a temporally specific manner. Hippocampus. 2002;12:495–504. doi: 10.1002/hipo.10029. [DOI] [PubMed] [Google Scholar]
- 29.Quirk MC, Wilson MA. Interaction between spike waveform classification and temporal sequence detection. J Neurosci Methods. 1999;94:41–52. doi: 10.1016/s0165-0270(99)00124-7. [DOI] [PubMed] [Google Scholar]
- 30.Ranck JB., Jr Studies on single neurons in dorsal hippocampal formation and septum in unrestrained rats. I. Behavioral correlates and firing repertoires. Exp Neurol. 1973;41:461–531. doi: 10.1016/0014-4886(73)90290-2. [DOI] [PubMed] [Google Scholar]
- 31.Rawlins JN. Associations across time: the hippocampus as a temporary memory store. Behav Brain Sci. 1985;8:479–496. [Google Scholar]
- 32.Rawlins JN, Tsaltas E. The hippocampus, time and working memory. Behav Brain Res. 1983;10:233–262. doi: 10.1016/0166-4328(83)90033-5. [DOI] [PubMed] [Google Scholar]
- 33.Rawlins JN, Winocur G, Gray JA. The hippocampus, collateral behavior, and timing. Behav Neurosci. 1983;97:857–872. doi: 10.1037//0735-7044.97.6.857. [DOI] [PubMed] [Google Scholar]
- 34.Rolls E. Principles underlying the representation and storage of information in neuronal networks in the primate hippocampus and cerebral cortex. In: Zornetzer SF, Davis JL, Lau C, editors. An introduction to neural and electronic networks. Academic; San Diego: 1990. pp. 73–90. [Google Scholar]
- 35.Rosner BA. Hypothesis testing: categorical data. In: Rosner BA, editor. Fundamentals of biostatistics, Ed 3. Duxbury; Belmont, CA: 1990. pp. 319–322. [Google Scholar]
- 36.Rudy JW, Sutherland RJ. Configural association theory and the hippocampal formation: an appraisal and reconfiguration. Hippocampus. 1995;5:375–389. doi: 10.1002/hipo.450050502. [DOI] [PubMed] [Google Scholar]
- 37.Solomon PR. Role of the hippocampus in blocking and conditioned inhibition of the rabbits' nictitating membrane response. J Comp Physiol Psychol. 1977;91:407–417. doi: 10.1037/h0077330. [DOI] [PubMed] [Google Scholar]
- 38.Solomon PR, Vander Schaaf ER, Thompson RF, Weisz DJ. Hippocampus and trace conditioning of the rabbit's classically conditioned nictitating membrane response. Behav Neurosci. 1986;100:729–744. doi: 10.1037//0735-7044.100.5.729. [DOI] [PubMed] [Google Scholar]
- 39.Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76:1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- 40.Wallenstein GV, Eichenbaum H, Hasselmo ME. The hippocampus as an associator of discontiguous events. Trends Neurosci. 1998;8:317–323. doi: 10.1016/s0166-2236(97)01220-4. [DOI] [PubMed] [Google Scholar]
- 41.Wilson MA, McNaughton BL. Dynamics of the hippocampal ensemble code for space. Science. 1993;261:1055–1058. doi: 10.1126/science.8351520. [DOI] [PubMed] [Google Scholar]
- 42.Young B, McNaughton N. Common firing patterns of hippocampal cells in a differential reinforcement of low rates of response schedule. J Neurosci. 2000;20:7043–7051. doi: 10.1523/JNEUROSCI.20-18-07043.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]