Abstract
We hypothesized that cessation of brainstem monoaminergic systems and an activation of brainstem inhibitory systems are both involved in pontine inhibitory area (PIA) stimulation-induced muscle atonia. In our previous study (Lai et al., 2001), we found a decrease in norepinephrine and serotonin release in motoneuron pools during PIA stimulation-induced muscle tone suppression. We now demonstrate an increase in inhibitory amino acid release in motor nuclei during PIA stimulation in the decerebrate cat using in vivomicrodialysis and HPLC analysis techniques. Microinjection of acetylcholine into the PIA elicited muscle atonia and simultaneously produced a significant increase in both glycine and GABA release in both the hypoglossal nucleus and the lumbar ventral horn. Glycine release increased by 74% in the hypoglossal nucleus and 50% in the spinal cord. GABA release increased by 31% in the hypoglossal nucleus and 64% in the spinal cord during atonia induced by cholinergic stimulation of the PIA. As with cholinergic stimulation, 300 msec train electrical stimulation of the PIA elicited a significant increase in glycine release in the hypoglossal nucleus and ventral horn. GABA release was significantly increased in the hypoglossal nucleus but not in the spinal cord during electrical stimulation of the PIA. Glutamate release in the motor nuclei was not significantly altered during atonia induced by electrical or acetylcholine stimulation of the PIA. We suggest that both glycine and GABA play important roles in the regulation of upper airway and postural muscle tone. A combination of decreased monoamine and increased inhibitory amino acid release in motoneuron pools causes PIA-induced atonia and may be involved in atonia linked to rapid eye-movement sleep.
Keywords: glycine, GABA, hypoglossal nucleus, spinal cord, upper airway, REM sleep, obstructive sleep apnea
Introduction
Activation of the pontine inhibitory area (PIA), the medial part of the nucleus pontis centralis oralis, generates muscle atonia in decerebrate animals (Lai and Siegel, 1999;Hajnik et al., 2000) and induces rapid eye-movement (REM) sleep in freely moving cats (Van Dongen et al., 1978). Application of acetylcholine (ACh) (George et al., 1964; Lai and Siegel, 1988;Kodama et al., 1990; Reinoso-Suarez et al., 1994), glutamate (Lai and Siegel, 1991; Onoe and Sakai, 1995), corticotropin-releasing factor (Lai and Siegel, 1992), and GABA antagonist (Xi et al., 2001) to the PIA can trigger muscle atonia and REM sleep.
At the level of the motor nuclei, the balance between excitatory and inhibitory neurotransmitter release determines motoneuronal excitability and muscle activity. The brainstem monoaminergic neuronal groups, the locus ceruleus and raphe nuclei, have been demonstrated to exert a facilitatory effect on motoneurons (Lai et al., 1989; White and Fung, 1989). In contrast, the medial portion of the pontomedullary reticular formation has been postulated to inhibit motoneuronal activity via an active inhibitory mechanism (Llinas and Terzuolo, 1964). Suppression of muscle tone could result from activation of inhibitory systems, inactivation of excitatory systems, or both. Our previous study showed a reduction of norepinephrine and serotonin release in both the spinal ventral horn and hypoglossal (XII) nucleus during PIA stimulation in the decerebrate cat (Lai et al., 2001). This result combined with other studies that showed a facilitatory effect of these monoamines on motoneurons (Takahashi and Berger, 1990; Parkis et al., 1995; Jelev et al., 2001) indicates that disfacilitation plays an important role in muscle tone suppression.
The physiological function of glycine in the control of REM sleep atonia and brainstem stimulation-induced motor inhibition has been well studied. Systemic application of strychnine suppresses pontine stimulation-induced inhibition of the monosynaptic reflex and reverses motoneuron IPSPs induced by pontine stimulation in the decorticated cat (Kawai and Sasaki, 1964). Chase and his colleagues found that iontophoretic application of strychnine, a glycine antagonist, into motor nuclei blocked pontine carbachol or natural REM sleep-induced IPSPs in the spinal (Chase et al., 1989), trigeminal (Soja et al., 1991), and hypoglossal (Yamuy et al., 1999) motoneurons. Thus, they concluded that glycine plays a critical role in the control of REM sleep atonia in the postural and upper airway muscles. In contrast,Kubin et al. (1993) hypothesized that inhibitory amino acids might not be important in the regulation of hypoglossal motoneuron activity during REM sleep and that pontine inhibition of respiratory motoneurons was attributable to disfacilitation via cessation of serotonin release rather than glycinergic inhibition.
GABA has also been shown to inhibit motoneuron activity (Curtis, 1959;ten Bruggencate and Sonnhof, 1972; Yajima and Hayashi, 1997). However, its role in the regulation of muscle tone across sleep cycle has not been identified. Therefore, our current study was designed to determine (1) the nature of the change in both glycine and GABA release in the motoneuron pools during PIA-induced muscle tone suppression and (2) whether there was a difference between the release of glycine in the ventral horn and hypoglossal nucleus during pontine-induced atonia.
Materials and Methods
All procedures were approved by the Animal Care Committee at the University of California, Los Angeles [Animal Research Committee (ARC) #01-042-01] and the Veterans Affairs Greater Los Angeles Healthcare System (ARC #9308-053 and ARC #9710-023). Experiments were performed in two male and six female cats. Surgical preparation was described previously (Lai et al., 2001). Briefly, tracheotomy, arterial and venous cannulations, and decerebration at the precollicular–postmammillary level were performed under halothane–oxygen anesthesia. Halothane was discontinued immediately after decerebration. The caudomedial cerebellum was removed by aspiration for implantation of dialysis probes. Laminectomy was performed at the L5–S1 segments to expose the spinal cord for insertion of dialysis probes. Electromyographic (EMG) activity was recorded from the genioglossus muscle, which opens the upper airway, diaphragm, the neck (occipitoscapularis and splenius), and the right gastrocnemius muscles using bipolar electrodes. Blood pressure was measured through a Gould-Statham blood pressure transducer. Mean arterial pressure was kept at >80 mmHg. Rectal temperature was monitored at 38 ± 0.5°C by a heating pad regulated by an automatically activated thermoregulator.
Chemical microinjection and electrical stimulation. Three of the eight cats received ACh injection into the PIA, whereas electrical stimulation of the PIA was performed in all eight cats. Chemical stimulation of the PIA after its identification by electrical stimulation (Lai and Siegel, 1999) was achieved by microinjection of ACh (1 m/0.5 μl) through a 25 small gauge 1 μl Hamilton microsyringe (model 7001). Three microinjections were performed on each PIA with at least 3 hr between microinjections. Electrical stimulation was delivered into the PIA through a stainless steel microelectrode (5712; A-M Systems, Carlsberg, WA), using 300 msec trains of 100 Hz, 0.2 msec, and 10–60 μA rectangular cathodal pulses, once every 10 sec over a period of 5 min. As with the ACh injection experiment, three electrical stimulations were administered to each PIA site with an interval of 3 hr between stimulations.
Microdialysis sampling. Microdialysis probes were aimed at the XII nucleus, which innervates genioglossus, and at L5–S1 ventral horn, whose motoneurons innervate postural musculature. Microdialysis probes were inserted into the targets bilaterally 3 hr before sampling. The probes inserted into the XII nucleus had a tip length of 1 mm (type A-1-01; Eicom, Kyoto, Japan), whereas the probes inserted into the spinal ventral horn had a tip length of 2 mm (59-7005; Harvard Apparatus, South Natick, MA). Microdialysis probes were perfused with artificial CSF (Harvard Apparatus) at a flow rate of 2 μl/min, using an infusion pump (EPS-64; Eicom). Dialysates were collected from the XII nucleus and spinal ventral horn for 5 min intervals. Each experiment included three dialysates collected immediately before the stimulation, one dialysate collected during the stimulation, and four dialysates collected during the poststimulation period. The collected dialysates were stored frozen at −80°C until analysis.
Amino acid assay. The concentration of amino acid in the perfusate was determined by HPLC (EDT-300; Eicom) with a fluorescence detector (excitation at 340 nm, emission at 440 nm; FLD-370; Eicom) and quantified with a PowerChrom (ADInstruments, Sydney, Australia), using an external amino acid standard (Sigma, St. Louis, MO). Precolumn derivatization was performed witho-phthaldialdehyde–2-merca-ptoethanol by an autosampler (model 231XL; Gilson, Villiers le Bel, France) at 10°C for 3 min. The derivatives were then separated in a liquid chromatography column (4.6 mm in diameter, 150 mm in length; MA-5ODS; Eicom) at 30°C with 30% methanol in 0.1 m phosphate buffer, pH 6.0, after being degassed by an on-line degasser (DG-100; Eicom). The detection limits for glutamate, GABA, and glycine were 20 fmol.
Histology. To mark the stimulation sites, a 50 μA anodal current was delivered through a stimulation electrode over a period of 20 sec into the electrical stimulation and chemical injection sites at the end of the experiment. Then, cats were deeply anesthetized (Nembutal, 100 mg/kg, i.v.) and perfused intracardially with saline, followed by 10% Formalin buffer solution. Serial coronal sections were cut at 60 μm and then stained with neutral red. Stimulation sites were identified with potassium ferrocyanide counterstaining. Dialysate collection sites were also identified by the tract of the dialysis probe.
Data analysis. Three samples per experiment (three experiments were conducted at each site) were collected in each animal before stimulation (baseline), one during stimulation and four after stimulation. The baseline and poststimulation values were averaged for each experiment, producing three values for each experiment: baseline, stimulation, and poststimulation. Statistics were performed on these data across experiments with Student's t test. ANOVA was used to evaluate the difference between levels of neurotransmitter release in the XII nucleus and ventral horn during the prestimulation, stimulation, and poststimulation periods. For graphical presentation, the changes in amino acid release in the motor nuclei were converted to a percentage of the baseline level, calculated as an average of the samples collected during each period. Baseline error values represent variation from the mean across animals.
Results
Stimulation and collecting sites in the brainstem and spinal cord
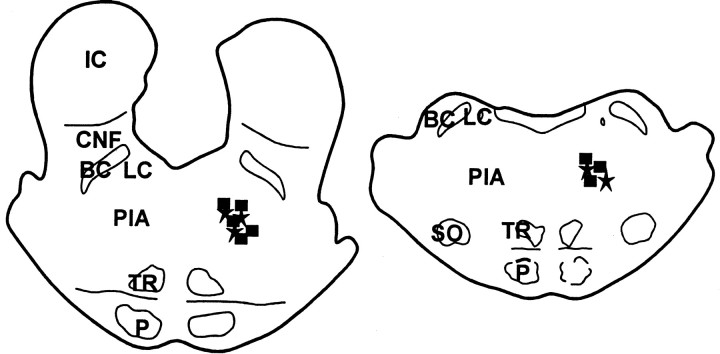
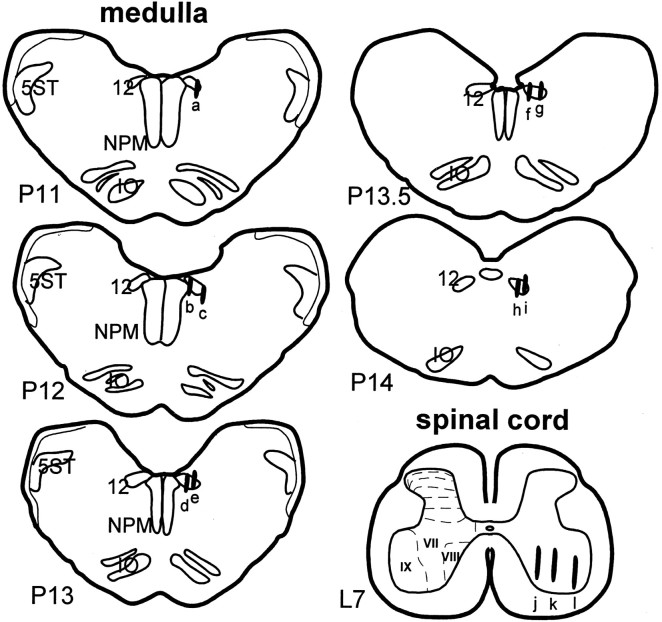
The sites of electrical stimulation and chemical injection were located exclusively in the PIA (Fig.1). In the electrical stimulation experiments, dialysate collecting sites in the XII nucleus (Fig.2) were found in the rostral (eight cases) and caudal (seven cases) parts of the nucleus. One probe was located in the caudal part of the medial longitudinal funiculus. Of the 15 probes in the XII nucleus, 11 were found in the lateral portion, two were located in the medial portion, and the remaining two were located in the center of the nucleus. The hypoglossal nucleus innervates the tongue musculature, an important component of the upper airway respiratory muscle control system. In the spinal cord, dialysate probes were located in the spinal ventral horn (15 cases) and ventrolateral funiculus (one case). This region innervates the gastrocnemius and related postural muscles, which are not involved in airway regulation. Of the 15 probes located in the ventral horn, 10 probes were located in lamina IX, three were found in lamina VIII, and the remaining two probes were located in lamina VII. In the ACh injection experiment, dialysis probes were located in either the rostral (four cases) or caudal (two cases) of the XII nucleus. Three probes were located in the lateral portion, two were found in the medial portion, and the remaining one probe was found in the center of the XII nucleus. Six dialysis probes were found in the ventral horn in PIA–ACh injection experiment. Among six probes seen in the ventral horn, three were located in lamina IX, two were found in lamina VII, and one probe was found in lamina VIII.
Fig. 1.
Histology showing electrical stimulation (squares) and acetylcholine injection (stars) sites. BC, Brachium conjunctivum;CNF, cuniformis nucleus; IC, inferior colliculus; LC, locus ceruleus; P, pyramidal tract; PIA, pontine inhibitory area;SO, superior olivary nucleus; TR, tegmental reticular nucleus.
Fig. 2.
Histology showing the dialysate collecting sites in the brainstem and spinal cord. Dialysates were collected from both sides of the motor nuclei with a total of 30 sites (1 case,h; 2 cases, a, b,d–f, i; 4 cases, c,g, j, k; 12 cases,l) in eight cats. Dialysates collected from the following sites were under both electrical and chemical stimulation administered into the PIA: a, b,d–f, and i–l. Collecting sites were reconstructed and presented on the right.L7, The seventh lumbar segment of the spinal cord;NPM, nucleus paramedianus; P11–P14, brainstem levels from 11–14 posterior to the interaural zero according to the atlas of Berman (1968); 5ST, spinal trigeminal tract; 12, hypoglossal nucleus; VII,VIII, IX, laminas VII, VIII, and IV in the ventral horn.
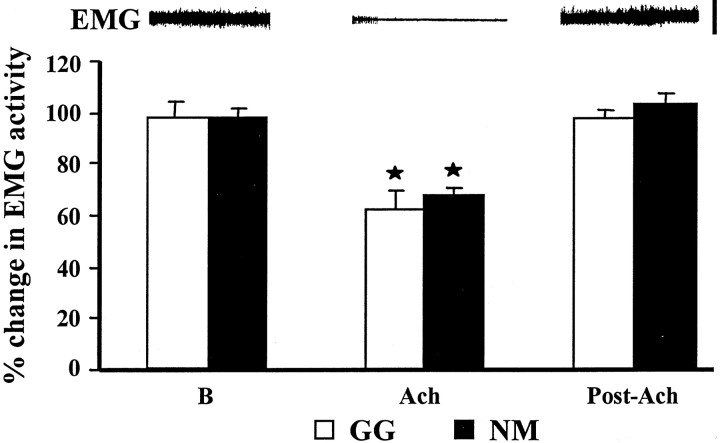
As reported previously (Lai et al., 2001), electrical stimulation and ACh injection into the PIA produced muscle tone suppression in both postural and respiratory muscles. The percentage decrease of integrated muscle activity in respiratory and postural muscles with PIA ACh injection was 62.5 and 67.3%, respectively (Fig.3, Table1). The duration of muscle tone suppression induced by ACh injection averaged 4.6 ± 0.7 min in both postural and respiratory muscles. In the genioglossus, electrical stimulation of the PIA generated either a brief suppression (12 of 20 cases) or a prolonged suppression (8 of 20 cases) of muscle activity. The percentage decrease of integrated genioglossus muscle activity over the period of 5 min stimulation averaged 18.4% from the baseline level. Three patterns of postural EMG change, a brief suppression, a prolonged suppression, and sustained atonia, were found during repetitive electrical stimulation of the PIA, as we reported previously (Lai et al., 2001). A brief suppression in which muscle tone returned to the baseline immediately after the cessation of stimulation was seen in 10 of 22 cases. A prolonged suppression of muscle tone lasting 1–5 sec after stimulation was found in 8 of 22 cases. Sustained muscle atonia induced by trains stimulation was seen in 4 of 22 cases. With electrical train stimulation at 10 sec intervals over a 5 min period, the percentage decrease of integrated muscle activity over the entire 5 min period ranged from 4.8 to 42.6%, with an average of 16.7% from the baseline amplitude.
Fig. 3.
Change in EMG activity by ACh injection into the pontine inhibitory area. Polygraphic recording of neck EMG activity during control baseline, ACh injection, and recovery is shown at thetop. A significant reduction of genioglossus (GG) and neck (NM) muscle activity was seen after pontine ACh injection. Both genioglossus and neck EMG returned to the pre-ACh injection level at an average of 4.6 min after injection. The error bars shown in this and subsequent figures represent the SEM. B, Baseline. Calibration for EMG activity, 200 μV. *p < 0.05,t test relative to baseline.
Table 1.
Percentage change in muscle activity and amino acid release in the motor nuclei during pontine stimulation-induced muscle tone suppression
| GG | NM | Glycine | GABA | Glutamate | |||||
|---|---|---|---|---|---|---|---|---|---|
| XII | SC | XII | SC | XII | SC | ||||
| ES | Mean | 78* | 80* | 121.8* | 126.2* | 112.7* | 111.5 | 100.9 | 99.2 |
| SEM | 3.5 | 10.2 | 5.8 | 16.6 | 5.9 | 10.9 | 4.1 | 15.2 | |
| ACh | Mean | 62* | 671-160 | 174.51-160 | 149.7* | 131.3* | 164.5* | 100.0 | 141.7 |
| SEM | 7.4 | 3.8 | 36.4 | 38.6 | 13.75 | 28.9 | 35.9 | 46.4 | |
Baseline level, 100%. ACh, Acetylcholine injection; ES, electrical stimulation; GG, genioglossus muscle; NM, neck muscle; SC, spinal cord; XII, hypoglossal nucleus. Student's t test, relative to baseline.
p < 0.05;
F1-160: p < 0.01.
Glycine release in motoneuron pools during pontine acetylcholine injection
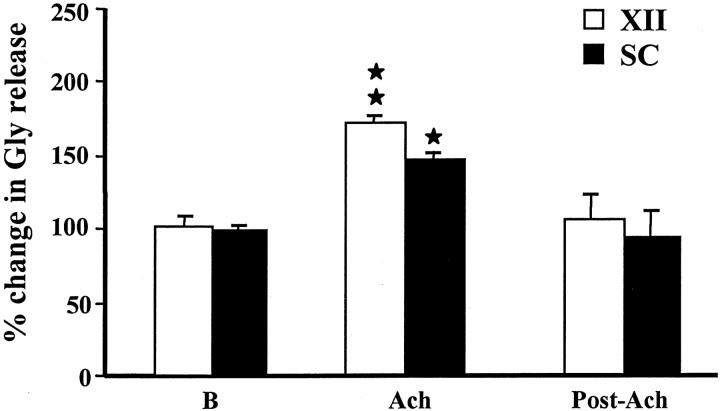
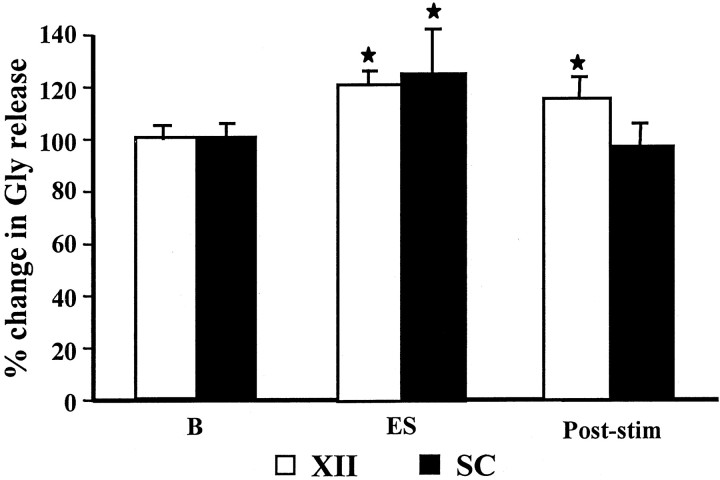
Acetylcholine microinjected into the PIA elicited an increase in glycine release from the baseline level in the hypoglossal nucleus (p = 0.005; df = 22; t = 3.09; t test) (Fig. 4) and lumbar ventral horn (p = 0.049; df = 18;t = 2.1; t test) (Fig. 4). The average level of glycine release in the XII nucleus was 174.5 ± 36.4% (mean ± SEM) (Table 1) of the baseline level during PIA ACh injection-induced muscle tone suppression. The increase in glycine release in the XII nucleus during PIA ACh injection did not differ between the sides ipsilateral and contralateral to the site of injection (p = 0.236; df = 5;t = 1.68; t test). The rostral and caudal portions of the XII nucleus also had the same pattern of increase in glycine release. In the spinal cord, the average glycine release during PIA ACh injection-induced muscle tone suppression was 149.7 ± 38.6% of the baseline level. Glycine release in the spinal cord ipsilateral and contralateral to the injection did not differ during PIA ACh injection-induced atonia, either.
Fig. 4.
Change in glycine release in the hypoglossal nucleus and spinal cord with pontine acetylcholine injection. A significant increase in glycine release in both hypoglossal nucleus and ventral horn was seen during pontine ACh injection-induced muscle tone suppression. SC, Spinal cord; XII, hypoglossal nucleus; B, baseline. *p< 0.05; **p < 0.01; t test relative to baseline.
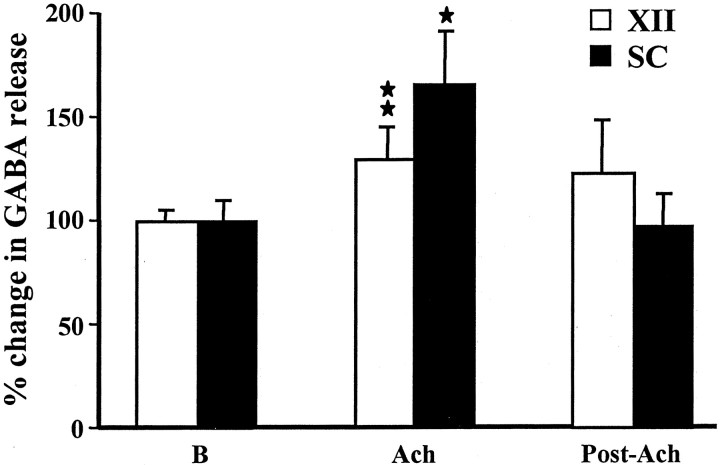
GABA release in motoneuron pools during pontine acetylcholine injection
The release of GABA in the motoneuron pools was also increased during PIA ACh injection-induced atonia (Table 1). The amplitude of GABA release in the XII nucleus (131.3 ± 13.7%) during pontine ACh injection was significantly different from the baseline levels (p = 0.009; df = 18; t = 2.89; t test) (Fig. 5). Similarly, the GABA release in the spinal cord (164.5 ± 28.9%) during pontine ACh injection was also significantly (p = 0.013; df = 18; t = 2.76; t test) (Fig. 5) higher than that of the baseline level. The ipsilateral and contralateral sides of the XII nucleus and spinal cord did not differ in their pattern of increase in GABA release during PIA ACh injection (p > 0.6; df = 9;t = 0.48; t test).
Fig. 5.
Change in GABA release in the hypoglossal nucleus and spinal cord with pontine acetylcholine injection. A significant increase in GABA release in both hypoglossal nucleus and ventral horn was seen during pontine ACh injection-induced muscle tone suppression.SC, Spinal cord; XII, hypoglossal nucleus; B, baseline.
Inhibitory amino acid release in the hypoglossal nucleus and spinal cord during pontine electrical stimulation
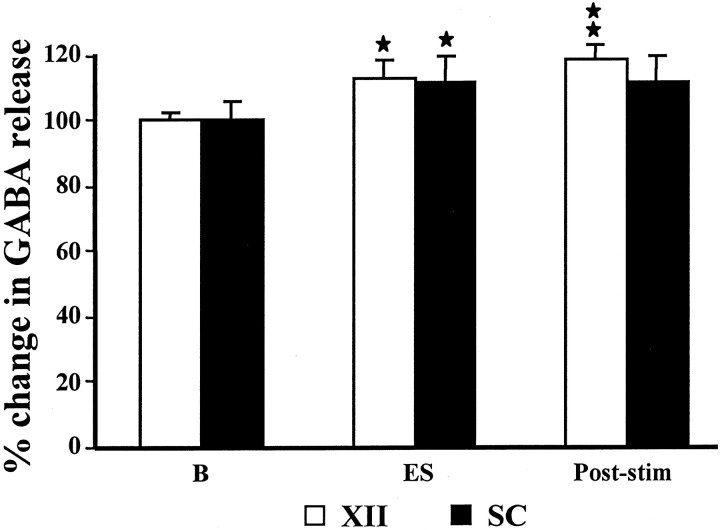
Release of inhibitory amino acids in the XII nucleus was also significantly increased during PIA electrical stimulation-induced muscle tone suppression. Electrical stimulation in the PIA elicited a significant increase in glycine (121.8 ± 5.8% of the baseline level; p = 0.016; df = 46; t = 2.50; t test) (Fig. 6) and GABA (112.7 ± 5.9% of the baseline level; p = 0.033; df = 46; t = 2.20; t test) (Fig.7) release in the XII nucleus. As with ACh injection, the glycine and GABA release increase ipsilateral and contralateral to the stimulation site, as well as in the rostral and caudal portions of the XII nucleus, did not significantly differ during electrical stimulation in the pons. As in the XII nucleus, glycine release in the spinal ventral horn was also significantly increased during electrical stimulation in the PIA (126.2 ± 16.6% of the baseline level; p = 0.030; df = 62;t = 2.24; t test) (Fig. 6). In contrast, GABA release in the ventral horn was not significantly different from the baseline level during PIA electrical stimulation-induced atonia (111.5 ± 10.9% of the baseline level; p = 0.32; df = 70; t = 1.01; t test) (Fig.7).
Fig. 6.
Change in glycine release in the hypoglossal nucleus and spinal cord during pontine electrical stimulation. Electrical stimulation (ES) in the PIA induces a significant increase in glycine release in both the hypoglossal nucleus and the ventral horn. SC, Spinal cord;XII, hypoglossal nucleus; B, baseline.
Fig. 7.
GABA release in the hypoglossal nucleus and spinal cord during electrical stimulation in the PIA. Train stimulations in the PIA elicit a significant increase in GABA release in the hypoglossal nucleus, whereas the same stimulation did not produce a significant change in GABA release in the ventral horn.SC, Spinal cord; XII, hypoglossal nucleus; B, baseline.
Comparison of GABA and glycine release in the hypoglossal nucleus and spinal cord during pontine stimulation-induced muscle tone suppression
We compared the pattern of glycine and GABA release in the XII nucleus and spinal cord during pontine stimulation-induced atonia. The increase in glycine release in the XII nucleus and spinal cord did not significantly differ over the 40 min observation period (F(7,72) = 0.25; p = 0.97; two-way ANOVA) after PIA ACh injection. Similarly, there was no difference in the increased GABA release in the XII nucleus and spinal cord during pontine ACh-induced muscle tone suppression (F(7,64) = 1.51; p = 0.18; two-way ANOVA).
Excitatory amino acid release in motoneuron pools during pontine electrical stimulation
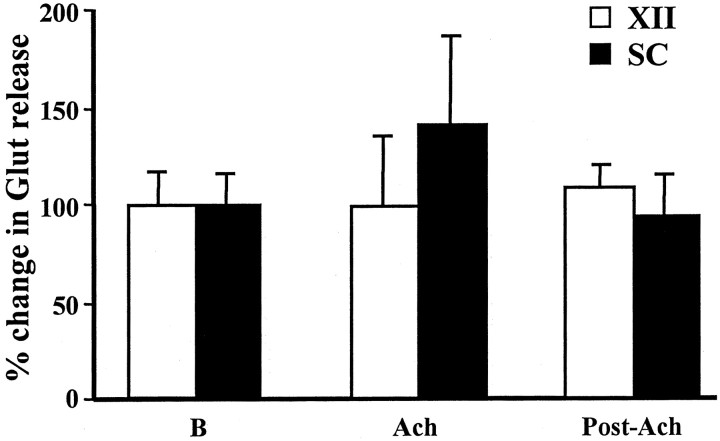
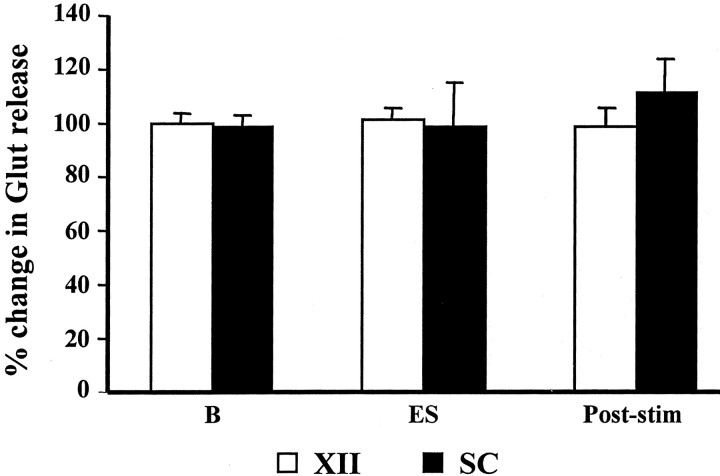
We analyzed glutamate release in the XII nucleus and spinal cord during PIA stimulation-induced muscle tone suppression. Unlike the inhibitory amino acids, glutamate release in the XII nucleus and spinal cord did not change significantly with either pontine electrical or chemical stimulation-induced atonia. Glutamate release in the XII nucleus and spinal cord was 100.0 ± 35.9% (p = 0.99; df = 18; t test) (Fig. 8) and 141.7 ± 46.4% of the baseline level (p = 0.33; df = 18;t test) (Fig. 8), respectively, after PIA ACh injection. Similarly, glutamate release in the XII nucleus (100.9 ± 4.1% of the baseline level; p = 0.93; df = 34;t test) (Fig. 9) and ventral horn (99.2 ± 15.2% of the baseline level; p = 0.99; df = 62; t test) (Fig. 9) was not significantly changed during pontine electrical stimulation-induced atonia.
Fig. 8.
Glutamate release in the hypoglossal nucleus and spinal cord was unaltered by pontine acetylcholine injection. A nonsignificant increase in glutamate release in the ventral horn was seen during ACh injection-induced muscle tone suppression.SC, Spinal cord; XII, hypoglossal nucleus; B, baseline.
Fig. 9.
Glutamate release in the hypoglossal nucleus and spinal cord was unaltered by electrical stimulation in the PIA. As with ACh injection, electrical stimulation (ES) in the PIA did not change glutamate release in the motor nuclei.SC, Spinal cord; XII, hypoglossal nucleus; B, baseline.
Discussion
We found that release of the inhibitory amino acids glycine and GABA in the hypoglossal nucleus and spinal cord was significantly increased during ACh injection in the PIA, which elicited suppression of tone in the postural and respiratory muscles (Lai et al., 2001). Electrical stimulation in the PIA also produced an increase in both glycine and GABA release in the XII nucleus. An increase in glycine release but no significant change in GABA release in the ventral horn was seen with electrical stimulation-induced atonia. Although Kubin et al. (1993) hypothesized that glycine and GABA did not have the same role in the XII nucleus as it did in the trigeminal motor nucleus and ventral horn during pontine carbachol-induced atonia, we observed a significant increase in glycine and GABA release of the XII nucleus of the same magnitude as seen in the ventral horn during PIA stimulation-induced atonia. Our results suggest that glycine and GABA have a similar role in the regulation of hypoglossal and spinal motoneuron activity during PIA stimulation-induced muscle tone suppression.
We found that electrical and chemical stimulation in the PIA elicited a different pattern of GABA release in the ventral horn. The lack of a significant change in GABA release in the ventral horn during PIA electrical stimulation in contrast to the pattern after acetylcholine stimulation of the PIA could be attributable to the short duration of muscle atonia elicited by electrical stimulation and therefore lower signal-to-noise ratio of correlated amino acid release. In contrast, ACh injection into the PIA generated prolonged muscle tone suppression and simultaneously induced a significant increase in both GABA and glycine release in the ventral horn.
Glycine has been postulated to inhibit motoneuronal activity during REM sleep. Using intracellular recording and microiontophoretic techniques, Chase and his colleagues found that IPSPs in both cranial and spinal motoneurons can be blocked by application of strychnine, a glycine antagonist, during natural (Chirwa et al., 1991; Soja et al., 1991) and pontine carbachol-induced (Kohlmeier et al., 1996; Yamuy et al., 1999) REM sleep in the cat. GABA has also been shown to inhibit motoneuron activity. Systemic infusion of GABA and glycine agonists produces a decrease, whereas antagonist perfusion generates an increase in the frequency and amplitude of hypoglossal nerve activity in the decerebrate rat (Hayashi and Lipski, 1992). Iontophoretic application of glycine and GABA into the spinal cord blocks motoneuron discharge induced by dl-homocysteic acid (Werman et al., 1968). ten Bruggencate and Sonnhof (1972) demonstrated that iontophoretic application of glycine and GABA generates IPSPs in hypoglossal motoneurons, and this hyperpolarization can be blocked by glycine and GABA antagonists, strychnine and picrotoxin, respectively. Studies using intracellular recording in rodent in vitro preparation showed that glycine and GABA are either colocalized and coreleased from the same presynaptic vesicle or released from the separate terminals onto spinal and cranial motoneurons (Jonas et al., 1998; O'Brien and Berger, 1999; Russier et al., 2002). Anatomical studies also demonstrated that input terminals on spinal motoneurons contain GABA, glycine, or both (Taal and Holstege, 1994; Ornung et al., 1996). The present study suggests that both glycine and GABA release contribute to the active inhibition of muscle tone during electrical stimulation and after ACh stimulation of PIA, as well as during the normal release of ACh on the PIA in REM sleep (Kodama et al., 1990). Although it is likely that a similar pattern of release of inhibitory amino acids would be seen during the atonia of natural REM sleep, a direct test of this hypothesis is required.
The sources of glycine and GABA inputs to the motoneurons could be local interneurons or supraspinal systems. Neurons containing inhibitory amino acids have been found in the hypoglossal nucleus (Takasu et al., 1987; Aldes et al., 1988) and spinal ventral horn (Shupliakov et al., 1993). On the other hand, immunohistochemistry combined with retrograde tracer injection technique revealed that GABAergic and glycinergic neurons in the nucleus gigantocellularis α and ventralis in the rat, which corresponds to the nucleus magnocellularis (NMC) in the cat, project to the spinal ventral horn (Holstege, 1991; Holstege and Bongers, 1991; Ellenberger, 1999) and hypoglossal nucleus (Li et al., 1997). GABAergic neurons in the PIA are reported to project to the hypoglossal nucleus (Li et al., 1997). Although inhibitory amino acid containing neurons are found in the PIA (Rampon et al., 1996; Maloney et al., 2000), the neuronal type projecting to the spinal cord from this region (Kuypers and Maisky, 1975; Tohyama et al., 1979; Matsuyama et al., 1999) remains unclear.
We suggest that stimulation in the PIA elicits an increase in glycine and GABA release in motor nuclei through one or more of three pathways. First, neuronal activity in the PIA may activate GABAergic and glycinergic interneurons in motor nuclei. Second, stimulation may activate descending GABAergic and glycinergic neurons in the PIA. Finally, stimulation in the PIA may activate reticulospinal GABAergic and glycinergic neuron activity in the NMC. Our previous study demonstrated that injection of carbachol into the PIA elicits muscle atonia, which can be reversed by microinjection of the glutamate antagonist γ-d-glutamylglycine into the NMC of the medulla in the decerebrate cat (Lai and Siegel, 1988), indicating that the PIA-induced muscle atonia relays through a glutamatergic projection to the NMC. Using orthodromic and antidromic stimulation and extracellular recording, Kohyama et al. (1998) found that PIA stimulation, which induces atonia, activates reticulospinal neuronal activity in the medullary gigantocellularis and magnocellularis nuclei in the decerebrate cat. A similar finding was also reported in the chronic cat during natural REM sleep (Kanamori et al., 1980).
Immunohistochemical studies demonstrated that there are glutamate receptors in spinal (Petralia et al., 1994; Neve et al., 1997) and hypoglossal (Watanabe et al., 1994; Garcia del Cano et al., 1999) motoneurons. Glutamate application induces EPSPs in feline spinal motoneurons (Engberg et al., 1979) and in the rodent hypoglossal motoneurons (O'Brien et al., 1997). However, we did not see a change in glutamate release in the motoneuron pools during PIA stimulation in our present study. This suggests that a disfacilitation caused by a reduction in glutamate release may not be involved in pontine stimulation-induced atonia. However, the high level of glutamate present in the interstitial tissue may make detection of a relatively small change in glutamate release as a result of synaptic activity relatively difficult to detect, so the current results do not rule out an important role for glutamate in the regulation of PIA-induced atonia.
In conclusion, stimulation in the PIA produced skeletal muscle tone suppression and increased glycine and GABA release in both the hypoglossal nucleus and lumbar ventral horn. The PIA-induced muscle tone suppression may be mediated through GABAergic and glycinergic mechanisms via medial medulla and/or interneurons in the motoneuron pools. Results from our previous (Lai et al., 2001) and present studies lead us to conclude that the coordinated activation of inhibitory amino acid-containing neuronal activity and simultaneous inactivation of brainstem monoaminergic neuronal activity contributes to the suppression of muscle tone during PIA activation. Manipulation of the action of these transmitters may alter sleep-related atonia and correlated pathologies of this atonia that result in REM sleep behavior disorder, cataplexy, and obstructive sleep apnea.
Footnotes
This study was supported by United States Public Health Service Grants HL 41370 and HL 60296 and the Medical Research Service of the Department of Veterans Affairs.
Correspondence should be addressed to Dr. Yuan-Yang Lai, Neurobiology Research (151A3), Veterans Affairs Medical Greater Los Angeles Healthcare System, 16111 Plummer Street, North Hills, CA 91343. E-mail:yylai@ucla.edu.
References
- 1.Aldes LD, Chronister RB, Marco LA. Distribution of glutamic acid decarboxylase and gamma-aminobutyric acid in the hypoglossal nucleus in the rat. J Neurosci Res. 1988;19:343–348. doi: 10.1002/jnr.490190309. [DOI] [PubMed] [Google Scholar]
- 2.Berman AL. The brain stem of the cat. University of Wisconsin; Madison, WI: 1968. [Google Scholar]
- 3.Chase MH, Soja PJ, Morales FR. Evidence that glycine mediates the postsynaptic potentials that inhibits lumbar motoneurons during the atonia of active sleep. J Neurosci. 1989;9:743–751. doi: 10.1523/JNEUROSCI.09-03-00743.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chirwa SS, Stafford-Segert I, Soja PJ, Chase MH. Strychnine antagonizes jaw-closer motoneuron IPSPs induced by reticular stimulation during active sleep. Brain Res. 1991;547:323–326. doi: 10.1016/0006-8993(91)90979-6. [DOI] [PubMed] [Google Scholar]
- 5.Curtis DR. Pharmacological investigations upon inhibition of spinal neurons. J Physiol (Lond) 1959;145:175–192. doi: 10.1113/jphysiol.1959.sp006134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ellenberger HH. Distribution of bulbospinal γ-aminobutyric acid-synthesizing neurons of the ventral respiratory group of the rat. J Comp Neurol. 1999;411:130–144. doi: 10.1002/(sici)1096-9861(19990816)411:1<130::aid-cne10>3.0.co;2-c. [DOI] [PubMed] [Google Scholar]
- 7.Engberg I, Flatman JA, Lambert JDC. The actions of excitatory amino acids on motoneurones in the feline spinal cord. J Physiol (Lond) 1979;288:227–261. [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia del Cano G, Martinez-Millan L, Gerrikagoitia I, Sarasa M, Matute C. Ionotropic glutamate receptor subunit distribution on hypoglossal motoneuronal pools in the rat. J Neurocytol. 1999;28:455–468. doi: 10.1023/a:1007001020700. [DOI] [PubMed] [Google Scholar]
- 9.George R, Haslett WL, Jenden DJ. A cholinergic mechanism in the brainstem reticular formation: induction of paradoxical sleep. Int J Neuropharmacol. 1964;3:541–552. doi: 10.1016/0028-3908(64)90076-0. [DOI] [PubMed] [Google Scholar]
- 10.Hajnik T, Lai YY, Siegel JM. Atonia-related regions in the rodent pons and medulla. J Neurophysiol. 2000;84:1942–1948. doi: 10.1152/jn.2000.84.4.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayashi F, Lipski J. The role of inhibitory amino acids in control of respiratory motor output in an arterially perfused rat. Respir Physiol. 1992;89:47–63. doi: 10.1016/0034-5687(92)90070-d. [DOI] [PubMed] [Google Scholar]
- 12.Holstege JC. Ultrastructural evidence for GABAergic brain stem projections to spinal motoneurons in the rat. J Neurosci. 1991;11:159–167. doi: 10.1523/JNEUROSCI.11-01-00159.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holstege JC, Bongers CMH. A glycinergic projection from the ventromedial lower brainstem to spinal motoneurons. An ultrastructural double labeling study in rat. Brain Res. 1991;566:308–315. doi: 10.1016/0006-8993(91)91715-d. [DOI] [PubMed] [Google Scholar]
- 14.Jelev A, Sood S, Liu H, Nolan P, Horner RL. Microdialysis perfusion of 5-HT into hypoglossal motor nucleus differentially modulates genioglossus activity across natural sleep-wake states in rats. J Physiol (Lond) 2001;532:467–481. doi: 10.1111/j.1469-7793.2001.0467f.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jonas P, Bischofberger J, Sandkuhler J. Corelease of two fast neurotransmitters at a central synapse. Science. 1998;281:419–424. doi: 10.1126/science.281.5375.419. [DOI] [PubMed] [Google Scholar]
- 16.Kanamori N, Sakai K, Jouvet M. Neural activity specific to paradoxical sleep in the ventromedial medullary reticular formation of unrestrained cats. Brain Res. 1980;189:251–255. doi: 10.1016/0006-8993(80)90024-4. [DOI] [PubMed] [Google Scholar]
- 17.Kawai I, Sasaki K. Effects of strychnine upon supraspinal inhibition. Jpn J Physiol. 1964;14:309–317. doi: 10.2170/jjphysiol.14.309. [DOI] [PubMed] [Google Scholar]
- 18.Kodama T, Takahashi Y, Honda Y. Enhancement of acetylcholine release during paradoxical sleep in the dorsolateral tegmental field of the cat brainstem. Neurosci Lett. 1990;98:159–165. doi: 10.1016/0304-3940(90)90576-u. [DOI] [PubMed] [Google Scholar]
- 19.Kohlmeier KA, Lopez-Rodriguez F, Liu R-H, Morales FR, Chase MH. State-dependent phenomena in cat masseter motoneurons. Brain Res. 1996;722:30–38. doi: 10.1016/0006-8993(96)00173-4. [DOI] [PubMed] [Google Scholar]
- 20.Kohyama J, Lai YY, Siegel JM. Reticulospinal systems mediate atonia with short and long latencies. J Neurophysiol. 1998;80:1839–1851. doi: 10.1152/jn.1998.80.4.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kubin L, Kimura H, Tojima H, Davies RO, Pack AI. Suppression of hypoglossal motoneurons during the carbachol-induced atonia of REM sleep is not caused by fast synaptic inhibition. Brain Res. 1993;611:300–312. doi: 10.1016/0006-8993(93)90517-q. [DOI] [PubMed] [Google Scholar]
- 22.Kuypers HGJM, Maisky VA. Retrograde axonal transport of horseradish peroxidase from spinal cord to brain stem cell groups in the cat. Neurosci Lett. 1975;1:9–14. doi: 10.1016/0304-3940(75)90004-x. [DOI] [PubMed] [Google Scholar]
- 23.Lai YY, Siegel JM. Medullary regions mediating atonia. J Neurosci. 1988;8:4790–4796. doi: 10.1523/JNEUROSCI.08-12-04790.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lai YY, Siegel JM. Pontomedullary glutamate receptors mediating locomotion and muscle tone suppression. J Neurosci. 1991;11:2931–2937. doi: 10.1523/JNEUROSCI.11-09-02931.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai YY, Siegel JM. Corticotropin-releasing factor mediated muscle atonia in pons and medulla. Brain Res. 1992;575:63–68. doi: 10.1016/0006-8993(92)90423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lai YY, Siegel JM. Muscle atonia and REM sleep. In: Mallick BN, Inoue S, editors. Rapid eye movement sleep. Narosa; New Dehli, India: 1999. pp. 69–90. [Google Scholar]
- 27.Lai YY, Strahlendorf HK, Fung SJ, Barnes CD. The actions of two monoamines on spinal motoneurons from stimulation of the locus coeruleus. Brain Res. 1989;484:268–272. doi: 10.1016/0006-8993(89)90369-7. [DOI] [PubMed] [Google Scholar]
- 28.Lai YY, Kodama T, Siegel JM. Changes in monoamine release in the ventral horn and hypoglossal nucleus linked to pontine inhibition of muscle tone: an in vivo microdialysis study. J Neurosci. 2001;21:7384–7391. doi: 10.1523/JNEUROSCI.21-18-07384.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li YQ, Takada M, Kaneko T, Mizuno N. Distribution of GABAergic and glycinergic premotor neurons projecting to the facial and hypoglossal nuclei in the rat. J Comp Neurol. 1997;378:283–294. doi: 10.1002/(sici)1096-9861(19970210)378:2<283::aid-cne10>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 30.Llinas R, Terzuolo CA. Mechanisms of supraspinal actions upon spinal cord activities. Reticular inhibitory mechanisms on alpha-extensor motoneurons. J Neurophysiol. 1964;27:579–591. doi: 10.1152/jn.1964.27.4.579. [DOI] [PubMed] [Google Scholar]
- 31.Maloney KJ, Mainville L, Jones BE. c-Fos expression in GABAergic, serotonergic and other neurons of the pontomedullary reticular formation and raphe after paradoxical sleep deprivation and recovery. J Neurosci. 2000;15:4669–4679. doi: 10.1523/JNEUROSCI.20-12-04669.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matsuyama K, Mori F, Kuze B, Mori S. Morphology of single pontine reticulospinal axons in the lumbar enlargement of the cat: a study using the anterograde tracer PHA-L. J Comp Neurol. 1999;410:413–430. [PubMed] [Google Scholar]
- 33.Neve RL, Howe JR, Hong S, Kalb RG. Introduction of the glutamate receptor subunit 1 into motor neurons in vitro and in vivo using recombinant herpes simplex virus. Neuroscience. 1997;79:435–447. doi: 10.1016/s0306-4522(96)00645-8. [DOI] [PubMed] [Google Scholar]
- 34.O'Brien JA, Berger AJ. Cotransmission of GABA and glycine to brain stem motoneurons. J Neurophysiol. 1999;82:1638–1641. doi: 10.1152/jn.1999.82.3.1638. [DOI] [PubMed] [Google Scholar]
- 35.O'Brien JA, Isaacson JS, Berger AJ. NMDA and non-NMDA receptors are co-localized at excitatory synapses of rat hypoglossal motoneurons. Neurosci Lett. 1997;227:5–8. doi: 10.1016/s0304-3940(97)00293-0. [DOI] [PubMed] [Google Scholar]
- 36.Onoe H, Sakai K. Kainate receptors: a novel mechanism in paradoxical (REM) sleep generation. NeuroReport. 1995;6:353–356. [PubMed] [Google Scholar]
- 37.Ornung G, Shupliakov O, Linda H, Ottersen OP, Storm-Mathisen J, Ulfhake B, Cullheim S. Qualitative and quantitative analysis of glycine- and GABA-immunoreactive nerve terminals on motoneuron cell bodies in the cat spinal cord: a postembedding electron microscopic study. J Comp Neurol. 1996;365:413–426. doi: 10.1002/(SICI)1096-9861(19960212)365:3<413::AID-CNE6>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 38.Parkis MA, Bayliss DA, Berger AJ. Actions of norepinephrine on rat hypoglossal motoneurons. J Neurophysiol. 1995;74:1911–1919. doi: 10.1152/jn.1995.74.5.1911. [DOI] [PubMed] [Google Scholar]
- 39.Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distribution of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptice antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rampon C, Luppi PH, Fort P, Peyron C, Jouvet M. Distribution of glycine-immunoreactive cell bodies and fibers in the rat brain. Neuroscience. 1996;75:737–755. doi: 10.1016/0306-4522(96)00278-3. [DOI] [PubMed] [Google Scholar]
- 41.Reinoso-Suarez F, de Andres I, Rodrigo-Angulo ML, Rodriguez-Veiga E. Location and anatomical connections of a paradoxical sleep induction site in the cat ventral pontine tegmentum. Eur J Neurosci. 1994;6:1829–1836. doi: 10.1111/j.1460-9568.1994.tb00575.x. [DOI] [PubMed] [Google Scholar]
- 42.Russier M, Kopysova IL, Ankri N, Ferrand N, Debanne D. GABA and glycine co-release optimizes functional inhibition in rat brainstem motoneurons in vitro. J Physiol (Lond) 2002;541:123–137. doi: 10.1113/jphysiol.2001.016063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shupliakov O, Ornung G, Brodin L, Ulfhake B, Ottersen OP, Storm-Mathisen J, Cullheim S. Immunocytochemical localization of amino acid neurotransmitter candidates in the ventral horn of the cat spinal cord: a light microscopic study. Exp Brain Res. 1993;96:404–418. doi: 10.1007/BF00234109. [DOI] [PubMed] [Google Scholar]
- 44.Soja PJ, Lopez-Rodriguez F, Morales FR, Chase MH. The postsynaptic inhibitory control of lumbar motoneurons during the atonia of active sleep: effect of strychnine on motoneuron properties. J Neurosci. 1991;11:2804–2811. doi: 10.1523/JNEUROSCI.11-09-02804.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Taal W, Holstege JC. GABA and glycine frequently colocalize in terminals on cat spinal motoneurons. NeuroReport. 1994;5:2225–2228. doi: 10.1097/00001756-199411000-00005. [DOI] [PubMed] [Google Scholar]
- 46.Takahashi T, Berger AJ. Direct excitation of rat spinal motoneurones by serotonin. J Physiol (Lond) 1990;423:63–76. doi: 10.1113/jphysiol.1990.sp018011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Takasu N, Nakatani T, Arikuni T, Kimura H. Immunocytochemical localization of γ-aminobutyric acid in the hypoglossal nucleus of the macaque monkey, Macaca fuscata: a light and electron microscopic study. J Comp Neurol. 1987;263:42–53. doi: 10.1002/cne.902630104. [DOI] [PubMed] [Google Scholar]
- 48.ten Bruggencate G, Sonnhof U. Effects of glycine and GABA, and blocking actions of strychnine and picrotoxin in the hypoglossus nucleus. Pflügers Arch. 1972;334:240–252. doi: 10.1007/BF00626226. [DOI] [PubMed] [Google Scholar]
- 49.Tohyama M, Sakai K, Salvert D, Touret M, Jouvet M. Spinal projections from the lower brain stem in the cat as demonstrated by the horseradish peroxidase technique. I. Origins of the reticulospinal tracts and their funicular trajectiories. Brain Res. 1979;173:383–403. doi: 10.1016/0006-8993(79)90237-3. [DOI] [PubMed] [Google Scholar]
- 50.Van Dongen PA, Broekkamp CL, Cools AR. Atonia after carbachol microinjections near the locus coeruleus in cats. Pharmacol Biochem Behav. 1978;8:527–532. doi: 10.1016/0091-3057(78)90382-9. [DOI] [PubMed] [Google Scholar]
- 51.Watanabe M, Mishina M, Inoue Y. Distinct distributions of five NMDA receptor channel subunit mRNAs in the brainstem. J Comp Neurol. 1994;343:520–531. doi: 10.1002/cne.903430403. [DOI] [PubMed] [Google Scholar]
- 52.Werman R, Davidoff RA, Aprison MH. Inhibitory action of glycine on spinal neurons in the cat. J Neurophysiol. 1968;31:81–95. doi: 10.1152/jn.1968.31.1.81. [DOI] [PubMed] [Google Scholar]
- 53.White SR, Fung SJ. Serotonin depolarizes cat spinal motoneurons in situ and decreases motoneuron afterhyperpolarizing potentials. Brain Res. 1989;502:205–213. doi: 10.1016/0006-8993(89)90615-x. [DOI] [PubMed] [Google Scholar]
- 54.Xi MC, Morales FR, Chase MH. Induction of wakefulness and inhibition of active (REM) sleep by GABAergic processes in the nucleus pontis oralis. Arch Ital Biol. 2001;139:125–145. [PubMed] [Google Scholar]
- 55.Yajima Y, Hayashi Y. GABAA receptor-mediated inhibition in the nucleus ambiguus motoneuron. Neuroscience. 1997;79:1079–1088. doi: 10.1016/s0306-4522(97)00012-2. [DOI] [PubMed] [Google Scholar]
- 56.Yamuy J, Fung SJ, Xi M, Morales FR, Chase MH. Hypoglossal motoneurons are postsynaptically inhibited during carbachol-induced rapid eye movement sleep. Neuroscience. 1999;94:11–15. doi: 10.1016/s0306-4522(99)00355-3. [DOI] [PubMed] [Google Scholar]