Abstract
An antibody against recoverin, the calcium-binding protein, labels photoreceptors, cone bipolar cells, and a subpopulation of cells in the ganglion cell layer. In the present study, we sought to establish the origin and identity of the cells expressing recoverin in the ganglion cell layer of the rat retina. By double labeling with rhodopsin, we demonstrate that early in development some of the recoverin-positive cells in the ganglion cell layer are photoreceptors. During the first postnatal week, these rhodopsin-positive cells are eliminated from the ganglion cell layer, but such neurons remain in the inner nuclear layer well into the first postnatal month. Another contingent of recoverin-positive cells, with morphological features equivalent to those of bipolar cells, is present in the postnatal retina, and ∼50% of these neurons survive to maturity. The incidence of such cells in the ganglion cell layer was not affected by early transection of the optic nerve, a manipulation that causes rapid loss of retinal ganglion cells. These recoverin-positive cells were not double-labeled by cell-specific markers expressed by photoreceptors, rod bipolar cells, or horizontal and amacrine cells. Based on their staining with recoverin and salient morphological features, these ectopic profiles in the ganglion cell layer are most likely cone bipolar cells. Collectively, the results provide evidence for photoreceptors in the ganglion cell and inner nuclear layers of the developing retina, and a more permanent subpopulation of cone bipolar cells displaced to the ganglion cell layer.
Keywords: ectopic cells, recoverin, bipolar cells, photoreceptors, retina, development
Introduction
A striking feature of the vertebrate retina is its distinct laminar organization. Cells in the three cellular layers have been extensively characterized on the basis of their morphological, functional, and molecular properties, and sound estimates for the total number of cell types present in the mammalian retina have been provided recently (Masland and Raviola, 2000). To attain their layer-specific distribution patterns, newborn neurons must migrate from the proliferative zone of the inner retina to their appropriate laminar destination. Unlike the temporal sequence that has been delineated in the other layered structures of the brain, the pattern of cell generation in the retina does not follow a spatially dependent order. Thus, ganglion cells and photoreceptors are generated during similar developmental periods (Morest, 1970; Barnstable et al., 1988; Treisman et al., 1988; Watanabe and Raff, 1990; Reese and Colello, 1992). Moreover, compared with other brain structures, our understanding of the mechanisms underlying cell migration in the developing retina is still rudimentary, but Müller cells as well as cell-to-cell interactions have been implicated in this process (Altshuler and Cepko, 1992; Reh, 1992; Wong and Godinho, 2003).
A cell marker that has proven particularly effective for studying the developing retina is recoverin, a 23 kDa calcium-binding protein originally purified from bovine rod outer segments (Dizhoor et al., 1991; Lambrecht and Koch, 1992). The anti-recoverin antibody labels cone and rod photoreceptors (Dizhoor et al., 1991; Korf et al., 1992;Milam et al., 1993; Wiechmann and Hammarback, 1993; Grunert et al., 1994; Johnson et al., 1999) as well as ON and OFF cone bipolar cells (Milam et al., 1993; Miller et al., 1999; Günhan-Agar et al., 2000; Günhan et al., 2002). Somas and processes are recognized by the anti-recoverin antibody at a relatively early stage of development, even before neurons have begun their migration. This has made it feasible to use recoverin as a marker to assess the development of photoreceptors (Johnson et al., 1999) as well as ON and OFF cone bipolar cells (Miller et al., 1999; Günhan-Agar et al., 2000).
Recoverin or its mRNA also has been localized in a small number of cells in the ganglion cell layer of several different species (Stepanik et al., 1993; Wiechmann and Hammarback, 1993; McGinnis et al., 1999;Günhan-Agar et al., 2000), including the human retina (Yan and Wiechmann, 1997). Neither the origin nor the identity of these recoverin-positive cells has been established, although it has been commonly assumed that these represent a subclass of ganglion cells and displaced amacrine cells.
In the present study, we provide evidence that the recoverin-positive cells in the ganglion cell layer are composed of two distinct populations: photoreceptors and cone bipolar cells. The photoreceptors survive in the ganglion cell layer for only a short time, being eliminated by the first postnatal week. In contrast, a significant number of cone bipolar cells remain in the ganglion cell layer into maturity, although the majority of these cells are also lost during postnatal development. Thus, in addition to ganglion cells and displaced amacrine cells, the ganglion cell layer of the mature retina contains displaced bipolar cells.
Materials and Methods
Animals and tissue preparation. Timed-pregnant and adult Long–Evans rats were obtained from Charles River Laboratories (New York, NY). The animals were housed and bred in accordance with University of California guidelines for the use of laboratory animals. The animals were killed by a lethal injection of sodium pentobarbital (0.6 mg/kg body weight, i.p.) at time points ranging from the day of birth [postnatal day 0 (P0)] to adult. The eyecups were removed from the embryos, hemisected, and fixed in 4% paraformaldehyde (PFA) for 1 hr. Postnatal animals were perfused transcardially with PBS followed by 4% PFA. The eyecups were removed, hemisected, and postfixed with 4% PFA for 30 min. The tissue was then immersed in 25% sucrose solution and embedded in tissue freezing medium (Tissue Tek, Torrance, CA), and 10–12 μm sections were made using a Leica (Bannockburn, IL) 1900 cryostat. The sections were mounted on poly-l-lysine-coated slides (Sigma, St Louis, MO) and kept frozen.
For the whole-mount preparations, a suture was placed in the connective tissue to mark the temporal edge before enucleation. After the retina was removed, a radial cut was made at the site of the suture and additional radial cuts were made to facilitate flattening.
To deplete retinal ganglion cells, a unilateral transection of the optic nerve was performed at P0 or P1. For this purpose, the pups were anesthetized by hypothermia and the frontal cortex and optic nerve were aspirated under visual control.
Antibodies. Photoreceptors, two types of cone bipolar cells, and some cells in the ganglion cell layer were labeled with a rabbit polyclonal antibody to recoverin (1:500–1:2500; a gift from Dr. Alexander Dizhoor Wayne State University, Detroit, MI). Rod photoreceptors were also labeled with a monoclonal antibody to rhodopsin (10 μg/ml; Chemicon, Temecula, CA). The immunogen was prepared using adult rat retina; this antibody reacts with a protein of 39 kDa identified as rhodopsin. Horizontal cells were stained with a monoclonal antibody to calbindin (1:1000; Sigma). A subpopulation of cone bipolar cells was identified with anti-Go antibody (10 μg/ml;Chemicon). Rod bipolar cells were stained with a monoclonal protein kinase C (PKC) antibody (1:10; Amersham Biosciences, Piscataway, NJ). AII amacrine cells were identified with a monoclonal antibody to parvalbumin (1:2000; Sigma). Dopaminergic amacrine cells were labeled using a polyclonal antibody to tyrosine hydroxylase (TH) (1:500, Chemicon), and cholinergic cells were labeled with antivesicular acetylcholine transporter (VAChT; 1:2500) or anti-choline acetyl transferase (ChAT; 1:50) antibodies (Chemicon). A monoclonal vimentin antibody was used to identify Müller cells (1:500; Dako, Carpinteria, CA).
Immunohistochemistry. Retinal sections were incubated in blocking solution containing 10% normal serum, 2% bovine serum albumin, and 0.3% Triton X-100 in PBS for 1 hr at room temperature (RT). Primary antibodies were diluted in blocking solution, and the sections were incubated in the primary antibody solution overnight at 4°C. Primary antibody incubation was omitted for control slides. After several washes with PBS, the sections were incubated with fluorescent secondary antibodies (1:500; Jackson ImmunoResearch, West Grove, PA; 3 μg/ml, Molecular Probes, Eugene, OR) diluted in PBS for 1 hr at RT, washed three times with PBS, and coverslipped with Vectashield mounting media (Vector Laboratories, Burlingame, CA). Other sections were incubated with biotinylated secondary antibodies (1:500; Jackson ImmunoResearch) for 1 hr at RT. After several washes with PBS, tissues were incubated for 1 hr in the Vectastain Elite ABC Kit (Vector Laboratories), and peroxidase was visualized with a 0.5 mg/ml diaminobenzidine (DAB) solution in the presence of H2O2. After final washes, the slides were coverslipped with Vectamount (Vector Laboratories). For double-labeling, sections were incubated in a mixture of two primary antibodies, rinsed with PBS, and incubated in a mixture of two secondary-antibody-conjugated fluorochromes that have different excitation ranges.
For retinal whole-mount processing, the dissected retina was washed in PBS, placed in 25% sucrose solution, and alternately frozen and thawed three times. The tissue was then placed on a glass slide, ganglion cell layer down, treated for endogenous peroxidase with 0.6% H2O2 for 30 min, and then washed thoroughly in PBS. Anti-recoverin antibody was diluted 1:500 in blocking solution. The tissue was incubated in the primary antibody at 4°C for 5 d in younger animals and for up to 7 d in adults. The retina was then washed in PBS, placed in biotinylated goat anti-rabbit secondary antibody diluted 1:300, and incubated for 3 d at 4°C. After washing in PBS, the retina was incubated with ABC solution (Vector Laboratories) overnight at 4°C and then treated with a 0.5 mg/ml DAB solution in the presence of H2O2 for 5–10 min. The retina was then coverslipped with Vectashield mounting media (Vector Laboratories).
Imaging. Confocal images for immunofluorescence were acquired by an Olympus Optical (Tokyo, Japan) upright confocal microscope equipped with an argon–krypton laser in the epifluorescence-confocal mode. A stack of images along thez-axis (0.2–2 μm steps) was collected for each slide. Bright-field photomicrographs were taken by a digital camera (Optronics International, Chelmsford, MA) attached to a Nikon (Tokyo, Japan) eclipse E600 microscope and viewed by differential interference contrast optics.
Statistical analysis. Recoverin-positive cells in the ganglion cell layer were counted using two different methods. In two postnatal retinas (P6 and P8), immunopositive cells were counted in retinal whole mounts using methods described previously (White and Chalupa, 1991; Hutsler and Chalupa, 1995). Briefly, a drawing tube mounted to an upright Nikon microscope was used to create a drawing of the flattened retina, and every immunopositive cell was counted and its locus marked by a dot on the retinal whole-mount drawing. The number of dots in each retina was then counted to obtain an estimate of the total number of recoverin-positive cells. It was problematic to obtain complete labeling of cells in whole-mounted adult retinas. To circumvent this problem, counts were made in sections of the adult retina using the optical volume fractionator method (Gundersen et al., 1988a,b; Bolender et al., 1993). Briefly, 12 μm vertical serial sections were obtained from whole hemisected eyes, and every 11th and 12th section was taken and immunostained with recoverin antibody using peroxidase staining for bright-field microscopy. All of the recoverin-positive cells were counted in the 11th section, and the cells that overlapped in the 11th and 12th sections were subtracted from the total number to avoid double-counting. After these counts were completed, the average number of cells in a single section was calculated and multiplied by the total number of sections to obtain the “unbiased” total number of recoverin-positive cells.
Soma size was measured in P4, P6, P8, P10, and adult whole-mount retinas. Images were captured using a color digital camera (Optronics International) attached to an upright, binocular microscope (Nikon Eclipse E600) using Adobe Photoshop (Adobe Systems, San Jose, CA), and transferred to a Dell computer (Workstation PWS530; Dell Computer Company, Round Rock, TX), where Neurolucida 2000 (MicroBrightField, Inc., Colchester, VT) was used to trace the contours of each soma. NeuroExplorer (MicroBrightField, Inc.) was used to analyze the data, which were then transferred to Microsoft (Seattle, WA) Excel for additional analysis.
Results
Recoverin labeling in the mature rat retina
In the mature retina, recoverin labels two major classes of cells: photoreceptors in the outer nuclear layer as well as ON and OFF cone bipolar cells in the inner nuclear layer (Milam et al., 1993;Euler and Wässle, 1995; Günhan-Agar et al., 2000). Recoverin-positive cells are also present in the ganglion cell layer [Günhan-Agar et al. (2000), their Figs. 2 and 3] (see Fig. 5and vimentin-recoverin labeling in Fig. 6 of the present study). In the present study counts, of these cells in the ganglion cell layer of two adult rats provided population estimates of 960 such neurons in one retina and 823 in another. Although recoverin-positive neurons have been noted previously in several species (Stepanik et al., 1993;Wiechmann and Hammarback, 1993; Yan and Wiechmann, 1997; McGinnis et al., 1999; Günhan-Agar et al., 2000), their origin and cell class have not been established. In an effort to resolve this issue, we examined recoverin-labeled cells that migrated toward and into the presumptive ganglion cell layer in the developing rat retina. We also sought to establish the identity of these recoverin-positive cells by immunocytochemical markers that label selectively different classes of retinal neurons.
Fig. 5.
Confocal images of recoverin-expressing cells (arrows) in the ganglion cell layer of adult retinas (Normal) and in retinas of comparable age in which all ganglion cells were depleted by an optic nerve transection (ONT) at P0 or P1. The presence of such recoverin-positive neurons after early ONT suggests that these were not ganglion cells with centrally projecting axons. In some cases, the axon of a recoverin-expressing cell was found to project upward toward the inner plexiform layer, as shown in the left middle panel. Scale bar, 20 μm.
Fig. 6.
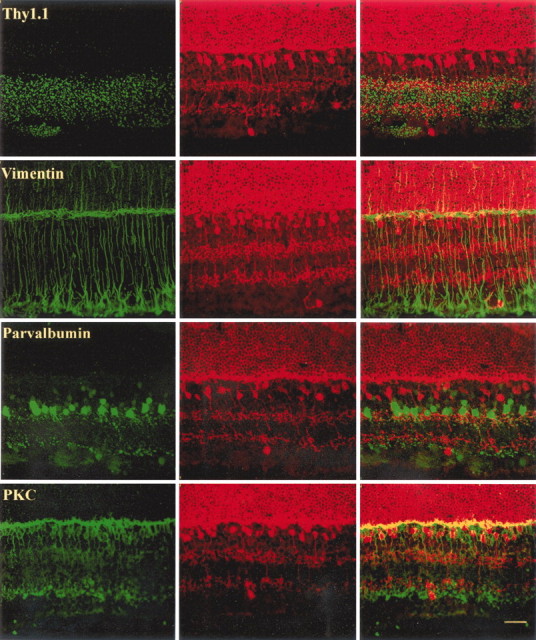
Confocal images of Thy1.1-, vimentin-, parvalbumin-, and PKC-labeled cells (left) and recoverin-labeled cells (middle) in the rat retina. Overlapping images are shown at the left. Note that none of the recoverin-positive cells in the ganglion cell layer are double-labeled. Note also that the axonal process of recoverin-positive cells in the ganglion cell layer is directed toward the inner plexiform layer in the vimentin–recoverin panel. Scale bar, 20 μm.
Recoverin labeling in the developing rat retina
Recoverin is expressed by developing cells in the ventricular zone of the rat retina as early as embryonic day 16 (E16) (data not shown). Recoverin-labeled cells can be observed migrating toward the inner part of the retina at E18, as shown in Figure1. On E20, ∼2 d before birth, recoverin-positive cells are already present in the presumptive ganglion cell layer. By P4, such neurons become more numerous, and by P10, the recoverin-labeled cells in the ganglion cell layer appear to be well differentiated. In many cases, dendritic and/or axonal processes could be seen to originate from particular somas, indicated by arrows in Figure 1.
Fig. 1.

Confocal images of recoverin expression patterns in the rat retina during prenatal and postnatal development. Ages are indicated in each photomicrograph. Gestation in the rat is 21 d. Recoverin is expressed by developing cells in the neuroblast layer of the rat retina as early as E16. The number of cells that express recoverin increases markedly during the last days of gestation and the first week of postnatal life. Note that the migration of these cells into the ganglion cell layer (GCL,arrows) precedes normal migration into the inner nuclear layer (INL). In this and all of the other figures, the ganglion cell layer is oriented down. NBL, Neuroblast layer; ONL, outer nuclear layer;OPL, outer plexiform layer; IPL, inner plexiform layer. Scale bars, 20 μm.
To assess the overall distribution of the recoverin-positive cells in the ganglion cell layer, we attempted to label the entire population of these neurons in P6 and P8 retinal whole mounts. Figure2 (top) shows the location of every recoverin-positive cell in the ganglion cell layer in these two retinas. Note that such cells are scattered throughout all retinal quadrants, and that their density appears relatively uniform. A detailed count revealed that there were 2038 cells at P8, whereas there were 2222 in the P6 retina. This is more than twice the number estimated to be present in the mature retina.
Fig. 2.
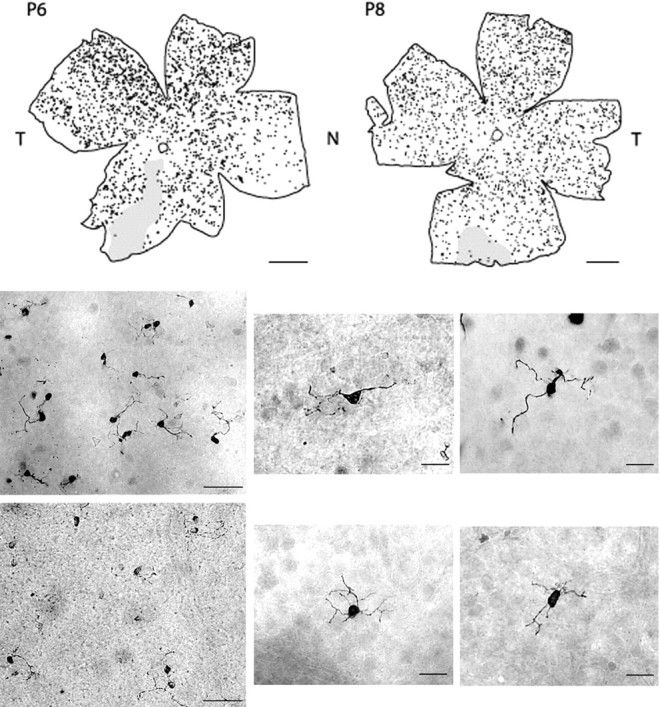
Top, Distribution of recoverin-positive cells in the ganglion cell layer of P6 and P8 whole-mounted retinas. Shaded regions had incomplete staining. Scale bar, 1 mm. Note that the P8 retina is sized to be comparable with the P6 retina. N, Nasal;T, temporal. Bottom, Selected low- and high-power images from P6 (top row) and P8 (bottom row) whole-mounted retinas. Scale bars, 50 μm in low-power images and 20 μm in high-power images.
The extensive dendritic processes of the recoverin-positive neurons could be observed in their entirety in these retinal whole mounts, as shown in the photomicrographs depicted in Figure 2, bottom. In older retinas, recoverin-positive processes were less extensive and the soma sizes were significantly smaller. At P4, P6, and P8, the average soma sizes were 58.20 ± 14.17, 58.24 ± 10.94, and 59.27 ± 12.6 μm2 (means ± SD), respectively. By P10, the soma sizes were smaller, 48.67 ± 8.74 μm2, comparable with the adult size of 46.08 ± 8.22 μm2. The age-related differences were found to be statistically significant (p < 0.01; one-way ANOVA). Additional analysis showed that there were no differences among ages P4, P6, and P8, nor was there a difference between P10 and adult retinas; however, there was a statistically significant difference between the P4–P6–P8 group and the P10 to adult group (p < 0.01; Tukey test).
Rhodopsin labeling of recoverin-positive cells
In the rat, photoreceptors are generated from approximately E16 to P5, with the majority of the rods developing after birth (Kuwabara and Weidman, 1974; Carter-Dawson and LaVail, 1979; Hinds and Hinds, 1979;Barnstable, 1981; Young, 1985). Rhodopsin expression, a visual pigment specific to differentiated rods, does not occur until P1–P2 (Treisman et al., 1988; Watanabe and Raff, 1990). Double-labeling developing retinas with antibodies against recoverin and rhodopsin revealed that some of the recoverin-positive cells outside the photoreceptor layer were rhodopsin-positive. Figure 3 depicts such double-labeled neurons at P0, P1, P3, and P7. At the youngest ages (P0–P3), these cells were situated in the presumptive ganglion cell layer (i.e., in the innermost retinal layer). Several days later, virtually all of these neurons were eliminated from the ganglion cell layer, but a significant number of cells could now be visualized in the inner nuclear layer (Fig. 3, P7 panels). Opsin-like immunoreactive cells have been noted previously in the inner nuclear layer of the developing rat retina (Barnstable, 1982; Araki et al., 1988), but not in the ganglion cell layer.
Fig. 3.
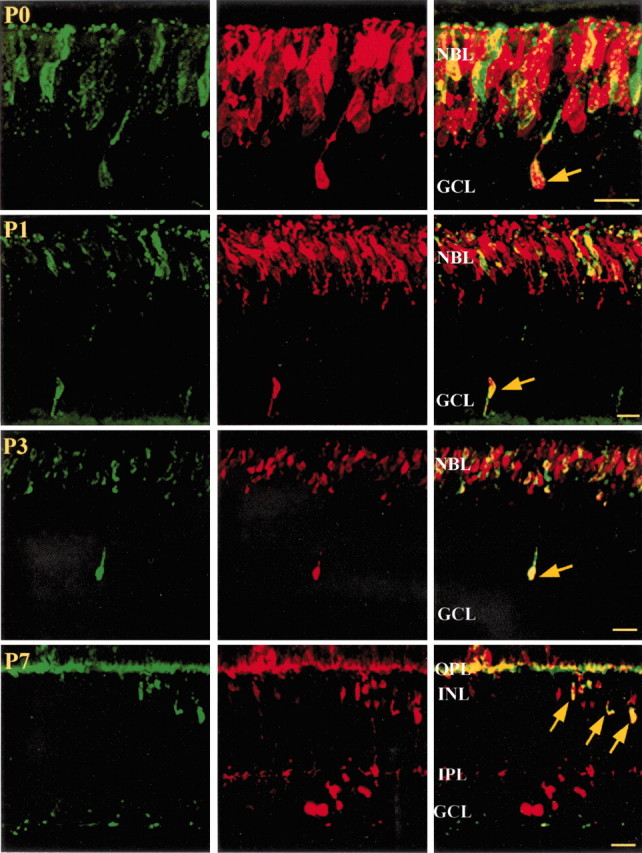
Confocal images of rhodopsin-labeled cells (left) and recoverin-labeled cells (middle) in the rat retina during postnatal development (P0–P7). As indicated by arrows in the right panels, some recoverin-positive cells are rods that have migrated beyond the photoreceptor layer. GCL, Ganglion cell layer; NBL, neuroblast layer; OPL, outer plexiform layer; IPL, inner plexiform layer;INL, inner nuclear layer. Scale bars, 20 μm.
The distribution of rhodopsin-positive cells within the inner nuclear layer at P20 is shown in Figure 4,top. In the mature retina, all rhodopsin labeling is confined to the photoreceptor layer (Fig. 4, bottom). Because rhodopsin-positive cells are eliminated from the ganglion cell layer a few days after birth, this means that such cells were not included in the distributions depicted in Figure 2.
Fig. 4.
Bright-field images of rhodopsin labeling in the P20 and adult retinas. Note that in the adult retina there are no rhodopsin-positive cells in the inner retina. Inset, High-power image of P20. Scale bars, 10 μm in low-power images and 5 μm in high-power images.
Identity of recoverin-positive cells in ganglion cell layer of adult retina
To determine whether the cells expressing recoverin in the ganglion cell layer of the mature retina might be ganglion cells, the optic nerve was transected at either P0 or P1. This manipulation causes a complete and rapid loss of retinal ganglion cells (Miller and Oberdorfer, 1981; Osborne and Perry, 1985; Beazley et al., 1987;Günhan-Agar et al., 2000). Examples of recoverin-positive profiles in the ganglion cell layer of the normal adult retina and in an adult retina that sustained optic nerve transection at P0 are shown in Figure 5. The morphological features and the incidence of such cells in normal retinas and those with early optic nerve sections were virtually identical. These results indicate that the recoverin-positive cells observed in the ganglion cell layer of the adult retina are not ganglion cells.
In retinal cross sections, the recoverin-positive cells in the ganglion cell layer appeared to have morphological features identical to those of bipolar cells. Note in the cells demarcated by arrows in Figure 5 the dense dendritic arbors of the recoverin-positive cells situated in the ganglion cell layer extending into the inner plexiform layer and in some cases the single axon stemming from the opposite pole of the soma. Moreover, axonal processes of some cells could be seen to make a sharp turn upward to innervate the inner plexiform layer (Figs.5, 6, vimentin–recoverin panel).
In an additional effort to establish the identity of these neurons, we also double-labeled retinas with recoverin and a variety of markers specific for different retinal cell types, including ChAT, VAChT, Thy1.1, vimentin, TH, calbindin, parvalbumin, Go, and PKC. Figure 6 depicts the resulting labeling patterns for four of these markers in conjunction with recoverin labeling. As can be seen, there was no evidence of cells double-labeled with recoverin and Thy1.1, vimentin, parvalbumin, or PKC. This was also the case for the five other cell-specific markers we used (data not shown).
Discussion
In the present study, we provide evidence for the presence of two distinct subpopulations of ectopic neurons in the developing and mature rat retina that express the calcium-binding protein recoverin. In the adult retina, the anti-recoverin antibody labels photoreceptors and ON and OFF cone bipolar cells, as well as a smaller number of neurons in the ganglion cell layer. Here we show that these atypical cells are photoreceptors and cone bipolar cells. By labeling retinas from E16 through the early postnatal period, separated at 1 d intervals, we were able to visualize recoverin-positive profiles migrating from the outer to the inner retina. Such cells were observed to exit the germinative layer at E18, and by E20 they were found in the presumptive ganglion cell layer. Thus, most likely, the presence of these neurons outside their normal retinal layers reflects migration errors during early development.
To identify ectopic photoreceptors, we relied on double-labeling with antibodies against recoverin and rhodopsin. Recoverin/rhodopsin-positive profiles were apparent in the presumptive ganglion cell layer as early as P0, but 1 week after birth they were present only in the inner nuclear layer, and at maturity none were found outside the photoreceptor layer. A possible explanation for this sequence of events is that this contingent of photoreceptors fails to recognize a signal to stop migrating when these cells arrive at the photoreceptor layer. The early elimination of these photoreceptors from the ganglion cell layer, and their later demise from the inner nuclear layer probably reflects a failure of these cells to form functional synapses in the inappropriate retinal layers.
The other population of recoverin-positive cells in the ganglion cell layer survives to maturity. Several lines of evidence indicate that these are cone bipolar cells. First, these neurons are recoverin-positive, as is the case with ON and OFF cone bipolar cells. Second, their salient morphological features, consisting of a relatively small cell body with dendritic and axonal processes emanating from opposite poles of the soma, are identical to those of retinal bipolar cells. Third, these cells survived early transection of the optic nerve, a manipulation that induces a rapid loss of developing ganglion cells but does not have an impact on the survival of recoverin-expressing cone bipolar cells (Günhan-Agar et al., 2000). Fourth, these recoverin-positive cells were not double-labeled with any of the 10 cell-specific markers we used, including Thy1.1 for ganglion cells (Beale and Osborne, 1982; Barnstable and Drager, 1984;Barres et al., 1988), rhodopsin for photoreceptors (Treisman et al., 1988; Watanabe and Raff, 1990), PKC for rod bipolar cells (Greferath et al., 1990), calbindin for horizontal cells (Pochet et al., 1991;Mitchell et al., 1995), vimentin for Müller cells (Okada et al., 1990), Go for ON-cone bipolar cells (Vardi, 1998), parvalbumin for AII amacrine cells (Endo et al., 1986; Wässle et al., 1993), TH for dopaminergic amacrine cells (Oyster et al., 1985; Martin-Martinelli et al., 1989), and ChAT (Eckenstein and Thoenen, 1982; Pourcho and Osman, 1986; Brandon, 1987;Wässle et al., 1987; Chun et al., 1988) and VAChT (Koulen, 1997) for cholinergic amacrine cells.
At ∼1 week after birth, the number of recoverin-positive cells in the ganglion cell layer was found to be >2000. In the mature retina, this number drops by >50%, to <1000. The long-term survival of such a sizable contingent of cone bipolar cells in the ganglion cell layer is intriguing, because it is generally assumed that ectopic cells must form functional connections to survive (Rakic, 1975, 1988).
What could be the functional significance of the surviving ectopic bipolar cells in the mature retina? One possibility is that they have formed synaptic connections appropriate for cone bipolar cells. This seems unlikely when one considers the inputs to these neurons. We never observed the dendrites of the recoverin-positive cells to extend into the outer plexiform layer, nor did we note exuberant photoreceptor processes projecting to the inner plexiform layer, as has been documented in the developing ferret retina (Johnson et al., 1999). With respect to the axonal connections of the ectopic bipolar cells, in at least some cases, these could have made appropriate contacts with the dendrites of retinal ganglion cells. In cases in which ostensibly the full extent of the axonal process could be visualized, it appeared that the axon was directed into the inner plexiform layer, in which contacts with the dendrites of retinal ganglion cells could have been established. Whether such functional contacts play a role in the processing of visual information remains to be established. Given the sizable contingent of displaced bipolar cells identified in the present study and the presence of recoverin-positive cells in the human retina, this issue is certainly worth pursuing.
Footnotes
This work was supported by National Eye Institute (NEI) Grant EY0339, an NEI Core Grant, and the Foundation to Prevent Blindness. We thank Dr. Alexander Dizhoor (Wayne State University, Detroit, MI) for the generous gift of the recoverin antibody and Dr. Handan Camdeviren (Mersin University, Mersin, Turkey) for statistical consultation.
Correspondence should be addressed to Dr. Leo M. Chalupa, Section of Neurobiology, Physiology, and Behavior, 1 Shields Avenue, University of California, Davis, CA 95616. E-mail: lmchalupa@ucdavis.edu.
E. Günhan's present address: Mersin University, School of Medicine, Yenisehir Campus, Yenisehir 33169, Mersin, Turkey.
References
- 1.Altshuler D, Cepko C. A temporally regulated, diffusible activity is required for rod photoreceptor development in vitro. Development. 1992;114:947–957. doi: 10.1242/dev.114.4.947. [DOI] [PubMed] [Google Scholar]
- 2.Araki M, Hanihara T, Saito T. Histochemical observations on unique rod-like cells in the developing retina of the normal rat. J Neurocytol. 1988;17:179–188. doi: 10.1007/BF01674205. [DOI] [PubMed] [Google Scholar]
- 3.Barnstable CJ. A gradient of membrane protein in the retina. Nature. 1981;292:13–14. doi: 10.1038/292013a0. [DOI] [PubMed] [Google Scholar]
- 4.Barnstable CJ. Immunological studies of the retina. In: Brockes J, editor. Neuroimmunology. Plenum; New York: 1982. pp. 183–214. [Google Scholar]
- 5.Barnstable CJ, Drager UC. Thy-1 antigen: a ganglion cell specific marker in rodent retina. Neuroscience. 1984;11:847–855. doi: 10.1016/0306-4522(84)90195-7. [DOI] [PubMed] [Google Scholar]
- 6.Barnstable CJ, Blum AS, Devoto SH, Hicks D, Morabito MA, Sparrow JR, Treisman JE. Cell differentiation and pattern formation in the developing mammalian retina. Neurosci Res Suppl. 1988;8:S27–S41. doi: 10.1016/0921-8696(88)90005-9. [DOI] [PubMed] [Google Scholar]
- 7.Barres BA, Silverstein BE, Corey DP, Chun LL. Immunological, morphological, and electrophysiological variation among retinal ganglion cells purified by panning. Neuron. 1988;1:791–803. doi: 10.1016/0896-6273(88)90127-4. [DOI] [PubMed] [Google Scholar]
- 8.Beale R, Osborne NN. Localization of the Thy-1 antigen to the surfaces of rat retinal ganglion cells. Neurochem Int. 1982;4:587–595. doi: 10.1016/0197-0186(82)90049-3. [DOI] [PubMed] [Google Scholar]
- 9.Beazley LD, Perry VH, Baker B, Darby JE. An investigation into the role of ganglion cells in the regulation of division and death of other retinal cells. Brain Res. 1987;430:169–184. doi: 10.1016/0165-3806(87)90151-9. [DOI] [PubMed] [Google Scholar]
- 10.Bolender RP, Hyde DM, Dehoff RT. Lung morphometry: a new generation of tools and experiments for organ, tissue, cell, and molecular biology. Am J Physiol. 1993;265:L521–L548. doi: 10.1152/ajplung.1993.265.6.L521. [DOI] [PubMed] [Google Scholar]
- 11.Brandon C. Cholinergic neurons in the rabbit retina: immunocytochemical localization, and relationship to GABAergic and cholinesterase-containing neurons. Brain Res. 1987;401:385–391. doi: 10.1016/0006-8993(87)91426-0. [DOI] [PubMed] [Google Scholar]
- 12.Carter-Dawson LD, LaVail MM. Rods and cones in the mouse retina. II. Autoradiographic analysis of cell generation using tritiated thymidine. J Comp Neurol. 1979;188:263–272. doi: 10.1002/cne.901880205. [DOI] [PubMed] [Google Scholar]
- 13.Chun MH, Wässle H, Brecha N. Colocalization of [3H]muscimol uptake and choline acetyltransferase immunoreactivity in amacrine cells of the cat retina. Neurosci Lett. 1988;94:259–263. doi: 10.1016/0304-3940(88)90027-4. [DOI] [PubMed] [Google Scholar]
- 14.Dizhoor AM, Ray S, Kumar S, Niemi G, Spencer M, Brolley D, Walsh KA, Philipov PP, Hurley JB, Stryer L. Recoverin: a calcium sensitive activator of retinal rod guanylate cyclase. Science. 1991;251:915–918. doi: 10.1126/science.1672047. [DOI] [PubMed] [Google Scholar]
- 15.Eckenstein F, Thoenen H. Production of specific antisera and monoclonal antibodies to choline acetyltransferase: characterization and use for identification of cholinergic neurons. EMBO J. 1982;1:363–368. doi: 10.1002/j.1460-2075.1982.tb01175.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Endo T, Kobayashi M, Kobayashi S, Onaya T. Immunocytochemical and biochemical localization of parvalbumin in the retina. Cell Tissue Res. 1986;243:213–217. doi: 10.1007/BF00221870. [DOI] [PubMed] [Google Scholar]
- 17.Euler T, Wässle H. Immunocytochemical identification of cone bipolar cells in the rat retina. J Comp Neurol. 1995;361:461–478. doi: 10.1002/cne.903610310. [DOI] [PubMed] [Google Scholar]
- 18.Greferath U, Grunert U, Wässle H. Rod bipolar cells in the mammalian retina show protein kinase-C-like immunoreactivity. J Comp Neurol. 1990;301:433–442. doi: 10.1002/cne.903010308. [DOI] [PubMed] [Google Scholar]
- 19.Grunert U, Martin PR, Wässle H. Immunocytochemical analysis of bipolar cells in the macaque monkey retina. J Comp Neurol. 1994;348:607–627. doi: 10.1002/cne.903480410. [DOI] [PubMed] [Google Scholar]
- 20.Gundersen HJ, Bendtsen TF, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sorensen FB, Vesterby A, West MJ. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. APMIS. 1988a;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- 21.Gundersen HJ, Bagger P, Bendtsen TF, Evans SM, Korbo L, Marcussen N, Moller A, Nielsen K, Nyengaard JR, Pakkenberg B, Sørensen FB, Vesterby A, West MJ. The new stereological tools: dissector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. APMIS. 1988b;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- 22.Günhan E, Choudary PV, Landerholm TE, Chalupa LM. Depletion of cholinergic amacrine cells by a novel immunotoxin does not perturb the formation of segregated on and off cone bipolar cell projections. J Neurosci. 2002;22:2265–2273. doi: 10.1523/JNEUROSCI.22-06-02265.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Günhan-Agar E, Kahn D, Chalupa LM. Segregation of on and off bipolar cell axonal arbors in the absence of retinal ganglion cells. J Neurosci. 2000;20:306–314. doi: 10.1523/JNEUROSCI.20-01-00306.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hinds JW, Hinds PL. Differentiation of photoreceptors and horizontal cells in the embryonic mouse retina: an electron microscopic, serial section analysis. J Comp Neurol. 1979;187:495–511. doi: 10.1002/cne.901870303. [DOI] [PubMed] [Google Scholar]
- 25.Hutsler JJ, Chalupa LM. Development of neuropeptide Y immunoreactive amacrine and ganglion cells in the pre- and postnatal cat retina. J Comp Neurol. 1995;361:152–164. doi: 10.1002/cne.903610112. [DOI] [PubMed] [Google Scholar]
- 26.Johnson PT, Williams RR, Cusato K, Reese BE. Rods and cones project to the inner plexiform layer during development. J Comp Neurol. 1999;414:1–12. [PubMed] [Google Scholar]
- 27.Korf HW, White BH, Schaad NC, Klein DC. Recoverin in pineal organs and retinae of various vertebrate species including man. Brain Res. 1992;595:57–66. doi: 10.1016/0006-8993(92)91452-k. [DOI] [PubMed] [Google Scholar]
- 28.Koulen P. Vesicular acetylcholine transporter (VAChT): a cellular marker in rat retinal development. NeuroReport. 1997;8:2845–2848. doi: 10.1097/00001756-199709080-00008. [DOI] [PubMed] [Google Scholar]
- 29.Kuwabara T, Weidman TA. Development of the prenatal rat retina. Invest Ophthalmol. 1974;13:725–739. [PubMed] [Google Scholar]
- 30.Lambrecht HG, Koch KW. Recoverin, a novel calcium-binding protein from vertebrate photoreceptors. Biochim Biophys Acta. 1992;1160:63–66. doi: 10.1016/0167-4838(92)90038-f. [DOI] [PubMed] [Google Scholar]
- 31.Martin-Martinelli E, Simon A, Vigny A, Nguyen-Legros J. Postnatal development of tyrosine-hydroxylase-immunoreactive cells in the rat retina: morphology and distribution. Dev Neurosci. 1989;11:11–25. doi: 10.1159/000111881. [DOI] [PubMed] [Google Scholar]
- 32.Masland RH, Raviola E. Confronting complexity: strategies for understanding the microcircuitry of the retina. Annu Rev Neurosci. 2000;23:249–284. doi: 10.1146/annurev.neuro.23.1.249. [DOI] [PubMed] [Google Scholar]
- 33.McGinnis JF, Stepanik PL, Chen W, Elias R, Cao W, Lerious V. Unique retina cell phenotypes revealed by immunological analysis of recoverin expression in rat retina cells. J Neurosci Res. 1999;55:252–260. doi: 10.1002/(SICI)1097-4547(19990115)55:2<252::AID-JNR13>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 34.Milam AH, Dacey DM, Dizhoor AM. Recoverin immunoreactivity in mammalian cone bipolar cells. Vis Neurosci. 1993;10:1–12. doi: 10.1017/s0952523800003175. [DOI] [PubMed] [Google Scholar]
- 35.Miller ED, Tran MN, Wong GK, Oakley DM, Wong RO. Morphological differentiation of bipolar cells in the ferret retina. Vis Neurosci. 1999;16:1133–1144. doi: 10.1017/s0952523899166136. [DOI] [PubMed] [Google Scholar]
- 36.Miller NM, Oberdorfer M. Neuronal and neuroglial responses following retinal lesions in the neonatal rats. J Comp Neurol. 1981;202:493–504. doi: 10.1002/cne.902020404. [DOI] [PubMed] [Google Scholar]
- 37.Mitchell CK, Rowerendleman C, Ashraf S, Redburn DA. Calbindin immunoreactivity of horizontal cells in the developing rabbit retina. Exp Eye Res. 1995;61:691–698. doi: 10.1016/s0014-4835(05)80020-x. [DOI] [PubMed] [Google Scholar]
- 38.Morest DK. The pattern of neurogenesis in the retina of the rat. Z Anat Entwicklungsgesch. 1970;131:45–67. doi: 10.1007/BF00518815. [DOI] [PubMed] [Google Scholar]
- 39.Okada M, Matsumura M, Ogino N, Honda Y. Müller cells in detached human retina express glial fibrillary acidic protein and vimentin. Graefes Arch Clin Exp Ophthalmol. 1990;228:467–474. doi: 10.1007/BF00927264. [DOI] [PubMed] [Google Scholar]
- 40.Osborne NN, Perry VH. Effect of neonatal optic nerve transection on some classes of amacrine cells in the rat retina. Brain Res. 1985;343:230–235. doi: 10.1016/0006-8993(85)90739-5. [DOI] [PubMed] [Google Scholar]
- 41.Oyster C, Takahashi E, Cilluffo M, Brecha N. Morphology and distribution of tyrosine hydroxylase-like immunoreactive neurons in the cat retina. Proc Natl Acad Sci USA. 1985;82:6335–6339. doi: 10.1073/pnas.82.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pochet R, Pasteels B, Setoohshima A, Bastianelli E, Kitajima S, Vaneldik LJ. Calmodulin and calbindin localization in retina from 6 vertebrate species. J Comp Neurol. 1991;314:750–762. doi: 10.1002/cne.903140408. [DOI] [PubMed] [Google Scholar]
- 43.Pourcho RG, Osman K. Cytochemical identification of cholinergic amacrine cells in cat retina. J Comp Neurol. 1986;247:497–504. doi: 10.1002/cne.902470409. [DOI] [PubMed] [Google Scholar]
- 44.Rakic P. Cell migration and neuronal ectopias in the brain. Birth Defects Orig Artic Ser. 1975;11:95–129. [PubMed] [Google Scholar]
- 45.Rakic P. Defects of neuronal migration and the pathogenesis of cortical malformations. Prog Brain Res. 1988;73:15–37. doi: 10.1016/s0079-6123(08)60494-x. [DOI] [PubMed] [Google Scholar]
- 46.Reese BE, Colello RJ. Neurogenesis in the retinal ganglion cell layer of the rat. Neuroscience. 1992;46:419–429. doi: 10.1016/0306-4522(92)90062-7. [DOI] [PubMed] [Google Scholar]
- 47.Reh TA. Cellular interactions determine neuronal phenotypes in rodent retinal cultures. J Neurobiol. 1992;23:1067–1083. doi: 10.1002/neu.480230811. [DOI] [PubMed] [Google Scholar]
- 48.Stepanik PL, Lerious V, McGinnis JF. Developmental appearance, species and tissue specificity of mouse 23-kDa, a retinal calcium-binding protein (recoverin). Exp Eye Res. 1993;57:189–197. doi: 10.1006/exer.1993.1114. [DOI] [PubMed] [Google Scholar]
- 49.Treisman JE, Morabito MA, Barnstable CJ. Opsin expression in the rat retina is developmentally regulated by transcriptional activation. Mol Cell Biol. 1988;8:1570–1579. doi: 10.1128/mcb.8.4.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vardi N. α subunit of Go localizes in the dendritic tips of ON bipolar cells. J Comp Neurol. 1998;395:43–52. [PubMed] [Google Scholar]
- 51.Wässle H, Chun MH, Muller F. Amacrine cells in the ganglion cell layer of the cat retina. J Comp Neurol. 1987;265:391–408. doi: 10.1002/cne.902650308. [DOI] [PubMed] [Google Scholar]
- 52.Wässle H, Grunert U, Rohrenbeck J. Immunocytochemical staining of AII-amacrine cells in the rat retina with antibodies against parvalbumin. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- 53.Watanabe T, Raff MC. Rod photoreceptor development in vitro: intrinsic properties of proliferating neuroepithelial cells change as development proceeds in the rat retina. Neuron. 1990;4:461–467. doi: 10.1016/0896-6273(90)90058-n. [DOI] [PubMed] [Google Scholar]
- 54.White CA, Chalupa LM. Subgroup of α ganglion cells in the adult cat retina is immunoreactive for somatostatin. J Comp Neurol. 1991;304:1–13. doi: 10.1002/cne.903040102. [DOI] [PubMed] [Google Scholar]
- 55.Wiechmann AF, Hammarback JA. Expression of recoverin mRNA in the human retina: localization by in situ hybridization. Exp Eye Res. 1993;57:763–769. doi: 10.1006/exer.1993.1184. [DOI] [PubMed] [Google Scholar]
- 56. Wong ROL, Godinho L. Development of vertebrate retina. The visual neurosciences Chalupa LM, Werner JS. 2003. MIT; Cambridge, MA, in press. [Google Scholar]
- 57.Yan XX, Wiechmann AF. Early expression of recoverin in a unique population of neurons in the human retina. Anat Embryol (Berl) 1997;195:51–63. doi: 10.1007/s004290050024. [DOI] [PubMed] [Google Scholar]
- 58.Young RW. Cell differentiation in the retina of the mouse. Anat Rec. 1985;212:199–205. doi: 10.1002/ar.1092120215. [DOI] [PubMed] [Google Scholar]




