Abstract
The importance of the basal ganglia in controlling motor function is well known. However, neuroimaging studies have failed to show either movement-rate dependence or different activation patterns caused by self-initiated (SI) and externally triggered (ET) movements in the basal ganglia–thalamo-motor loop. We herein report the functional magnetic resonance image (fMRI) mapping of sequential left-hand finger movements at five different rates under SI and ET conditions. Significant movement-rate dependence was found in the whole right basal ganglia–thalamo-motor loop only during the SI task. Network analysis also showed strong interactions within this loop during SI movement, whereas interactions were present only from the premotor cortex to the putamen via the sensorimotor cortex during the ET task. Furthermore, psychophysiological interaction analysis confirmed the different modulation between the two tasks in the putamen. fMRI provides evidence that the basal ganglia–thalamo-motor loop plays a key role in controlling the rate of sequential finger movements in SI movement but not in ET movement.
Keywords: basal ganglia, fMRI, motor control, self-initiated movement, externally triggered movement, movement rate, path analysis
Introduction
The role of the basal ganglia in controlling motor function remains enigmatic despite considerable advances in the understanding of the pathophysiology of movement disorders (Brooks, 2000). The basal ganglia have been assumed to control the velocity of movement partly because of the bradykinesia seen in patients with Parkinson's disease (PD) (Hallett and Khoshbin, 1980) and electrophysiological studies in nonhuman primates (Georgopoulos et al., 1983; Mitchell et al., 1987; Hamada et al., 1990). Previous imaging studies, however, failed to show a correlation between movement rate and activity of the basal ganglia (Blinkenberg et al., 1996; Sadato et al., 1996; Jenkins et al., 1997). These findings provide evidence that basal ganglia do not play a significant role in the decision of movement rate (Brooks, 1995, 2000). The tasks used in previous studies were externally cued movements rather than self-paced sequential movements. PD patients experience great difficulty in volitional sequential movements (Benecke et al., 1987), although external cues improve their performance (Georgiou et al., 1994). Consequently, the basal ganglia seem to be activated more by sequential or internally cued movement than by repetitive or externally cued movement. Non-dominant hand movement caused a greater recruitment of striatum than that of dominant hand in sequential finger movement (Mattay et al., 1998). Thus, the effects of sequential and internally cued movement in the non-dominant hand must be investigated to elucidate the physiological role of the basal ganglia in movement rate and volitional movement.
Discrepancies in the performance of PD patients suggest that the basal ganglia may be differently involved in self-initiated (SI) and externally triggered (ET) movements (Benecke et al., 1987; Georgiou et al., 1994). Electrophysiological studies in monkeys have suggested that the basal ganglia–supplementary motor area (SMA) projection is more involved in SI movement, whereas the cerebellum–lateral premotor cortex (PM) projection is more important in ET movement (Romo and Schultz, 1987; Mushiake et al., 1991; Middleton and Strick, 2000). However, only a few imaging studies have reported the differential activation of the basal ganglia between SI and ET movements (Menon et al., 1998; Cunnington et al., 2002). Neither study investigated the function of the cortico–subcortical loops, nor did they describe the role of basal ganglia in the movement. Thus, the role of the basal ganglia in SI movement remains unknown.
In this study, we investigated the regional cerebral activation by functional magnetic resonance image (fMRI) during sequential left-hand finger movements at five different rates in SI or ET movement. We focused on motor-related regions, particularly the basal ganglia–thalamo-motor loop. The aims of the present study were (1) to study the correlation between the activity of the basal ganglia and rates of sequential finger movements during SI and ET movements; (2) to construct a functional network to account for correlation in the basal ganglia–thalamo-motor loop; and (3) to compare the functional network model of SI movement with that of ET movement to elucidate the physiological role of the basal ganglia.
Materials and Methods
Participants. Ten healthy male volunteers (age range, 24–29 years) participated in the study. All subjects were strongly right-handed as assessed by a modified version of the Edinburgh handedness inventory (Oldfield, 1971). All subjects gave informed written consent.
Experimental design. We showed the subjects how to move each finger with changing movement rates. They were required to practice the task before the scan until they were able to perform it at constant amplitude without error. They were also instructed to keep their eyes closed during MRI. The activation paradigm consisted of a sequential movement performed with the left hand. To perform this task, the subjects had to (1) make finger-to-thumb opposition movements in the order of index, middle, ring, and little finger; (2) open and clench the fist twice; (3) complete finger-to-thumb opposition in the opposite order (i.e., little, third, middle, and index finger); (4) open and clench the fist twice again; and (5) repeat the same series of movements during the 40 sec of data acquisition (Sabatini et al., 2000). Each finger-to-thumb opposition movement and a pair of open and clench the fist movements was counted as a single movement. In the SI task, movement rates were set at very slow (as slow as possible), slow, moderate (comfortable pace), fast, very fast (as fast as possible). During the practice session, movement rates were ∼0.5 Hz at very slow and near 4.0 Hz at very fast in most of the subjects. In the ET task, therefore, movement rates were set at 0.5, 1, 2, 3, or 4 Hz. Subjects paced their movements in response to a metronome, which consisted of a clicking sound at precise time intervals and was delivered binaurally to the subject via air conduction through a pair of plastic tubes 2.5 m in length. During the rest condition, subjects lay down while listening to the metronome used in the ET session. Movements were performed for 40 sec (activation) at a constant rate, followed by 40 sec of rest (baseline), and were switched by a voice signal. Movement rate conditions were presented in pseudorandom order within an imaging series. As a consequence, there were a total of five baseline/activation (five different rates) cycles per imaging series. Four consecutive imaging series (two SI and two ET) were conducted per subject. Finger movements were analyzed visually through a TV monitor, and exact movement rates were determined.
fMRI methods. Images were acquired on a 1.5 tesla Magnetom SYMPHONY (Siemens, Enlangen, Germany) whole-body MRI system equipped with a circular polarized volume head coil. Initially, a set of localized images was acquired to position the image slice. For each session, 100 EPI multislice data sets were acquired (echo time 50 msec; repetition time 4 sec; flip angle 90°; acquisition time for the whole paradigm, 400 sec). Each multislice data set contained 32 transverse slices (slice thickness 3.0 mm; interslice gap 1.0 mm; matrix 64 × 64; field of view 23 cm). All images were analyzed using Statistical Parametric Mapping 99 Software (Wellcome Department of Cognitive Neurology, London, UK) (Friston et al., 1995). The first three data sets of each time series were discarded, and the remaining EPI volumes were then realigned to the first volume. Data sets (one SI and one ET from each subject) were then chosen for their high quality as demonstrated by small motion corrections (<1 mm along each axis). The images were spatially normalized to a standard template and smoothed with a Gaussian kernel of 8 mm full-width at half-maximum. The design matrix was set using the box-car reference waveform (40 sec epoch). The time series in each voxel were high-pass filtered (160 sec cutoff) and scaled to a grand mean of 100 over voxels and scans within each session. Using a parametric approach, three different rate-response relationships could be identified: (1) categorical on–off responses based on the difference between finger movement and resting conditions regardless of movement rate (zero order term), (2) linear responses in parallel with movement rate (first order term), and (3) nonlinear relationships (second order term) (Buchel et al., 1996,1998).
A general linear model was applied to reveal the voxel-wise effects of tasks (zero order term) and the changes in these effects across scans (first and second order). A statistical parametrical map (SPM) of theF-static SPM{F} for the group data were generated for this general linear model. For group analysis, we used multi-subject, fixed-effect analysis. The threshold for significance was set at p < 0.05 corrected (F(1,623.7) > 25.05). fMRI signal change was calculated at the local maxima (the voxel with peakF value) in the activated regions within the basal ganglia–thalamo-motor loop from the conjunction analysis. For the voxels thus identified, the percentage signal changes were extracted for different order terms per movement rate across each subject and were plotted against the rates of movement. Pearson's correlation coefficient was calculated to examine a linear correlation between the movement rate and fMRI signal change at each assigned activated region.
Network analysis. To construct a functional network to account for correlation in the basal ganglia–thalamo-motor loop, interregional correlation was calculated on the basis of data for the correlation between movement rate and fMRI signal change. Individual data of signal changes from five different movement rates were cross-correlated on a region by region basis using a Pearson product moment correlation. Each of the selected regions was centered on the stereotactic coordinates of the local SPM{F} maximum detected in the relevant anatomical region [i.e., right putamen, right thalamus, SMA, right sensory motor cortex (SMC), and right PM] as described above. The selected regions are presented in Table 2. The connections between these areas are based on the modified model reported previously (Grafton et al., 1994). The movement-related correlation matrices were used to calculate path coefficients as defined in Figure 4 using a path analysis, implemented with the software LISREL (Scientific Software, Chicago IL), as described previously (Grafton et al., 1994; McIntosh and Gonzalez-Lima, 1994; McIntosh et al., 1994). A maximum likelihood algorithm was used to fit parameters. Typical fits required only 4 or 14 iterations to obtain stable solutions. The model fit was good because Bollen's fit index was 0.938 in SI and 0.998 in ET, respectively (Bullmore et al., 2000).
Table 2.
Inter-regional correlation coefficients (Pearson product-moment correlation)
| Rt. putamen | Rt. thalamus | SMA | Rt. SMC | Rt. PM | |
|---|---|---|---|---|---|
| Self-initiated | |||||
| Rt. putamen | 1.000 | ||||
| Rt. thalamus | 0.776 | 1.000 | |||
| SMA | 0.622 | 0.459 | 1.000 | ||
| Rt. SMC | 0.529 | 0.591 | 0.390 | 1.000 | |
| Rt. PM | 0.236 | 0.236 | 0.199 | 0.387 | 1.000 |
| Externally triggered | |||||
| Rt. putamen | 1.000 | ||||
| Rt. thalamus | 0.188 | 1.000 | |||
| SMA | 0.209 | 0.153 | 1.000 | ||
| Rt. SMC | 0.417 | 0.127 | 0.088 | 1.000 | |
| Rt. PM | 0.298 | 0.057 | 0.024 | 0.764 | 1.000 |
SMA, Supplementary motor area; SMC, sensorimotor cortex; PM, premotor cortex; Rt., right. Abbreviations also apply to Table 3.
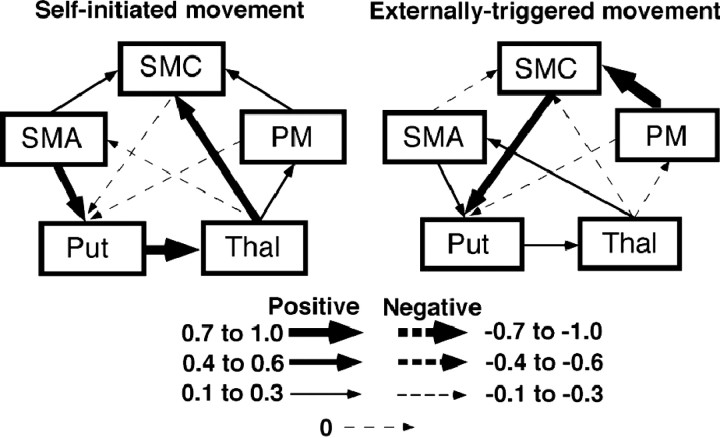
Fig. 4.
Results of LISREL parameter estimates during self-initiated and externally triggered movements. Positive coefficients (solid arrows) indicate interactions in which an increase of activity in one area is associated with an increase of activity in the other area. Negative coefficients (broken arrows) indicate an opposite interaction. Compared with externally triggered movement, self-initiated movement is associated with strong positive interaction of SMA–basal ganglia–thalamocortical projections to the SMC. Put, Right putamen; Thal, right thalamus; SMA, supplementary motor area; SMC, right sensorimotor cortex; PM, right premotor cortex.
Psychophysiological interaction analysis. To confirm the results of network analysis, psychophysiological interaction analysis was used (Friston et al., 1997). The design matrix represented a factorial design in a parametric context with two factors (SI versus ET and five rates of movements). Movement rates were set as typical mirror symmetry (i.e., positive linear for SI and negative linear for ET) between SI and ET. We selected a reference region, which showed significant positive linearity between movement rates and signal change during SI in the design matrix as described above: the right putamen (x, y, z = 26, −6, 12;Z = 4.02; p < 0.0001). The covariates consisted of the group effect (mirror symmetry between SI and ET) and signal change of the reference region. The analysis looked for the brain regions within SMA and right thalamus with activities that were modulated differently in SI compared with ET. Multi-subject, fixed-effect analysis was used for group analysis. The resulting set of voxel values for each contrast, constituting an SPM of thet-static SPM{t}, was transformed to the unit normal distribution SPM{Z} and thresholded at p = 0.05, corrected (Z > 4.80). If the activities in these regions were modulated separately for the two tasks, there should be an interaction in term of the difference in the regression slope between the two tasks. Analysis of covariance (ANCOVA) was used to compare the slope by task.
Results
Performance of subjects
In general, the subjects showed fairly good performance in SI movements. Very slow movements were performed at the frequency of 0.64 ± 0.24 Hz (mean ± SD), slow movements at 1.02 ± 0.32 Hz, moderate movements at 1.79 ± 0.47 Hz, fast movements at 3.05 ± 0.61 Hz, and very fast movements at 4.27 ± 0.73 Hz, respectively. ET movement rates were almost identical to the rate of the auditory trigger (0.5 Hz trigger, 0.50 ± 0.00 Hz; 1.0 Hz trigger, 1.00 ± 0.01 Hz; 2.0 Hz trigger, 2.00 ± 0.04 Hz; 3.0 Hz trigger, 3.00 ± 0.03 Hz; 4.00 Hz trigger; 4.00 ± 0.04 Hz). The rates of the SI and ET movement were very similar without statistically significant differences (p = 0.438; two-way ANOVA with repeated measures).
Foci of activation
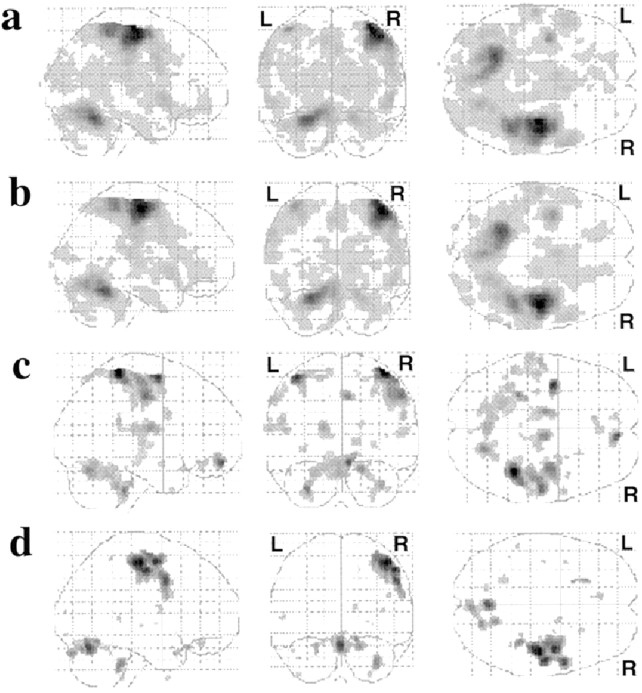
To separate the regional activity within the same task but at different rate-response functions, a parametric approach based on orthogonal basis functions up to the second order was used. In the study of categorical on–off response, both tasks caused significant activation at the right posterior putamen, right thalamus, SMA, right primary SMC, and right PM (Table 1; Figs.1a,b,2a,b).
Table 1.
Parametric analysis of rate-dependent BOLD effects
| Region | Categorical on–off responses | First order term linear effect | ||
|---|---|---|---|---|
| F values | Talairach coordinates (x,y, z) | F values | Talairach coordinates (x, y, z) | |
| Self-initiated movement | ||||
| Rt Putamen | 118.69 | (26, −2, −12) | 29.89 | (28, −12, 0) |
| Rt Thalamus | 151.26 | (12, −16, 6) | 37.23 | (14, −18, 4) |
| SMA | 203.3 | (−6, 2, 50) | 62.49 | (6, −14, 44) |
| Rt SMC | 1133.05 | (38, −14, 62) | 114.93 | (38, −40, 64) |
| Rt PM | 261.44 | (56, 6, 36) | 71.65 | (34, −8, 62) |
| Externally triggered movement | ||||
| Rt Putamen | 83.27 | (28, −4, −10) | n.s. | |
| Rt Thalamus | 137.41 | (12, −20, 4) | n.s. | |
| SMA | 166.91 | (−4, −2, 48) | n.s. | |
| Rt SMC | 1039.56 | (42, −16, 56) | 67.01 | (44, −20, 56) |
| Rt PM | 151.92 | (54, 4, 36) | 59.09 | (40, −6, 56) |
F values refer to activation maximum within the respective region, p < 0.05 corrected, height thresholdF(1,623.7)> 25.05; SPM coordinates in parentheses. n.s., Activation below correctedp < 0.05 values; SMA, supplementary motor area; SMC, sensorimotor cortex; PM, premotor cortex; Rt, right.
Fig. 1.
Statistical parametric maps showing the sites of significant hemodynamic responses (threshold at p< 0.05, corrected). These areas are projected onto single sagittal, coronal, and transverse planes. a,b, Categorical on–off responses across all movement rates and subjects. c, d, Parametric responses characterized by linear effect. a,c, Self-initiated movement. b,d, Externally triggered movement. R, Right; L, left.
Fig. 2.
Areas of subcortical activation (p < 0.05, corrected) located within the right putamen for self-initiated (a, c) and externally triggered movements (b,d). a, b, Categorical on–off responses across all movement rates and subjects.c, d, Parametric responses characterized by linear effect. R, Right; L, left.
A significant linear relationship between the measured hemodynamic responses and the movement rate was evident at the right posterior putamen, right thalamus including the ventrolateral and ventroanterior nuclei, SMA, right SMC, and right PM during the SI task. In the ET task, a significant linear relationship was found only at the right SMC and right PM (Table 1; Figs. 1c,d,2c,d).
A significant nonlinear rate-response function was observed only at the cingulate cortex (F(1,614) = 36.28;p < 0.001; x, y,z = −4, 8, 34) in the ET task.
Movement rate and fMRI signal change
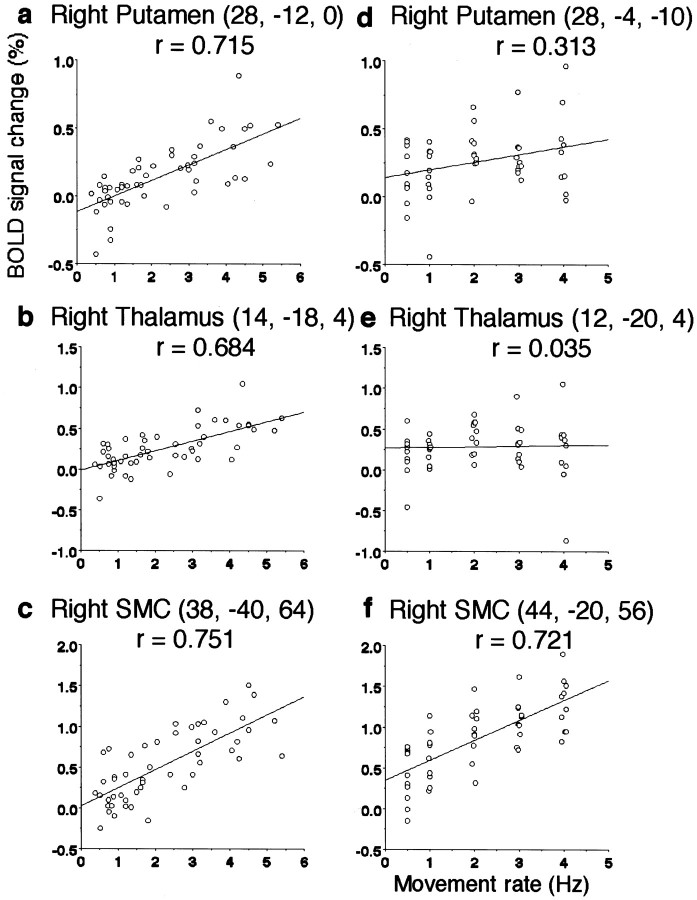
To confirm the linear relationship between the activation of the basal ganglia–thalamocortical loop and the rate of sequential finger movement, fMRI signal changes were plotted against the rate of finger movement for activated areas identified from the first order term (Fig.3). This was done for the right putamen, right thalamus, SMA, right SMC, and right PM during the SI task, and for the right SMC and right PM during the ET task. In other structures, the fMRI signal changes were plotted for the areas from the zero order term. During the SI task, a strong positive correlation was found at the right posterior putamen (r = 0.715;p < 0.001), right thalamus (r = 0.684;p < 0.001), and right SMC (r = 0.751;p < 0.001). A moderate positive correlation was observed at the SMA (r = 0.510; p < 0.001), whereas a weak positive correlation was noted at the right PM (r = 0.393; p = 0.005). During the ET task, a strong positive correlation was found at the right SMC (r = 0.721; p < 0.001) and right PM (r = 0.677; p < 0.001). There was a weak positive correlation at the right putamen (r = 0.313; p = 0.027). No significant linear correlation was found at the right thalamus (r = 0.034;p = 0.810) and SMA (r = 0.164;p = 0.256).
Fig. 3.
Relationship between finger movement rates and fMRI signal change across each subject.a–c, Self-initiated movement.d–f, Externally triggered movement. SMC, Sensorimotor cortex.
Functional network analysis
During the SI movement task, there was a strong positive correlation between movement rate and fMRI signal change within the basal ganglia–thalamo-motor loop; therefore, this loop appeared to play a significant role in rate-dependent motor processing. To confirm this hypothesis, functional network analysis was performed within the loop.
First, we constructed the correlation matrix of fMRI signal changes. Among the activated areas, Table 2 shows the inter-regional cross-correlation within the basal ganglia–thalamo-motor loop obtained from both tasks. Estimates of path coefficients during SI and ET finger movements are summarized in Table3. Regional interactions can be characterized by a path diagram as shown in Figure4. In this diagram, the width of the arrows and the direction of arrowheads correspond to the strength of uni-directional coefficients but not bi-directional correlation. During SI finger movement, there was a strong positive interaction in the projection from the right putamen to the right thalamus. Moderate positive interactions were found in the projection from the SMA to the right putamen and from the right thalamus to the right SMC. The overall pattern of regional interactions showed a dramatic change during ET finger movement. There was a strong positive interaction from the right PM to the right SMC and a moderate interaction from the SMC to the putamen. There were weak or no interactions among other structures.
Table 3.
Path coefficients during two different tasks
| Rt. putamen | Rt. thalamus | SMA | Rt. SMC | Rt. PM | |
|---|---|---|---|---|---|
| Self-initiated | |||||
| Rt. putamen | 0.627 | 0.081 | -0.029 | ||
| Rt. thalamus | 0.780 | ||||
| SMA | −0.069 | ||||
| Rt. SMC | 0.430 | 0.146 | 0.248 | ||
| Rt. PM | 0.242 | ||||
| Externally triggered | |||||
| Rt. putamen | 0.159 | 0.412 | -0.028 | ||
| Rt. thalamus | 0.140 | ||||
| SMA | 0.128 | ||||
| Rt. SMC | 0.051 | 0.059 | 0.759 | ||
| Rt. PM | 0.016 |
Definitions of abbreviations provided in Table 2 footnote.
Psychophysiological interaction analysis
Because parametric analysis showed a task-related difference in the right putamen, the right thalamus, and the SMA, psychophysiological interaction analysis was performed among those regions. We demonstrated significant alteration in functional connectivity between the right putamen and SMA (x, y, z = 8, −14, 50; Z = 5.94) and also between right putamen and the right thalamus (14, −10, 4; Z = 5.06) (Fig.5). A comparison of regression slope showed a different functional relationship between the SMA and right putamen (p = 0.0135 by ANCOVA) and also between the right putamen and the right thalamus (p = 0.0055) during SI compared with ET (Fig. 5).
Fig. 5.
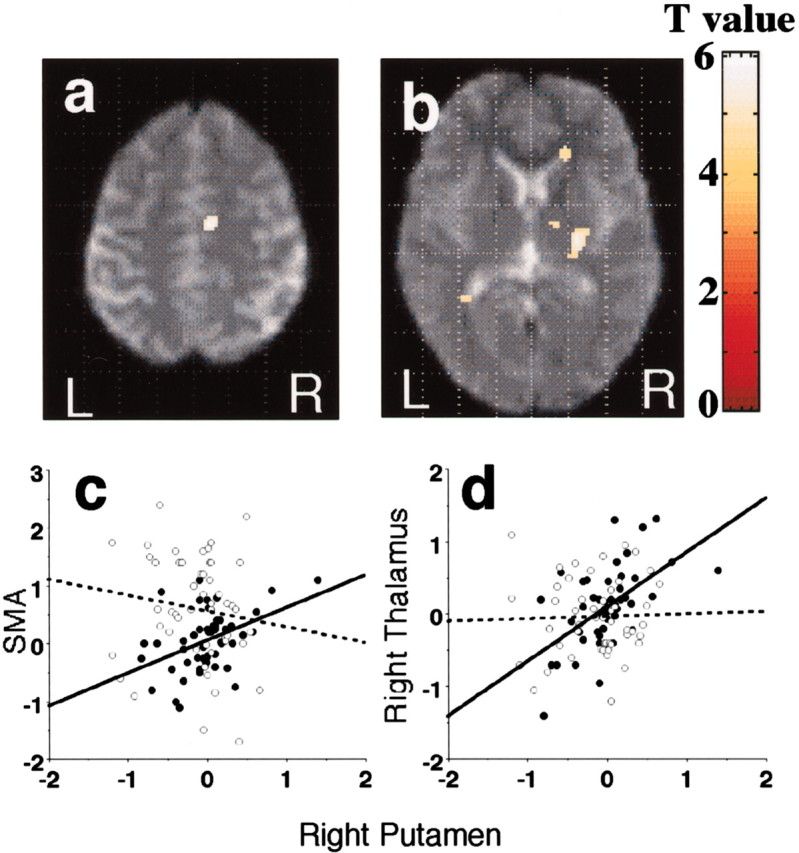
SMA (a) and right thalamus (b) showing a significant increase in functional connectivity with right putamen in SI movement versus ET movement. R, Right; L, left. c, d, Scatterplots of the fMRI percentage signal changes in the right putamen (x,y, z = 26, −6, 12) versus SMA (8, −14, 50) (c) and the right putamen versus the right thalamus (14, −10, 4) (d) during SI (●) and ET (○). Two regression lines (a solid line for SI and a broken line for ET) are overlaid in each group, and each line shows the percentage signal change per movement rate across each subject. Note that the slopes of regression lines are significantly different.
Discussion
In an attempt to elucidate the motor role of the basal ganglia, we measured the regional activation of the basal ganglia–thalamo-motor loop using fMRI during sequential finger movements of SI and ET tasks. Our major new findings were as follows: (1) the signal intensity of the right posterior putamen increased in parallel with the movement rate during the SI task; (2) path analysis based on the rate-dependent fMRI signal changes showed strong interactions in the basal ganglia–thalamo-motor loop during SI movement; and (3) interactions of the basal ganglia–thalamo-motor loop differed between SI and ET finger movements, which were confirmed by psychophysiological interaction analysis.
Putaminal activation and movement rate
Partly on the basis of the observation that PD patients have bradykinesia, it has been suggested that the basal ganglia play an important role in controlling the velocity of movements (Hallett and Khoshbin, 1980). One approach for determining whether specific brain areas play a key role in mediating a specific function is to examine whether they show a significant correlation with the levels of blood flow when such function is performed at different intensities. Previous neuroimaging studies showed no correlation of lentiform regional cerebral blood flow (rCBF) with increasing movement rates (Blinkenberg et al., 1996; Sadato et al., 1996; Jenkins et al., 1997). This suggests that the basal ganglia do not determine basic movement parameters (Brooks, 1995, 2000). Our current results showed that ET movement produced only weak linear correlation between movement rate and activation of the basal ganglia. In SI movement, however, we found a strong positive linear relation between movement rate and putaminal activation for the first time. This is consistent with the electrophysiological data (Georgopoulos et al., 1983; Mitchell et al., 1987; Hamada et al., 1990) and clinical observations in PD (Hallett and Khoshbin, 1980) and supports the hypothesis that the basal ganglia play a significant role in determining the rate of SI sequential movement.
Previous neuroimaging studies have been performed using stereotyped finger-to-thumb oppositions (Sadato et al., 1996), finger tapping (Blinkenberg et al., 1996), or joystick movements (Jenkins et al., 1997), ranging in frequency from 0.25–4 Hz. The tasks were externally cued, nonsequential movements. The different tasks between the studies could explain the absence of rate-related activation of the basal ganglia in the previous studies. Another important factor could be the anatomical difference of the activated area in the putamen. In our current results, the location of the rate-related activation in the right putamen corresponds fairly well with the posterior part of putamen related to somatomotor function (Alexander et al., 1990). Previous studies (Sadato et al., 1996; Jenkins et al., 1997), on the contrary, showed task-related activation in the anterior part of putamen, which belongs to the non-motor loop (Alexander et al., 1990).
Because our tasks were rather complicated, we speculated that not only the rate of movement but also the complexity of the task could affect our results. Increasing sequence complexity showed a positive correlation of rCBF in the anterior globus pallidus (GP), contralateral thalamus, pre-SMA, premotor cortex, parietal cortex, ipsilateral SMC, and cerebellum, but not in the contralateral SMC and posterior putamen (Boecker et al., 1998; Catalan et al., 1998; Haslinger et al., 2002). Thus, the activation of the contralateral posterior putamen, shown in our current results, is unlikely to be caused by the complexity of the task. We conclude that a task with sequential and internally cued movement in the nondominant hand is useful to map the movement rate-dependent changes in the basal ganglia.
Basal ganglia–thalamo-motor loop
We investigated interactions among the basal ganglia–thalamo-motor loop during SI finger movements using path analysis. This method has provided new insight into task-specific functional networks (Grafton et al., 1994; McIntosh and Gonzalez-Lima, 1994; McIntosh et al., 1994). In addition, we used correlation data based on rate-dependent movement. This approach provides additional information about brain physiology such as rhythm formation, motor preparation, or motor execution that is not always apparent in the results of categorical comparisons of fMRI data. At least five distinct parallel loops involving the basal ganglia are known to exist (Alexander et al., 1990). These include a “motor” loop involving the SMA, primary motor cortex, putamen, GP, and ventrolateral thalamus. Recent studies in monkeys have shown that the motor loop appears to be divided into projections to (Middleton and Strick, 2000) or subloops in (Nakano et al., 2000) the SMC, SMA, and PM. These subloops arise from the cortical region, project into the internal part of the GP via the putamen, and finally return to the cortex via the thalamus (Nakano et al., 2000). On the basis of this neuroanatomy, we constructed a modified functional model of the reported network (Grafton et al., 1994) except for the GP and subregions within the basal ganglia and thalamus, because the cluster of the right putamen was too large to discriminate.
To our knowledge, we are the first to show a strong interaction in the whole loop during an SI task. There were strong positive interactions in the projection from the SMA to the putamen, from the putamen to the thalamus, and from the thalamus to the SMC. The SMA has been shown to be active during internally generated automated movements, movement preparation, movement sequencing, or performance of complex movements (Roland et al., 1980; Deiber et al., 1991; Halsband et al., 1993;Boecker et al., 1998). The basal ganglia can focus on and filter desired motor patterns during movement, optimizing them and inhibiting unwanted movements (Mink and Thach, 1991; Marsden and Obeso, 1994), and the SMC has been thought to play a primary role in motor execution. Therefore, our results indicate that the SMA–putamen–thalamus–SMC pathway plays a significant role in rate-dependent movement during SI movement. In other words, the basal ganglia–thalamo-motor loop appears to play a primary role in timing SI movement.
SI versus ET movements
Because patients with PD have difficulty especially with SI or volitional movements (Benecke et al., 1987), a potentially powerful method to investigate the motor function of the basal ganglia is to examine the differences between SI and ET movements. Several imaging studies have examined the cerebral activation during index finger extensions or arm movements that involved SI or ET movements in neurologically normal subjects and showed no significant difference (Jahanshahi et al., 1995; Jenkins et al., 2000). A recent event-related fMRI study showed activation of the lentiform nucleus only during SI movement (Cunnington et al., 2002). Although the activation was bilateral, it was seen more on the ipsilateral side, and the study failed to show clear activation of the motor loop. Our network analysis was based on the rate dependence; however, it showed that different systems operate during the two tasks. There were moderate to strong interactions from the SMA to SMC via the putamen and thalamus on SI movement. In ET movement, apparent interaction was found only from the PM to SMC and from the SMC to putamen. Psychophysiological interaction analysis confirmed the different modulation between the tasks in the basal ganglia. These results demonstrate that the basal ganglia–thalamo-motor loop plays a significant role in SI movement but not in ET movement.
Many unit-recording studies in monkeys have been performed to compare SI and ET movements. Simple motor tasks caused movement-related activity in the neurons of SMA, PM, and putamen during both SI and ET tasks. Pre-movement activities were observed mainly before the SI movement in SMA and putamen (Okano and Tanji, 1987; Romo et al., 1992). During the sequential movement, most of the SMA neurons were active in relation to SI during both pre-movement and movement periods, whereas PM neurons were more active in ET (Mushiake et al., 1991). Studies in the motor thalamus suggested that specific subcircuits within the basal ganglia–thalamocortical and cerebella–thalamocortical pathways were clearly differentiated by using SI and ET tasks (van Donkelaar et al., 1999, 2000). Therefore, strong interaction within the basal ganglia−thalamo-motor loop, which was only seen in SI, indicates the internal movement generation process from preparation to execution. In contrast, the interaction from PM to SMC observed in ET suggests motor preparation and execution of the neuronal process through which the reception of stimulus is translated into the execution of a movement.
We may need further study on the effect of right-hand movements because of the limited generality of the use of the left hand. In conclusion, however, out results support the findings of non-primate electrophysiological studies (Georgopoulos et al., 1983; Mitchell et al., 1987; Hamada et al., 1990; Mushiake and Strick, 1995; Middleton and Strick, 2000) by fMRI for the first time. Our data also clearly explain the dissociation between the two tasks in PD (Benecke et al., 1987; Georgiou et al., 1994; Martin et al., 1994). The combined use of sequential finger movement, fMRI, and path analysis made it possible to visualize the functional network of the basal ganglia motor loop and allowed us to accomplish in vivo neuroimaging for movement disorders in which different activation patterns of the basal ganglia–thalamo-motor loop are predicted by the results in vitro (Delong, 1990).
T.T. and A.O. contributed equally to this work.
This research was supported by grants from the Ministry of Education, Science, Sports and Culture, Japan (No. 12670608), the Casio Science Promotion Foundation, the Japanese Neurological Foundation, and the Magnetism and Health Science Promotion Foundation. We thank Dr. Hideaki Kawabata (Department of Psychology, Faculty of Literature, Kagoshima University) and Dr. Naoko Kinukawa (Department of Medical Informatics, Graduate School of Medical Sciences, Kyushu University) for helpful comments on this manuscript.
Correspondence should be addressed to Takayuki Taniwaki, Department of Clinical Neurophysiology, Neurological Institute, Graduate School of Medical Sciences, Kyushu University, 3-1-1, Maidashi, Fukuoka 812-8582, Japan. E-mail:ttaniwa@neuro.med.kyushu-u.ac.jp.
References
- 1.Alexander GE, Crutcher MD, Delong MR. Basal ganglia thalamo-cortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- 2.Benecke R, Rothwell JC, Dick JPR, Day BL, Marsden CD. Disturbance of sequential movements in patients with Parkinson's disease. Brain. 1987;110:361–379. doi: 10.1093/brain/110.2.361. [DOI] [PubMed] [Google Scholar]
- 3.Blinkenberg M, Bonde C, Holm S, Svarer C, Andersen J, Paulson OB, Law I. Rate dependence of regional cerebral activation during performance of a repetitive motor task: a PET study. J Cereb Blood Flow Metab. 1996;16:794–803. doi: 10.1097/00004647-199609000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Boecker H, Dagher A, Ceballos-Baumann AO, Passingham RE, Samuel M, Friston KJ, Poline J-B, Dettmers C, Conrad B, Brooks DJ. Role of the human supplementary motor area and the basal ganglia in motor sequence control: investigations with H215O PET. J Neurophysiol. 1998;79:1070–1080. doi: 10.1152/jn.1998.79.2.1070. [DOI] [PubMed] [Google Scholar]
- 5.Brooks DJ. The role of the basal ganglia in motor control: contributions from PET. J Neurol Sci. 1995;128:1–13. doi: 10.1016/0022-510x(94)00206-4. [DOI] [PubMed] [Google Scholar]
- 6.Brooks DJ. Imaging basal ganglia function. J Anat. 2000;196:543–554. doi: 10.1046/j.1469-7580.2000.19640543.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchel C, Wise RJS, Mummery CJ, Poline J-B, Friston KJ. Nonlinear regression in parametric activation studies. NeuroImage. 1996;4:60–66. doi: 10.1006/nimg.1996.0029. [DOI] [PubMed] [Google Scholar]
- 8.Buchel C, Holmes AP, Rees G, Friston KJ. Characterizing stimulus-response functions using nonlinear regressors in parametric fMRI experiments. NeuroImage. 1998;8:140–148. doi: 10.1006/nimg.1998.0351. [DOI] [PubMed] [Google Scholar]
- 9.Bullmore E, Horwitz B, Honey G, Brammer M, Williams S, Sharma T. How good is good enough in path analysis of fMRI data. NeuroImage. 2000;11:289–301. doi: 10.1006/nimg.2000.0544. [DOI] [PubMed] [Google Scholar]
- 10.Catalan MJ, Honda M, Weeks RA, Cohen LG, Hallett M. The functional neuroanatomy of simple and complex sequential finger movements: a PET study. Brain. 1998;121:253–264. doi: 10.1093/brain/121.2.253. [DOI] [PubMed] [Google Scholar]
- 11.Cunnington R, Windischberger C, Deecke L, Moser E. The preparation and execution of self-initiated and externally-triggered movement: a study of event-related fMRI. NeuroImage. 2002;15:373–385. doi: 10.1006/nimg.2001.0976. [DOI] [PubMed] [Google Scholar]
- 12.Deiber M-P, Passingham RE, Colebatch JG, Friston KJ, Nixon PD, Frackowiak RSJ. Cortical areas and the selection of movement: a study with positron emission tomography. Exp Brain Res. 1991;84:393–402. doi: 10.1007/BF00231461. [DOI] [PubMed] [Google Scholar]
- 13.Delong MR. Primate models of movement disorders of basal ganglia origin. Trends Neurosci. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- 14.Friston KJ, Holmes AP, Worsley KJ, Poline J-P, Frith CD, Frackowiak RSJ. Statistical parametric maps in functional imaging: a general linear approach. Hum Brain Mapp. 1995;2:189–210. [Google Scholar]
- 15.Friston KJ, Buechel C, Fink GR, Morris J, Rolls E, Dolan RJ. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6:218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- 16.Georgiou N, Bradshaw JL, Iansek R, Phillips JG, Mattingley JB, Bradshaw JA. Reduction in external cues and movement sequencing in Parkinson's disease. J Neurol Neurosurg Psychiatry. 1994;57:368–370. doi: 10.1136/jnnp.57.3.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Georgopoulos AP, DeLong MR, Crutcher MD. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–1598. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grafton ST, Sutton J, Couldwell W, Lew M, Waters C. Network analysis of motor system connectivity in Parkinson's disease: modulation of thalamocortical interactions after pallidotomy. Hum Brain Mapp. 1994;2:45–55. [Google Scholar]
- 19.Hallett M, Khoshbin S. A physiological mechanism of bradykinesia. Brain. 1980;103:301–314. doi: 10.1093/brain/103.2.301. [DOI] [PubMed] [Google Scholar]
- 20.Halsband U, Ito N, Tanji J, Freund H-J. The role of premotor cortex and the supplementary motor area in the temporal control of movement in man. Brain. 1993;116:243–266. doi: 10.1093/brain/116.1.243. [DOI] [PubMed] [Google Scholar]
- 21.Hamada I, Delong MR, Mano N. Activity of identified wrist-related pallidal neurons during step and ramp wrist movements in the monkey. J Neurophysiol. 1990;64:1892–1906. doi: 10.1152/jn.1990.64.6.1892. [DOI] [PubMed] [Google Scholar]
- 22.Haslinger B, Erhard P, Weilke F, Ceballos-Baumann AO, Bartenstein P, von Einsiedel HG, Schwaiger M, Conrad B, Boecker H. The role of lateral premotor-cerebellar-parietal circuits in motor sequence control: a parametric fMRI study. Cognit Brain Res. 2002;13:159–168. doi: 10.1016/s0926-6410(01)00104-5. [DOI] [PubMed] [Google Scholar]
- 23.Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. I. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson's disease subjects. Brain. 1995;118:913–933. doi: 10.1093/brain/118.4.913. [DOI] [PubMed] [Google Scholar]
- 24.Jenkins IH, Passingham RE, Brooks DJ. The effect of movement frequency on cerebral activation: a positron emission tomography study. J Neurol Sci. 1997;151:195–205. doi: 10.1016/s0022-510x(97)00145-7. [DOI] [PubMed] [Google Scholar]
- 25.Jenkins IH, Jahanshahi M, Jueptner M, Passingham RE, Brooks DJ. Self-initiated versus externally triggered movements. II. The effect of movement predictability on regional cerebral blood flow. Brain. 2000;123:1216–1228. doi: 10.1093/brain/123.6.1216. [DOI] [PubMed] [Google Scholar]
- 26.Marsden CD, Obeso JA. The functions of the basal ganglia and the paradox of stereotaxic surgery in Parkinson's disease. Brain. 1994;117:877–897. doi: 10.1093/brain/117.4.877. [DOI] [PubMed] [Google Scholar]
- 27.Martin KE, Phillips JG, Iansek R, Bradshaw JL. Inaccuracy and instability of sequential movements in Parkinson's disease. Exp Brain Res. 1994;102:131–140. doi: 10.1007/BF00232445. [DOI] [PubMed] [Google Scholar]
- 28.Mattay VS, Callicott JH, Bertolino A, Santha AKS, van Horn JD, Tallent KA, Frank JA, Weinberger DR. Hemispheric control of motor function: a whole brain echo planar fMRI study. Psychiatry Res. 1998;83:7–22. doi: 10.1016/s0925-4927(98)00023-7. [DOI] [PubMed] [Google Scholar]
- 29.McIntosh AR, Gonzalez-Lima F. Structural equation modeling and its application to network analysis in functional brain imaging. Hum Brain Mapp. 1994;2:2–22. [Google Scholar]
- 30.McIntosh AR, Grady CL, Ungerleider LG, Haxby JV, Rapoport SI, Horwitz B. Network analysis of cortical visual pathways mapped with PET. J Neurosci. 1994;14:655–666. doi: 10.1523/JNEUROSCI.14-02-00655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Menon V, Glover GH, Pfefferbaum A. Differential activation of dorsal basal ganglia during externally and self paced sequences of arm movements. NeuroReport. 1998;9:1567–1573. doi: 10.1097/00001756-199805110-00058. [DOI] [PubMed] [Google Scholar]
- 32.Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Rev. 2000;31:236–250. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- 33.Mink JW, Thach WT. Basal ganglia motor control. III. Pallidal ablation: normal reaction time, muscle cocontraction, and slow movement. J Neurophysiol. 1991;65:330–351. doi: 10.1152/jn.1991.65.2.330. [DOI] [PubMed] [Google Scholar]
- 34.Mitchell SJ, Richardson RT, Baker FH, Delong MR. The primate globus pallidus: neuronal activity related to direction of movement. Exp Brain Res. 1987;68:491–505. doi: 10.1007/BF00249793. [DOI] [PubMed] [Google Scholar]
- 35.Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. J Neurophysiol. 1995;74:2754–2758. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- 36.Mushiake H, Inase M, Tanji J. Neuronal activity in the primate premotor, supplementary, and precentral motor cortex during visually guided and internally determined sequential movements. J Neurophysiol. 1991;66:705–718. doi: 10.1152/jn.1991.66.3.705. [DOI] [PubMed] [Google Scholar]
- 37.Nakano K, Kayahara T, Tsutsumi T, Ushiro H. Neural circuits and functional organization of the striatum. J Neurol. 2000;247[Suppl 5]:V1–V15. doi: 10.1007/pl00007778. [DOI] [PubMed] [Google Scholar]
- 38.Okano K, Tanji J. Neuronal activities in the primate motor fields of the agranular frontal cortex preceding visually triggered and self-paced movement. Exp Brain Res. 1987;66:155–166. doi: 10.1007/BF00236211. [DOI] [PubMed] [Google Scholar]
- 39.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 40.Roland PE, Larsen B, Lassen NA, Skinhoj E. Supplementary motor area and other cortical areas in organization of voluntary movements in man. J Neurophysiol. 1980;43:118–136. doi: 10.1152/jn.1980.43.1.118. [DOI] [PubMed] [Google Scholar]
- 41.Romo R, Schultz W. Neuronal activity preceding self-initiated or externally timed arm movements in area 6 of monkey cortex. Exp Brain Res. 1987;67:656–662. doi: 10.1007/BF00247297. [DOI] [PubMed] [Google Scholar]
- 42.Romo R, Scarnati E, Schultz W. Role of primate basal ganglia and frontal cortex in the internal generation of movements. II. Movement-related activity in the anterior striatum. Exp Brain Res. 1992;91:385–395. doi: 10.1007/BF00227835. [DOI] [PubMed] [Google Scholar]
- 43.Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O. Cortical motor reorganization in akinetic patients with Parkinson's disease: a functional MRI study. Brain. 2000;123:394–403. doi: 10.1093/brain/123.2.394. [DOI] [PubMed] [Google Scholar]
- 44.Sadato N, Ibanez V, Deiber M-P, Campbell G, Leonardo M, Hallett M. Frequency-dependent changes of regional cerebral blood flow during finger movements. J Cereb Blood Flow Metab. 1996;16:23–33. doi: 10.1097/00004647-199601000-00003. [DOI] [PubMed] [Google Scholar]
- 45.van Donkelaar P, Stein JF, Passingham RE, Miall RC. Neuronal activity in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 1999;82:934–945. doi: 10.1152/jn.1999.82.2.934. [DOI] [PubMed] [Google Scholar]
- 46.van Donkelaar P, Stein JF, Passingham RE, Miall RC. Temporary inactivation in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 2000;83:2780–2790. doi: 10.1152/jn.2000.83.5.2780. [DOI] [PubMed] [Google Scholar]