Abstract
α-Synuclein is likely to play a role in neurodegenerative processes, including the degeneration of nigrostriatal dopaminergic neurons that underlies Parkinson's disease. However, the toxicological properties of α-synuclein remain relatively unknown. Here, the relationship between α-synuclein expression and neuronal injury was studied in mice exposed to the herbicide paraquat. Paraquat neurotoxicity was compared in control animals versus mice with transgenic expression of human α-synuclein driven by the tyrosine hydroxylase (TH) promoter. In control mice, paraquat caused both the formation of α-synuclein-containing intraneuronal deposits and the degeneration of nigrostriatal neurons, as demonstrated by silver staining and a reduction of the counts of TH-positive and Nissl-stained cells. Mice overexpressing α-synuclein, either the human wild-type or the Ala53Thr mutant form of the protein, displayed paraquat-induced protein aggregates but were completely protected against neurodegeneration. These resistant animals were also characterized by increased levels of HSP70, a chaperone protein that has been shown to counteract paraquat toxicity in other experimental models and could therefore contribute to neuroprotection in α-synuclein transgenic mice. The results indicate a dissociation between toxicant-induced α-synuclein deposition and neurodegeneration. They support a role of α-synuclein against toxic insults and suggest that its involvement in human neurodegenerative processes may arise not only from a gain of toxic function, as previously proposed, but also from a loss of defensive properties.
Keywords: Parkinson, pesticide, inclusion, substantia nigra, HSP70, chaperone
Introduction
The tendency of α-synuclein to aggregate into nonfibrillar and fibrillar structures (Conway et al., 1998; Giasson et al., 1999) is likely to explain, at least in part, its involvement in the formation of intracellular inclusions typical of diseases such as Parkinson's disease (PD), dementia with Lewy bodies, and multiple system atrophy (Spillantini et al., 1998; Tu et al., 1998). Evidence also suggests that α-synuclein may play a role in the neurodegenerative processes that underlie these diseases (Polymeropoulos et al., 1997; Spira et al., 2001). However, the toxicological properties of α-synuclein remain relatively unknown, and studies in various experimental models have failed to show a consistent relationship between α-synuclein expression and neuronal injury (Masliah et al., 2000; Ostrerova-Golts et al., 2000; Matsuoka et al., 2001; Hashimoto et al., 2002). It has been hypothesized that pathological changes may arise from interactions of α-synuclein with toxic agents, providing a mechanism by which environmental risk factors could contribute to the pathogenesis of PD (Di Monte et al., 2002). In support of this hypothesis, in vitro findings have revealed that incubations of recombinant α-synuclein in the presence of pesticides, such as the herbicide paraquat, result in a dramatic acceleration of protein fibrillation (Uversky et al., 2001;Manning-Boğ et al., 2002). Furthermore, exposure of mice to either paraquat or the parkinsonism-inducing toxicant 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is followed by an upregulation of α-synuclein that appears to be part of a neuronal response to toxic insults (Vila et al., 2000; Manning-Boğ et al., 2002). Finally, α-synuclein-containing aggregates have been observed in two animal models of selective nigrostriatal degeneration, one induced by exposure of rats to rotenone (Betarbet et al., 2000) and the other caused by injections of mice with paraquat (Manning-Boğ et al., 2002; McCormack et al., 2002).
The features of dopaminergic cell injury accompanied by α-synuclein upregulation and deposition, which characterize mice exposed to paraquat, make this experimental model particularly suitable for investigation into the role of α-synuclein in neurotoxic processes. In the present study, we tested the hypothesis that increased α-synuclein levels may affect paraquat neurodegeneration by comparing paraquat-induced dopaminergic cell loss and protein aggregation in mice with and without transgenic α-synuclein overexpression.
Materials and Methods
Animals. Experiments were performed using transgenic mice that overexpressed either human wild-type α-synuclein or a mutant form of the human protein with an Ala53Thr substitution in the amino acid sequence. This mutation is associated with parkinsonism in rare familial cases of the disease (Polymeropoulos et al., 1997). Both lines of transgenic animals have been characterized previously (Matsuoka et al., 2001). They express high levels of α-synuclein within catecholaminergic neurons under the regulatory control of the tyrosine hydroxylase (TH) promoter and do not develop any overt spontaneous pathology. Transgenic animals of 3–4 months of age were injected intraperitoneally with either saline or paraquat at the dose of 10 mg/kg once a week for 3 consecutive weeks. Control littermates were also treated with saline or paraquat. Animals were killed by cervical dislocation at 1 week after the last injection. Experimental protocols were in accordance with the National Institutes of Health guidelines for use of live animals and were approved by the Animal Care and Use Committee at The Parkinson's Institute.
Histochemistry and cell counting. After removal of the brains, midbrain blocks were immersion fixed in 4% paraformaldehyde and cryoprotected in sucrose. Serial coronal sections (40 μm) were cut on a cryostat, collected in cryopreservative, and stored. Midbrain sections containing the substantia nigra at the level of the third nerve were used for silver staining. Sections were rinsed of cryoprotectant solution and then incubated in proprietary reagents per manufacturers instructions (FD Neurotechnologies, Ellicott City, MD), mounted on gelatin-coated slides, dehydrated, and coverslipped. For stereological cell counting, coronal midbrain sections were immunostained with an antibody against TH (1:800; Pel Freez Biologicals, Rogers, AR) and counterstained with 0.5% Cresyl violet. After delineation at low magnification, every sixth section throughout the substantia nigra pars compacta was sampled at high magnification (cast grid system; Olympus, Albertslund, Denmark). Neurons were counted using the optical fractionator method, an unbiased quantitative technique that is independent of neuronal size and shape and any conformational changes in the tissue (West et al., 1991;McCormack et al., 2002). In experiments in which the number of neurons containing α-synuclein aggregates was estimated, sections at the level of the third nerve were incubated with anti-α-synuclein (1:200;Chemicon International, Temecula, CA) and anti-neuron-specific nuclear protein (NeuN) (1:200; Chemicon International), followed by rabbit anti-sheep-FITC (Jackson ImmunoResearch Laboratories, West Grove, PA) and goat anti-mouse-Cy3 (Chemicon International), and mounted in Anti-Fade medium (Molecular Probes, Eugene, OR). Sections were observed using a Nikon light microscope equipped for epifluorescence, and neurons were counted in the substantia nigra pars compacta. Data were expressed as number of NeuN-positive cells containing α-synuclein-immunoreactive aggregates/total number of NeuN-positive cells × 100. For confocal microscopy, tissue sections were blocked in Mouse on Mouse Monoclonal blocking reagent (Vector Laboratories, Burlingame, CA) before overnight incubation at 4°C with anti-α-synuclein (1:200; Transduction Laboratories, Lexington, KY), followed by immersion in goat anti-mouse-FITC (Jackson ImmunoResearch Laboratories). Sections were counterstained with Hoechst bisbenzimide and observed using theZeiss-LSM5 Pascal confocal microscopy system. For dual-labeled immunofluorescence, overnight incubation at 4°C with anti-α-synuclein (1:200; Transduction Laboratories) and anti-HSP70 (SPA-812 1:200; StressGen, Vancouver, Canada) was followed by immersion in goat anti-mouse-FITC and goat anti-rabbit-Cy-3 (both from Jackson ImmunoResearch Laboratories).
Immunoblotting. Brain samples from the ventral mesencephalon and the cerebellum were dissected on ice, sonicated in lysis buffer with protease inhibitors, and after centrifugation, the supernatant fraction was decanted. Protein extracts were separated by SDS gel electrophoresis, and proteins were transferred to nitrocellulose. Blots were blocked and incubated overnight with anti-HSP70 (sc-24 1:500; Santa Cruz Biotechnology, Santa Cruz, CA) or SPA-812 (1:1000; StressGen) or anti-synaptophysin (1:10,000; Dako, Carpinteria, CA). Synaptophysin immunoreactivity was used for assessing equal protein loading.
Statistical analysis. Differences among means were analyzed using one-way ANOVA. Newman–Keuls post hoc analysis was used when differences were observed in ANOVA testing (p < 0.05).
Results
Effect of α-synuclein overexpression on paraquat-induced neurodegeneration
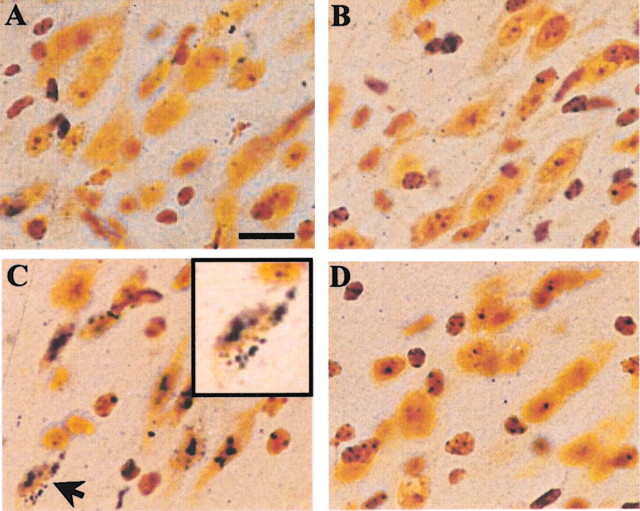
Silver staining was used as a marker for degenerating neurons to compare paraquat neurotoxicity in overexpressing mice versus controls. In control animals, no silver staining was observed after saline injections, whereas scattered injured neurons containing silver grains were present throughout the substantia nigra pars compacta of animals exposed to paraquat (Fig.1A,C). Results in overexpressing mice were substantially different in that midbrain sections showed no silver staining regardless of whether these transgenic animals had been exposed to paraquat or injected with saline (Fig. 1B,D). This lack of pathology provided initial evidence that increased α-synuclein expression conferred resistance to paraquat-induced neurodegeneration.
Fig. 1.
Effect of α-synuclein overexpression on paraquat-induced neurodegeneration. Silver staining of the substantia nigra pars compacta of control (A, C) and α-synuclein-overexpressing (B, D) mice injected with saline (A, B) or paraquat (C, D). Animals were treated once a week for 3 consecutive weeks and killed at 7 d after the last injection of saline or paraquat. The arrow inC indicates silver grain deposition in a degenerating neuron, and the inset represents a higher magnification image of this neuron. Sections shown in this figure were obtained from mice overexpressing human wild-type α-synuclein and corresponding controls. Similar results, however, were observed when saline or paraquat was administered to mice overexpressing human Ala53Thr mutant α-synuclein and littermate controls. Scale bar, 25 μm.
The effect of paraquat on the survival of dopaminergic cells was then evaluated directly by stereological counts of TH-immunoreactive neurons. Consistent with previously reported findings in C57BL/6 mice (McCormack et al., 2002), the number of TH-positive cells was significantly reduced by 25–35% in the substantia nigra pars compacta of control mice at 7 d after the last of three weekly paraquat injections (Table 1). That this loss of TH-immunoreactive cells was caused by actual degeneration of dopaminergic neurons rather than a mere downregulation of the TH marker was demonstrated by counting the number of nigral Nissl-stained neurons and showing that this number was also significantly decreased by paraquat in non-overexpressing mice (Table 1). Quite in contrast, when TH-positive and Nissl-stained neurons were evaluated in the substantia nigra pars compacta of overexpressing mice, no cell loss was found, and nigral counts were similar in animals treated with paraquat as compared with saline (Table 1). It is noteworthy that complete protection against paraquat-induced neurodegeneration, as assessed by silver staining and cell counting, was observed in both lines of transgenic mice used for these studies, i.e., animals overexpressing human wild-type α-synuclein and transgenic mice with human Ala53Thr mutant α-synuclein (Fig. 1, Table 1).
Table 1.
Overexpression of α-synuclein protects against paraquat-induced neurodegeneration
| Wild-type | Ala53Thr mutant | |||
|---|---|---|---|---|
| TH | Nissl | TH | Nissl | |
| Saline | ||||
| Controls | 12,084 ± 143 | 16,468 ± 476 | 12,576 ± 674 | 15,632 ± 668 |
| Overexpressors | 12,068 ± 184 | 16,046 ± 305 | 12,115 ± 138 | 15,982 ± 300 |
| Paraquat | ||||
| Controls | 9,462 ± 296** | 13,522 ± 493* | 8,010 ± 330** | 12,192 ± 772* |
| Overexpressors | 13,012 ± 304 | 17,270 ± 112 | 10,904 ± 274 | 15,966 ± 780 |
The number of TH-immunoreactive and Nissl-stained neurons was counted in the substantia nigra pars compacta of mice injected with either saline or paraquat. Data (means ± SEM; n = 5) were compared in transgenic animals overexpressing human α-synuclein versus non-overexpressing controls. Two lines of transgenic mice were used: one expressed the wild-type protein and the other expressed Ala53Thr mutant α-synuclein. *p < 0.05 and **p < 0.01 versus the corresponding saline-treated group.
Paraquat-induced α-synuclein aggregation
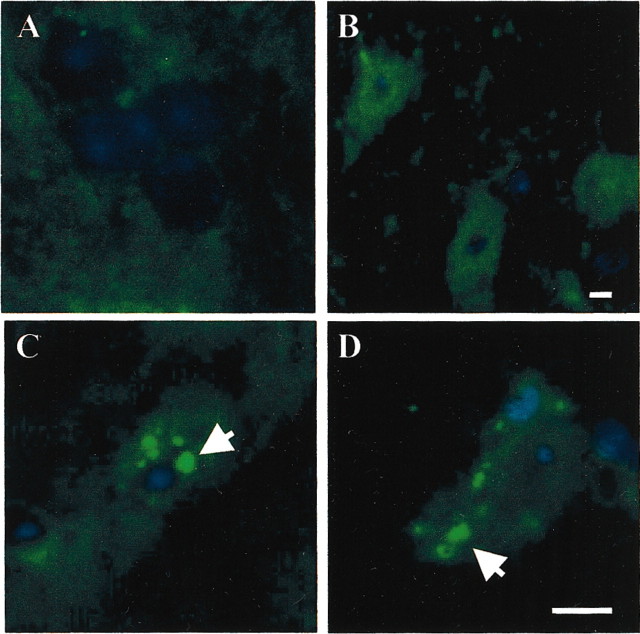
In C57BL/6 mice, paraquat has been reported to cause not only dopaminergic cell degeneration but also the intraneuronal deposition of α-synuclein-containing aggregates (Manning-Boğ et al., 2002). We therefore compared paraquat-induced α-synuclein assembly in control and overexpressing mice and assessed whether protection against neurodegeneration in the latter experimental group was accompanied by significant changes in protein deposition. In midbrain sections from control mice injected with saline, α-synuclein immunoreactivity was diffuse throughout the substantia nigra and stained mostly neuronal fibers (Fig.2A). In saline-treated overexpressing animals, confocal microscopy showed α-synuclein immunoreactivity within nigral cell bodies (Fig. 2B). This effect was most likely a consequence of α-synuclein overexpression driven by the TH promoter because colocalization was found after labeling these cells with antibodies against α-synuclein and TH (data not shown). Paraquat exposure caused the accumulation of intracellular α-synuclein-immunoreactive deposits. These paraquat-induced aggregates were observed in both control and overexpressing mice with no overt differences in their histological characteristics and distribution (Fig. 2C,D). The number of neurons containing aggregates was counted in the substantia nigra pars compacta at the level of the third nerve and found to be 29.5 ± 2.2 (SEM) and 36.3 ± 3.0% of the total neuronal count in paraquat-exposed control and overexpressing mice, respectively.
Fig. 2.
Paraquat-induced α-synuclein deposition in control and α-synuclein-overexpressing mice. Confocal images of neurons in the substantia nigra pars compacta of control (A, C) and α-synuclein-overexpressing (B, D) mice injected with saline (A, B) or paraquat (C, D). Midbrain sections were stained with an antibody against α-synuclein and counterstained with Hoechst bisbenzimide. Arrows inC and D indicate α-synuclein-positive deposits. Sections shown in this figure were obtained from mice overexpressing human Ala53Thr mutant α-synuclein and corresponding controls. Similar results, however, were observed when saline or paraquat was administered to mice overexpressing human wild-type α-synuclein and littermate controls. Scale bars, 10 μm.
α-Synuclein overexpression and HSP70
Chaperone proteins and, in particular, HSP70 have been reported to play a protective role against paraquat toxicity (Ding and Keller, 2001; Minois, 2001). We therefore tested the hypothesis that chaperones may also contribute to the resistance of transgenic mice to paraquat by comparing HSP70 levels in α-synuclein-overexpressing mice versus control animals. In the former, more pronounced HSP70-immunorective bands were observed by Western blot analysis of midbrain homogenates (Fig. 3A). This increase in HSP70 was observed only in catecholaminergic brain regions (e.g., in the ventral mesencephalon, but not in the cerebellum), thus supporting its association with the TH-driven α-synuclein transgenic expression (Fig. 3A,B). Additional evidence of α-synuclein-dependent changes in HSP70 was obtained from histological observations. Midbrain sections from overexpressing and control animals were immunostained with antibodies against HSP70 and α-synuclein. Transgenic mice showed enhanced HSP70 immunoreactivity and colocalization of α-synuclein and HSP70 within neurons of the substantia nigra (Fig.3C–H).
Fig. 3.
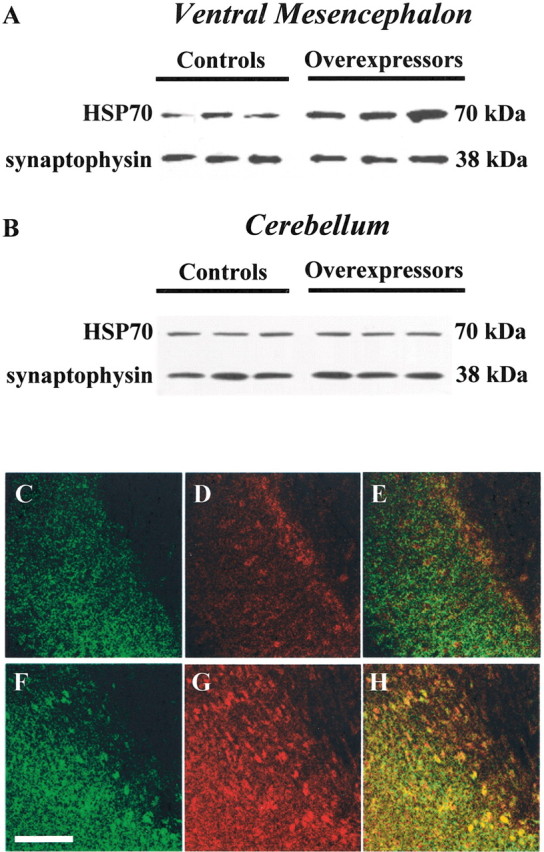
Enhanced HSP70 levels in α-synuclein overexpressing mice. Western blot analysis of HSP70 and synaptophysin immunoreactivities in homogenates from the ventral mesencephalon (A) and cerebellum (B) of control animals and transgenic mice overexpressing human wild-type α-synuclein is shown. C–H, Midbrain sections from control animals (C–E) and mice overexpressing human Ala53Thr mutant α-synuclein (F–H) were immunostained with antibodies against α-synuclein (C, F) and HSP70 (D, G). Merged images (E, H) show colocalization within nigral cell bodies of overexpressing animals (H). Scale bar, 100 μm.
Discussion
Results of this study provide the first evidence in an animal model that increased expression of α-synuclein can prevent toxicant-induced dopaminergic cell degeneration. This neuroprotective effect is apparently at odds with findings in other experimental systems that indicated a relationship between α-synuclein and neuronal injury. For example, transgenic α-synuclein expression has been reported to cause both pathological inclusions and neurodegeneration in Drosophila (Feany and Bender, 2000;Auluck et al., 2002), and consistent with a deleterious role of the protein, lack of α-synuclein protected knock-out mice against the neuronal loss triggered by the toxicant MPTP (Dauer et al., 2002). Earlier evidence, however, also suggests that increased levels of α-synuclein do not necessarily lead to neurotoxicity, because α-synuclein overexpression in transgenic mice does not consistently induce neuronal damage (Masliah et al., 2000; Matsuoka et al., 2001), nor does it exacerbate neurodegeneration caused by MPTP (Rathke-Hartlieb et al., 2001). Finally, findings in vitrohave raised the possibility that α-synuclein may play a protective role. Neuronal cell lines transfected with α-synuclein were resistant to hydrogen peroxide-induced oxidative stress as well as to apoptotic stimuli (da Costa et al., 2000; Hashimoto et al., 2002), and primary mesencephalic cultures isolated from α-synuclein null mice were more susceptible to dopaminergic cell loss caused by rotenone (Dauer et al., 2002). The involvement of α-synuclein in protective mechanisms would also be in line with observations during brain development (Clayton and George, 1998; Hsu et al., 1998) and in models of developmental target injury (Kholodilov et al., 1999a,b), suggesting a relationship between α-synuclein expression and neuronal plasticity and recovery. Taken together, our current results and previous data indicate that the toxic consequences of α-synuclein expression may vary quite significantly under different conditions. Because of this variability, it is conceivable that α-synuclein participates in both toxic and protective pathways and that its involvement in human neurodegenerative processes may arise from a gain of toxic function as well as a loss of defensive properties.
A comparison of paraquat neurotoxicity in mice with transgenic expression of human wild-type versus Ala53Thr mutant α-synuclein revealed no differences, because both lines of overexpressing animals displayed complete absence of dopaminergic cell death. This finding supports the interpretation that, at least in the mouse model, neuroprotection is associated more strictly with increased protein levels and is less dependent on the expression of different forms of α-synuclein. As a possible mechanism of α-synuclein neuroprotection, we also assessed the relationship between dopaminergic cell damage and α-synuclein aggregation. Paraquat exposure induced the formation of α-synuclein-containing deposits. This effect, however, was seen in both control and overexpressing mice, i.e., in the presence and absence of neurodegeneration, thus suggesting that neuroprotection is not a mere consequence of lack of protein deposition.
The precise mechanisms involved in α-synuclein neuroprotection remain unclear. α-Synuclein itself may possess properties that counteract toxic injury, and its expression could affect specific stress-signaling pathways linked to neuronal survival. For example, Hashimoto and colleagues (2002) have recently suggested that α-synuclein expression could confer resistance to in vitro hydrogen peroxide toxicity via the inactivation of c-Jun N-terminal kinase, a member of the mitogen-activated protein kinase family. A consistent feature associated with the TH-driven expression of either human wild-type or mutant α-synuclein in our resistant mice was an increased level of HSP70 within dopaminergic neurons. Because this chaperone plays a role in protein-induced stress responses (Bukau and Horwich, 1998), it is quite possible that its increase may represent an adaptive change to high intraneuronal α-synuclein concentrations. Enhanced expression of HSP70 has been shown to be neuroprotective in a Drosophilamodel of Parkinson-like pathology (Auluck et al., 2002). In this model, HSP70 suppressed neurodegeneration without affecting the formation of α-synuclein-containing inclusions, a finding that bears interesting similarities to our current observation of neuronal survival in the presence of protein deposition. Transgenic flies carrying extra copies of HSP70 have also been found to be relatively resistant to paraquat toxicity (Minois, 2001). Moreover, a role of chaperones against the damaging effects of the herbicide is supported by in vitrostudies in which neuronal cell lines stably transfected with heat-shock proteins were less vulnerable to paraquat-induced impairment of proteasomal activity and decrease in cell viability (Ding and Keller, 2001). Overall, these data indicate that higher levels of HSP70 could conceivably contribute to neuroprotection after paraquat exposure to α-synuclein transgenic mice. Paraquat is known to trigger toxic oxidative reactions (Cohen, 1994), and chaperones like HSP70 could prevent its toxicity by counteracting oxidative damage to proteins and facilitating the degradation of oxidized proteins through the proteasomal system (Grune et al., 1997; Okada et al., 1999; Ding and Keller, 2001).
Footnotes
This work was supported by National Institutes of Health Grants ES10442 and ES10806, the Backus Foundation, the Lookout Fund, and Mylan Pharmaceuticals. We thank Drs. John Hardy, Matthew Farrer, and Karen Duff for their kind donation of α-synuclein transgenic founders, Drs. Serge Przedborski and Michael Lee for helpful discussions, and Su Cumine and Mitra Lavasani for technical assistance.
Correspondence should be addressed to D. A. Di Monte, The Parkinson's Institute, 1170 Morse Avenue, Sunnyvale, CA 94089-1605. E-mail: ddimonte@parkinsonsinstitute.org.
References
- 1.Auluck PK, Chan HY, Trojanowski JQ, Lee VM, Bonini NM. Chaperone suppression of α-synuclein toxicity in a Drosophila model for Parkinson's disease. Science. 2002;295:865–868. doi: 10.1126/science.1067389. [DOI] [PubMed] [Google Scholar]
- 2.Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT. Chronic systemic pesticide exposure reproduces features of Parkinson's disease. Nat Neurosci. 2000;3:1301–1306. doi: 10.1038/81834. [DOI] [PubMed] [Google Scholar]
- 3.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 4.Clayton DF, George JM. The synucleins: a family of proteins involved in synaptic function, plasticity, neurodegeneration and disease. Trends Neurosci. 1998;21:249–254. doi: 10.1016/s0166-2236(97)01213-7. [DOI] [PubMed] [Google Scholar]
- 5.Cohen G. Enzymatic/nonenzymatic sources of oxyradicals and regulation of antioxidant defenses. Ann NY Acad Sci. 1994;738:8–14. doi: 10.1111/j.1749-6632.1994.tb21784.x. [DOI] [PubMed] [Google Scholar]
- 6.Conway KA, Harper JD, Lansbury PT. Accelerated in vitro fibril formation by a mutant α-synuclein linked to early-onset Parkinson disease. Nat Med. 1998;4:1318–1320. doi: 10.1038/3311. [DOI] [PubMed] [Google Scholar]
- 7.da Costa CA, Ancolio K, Checler F. Wild-type but not Parkinson's disease-related ala-53 → Thr mutant α-synuclein protects neuronal cells from apoptotic stimuli. J Biol Chem. 2000;275:24065–24069. doi: 10.1074/jbc.M002413200. [DOI] [PubMed] [Google Scholar]
- 8.Dauer W, Kholodilov N, Vila M, Trillat AC, Goodchild R, Larsen KE, Staal R, Tieu K, Schmitz Y, Yuan CA, Rocha M, Jackson-Lewis V, Hersch S, Sulzer D, Przedborski S, Burke R, Hen R. Resistance of α-synuclein null mice to the parkinsonian neurotoxin MPTP. Proc Natl Acad Sci USA. 2002;99:14524–14529. doi: 10.1073/pnas.172514599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Di Monte DA, Lavasani M, Manning-Boğ AB. Environmental factors in Parkinson's disease. Neurotoxicology. 2002;23:487–502. doi: 10.1016/s0161-813x(02)00099-2. [DOI] [PubMed] [Google Scholar]
- 10.Ding Q, Keller JN. Proteasome inhibition in oxidative stress neurotoxicity: implications for heat shock proteins. J Neurochem. 2001;77:1010–1017. doi: 10.1046/j.1471-4159.2001.00302.x. [DOI] [PubMed] [Google Scholar]
- 11.Feany MB, Bender WW. A Drosophila model of Parkinson's disease. Nature. 2000;404:394–398. doi: 10.1038/35006074. [DOI] [PubMed] [Google Scholar]
- 12.Giasson BI, Uryu K, Trojanowski JQ, Lee VM. Mutant and wild type human α-synucleins assemble into elongated filaments with distinct morphologies in vitro. J Biol Chem. 1999;274:7619–7622. doi: 10.1074/jbc.274.12.7619. [DOI] [PubMed] [Google Scholar]
- 13.Grune T, Reinheckel T, Davies KJ. Degradation of oxidized proteins in mammalian cells. FASEB J. 1997;11:526–534. [PubMed] [Google Scholar]
- 14.Hashimoto M, Hsu LJ, Rockenstein E, Takenouchi T, Mallory M, Masliah E. α-Synuclein protects against oxidative stress via inactivation of the c-Jun N-terminal kinase stress-signaling pathway in neuronal cells. J Biol Chem. 2002;277:11465–11472. doi: 10.1074/jbc.M111428200. [DOI] [PubMed] [Google Scholar]
- 15.Hsu L, Mallory M, Xia Y, Veinbergs I, Hashimoto M, Yoshimoto M, Thal L, Saitoh T, Masliah E. Expression pattern of synucleins (non-Aβ component of Alzheimer's disease amyloid precursor protein/alpha-synuclein) during murine brain development. J Neurochem. 1998;71:338–344. doi: 10.1046/j.1471-4159.1998.71010338.x. [DOI] [PubMed] [Google Scholar]
- 16.Kholodilov NG, Neystat M, Oo TF, Lo SE, Larsen KE, Sulzer D, Burke RE. Increased expression of rat synuclein in the substantia nigra pars compacta identified by mRNA differential display in a model of developmental target injury. J Neurochem. 1999a;73:2586–2599. doi: 10.1046/j.1471-4159.1999.0732586.x. [DOI] [PubMed] [Google Scholar]
- 17.Kholodilov NG, Oo TF, Burke RE. Synuclein expression is decreased in rat substantia nigra following induction of apoptosis by intrastriatal 6-hydroxydopamine. Neurosci Lett. 1999b;275:105–108. doi: 10.1016/s0304-3940(99)00740-5. [DOI] [PubMed] [Google Scholar]
- 18.Manning-Boğ AB, McCormack AL, Li J, Uversky VN, Fink AL, Di Monte DA. The herbicide paraquat causes up-regulation and aggregation of α-synuclein in mice. J Biol Chem. 2002;277:1641–1644. doi: 10.1074/jbc.C100560200. [DOI] [PubMed] [Google Scholar]
- 19.Masliah E, Rockenstein E, Veinbergs I, Mallory M, Hashimoto M, Takeda A, Sagara Y, Sisk A, Mucke L. Dopaminergic loss and inclusion body formation in α-synuclein mice: implications for neurodegenerative disorders. Science. 2000;287:1265–1269. doi: 10.1126/science.287.5456.1265. [DOI] [PubMed] [Google Scholar]
- 20.Matsuoka Y, Vila M, Lincoln S, McCormack A, Picciano M, LaFrancois J, Yu X, Dickson D, Langston JW, McGowan E, Farrer M, Hardy J, Duff K, Przedborski S, Di Monte DA. Lack of nigral pathology in transgenic mice expressing human α-synuclein driven by the tyrosine hydroxylase promoter. Neurobiol Dis. 2001;8:535–539. doi: 10.1006/nbdi.2001.0392. [DOI] [PubMed] [Google Scholar]
- 21.McCormack AL, Thiruchelvam M, Manning-Boğ AB, Thiffault C, Langston JW, Cory-Slechta DA, Di Monte DA. Environmental risk factors and Parkinson's disease: selective degeneration of nigral dopaminergic neurons caused by the herbicide paraquat. Neurobiol Dis. 2002;10:119–127. doi: 10.1006/nbdi.2002.0507. [DOI] [PubMed] [Google Scholar]
- 22.Minois N. Resistance to stress as a function of age in transgenic Drosophila melanogaster overexpressing Hsp70. J Insect Physiol. 2001;47:1007–1012. doi: 10.1016/s0022-1910(01)00076-2. [DOI] [PubMed] [Google Scholar]
- 23.Okada K, Wangpoengtrakul C, Osawa T, Toyokuni S, Tanaka K, Uchida K. 4-Hydroxy-2-nonenal-mediated impairment of intracellular proteolysis during oxidative stress. Identification of proteasomes as target molecules. J Biol Chem. 1999;274:23787–23792. doi: 10.1074/jbc.274.34.23787. [DOI] [PubMed] [Google Scholar]
- 24.Ostrerova-Golts N, Petrucelli L, Hardy J, Lee JM, Farrer M, Wolozin B. The A53T α-synuclein mutation increases iron-dependent aggregation and toxicity. J Neurosci. 2000;20:6048–6054. doi: 10.1523/JNEUROSCI.20-16-06048.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polymeropoulos MH, Lavedan C, Leroy E, Ide SE, Dehejia A, Dutra A, Pike B, Root H, Rubenstein J, Boyer R, Stenroos ES, Chandrasekharappa S, Athanassiadou A, Papapetropoulos T, Johnson WG, Lazzarini AM, Duvoisin RC, Di Iorio G, Golbe LI, Nussbaum RL. Mutation in the α-synuclein gene identified in families with Parkinson's disease. Science. 1997;276:2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 26.Rathke-Hartlieb S, Hahle PJ, Neumann M, Ozmen L, Haid S, Okochi M, Haass C, Schulz JB. Sensitivity to MPTP is not increased in Parkinson's disease-associated mutant α-synuclein transgenic mice. J Neurochem. 2001;77:1181–1184. doi: 10.1046/j.1471-4159.2001.00366.x. [DOI] [PubMed] [Google Scholar]
- 27.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson's disease and dementia with Lewy bodies. Proc Natl Acad Sci USA. 1998;95:6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Spira PJ, Sharpe DM, Halliday G, Cavanagh J, Nicholson GA. Clinical and pathological features of a Parkinsonian syndrome in a family with an Ala53Thr α-synuclein mutation. Ann Neurol. 2001;49:313–319. [PubMed] [Google Scholar]
- 29.Tu PH, Galvin JE, Baba M, Giasson B, Tomita T, Leight S, Nakajo S, Iwatsubo T, Trojanowski JQ, Lee VM. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble α-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- 30.Uversky VN, Li J, Fink AL. Pesticides directly accelerate the rate of α-synuclein fibril formation: a possible factor in Parkinson's disease. FEBS Lett. 2001;500:105–108. doi: 10.1016/s0014-5793(01)02597-2. [DOI] [PubMed] [Google Scholar]
- 31.Vila M, Vukosavic S, Jackson-Lewis V, Neystat M, Jakowec M, Przedborski S. α-Synuclein up-regulation in substantia nigra dopaminergic neurons following administration of the parkinsonian toxin MPTP. J Neurochem. 2000;74:721–729. doi: 10.1046/j.1471-4159.2000.740721.x. [DOI] [PubMed] [Google Scholar]
- 32.West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]