Abstract
Recent studies indicate that nicastrin (NCT) and presenilins form functional components of a multimeric γ-secretase complex required for the regulated intramembraneous proteolysis of Notch and β-amyloid (Aβ) precursor protein (APP). To determine whether nicastrin is required for proteolytic processing of Notch and APP in mammals and the role of nicastrin in presenilin/γ-secretase complex assembly, we generated nicastrin-deficient (NCT−/−) mice and derived fibroblasts from NCT−/− embryos. Nicastrin-null embryos died by embryonic day 10.5 and exhibited several patterning defects, including abnormal somite segmentation, phenotypes that are reminiscent of embryos lacking Notch1 or both presenilins. Importantly, secretion of Aβ peptides is abolished inNCT−/− fibroblasts, whereas it is reduced by ∼50% in NCT+/− cells; the failure to generate Aβ peptides inNCT−/− cells is accompanied by destabilization of the presenilin/γ-secretase complex and accumulation of APP–C-terminal fragments. Moreover, APP trafficking analysis in NCT−/−fibroblasts revealed a significant delay in the rate of APP reinternalization compared with that of control cells. Together, these results establish that nicastrin is an essential component of the multimeric γ-secretase complex in mammals required for both γ-secretase activity and APP trafficking and suggest that nicastrin may be a valuable therapeutic target for Alzheimer's disease.
Keywords: nicastrin knock-out mice, nicastrin-deficient fibroblasts, presenilin/γ-secretase complex, Notch signaling, APP processing and trafficking, Alzheimer's disease
Introduction
Alzheimer's disease (AD), a progressive neurodegenerative disorder of the elderly, is characterized by dementia, the deposition of β-amyloid (Aβ), and the presence of neurofibrillary tangles in the hippocampus and cortex (Price and Sisodia, 1998). Endoproteolytic cleavages of β-amyloid precursor protein (APP) by the activities of β- and γ-secretase result in the generation of Aβ peptides that are believed to be neurotoxic (Hardy and Selkoe, 2002; Wong et al., 2002). The presenilins (PSs), which when mutated cause autosomal dominant AD (Sisodia and George-Hyslop, 2002), are essential for the regulated intramembraneous proteolysis of a variety of transmembrane proteins, including APP (De Strooper et al., 1998; Naruse et al., 1998), Notch (De Strooper et al., 1999; Saxena et al., 2001), ErbB4 (Ni et al., 2001), and E-cadherin (Marambaud et al., 2002). Although it is not completely clear whether PSs themselves act as aspartal proteases (Wolfe et al., 1999; Esler et al., 2000; Li et al., 2000a,b), function as cofactors critical for the activity of γ-secretase, or exert their influence by playing a role in the trafficking of substrates (Naruse et al., 1998;Kim et al., 2001), recent studies support the view that presenilins play a dual role in both the processing by γ-secretase and the trafficking of substrates (Kaether et al., 2002). An emerging view is that PSs form high molecular weight complexes with several other transmembrane proteins critical for the generation of functional γ-secretase complexes.
One important member of the γ-secretase complex is nicastrin (NCT), a type I transmembrane glycoprotein, which forms high molecular weight complexes with presenilins (Yu et al., 2000) and binds to the membrane-tethered form of Notch1 (Chen et al., 2001). Recent studies have indicated that nicastrin is required for Notch signaling and APP processing (Yu et al., 2000; Chung and Struhl, 2001; Edbauer et al., 2002; Hu et al., 2002; Lopez-Schier and St Johnston, 2002). Studies in Drosophila have shown that nicastrin may function to stabilize PSs and appears to be critical for the trafficking of PSs to the cell surface. Likewise, PS is required for the maturation and cell surface accumulation of nicastrin (Edbauer et al., 2002; Kaether et al., 2002; Leem et al., 2002). These results have suggested that nicastrin and PSs form functional components of a multimeric complex required for the intramembraneous proteolysis of both Notch and APP. To determine the physiological role of nicastrin in mammals and to clarify the mechanism whereby nicastrin facilitates the assembly of PSs into a functional γ-secretase complex, we generated and analyzed nicastrin-deficient (NCT−/−) mice andNCT−/− fibroblasts.NCT−/−embryos show phenotypes that resemble those ofNotch1−/− (Swiatek et al., 1994;Conlon et al., 1995; Huppert et al., 2000) orPS−/− (Donoviel et al., 1999; Herreman et al., 1999) embryos. Significantly, secretion of Aβ peptides is abolished in NCT−/− fibroblasts, whereas it is reduced by ∼50% in NCT+/− cells. In addition, cell surface reinternalization of APP is markedly delayed in NCT−/− fibroblasts. Our data support the view that nicastrin is an essential component of the γ-secretase complex that controls PS assembly and APP trafficking in mammals and, as such, represents a potential therapeutic target for the treatment of Alzheimer's disease.
Materials and Methods
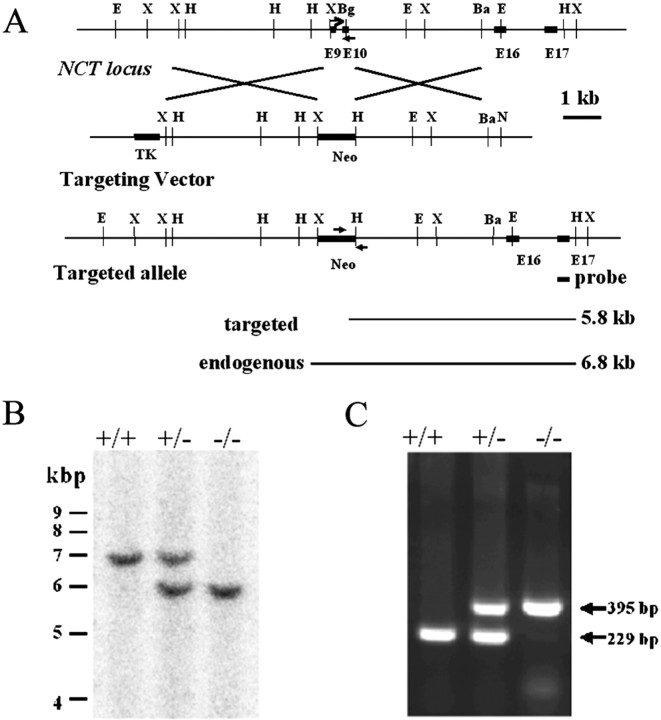
Generation and analysis ofNCT−/−mice. TheNCT gene, isolated from a murine genomic 129/Sv library (Stratagene, La Jolla, CA) using a murine NCTcDNA probe was characterized by DNA blotting and sequencing. In theNCT targeting vector, a 0.5 kb fragment containing exon 9, intron 9, and part of exon 10 of the NCT gene was replaced with a neomycin-resistant (Neo) gene. The linearizedNCT targeting vector was electroporated into 129/suj embryonic stem (ES) cells, and targeted clones were selected by DNA blot analysis. Four independent targeted clones were injected into C57BL/6 blastocysts to generate NCT chimeric mice. Mating of chimeric mice to C57BL/6 mice produced offspring bearing one inactivated NCT allele (NCT+/−mice) and intercrosses of NCT+/− mice generated NCT−/− mice. DNA extracted from tail clips of mice or yolk sacs of embryos were genotyped by PCR using primers for the NCT exon 10 (5′-CTG AGA CAT GGG ATC TGT GTG CAT CC), NCT intron 9 (5′-CGG CTC GAG AAC ATC GAC TCC TTC G), and Neo gene (5′-CT TCC ATT GCT CAG CGG TGC TGT C). Embryos were dissected and analyzed using light microscopy or fixed in 4% paraformaldehyde, embedded in paraffin, sectioned, and stained with hematoxylin and eosin.
Generation of NCT−/−fibroblasts. Fibroblast cultures were established from wild-type, NCT+/−, andNCT−/− embryos at embryonic day 9.5 (E9.5). Briefly, the embryos were minced and suspended in HEPES-buffered DMEM supplemented with 0.25% trypsin and 0.01% DNase I and incubated at 37°C for 20 min. The tissues were then transferred to DMEM supplemented with 10% fetal bovine serum and dissociated by repeated trituration. The dispersed cells were plated in one well of a 24-well plate. Cells were subsequently immortalized with large T antigen.
APP internalization analysis. APP uptake experiments were performed essentially as described previously (Kaether et al., 2002). Briefly, cells plated on slide chambers were transiently transfected with expression vector encoding human APP695. Twenty-four hours after transfection, the cells were washed in ice-cold PCM (PBS supplemented with 1 mm CaCl2 and 0.5 mm MgCl2) and incubated on ice with P2-1 antibody (1:200 dilution). After 20 min incubation, cells were washed twice with PCM on ice, changed to prewarmed culture medium, and finally incubated for various times at 37°C. Slides were then fixed in 4% paraformaldehyde with 4% sucrose in PBS for 20 min and processed for standard immunofluorescence using Alexa 594-coupled secondary anti–mouse antibodies (Molecular Probes, Eugene, OR).
Blue native-PAGE analysis. To prepare the membrane fraction, cells were washed with PBS, resuspended in 20 mmHEPES, pH 7.2, 150 mm NaCl, 2 mm dithioreitol, 2 mm EDTA, and 10% glycerol with a mixture of protease inhibitor (Boehringer Mannheim, Indianapolis, IN), and homogenized with 30 strokes in a glass Dounce homogenizer. Cell debris and nuclei were removed by centrifugation at 800 × g for 10 min. The supernatants were centrifuged at 100,000 × g for 60 min to collect the membrane fractions. Membrane preparations were resuspended in 0.5% N-dodecyl β-d-maltoside (DDM), 500 mm e-amino caproic acid, 20 mm Tris HCl, pH 7.4, 2 mmEDTA, and 10% glycerol. The supernatants were centrifuged at 100,000 × g for 60 min. The membrane protein extracts were subjected to blue native (BN)-PAGE essentially as described previously (Schagger and von Jagow, 1991). Marker proteins were BSA (66 kDa), β-amylase (200 kDa), apoferritin (443 kDa), and thyroglobulin (669 kDa) (Sigma, St. Louis, MO). After electrophoresis, Western blotting was performed, and protein complexes were analyzed by immunoblotting with enhanced chemiluminescence.
Western blotting. Samples were resolved on either 4–20% Tris-glycine PAGE gels [for PS1–N-terminal fragment (NTF), PS1–C-terminal fragment (CTF), and nicastrin] or 10–20% Tris-tricine PAGE gels (APP-CTFs), transferred to polyvinylidene difluoride, and probed with rabbit polyclonal antisera raised against the C-terminal 19 amino acid of NCT (NCT-3925, 1:2000) or previously characterized antisera specific for PS1 (Thinakaran et al., 1996) (PS1-NTF, 1:5000; PS1-loop, 1:2500). Antiserum 7523 against the N terminus of β-secretase (BACE1) was as described previously (Cai et al., 2001). APP and APP-CTFs were detected using antibodies CT-15 (Chemicon, Temecula, CA). Monoclonal antibody against actin was purchased from Chemicon. Antibody against APP N-terminal P2-1 was from BioSource International (Camarillo, CA).
Aβ assays. APP recombinant adenovirus was constructed as described previously (Cai et al., 2001). To assay the APP processing, fibroblasts were infected with 5 × 106 plaque-forming units of adenovirus expressing human APPswe for 24–48 hr. Aβ1–42 and Aβ1–40levels from culture supernatants of cells were measured using a quantitative sandwich ELISA kit (BioSource International) that specifically detects human Aβ.
Mass spectrometric analysis. The β-amyloid peptides in cultured medium were captured with 6E10 monoclonal antibody on preactivated PS20 ProteinChip (Ciphergen Biosystems, Palo Alto, CA). ProteinChip array was analyzed by surface-enhanced laser desorption/ionization time-of-flight mass spectrometry (MS) in the presence of α-cyano-4-hydroxy cinnamic acid (CHCA) matrix solution (Ciphergen Biosystems). External standards were used for calibration.
Results
Targeted inactivation of mouse nicastrin gene
To determine whether nicastrin is required for presenilin-mediated Notch signaling and APP processing in mammals and to examine the mechanism whereby nicastrin facilitates the assembly of the γ-secretase complex, we began by examining the functional consequence of ablating the NCT gene in mice. We used a homologous recombination strategy in ES cells to inactivate the mouseNCT gene. We screened a mouse genomic 129/Sv library with a mouse NCT cDNA as probe. To generate the NCTtargeting vector, a 0.5 kb fragment containing exon 9, intron 9, and part of exon 10 of the NCT gene was replaced with a neomycin-resistant gene (Fig.1A). 129/suj ES cells were transfected with the linearized NCT targeting vector, and 16 clones (of 200 screened) were targeted at the NCTlocus. Of the 16 targeted clones, 4 ES cell clones with a targetednicastrin allele (NCT+/−) were injected into C57BL/6 blastocysts to generate NCT-chimeric mice. Mating of chimeric mice to C57BL/6 mice produced offspring bearing one inactivated NCT allele (NCT+/− mice), and intercrosses ofNCT+/− mice producedNCT−/− mice (Fig.1B,C). Genotypic analysis of postnatal progeny from intercrosses ofNCT+/− mice revealed onlyNCT+/+ andNCT+/− pups, an observation consistent with the concept that deletion of nicastrin may lead to embryonic lethality. To determine the age and stage at which embryos die, we collected embryos from E8 to E14. WhereasNCT+/+ andNCT+/− embryos were identified at these time points, no NCT−/− embryos were recovered beyond E10.5 (Table 1).
Fig. 1.
Targeted disruption of nicastringene by homologous recombination. A, Maps of the wild-type NCT locus, the targeting vector, and the disrupted NCT allele. Exon 9 (E9) and exon 10 are indicated by black boxes. The targeting vector shows the replacement of the exon 9, part of exon 10, and the intron 9 sequences by neomycin gene (Neo). Arrows indicate the sites within the targeted and the wild-type alleles from which PCR primers were chosen for genotyping. Lines below denote expected sizes forHindIII-digested fragments detected by a 3′-flanking probe (a 0.3 kb PCR fragment; black bar) from targeted and endogenousNCT alleles. B, Analysis of genomic DNA from mouse embryos by Southern blot. TheHindIII fragment detected for wild-type (6.8 kb) and targeted (5.8 kb) NCT alleles with the 3′ probe are indicated. C, PCR analysis of DNA extracted from embryos using primers indicated in Figure 1A. The 395 or 229 bp fragment is specific to the targeted or endogenousNCT allele, respectively. E, EcoRI; X,XbaI; BG, BglII; BA, BamHI; N,NotI; TK, thymidine kinase; +/+, wild type; +/−, Nicastrin heterozygous; −/−, Nicastrin knock-out.
Table 1.
Progenies of crosses of NCT+/−mice
| Age | No. of litters | Total no. of pups | NCT+/+ | NCT+/− | NCT−/− |
|---|---|---|---|---|---|
| E8–E8.5 | 10 | 80 | 19 | 43 | 18 |
| E9–E9.5 | 6 | 58 | 15 | 28 | 15 |
| E10–E10.5 | 3 | 19 | 6 | 9 | 4 |
| E11–E14 | 9 | 55 | 19 | 36 | 0 |
| Adult | 9 | 56 | 21 | 35 | 0 |
Notch signaling abnormalities inNCT−/− mice
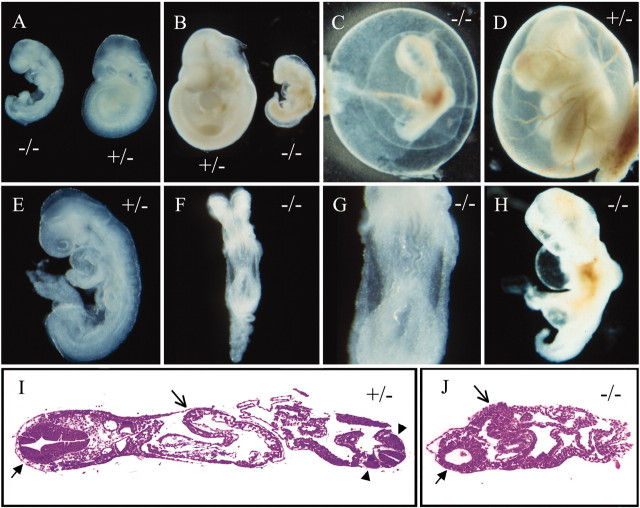
To determine whether the nicastrin-null phenotype resembles that of the PS1+PS2 double knock-out (Donoviel et al., 1999; Herreman et al., 1999) or Notch1-null (Swiatek et al., 1994;Conlon et al., 1995; Huppert et al., 2000) embryos, we undertook a series of morphological studies to characterize the embryonic phenotypes of NCT−/− embryos. The development of NCT−/− embryos was dramatically retarded by E9.5 compared with heterozygous or wild-type littermates (Fig.2A). Notably,NCT−/− embryos exhibited defects in the development of caudal parts of the embryo and in somite segmentation (Fig. 2B); in addition, there were defects in angiogenic vascular morphogenesis in the yolk sac (Fig.2E,F), kinks in the neural tube (Fig. 2F,G), and distention of the pericardial sac (Fig. 2H). Histological analysis revealed patterning defects in the neural tube and heart (Fig.2I,J). Because the defects observed in NCT−/− embryos essentially resemble that of the Notch1−/− orPS−/− embryos, our results establish that NCT is required for Notch signaling and that NCT is necessary for both PS1- and PS2-dependent γ-secretase activities in mammals.
Fig. 2.
Phenotype of nicastrin mutant embryos.NCT−/− embryos at E9 (A) and E9.5 (B) display severe growth retardation and abnormal somite segmentation compared with NCT+/− orNCT+/+ embryos. E8NCT−/− embryos show a twisted neural tube and no somite segmentation (F, G), whereas the E8NCT+/− orNCT+/+ embryos show clear somite segmentation (E). Although the yolk sac vasculature is well formed in the E10NCT+/− embryo (D), vascular morphogenesis is abnormal in theNCT−/− embryo (C). TheNCT−/− embryos also show an underdeveloped heart (H). Transverse sections through E9.5 embryos show disorganization of the trunk and the ventral neural tube and small unlooped hearts in theNCT−/− embryo (J), whereas in theNCT+/− embryo (I), segmented somites and a well developed heart are observed (note that I andJ are shown in different scales). Solid arrow, Neural tube; arrow, heart; arrowhead, somite.
Nicastrin is required for assembly of PS into γ-secretase complex
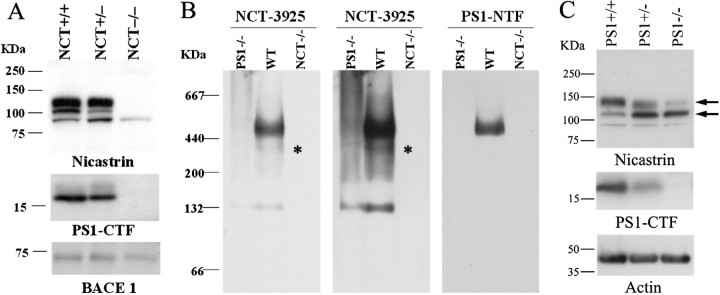
To test the role of nicastrin in presenilin/γ-secretase complex formation and APP processing, we established primary fibroblasts from E9.5 control, NCT+/−, andNCT−/− embryos. Protein-blotting analysis of fibroblast extracts with a highly specific NCT antiserum confirmed that NCT was undetectable inNCT−/− fibroblasts, whereas inNCT+/− fibroblasts, NCT accumulated to ∼50% of the level of theNCT+/+ fibroblast, (Fig.3A). Because initial studies in Drosophila (Hu et al., 2002) indicated that nicastrin might be required for stabilization of PS, we examined the level of PS1 in NCT−/−fibroblasts. Using an antibody that detects the C-terminal fragment of PS1 (Thinakaran et al., 1996), we observed that, whereas there are significant reductions of PS1 CTFs inNCT+/− cells compared with those of controls, PS1 CTFs are undetectable inNCT−/− fibroblasts (Fig. 3A). Moreover, formation of PS1 high molecular weight complexes are abolished in NCT−/− cells (Fig.3B) as judged by blotting of blue native gel (Schagger and von Jagow, 1991) using an antibody against PS1 (Thinakaran et al., 1996). However, it is interesting to note that there appear to be nicastrin high molecular weight complexes devoid of PS1 (Fig.3B), suggesting that nicastrin may initially form an intermediate complex with other members of the γ-secretase complex such as Aph-1 (anterior pharynx defective) or Pen-2 (presenilin enhancer) (Francis et al., 2002; Goutte et al., 2002; Lee et al., 2002; Steiner et al., 2002). Nevertheless, our results establish that nicastrin is required for the assembly of PS into the γ-secretase complex, consistent with the results observed using RNA interference (RNAi) approaches in insect and mammalian cells (Edbauer et al., 2002; Hu et al., 2002; Lopez-Schier and St Johnston, 2002). In addition, we have also confirmed that PS is necessary for the maturation and cell surface trafficking of nicastrin (Fig.3C and data not shown), as demonstrated by several other groups (Edbauer et al., 2002; Leem et al., 2002).
Fig. 3.
Nicastrin is required for assembly of PS1 into the γ-secretase complex. A, Cell lysates fromNCT+/+,NCT+/−, andNCT−/− embryonic fibroblasts were immunoblotted using an antiserum specific for nicastrin and reprobed with antiserum against PS1-CTF or BACE1. Note that nicastrin and PS1 levels are markedly reduced inNCT+/− cells, whereas they are absent in NCT−/− fibroblasts.B, Lysates from membrane fractions of control,PS1−/−, andNCT−/− cells were solubilized with DDM, subjected to BN-PAGE, and analyzed by immunoblotting using antibodies specific to either PS1-NTF or NCT. Note that cells deficient in PS1 or NCT fail to form a high molecular weight complex, and the asterisk denotes an apparent intermediate NCT-containing complex independent of PS1. The middle panel is a darker exposure of the left panel. WT, Wild type. C, Lysates of wild-type (PS1+/+),PS1+/−, andPS1−/− cells were immunoblotted using antisera specific for NCT. The same blot was reprobed with antisera specific for PS1 (PS1-loop) or actin. Note that the ratio of immature to mature NCT in PS1+/− andPS1−/− cells is markedly altered compared with that in control fibroblasts. PS1-CTF, PS1 C-terminal fragment.
APP processing and trafficking defects inNCT−/− fibroblasts
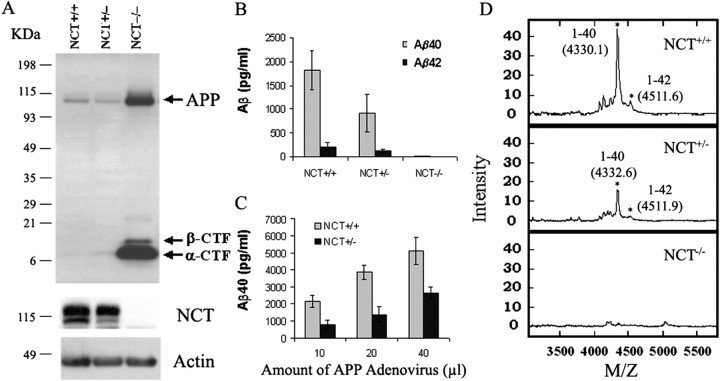
To examine the influences of nicastrin in APP processing and Aβ secretion, we infected control, NCT+/−, and NCT−/− cells with recombinant adenovirus expressing a humanized APP cDNA bearing the Swedish variant (APPswe) (Cai et al., 2001). Protein blot analyses using CT15, an antibody specific for the C terminus of APP, revealed the accumulation of full-length APP and APP-CTFs (Fig.4A) inNCT−/− cells, results that are reminiscent of those obtained in studies of PS1 or PS1+PS2 null cells (De Strooper et al., 1998; Naruse et al., 1998; Herreman et al., 2000;Zhang et al., 2000). Quantitative sandwich ELISA analyses from conditioned media of NCT−/− cultures expressing APPswe showed undetectable levels of Aβ1–40 and Aβ1–42(Fig. 4B); significantly, Aβ1–40 and Aβ1–42peptides were markedly reduced in NCT+/−cells compared with controls (Fig. 4B). Additional analysis of NCT+/− cells infected with different amounts of APP-expressing adenovirus showed an ∼50% reduction of Aβ1–40 compared with that of control fibroblasts (Fig. 4C). Similarly, immunoprecipitation (IP)-MS analysis of conditioned culture media from control fibroblasts using an antiserum (6E10) specific to the N terminus of human Aβ revealed two prominent Aβ species with mass values corresponding to those of human Aβ1–40and Aβ1–42, respectively (Fig.4D, top panel). Importantly, secretion of these Aβ species is abolished from NCT−/−fibroblasts (Fig. 4D, bottom panel), whereas they are significantly reduced in NCT+/− cells (Fig. 4D, middle panel). Together, these data establish that NCT is required for γ-secretase cleavage of APP-CTFs to release the Aβ peptides and suggest that NCT is a potential therapeutic target for anti-amyloidogenic therapies.
Fig. 4.
APP processing and trafficking defects inNCT−/− fibroblasts.A, Cell lysates fromNCT+/+,NCT+/−, andNCT−/− embryonic fibroblasts infected with recombinant adenovirus expressing APPswe were immunoblotted using CT15, an antiserum recognizing the C terminus of APP, and reprobed with antisera against NCT and actin. The cells were harvested 48 hr after infection. B, Conditioned media collected from NCT+/+,NCT+/−, andNCT−/− fibroblast cultures infected with adenovirus expressing APPswe for 48 hr were subjected to Aβ40 and Aβ42 ELISA assays. Bars represent average of eight determinations ± SEM. C, Conditioned media collected from NCT+/+ andNCT+/− fibroblast cultures infected with different doses of adenovirus expressing APPswe for 24 hr were subjected to Aβ40 ELISA assays. Bars represent average of four determinations ± SEM. D, IP-mass spectrometry analysis of secreted Aβ peptides fromNCT+/+,NCT+/−, andNCT−/− fibroblasts expressing APPswe. Asterisks denote peaks corresponding to human Aβ peptides; the mass of each peptide is in parentheses. M/Z, Mass-to-charge ratio.
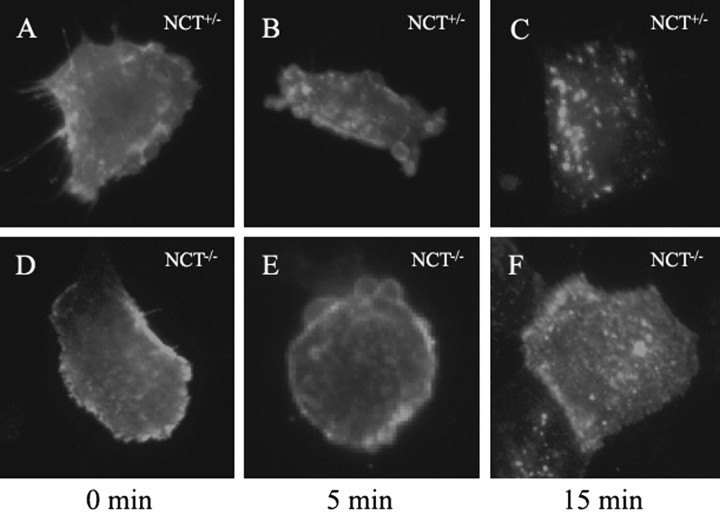
To confirm that the accumulation of APP inNCT−/− cells (Fig. 4A) might be caused by defects in APP reinternalization, we compared the rate of APP reinternalization in control andNCT−/− fibroblasts. Labeling with P2-1, an antibody recognizing the ectodomain of APP, showed that the rate of APP reinternalization was markedly decreased inNCT−/− cells (Fig.5D–F) compared with that of controls (Fig. 5A–C). These results indicate that the increased accumulation of APP inNCT−/− cells reflects defects in PS-dependent APP trafficking, an outcome consistent with the view that the nicastrin/PS complex facilitates the trafficking of APP (Kim et al., 2001; Kaether et al., 2002).
Fig. 5.
APP trafficking defects inNCT−/− fibroblasts. Endocytosis of APP is delayed in NCT−/− fibroblasts (D–F) compared withNCT+/+ cells (A–C). NCT+/+and NCT−/− cells were transiently expressing APPswe, labeled with antiserum P2-1, chased for 5 and 15 min at 37°C, and fixed for immunofluorescence.
Discussion
Although initial studies in Caenorhabditis elegans andDrosophila established that nicastrin is critical for Notch signaling (Yu et al., 2000; Chung and Struhl, 2001; Hu et al., 2002;Lopez-Schier and St Johnston, 2002), it remained unresolved whether the deletion of nicastrin will lead to a complete or partial (Shen et al., 1997; Wong et al., 1997; De Strooper et al., 1998) Notch-null phenotype in mammals. Our results demonstrating that the phenotype of nicastrin knock-out mice (Fig. 2) resembles that of the Notch1-null (Swiatek et al., 1994; Conlon et al., 1995; Huppert et al., 2000) or PS-null (Donoviel et al., 1999; Herreman et al., 1999) embryos now establish that, in mammals, nicastrin is required for both PS1- and PS2-mediated Notch signaling during embryogenesis and that nicastrin is an essential component of the PS/γ-secretase complex. Although studies inDrosophila using reporter constructs indicated that nicastrin is required for the processing of APP-CTFs (Chung and Struhl, 2001), it was not clear whether the secretion of Aβ was totally dependent on nicastrin. In addition, the finding of nicastrin RNAi studies showing an ∼70% reduction of Aβ in mammalian cells (Edbauer et al., 2002) raised the possibility that Aβ secretion may not be completely dependent on nicastrin. However, the present investigation shows that fibroblasts deficient in nicastrin do not secrete Aβ peptides and accumulate high levels of APP-CTFs (Fig. 4), a result observed in studies of PS-null fibroblasts (Herreman et al., 2000; Zhang et al., 2000). This work established that nicastrin is required for the PS/γ-secretase processing of APP and secretion of Aβ peptides. Interestingly, our observations suggest that there is a nicastrin dose-dependent decrease of Aβ secretion fromNCT+/− fibroblasts, a finding supporting the view that nicastrin is one limiting factor critical for the assembly of the PS/γ-secretase complex (Thinakaran et al., 1996). It is encouraging that NCT+/− mice show no overt pathology (up to 1.5 years of age) or obvious clinical phenotype (data not shown) in the face of marked reduction in secretion of Aβ from NCT+/− fibroblasts. These results suggest that nicastrin may be a valuable anti-Aβ drug target, and studies to test whether the decrease in nicastrin ameliorates Aβ deposition in transgenic mouse models of AD should be instructive.
Although recent cell culture studies from several laboratories as well as our present work have established that nicastrin is required for the stability of PS (Edbauer et al., 2002; Francis et al., 2002; Hu et al., 2002), the mechanisms whereby nicastrin facilitates stability or assembly of PS into a functional γ-secretase complex remain poorly understood. Whether nicastrin facilitates PS assembly through a direct interaction between nicastrin and PS remains to be established; however, the recent identification of Aph-1 and Pen-2 as critical components of the γ-secretase complex (Francis et al., 2002; Goutte et al., 2002; Lee et al., 2002; Steiner et al., 2002) raises the possibility that nicastrin may be acting through these or other as-yet-unidentified members. The findings that nicastrin, Aph-1, and Pen-2 are required for PS stability and that these components along with PS are localized to high molecular weight complexes are consistent with the view that these components are assembled into the mature active γ-secretase complex. It is interesting to note that there appear to be intermediate nicastrin-containing complexes that are independent of PS (Fig.3). Whether these intermediate complexes are composed of Aph-1 or Pen-2 remains to be determined. Whether nicastrin, PS, Aph-1, and Pen-2 are sufficient for the formation of functional γ-secretase complexes remains to be established, and future investigations using our nicastrin-null cells should facilitate additional clarification of the mechanism of complex assembly.
Although the functional roles of PS remain incompletely elucidated, recent findings showing that PSs are targeted in a complex with nicastrin to the plasma membrane (Kaether et al., 2002) support dual roles for PSs in both γ-secretase processing (Wolfe et al., 1999) and trafficking of APP (Kim et al., 2001) and nicastrin (Leem et al., 2002). Our demonstration that the reinternalization of cell surface APP is markedly delayed (Fig. 5) and coupled with very substantial increases in APP as well as APP-CTFs in nicastrin-deficient fibroblasts compared with that in control cells (Fig. 4) is consistent with the view that a functional nicastrin/PS complex is necessary not only for γ-secretase activity but also for facilitating the trafficking of APP. The observation that nicastrin binds to both full-length APP and APP-CTFs as well as to PS (Yu et al., 2000; Kimberly et al., 2002; Yang et al., 2002) is also consistent with the notion that the nicastrin/PS complex plays a pivotal role in the trafficking of substrates.
In summary, the present investigation establishes that, in mammals, nicastrin is an essential component of the PS/γ-secretase complex required for processing of APP and Notch as well as for trafficking of APP. The discoveries that NCT+/− mice are viable and without an overt phenotype and that a partial decrease in nicastrin leads to a marked reduction in Aβ secretion suggest that nicastrin may be a valuable therapeutic target for AD. This concept can be evaluated in transgenic mouse models of Aβ amyloidosis.
Footnotes
This work was supported by grants from the National Institutes of Health (NS41438 and NS45150), the Rotary CART Fund, Adler Foundation, and Bristol-Myers Squibb Foundation. We thank Y. Wang, M. Estevez, E. Corpus, J. Peck, F. Davenport, E. Ruch, and G. Cristostomo for technical support.
Correspondence should be addressed to Dr. Philip C. Wong, Department of Pathology, The Johns Hopkins University School of Medicine, 558 Ross Research Building, 720 Rutland Avenue, Baltimore, MD 21205-2196. E-mail: wong@jhmi.edu.
References
- 1.Cai H, Wang Y, McCarthy D, Wen H, Borchelt DR, Price DL, Wong PC. BACE1 is the major β-secretase for generation of Aβ peptides by neurons. Nat Neurosci. 2001;4:233–234. doi: 10.1038/85064. [DOI] [PubMed] [Google Scholar]
- 2.Chen F, Yu G, Arawaka S, Nishimura M, Kawarai T, Yu H, Tandon A, Supala A, Song YQ, Rogaeva E, Milman P, Sato C, Yu C, Janus C, Lee J, Song L, Zhang L, Fraser PE, George-Hyslop PH. Nicastrin binds to membrane-tethered Notch. Nat Cell Biol. 2001;3:751–754. doi: 10.1038/35087069. [DOI] [PubMed] [Google Scholar]
- 3.Chung HM, Struhl G. Nicastrin is required for presenilin-mediated transmembrane cleavage in Drosophila. Nat Cell Biol. 2001;3:1129–1132. doi: 10.1038/ncb1201-1129. [DOI] [PubMed] [Google Scholar]
- 4.Conlon RA, Reaume AG, Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121:1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- 5.De Strooper B, Saftig P, Craessaerts K, Vanderstichele H, Guhde G, Annaert W, Von Figura K, Van Leuven F. Deficiency of presenilin-1 inhibits the normal cleavage of amyloid precursor protein. Nature. 1998;391:387–390. doi: 10.1038/34910. [DOI] [PubMed] [Google Scholar]
- 6.De Strooper B, Annaert W, Cupers P, Saftig P, Craessaerts K, Mumm JS, Schroeter EH, Schrijvers V, Wolfe MS, Ray WJ, Goate A, Kopan R. A presenilin-1-dependent γ-secretase-like protease mediates release of Notch intracellular domain. Nature. 1999;398:518–522. doi: 10.1038/19083. [DOI] [PubMed] [Google Scholar]
- 7.Donoviel DB, Hadjantonakis AK, Ikeda M, Zheng H, Hyslop PS, Bernstein A. Mice lacking both presenilin genes exhibit early embryonic patterning defects. Genes Dev. 1999;13:2801–2810. doi: 10.1101/gad.13.21.2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edbauer D, Winkler E, Haass C, Steiner H. Presenilin and nicastrin regulate each other and determine amyloid β-peptide production via complex formation. Proc Natl Acad Sci USA. 2002;99:8666–8671. doi: 10.1073/pnas.132277899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esler WP, Kimberly WT, Ostaszewski BL, Diehl TS, Moore CL, Tsai JY, Rahmati T, Xia W, Selkoe DJ, Wolfe MS. Transition-state analogue inhibitors of γ-secretase bind directly to presenilin-1. Nat Cell Biol. 2000;2:428–434. doi: 10.1038/35017062. [DOI] [PubMed] [Google Scholar]
- 10.Francis R, McGrath G, Zhang J, Ruddy DA, Sym M, Apfeld J, Nicoll M, Maxwell M, Hai B, Ellis MC, Parks AL, Xu W, Li J, Gurney M, Myers RL, Himes CS, Hiebsch R, Ruble C, Nye JS, Curtis D. aph-1 and pen-2 are required for Notch pathway signaling, γ-secretase cleavage of βAPP, and presenilin protein accumulation. Dev Cell. 2002;3:85–97. doi: 10.1016/s1534-5807(02)00189-2. [DOI] [PubMed] [Google Scholar]
- 11.Goutte C, Tsunozaki M, Hale VA, Priess JR. APH-1 is a multipass membrane protein essential for the Notch signaling pathway in Caenorhabditis elegans embryos. Proc Natl Acad Sci USA. 2002;99:775–779. doi: 10.1073/pnas.022523499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardy J, Selkoe DJ. The amyloid hypothesis of Alzheimer's disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 13.Herreman A, Hartmann D, Annaert W, Saftig P, Craessaerts K, Serneels L, Umans L, Schrijvers V, Checler F, Vanderstichele H, Baekelandt V, Dressel R, Cupers P, Huylebroeck D, Zwijsen A, Van Leuven F, De Strooper B. Presenilin 2 deficiency causes a mild pulmonary phenotype and no changes in amyloid precursor protein processing but enhances the embryonic lethal phenotype of presenilin 1 deficiency. Proc Natl Acad Sci USA. 1999;96:11872–11877. doi: 10.1073/pnas.96.21.11872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Herreman A, Serneels L, Annaert W, Collen D, Schoonjans L, De Strooper B. Total inactivation of γ-secretase activity in presenilin-deficient embryonic stem cells. Nat Cell Biol. 2000;2:461–462. doi: 10.1038/35017105. [DOI] [PubMed] [Google Scholar]
- 15.Hu Y, Ye Y, Fortini ME. Nicastrin is required for γ-secretase cleavage of the Drosophila Notch receptor. Dev Cell. 2002;2:69–78. doi: 10.1016/s1534-5807(01)00105-8. [DOI] [PubMed] [Google Scholar]
- 16.Huppert SS, Le A, Schroeter EH, Mumm JS, Saxena MT, Milner LA, Kopan R. Embryonic lethality in mice homozygous for a processing-deficient allele of Notch1. Nature. 2000;405:966–970. doi: 10.1038/35016111. [DOI] [PubMed] [Google Scholar]
- 17.Kaether C, Lammich S, Edbauer D, Ertl M, Rietdorf J, Capell A, Steiner H, Haass C. Presenilin-1 affects trafficking and processing of βAPP and is targeted in a complex with nicastrin to the plasma membrane. J Cell Biol. 2002;158:551–561. doi: 10.1083/jcb.200201123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim SH, Leem JY, Lah JJ, Slunt HH, Levey AI, Thinakaran G, Sisodia SS. Multiple effects of aspartate mutant presenilin 1 on the processing and trafficking of amyloid precursor protein. J Cell Biol. 2001;277:43343–43350. doi: 10.1074/jbc.M108245200. [DOI] [PubMed] [Google Scholar]
- 19.Kimberly WT, LaVoie MJ, Ostaszewski BL, Ye W, Wolfe MS, Selkoe DJ. Complex N-linked glycosylated nicastrin associates with active γ-secretase and undergoes tight cellular regulation. J Biol Chem. 2002;277:35113–35117. doi: 10.1074/jbc.M204446200. [DOI] [PubMed] [Google Scholar]
- 20.Lee SF, Shah S, Li H, Yu C, Han W, Yu G. Mammalian APH-1 interacts with presenilin and nicastrin and is required for intramembrane proteolysis of amyloid-β precursor protein and Notch. J Biol Chem. 2002;277:45013–45019. doi: 10.1074/jbc.M208164200. [DOI] [PubMed] [Google Scholar]
- 21.Leem JY, Vijayan S, Han P, Cai D, Machura M, Lopes KO, Veselits ML, Xu H, Thinakaran G. Presenilin 1 is required for maturation and cell surface accumulation of nicastrin. J Biol Chem. 2002;277:19236–19240. doi: 10.1074/jbc.C200148200. [DOI] [PubMed] [Google Scholar]
- 22.Li YM, Lai MT, Xu M, Huang Q, DiMuzio-Mower J, Sardana MK, Shi XP, Yin KC, Shafer JA, Gardell SJ. Presenilin 1 is linked with γ-secretase activity in the detergent solubilized state. Proc Natl Acad Sci USA. 2000a;97:6138–6143. doi: 10.1073/pnas.110126897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li YM, Xu M, Lai MT, Huang Q, Castro JL, DiMuzio-Mower J, Harrison T, Lellis C, Nadin A, Neduvelil JG, Register RB, Sardana MK, Shearman MS, Smith AL, Shi XP, Yin KC, Shafer JA, Gardell SJ. Photoactivated γ-secretase inhibitors directed to the active site covalently label presenilin 1. Nature. 2000b;405:689–694. doi: 10.1038/35015085. [DOI] [PubMed] [Google Scholar]
- 24.Lopez-Schier H, St Johnston D. Drosophila nicastrin is essential for the intramembranous cleavage of Notch. Dev Cell. 2002;2:79–89. doi: 10.1016/s1534-5807(01)00109-5. [DOI] [PubMed] [Google Scholar]
- 25.Marambaud P, Shioi J, Serban G, Georgakopoulos A, Sarner S, Nagy V, Baki L, Wen P, Efthimiopoulos S, Shao Z, Wisniewski T, Robakis NK. A presenilin-1/γ-secretase cleavage releases the E-cadherin intracellular domain and regulates disassembly of adherens junctions. EMBO J. 2002;21:1948–1956. doi: 10.1093/emboj/21.8.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Naruse S, Thinakaran G, Luo JJ, Kusiak JW, Tomita T, Iwatsubo T, Qian X, Ginty DD, Price DL, Borchelt DR, Wong PC, Sisodia SS. Effects of PS1 deficiency on membrane protein trafficking in neurons. Neuron. 1998;21:1213–1221. doi: 10.1016/s0896-6273(00)80637-6. [DOI] [PubMed] [Google Scholar]
- 27.Ni CY, Murphy MP, Golde TE, Carpenter G. γ-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179–2181. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 28.Price DL, Sisodia SS. Mutant genes in familial Alzheimer's disease and transgenic models. Annu Rev Neurosci. 1998;21:479–505. doi: 10.1146/annurev.neuro.21.1.479. [DOI] [PubMed] [Google Scholar]
- 29.Saxena MT, Schroeter EH, Mumm JS, Kopan R. Murine Notch homologs (N1-4) undergo presenilin-dependent proteolysis. J Biol Chem. 2001;276:40268–40273. doi: 10.1074/jbc.M107234200. [DOI] [PubMed] [Google Scholar]
- 30.Schagger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 31.Shen J, Bronson RT, Chen DF, Xia W, Selkoe DJ, Tonegawa S. Skeletal and CNS defects in presenilin-1-deficient mice. Cell. 1997;89:629–639. doi: 10.1016/s0092-8674(00)80244-5. [DOI] [PubMed] [Google Scholar]
- 32.Sisodia SS, George-Hyslop PH. γ-Secretase, Notch, Aβ and Alzheimer's disease: where do the presenilins fit in? Nat Rev Neurosci. 2002;3:281–290. doi: 10.1038/nrn785. [DOI] [PubMed] [Google Scholar]
- 33.Steiner H, Winkler E, Edbauer D, Prokop S, Basset G, Yamasaki A, Kostka M, Haass C. PEN-2 is an integral component of the γ-secretase complex required for coordinated expression of presenilin and nicastrin. J Biol Chem. 2002;277:39062–39065. doi: 10.1074/jbc.C200469200. [DOI] [PubMed] [Google Scholar]
- 34.Swiatek PJ, Lindsell CE, del Amo FF, Weinmaster G, Gridley T. Notch1 is essential for postimplantation development in mice. Genes Dev. 1994;8:707–719. doi: 10.1101/gad.8.6.707. [DOI] [PubMed] [Google Scholar]
- 35.Thinakaran G, Borchelt DR, Lee MK, Slunt HH, Spitzer L, Kim G, Ratovitsky T, Davenport F, Nordstedt C, Seeger M, Hardy J, Levey AI, Gandy SE, Jenkins NA, Copeland NG, Price DL, Sisodia SS. Endoproteolysis of presenilin 1 and accumulation of processed derivatives in vivo. Neuron. 1996;17:181–190. doi: 10.1016/s0896-6273(00)80291-3. [DOI] [PubMed] [Google Scholar]
- 36.Wolfe MS, Xia W, Ostaszewski BL, Diehl TS, Kimberly WT, Selkoe DJ. Two transmembrane aspartates in presenilin-1 required for presenilin endoproteolysis and γ-secretase activity. Nature. 1999;398:513–517. doi: 10.1038/19077. [DOI] [PubMed] [Google Scholar]
- 37.Wong PC, Zheng H, Chen H, Becher MW, Sirinathsinghji DJ, Trumbauer ME, Chen HY, Price DL, Van der Ploeg LH, Sisodia SS. Presenilin 1 is required for Notch1 and DII1 expression in the paraxial mesoderm. Nature. 1997;387:288–292. doi: 10.1038/387288a0. [DOI] [PubMed] [Google Scholar]
- 38.Wong PC, Cai H, Borchelt DR, Price DL. Genetically engineered mouse models of neurodegenerative diseases. Nat Neurosci. 2002;5:633–639. doi: 10.1038/nn0702-633. [DOI] [PubMed] [Google Scholar]
- 39.Yang DS, Tandon A, Chen F, Yu G, Yu H, Arawaka S, Hasegawa H, Duthie M, Schmidt SD, Ramabhadran TV, Nixon RA, Mathews PM, Gandy SE, Mount HT, George-Hyslop P, Fraser PE. Mature glycosylation and trafficking of nicastrin modulate its binding to presenilins. J Biol Chem. 2002;277:28135–28142. doi: 10.1074/jbc.M110871200. [DOI] [PubMed] [Google Scholar]
- 40.Yu G, Nishimura M, Arawaka S, Levitan D, Zhang L, Tandon A, Song YQ, Rogaeva E, Chen F, Kawarai T, Supala A, Levesque L, Yu H, Yang DS, Holmes E, Milman P, Liang Y, Zhang DM, Xu DH, Sato C. Nicastrin modulates presenilin-mediated notch/glp-1 signal transduction and βAPP processing. Nature. 2000;407:48–54. doi: 10.1038/35024009. [DOI] [PubMed] [Google Scholar]
- 41.Zhang Z, Nadeau P, Song W, Donoviel D, Yuan M, Bernstein A, Yankner BA. Presenilins are required for γ-secretase cleavage of β-APP and transmembrane cleavage of Notch-1. Nat Cell Biol. 2000;2:463–465. doi: 10.1038/35017108. [DOI] [PubMed] [Google Scholar]