Abstract
Aims
We aimed to provide reference values for speckle-tracking derived systolic and diastolic myocardial deformation markers, and to determine their relation with age, sex, and cardiovascular risk factors.
Methods and results
The Characteristics and Course of Heart Failure STAges A/B and Determinants of Progression (STAAB) cohort study recruited a representative sample of the population of Würzburg, Germany, aged 30–79 years. In a sample of 1818 participants (52% female, mean age 54±12 years) global longitudinal peak systolic strain (GL-PSS, n = 1218), systolic (GL-SSR, n = 1506), and early (GL-EDSR, n = 1506) and late diastolic strain rates (GL-LDSR, n = 1500) were derived from 2D speckle tracking analysis. From a subgroup of 323 individuals without any cardiovascular risk factor, sex- and age-specific reference values were computed. GL-PSS, GL-SSR, and GL-EDSR were associated with sex, GL-EDSR decreased and GL-LDSR increased with age. In the total sample, dyslipidemia was associated with altered GL-PSS, GL-SSR, and GL-EDSR in women but not in men, whereas obesity was associated with less favorable GL-PSS and GL-EDSR in either sex. Hypertension impacted more adversely on systolic and diastolic myocardial deformation in women compared to men (all p<0.01).
Conclusion
The female myocardium appeared more vulnerable to high blood pressure and dyslipidemia when compared to men, while obesity was associated with adverse myocardial deformation in either sex. The reference values for echocardiographic myocardial deformation provided for a non-diseased population and their here reported associations with cardiovascular risk factors will inform future observational and intervention studies regarding i) effect sizes and power calculation, ii) cross-study comparisons, and iii) categorization of myocardial deformation in specific patient groups.
Introduction
Echocardiography is the most frequently used method in the assessment of cardiac function. Conventional measurements like left ventricular (LV) ejection fraction are of limited utility to detect changes over time, hence, more sensitive methods are required. Strain as a measure of myocardial deformation carries incremental information on the change of the LV shape during the cardiac cycle [1]. Strain imaging may detect subtle alterations in cardiac function [2]. Two-dimensional speckle-tracking assesses myocardial motion by tracking speckles in the ultrasonic image. This method determines strain and strain rates avoiding Doppler-associated angulation errors and tethering artifacts with a good correlation to sonomicrometry and tagged magnetic resonance imaging (r = 0.87) [3]. Typically, the impairment in longitudinal deformation precedes deterioration of radial and/or circumferential deformation [4, 5].
The ability to quantify abnormal function relies on the definition of “normal”. Longitudinal systolic strain has consistently been reported more negative in women compared to men [6–13] indicating the necessity to apply sex-specific normal values. In contrast, the association of systolic strain patterns with age are contradictory [6–14], and knowledge on the association of diastolic myocardial deformation with age and sex is scarce. Importantly, there are no reference values available for speckle-tracking derived diastolic strain rates.
The adjustment of LV function to physiologic ageing is heavily influenced by the presence and individual expression of cardiovascular (CV) risk factors [15]. However, knowledge on their age-modifying effect on systolic and/or diastolic myocardial deformation is scarce. These small-scaled studies predominantly investigated selected age groups and isolated risk factors [5, 16–28]
We therefore aimed a) to establish speckle tracking derived sex- and age-specific normal values for systolic and diastolic myocardial deformation from a carefully selected group of individuals in sinus rhythm free of CV risk factors, and b) to determine the impact of age, sex, and classical CV risk factors on myocardial deformation.
Methods
Study population and recruitment
This is a prospectively planned analysis of the Characteristics and Course of Heart Failure Stages A-B and Determinants of Progression (STAAB) Cohort Study, based on consecutive participants from the general population of Würzburg, Germany, enrolled up to December 31, 2015. The detailed study design and methodology has been published [29]. A brief description is given in the supporting information.
Cardiovascular risk factors
Prevalence of diabetes mellitus, CV disease (previous myocardial infarction, coronary artery disease, stroke, peripheral artery disease), and current pharmacotherapy was assessed by physician-led face-to-face interview. Assessment of smoking status, height, weight, and blood pressure, and an oral glucose tolerance test were performed according to standard operating procedures by trained and certified personnel [29]. Fasting lipid profile and glycosylated hemoglobin (HbA1c) were measured at the central laboratory of the University Hospital Würzburg. CV risk factors were defined according to current recommendations as follows: hypertension = blood pressure ≥140/90 mmHg [30] or anti-hypertensive pharmacotherapy; dyslipidemia = low density lipoprotein ≥190 mg/dl [31] or lipid-lowering pharmacotherapy; obesity = body mass index >30 kg/m2 [32]; diabetes mellitus = HbA1c >6.5%, fasting plasma glucose >7.0 mmol/l or 2h-plasma glucose >11.1 mmol/l [33] or anti-diabetic medication; smoking = current or ex-smoker.
All individuals with valid assessment of myocardial deformation entered the analyses regarding the impact of CV risk factors on myocardial deformation. For determination of normal values, we defined a sub-sample of healthy individuals, i.e. subjects in sinus rhythm and free from CV risk factors or CV disease.
Echocardiography
The characteristics and effectiveness of performance measures of the echocardiographic quality assurance program established for the STAAB cohort study have been published [29, 34]. Image acquisition was performed by trained and certified sonographers on one echocardiography machine (Vivid S6®, M4S Sector Array Transducer operating at 1.5–4.3 MHz, GE Healthcare, Horten, Norway) with consistent system presets according to a pre-specified protocol [29, 34]. A minimum of three cardiac cycles was recorded. Standard LV apical views were acquired avoiding LV foreshortening with a frame rate of 50 to 80s-1, thus compatible with speckle tracking analysis. For tissue Doppler imaging (TDI) based reference assessments of myocardial deformation, additionally, small-angled images with high frame rates (80–100 s-1) were collected from the LV septal and lateral walls.
LV myocardial deformation was assessed offline using Q-Analysis (EchoPAC® PC Version 113, GE Healthcare, Buckinghamshire, Great Britain). Timing of aortic valve closure was determined using continuous-wave Doppler across the aortic valve. Systolic as well as early and late diastolic SR at the time of peak S, peak E and peak A, respectively, were measured in each apical view and averaged to generate global longitudinal systolic (GL-SSR) as well as early diastolic (GL-EDSR) and late diastolic SR (GL-LDSR). Global longitudinal peak systolic strain (GL-PSS) was automatically averaged from individually calculated segmental strain values. If more than two out of 18 LV segments were insufficiently tracked, the individual was excluded from GL-PSS analysis. Nevertheless, all LV segments that could be analyzed entered segment-specific analyses. For variability assessment and in accordance with standard operating procedures of the quality control program [29, 34], 10 recordings were interpreted by two observers and by one observer twice, 10–14 days apart, blinded to the previous results. For validation of speckle tracking versus TDI based strain imaging, TDI based GL-EDSR of the LV mid-septum and mid-lateral wall was determined in 25 random subjects (detailed description provided in supporting information).
Data analysis
Statistical analysis was performed using SPSS (Version 23, SPSS Inc., Chicago, USA). Descriptives of quantitative data are provided as mean and standard deviation. The relationship of global strain and SR with age and risk factors was examined by analysis of covariance. Main and interaction effects of CV risk factors on GL-PSS and SR were assessed using a general linear model. Age and sex were defined as main effects for analyses in the healthy sub-sample, and “no CV risk factor” plus individual CV risk factor for analyses in the total sample, respectively. P-values <0.05 were considered statistically significant. Observer variability was assessed using Bland-Altman 95% limits of agreement.
Results
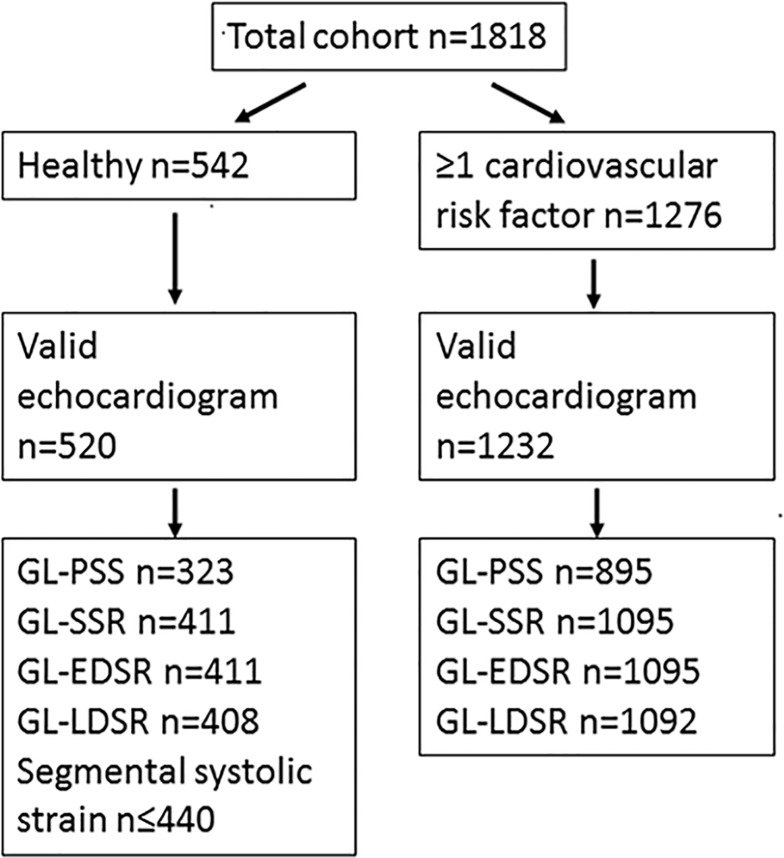
In the frame of the first planned interim analysis, we analyzed 1818 STAAB participants (mean age 54±12 years, 51.5% women). Of those, 542 (30%) participants qualified for the sub-sample of healthy individuals (49±11 years, 58% women) and 1276 exhibited at least one CV risk factor (Table 1, Fig 1).
Table 1. Clinical and echocardiographic characteristics of study participants, and subgroups without and with cardiovascular risk factors.
| Total | Healthy | With cardiovascular risk factor | P | |
|---|---|---|---|---|
| n | 1818 | 542 | 1276 | |
| Age [years], mean (SD) | 54 (12) | 49 (11) | 56 (12) | <0.001 |
| Female sex, n (%) | 937 (52) | 313 (58) | 624 (49) | <0.001 |
| BMI [kg/m2], mean (SD) | 26 (9) | 24 (3) | 28 (11) | <0.001 |
| Systolic BP [mmHg], mean (SD) | 124 (15) | 118 (11) | 127 (16) | <0.001 |
| Diastolic BP [mmHg], mean (SD) | 75 (10) | 72 (9) | 76 10) | <0.001 |
| Heart rate [min-1], mean (SD) | 62 (13) | 62 (9) | 63 (15) | 0.929 |
| Total cholesterol [mg/dl], mean (SD) | 20 (38) | 202 (35) | 208 (39) | 0.001 |
| HDL cholesterol [mg/dl], mean (SD) | 64 (19) | 68 (18) | 62 (19) | <0.001 |
| LDL cholesterol [mg/dl], mean (SD) | 121 (35) | 117 (31) | 123 (36) | <0.001 |
| Triglycerides [mg/dl], mean (SD) | 108 (81) | 84 (43) | 119 (91) | <0.001 |
| HbA1c [%], mean (SD) | 5.5 (0.6) | 5.3 (0.3) | 5.6 (0.6) | <0.001 |
| Echocardiography | ||||
| Frame Rate [s-1] | ||||
| N | 1752 | 520 | 1232 | |
| mean (SD) | 53 (11) | 53 (10) | 53 (11) | 0.387 |
| GL-PSS [%] | ||||
| N | 1218 | 323 | 895 | |
| mean (SD) | -19.1 (2.4) | -19.7 (2.2) | -18.9 (2.5) | <0.001 |
| Systolic SR [s-1] | ||||
| N | 1506 | 411 | 1095 | |
| mean (SD) | -0.95 (0.15) | -0.98 (0.14) | -0.94 (0.15) | <0.001 |
| Early diastolic SR [s-1] | ||||
| N | 1506 | 411 | 1095 | |
| mean (SD) | 1.19 (0.37) | 1.32 (0.36) | 1.13 (0.36) | <0.001 |
| Late diastolic SR [s-1] | ||||
| N | 1500 | 408 | 1092 | |
| mean (SD) | 0.80 (0.21) | 0.82 (0.21) | 0.76 (0.19) | <0.001 |
| LVEDD [mm] | ||||
| N | 1752 | 520 | 1232 | |
| mean (SD) | 48.4 (4.7) | 47.6 (4.5) | 48.8 (4.8) | <0.001 |
| IVSd [mm] | ||||
| N | 1711 | 482 | 1229 | |
| mean (SD) | 8.7 (1.3) | 8.1 (1.1) | 8.9 (1.3) | <0.001 |
| LVPWd [mm] | ||||
| N | 1710 | 482 | 1228 | |
| mean (SD) | 8.2 (1.2) | 7.5 (1.1) | 8.4 (1.2) | <0.001 |
| LA area [cm2] | ||||
| N | 1689 | 499 | 1190 | |
| mean (SD) | 16.8 (3.1) | 15.7 (2.7) | 17.2 (3.1) | <0.001 |
| LVEF [%] | ||||
| N | 1729 | 517 | 1212 | |
| mean (SD) | 60.4 (4.5) | 61.0 (4.1) | 60.1 (4.6) | <0.001 |
| e´ [m/s] | ||||
| N | 1720 | 516 | 1204 | |
| mean (SD) | 0.11 (0.03) | 0.12 (0.03) | 0.10 (0.03) | <0.001 |
| E/e´ | ||||
| N | 1714 | 515 | 1199 | |
| mean (SD) | 7.0 (2.3) | 6.3 (1.7) | 7.3 (2.5) | <0.001 |
Values are given as mean ± standard deviation. P values refer to the comparison of healthy individuals versus individuals with cardiovascular risk factors.
BP = blood pressure, HDL = high density lipoprotein, LDL = low density lipoprotein, HbA1c = hemoglobin A1c, GL-PSS = global longitudinal peak systolic strain, SR = strain rate, LVEDD = left ventricular end-diastolic diameter, IVSd = interventricular septum end-diastolic, LVPWd = left ventricular posterior wall end-diastolic, LA = left atrium, LVEF = left ventricular ejection fraction, e´ = PW-Doppler derived early diastolic myocardial lengthening velocity, E = early mitral inflow velocity
Fig 1. Number of individuals in each sub-sample in whom strain parameters could be derived.
GL-PSS = global longitudinal peak systolic strain, GL-SSR = global longitudinal systolic strain rate, GL-EDSR = global longitudinal early diastolic strain rate, and GL-LDSR = global longitudinal late diastolic strain rate.
Owing to the preselection on risk, participants with CV risk factors featured numerous differences compared to the healthy group: they were older, had higher body mass index, blood pressure, cholesterol values, and also a higher HbA1c (Table 1). Accordingly, most echocardiographic markers matched with this adverse profile. Participants with CV risk factors had lower values for LVEF, GL-PSS, and all types of SR, but higher values for E/e´, LV end-diastolic diameter, septal and posterior wall thickness, and left atrial size. Of note, heart rate and frame rate of echocardiographic image acquisition were similar between groups (Table 1).
Although the distribution of sex was balanced across the five age categories in the total sample (p = 0.41), subjects with CV risk factors were expectedly older than healthy subjects.
In a total of 1752 individuals with valid echocardiograms, feasibility was 70% for GL-PSS and 86% for strain rates, respectively. Age, body mass index, heart rate, and frame rate had no impact on feasibility to derive GL-PSS measurement, but individuals with valid GL-PSS were significantly more often male (624 men vs. 594 women, p = 0.01). The feasibility to derive any modality of SR was significantly associated with younger age, male sex, and lower body mass index (all p<0.05).
For GL-PSS, GL-SSR, GL-EDSR, and GL-LDSR, the 90th percentiles of the absolute difference of two interpretations were 0.8%, 0.05 s-1, 0.08 s-1, and 0.04 s-1 for repeated interpretation by the same observer, and 2.6%, 0.16 s-1, 0.01 s-1, and 0.03 s-1 for the interpretation by two observers, respectively. GL-EDSR derived by speckle tracking and TDI was 1.40±0.68 s-1 and 1.89±0.56 s-1, respectively; the correlation coefficient for both methods was r = 0.70 [95%CI 0.59–0.80] (Figure A in S1 File).
Normal values for myocardial deformation in individuals free from CV risk factors and CV disease
Systolic strain
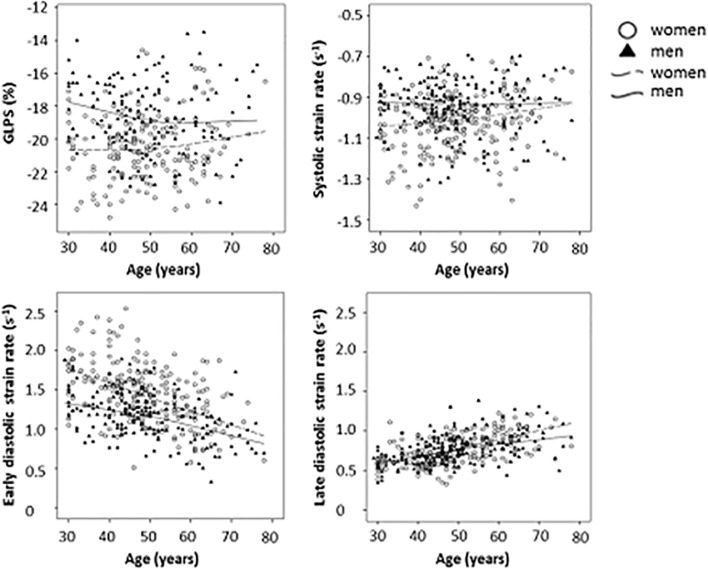
GL-PSS values could be assessed in 323 healthy individuals and were normally distributed (Figure B in S1 File). In a linear model, there was a non-significant change of GL-PSS of -0.23% per age decade in men (p = 0.131) and of +0.29% per age decade in women (p = 0.054); however, the slopes for both sexes differed significantly (p = 0.015; Fig 2). Overall, regardless of age, GL-PSS was by 1.74% more negative in women compared to men (p<0.001). Sex-specific normal values per age decades are given in Tables 2 and 3.
Fig 2.
Combined averaged a) global longitudinal peak systolic strain (GL-PSS, n = 323), b) systolic strain rate (GL-SSR, n = 410), c) early diastolic strain rate (GL-EDSR, n = 410), and d) late diastolic strain rate (GL-LDSR, n = 407) according to age and sex in individuals in sinus rhythm free from cardiovascular risk factors and cardiovascular disease (mean age 49±11 years, 55% females).
Table 2. Speckle tracking derived markers of left ventricular myocardial deformation in male participants by age group.
| Age group (years) | 30–39 | 40–49 | 50–59 | 60–79 | P |
|---|---|---|---|---|---|
| GL-PSS [%] | |||||
| N | 25 | 53 | 38 | 30 | |
| mean (SD) | -17.8 (1.7) | -18.9 (2.0) | -19.0 (2.3) | -18.8 (2.5) | 0.160 |
| 2SD range | -21.3; -14.4 | -22.9; -14.9 | -23.6; -14.4 | -23.8; -13.8 | |
| Systolic strain rate [s-1] | |||||
| N | 34 | 70 | 39 | 40 | |
| mean (SD) | -0.93 (0.10) | -0.95 (0.13) | -0.95 (0.18) | -0.93 (0.14) | 0.691 |
| 2SD range | -1.13; -0.73 | -1.21; -0.69 | -1.31; -0.59 | -1.21; -0.65 | |
| Early diastolic strain rate [s-1] | |||||
| N | 34 | 70 | 39 | 40 | |
| mean (SD) | 1.30 (0.26) | 1.23 (0.24) | 1.16 (0.28) | 0.95 (0.27) | <0.001 |
| 2SD range | 0.78; 1.82 | 0.75; 1.71 | 0.60; 1.72 | 0.41; 1.49 | |
| Late diastolic strain rate [s-1] | |||||
| N | 34 | 69 | 39 | 40 | |
| mean (SD) | 0.62 (0.13) | 0.75 (0.18) | 0.82 (0.17) | 0.87 (0.21) | <0.001* |
| 2SD range | 0.36; 0.88 | 0.39; 1.11 | 0.48; 1.16 | 0.45; 1.29 |
Values are given as mean ± standard deviation. P values refer to the comparison of age groups using ANCOVA or *Welch´s test, depending on equality of variance in Levene´s test. To increase statistical power, the two top decades were combined.
SD = standard deviation, CI = confidence interval, GL-PSS = global longitudinal peak systolic strain
Table 3. Speckle tracking derived markers of left ventricular myocardial deformation in female participants by age group.
| Age group (years) | 30–39 | 40–49 | 50–59 | 60–79 | P |
|---|---|---|---|---|---|
| GL-PSS [%] | |||||
| N | 28 | 73 | 42 | 34 | |
| mean (SD) | -20.7 (2.2) | -20.5 (1.8) | -20.7 (1.6) | -19.6 (2.4) | 0.120* |
| 2SD range | -25.1; -16.3 | -24.1; -16.9 | -24.1; -17.3 | -24.4; -14.8 | |
| Systolic strain rate [s-1] | |||||
| N | 38 | 97 | 53 | 40 | |
| mean (SD) | -1.06 (0.15) | -1.02 (0.13) | -1.02 (0.10) | -0.98 (0.15) | 0.174* |
| 2SD range | -1.36; -0.76 | -1.28; -0.76 | -1.22; -0.82 | -1.28; -0.68 | |
| Early diastolic strain rate [s-1] | |||||
| N | 38 | 97 | 53 | 40 | |
| mean (SD) | 1.69 (0.35) | 1.52 (0.35) | 1.35 (0.27) | 1.15 (0.36) | <0.001 |
| 2SD range | 0.99; 2.39 | 0.82; 2.22 | 0.81; 1.89 | 0.43; 1.87 | |
| Late diastolic strain rate [s-1] | |||||
| N | 37 | 97 | 52 | 40 | |
| mean (SD) | 0.65 (0.15) | 0.69 (0.15) | 0.82 (0.15) | 0.94 (0.16) | <0.001 |
| 2SD range | 0.35; 0.95 | 0.39; 0.99 | 0.52; 1.12 | 0.62; 1.26 |
Values are given as mean ± standard deviation. P values refer to the comparison of age groups using ANCOVA or *Welch´s test, depending on equality of variance in Levene´s test. To increase statistical power, the two top decades were combined. SD = standard deviation, CI = confidence interval, GL-PSS = global longitudinal peak systolic strain
We provide sex-specific percentiles for GL-PSS (Figures C and D in S1 File) as well as age- and sex specific systolic strain values for each left ventricular segment (Tables A and B in S1 File). In women, basal septal, mid septal, basal inferior, as well as all anteroseptal segments showed a significantly less negative strain with increasing age, whereas in men systolic strain remained unchanged in all segments.
Strain rate
Sex-specific normal values per age decades are given in Tables 2 and 3. GL-SSR changed by -0.003 s-1 per age decade in men (p = 0.741), and +0.023 s-1 per decade in women (p = 0.007), with a significant difference between slopes (p = 0.032). Regardless of age, women had 0.072 s-1 more negative values compared to men (p<0.001; Fig 2).
GL-EDSR changed by -0.106 s-1 per age decade in men (p<0.001), and -0.175 s-1 per age decade in women (p<0.001), with a significant difference between slopes (p = 0.011). Regardless of age, women had 0.275/s-1 more positive values compared to men (p<0.001).
GL-LDSR changed by +0.074 s-1 per age decade in men (p<0.001), and +0.100 s-1 per age decade in women (p<0.001,) without a significant difference between slopes (p = 0.080). Regardless of age, women had 0.006 s-1 less positive values compared to men (p = 0.747).
Sex- and age-specific percentiles of GL-SSR, GL-EDSR, and GL-LDSR are detailed in Figures E-J in S1 File.
Impact of CV risk factors on myocardial deformation
1276 individuals exhibited at least one CV risk factor (56±12 years, 49% women). The number of individuals decreased with increasing number of prevalent CV risk factors and was evenly distributed over the decades (Figures K and L in S1 File).
In the total sample, GL-PSS was adversely affected by obesity in either sex (p<0.001), whereas an adverse effect of hypertension and dyslipidemia on GL-PSS was selectively observed in women (Table 4, Table C in S1 File). An adverse effect of dyslipidemia, hypertension, and obesity on GL-SSR was consistently observed in women only (Table 4, Table D in S1 File). GL-EDSR was negatively affected by hypertension and dyslipidemia, with a significantly more adverse effect in women, and by obesity in either sex (Table 4, Table E in S1 File). GL-LDSR was significantly increased in individuals with hypertension in either sex, with a significantly more adverse effect in women (Table 3, Table F in S1 File). Diabetes mellitus and smoking had no significant adverse effect on myocardial deformation (Tables C-F in S1 File)
Table 4. Impact of cardiovascular risk factors on myocardial deformation in the total cohort and according to sex.
| CV risk factor | Impact of CV risk factor | P | Effect size women vs. men | P for effect in women | P for effect in men | P for interaction | |
|---|---|---|---|---|---|---|---|
| GL-PSS | Obesity | +0.7% | <0.001 | +0.9% vs. +0.7% | <0.01 | <0.01 | 0.69 |
| Hypertension | +0.3% | ns | +0.7% vs. -0.1% | <0.01 | ns | 0.004 | |
| Dyslipidemia | +0.3% | <0.001 | +1.2% vs. +0.3% | <0.001 | ns | 0.03 | |
| GL-SSR | Obesity | +0.04 s-1 | <0.001 | +0.06 s-1 vs. +0.01 s-1 | <0.001 | ns | 0.047 |
| Hypertension | +0.03 s-1 | <0.01 | +0.06 s-1 vs. +0.02 s-1 | <0.001 | ns | 0.02 | |
| Dyslipidemia | +0.03 s-1 | <0.05 | +0.05 s-1 vs. +0.01 s-1 | <0.01 | ns | 0.07 | |
| GL-EDSR | Obesity | -0.12 s-1 | <0.001 | -0.14 s-1 vs. -0.12 s-1 | <0.001 | <0.001 | 0.72 |
| Hypertension | -0.72 s-1 | <0.001 | -0.24 s-1 vs. -0.10 s-1 | <0.001 | <0.001 | <0.001 | |
| Dyslipidemia | -0.12 s-1 | <0.001 | -0.19 s-1 vs. -0.2 s-1 | <0.001 | ns | 0.001 | |
| GL-LDSR | Hypertension | +0.09 s-1 | <0.001 | +0.11 s-1 vs. +0.06 s-1 | <0.001 | <0.001 | 0.02 |
The impact of a CV risk factor on a specific strain marker is expressed as absolute change for the total sample and per sex group. Interaction effects computed from general linear models (see Methods).
CV = cardiovascular, vs. = versus, GL-PSS = global longitudinal peak systolic strain (n = 1218), GL-SSR = global longitudinal systolic strain rate (n = 1506), GL-EDSR = global longitudinal early diastolic strain rate (n = 1506), GL-LDSR = global longitudinal late diastolic strain rate (n = 1500), ns = not significant; Hypertension = blood pressure ≥140/90 mmHg or antihypertensive pharmacotherapy, dyslipidemia = low density lipoprotein ≥190 mg/dl or lipid-lowering pharmacotherapy, obesity = body mass index >30 kg/m2.
Discussion
From a well-characterized, population-based cohort balanced for age and sex, we defined a sub-sample of healthy individuals (in sinus rhythm and free of CV risk factors and CV disease) and established reference values for global and segmental peak systolic strain and systolic SR of the LV. To the best of our knowledge, the current report is first to provide speckle-tracking derived reference values for early and late diastolic SR. Systolic and early, but not late, diastolic myocardial deformation showed a strong association with sex. Additionally, in contrast to systolic deformation parameters, diastolic SR markers were strongly affected by age: GL-EDSR decreased, while GL-LDSR increased with age.
In the total sample, CV risk factors differentially affected the various aspects of myocardial deformation. Further, sex-specific effects of CV risk factors on myocardial deformation were observed. This is compatible with the hypothesis that the myocardial sensitivity to individual risk factors is determined by sex.
Quality assurance
Assessment of acquisition variability and interpretation variability confirmed sound agreement between observers. Applying high quality standards to image and tracking quality, feasibility of GL-PSS in the total sample was 70%, which is comparable to other larger studies [11, 12]. Compared to TDI, speckle tracking derived GL-EDSR, which due to the highest velocity is most prone to undersampling by lower frame rates, indeed yielded a systematic deviation exhibiting slightly lower values (factor 0.7). Nevertheless, the good correlation between both methods and comparable standard deviation (1.40±0.68 s-1 versus 1.89±0.56 s-1) justify the clinical application of speckle tracking derived GL-EDSR. These findings emphasize the need for population-based normal values specifically derived from speckle tracking based strain imaging.
Systolic myocardial deformation in healthy individuals
In healthy individuals, GL-PSS and SR were found more negative in women compared to men [11], with disparate results regarding their association with age [11]. As age advances, the relationship of cardiac structure and function with age is confounded by the accumulation of traditional risk factors [15]. Most echocardiographic studies describing an association of GL-PSS with age did not systematically exclude individuals with CV risk factors or overt CV disease [11, 35, 36], which is the likely reason for these incongruent results.
We performed a detailed, physician-based assessment and thus established a well selected sub-sample of “truly healthy” individuals, i.e. in sinus rhythm and free from CV risk factors and CV disease. We here confirmed a more negative GL-PSS in women compared to men. Further, we found no significant change of systolic myocardial deformation with age in men, but significantly less positive GL-SSR with advancing age and a trend towards less negative GL-PSS accompanied by a significant impairment of segmental GL-PSS in septal and anteroseptal segments in women. This is in line with results from the EACVI NORRE study, where the pattern of worse systolic longitudinal LV function with advancing age in women was associated with more negative values of circumferential strain [37]. More detailed assessment of the underlying pathomechanisms including hormonal analyses and the evaluation of other than the conventional cardiovascular risk factors will be subjected to further research.
Diastolic myocardial deformation in healthy individuals
GL-EDSR is considered a comprehensive measure of early active LV relaxation. Importantly, diastolic SR yielded higher accuracy regarding the estimation of LV filling pressures compared to indices including the broadly used but angle dependent and mono-dimensional TDI measurement e´ [38, 39]. Further, GL-LDSR is considered a measure of late diastolic LV filling induced by active atrial contraction. Our analyses extend previous knowledge, as we found that GL-EDSR was significantly higher in women compared to men, whereas no sex-related difference was apparent regarding GL-LDSR. Further, GL-EDSR significantly decreased, whereas GL-LDSR significantly increased with age. This implies an increase of active atrial contribution to LV filling with advancing age, thus possibly compensating for the described decrease in active LV relaxation.
Impact of CV risk factors on systolic and diastolic myocardial deformation
A recent report from the MESA study employing cardiac magnet resonance tomography reported that CV risk status–besides sex and ethnicity–were major drivers of the progression of LV measures [15]. Using echocardiography, hypertensive heart disease with normal ejection fraction has been associated with reduced myocardial velocities and reduced regional function [40], and diabetes mellitus with worse LV remodeling and function [26, 28]. Results regarding the impact of obesity on myocardial function are inconsistent, reporting negative, positive or neutral associations of LV diastolic function patterns with the degree of obesity [5, 20, 22, 24, 25, 27, 41]. Two larger studies reported neutral findings [24, 25] and emphasized the importance of factors defining the metabolic syndrome rather than obesity itself. According to one report comparing 40 otherwise healthy smokers with age-matched controls, smoking intensity gradually impaired systolic and diastolic myocardial deformation patterns of both the left and the right ventricle [23]. Hence, evidence of the negative impact of CV risk factors on myocardial deformation is inconsistent, mainly due to heterogeneous study quality and relatively small samples looking at restricted age ranges and risk profiles.
To our knowledge, the current study is first to assess the individual impact of each of the established CV risk factors on systolic and diastolic myocardial deformation in a well-controlled representative cohort. Our results suggest a sex-specific sensitivity of the myocardium to individual CV risk factors. The vulnerability of the female myocardium to high blood pressure with subsequent alteration of the active early diastolic myocardial relaxation, for example, might be an explanation for the preponderance of females in HF with preserved ejection fraction. We did not observe any direct negative impact of smoking and diabetes mellitus on myocardial deformation at rest. These risk factors might act as dormant harmful factors affecting the vasculature, i.e. not affecting the myocardial function at rest until an ischemic damage has occurred. Further, all individuals with diabetes mellitus also exhibited at least one additional CV risk factor, which might have had a stronger impact on longitudinal LV function than diabetes mellitus.
Strengths and limitations
Strain imaging depends on optimal image quality. Hence, feasibility is lower compared to routine echocardiography. Nevertheless, applying high quality standards, we achieved a feasibility comparable to other large cohort studies [11, 36]. The current analysis omitted radial and circumferential deformation as we focused on longitudinal myocardial deformation, which is affected first along the pathophysiological cascade [4]. CV risk factors were assessed very carefully. Nevertheless, more detailed analyses including pharmacotherapy and quality of cardiovascular risk factor control were not performed due to the sample size.
Clinical impact and conclusion
Healthy aging seems to be associated with a selective decrease in systolic function in women. By contrast, active LV relaxation decreases with advancing age in either sex, necessitating the left atrium to contribute increasingly more to left ventricular filling. Further, the female myocardium appears more vulnerable to high blood pressure and dyslipidemia when compared to men, while obesity might reduce myocardial deformation to a similar extent in either sex.
The here presented sex- and age-specific speckle-tracking derived reference values for systolic and, importantly, also for diastolic myocardial deformation, will help to classify myocardial deformation in patients more reliably. Reference values of strain and strain rates and their here reported association with CV risk factors will inform future observational and intervention studies regarding i) effect sizes and power calculation, ii) cross-study comparisons, and iii) categorization of myocardial deformation in specific patient groups.
Supporting information
Supplementary tables: Table A. Left ventricular global and segmental peak systolic longitudinal strain in males. Table B. Left ventricular global and segmental peak systolic longitudinal strain in females. Table C. Impact of cardiovascular risk factors on global longitudinal peak systolic strain in the total cohort and according to sex. Table D. Impact of cardiovascular risk factors on systolic strain rate in the total cohort and according to sex. Table E. Impact of cardiovascular risk factors on early diastolic strain rate in the total cohort and according to sex. Table F. Impact of cardiovascular risk factors on late diastolic strain rate in the total cohort and according to sex. Supplementary figures: Figure A. Correlation of speckle tracking and tissue Doppler imaging derived global early diastolic strain rate. Figure B. Distribution of GL-PSS values in individuals without CVRF. Figure C. Percentiles of global longitudinal peak systolic strain in men. Figure D. Percentiles of global longitudinal peak systolic strain in women. Figure E. Percentiles of systolic strain rate in men. Figure F. Percentiles of systolic strain rate in women. Figure G. Percentiles of early diastolic strain rate in men. Figure H. Percentiles of early diastolic strain rate in women. Figure I. Percentiles of late diastolic strain rate in men. Figure J. Percentiles of late diastolic strain rate in women. Figure K. Number of male individuals with 0–5 cardiovascular risk factors by age. Figure L. Number of female individuals with 0–5 cardiovascular risk factors by age.
(DOCX)
Acknowledgments
The STAAB Consortium: S. Frantz (Dept. of Medicine I, Div. of Cardiology, University Hospital Würzburg); C. Maack (Comprehensive Heart Failure Center, University Hospital and University of Würzburg); G. Ertl (University Hospital Würzburg); M. Fassnacht (Dept. of Medicine I, Div. of Endocrinology, University Hospital Würzburg); C. Wanner (Dept. of Medicine I, Div. of Nephrology, University Hospital Würzburg); R. Leyh (Dept. of Cardiovascular Surgery, University Hospital Würzburg); J. Volkmann (Dept. of Neurology, University Hospital Würzburg); J. Deckert (Dept. of Psychiatry, Psychosomatics and Psychotherapy, Center of Mental Health, University Hospital Würzburg); H. Faller (Dept. of Medical Psychology, University of Würzburg); R. Jahns (Interdisciplinary Bank of Biomaterials and Data Würzburg, University Hospital Würzburg).
Abbreviations
- CV
Cardiovascular
- GL-EDSR
Global longitudinal early diastolic strain rate
- GL-LDSR
Global longitudinal late diastolic strain rate
- GL-PSS
Global longitudinal peak systolic strain
- GL-SSR
Global longitudinal systolic strain rate
- LV
Left ventricle/ventricular
- STAAB
Characteristics and Course of Heart Failure STAges A/B and Determinants of Progression cohort study
- SR
Strain rate
- TDI
Tissue Doppler Imaging
Data Availability
The data underlying the results presented in the study are available from 10.5281/zenodo.3357311.
Funding Statement
This work was supported by the German Ministry of Research and Education within the Comprehensive Heart Failure Center Würzburg (BMBF 01EO1004 and 01EO1504). https://www.bmbf.de/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Pedrizzetti G, Mangual J and Tonti G. On the geometrical relationship between global longitudinal strain and ejection fraction in the evaluation of cardiac contraction. J Biomech. 2014;47:746–9. 10.1016/j.jbiomech.2013.12.016 [DOI] [PubMed] [Google Scholar]
- 2.Stampehl MR, Mann DL, Nguyen JS, Cota F, Colmenares C and Dokainish H. Speckle strain echocardiography predicts outcome in patients with heart failure with both depressed and preserved left ventricular ejection fraction. Echocardiography. 2015;32:71–8. 10.1111/echo.12613 [DOI] [PubMed] [Google Scholar]
- 3.Perk G, Tunick PA and Kronzon I. Non-Doppler two-dimensional strain imaging by echocardiography—from technical considerations to clinical applications. J Am Soc Echocardiogr. 2007;20:234–43. [DOI] [PubMed] [Google Scholar]
- 4.Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N and Oki T. The functional role of longitudinal, circumferential, and radial myocardial deformation for regulating the early impairment of left ventricular contraction and relaxation in patients with cardiovascular risk factors: a study with two-dimensional strain imaging. J Am Soc Echocardiogr. 2008;21:1138–44. 10.1016/j.echo.2008.07.016 [DOI] [PubMed] [Google Scholar]
- 5.Share BL, La Gerche A, Naughton GA, Obert P and Kemp JG. Young Women With Abdominal Obesity Have Subclinical Myocardial Dysfunction. Can J Cardiol. 2015;31:1195–201. 10.1016/j.cjca.2015.02.004 [DOI] [PubMed] [Google Scholar]
- 6.Yingchoncharoen T, Agarwal S, Popovic ZB and Marwick TH. Normal ranges of left ventricular strain: a meta-analysis. J Am Soc Echocardiogr. 2013;26:185–91. 10.1016/j.echo.2012.10.008 [DOI] [PubMed] [Google Scholar]
- 7.Kleijn SA, Pandian NG, Thomas JD, Perez de Isla L, Kamp O, Zuber M, Nihoyannopoulos P, Forster T, Nesser HJ, Geibel A, Gorissen W and Zamorano JL. Normal reference values of left ventricular strain using three-dimensional speckle tracking echocardiography: results from a multicentre study. Eur Heart J Cardiovasc Imaging. 2015;16:410–6. 10.1093/ehjci/jeu213 [DOI] [PubMed] [Google Scholar]
- 8.Bernard A, Addetia K, Dulgheru R, Caballero L, Sugimoto T, Akhaladze N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Ilardi F, Lopez T, de la Morena G, Popescu BA, Penicka M, Ozyigit T, David Rodrigo Carbonero J, van de Veire N, Stephan Von Bardeleben R, Vinereanu D, Luis Zamorano J, Martinez C, Magne J, Cosyns B, Donal E, Habib G, Badano LP, Lang RM and Lancellotti P. 3D echocardiographic reference ranges for normal left ventricular volumes and strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:475–483. 10.1093/ehjci/jew284 [DOI] [PubMed] [Google Scholar]
- 9.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD and Vasan RS. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–9. 10.1161/CIRCIMAGING.112.000627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menting ME, McGhie JS, Koopman LP, Vletter WB, Helbing WA, van den Bosch AE and Roos-Hesselink JW. Normal myocardial strain values using 2D speckle tracking echocardiography in healthy adults aged 20 to 72 years. Echocardiography. 2016;33:1665–1675. 10.1111/echo.13323 [DOI] [PubMed] [Google Scholar]
- 11.Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ and Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–83. 10.1093/ejechocard/jep194 [DOI] [PubMed] [Google Scholar]
- 12.Moreira HT, Nwabuo CC, Armstrong AC, Kishi S, Gjesdal O, Reis JP, Schreiner PJ, Liu K, Lewis CE, Sidney S, Gidding SS, Lima JAC and Ambale-Venkatesh B. Reference Ranges and Regional Patterns of Left Ventricular Strain and Strain Rate Using Two-Dimensional Speckle-Tracking Echocardiography in a Healthy Middle-Aged Black and White Population: The CARDIA Study. J Am Soc Echocardiogr. 2017;30:647–658 e2. 10.1016/j.echo.2017.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Park JH, Lee JH, Lee SY, Choi JO, Shin MS, Kim MJ, Jung HO, Park JR, Sohn IS, Kim H, Park SM, Yoo NJ, Choi JH, Kim HK, Cho GY, Lee MR, Park JS, Shim CY, Kim DH, Shin DH, Shin GJ, Shin SH, Kim KH, Kim WS and Park SW. Normal 2-Dimensional Strain Values of the Left Ventricle: A Substudy of the Normal Echocardiographic Measurements in Korean Population Study. J Cardiovasc Ultrasound. 2016;24:285–293. 10.4250/jcu.2016.24.4.285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaku K, Takeuchi M, Tsang W, Takigiku K, Yasukochi S, Patel AR, Mor-Avi V, Lang RM and Otsuji Y. Age-related normal range of left ventricular strain and torsion using three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2014;27:55–64. 10.1016/j.echo.2013.10.002 [DOI] [PubMed] [Google Scholar]
- 15.Liu CY, Lai S, Kawel-Boehm N, Chahal H, Ambale-Venkatesh B, Lima JAC and Bluemke DA. Healthy aging of the left ventricle in relationship to cardiovascular risk factors: The Multi-Ethnic Study of Atherosclerosis (MESA). PLoS One. 2017;12:e0179947 10.1371/journal.pone.0179947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bjornstad P, Truong U, Pyle L, Dorosz JL, Cree-Green M, Baumgartner A, Coe G, Regensteiner JG, Reusch JE and Nadeau KJ. Youth with type 1 diabetes have worse strain and less pronounced sex differences in early echocardiographic markers of diabetic cardiomyopathy compared to their normoglycemic peers: A RESistance to InSulin in Type 1 ANd Type 2 diabetes (RESISTANT) Study. J Diabetes Complications. 2016;30:1103–10. 10.1016/j.jdiacomp.2016.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Szelenyi Z, Fazakas A, Szenasi G, Tegze N, Fekete B, Molvarec A, Hadusfalvy-Sudar S, Janosi O, Kiss M, Karadi I and Vereckei A. The mechanism of reduced longitudinal left ventricular systolic function in hypertensive patients with normal ejection fraction. J Hypertens. 2015;33:1962–9; discussion 1969. 10.1097/HJH.0000000000000624 [DOI] [PubMed] [Google Scholar]
- 18.Huang J, Yan ZN, Rui YF, Fan L, Shen D and Chen DL. Left Ventricular Systolic Function Changes in Primary Hypertension Patients Detected by the Strain of Different Myocardium Layers. Medicine (Baltimore) 2016;95:e2440 10.1097/MD.0000000000002440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Almeida AL, Teixido-Tura G, Choi EY, Opdahl A, Fernandes VR, Wu CO, Bluemke DA and Lima JA. Metabolic syndrome, strain, and reduced myocardial function: multi-ethnic study of atherosclerosis. Arq Bras Cardiol. 2014;102:327–35. 10.5935/abc.20140040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pascual M, Pascual DA, Soria F, Vicente T, Hernandez AM, Tebar FJ and Valdes M. Effects of isolated obesity on systolic and diastolic left ventricular function. Heart. 2003;89:1152–6. 10.1136/heart.89.10.1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wong CY, O'Moore-Sullivan T, Leano R, Byrne N, Beller E and Marwick TH. Alterations of left ventricular myocardial characteristics associated with obesity. Circulation. 2004;110:3081–7. [DOI] [PubMed] [Google Scholar]
- 22.Wierzbowska-Drabik K, Chrzanowski L, Kapusta A, Uznanska-Loch B, Plonska E, Krzeminska-Pakula M, Kurpesa M, Rechcinski T, Trzos E and Kasprzak JD. Severe obesity impairs systolic and diastolic heart function—the significance of pulsed tissue Doppler, strain, and strain rate parameters. Echocardiography. 2013;30:904–11. 10.1111/echo.12164 [DOI] [PubMed] [Google Scholar]
- 23.Eroglu E, Aydin S, Yalniz F, Kalkan AK, Bayrak F and Degertekin M. Chronic cigarette smoking affects left and right ventricular long-axis function in healthy young subjects: a Doppler myocardial imaging study. Echocardiography. 2009;26:1019–25. 10.1111/j.1540-8175.2009.00924.x [DOI] [PubMed] [Google Scholar]
- 24.Dobson R, Burgess MI, Sprung VS, Irwin A, Hamer M, Jones J, Daousi C, Adams V, Kemp GJ, Shojaee-Moradie F, Umpleby M and Cuthbertson DJ. Metabolically healthy and unhealthy obesity: differential effects on myocardial function according to metabolic syndrome, rather than obesity. Int J Obes (Lond) 2016;40:153–61. [DOI] [PubMed] [Google Scholar]
- 25.Russo C, Sera F, Jin Z, Palmieri V, Homma S, Rundek T, Elkind MS, Sacco RL and Di Tullio MR. Abdominal adiposity, general obesity, and subclinical systolic dysfunction in the elderly: A population-based cohort study. Eur J Heart Fail. 2016;18:537–44. 10.1002/ejhf.521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kishi S, Gidding SS, Reis JP, Colangelo LA, Venkatesh BA, Armstrong AC, Isogawa A, Lewis CE, Wu C, Jacobs DR, Jr., Liu K and Lima JA. Association of Insulin Resistance and Glycemic Metabolic Abnormalities With LV Structure and Function in Middle Age: The CARDIA Study. JACC Cardiovasc Imaging. 2017;10:105–114. 10.1016/j.jcmg.2016.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zarich SW, Kowalchuk GJ, McGuire MP, Benotti PN, Mascioli EA and Nesto RW. Left ventricular filling abnormalities in asymptomatic morbid obesity. Am J Cardiol. 1991;68:377–81. [DOI] [PubMed] [Google Scholar]
- 28.Jensen MT, Sogaard P, Andersen HU, Bech J, Fritz Hansen T, Biering-Sorensen T, Jorgensen PG, Galatius S, Madsen JK, Rossing P and Jensen JS. Global longitudinal strain is not impaired in type 1 diabetes patients without albuminuria: the Thousand & 1 study. JACC Cardiovasc Imaging. 2015;8:400–10. 10.1016/j.jcmg.2014.12.020 [DOI] [PubMed] [Google Scholar]
- 29.Wagner M, Tiffe T, Morbach C, Gelbrich G, Stork S, Heuschmann PU and Consortium S. Characteristics and Course of Heart Failure Stages A-B and Determinants of Progression—design and rationale of the STAAB cohort study. Eur J Prev Cardiol. 2017;24:468–479. 10.1177/2047487316680693 [DOI] [PubMed] [Google Scholar]
- 30.Mancia G, Fagard R, Narkiewicz K, Redon J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchhof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F, Redon J, Dominiczak A, Narkiewicz K, Nilsson PM, Burnier M, Viigimaa M, Ambrosioni E, Caufield M, Coca A, Olsen MH, Schmieder RE, Tsioufis C, van de Borne P, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Clement DL, Coca A, Gillebert TC, Tendera M, Rosei EA, Ambrosioni E, Anker SD, Bauersachs J, Hitij JB, Caulfield M, De Buyzere M, De Geest S, Derumeaux GA, Erdine S, Farsang C, Funck-Brentano C, Gerc V, Germano G, Gielen S, Haller H, Hoes AW, Jordan J, Kahan T, Komajda M, Lovic D, Mahrholdt H, Olsen MH, Ostergren J, Parati G, Perk J, Polonia J, Popescu BA, Reiner Z, Ryden L, Sirenko Y, Stanton A, Struijker-Boudier H, Tsioufis C, van de Borne P, Vlachopoulos C, Volpe M and Wood DA. 2013 ESH/ESC guidelines for the management of arterial hypertension: the Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC). Eur Heart J. 2013;34:2159–219. 10.1093/eurheartj/eht151 [DOI] [PubMed] [Google Scholar]
- 31.European Association for Cardiovascular P, Rehabilitation, Reiner Z, Catapano AL, De Backer G, Graham I, Taskinen MR, Wiklund O, Agewall S, Alegria E, Chapman MJ, Durrington P, Erdine S, Halcox J, Hobbs R, Kjekshus J, Filardi PP, Riccardi G, Storey RF, Wood D, Guidelines ESCCfP and Committees. ESC/EAS Guidelines for the management of dyslipidaemias: the Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). Eur Heart J. 2011;32:1769–818. 10.1093/eurheartj/ehr158 [DOI] [PubMed] [Google Scholar]
- 32.Fruhbeck G, Toplak H, Woodward E, Yumuk V, Maislos M, Oppert JM and Executive Committee of the European Association for the Study of O. Obesity: the gateway to ill health—an EASO position statement on a rising public health, clinical and scientific challenge in Europe. Obes Facts. 2013;6:117–20. 10.1159/000350627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Authors/Task Force M, Ryden L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, Guidelines ESCCfP, Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document R, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C and Xuereb RG. ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013;34:3035–87. 10.1093/eurheartj/eht108 [DOI] [PubMed] [Google Scholar]
- 34.Morbach C, Gelbrich G, Breunig M, Tiffe T, Wagner M, Heuschmann PU and Stork S. Impact of acquisition and interpretation on total inter-observer variability in echocardiography: results from the quality assurance program of the STAAB cohort study. Int J Cardiovasc Imaging. 2018. [DOI] [PubMed] [Google Scholar]
- 35.Kuznetsova T, Herbots L, Richart T, D'Hooge J, Thijs L, Fagard RH, Herregods MC and Staessen JA. Left ventricular strain and strain rate in a general population. Eur Heart J. 2008;29:2014–23. 10.1093/eurheartj/ehn280 [DOI] [PubMed] [Google Scholar]
- 36.Hung CL, Goncalves A, Shah AM, Cheng S, Kitzman D and Solomon SD. Age- and Sex-Related Influences on Left Ventricular Mechanics in Elderly Individuals Free of Prevalent Heart Failure: The ARIC Study (Atherosclerosis Risk in Communities). Circ Cardiovasc Imaging. 2017;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugimoto T, Dulgheru R, Bernard A, Ilardi F, Contu L, Addetia K, Caballero L, Akhaladze N, Athanassopoulos GD, Barone D, Baroni M, Cardim N, Hagendorff A, Hristova K, Lopez T, de la Morena G, Popescu BA, Moonen M, Penicka M, Ozyigit T, Rodrigo Carbonero JD, van de Veire N, von Bardeleben RS, Vinereanu D, Zamorano JL, Go YY, Rosca M, Calin A, Magne J, Cosyns B, Marchetta S, Donal E, Habib G, Galderisi M, Badano LP, Lang RM and Lancellotti P. Echocardiographic reference ranges for normal left ventricular 2D strain: results from the EACVI NORRE study. Eur Heart J Cardiovasc Imaging. 2017;18:833–840. 10.1093/ehjci/jex140 [DOI] [PubMed] [Google Scholar]
- 38.Dokainish H, Sengupta R, Pillai M, Bobek J and Lakkis N. Usefulness of new diastolic strain and strain rate indexes for the estimation of left ventricular filling pressure. Am J Cardiol. 2008;101:1504–9. 10.1016/j.amjcard.2008.01.037 [DOI] [PubMed] [Google Scholar]
- 39.Ersboll M, Andersen MJ, Valeur N, Mogensen UM, Fakhri Y, Thune JJ, Moller JE, Hassager C, Sogaard P and Kober L. Early diastolic strain rate in relation to systolic and diastolic function and prognosis in acute myocardial infarction: a two-dimensional speckle-tracking study. Eur Heart J. 2014;35:648–56. 10.1093/eurheartj/eht179 [DOI] [PubMed] [Google Scholar]
- 40.Narayanan A, Aurigemma GP, Chinali M, Hill JC, Meyer TE and Tighe DA. Cardiac mechanics in mild hypertensive heart disease: a speckle-strain imaging study. Circ Cardiovasc Imaging. 2009;2:382–90. 10.1161/CIRCIMAGING.108.811620 [DOI] [PubMed] [Google Scholar]
- 41.Peterson LR, Waggoner AD, Schechtman KB, Meyer T, Gropler RJ, Barzilai B and Davila-Roman VG. Alterations in left ventricular structure and function in young healthy obese women: assessment by echocardiography and tissue Doppler imaging. J Am Coll Cardiol. 2004;43:1399–404. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary tables: Table A. Left ventricular global and segmental peak systolic longitudinal strain in males. Table B. Left ventricular global and segmental peak systolic longitudinal strain in females. Table C. Impact of cardiovascular risk factors on global longitudinal peak systolic strain in the total cohort and according to sex. Table D. Impact of cardiovascular risk factors on systolic strain rate in the total cohort and according to sex. Table E. Impact of cardiovascular risk factors on early diastolic strain rate in the total cohort and according to sex. Table F. Impact of cardiovascular risk factors on late diastolic strain rate in the total cohort and according to sex. Supplementary figures: Figure A. Correlation of speckle tracking and tissue Doppler imaging derived global early diastolic strain rate. Figure B. Distribution of GL-PSS values in individuals without CVRF. Figure C. Percentiles of global longitudinal peak systolic strain in men. Figure D. Percentiles of global longitudinal peak systolic strain in women. Figure E. Percentiles of systolic strain rate in men. Figure F. Percentiles of systolic strain rate in women. Figure G. Percentiles of early diastolic strain rate in men. Figure H. Percentiles of early diastolic strain rate in women. Figure I. Percentiles of late diastolic strain rate in men. Figure J. Percentiles of late diastolic strain rate in women. Figure K. Number of male individuals with 0–5 cardiovascular risk factors by age. Figure L. Number of female individuals with 0–5 cardiovascular risk factors by age.
(DOCX)
Data Availability Statement
The data underlying the results presented in the study are available from 10.5281/zenodo.3357311.