Abstract
Wolbachia are alpha-proteobacteria known to infect arthropods, which are of interest for disease control since they have been associated with improved resistance to viral infection. Although several genomes for different strains have been sequenced, there is little knowledge regarding the relationship between this bacterium and their hosts, particularly on their dependency for survival. Motivated by the potential applications on disease control, we developed genome-scale models of four Wolbachia strains known to infect arthropods: wAlbB (Aedes albopictus), wVitA (Nasonia vitripennis), wMel and wMelPop (Drosophila melanogaster). The obtained metabolic reconstructions exhibit a metabolism relying mainly on amino acids for energy production and biomass synthesis. A gap analysis was performed to detect metabolic candidates which could explain the endosymbiotic nature of this bacterium, finding that amino acids, requirements for ubiquinone precursors and provisioning of metabolites such as riboflavin could play a crucial role in this relationship. This work provides a systems biology perspective for studying the relationship of Wolbachia with its host and the development of new approaches for control of the spread of arboviral diseases. This approach, where metabolic gaps are key objects of study instead of just additions to complete a model, could be applied to other endosymbiotic bacteria of interest.
Author summary
The expansion of the geographic distribution of arthropods has led to an increase in the number of infections of Zika and Dengue. This motivates the search for new alternative approaches for disease control. Wolbachia pipientis, an obligate intracellular bacteria known to provide pathogen-blocking capabilities to its host, has been used to this end. Wolbachia-infected mosquitoes have been released into the environment as a strategy for controlling the mosquito population and hence the spread of arboviral-derived diseases. However, there is little knowledge regarding the specific interactions that occur between Wolbachia and its host, particularly those associated with its obligate intracellular nature. In this work we studied Wolbachia endosymbiosis from a systems biology perspective. By analyzing the gaps in the metabolic networks of four Wolbachia strains we were able to identify key interaction points between this intracellular bacteria and its host, which play a crucial role in their metabolic relationship. This approach focuses on what is missing to allow unveiling new information regarding the interplay between interacting metabolic networks, and it is especially useful in the case of intracellular bacteria where experimental information is scarce. This additional insight on the metabolic interactions between host and parasite hold great potential for the development of arboviral disease control strategies.
Introduction
Wolbachia are obligate intracellular alpha-proteobacteria, member of the Rickettsiales order known to infect nematodes and arthropods by developing diverse complex interactions with their hosts, such as supplementation with vitamins, cytoplasmic incompatibility and parthenogenesis [1–4].
The nature of these interactions is influenced by the strain and organism involved and they have been reviewed extensively [4]. In some organisms, Wolbachia has shown to impart a fitness advantage to arthropod host such as better reproductive traits or improved resistance to virus infection [5]. Particularly, interactions between this endosymbiont with its hosts range from metabolite supplementation, particularly biotin [6], riboflavin [7], to pathogenic interactions such as those of the strain wMelPop [8].
Several studies have been developed towards the understanding of Wolbachia interactions with their hosts driven by potential applications in development of novel control strategies for the spread of arbovirus-derived diseases such as Yellow fever, Zika, Chikungunya and Dengue [9]. Genomes of several Wolbachia strains have been sequenced [8,10–12] and comparatively analyzed [8] in order to explain host-symbiont features of interest such as cytoplasmic incompatibility.
Wolbachia are known to have a reduced genome size as a result of their adaptation to depend on other organism to their survival. Thus, it is expected that they exhibit a small and rather incomplete metabolic network, as it has been previously observed for other endosymbiotic bacteria [13,14]. We hypothesized that a thorough analysis of Wolbachia metabolism could be achieved by analyzing a representation of their wide metabolic capabilities given by genome-scale models.
Genome-scale models (GEMs) have emerged as a powerful tool for studying cellular metabolism since they provide a global representation of all biochemical transformations that could be carried out by a specific organism based on their genome annotation [15].
In this work, we developed genome-scale models for four Wolbachia strains found in insect arthropods, wAlbB (from Aedes albopictus) [11], wVitA (from Nasonia vitripennis) [10], wMel [12] and wMelPop [8] (both from Drosophila melanogaster). We hypothesized that the analysis of the metabolic gaps in the curation stage could reveal potential candidates to explain the endosymbiotic nature of Wolbachia as shared metabolites between both species.
Materials and methods
Four strains of Wolbachia pipientis were selected based on their characteristics, such as: their ability of causing cytoplasmic incompatibility, to infect mosquito species and pathogenicity. wAlbB (Aedes albopictus), wVitA (Nasonia vitripennis) and wMel and wMelPop (Drosophila melanogaster) genomes were used to generate draft genome-scale models using modelSEED [13]. Gap filling was performed considering a complete media extracellular environment, which means that the model can consume any nutrient for which a transport reaction is available to the model. This gap filling was analyzed to determine potential gene gaps which could putatively explain Wolbachia pipientis' symbiotic relationship with its hosts.
We proposed a draft biomass composition for Wolbachia based on phylogenetic information and predicted pathways in the annotation process [13]. The obtained composition was modified based on DNA and amino acid composition and updated fatty acid content based on reported concentration of phospholipids in Wolbachia, Rickettsia and Escherichia coli [16–19]. Computation of the stoichiometric coefficients associated with each component in the biomass function was achieved based on the protocol proposed by Thiele et al., (2010) [15].
Integration of the four obtained Wolbachia metabolic reconstructions was achieved using COBRApy [20]. Gene protein reaction (GPR) associations are modified to integrate gene identifiers of all the studied strains. The obtained gaps were confirmed using BlastP to detect top hits to unannotated gene products, which were selected and incorporated into the genome-scale model when E-values were lower than 0.001 and coverage was above 85%. Flux Balance Analysis (FBA) simulations were achieved using COBRApy [20].
Results
Four Wolbachia strains were selected based on the following characteristics: wVitA due to its reported ability to cause cytoplasmic incompatibility, wAlbB was known to infect the mosquito Aedes albopictus, wMel due to its reported effects on pathogen virus blocking and wMelPop because of its pathogenicity to their host. Based on their genome annotation, four genome-scale models were obtained (Table 1, S1 File, S2 File).
Table 1. Wolbachia pipientis genome-scale models.
| wAlbB | wVitA | wMel | wMelPop | |
|---|---|---|---|---|
| Genes | 324 | 334 | 316 | 329 |
| Reactions | 756 | 752 | 766 | 775 |
| Metabolites | 830 | 821 | 840 | 849 |
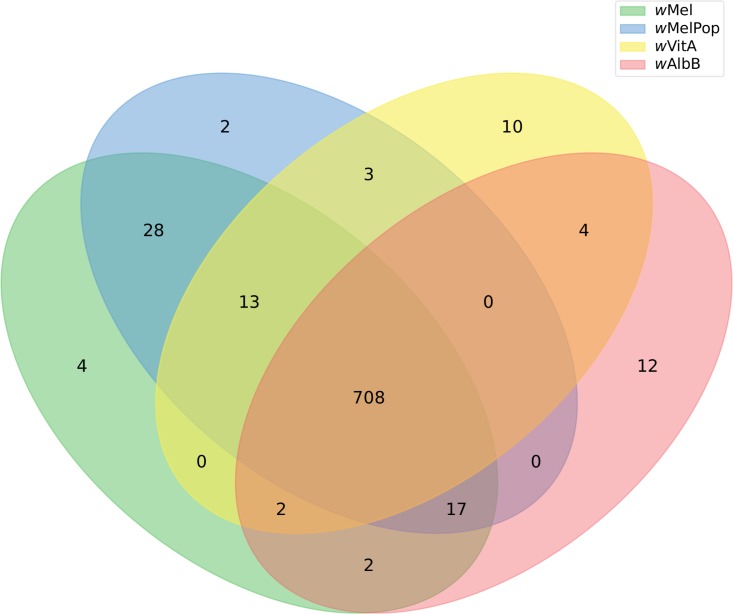
All four Wolbachia metabolic reconstructions share 708 common reactions. Strains wAlbB and wVitA have 4 reactions in common which are absent in wMel and wMelPop, wVitA has 10 unique reactions and wAlbB includes 12 reactions that are not present on the model of the strain that infects other hosts. On the other hand, wMel and wMelPop have 28 common reactions that are not present in the other two strains, while wMel includes 4 unique reactions and wMelPop 2 unique reactions of their own (Fig 1).
Fig 1. Wolbachia metabolic reconstructions.
Reactions shared amongst the Wolbachia metabolic reconstructions for the strains causing cytoplasmic incompatibility (wMel, wVitA), pathogen blocking (wMel and wMelPop), the pathogenic strain wMelPop, and the Aedes albopictus strain wAlbB.
wVitA presents 10 unique reactions not shared with the other 3 analyzed strains, including wMel and wAlbB which are also known to induce cytoplasmic incompatibility. These reactions are associated with the use of deoxyguanosine as a precursor of dGTP and thioredoxin oxidation and are not associated with this phenotype. Cytoplasmic incompatibility has been studied and associated with two genes: cifA and cifB in Drosophila melanogaster [21] which have been associated with regulatory processes that are not represented by this approach.
On the other hand, wAlbB has 12 reactions exclusive to this strain involving protocatehuic acid (PCA), a component known to be part of the protein fraction of the insect cuticule and wMelPop includes 2 unique reactions associated with tetrahydrofolate metabolism, which are not associated with its pathogenic nature and present gaps of its metabolic nature. We then propose that wMelPop detrimental effect on its host lies in regulatory processes that determine cell density inside its host rather than in specific metabolic reactions that could be associated with pathogenicity.
Wolbachia metabolism
We analyzed previously reported metabolic features for the wMel strain, which has been described to rely mainly on amino acids to support their energetic requirements, having limited carbohydrate synthesis capacity and being unable to transport ATP directly from its host and to synthesize Lipid A [12,22]. Our findings support these affirmations, with a Wolbachia metabolic reconstruction exhibiting a complete glycolysis pathway starting from fructose 6 phosphate towards phosphoenolpyruvate and a complete TCA (tricarboxylic acid) cycle.
Additionally, Wolbachia presents a highly conserved pentose phosphate pathway (PPP) for sugar nucleotide synthesis, contrary to what has been reported for close organisms such as Rickettsia [14] and an amino acid metabolism, characterized by the presence of amino dipeptidases. This suggests that dipeptide transport is required by this endosymbiont for acquiring amino acids derived from its host metabolism.
The riboflavin synthesis pathway, another putative symbiosis-determining pathway, is highly conserved with one missing step that is common to all studied strains, FMN hydrolase/5-amino-6-(5-phospho-D-ribitylamino) uracil phosphatase. However, this reaction has been found to be associated with the gene ribD reported to be found in wMel, wRi, wHa, and wAu [7]. Therefore, this is not an incomplete metabolic pathway in any of the studied strains.
We also analyzed the biomass reaction given by modelSEED which is generated from phylogenetic information and the predicted pathways based on their genome annotation [13]. Particularly, this stoichiometric composition is derived from the Gram-negative bacteria template and the presence of predicted metabolic pathways such as ubiquinone biosynthesis, fatty acid biosynthesis, and polyamine metabolism among others.
However, Wolbachia is closer to the Rickettsia genus rather than Escherichia coli, the exemplary Gram-negative species. Subsequently, the biomass composition was modified to include specific phospholipid and fatty acid composition derived from studies made in Escherichia coli and particularly in Rickettsia prowazekii [16–19], in order to find specific network spots where the symbiotic nature of the predicted gaps in this metabolic reconstruction could be found.
Gap analysis
We performed an analysis of the gap filling process to determine candidates for the metabolic relationship between Wolbachia and its hosts (S3 File). In this analysis all four metabolic reconstructions shared the same 127 gap fill reactions (75%) which are presumably requirements for synthesis of biomass components that no Wolbachia strain is able to perform.
The added reactions correspond approximately to 20% of the total number of reactions in the analyzed metabolic reconstructions. This high proportion as a single fact putatively explains single handedly the obligate endosymbiotic nature of Wolbachia, as it has been discussed for other organisms of similar nature [13].
Most of these reactions are associated with transport of essential metabolites for cell growth instead of de novo synthesis since internalization of external metabolites requires the addition of a lower number of reactions to the model, a feature that was preferred in our reconstruction framework (Fig 2). However, it is worth mentioning that transport reactions are only about one third of the gap reactions of the model, which means that real gaps are also present in our reconstructions that are not solved by importing metabolites into the cell.
Fig 2. Metabolic gaps in Wolbachia pipientis.
Functionality of the metabolic gaps in the obtained Wolbachia metabolic reconstruction.
An inspection of the added reactions for wAlbB and wVitA showed that potential metabolic candidates for explaining their symbiotic relationship with their hosts are mainly associated with fatty acid, lipopolysaccharide and amino acid metabolism. Arginine, glutamine and alanine are particularly important for wAlbB and asparagine, glutamine, histidine, isoleucine and leucine for wVitA. Additionally, wAlbB requires transport of TTP, hexadecanoate and dGTP for survival while wVitA requires transport of histidine and CTP.
wMel and wMelPop share over 90% of their metabolic reactions, which is expected due to their phylogenetic closeness. An analysis on these reactions showed that biosynthesis of antibiotic precursors, lipopolysaccharide biosynthesis and alanine, serine and glycine metabolism are metabolite candidates to explain Wolbachia interaction with its hosts.
Wolbachia pipientis is known to be highly dependent on amino acid metabolism of its hosts [12], which is consistent with the obtained gaps in the analyzed strains. In support of this, transport of dipeptides and amino acids has been added in the gap filling process, which can be another factor to explain the symbiotic relationship of Wolbachia with their hosts.
The obtained metabolic reconstruction also includes gaps associated with lipid A synthesis, which has previously been hypothesized to be absent in wMel and wBm [22]. In fact, transformations of amino acids initially found to be gaps in this model are part of the synthesis of this precursor of lipopolysaccharide (LPS) synthesis.
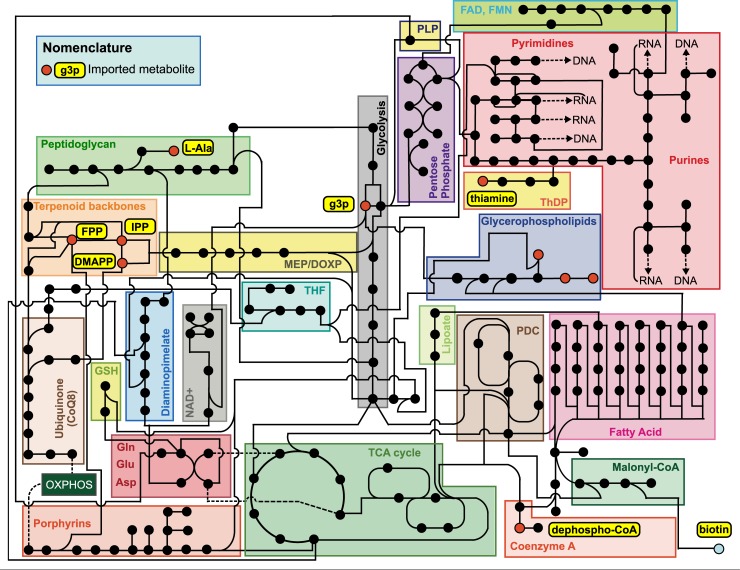
Given the phylogenetic closeness of genera Wolbachia and Rickettsia, the obtained metabolic gaps for the different Wolbachia strains were compared to the ones reported for Rickettsia [14]. An analysis showing metabolic features of Wolbachia pipientis and predicted imported metabolites derived from gap analysis of this genome-scale model (Fig 3) that although both are members of the Anaplasmataceae family, the reductive genome evolution has led to the loss of different functionalities in each phylogenetic branch.
Fig 3. Wolbachia metabolism.
Wolbachia presents highly conserved pathways for nucleotide, peptidoglycan and fatty acid synthesis but depends highly on transport of carbon sources such as glycerate 3 phosphate, amino acids such as alanine, and phospholipid transport to support cell growth. g3p: glycerate 3 phosphate, FPP: farnesyl pyrophosphate, IPP: isopentenyl phosphate, DMAPP: dimethylallyl pyrophosphate. Adapted from [14].
Rickettsia shows depleted metabolic pathways for B vitamins, several cofactors and the pentose phosphate pathway [14], contrary to what has been obtained in this work, where these metabolic pathways are highly conserved (Fig 3). The obtained metabolic reconstruction is able to synthesize S-adenosyl methionine (SAM) from methionine but includes gaps associated with SAM metabolization for heme synthesis, which was initially reported to also be missing in the nematode infecting strain wOo [23] but then experimentally found to be fully present in Wolbachia [24].
On the other hand, common metabolic gaps between Wolbachia and Rickettsia have also been identified. The obtained Wolbachia model has reactions without associated genes involved in the synthesis of isopentenyl phosphate (IPP) and 4-hydroxybenzoate from lanosterol (S2 and S3 Files) while Rickettsia is known to import IPP and 4-hydroxybenzoate so we infer that Wolbachia must also acquire these metabolites from its host. Tetrahydrofolate has also been proposed as an imported metabolite in Rickettsia, which is consistent with our findings for Wolbachia in the analysis of Wolbachia's metabolism.
Discussion
Wolbachia are endosymbiotic bacteria of interest due to their interactions with their arthropod hosts, such as pathogen blocking, which includes blocking of human arboviral pathogen spread, and cytoplasmic incompatibility. In this work we present metabolic reconstruction of four Wolbachia strains known to infect arthropods, wMel, wMelPop, wVitA and wAlbB. An analysis of these metabolic reconstructions is focused on their metabolic gaps as these missing reactions may be the key interactions with its hosts that explain their obligate endosymbiont nature and the difficulty found to cultivate these bacteria in the laboratory outside their host cell.
Wolbachia are known to have a metabolism that is highly dependent on amino acids for cell growth [12,25]. The presence of amino peptidases suggests that this endosymbiont is able to feed on peptides derived from proteolytic processes inside their host. The obtained metabolic reconstruction predicts the lack of genes associated with lipid A synthesis as it has been proposed for the strain wMel and wBm [22].
A series of confirmed gaps are associated with IPP and 4-hydroxybenzoate synthesis which are metabolic requirements for ubiquinone 8 synthesis, a highly conserved pathway in Wolbachia. Since these metabolites have been previously predicted to be imported in Rickettsia, we propose that similar mechanisms exist for their import in this alpha-proteobacteria.
Regarding our proposed methodology to find metabolites linked to the endosymbiotic nature of Wolbachia, several factors are known to affect the gap filling process in genome-scale models, such as media and biomass composition. Different extracellular environments result into different sets of metabolites that could be added, as transport reactions, into the intracellular compartment of the obtained model. On the other hand, the artificial inclusion of components that are not part of the modeled organism's biomass, result in the addition of metabolic capabilities observed to be absent experimentally.
In this work, gap analysis was performed based on the obtained models for four different Wolbachia strains. The media composition was assumed to be rich in nutrients due to the obligate intracellular location of Wolbachia. Additionally, the biomass function was updated to represent the experimentally determined Wolbachia phospholipid and fatty acid content. The actual existence of the obtained gaps was confirmed by searches using BlastP against non-redundant protein and translated nucleotide databases. Their essentiality was assessed based on the upgraded Wolbachia biomass composition, as well as the composition of their surrounding environment to guarantee an improved quality of the predictions made by this approach.
Alternative strategies to study metabolism of endosymbionts have been published recently. Driscoll et al., (2017) [14] reconstructed Rickettsia metabolism and predicted transported compounds based solely on observed gaps in their metabolic network without information on the requirements for cell growth given by their biomass composition. Rickettsia exhibits depleted metabolic pathways for B vitamins, and the pentose phosphate pathway contrary to our findings in Wolbachia, which presents highly conserved pathways for synthesis of vitamins and nucleotides. These differences can be associated with the endosymbiotic and rather mutualistic behavior of Wolbachia versus Rickettsia which has a pathogenic nature. Accordingly, Rickettsia presents a metabolism oriented to the import of metabolites (SAM, ATP) whilst Wolbachia conserves metabolic pathways for metabolic provisioning to its hosts.
There is no available automatic method to distinguish between true annotation gaps, which have biological meaning and correspond to gene losses that explain the dependency of Wolbachia on its host for their survival, from annotation gaps originated from experimental errors in the process of sequencing and annotation of the DNA of the analyzed strains. Particularly, of the 1,174 genes found originally in the annotation process for wMel [12], only 316 were linked to metabolic functions represented in our genome-scale model, showing that although there is valuable information regarding metabolic interactions derived from Wolbachia gene annotation, there is a considerable number of genes associated with regulatory processes that are not represented by this approach. Widely studied Wolbachia genes as the cytoplasmic incompatibility genes (cifA, cifB) [21] and ankyrin repeat proteins [12] are not included in genome-scale models, given that they are not associated with chemical reactions that this organism can carry out.
We propose that the inclusion of information of multiple strains could help minimize this problem and provide the scientific community with a robust tool for metabolic studies of this intracellular bacteria. Additionally, this approach, where metabolic gaps are key objects of study instead of just additions to complete a model, could be expanded for other endosymbiotic bacteria of interest.
Conclusion
In this work we present Wolbachia genome-scale model for four strains known to infect arthropods. We propose that the metabolic gaps present in this model are key to study metabolic interactions with their hosts and could potentially influence processes that lead to cytoplasmic incompatibility and pathogen blocking.
Wolbachia metabolism is characterized by the import of amino acids for energy and growth, and also particularly the import of isopentenyl phosphate and 4-hydroxybenzoate for ubiquinone 8 biosynthesis. Although, Wolbachia share several characteristics with Rickettsia, the metabolism of the analyzed Wolbachia strains presents highly conserved pathways for nucleotide synthesis and riboflavin, which can be ascribed to their mutualistic rather than parasitic behavior.
We have provided a systems biology analysis framework to study Wolbachia metabolism to improve the understanding on the relationship with its host and the development of new approaches towards the control of spread of arboviral diseases.
Supporting information
Wolbachia genome-scale models in SBML for the four analyzed strains wMel, wMelPop, wAlbB, and wVitA.
(ZIP)
Spreadsheet (xlsx format) listing all the included reactions and gene associations for the four Wolbachia genome-scale models.
(XLSX)
Spreadsheet (xlsx format) listing new annotated genes and filled metabolic gaps derived from this work.
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This work was funded by MOSTICAW STIC-AmSud, PIA Fellowship AFB170001 (CMM) and PFBasal-0001 (CeBiB), Conicyt, Chile. The latter also funded publication costs associated to this article. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Werren JH, Baldo L, Clark ME. Wolbachia: master manipulators of invertebrate biology. Nature Reviews Microbiology. 2008;6: 741 10.1038/nrmicro1969 [DOI] [PubMed] [Google Scholar]
- 2.Werren JH. Biology of Wolbachia. Annual Review of Entomology. 1997;42: 587–609. 10.1146/annurev.ento.42.1.587 [DOI] [PubMed] [Google Scholar]
- 3.Hosokawa T, Koga R, Kikuchi Y, Meng X-Y, Fukatsu T. Wolbachia as a bacteriocyte-associated nutritional mutualist. PNAS. 2010;107: 769–774. 10.1073/pnas.0911476107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zug R, Hammerstein P. Bad guys turned nice? A critical assessment of Wolbachia mutualisms in arthropod hosts. Biological Reviews. 2015;90: 89–111. 10.1111/brv.12098 [DOI] [PubMed] [Google Scholar]
- 5.Frentiu FD, Zakir T, Walker T, Popovici J, Pyke AT, Hurk A van den, et al. Limited Dengue Virus Replication in Field-Collected Aedes aegypti Mosquitoes Infected with Wolbachia. PLOS Neglected Tropical Diseases. 2014;8: e2688 10.1371/journal.pntd.0002688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nikoh N, Hosokawa T, Moriyama M, Oshima K, Hattori M, Fukatsu T. Evolutionary origin of insect–Wolbachia nutritional mutualism. PNAS. 2014;111: 10257–10262. 10.1073/pnas.1409284111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moriyama M, Nikoh N, Hosokawa T, Fukatsu T. Riboflavin Provisioning Underlies Wolbachia’s Fitness Contribution to Its Insect Host. mBio. 2015;6 10.1128/mBio.01732-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woolfit M, Iturbe-Ormaetxe I, Brownlie JC, Walker T, Riegler M, Seleznev A, et al. Genomic Evolution of the Pathogenic Wolbachia Strain, wMelPop. Genome Biol Evol. 2013;5: 2189–2204. 10.1093/gbe/evt169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Christodoulou M. Biological vector control of mosquito-borne diseases. Lancet Infect Dis. 2011;11: 84–85. [DOI] [PubMed] [Google Scholar]
- 10.Kent BN, Salichos L, Gibbons JG, Rokas A, Newton ILG, Clark ME, et al. Complete Bacteriophage Transfer in a Bacterial Endosymbiont (Wolbachia) Determined by Targeted Genome Capture. Genome Biol Evol. 2011;3: 209–218. 10.1093/gbe/evr007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mavingui P, Moro CV, Tran-Van V, Wisniewski-Dyé F, Raquin V, Minard G, et al. Whole-Genome Sequence of Wolbachia Strain wAlbB, an Endosymbiont of Tiger Mosquito Vector Aedes albopictus. J Bacteriol. 2012;194: 1840–1840. 10.1128/JB.00036-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu M, Sun LV, Vamathevan J, Riegler M, Deboy R, Brownlie JC, et al. Phylogenomics of the Reproductive Parasite Wolbachia pipientis wMel: A Streamlined Genome Overrun by Mobile Genetic Elements. PLOS Biology. 2004;2: e69 10.1371/journal.pbio.0020069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henry CS, DeJongh M, Best AA, Frybarger PM, Linsay B, Stevens RL. High-throughput generation, optimization and analysis of genome-scale metabolic models. Nat Biotechnol. 2010;28: 977–982. 10.1038/nbt.1672 [DOI] [PubMed] [Google Scholar]
- 14.Driscoll TP, Verhoeve VI, Guillotte ML, Lehman SS, Rennoll SA, Beier-Sexton M, et al. Wholly Rickettsia! Reconstructed Metabolic Profile of the Quintessential Bacterial Parasite of Eukaryotic Cells. mBio. 2017;8: e00859–17. 10.1128/mBio.00859-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thiele I, Palsson B \O. A protocol for generating a high-quality genome-scale metabolic reconstruction. Nat Protoc. 2010;5: 93–121. 10.1038/nprot.2009.203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Orth JD, Conrad TM, Na J, Lerman JA, Nam H, Feist AM, et al. A comprehensive genome-scale reconstruction of Escherichia coli metabolism—2011. Mol Syst Biol. 2011;7: 535 10.1038/msb.2011.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reed JL, Vo TD, Schilling CH, Palsson BO. An expanded genome-scale model of Escherichia coli K-12 (iJR904 GSM/GPR). Genome Biology. 2003;4: R54 10.1186/gb-2003-4-9-r54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Winkler HH, Miller ET. Phospholipid composition of Rickettsia prowazeki grown in chicken embryo yolk sacs. J Bacteriol. 1978;136: 175–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wood WH, Wisseman CL. The Cell Wall of Rickettsia mooseri I. Morphology and Chemical Composition. J Bacteriol. 1967;93: 1113–1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ebrahim A, Lerman JA, Palsson BO, Hyduke DR. COBRApy: COnstraints-Based Reconstruction and Analysis for Python. BMC Systems Biology. 2013;7: 74 10.1186/1752-0509-7-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.LePage DP, Metcalf JA, Bordenstein SR, On J, Perlmutter JI, Shropshire JD, et al. Prophage WO genes recapitulate and enhance Wolbachia-induced cytoplasmic incompatibility. Nature. 2017;543: 243–247. 10.1038/nature21391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Foster J, Ganatra M, Kamal I, Ware J, Makarova K, Ivanova N, et al. The Wolbachia Genome of Brugia malayi: Endosymbiont Evolution within a Human Pathogenic Nematode. PLOS Biology. 2005;3: e121 10.1371/journal.pbio.0030121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darby AC, Armstrong SD, Bah GS, Kaur G, Hughes MA, Kay SM, et al. Analysis of gene expression from the Wolbachia genome of a filarial nematode supports both metabolic and defensive roles within the symbiosis. Genome Res. 2012;22: 2467–2477. 10.1101/gr.138420.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gill AC, Darby AC, Makepeace BL. Iron Necessity: The Secret of Wolbachia’s Success? PLOS Neglected Tropical Diseases. 2014;8: e3224 10.1371/journal.pntd.0003224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Caragata EP, Rancès E, O’Neill SL, McGraw EA. Competition for Amino Acids Between Wolbachia and the Mosquito Host, Aedes aegypti. Microb Ecol. 2014;67: 205–218. 10.1007/s00248-013-0339-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Wolbachia genome-scale models in SBML for the four analyzed strains wMel, wMelPop, wAlbB, and wVitA.
(ZIP)
Spreadsheet (xlsx format) listing all the included reactions and gene associations for the four Wolbachia genome-scale models.
(XLSX)
Spreadsheet (xlsx format) listing new annotated genes and filled metabolic gaps derived from this work.
(XLSX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.