Abstract
Transcriptional signatures and immune cell infiltrates associated with immune activation distinguish patients with glioblastoma who initially respond to immune checkpoint blockade from those who do not.
Gliomas, such as glioblastoma (GBM), are primary cancers of the brain that affect approximately 20,000 US residents per year1. Standard-of-care therapies include not only surgical resection with chemoradiation, but also other Food and Drug Administration (FDA)-approved treatments (such as alternating electrical fields, bevacizumab, and intracavitary chemotherapy wafers)2. Almost all GBMs recur within the first year following diagnosis, and the median survival time from initial diagnosis is less than 15 months. Once GBMs recur, they can be surgically resected again. The patient can also be treated with one of the FDA-approved therapies listed above, receive an ‘off-label’ chemotherapy, or participate in a clinical trial. However, there has been a high rate of failure in clinical trials for GBM over the last 50 years3,4. A major reason for this is the relative inability to easily access this type of brain tumor, either by additional resections and biopsies during treatment or by postmortem acquisition, to analyze it for molecular and cellular evidence of response to therapy. Without this data, the clinician is unable to understand why a patient responds or does not respond to the therapy in a clinical trial.
Immune checkpoint blockade (ICB) consists of humanized monoclonal antibodies that inhibit signaling between the receptor programmed cell death-1 (PD-1), which is expressed on the surface of anticancer CD8 + T cells, and its ligand programmed cell death ligand-1 (PD-L1), which is expressed on the surface of tumor cells, macrophages, and microglia. This signaling leads to downregulation of cytotoxic CD8 + T cell activation and is a ‘hallmark’ of T cell ‘exhaustion’ and cancer immune evasion5. In this issue of Nature Medicine, three reports analyze specimens from recurrent GBMs that were treated with ICB with the aim of identifying molecular and cellular markers of response. All three studies focus their analyses on tumor and immune cells found within the GBM microenvironment.
Zhao et al.6 carried out a retrospective analysis of individuals with recurrent GBM, 17 of whom were ‘long-term’ responders who had stable disease for at least 6 months and 49 of whom were nonresponders to anti-PD-1 therapy. By the authors’ criteria for responder/nonresponder status, the median survival of responders was 14.3 months versus 10.1 months for nonresponders. Genomic and transcriptomic analyses of tumor tissue showed a significant enrichment of PTEN mutations associated with immunosuppressive gene signatures in nonresponders, while there was enrichment of MAPK pathway alterations (PTPN11, BRAF) in responders. Single-cell RNA sequencing of one of the PTEN-mutant, nonresponder tumors showed an immunosuppressive signature primarily from CD44 + tumor cells. CD44 has previously been shown to be a marker of GBM cells associated with invasion and migration into normal brain7. PTEN-mutant tumors were enriched with T regulatory cells, macrophages, microglia, and neutrophils, indicative of an immunosuppressive microenvironment. Ultimately, even in responders, therapy failed. The authors attributed this to immunoediting. That is, their analysis of the clonal evolution of mutations in a small subset of responders (n = 3) and nonresponders (n = 2) shows evidence for neoantigenic mutations becoming lost and gene sets related to immunosuppression becoming enriched after therapy, preventing the continuation of an effective anticancer immune response.
Schalper et al.8 and Cloughesy et al.9 both carry out prospective, relatively early-phase clinical trials in which anti-PD-1 therapy was administered in a neoadjuvant setting (that is, anti-PD1 was administered before and after surgical resection of recurrent GBM). Schalper et al. analyzed 30 GBMs, 27 of which were recurrent and 3 newly diagnosed. All patients received preoperative and postoperative nivolumab. The efficacy outcome data for the therapy were consistent with the disheartening natural history of the disease, and two out of the three long-term survivors had GBMs with known biomarkers of survival (such as MGMT promoter methylation and IDH mutations)10,11. Molecular analyses of GBMs from all trial subjects revealed more chemokine transcripts (CXCL10, CCL4, and CCL3L1) and T cell infiltrates when compared with a historical group of GBM samples. Interestingly, T cell receptor (TCR) clonality analyses also showed more diversity in the group treated with nivolumab with an association between TCR clonotype diversity and survival. Using an immunofluorescence assay, the authors carried out studies of immune cells in the GBM microenvironment before and after nivolumab. Somewhat surprisingly, nivolumab treatment did not change the immune cell microenvironment of the GBMs; the number of lymphoid and myeloid cells remained the same. There were also no significant changes in T cell function (Ki67 for proliferation and Granzyme B for cytolytic activity) before versus after nivolumab treatment. In contrast, GBMs from the historical control group showed trends toward reduction of both lymphoid and myeloid cell populations throughout the disease course. Because GBM is known to contain few immune cells in its microenvironment12, these findings suggest that nivolumab may prevent immune cell loss in the tumor.
Cloughesy et al. carry out a randomized prospective trial comparing two schedules of pembrolizumab: before and after (neoadjuvant) surgery versus only after (adjuvant) surgery for 35 recurrent GBMs. The neoadjuvant PD-1 regimen significantly improved both overall (13.7 versus 7.5 months, P = 0.04) and progression-free survival (3.3 versus 2.4 months, P = 0.03). GBMs treated with neoadjuvant pembrolizumab showed a significant increase in a gene signatures related to interferon-γ (IFN-γ) responsiveness and a significant decrease in the number of tumors with cell cycle gene expression signatures (3 of 14 tumors treated with neoadjuvant therapy versus 11 of 15 treated with adjuvant). The authors concluded that anti-PD-1 therapy induces immune cell activation that represses the cell cycle proliferation signature of GBM cells. There was also high overlap in TCR clonotypes between tumor and blood in the group treated with the neoadjuvant strategy compared to the group treated with adjuvant alone, a finding also shown in a recently published immunotherapy trial12. Like Xiao et al., Cloughesy et al. evaluated whether there was evidence of immune checkpoint blockade treatment prompting immune evasion by GBMs and found that more neoadjuvant tumors showed spatially focal induction of PD-L1 expression when compared with adjuvant tumors.
These three studies indicate that the timing of ICB matters, and this may be a reason why in the Checkmate-143 trial, a randomized phase 3 clinical trial for In all three of the studies, therapies were administered in a neoadjuvant regimen, which led to more consistent immune activation. The neoadjuvant treatment could be further harnessed therapeutically via combination with other therapies that increase T cell tumor infiltration, such as chimeric antigen receptor–modificed (CAR) T cells or oncolytic viruses, or by adding agents that may restore PTEN function and/or reduce the number of tumor cells in the cell cycle. In spite of limitations, including the use of historical controls, inability to control for other treatment variables, such as bevacizumab, that are known to affect the immune response14 limited sample size, and limited tissue selection, these trials also suggest that some of the uncovered markers of response—like PTEN status, cell cycle proliferative status, and IFN-γ responsiveness—may be leveraged to identify subsets of patients that would benefit from ICB. However, it is not clear which of these markers may be more relevant. There were also contradictory findings: for instance, Cloughesy et al. report that neoadjuvant treatment did not lead to more TCR diversity, while Schalper et al and Xiao et al. report that it did. So, does neoadjuvant anti-PD-1 expand few clonotypes against a few tumor antigens, or does it increase the diversity of TCR clonotypes, allowing more ‘shots’ on multiple tumor targets?
Ultimately, it is highly encouraging that the design of clinical trials for GBM is resulting in the collection of tumors before and after treatment. The next step is to figure out how to collect GBM post-treatment at more than one time point. This is a huge challenge because of ethical/regulatory, financial, and technical concerns. Yet, only this type of approach can permit understanding the longitudinal evolution of the complexity of the GBM microenvironment in response to the therapies that we design against it Fig.1.
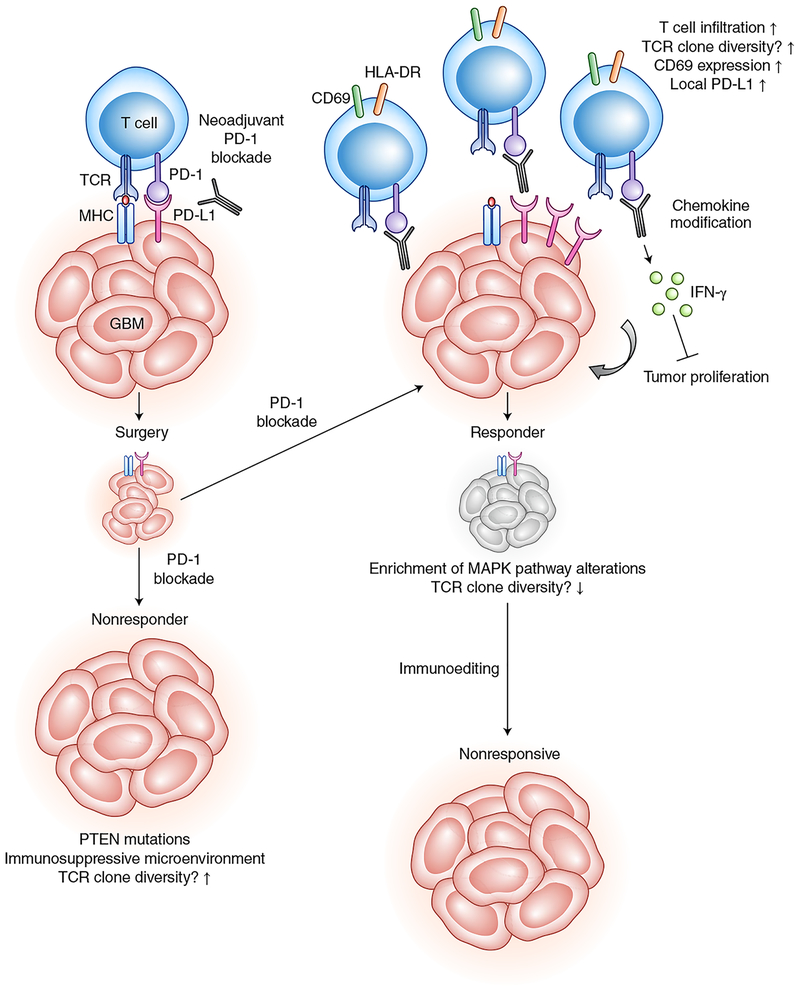
Fig. 1 |. The molecular responses of GBM to checkpoint blockade.
GBM cells blockade T cell activity by PD-1 and/or PD-L1 signaling. PD-1 blockade, shown in the figure as the gray antibodies, interrupts this signaling, leading to relief of T cell dysfunction. Administration of this therapy before surgery is known as neoadjuvant treatment. Surgical treatment enhances activated T cell infiltration into tumors. Examining continued PD-1 blockade treatment after surgery, the studies in this issue show that therapy-responding GBMs are associated with molecular and cellular markers that are different from those of nonresponsive GBMs. However, over time, even responsive GBMs become nonresponsive because of ongoing immunoediting in which tumor antigens are selected against. TCR, T cell receptor; MHC, major histocompatibility complex.
Footnotes
Competing interests
E.A.C is currently an advisor to Advantagene Inc., Alcyone Biosciences, Insightec, Inc., Sigilon Therepeutics and DNAtrix Inc. and has equity interest in DNAtrix; he has also advised Oncorus, Merck, Tocagen, Ziopharm, Stemgen, NanoTx., Ziopharm Oncology, Cerebral Therapeutics, Genenta, Merck, Janssen, Karcinolysis, and Shanaghai Biotech. He has received research support from the National Institutes of Health, the US Department of Defense, American Brain Tumor Association, National Brain Tumor Society, Alliance for Cancer Gene Therapy, Neurosurgical Research Education Foundation, Advantagene, NewLink Genetics, and Amgen. He also is a named inventor on patents related to oncolytic HSV1.
References
- 1.Ostrom QT et al. Neuro. Oncol 20, iv1–iv86 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nam JY & de Groot JF J. Oncol. Pract 13, 629–638 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Chiocca EA, Nassiri F, Wang J, Peruzzi P & Zadeh G Neuro. Oncol 21, 14–25 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kamiya-Matsuoka C & Gilbert MR CNS Oncol 4, 91–104 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyck L & Mills KH G. Eur. J. Immunol 47, 765–779 (2017). [DOI] [PubMed] [Google Scholar]
- 6.Zhao J Nat. Med 10.1038/s41591-019-0349-y (2019). [DOI] [Google Scholar]
- 7.Mooney KL et al. J. Clin. Neurosci 34, 1–5 (2016). [DOI] [PubMed] [Google Scholar]
- 8.Schalper KA Nat. Med 10.1038/s41591-018-0339-5 (2019). [DOI] [Google Scholar]
- 9.Cloughsey TF Nat. Med 10.1038/s41591-018-0337-7 (2019). [DOI] [Google Scholar]
- 10.Hegi ME et al. N. Engl. J. Med 352, 997–1003 (2005). [DOI] [PubMed] [Google Scholar]
- 11.Yan H et al. N. Engl. J. Med 360, 765–773 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keskin DB et al. Nature 565, 234–239 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kurz SC & Wen PY Curr. Treat. Options Neurol 20, 14 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Thomas AA et al. Cancer Immunol. Immunother 66, 379–389 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]