Abstract
Sarcomas are connective tissue tumors accounting for only 1% of all adult malignancies. Leiomyosarcoma (LMS) is a sarcoma arising from smooth muscle cells, and accounts for 10–20% of all sarcomas. A subtype of LMS are those originating from the smooth muscle of blood vessels. Leiomyosarcoma of the inferior vena cava is a sarcomatous tumor, with less than 350 cases described in the literature. It carries a poor prognosis, with 5- and 10-year survival rates of 31.4% and 7.4%, respectively. We present a case of a 46-year-old female with no significant past medical history presented to the emergency department with mild abdominal pain and distention, early satiety, and weight loss for three weeks, found to have unresectable metastatic leiomyosarcoma of the inferior vena cava.
Keywords: Leiomyosarcoma of the Inferior Vena Cava, Vascular Leiomyosarcoma, metastatic leiomyosarcoma, Inferior Vena Cava (IVC) tumors, Leiomyosarcoma, Sarcoma
CASE REPORT
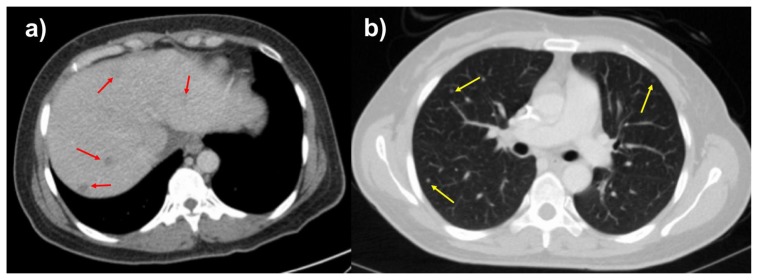
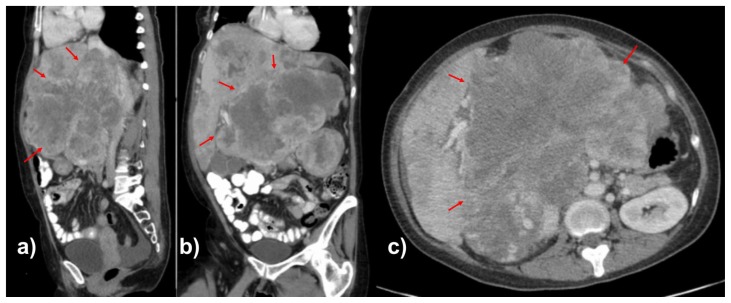
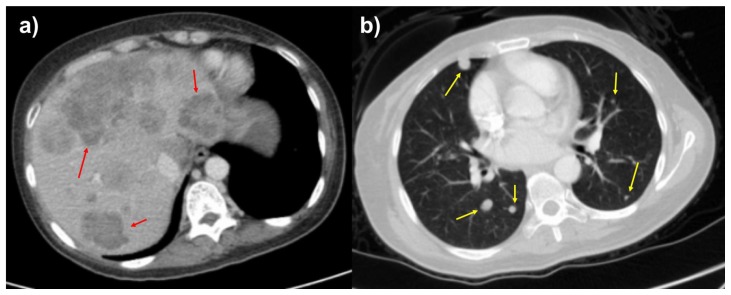
A 46-year-old female with no significant past medical history presented with a 3-week history of non-radiating, dull but progressively worsening right upper quadrant abdominal pain, as well as early satiety, nausea, vomiting, productive cough, and 15% unintentional weight loss. Contrast enhanced computed tomography (CT) was performed to assess for a cause of the patient’s symptoms. CT scan revealed a 15.4 cm heterogeneously enhancing mass, arising from the right retroperitoneum, although without clear origin of the mass. There was involvement the sub-hepatic inferior vena cava as well as mass effect and anterior displacement of upon adjacent solid organs (Figure 1). Additional imaging findings included several hypoenhancing liver lesions and multiple solid pulmonary nodules, consistent with hepatic and pulmonary metastases (Figure 2).
Figure 1.
46-year-old female with leiomyosarcoma of the IVC, initial scan.
FINDINGS: Contrast enhanced CT scan of the abdomen in the portal venous phase in the sagittal (1a), coronal (1b), and axial (1c) planes demonstrate heterogeneously enhancing mass in the retroperitoneum involving the subhepatic IVC with significant mass effect on the liver, right kidney, and surrounding vasculature. The mass measures up to 15.4 cm in the largest dimension.
TECHNIQUE: Axial CT with sagittal and coronal reconstructions, 158 mAs, 120 kV, 3 mm slice thickness, 80 mL Omnipaque 350 intravenous contrast.
Figure 2.
46-year-old female with leiomyosarcoma of the IVC, initial scan.
FINDINGS: Axial contrast enhanced of the abdomen in the portal venous phase (2a) and CT of the chest in the arterial phase (2b) demonstrate hypoattenuating, hypoenhancing, well-circumscribed liver lesions and solid, well-circumscribed, randomly distributed lung nodules suspicious for metastases. The largest liver lesion measures up to 1.0 cm, and the largest pulmonary nodule measures 0.5 cm.
TECHNIQUE: Axial CT with sagittal and coronal reconstructions, 158 mAs (Figure 2a), 181 mAs (figure 2b), 120 kV, 3 mm slice thickness, 80 mL Omnipaque 350 intravenous contrast.
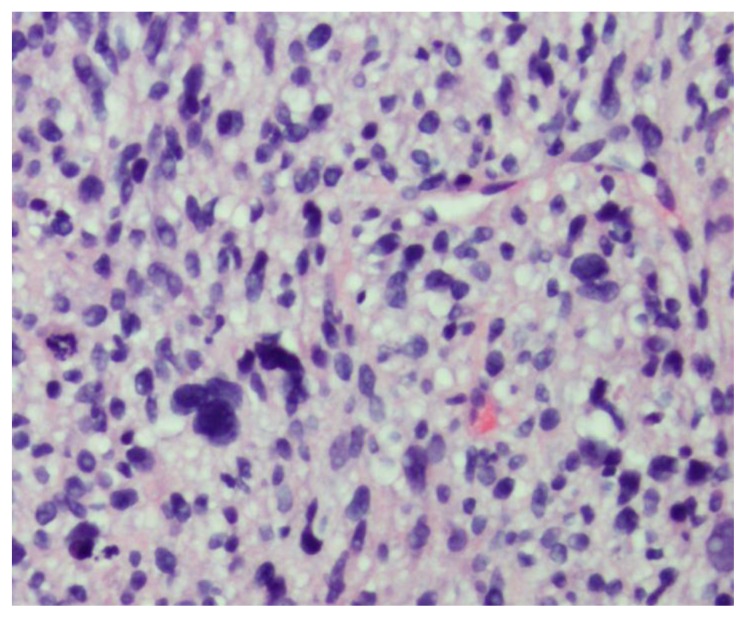
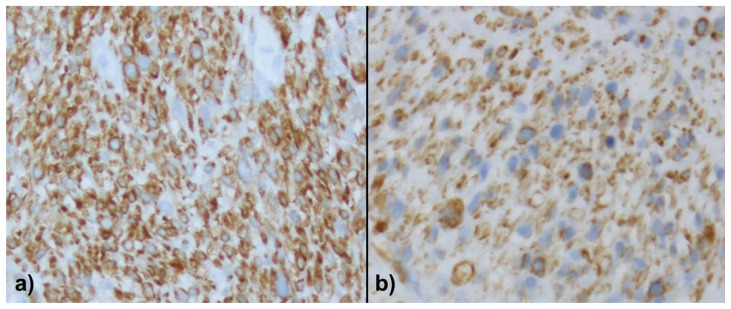
Due to the uncertain origin of the mass and nonspecific imaging findings, a CT guided biopsy was performed for pathological diagnosis. Histologic analysis revealed malignant proliferation of spindle cells with frequent pleomorphic nuclei, frequent mitoses, and areas of necrosis (Figure 3). Immunohistochemical stains were diffusely positive for vimentin, calponin, and caldesmon, weakly positive for actin, and partially positive for desmin expression (Figure 4). The constellation of histologic features, combined with imaging findings of a retroperitoneal mass involving the IVC, a diagnosis of primary leiomyosarcoma of the inferior vena cava (IVC) was rendered.
Figure 3.
46-year-old female with leiomyosarcoma of the IVC, initial biopsy. A histologic specimen of the IVC heterogeneous tumor on Hematoxillin and Eosin stain. The tumor is composed of spindle cells with eosinophilic cytoplasm arranged in intersecting fascicles. There is marked nuclear pleomorphic and frequent mitotic activity.
Figure 4.
46-year-old female with leiomyosarcoma of the IVC, initial biopsy. A histologic specimen of the IVC heterogeneous tumor on calponin (a) and caldesmon (b). The tumor is strongly and diffusely immunoreactive for calponin (a) and diffusely stained by caldesmon (b) demonstrating the smooth muscle differentiation.
Due to the presence of metastatic disease at initial diagnosis, surgical resection was deferred, and the patient was referred to medical oncology for chemotherapy. Several chemotherapy regimens were employed, with several lines of treatment. Chemotherapy included four cycles of MAI (ifosfamide, doxorubicin, and mesna), three cycles of docetaxel, gemcitabine, and olaratumab, oral pazobanib, and six cycles of trabectedin. Despite several lines and cycles of chemotherapy, there was continued disease progression (Figures 5–6). Ultimately, hospice care was recommended with palliative dacarbazine. The patient survived for 29 months after initial diagnosis.
Figure 5.
48-year-old female with leiomyosarcoma of the IVC, two-year follow-up scan.
FINDINGS: Contrast enhanced CT scan of the abdomen in the portal venous phase in the sagittal (5a), coronal (5b), and axial (5c) planes demonstrate worsening heterogeneously enhancing mass in the retroperitoneum involving the subhepatic IVC with significant mass effect on the liver, kidneys, bowel, and surrounding vasculature. The mass measures up to 20.0 cm (initially 15.4 cm) in the largest dimension.
TECHNIQUE: Axial CT with sagittal and coronal reconstructions, 151 mAs, 120 kV, 3 mm slice thickness, 80 mL Omnipaque 350 intravenous contrast and 300 mL of Gastroview oral contrast.
Figure 6.
48-year-old female with leiomyosarcoma of the IVC, two-year follow-up scan.
FINDINGS: Axial contrast enhanced two-year follow-up CT of the abdomen in the portal venous phase (6a) and CT of the chest in the arterial phase (6b) demonstrate worsening liver and lung metastases. The largest liver lesion measures up to 9.0 cm (initially 3.0 cm), and the largest pulmonary nodule measures up to 1.2 cm (initially 0.5 cm).
TECHNIQUE: Axial CT with sagittal and coronal reconstructions, 151 mAs (Figure 6a), 133 mAs (figure 6b), 120 kV, 3 mm slice thickness, 80 mL Omnipaque 350 intravenous contrast and 300 mL of Gastroview oral contrast.
DISCUSSION
Etiology & Demographics
Sarcomas are connective tissue tumors accounting for only 1% of all adult malignancies [1]. Leiomyosarcoma (LMS) is a sarcomatous tumor accounts for 10–20% of all sarcomas, which arises from smooth muscle cell lineage and can affect various organs, such as the skin, gastrointestinal tract, uterus, and blood vessels [2–4]. Vascular leiomyosarcomas are LMS arising from blood vessel walls and represent 1–2% of all LMS, rarely affecting the IVC [5]. In fact, LMS of the IVC accounts for only 5% of all vascular LMS with less than 350 cases described in the literature [1–4]. These vascular LMS tumors are generally chemotherapy resistant and carry a poor prognosis. LMS of the IVC has a higher female incidence (3:1) and an average age of 54 years at the time of diagnosis [4–7].
When originating in veins, including the IVC, LMS typically forms in the tunica media and can have different growth patterns: extraluminal, intraluminal, or combined [7]. For surgical planning, LMS of the IVC is usually classified based on the location of the primary tumor into three segments, describing the location and extension of the tumor [8]. Segment I am classified as an infrarenal extension; segment II spares the suprahepatic veins but includes the inter- and suprarenal veins; and segment III includes the suprahepatic veins with possible cardiac extension, which carries the worst prognosis [7, 8].
Clinical & Imaging Findings
The most common presenting symptom reported in the literature is nonspecific abdominal pain among a variety of other symptoms and clinical presentations, such as Budd-Chiari syndrome, lower extremity edema, hepatomegaly, ascites, and jaundice secondary to hepatic metastases [1, 2]. In the later stages of the disease, metastases occur in less than 50% of patients with LMS of the IVC, commonly in the liver, lung, lymph nodes, or bone [1]. Pathologic examination showing fascicles of spindle cells with hyperchromatic nuclei and characteristic immunohistochemical stain pattern is necessary for appropriate diagnosis, with the use of imaging aiding in management [3, 9]. Establishment of LMS of the IVC as the site of origin requires an evaluation of the size, the pattern of growth, and the extent of luminal narrowing of the IVC on cross sectional images, e.g., CT or MRI. On CT, LMS of the IVC usually appears as heterogeneously enhancing mass with central necrosis in the retroperitoneum involving the perihepatic IVC, sometimes with mass effect on surrounding organs. CT is also helpful to show internal calcification secondary to necrosis, although this is an uncommon feature. MRI of LMS usually demonstrates a soft tissue mass involving the IVC, which is homogeneously hypointense on T1, intermediate T2 signal intensity, and progressive avid enhancement on post-contrast T1 sequence. MRI is particularly helpful is assessing intraluminal tumors, which may be difficult to delineate on CT, especially on unenhanced CT. MRI sequences can differentiate T2 hypointense patent blood vessels from the higher signal intensity solid tumor. Because these tumors enhance, both CT and MR are useful in delineating tumor from bland thrombus. On ultrasound (US), LMS of the IVC appears as heterogeneous hypoechoic retroperitoneal mass with internal color flow [10]. Imaging also guides surgical and medical management, as it allows for tumor characterization, tumor localization, and evaluating for metastases.
Treatment & Prognosis
Normative treatment of LMS of the IVC has been wide and complete surgical resection of the IVC, with or without adjuvant chemotherapy [3, 5, 11]. Kieffer et al. reported a mean follow-up period of 43.7 months before death after surgical resection due to local recurrence and/or distant metastasis [2].
In general, LMS of the IVC is considered to be chemo-resistant malignancy without significant clinical data on the value of post-operative chemotherapy or radiation after surgical resection [3, 4, 5, 11]. When surgical resection is not indicated due to the presence of metastatic disease or large tumor size, the goal of treatment is to prolong survival, control the symptoms, and prevent further progression [1]. For patients who are deemed unresectable, chemotherapy is the mainstay of treatment. Traditional first line therapy includes gemcitabine and docetaxel, with doxorubicin and ifosfamide as two widely accepted alternatives [4, 11–12]. More recent studies have shown gemcitabine-docetaxel, trabectedin, dacarbazine, or pazopanib to have superior activity compared to ifosfamide in prolonging survival [10]. The 5-year and 10-year survival rates after surgical resection have been reported to be 31.4% and 7.4%, respectively, with near 0% 5-year survival rate observed with incomplete resection and inoperable cases that received chemotherapy and/or radiation [2, 3].
Differential Diagnosis
LMS of the IVC is a rare tumor that can mimic or be mistaken for many other retroperitoneal pathologies [13, 14]. Examples of solid organ tumors that can mimic LMS of the IVC are renal nephroblastoma (Wilms tumor), which originates from the kidney, or adrenal neuroblastoma, which tends to displace the kidney inferiorly, cross midline, and displace the abdominal aorta anteriorly. Both entities are commonly seen in pediatric patients as heterogeneously enhancing retroperitoneal mass on CT and MRI [15]. In adults, hilar cholangiocarcinoma is a peritoneal malignancy of the liver that can extend into the retroperitoneum and mimic LMS of the IVC, which appears on CT as a hypoenhancing liver mass, with associated intrahepatic ductal dilatation [16]. The duodenum is a retroperitoneal visceral organ with proximity to the IVC, which can be affected by a variety of benign and malignant processes; e.g., epithelial, mesenchymal, smooth muscle, lymphoproliferative, and neuroendocrine tumors. The predominant presenting symptoms of duodenal malignancy are abdominal pain, weight loss, bowel obstruction, nausea and vomiting [17]. Adrenal pheochromocytoma is also a retroperitoneal solid organ tumor presents as liable blood pressure and headache, and can be seen on CT or MRI images as avidly enhancing solid and cystic mass (low density on CT and T2 hyperintense signal on MRI) with central hemorrhage, necrosis, and calcification [18].
Non-solid organ tumors of the retroperitoneum may be benign or malignant, and are classified by their respective cell lineage, including: mesodermal, neurogenic, germinal, or lymphoid origins [14]. Lipomatosis and Erdheim-Chester disease (a non-Langerhans form of histiocytosis) are examples of mesodermal retroperitoneal non-solid organ tumors. Lipomatosis may lead to asymptomatic abdominal distention. On CT, lipomatosis shows fatty proliferation with fibrous strands appearance on CT surrounding anatomic structures in the pelvis. On MRI, retroperitoneal lipomatosis demonstrates signal loss on fat suppression sequences. Cystic teratoma is an asymptomatic germ cell tumor, which appears on CT as a complex cystic mass, consisting of fat and calcification on CT, US, and MRI [19]. Lymphangiomatosis, or invasive lymphangioma, is an asymptomatic retroperitoneal nonenhancing cystic mass with thick septation and minimal calcifications that can extend along tissue planes on CT with T2 hyperintense signal on MRI [20]. On the other hand, extrapulmonary lymphangioleiomyomatosis (LAM), commonly seen in patients with history of tuberous sclerosis, is a lymphatic obstruction caused by the proliferation of smooth muscle cells that can affect lungs, kidneys, and retroperitoneal lymph nodes leading to a formation of a retroperitoneal homogenously hypoattenuating cystic mass encasing the IVC without invasion on CT, and T2 hyperintense signal on MRI with normal flow void of the IVC [21]. Extramedullary hematopoiesis is hematopoietic tissue outside of bone marrow appears on CT imaging as a paravertebral hyperattenuating or isoattenuating round or lobulated mass, which can be confirmed with liver-spleen sulfur colloid nuclear medicine scan [22].
Several malignant non-solid organ tumors of the retroperitoneum are also included in the differential diagnosis. Liposarcoma, myxoid liposarcoma, and lipomyosarcoma are the most common types of retroperitoneal soft tissue sarcomas that are usually asymptomatic and appears as lipid rich lesions similar to lipomatosis but with mural enhancement on CT and MRI [23–27]. Malignant fibrous histiocytoma is the third most common retroperitoneal sarcomas, usually asymptomatic and appears on CT and MRI as an infiltrating, avidly enhancing soft-tissue mass with areas of necrosis and hemorrhage, and invasion of adjacent organs [28, 29]. Other uncommon sarcomas of the retroperitoneum are rhabdomyosarcoma, which is a pediatric mesenchymal tumor with intense enhancement on CT and MRI; extra skeletal chondrosarcoma of the retroperitoneum, which is a rare tumor usually seen on CT and MRI as a heterogeneously dense mass with arc type of calcifications; and synovial cell sarcoma—a rare tumor presents as hypodense soft tissue mass on CT, T2 hyperintense on MRI, and tends to occur near major joints [30–32].
Malignant vascular tumors of the retroperitoneum, besides LMS of the IVC, are angiosarcoma and perivascular epithelioid cell tumor, also known as PEComa. Angiosarcoma involves the endothelial layer of vessels causing expansion and hypodense, hypoenhancing filling defect on CT that can mimic a bland thrombus [33–35]. The major MRI features of angiosarcoma are T1 isointense to hyperintense signal and T2 hyperintense signal with minimal enhancement. The presence of high-flow serpentine vessels (T2 flow void signal on MRI) in a vascular-based soft tissue mass is highly specific for angiosarcoma [35]. PEComa, such as clear cell and myomelanocytic tumors, pigmented melanotic tumors, and sarcoma of perivascular cells are perivascular tumors that can develop around a vascular lumen with variable heterogeneously enhancing CT and MRI appearance [36–38].
Neurogenic tumors are asymptomatic tumors that can mimic masses of the retroperitoneum [39]. Schwannoma appears as solid well-circumscribed low density minimally enhancing mass on CT and MRI [40, 41]. Neurofibroma and plexiform neurofibroma are hypoenhancing retroperitoneal masses encasing the surrounding vessels on CT, which are commonly seen in type 1 neurofibromatosis [42, 43]. Malignant nerve sheath tumors, ganglioneuroma, and paraganglioma are usually symptomatic tumors present as dull flank or abdominal pain. On CT, they appear as well-margined hypodense, hypoenhacning retroperitoneal masses with internal feeding vessels [44–46].
Isolated or mixed germ cell-sex cord-stromal tumors of the retroperitoneum such as seminoma, malignant teratoma, embryonal carcinoma, yolk sac tumor, choriocarcinoma, and mixed germ cell tumor are frequently asymptomatic tumors with nonspecific imaging findings on CT, usually appear as solid hypoattenuating, heterogeneously enhancing masses with cystic changes and retroperitoneal lymphadenopathy on contrast enhanced CT or MRI [47–49].
Retroperitoneal fibrosis and retroperitoneal lymphoma can share similar features with LMS of the IVC. Retroperitoneal fibrosis is collagen vascular disease that is generally idiopathic, thought to be autoimmune-mediated, and can appear as a para-aortocaval hypoattenuating homogenously hypoenhancing soft tissue mass, encasing the surrounding vasculature, with associated minimal aortic displacement, medial deviation of the ureters, vascular thrombus, and obstructive uropathy [50–52]. The most common presenting symptoms for retroperitoneal fibrosis are back pain, abdominal pain, fatigue, fever, high erythrocyte sedimentation rate, and proteinuria, and the treatment is usually steroids, disease-modifying antirheumatic drugs, tamoxifen, or surgery [53, 54]. Retroperitoneal lymphoma can present as abdominal pain, fatigue, abdominal swelling, fever, high erythrocyte sedimentation rate, or anemia, and CT features of retroperitoneal lymphoma are enhancing soft tissue with relatively smooth margins, marked anterior displacement of the aorta, lymphadenopathy, and splenomegaly [55]. Zhang et al. reported that the most specific CT finding for retroperitoneal fibrosis is medial deviation of the ureters, while the most specific imaging finding for retroperitoneal lymphoma is the presence of lymphadenopathy, followed by splenomegaly, and anterior aortic displacement [50].
TEACHING POINT
Leiomyosarcoma of the inferior vena cava is a rare subtype of soft tissue sarcoma, with only a few hundred cases described in the literature. These tumors carry a poor prognosis, particularly when surgical resection is not possible. Leiomyosarcoma of the IVC is typically resistant to chemotherapy and radiation with palliative chemotherapy employed solely to prolong survival.
Table 1.
Differential diagnosis table for Leiomyosarcoma of the IVC.
| Diagnosis | Symptoms | CT findings | Additional Imaging Features |
|---|---|---|---|
| Leiomyosarcoma of the IVC | Right upper quadrant abdominal pain. | Heterogenous mass arising from the IVC; small tumors are solid, larger tumors may show necrosis or hemorrhage. | T1 hypointense signal, T2 intermediate signal, and progressive avid enhancement on MRI. Heterogeneous mass with internal color flow on US. |
| Adrenal Pheochromocytoma | Liable blood pressure and headache. | Heterogeneously enhancing mass arising from the adrenal gland with central cystic changes, necrosis, and calcifications. | T2 hyperintense signal, T1 variable signal (hyperintense if hemorrhagic), and heterogeneous enhancement. |
| Extrapulmonary Lymphangioleiomyoma (LAM) | Pulmonary symptoms due to lung involvement, history of tuberous sclerosis. | Homogenously hypoattenuating hypoenhancing cystic mass encasing the IVC, without invasion of the IVC wall or mucosa. | T1 hypointense signal, T2 hyperintense signal and low enhancement on MRI. |
| Extramedullary Hematopoiesis | History of anemia or sickle cell disease. | Paravertebral iso- or hyperenhancing round or lobulated mass. | Intense uptake on liver-spleen sulfur colloid nuclear medicine scan. |
| Retroperitoneal Liposarcoma | Usually asymptomatic. | Heterogeneously hypodense due to fat content. Dedifferentiated tumors may have calcification (in up to 30% of cases). | T1 high signal, T2 intermediate signal, and loss of signal on fat suppression sequences on MRI. |
| Retroperitoneal Malignant Fibrous Histiocytoma | Usually asymptomatic. | Infiltrating, heterogeneously enhancing soft-tissue mass with areas of necrosis, hemorrhage, and invasion of adjacent organs. | T1 hyperintense if hemorrhagic, T2 hypointense signal, and avid enhancement on MRI. |
| Angiosarcoma of the IVC | Usually asymptomatic. | Low attenuation with no appreciable enhancement on CT, which can mimic an intraluminal thrombus of the great vessels: pulmonary vessels, IVC, or the aorta. | T1 isointense to hyperintense and T2 hyperintense with minimal enhancement on MRI. The presence of internal serpentine vessels (T2 hypointense signal on MRI) is characteristic for this tumor. |
| PEComa of the IVC | Usually asymptomatic. | Perivascular tumors that can develop around a vascular lumen with variable CT appearance. | Variable T1 and T2 signal with post contrast enhancement on MRI. |
| Retroperitoneal Neurogenic Tumors | Dull flank or abdominal pain. | Hypoenhancing or hyperenhancing paravertebral soft tissue lesions. | T1 hyperintense, T2 hypointense, with avid enhancement on MRI. |
| Retroperitoneal Germ Cell Tumors | Usually asymptomatic. | Heterogeneously hypoenhancing masses with cystic changes. | Variable T1 and T2 signal with heterogeneous enhancement on MRI. |
| Retroperitoneal Lymphoma | Abdominal pain, fatigue, fever, high erythrocyte sedimentation rate, and anemia. | Poorly defined homogeneous infiltrating mass, with mild enhancement, encasing vasculature. | T1 isointense and T2 hypointense infiltrating mass with avid enhancement and restricted diffusion on MRI. |
| Retroperitoneal Fibrosis | Back pain, abdominal pain, fatigue, fever, high erythrocyte sedimentation rate, and proteinuria. | Mildly enhancing soft tissue mass encasing the aorta, and sometimes ureters, and cause ureteral obstruction and venous thrombosis of the IVC. | T1 isointense and T2 hypointense mass encasing vasculature and retroperitoneal structures on MRI. |
Table 2.
Summary table of Leiomyosarcoma of the IVC.
| Etiology | No known cause. The majority of reported cases in the literature are sporadic. |
| Incidence | LMS of the IVC accounts for 5% of all vascular LMS. Vascular LMS tumors represent about 1–2% of all LMS, which accounts for 10–20% of all sarcomas. Sarcoma represents 1% of all adult malignancies. |
| Symptoms | Most patients present with nonspecific right upper quadrant pain. |
| Gender and Age | 3:1 female to male ratio with a mean age of 54 years. |
| Treatment | Surgical treatment is preferred if localized to the wall of the IVC. Chemotherapy is the mainstay of treatment for cases with metastatic disease. |
| Prognosis | The 5-year and 10-year survival rates after surgical resection have been reported to be 31.4% and 7.4%, respectively, with 0% survival observed with incomplete resection or inoperable cases, with or without the chemoradiation therapy. |
| Findings on Imaging |
On CT, LMS of the IVC usually appears as heterogeneously enhancing mass with central necrosis and calcification in the retroperitoneum involving the perihepatic IVC, sometimes with mass effect on surrounding organs. On MRI, LMS of the IVC usually demonstrates a soft tissue mass involving the IVC, which is homogeneously hypointense on T1, intermediate T2 signal intensity, and progressive avid enhancement on post-contrast T1 sequence. On US, LMS of the IVC appears as heterogeneous hypoechoic retroperitoneal mass with internal color flow. |
ABBREVIATIONS
- CT
Computed tomography
- IVC
Inferior vena cava
- LAM
Lymphangioleiomyoma
- LMS
Leiomyosarcoma
- MRI
Magnetic resonance imaging
- US
Ultrasound
REFERENCES
- 1.Hasheminasab Zavareh R, Riahi Beni H, Iranpour A, Alam Samimi M, Sadeghipour A, Alavi Niakou SN. Leiomyosarcoma of Inferior Vena Cava and Right Atrium with Ascites and Jaundice: A Case Report. Int J Hematol Oncol Stem Cell Res. 2016;10(4):232–5. [PMC free article] [PubMed] [Google Scholar]
- 2.Kieffer E, Alaoui M, Piette JC, Cacoub P, Chiche L. Leiomyosarcoma of the inferior vena cava: experience in 22 cases. Ann Surg. 2006;244(2):289–95. doi: 10.1097/01.sla.0000229964.71743.db. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Teixeira FJR, Jr, do Couto Netto SD, Perina ALF, Torricelli FCM, Ragazzo Teixeira L, Zerati AE, et al. Leiomyosarcoma of the inferior vena cava: Survival rate following radical resection. Oncology letters. 2017;14(4):3909–16. doi: 10.3892/ol.2017.6706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin-Liberal J. Leiomyosarcoma: Principles of management. Intractable & rare diseases research. 2013;2(4):127–9. doi: 10.5582/irdr.2013.v2.4.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monteagudo Cortecero J, Guirau Rubio MD, Paya Roma A. Leiomyosarcoma of the inferior vena cava: AIRP best cases in radiologic-pathologic correlation. Radiographics : a review publication of the Radiological Society of North America, Inc. 2015;35(2):616–20. doi: 10.1148/rg.352130105. [DOI] [PubMed] [Google Scholar]
- 6.Lee HM, Jeong DS, Park PW, Kim WS, Sung K, Lee YT. Surgical treatment for an invasive leiomyosarcoma of the inferior vena cava. The Korean journal of thoracic and cardiovascular surgery. 2013;46(5):373–6. doi: 10.5090/kjtcs.2013.46.5.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brewer K, Attalla K, Husain F, Tsao C-K, Badani KK, Sfakianos JP. Leiomyosarcoma of the Inferior Vena Cava With Kidney Invasion. Urology Case Reports. 2016;9:33–6. doi: 10.1016/j.eucr.2016.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chetty R, Kalimuthu SN, Heinonen HR. Primary inferior vena cava smooth muscle tumor with diffuse bizarre giant nuclei and low mitotic rate: a nomenclatural conundrum. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2017;30:1–5. doi: 10.1016/j.carpath.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 9.Filho W, Melo SMC, de Brito Alves R, Junior LAS, da Fonseca Reis Silva D. Retromammary fat, axillary and arm metastases from a retroperitoneal leiomyosarcoma: report of a case with an indolent behaviour. Ecancermedicalscience. 2017;11:778. doi: 10.3332/ecancer.2017.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sessa B, Iannicelli E, Caterino S, D’Angelo F, Milione M, Ziparo V, et al. Imaging of leiomyosarcoma of the inferior vena cava: comparison of 2 cases and review of the literature. Cancer imaging : the official publication of the International Cancer Imaging Society. 2010;10(1):80–4. doi: 10.1102/1470-7330.2010.0009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cananzi FC, Mussi C, Bordoni MG, Marrari A, De Sanctis R, Colombo P, et al. Role of surgery in the multimodal treatment of primary and recurrent leiomyosarcoma of the inferior vena cava. Journal of surgical oncology. 2016;114(1):44–9. doi: 10.1002/jso.24244. [DOI] [PubMed] [Google Scholar]
- 12.Hensley ML, Maki R, Venkatraman E, Geller G, Lovegren M, Aghajanian C, et al. Gemcitabine and docetaxel in patients with unresectable leiomyosarcoma: results of a phase II trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2002;20(12):2824–31. doi: 10.1200/JCO.2002.11.050. [DOI] [PubMed] [Google Scholar]
- 13.Abdullah A, Williamson K, Lewis T, Elsamaloty H. Variant ventral intrahepatic course of inferior vena cava: volume-rendering and maximum intensity projection CT findings. The British journal of radiology. 2011;84(1003):e135–7. doi: 10.1259/bjr/51830082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rajiah P, Sinha R, Cuevas C, Dubinsky TJ, Bush WH, Jr, Kolokythas O. Imaging of uncommon retroperitoneal masses. Radiographics : a review publication of the Radiological Society of North America, Inc. 2011;31(4):949–76. doi: 10.1148/rg.314095132. [DOI] [PubMed] [Google Scholar]
- 15.Dumba M, Jawad N, McHugh K. Neuroblastoma and nephroblastoma: a radiological review. Cancer imaging : the official publication of the International Cancer Imaging Society. 2015;15(1):5. doi: 10.1186/s40644-015-0040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olthof SC, Othman A, Clasen S, Schraml C, Nikolaou K, Bongers M. Imaging of Cholangiocarcinoma. Visceral medicine. 2016;32(6):402–10. doi: 10.1159/000453009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bal A, Joshi K, Vaiphei K, Wig JD. Primary duodenal neoplasms: a retrospective clinico-pathological analysis. World journal of gastroenterology. 2007;13(7):1108–11. doi: 10.3748/wjg.v13.i7.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herr K, Muglia VF, Koff WJ, Westphalen AC. Imaging of the adrenal gland lesions. Radiologia brasileira. 2014;47(4):228–39. doi: 10.1590/0100-3984.2013.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SB, Cho KS, Kim JK. CT findings of mature cystic teratoma with malignant transformation: comparison with mature cystic teratoma. Clinical imaging. 2011;35(4):294–300. doi: 10.1016/j.clinimag.2010.08.016. [DOI] [PubMed] [Google Scholar]
- 20.Wunderbaldinger P, Paya K, Partik B, Turetschek K, Hormann M, Horcher E, et al. CT and MR imaging of generalized cystic lymphangiomatosis in pediatric patients. AJR American journal of roentgenology. 2000;174(3):827–32. doi: 10.2214/ajr.174.3.1740827. [DOI] [PubMed] [Google Scholar]
- 21.Avila NA, Dwyer AJ, Moss J. Imaging features of lymphangioleiomyomatosis: diagnostic pitfalls. AJR American journal of roentgenology. 2011;196(4):982–6. doi: 10.2214/AJR.10.4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Georgiades CS, Neyman EG, Francis IR, Sneider MB, Fishman EK. Typical and atypical presentations of extramedullary hemopoiesis. AJR American journal of roentgenology. 2002;179(5):1239–43. doi: 10.2214/ajr.179.5.1791239. [DOI] [PubMed] [Google Scholar]
- 23.Messiou C, Morosi C. Imaging in retroperitoneal soft tissue sarcoma. Journal of surgical oncology. 2018;117(1):25–32. doi: 10.1002/jso.24891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Messiou C, Moskovic E, Vanel D, Morosi C, Benchimol R, Strauss D, et al. Primary retroperitoneal soft tissue sarcoma: Imaging appearances, pitfalls and diagnostic algorithm. European journal of surgical oncology : the journal of the European Society of Surgical Oncology and the British Association of Surgical Oncology. 2017;43(7):1191–8. doi: 10.1016/j.ejso.2016.10.032. [DOI] [PubMed] [Google Scholar]
- 25.Marko J, Wolfman DJ. Retroperitoneal Leiomyosarcoma From the Radiologic Pathology Archives. Radiographics : a review publication of the Radiological Society of North America, Inc. 2018;38(5):1403–20. doi: 10.1148/rg.2018180006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheah K, Ouellette HA, Torriani M, Nielsen GP, Kattapuram S, Bredella MA. Metastatic myxoid liposarcomas: imaging and histopathologic findings. Skeletal radiology. 2008;37(3):251–8. doi: 10.1007/s00256-007-0424-1. [DOI] [PubMed] [Google Scholar]
- 27.Hong SH, Kim KA, Woo OH, Park CM, Kim CH, Kim MJ, et al. Dedifferentiated liposarcoma of retroperitoneum: spectrum of imaging findings in 15 patients. Clinical imaging. 2010;34(3):203–10. doi: 10.1016/j.clinimag.2009.12.025. [DOI] [PubMed] [Google Scholar]
- 28.Karki B, Xu YK, Wu YK, Zhang WW. Primary malignant fibrous histiocytoma of the abdominal cavity: CT findings and pathological correlation. World journal of radiology. 2012;4(4):151–8. doi: 10.4329/wjr.v4.i4.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hsiao PJ, Chen GH, Chang YH, Chang CH, Chang H, Bai LY. An unresectable retroperitoneal malignant fibrous histiocytoma: A case report. Oncology letters. 2016;11(4):2403–7. doi: 10.3892/ol.2016.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Naniwadekar RG, Vekariya MA, Kulkarni SR, Pednekar AS, Gupta V. Embryonal Rhabdomyosarcoma (RMS) of Retroperitoneum in Young Child. Journal of clinical and diagnostic research : JCDR. 2015;9(3):Pj03–4. doi: 10.7860/JCDR/2015/11933.5701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Taori K, Patil P, Attarde V, Chandanshive S, Rangankar V, Rewatkar N. Primary retroperitoneal extraskeletal mesenchymal chondrosarcoma: a computed tomography diagnosis. The British journal of radiology. 2007;80(959):e268–70. doi: 10.1259/bjr/13711118. [DOI] [PubMed] [Google Scholar]
- 32.Alhazzani AR, El-Sharkawy MS, Hassan H. Primary retroperitoneal synovial sarcoma in CT and MRI. Urology annals. 2010;2(1):39–41. doi: 10.4103/0974-7796.62916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noguchi G, Ota J, Ishigaki H, Onuki T, Kato Y, Moriyama M. [A case of retroperitoneal angiosarcoma effectively treated with recombinant interleukin-2]. Nihon Hinyokika Gakkai zasshi The japanese journal of urology. 2012;103(6):697–703. doi: 10.5980/jpnjurol.103.697. [DOI] [PubMed] [Google Scholar]
- 34.Scialpi M, Galasso C, Di Maggio A, Mancini A, Resta M, Angelelli G, et al. Primary retroperitoneal angiosarcoma: MR imaging features. European radiology. 2001;11(5):791–5. doi: 10.1007/s003300000635. [DOI] [PubMed] [Google Scholar]
- 35.Gaballah AH, Jensen CT, Palmquist S, Pickhardt PJ, Duran A, Broering G, et al. Angiosarcoma: clinical and imaging features from head to toe. The British journal of radiology. 90(1075) doi: 10.1259/bjr.20170039. 20170039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gkizas CV, Tsili AC, Katsios C, Argyropoulou MI. Perirenal PEComa: Computed Tomography Findings and Differential Diagnosis. Journal of clinical imaging science. 2015;5:69. doi: 10.4103/2156-7514.172977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sun L, Sun X, Li Y, Xing L. The role of (18)F-FDG PET/CT imaging in patient with malignant PEComa treated with mTOR inhibitor. OncoTargets and therapy. 2015;8:1967–70. doi: 10.2147/OTT.S85444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hekimoglu K, Haberal M. Liver Perivascular Epithelioid Cell Tumor with an Unusual Location: Diagnostic Characteristics with Multidetector Computed Tomography and Magnetic Resonance Imaging. Journal of clinical imaging science. 2017;7:36. doi: 10.4103/jcis.JCIS_43_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rha SE, Byun JY, Jung SE, Chun HJ, Lee HG, Lee JM. Neurogenic tumors in the abdomen: tumor types and imaging characteristics. Radiographics : a review publication of the Radiological Society of North America, Inc. 2003;23(1):29–43. doi: 10.1148/rg.231025050. [DOI] [PubMed] [Google Scholar]
- 40.Harada TL, Nagao G, Aoyagi T, Kuroda I, Tokuyama N, Takahashi M, et al. Giant retroperitoneal schwannoma in a 52-year-old man. Radiology case reports. 2018;13(4):810–4. doi: 10.1016/j.radcr.2018.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kalayc? M, Akyüz U, Demira? A, Gürses B, Ozkan F, Gökçe O. Retroperitoneal schwannoma: a rare case. Case reports in gastrointestinal medicine. 2011;2011 doi: 10.1155/2011/465062. 465062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bass JC, Korobkin M, Francis IR, Ellis JH, Cohan RH. Retroperitoneal plexiform neurofibromas: CT findings. AJR American journal of roentgenology. 1994;163(3):617–20. doi: 10.2214/ajr.163.3.8079855. [DOI] [PubMed] [Google Scholar]
- 43.Khandwala K, Sajjad Z, Abbasi SU, Tariq MU. Hepatic, Periportal, Retroperitoneal, and Mesenteric Neurofibromatosis in von Recklinghausen’s Disease. Cureus. 2018;10(2):e2248. doi: 10.7759/cureus.2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Otal P, Mezghani S, Hassissene S, Maleux G, Colombier D, Rousseau H, et al. Imaging of retroperitoneal ganglioneuroma. European radiology. 2001;11(6):940–5. doi: 10.1007/s003300000698. [DOI] [PubMed] [Google Scholar]
- 45.Yu YH, Wu JT, Ye J, Chen MX. Radiological findings of malignant peripheral nerve sheath tumor: reports of six cases and review of literature. World journal of surgical oncology. 2016;14:142. doi: 10.1186/s12957-016-0899-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji XK, Zheng XW, Wu XL, Yu ZP, Shan YF, Zhang QY, et al. Diagnosis and surgical treatment of retroperitoneal paraganglioma: A single-institution experience of 34 cases. Oncology letters. 2017;14(2):2268–80. doi: 10.3892/ol.2017.6468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Choyke PL, Hayes WS, Sesterhenn IA. Primary extragonadal germ cell tumors of the retroperitoneum: differentiation of primary and secondary tumors. Radiographics : a review publication of the Radiological Society of North America, Inc. 1993;13(6):1365–75. doi: 10.1148/radiographics.13.6.8290730. quiz 77–8. [DOI] [PubMed] [Google Scholar]
- 48.Pang S, Zhang L, Shi Y, Liu Y. Unclassified mixed germ cell-sex cord-stromal tumor with multiple malignant cellular elements in a young woman: a case report and review of the literature. International journal of clinical and experimental pathology. 2014;7(8):5259–66. [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y, Jiang J, Liu Q. Extragonadal malignant germ cell tumors: a clinicopathological and immunohistochemical analysis of 48 cases at a single Chinese institution. International journal of clinical and experimental pathology. 2015;8(5):5650–7. [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang S, Chen M, Li CM, Song GD, Liu Y. Differentiation of Lymphoma Presenting as Retroperitoneal Mass and Retroperitoneal Fibrosis: Evaluation with Multidetector-row Computed Tomography. Chinese medical journal. 2017;130(6):691–7. doi: 10.4103/0366-6999.201606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan N, Jiao Y. Non-Hodgkin lymphoma mimics retroperitoneal fibrosis. BMJ case reports. 2013:2013. doi: 10.1136/bcr-2013-010433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rosenkrantz AB, Spieler B, Seuss CR, Stifelman MD, Kim S. Utility of MRI features for differentiation of retroperitoneal fibrosis and lymphoma. AJR American journal of roentgenology. 2012;199(1):118–26. doi: 10.2214/AJR.11.7822. [DOI] [PubMed] [Google Scholar]
- 53.Liang B, Yin Z, Guo Q, Wei Y, Liu L, Yang J. Diagnosis and treatment of retroperitoneal fibrosis: A case report. Experimental and therapeutic medicine. 2013;5(4):1236–8. doi: 10.3892/etm.2013.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vaglio A, Maritati F. Idiopathic Retroperitoneal Fibrosis. Journal of the American Society of Nephrology : JASN. 2016;27(7):1880–9. doi: 10.1681/ASN.2015101110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ayari Y, Taktak T, Boussaffa H, Ghorbel Z, Zehani A, Sellami A, et al. Retroperitoneal extra-adrenal non-Hodgkin lymphoma: An uncommon presentation. Urol Case Rep. 2019;23:34–6. doi: 10.1016/j.eucr.2018.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]