Abstract
Objective
We aimed to investigate which clinical and metabolic tests offer optimal accuracy and acceptability to help diagnose diabetes among a large sample of people with serious mental illness in receipt of antipsychotic medication.
Methods
A prospective observational study design of biochemical and clinical factors was used. Biochemical measures were fasting glucose, insulin and lipids, oral glucose tolerance testing (OGTT), hemoglobin A1c, and insulin resistance assessed with the homeostatic model (HOMA-IR) were determined in a consecutive cohort of 798 adult psychiatric inpatients receiving antipsychotics. Clinical variables were gender, age, global assessment of functioning (GAF), mental health clinicians’ global impression (CGI), duration of severe mental illness, height, weight, BMI and waist/hip ratio. In addition, we calculated the risk using combined clinical predictors using the Leicester Practice Risk Score (LPRS) and the Topics Diabetes Risk Score (TDRS). Diabetes was defined by older criteria (impaired fasting glucose (IFG) or OGTT) as well as2010 criteria (IFG or OGTT or Glycated haemoglobin (HBA1c)) at conventional cut-offs.
Results
Using the older criteria, 7.8% had diabetes (men: 6.3%; women: 10.3%). Using the new criteria, 10.2% had diabetes (men: 8.2%, women: 13.2%), representing a 30.7% increase (p = 0.02) in the prevalence of diabetes. Regarding biochemical predictors, conventional OGTT, IFG, and HbA1c thresholds used to identify newly defined diabetes missed 25%, 50% and 75% of people with diabetes, respectively. The conventional HBA1c cut-point of ≥6.5% (48 mmol/mol) missed 7 of 10 newly defined cases of diabetes while a cut-point of ≥5.7% improved sensitivity from 44.4% to up to 85%. Specific algorithm approaches offered reasonable accuracy. Unfortunately no single clinical factor was able to accurately rule-in a diagnosis of diabetes. Three clinical factors were able to rule-out diabetes with good accuracy namely: BMI, waist/hip ratio and height. A BMI < 30 had a 92% negative predictive value in ruling-out diabetes. Of those not diabetic, 20% had a BMI ≥ 30. However, for complete diagnosis a specific biochemical protocol is still necessary.
Conclusions
Patients with SMI maintained on antipsychotic medication cannot be reliably screened for diabetes using clinical variables alone. Accurate assessment requires a two-step algorithm consisting of HBA1c ≥5.7% followed by both FG and OGTT which does not require all patients to have OGTT and FG.
Introduction
A rising population rate of overweight and obesity has contributed to a global diabetes epidemic, with harmful effects on mortality and morbidity worldwide [1]. Diabetes afflicts an estimated 382 million people and by 2035 this will rise to 592 million but many remain undiagnosed [2]. Diabetes implies a significant abnormality in glucose homeostasis with persistent hyperglycaemia. However, the exact definition of diabetes offered by expert committees has varied over time. In 1997, diabetes was defined by either an impaired fasting glucose (IFG) >125 mg/dL (≥7.0 mol/L) or a two-hour oral glucose tolerance test (OGTT) >199 mg/dL (>11 mmol/L). In 2010, Glycated haemoglobin (HBA1c) had been added to the qualifying criteria, such that diabetes mellitus can now be defined by one of three of the following: elevated fasting glucose (?7.0 mol/L), 2-hour OGTT (>11 mmol/L) or HBA1c (>6.4%; 46 mmol/mol)[3].Usually the abnormal test is repeated unless there is “unequivocal hyperglycemia” [3,4]. The addition of HbA1c reflects its contribution as an independent risk of morbidity and mortality [5]. Moreover, HBA1c is convenient, as it can be measured at any time of the day without fasting. However, it is unclear whether HbA1c is as good as blood glucose for predicting diabetes complications, such as retinopathy. Certainly, higher HBA1c is a risk for future diabetes. In a systematic review of 16 cohort studies with a follow-up interval averaging 5.6 years (range: 2.8–12 years), those with an HBA1c between 6.0 to 6.5% (42 mmol/mol—48 mmol/mol) had a 5-year risk of diabetes of 25% to 50% [6]. HBA1c has a modest-to-strong correlation with IFG and OGTT. For example, up to half of people with diabetes would not be diagnosed using HbA1c, and half of those diagnosed using HbA1c would not currently be diagnosed using IFG [7, 8]. The new definition of diabetes, which incorporates HBA1c, has increased the prevalence of diabetes in all populations,[9] but has never previously been studied in patients with severe mental illness (SMI), or those maintained on antipsychotic medications.
Observational studies have reported a clear association between patients with SMI and diabetes [10]. This is concerning as diabetes is associated with a reduced quality of life and increased mortality in people with SMI [11].The risk appears particularly severe in those maintained on antipsychotics, notably most second-generation antipsychotics [12, 13]. The risk conferred by the illness and/or antipsychotics extends to other components of the metabolic syndrome. For example, De Hert et al (2007) found that 27.8% of those started on second-generation antipsychotics had new (incident) metabolic syndrome (MetS) within three years, compared to 9.8% of those treated with first-generation antipsychotic agents [14]. In studies that have assessed metabolic abnormalities in drug-naïve, first-episode patients, some have found impaired glucose tolerance or insulin resistance but others found no appreciable effect and a recent meta-analysis found only modest abnormalities in metabolic syndrome or metabolic risk factors in drug-naïve patients (exceptions may be smoking, fitness and diet)[15]. Therefore, it is assumed that antipsychotics indirectly worsen glucose regulation by promoting obesity or directly by affecting glucose regulation through insulin resistance, [16] decreased secretion of glucose-dependent insulinotropic polypeptide (GIP), increased glucagon secretion, or by impairing beta cell function [17]. In addition, antipsychotics also contribute to dyslipidemia [18].
Despite these concerns about metabolic abnormalities in patients treated with antipsychotics, only a few studies examined screening procedures for diabetes/prediabetes in this population. In a modest sample of 100 patients, De Hert et al (2006) noted that a monitoring protocol based only on fasting glucose would detect only 63.6% of patients with glucose abnormalities. They suggested combining fasting glucose with fasting insulin [19]. This sample was later expanded to 415 patients and testing procedures were re-examined [20]. Against an OGTT definition of diabetes, IFG had a sensitivity of 46.2%, but a two-step procedure of impaired fasting glucose >100mg/dl and then an OGTT only for patients positive in step 1 gave a sensitivity of 96.2%, yet both retained 100% specificity. Manu et al (2012) used the new 2010 American Diabetes Association diagnostic criteria for prediabetes and found 37% of patients treated with antipsychotics met criteria[21].Among patients with prediabetes, HBA1c (5.7–6.4 mmol/mol) was the sole defining abnormality in 41%. Agarwal (2012) found that 48% of patients with schizophrenia had high HBA1c levels ≥ 5.7[22]. Only one study, however, has investigated HBA1c in diagnosing diabetes. Hanssens et al (2006) reported a limited value of HBA1c in diagnosing diabetes in those taking antipsychotics due to low sensitivity [23]. Since that time, the definition of diabetes has been updated and research is required to identify the role of HBA1c in diagnosing diabetes. Therefore we aimed to 1. fully examine the accuracy and clinical utility of HBA1c and other markers of glucose regulation in the diagnosis of diabetes in patients taking antipsychotics and 2. to develop an algorithm for clinicians to use in clinical practice to detect diabetes in mental health populations.
Whilst the addition of non-fasting HBA1c simplifies the biochemical diagnosis of diabetes in many non-specialist settings the diagnosis of diabetes remains a challenge due to the inconvenience of biochemical testing. Necessity for biochemical tests reduces the acceptability and uptake of testing for many clinicians and patients. The European evidence based guidelines for the prevention of type 2 diabetes[24] and the International Diabetes Federation[25] have recommend the use of simple risk scoring systems to identify people at high risk of future diabetes. However, these still rely on conventional testing. Recently, several groups have developed and tested the accuracy of clinical variables to diagnose diabetes (and to a lesser extent pre-diabetes) without recourse to blood tests. These could be valuable in clinical practice if they were sufficiently accurate. Usually these clinical variables have been combined in clinical prediction algorithms or risk models [26, 27, 28, 29, 30, 31].Abbasi et al (2012) reviewed 12 such models (an additional 13 required biochemical testing)[27]. Collins et al (2011) reviewed 43 risk prediction models of which 17 were purely clinical [24]. Noble et al (2012) evaluated 94 risk prediction models and identified 7 as being the most promising for adaptation and use in routine clinical practice [26]. Risk models vary from simple to complex. The majority have been validated in North American or European study populations. It has been suggested that simple models, derived from clinical history alone could be useful in clinical practice and could reduce the cost and inconvenience of screening. For example, the Diabetes Risk Calculator derived from the National Health and Nutrition Examination Survey (NHANES) III [32] includes questions about patient age, waist circumference, history of gestational diabetes, height, race/ethnicity, hypertension, family history, and exercise. Other models include the Atherosclerosis Risk in Communities (ARIC) risk calculator, the Australian Diabetes Risk Assessment Tool (AusDrisk), the Cambridge Risk Score, FINDRISC and CANRISK (see Table 1). Even clinical models vary in complexity of risk factors and scoring. Two particularly simple models may be suitable for application in mental health settings. These are the Leicester Practice Risk Score for Diabetes (LPRS) 33 and the Topics Diabetes Risk Score (TDRS) [34]. The accuracy of these clinical models is summarized in Table 1. One challenge of these models is to yield high clinical utility in the face of a relatively low prevalence of diabetes, typically 3–6% in the general population. The value of such tools is that they can potentially be used as a simpler form of screening which might increase the uptake of screening and ultimately reduce the incidence or complications of type 2 diabetes [35, 36, 37].
Table 1. Summary diabetic risk clinical models from general population studies.
| Study | Country | Model Description | Sensitivity | Specificity | Area under ROC | Sample Size | Youden |
|---|---|---|---|---|---|---|---|
| Prospective (incident cases) | |||||||
| Aekplakorn et al (2006) [65] | Thailand | Thai Risk: Age, sex, BMI, abdominal obesity (waist circumference), hypertension, family history of diabetes | 77 | 61.9 | 0.75 | 2420 | 0.389 |
| Balkau et al (2008) [66] | France |
Men: waist circumference, smoking status, hypertension Women: waist circumference, family history of diabetes, hypertension. |
50 | 74 | 66 | 3817 | 0.24 |
| Gao et al (2009) [67] | Mauritius | Qingdao Score:Age, sex, BMI, waist circumference, family history of diabetes | 74.5 | 48.5 | 0.63 | 3094 | 0.23 |
| Heianza et al (2013) [34] | Japan | TDRS: age, sex, family history of diabetes, current smoking habit, BMI, and hypertension | 72.7 | 68.1 | 0.77 | 33,335 | 0.408 |
| Kahn et al (2009) [68] | USA | Diabetes family history, hypertension, ethnicity, age, smoking status, waist circumference, height, resting pulse, weight | 69 | 64 | 0.71 | 3142 | 0.33 |
| Lindström et al (2003) [69] | Finland | FINDRISC: Age, BMI, waist circumference, use of blood pressure medication, history of high blood glucose, physical activity, daily consumption of vegetables | 78 | 77 | 0.85 | 4,435 | 0.55 |
| Robinson et al (2011) [70] | Canada | CANRISK: Age, BMI, waist circumference, use of blood pressure medication, history of high blood glucose, physical activity, daily consumption of vegetables | 70 | 67 | 0.75 | 1676 | 0.37 |
| Schulze et al (2007) [71] | Germany | German Diabetes Risk Score: Waist circumference, height, age, hypertension, diet, alcohol consumption, physical activity, former or current smoker | 83 | 68 | 0.83 | 25,167 | 0.51 |
| Cross-sectional (Prevalent Cases) | |||||||
| Al-Lawati et al (2007) [72] | Oman | Oman Diabetes Risk Score: Age, waist circumference, BMI, family history of diabetes, hypertension | 78.6 | 73.4 | 0.83 | 4881 | 0.52 |
| Baan et al (1999) [73] | The Netherlands | Rotterdam Score: Age, sex, use of antihypertensive medication, obesity (BMI ≥ 30) | 78 | 55 | 0.7 | 2364 | 0.33 |
| Bang et al (2009) [74] | USA | Patient Self-Assessment Score: Age, sex, family history of diabetes, history of hypertension, obesity (BMI or waist circumference), physical activity | 82 | 63 | 0.79 | 5258 | 0.45 |
| Gao et al (2010) [75] | China | Chinese Risk Score: Age, waist circumference, family history of diabetes | 89 | 27 | 0.64 | 6322 | 0.16 |
| Glümer et al (2004) [76] | Denmark | Danish Risk Score: Age, BMI, sex, known hypertension, physical activity, family history of diabetes | 76 | 72 | 0.81 | 6,784 | 0.48 |
| Gray et al (2010) [Addition cohort] [33] | UK | LPRS: age, ethnicity, sex, first degree family history of diabetes, hypertension, waist circumference and BMI | 81.1 | 41 | 0.69 | 6390 | 0.221 |
| Gray et al (2010) [Validation cohort] [33] |
UK | LPRS: age, ethnicity, sex, first degree family history of diabetes, hypertension, waist circumference and BMI | 91.5 | 32.4 | 0.72 | 3171 | 0.239 |
| Pires de Sousa et al (2009)[77] | Brazil | Brazilian Simple Prediction Model: Age, BMI, hypertension | 76 | 67 | 0.77 | 1224 | 0.43 |
| Ramachandran et al (2005)[78] | India | Age, family history of diabetes, BMI, waist circumference, physical activity | 76 | 59 | 0.73 | 10,003 | 0.35 |
Footer: Leicester Practice Risk Score (LPRS); Topics Diabetes Risk Score (TDRS); Finish Risk Score (FINDRISC), Canadian Risk Score (CANRISK)
Methods
Setting
In an observational study between November 2003 and July 2007, psychiatric patients were asked by their treating psychiatrist to agree to a standardized battery of tests to identify MetS and insulin resistance. All subjects gave written informed consent and the study was approved by the University Psychiatric Center’s Ethics Committee KU Leuven campus, Kortenberg.
Clinical and laboratory measurements
The procedure included measurements of height, weight, body mass index (BMI), waist circumference, arterial blood pressure, fasting blood glucose, insulin and lipids, Glycated hemoglobin (A1c), and a 2-hour oral glucose tolerance test (OGTT) after the ingestion of 75g of glucose. As described previously, all tests were performed in the same laboratory and using the same robust methods throughout the study period [13]. Fasting glucose, 2-hour postprandial glucose during OGGT and HBA1c data were used to define diabetes mellitus according to new and old (conventional) criteria. The older criteria were either a fasting glucose >125 mg/dl or a 2-hour postprandial glucose >199 mg/dL. The new criteria are a fasting glucose >125 mg/dL or a 2-hour postprandial glucose >199 mg/dLor an HBA1c ≥6.5% (48 mmol/mol); any of which should be repeated unless there is unequivocal hyperglycaemia. In addition, in a patient with classical symptoms of hyperglycaemia a random glucose >199 mg/dL is an acceptable test. The fasting glucose and insulin data were used for the homeostatic model assessment of insulin resistance (HOMA-IR) [38]. The weight and height were used to calculate the body mass index (BMI). The waist circumference, arterial blood pressure, and fasting glucose, triglycerides and high-density lipoprotein (HDL) cholesterol levels were also measured.
Psychiatric diagnoses were established according to DSM-IV by experienced psychiatrists who were qualified and familiar with the management of psychiatric patients taking antipsychotics. They were blinded to the study aims and affiliated with the University Psychiatric Center and responsible for the patient’s treatment. The treating psychiatrists assessed the severity of symptoms and rated them using the Global Assessment of Function (GAF) from 0 (worst) to 100 (best)[39] and the Clinical Global Impressions Severity (CGI-S) Scale from 1 (normal) to 7 (extremely ill)[40].
Diabetes modelling
Of the many clinical models available we chose to examine two popular models: the Leicester Practice Risk Score for Diabetes (LPRS) [30] and the Topics Diabetes Risk Score (TDRS) [31]. The LPRS encompasses the following risk factors: age, ethnicity, gender, first degree family history of diabetes, hypertension, waist circumference and BMI and has been validated in an ethnically diverse European population (sensitivity 91.5%, specificity 32.4, area under the curve 0.72). The TDRS includes age, sex, family history of diabetes, current smoking habit, BMI, and hypertension and has been validated in a Japanese sample and is currently the largest study of its type (sensitivity 72.7%, specificity 68.1%, area under the curve 0.77). In addition to these combination models, we examined the diagnostic accuracy of each individual clinical risk factor.
Inclusion and exclusion criteria
We included psychiatric patients without diabetes at baseline consecutively admitted to a single institution in Belgium. We excluded patient unable or unwilling to consent and those not taking antipsychotic medication. Thus the study cohort comprised 820 patients but 22 were excluded as they were not treated with antipsychotic drugs leaving 798 eligible patients. This provided sufficient power to analyse at least 8 predictor variables. Patients were tested and found to be free of diabetes prior to starting antipsychotics; therefore, the diabetes cases are incident cases. Altogether, 49.1% were taking antipsychotics <3 months, 3.9% 3–6 months, 5.9% 6–9 months, and 41.1% more >1 year. The proportions of patients treated with the same antipsychotic drug for more than 3 months were 81.4% for those receiving first-generation drugs, 76.0% for clozapine, 56.6% for amisulpride, 46.2% for risperidone, 46.2% for olanzapine, 38.7% for quetiapine and 12.2% for aripiprazole.
Statistical analyses
A receiver operator characteristic (ROC) curve analysis was conducted equally weighted for false positives and false negatives tested the best single markers for ruling-in (case-finding) or ruling out (screening). We also examined sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), likelihood ratios and clinical utility index (CUI) and relevant confidence intervals for all tests (all available from www.clinicalutlity.co.uk). In addition, we used ROC curve to calculate the optimal cut-off points for each test. For overall accuracy we used fraction correct (also known as overall accuracy = true positives plus true negatives / all cases). In order to calculate clinical utility, we used the clinical utility index. The CUI allows calculation of qualitative as well as quantitative value of a test [41, 42]. The clinical utility index takes into account both discriminatory ability and occurrence for case-finding (CUI+) and screening (CUI-) such that the positive utility index (CUI+) = sensitivity x positive predictive value and the negative utility index (CUI-) = specificity x negative predictive value. Further details are available here: www.clinicalutility.co.uk There commended qualitative grades of diagnostic accuracy were applied according to previous publications [43]. Namely the grades of the clinical utility index were > = 0.81: excellent, > = 0.64: good and > = 0.49: fair > = 0.36: poor; <0.36: very poor. Finally, algorithm approaches were investigated. Algorithm approaches attempt to improve upon acceptability / test burden. They usually start with a simple test, acceptability to the population as a whole (such as the HBA1c) and then advise a fasting test, or OGTT only if needed.
Results
Demographic and psychiatric characteristics
The total sample comprised 798 patients taking antipsychotic medications with a mean age of 37.7years. 61.1% were male and the most common diagnosis was schizophrenia (67.2%). 62% were smokers. Full details of demographic and psychiatric characteristics are presented in Table 2.
Table 2. Demographic and psychiatric characteristics.
| Total Sample (n = 798) |
New Definition of Diabetes (n = 80) |
No Diabetes(n = 718) | |
|---|---|---|---|
| Age (years) | 37.7 | 47.0 | 36.7 |
| Male Gender | 61.1% | 49.4% | 62.5% |
| Duration (years) | 11.1 | 16.7 | 10.5 |
| Schizophrenia | 67.2% | 45.6% | 68.5% |
| Bipolar | 14.3% | 17% | 13.9% |
| Depression | 2.4% | 7% | 2% |
| GAF (mode) | 55 | 60 | 55 |
| CGI | 4 | 4 | 4 |
| Weight | 79.3kg | 85.0kg | 78.6kg |
| BMI | 26.4 | 29.4 | 26.1 |
| Smokers | 62% | 66.7% | 61.6% |
Prevalence of diabetes and characteristics
Using the old definition of diabetes incorporating IFG and OGTT, 62/798 (7.8%) had diabetes. The rate was 30/474 (6.3%) in men and 32/311 (10.3%) in women (Chi2 = 4.0, p = 0.04). Using a definition of diabetes, incorporating IFG, OGTT and HBA1c, 81/798 (10.2%) had diabetes; 8.2% in men and 13.2% in women (Chi2 = 5.3, p = 0.02). Compared to the non-diabetic group (n = 717, 62.5% male), there was a lower percentage of men in both the old (43.5%) and new definitions (49.4%). In addition, the patients in the old and new diabetes criteria both had higher BMI (29.7 and 29.4 respectively) than the non-diabetic patient group (26.1). There was a higher percentage of people with depression and bipolar disorder in the old and new diabetes criteria compared to the non-diabetic group but a higher percentage of people with schizophrenia in the non-diabetic group. Of 536 patients diagnosed with schizophrenia, diabetes was present in 6.3% (old definition) and 8.4% (new definition). The Cohen’s kappa agreement between the two definitions was 0.86 (95% CI = 0.78 to 0.93). The general European population rate of diabetes is approximately 3.3% (old definition) and 6.8% (new definition), but the patient group reported here has a significantly younger mean age.7 Correcting for this, the expected population rate would be 1.1% (old definition) and 1.4% (new definition) suggesting a relative risk of 7.1 and 7.3, respectively.
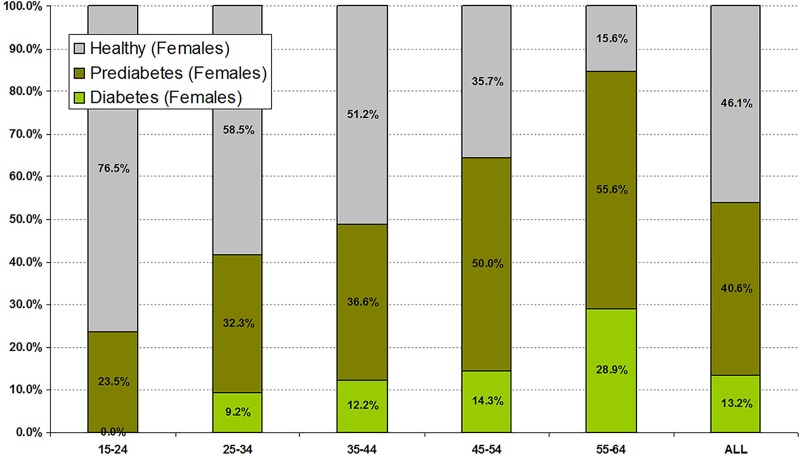
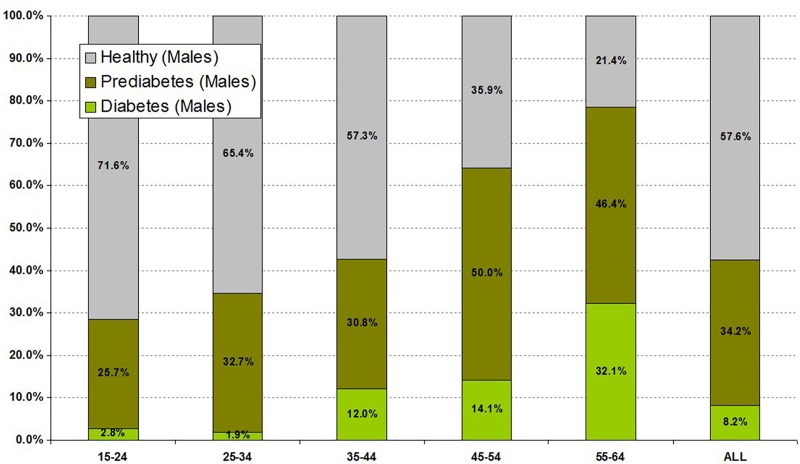
Particularly high rates of diabetes were seen in some subgroups. The rate of old and newly defined diabetes in those taking antipsychotic drugs less than 3 months was 8.9% and 7.9%, 3–6 months 12.9% and 12.9%, 6–9 months 21.3% and 6.3%, and >1 year 10.4% and 13.1%, respectively. However, the highest rates related to age. In males and females aged 45–55 years old, diabetes was present in 14.1 and 14.3%, respectively, and in those aged 56–64, diabetes was present in 32.1% and 28.9% respectively (Figs 1 and 2).
Fig 1. Prevalence rate of diabetes and prediabetes by age in women taking antipsychotics (n = 310).
Fig 2. Prevalence rate of diabetes and prediabetes by age in men taking antipsychotics (n = 488).
Glucose metabolism measurements
Mean cholesterol, trigyceride, HDL and LDL levels were 216 mg/dL, 229 mg/dL, 48 mg/dL, 124 mg/dL in the cohort of patients with diabetes using the old definition and 213 mg/dL, 211 mg/dL, 48 mg/dL and 124 mg/dL using the new definition. Using the old and new definitions of diabetes mean fasting glucose was 125.1 mg/dL vs. 117.4 mg/dL, mean fasting insulin was 20.9 mIU/Lvs. 20.1 mIU/L and mean HOMA-IR was 6.8 vs. 6.2, respectively. In short, the old definition of diabetes identified a more strongly at risk cohort according to their biochemical profile but the new definition identified a larger cohort at risk.
Predictive accuracy of clinical variables in the diagnosis of diabetes
The performance of each individual clinical variable against biochemically defined diabetes is shown in Table 3. No clinical variable was particularly accurate. Judging by area under the curve, the most accurate variable was age (ROC = 0.742), followed by BMI (ROC = 0.653) and illness duration (ROC = 0.639). Judging by overall correct (defined as true positives + true negatives / all cases) the most accurate were BMI (76.6% correct), waist/hip ratio (74.2% correct) and height (68.7% correct). Rule-in and rule-out accuracy may be best considered separately. The optimal individual clinical variables to confirm (rule-in with minimal false positives) a diagnosis of diabetes were 1.) age and 2.) illness duration. However, no variable performed well, all were very poor at confirming a correct diagnosis. The optimal individual clinical variables to refute (rule-out with minimal false negatives) a diagnosis of diabetes were 1.) BMI 2.) waist/hip ratio and 3.) height. These three variables all performed well in this capacity and could be considered as initial screening questions to rule out people unlikely to have diabetes. For example, a BMI less than 30 would correctly identify non- diabetic patients with SMI with 92.5% accuracy (NPV) with 7.5% false negative rate. Of those non- diabetic patients with SMI 80% had a low BMI, but 20% had a high BMI despite being non-diabetic. However, a high BMI could not confirm a diagnosis. Only 45% of those with diabetes had a high BMI and of all patients with a high BMI, only 21.3% would actually be diabetic (PPV).
Table 3. Clinical factors in the diagnosis of diabetes in patients receiving antipsychotic medication.
| Sensitivity | Specificity | PPV | NPV | Clinical Utility (+) | Clinical Utility (-) | Overall Correct | AUC | Optimal Cut-Off ≥ | LR(+) | LR - |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| Symptom | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| Individual Clinical Risk Factors | |||||||||||
| Gender | 54.0% (43.6%-64.5%) |
62.3% (58.8%-65.9%) |
14.6% (10.4%-18.7%) |
92.0% (89.6%-94.3%) |
Very poor (0.079, 0.076–0.081) |
Fair (0.573, 0.572–0.574) |
61.46 | 0.581 (0.526 to 0.637) |
2 | 1.43 (1.16–178) |
0.74 (0.58–0.93) |
| Age | 80.5% (72.1%-88.8%) |
58.7% (55.1%-62.2%) |
18.8% (14.4%-23.2%) |
96.2% (94.4%-98.0%) |
Very poor (0.151, 0.148–0.154) |
Fair (0.564, 0.563–0.565) |
60.98 | 0.742 (0.689 to 0.794) |
38.9 | 1.95 (1.70–2.23) |
0.33 (0.22–0.51) |
| Mental health GAF | 87.4% (80.4% - 94.3%) |
20.2% (17.3%-23.1%) |
11.5% (8.9%-14.1%) |
93.1% (89.1%-97.0%) |
Very poor (0.100, 0.099–0.102) |
Very poor (0.188, 0.186–0.189) |
27.32 | 0.504 (0.445 to 0.562) |
45 | 1.09 (1.00–1.20) |
0.63 (0.35–1.11) |
| Mental health CGI | 93.1% (87.8%-98.4%) |
13.0% (10.5%-15.4%) |
11.3% (8.8%-13.7%) |
94.1% (89.4%-98.7%) |
Very poor (0.105, 0.103–0.106) |
Very poor (0.122, 0.120–0.123) |
21.46 | 0.508 (0.450 to 0.565) |
5 | 1.07 (1.00–1.14) |
0.53 (0.24–1.18) |
| SMI Duration Illness | 72.4% (63.0%-81.8%) |
56.9% (53.3%-60.5%) |
16.6% (12.5%-20.7%) |
94.6% (92.4%-96.7%) |
Very poor (0.120, 0.118–0.123) |
Fair (0.538, 0.537–0.539) |
58.54 | 0.639 (0.571 to 0.706) |
9.4 | 1.68 (1.44–1.96) |
0.48 (0.34–0.69) |
| Height | 49.4% (38.9%-59.9%) |
70.9% (67.7%-74.2%) |
16.8% (11.8%-21.8%) |
92.2% (90.0%-94.4%) |
Very poor (0.083, 0.080–0.086) |
Good (0.654, 0.653–0.655) |
68.66 | 0.621 (0.539 to 0.703) |
1.68 | 1.70 (1.34–2.16) |
0.71 (0.58–0.88) |
| Weight | 71.3% (61.8%-80.8%) |
42.0% (38.4%-45.6%) |
12.7% (9.6%-15.9%) |
92.5% (89.7%-95.3%) |
Very poor (0.091, 0.089–0.093( |
Poor (0.389, 0.387–0.390) |
45.12 | 0.575 (0.511 to 0.639) |
74.2 | 1.23 (1.06–1.42) |
0.68 (0.49–0.96) |
| BMI | 44.8% (34.4%-55.3%) |
80.4% (77.5%-83.2%) |
21.3% (14.6%-28.0%) |
92.5% (90.4%-94.5%) |
Very poor (0.096, 0.091–0.100) |
Good (0.743, 0.742–0.744) |
76.59 | 0.653 (0.590 to 0.716) |
29.6 | 2.28 (1.73–3.00) |
0.69 (0.57–0.83) |
| Waist/Hip Ratio | 39.1% (28.8%-49.3%) |
78.3% (75.3%-81.3%) |
17.6% (11.7%-23.5%) |
91.5% (89.4%-93.7%) |
Very poor (0.069, 0.065–0.072) |
Good (0.717, 0.716–0.718) |
74.15 | 0.594 (0.528 to 0.661) |
1 | 1.80 (1.34–2.42) |
0.78 (0.65–0.92) |
| Combined Factors (Models) | |||||||||||
| Leicester Practice Risk Score (LPRS) [cut off ≥ 14] |
74.1% (64.5%-83.6%) |
64.2% (60.6%-67.7%) |
18.9% (14.1%-23.7%) |
95.6% (93.8%-97.5%) |
Very poor (0.140, 0.137–0.145) |
Fair (0.614, 0.613–0.615) |
65.16 | 0.756 (0.705 to 0.807) |
14 | 2.07 (1.76–2.43) |
0.40 (0.28–0.59) |
| TOPICS Model (TDRS) [cut off ≥ 8] |
71.6% (61.8%-81.4%) |
67.2% (63.8%-70.7%) |
19.8% (14.7%-24.9%) |
95.4% (93.6%-97.3%) |
Very poor (0.142; 0.138–0.145) |
Good (0.643, 0.641–0.643) |
67.67 | 0.765 (0.712 to 0.817) |
8 | 2.18 (1.84–2.60) |
0.42 (0.30–0.60) |
Footnote: AUC- Area under receiver operator characteristic curve; PPV–Positive predictive value; NPV—Negative predictive value; UI = Clinical utility index. The positive clinical utility index (UI+ = sensitivity x PPV) measures rule-in value and the negative clinical utility index (UI- = specificity x NPV) measures rule-out value. The following qualitative grades of diagnostic accuracy have been applied to the clinical utility index were > = 0.81: excellent, <0.81 good > = 0.64; <0.64 fair> = 0.49; <0.49 poor. > = 0.36; <0.36 very poor
Predictive accuracy of clinical models in the diagnosis of diabetes
The performance of combined clinical variables in the Leicester Practice Risk Score (LPRS) and the Topics Diabetes Risk Score (TDRS) were evaluated against biochemically defined diabetes (Table 4). These clinical models were not particularly accurate but they were no less accurate than shown in their parent (non-SMI) studies (Table 1). Judging by area under the curve and by overall correct, the most accurate model was TDRS followed by LPRS. When rule-in and rule-out accuracy were considered separately, the optimal model to refute (rule-out with minimal false negatives) a diagnosis of diabetes was TDRS. When negative at a score of <8, TDRS had 95.4% negative predictive value, meaning only about 5% with a low score would be missed (false negatives). Although neither clinical model was satisfactory at confirming a diagnosis, either could be used as an initial screening model in order to rule out people unlikely to have diabetes. There was one limitation of this approach, TDRS and LPRS would only be negative (under threshold) in 67% and 64% of non-diabetic patients respectively. In this respect these combination models perform significantly worse than just considering BMI alone. A low BMI was almost as accurate as a low score on TDRS (92.5% vs 95.4%) but with the advantage that 80% of non-diabetic patients would have a relevant negative (under threshold) score compared with 67% for the TDRS. Thus, we conclude that these clinical prediction models have little if any advantage over the individual clinical variables used alone. For completeness, we finally examined the accuracy of the clinical models to identify either pre-diabetes or diabetes. Accuracy was inferior when identifying both pre-diabetes and diabetes compared with diabetes alone. For either pre-diabetes or diabetes the AUC for TDRS was 0.661 (CI = 0.624 to 0.699) compared with 0.765 (CI = 0.712 to 0.817) for diabetes alone. For either pre-diabetes or diabetes the AUC for LPRS was 0.647 (CI = 0.609 to 0.685) compared with 0.756 (CI = 0.705 to 0.807) for diabetes alone. This confirms that these models were not particularly suitable for the identification of pre-diabetes or diabetes in SMI.
Table 4. Optimal biochemical measure vs old definition of diabetes.
| Sensitivity | Specificity | PPV | NPV | Clinical Utility (+) | Clinical Utility (-) | Overall Correct | AUC | Optimal Cut-Off ≥ | LR(+) | LR - |
|
|---|---|---|---|---|---|---|---|---|---|---|---|
| symptom | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | (95% CI) | |||
| HBA1C | 80.6% (70.8–90.5) |
71.3% (68.1–74.6) |
19.2% (13.9–24.5) |
97.8% (96.5–99.0) |
Very Poor 0.154 (0.150–0.159) |
Good 0.697 (0.697–0.698) |
72.06 | 0.832 (0.778–0.887) |
5.8 | 2.81 | 0.27 (0.16–0.45) |
| Fasting Glucose | 91.9% (85.5–98.7) |
82.5% (79.7–85.2) |
30.6% (22.7–38.6) |
99.2% (98.5–99.9) |
Very Poor 0.282 (0.267–0.288) |
Excellent 0.818 (0.818–0.818) |
83.21 | 0.935 (0.894–0.975) |
98 mg/dl | 5.25 | 0.10 (0.04–0.23) |
| OGTT 30min | 85.5% (76.7–94.3) |
76.7% (73.6–79.8) |
24.0% (17.5–30.4) |
98.4% (97.4–99.4) |
Very Poor 0.205 (0.200–0.210) |
Good 0.755 (0.754–0.755) |
77.39 | 0.867 (0.817–0.918) |
179 mg/dl | 3.67 | 0.19 (0.10–0.35) |
| OGTT 60min | 91.9% (85.2–98.7) |
83.5% (80.8–86.2) |
32.4% (24.0–40.8) |
99.2% (98.5–99.9) |
Very Poor 0.298 (0.291–0.304) |
Excellent 0.828 (0.828–0.829) |
84.16 | 0.936 (0.900–0.971) |
181 mg/dl | 1.10 | 0.10 (0.04–0.22) |
| OGTT 120min | 88.7% (80.8–96.6) |
91.1% (89.0–93.2) |
46.2% (34.1–58.4) |
98.9% (98.2–99.7) |
Poor 0.410 (0.402–0.418) |
Excellent 0.902 (0.901–0.902) |
90.93 | 0.947329 (0.908–0.986) |
145 mg/dl | 0.97 | 0.12 (0.06–0.25) |
| Fasting Insulin | 56.5% (44.1–68.8) |
81.9% (79.1–84.7) |
20.8% (13.9–27.7) |
95.7% (94.1–97.3) |
Very Poor 0.118 (0.112–0.123) |
Good 0.784 (0.784–0.785) |
79.95 | 0.722 (0.645–0.799) |
14.7 | 3.12 | 0.53 (0.40–0.71) |
| HOMA-IR | 66.1% (54.3–77.9) |
86.7% (84.2–89.1) |
29.5% 20.5–38.5) |
96.8% (95.5–98.2) |
Very Poor 0.195 (0.188–0.202) |
Excellent 0.839 (0.839–0.840) |
85.09 | 0.800 (0.733–0.866) |
3.38 | 4.97 | 0.39 (0.28–0.55) |
| Apriori Thresholds | |||||||||||
| HBA1C ≥6.5 | 27.4% (16.3–38.5) |
97.4% (96.3–98.6) |
47.2 (24.2–69.6) |
94.1% (92.4–97.2) |
Very Poor 0.129 (0.117–0.142) |
Excellent 0.917 (0.916–0.917) |
91.98 | 0.624 (0.568–0.680) |
≥6.5 | N/A | 0.75 (0.64–0.87) |
| Fasting Glucose >125mg/dl | 48.4% (35.9–60.8) |
100.0% (100–100) |
100.0% (100–100) |
95.8% (94.4–97.2) |
Poor 0.484 (0.469–0.499) |
Excellent 0.958 (0.958–0.958) |
95.99 | 0.742 (0.679–0.804) |
>125mg/dl | N/A | 0.52 (0.41–0.66) |
| OGTT 120min ≥199mg/dl | 74.2% (63.3–85.1) |
100.0% (100–100) |
100.0% (100–100) |
97.9% (96.8–98.9) |
Good 0.742 (0.734–0.750) |
Excellent 0.979 (0.979–0.979) |
97.99 | 0.871 (0.816–0.925) |
≥199mg/dl | N/A | 0.26 (0.17–0.39) |
| Algorithm Approaches | |||||||||||
| Van Winkel (FG then OGTT) | 59.7% (47.5–71.9) |
100.0% (100–100) |
100.0% (100–100) |
96.7% (95.4–98.0) |
Fair 0.597 (0.584–0.609) |
Excellent 0.967 (0.967–0.967) |
96.87 | 0.798 (0.736–0.860) |
As per algorithm | N/A | 0.40 (0.30–0.55) |
| Mitchell(a) (HBA1c > FG AND OGTT) | 71.0% (59.7–82.3) |
100.0% (100–100) |
100.0% (100–100) |
97.6% (96.5–98.7) |
Good 0.710 (0.701–0.719) |
Excellent 0.976 (0.976–0.976) |
97.74 | 0.855 (0.798–0.911) |
As per algorithm | N/A | 0.29 (0.20–0.43) |
| Mitchell(b) (HBA1c > OGTT) | 54.8% (42.5–67.2) |
100.0% (100–100) |
100.0% (100–100) |
96.3% (95.0–97.7) |
Fair 0.548 (0.535–0.562) |
Excellent 0.963 (0.963–0.963) |
96.49 | 0.774 (0.712–0.837) |
As per algorithm | N/A | 0.45 (0.34–0.59) |
| Mitchell(c) (HBA1c> FG) | 33.9% (22.1–45.7) |
100.0% (100–100) |
100.0% (100–100) |
94.7% (93.2–96.3) |
Very Poor 0.339 (0.321–0.357) |
Excellent 0.947 (0.947–0.947) |
94.86 | 0.669 (0.610–0.729) |
As per algorithm | N/A | 0.66 (0.55–0.79) |
| Mitchell(d)(HBA1c > FG or OGTT) | 45.2% (32.8–57.5) |
100.0% (100–100) |
100.0% (100–100) |
95.6% (94.1–97.0) |
Poor 0.453 (0.436–0.468 |
Excellent 0.956 (0.956–0.956) |
95.74 | 0.589 (0.541–0.637) |
As per algorithm | N/A | 0.55 (0.44–0.69) |
| Mitchell(e)(HBA1c5.8 >ALL) | 71.0% (59.7–82.3) |
100.0% (100–100) |
100.0% (100–100) |
97.6% (96.4–98.7) |
Good 0.710 (0.701–0.719) |
Excellent 0.976 (0.975–0.976) |
97.69 | 0.854 (0.798–0.912) |
As per algorithm | N/A | 0.29 (0.20–0.43) |
| Mitchell(f)(HBA1c5.7 >ALL) | 80.6% (70.8–90.5) |
100.0% (100–100) |
100.0% (100–100) |
98.4% (97.5–99.3) |
Good 0.806 (0.800–0.813) |
Excellent 0.984 (0.984–0.984) |
98.5 | 0.903 (0.854 to 0.953) |
As per algorithm | N/A | 0.19 (0.12–0.32) |
Footnote: AUC- Area under receiver operator characteristic curve; PPV–Positive predictive value; NPV—Negative predictive value; UI = Clinical utility index. The positive clinical utility index (UI+ = sensitivity x PPV) measures rule-in value and the negative clinical utility index (UI- = specificity x NPV) measures rule-out value. The following qualitative grades of diagnostic accuracy have been applied to the clinical utility index were > = 0.81: excellent, > = 0.64: good and > = 0.49: fair <0.49 = poor.OGTT–oral glucose tolerance test. Algorithm approaches are as follows:Van Winkel (FG >100mg/dl then OGTT?199mg/dl); Mitchell(a) (HBA1c >5.8 then IFG AND OGTT); Mitchell(b) (HBA1c >5.8 then OGTT); Mitchell(c) (HBA1c >5.8 then IFG); Mitchell(d) (HBA1c >5.8 then IFG or OGTT); Mitchell(e) (HBA1c >5.8 then IFG and OGTT and HBA1c)
Predictive accuracy of HBA1c and related variables for Pre2010 diabetes definition
Single metabolic tests
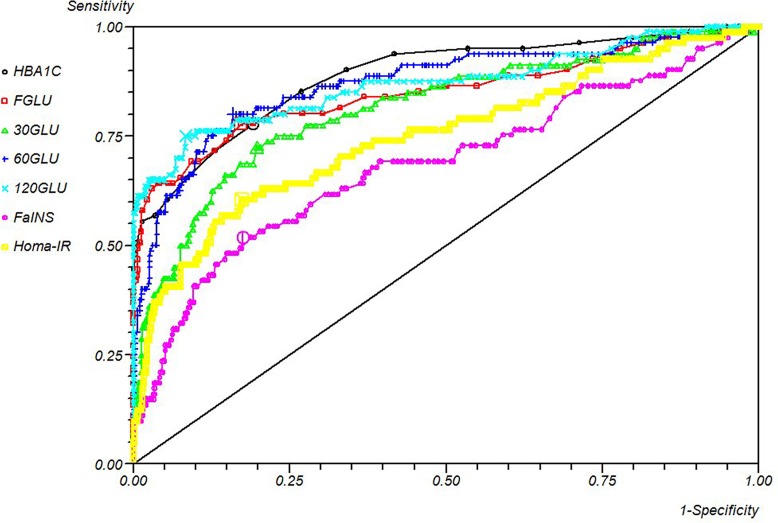
The performance of metabolic biochemical markers against the pre-2010 (conventional) definition of diabetes is shown in Table 2 and Fig 3. After choosing the optimal cut, no test was satisfactory for confirming diabetes. The best single test that could be used to confirm a diagnosis of diabetes with minimal false positives was the two-hour OGTT which had a positive predictive value (PPV) of 46%. All tests performed better in a rule-out capacity, which excludes those without a diagnosis of diabetes with minimal false negatives. All tests had a negative predictive value (NPV) above 95%, but the one-hour response to OGTT and fasting glucose (at a cut-point of 98mg/dl) both had NPV’s above 99%. However, both these tests suffered from a relative lack of specificity. When seeking the optimal rule-out test, the discrimination and occurrence of a negative test in those without the condition should be considered equally. With this in mind, the optimal rule-out test was the two-hour OGTT result, followed by HOMA-IR, fasting glucose level and one-hour OGTT. HBA1c offered a very poor confirmation of diabetes with only 19% PPV, but it could be used as part of a screening algorithm (see below), as it possessed 97.8% NPV.
Fig 3. ROC curve of metabolic measures vs old definition of diabetes.
Single metabolic tests (Apriori thresholds)
Using conventional cut-offs proposed for the diagnosis of diabetes in the general population revealed some unexpected findings. Conventional cut-offs in HBA1c, fasting glucose or OGTT all produced excellent rule-out statistics with few false negatives, suitable for use as an initial screening step. However, only two-hour OGTT (at >199mg/dl) was satisfactory for case-finding of diabetes. Even in this case, only 3 in 4 of subjects with diabetes had an OGTT >199mg/dl; 1 in 4 would be missed (sensitivity was 74%). HBA1c at the conventional cut-point of ≥6.5 proved wholly unsatisfactory as a method of confirming (older) diabetes. Only about 1 in 4 with diabetes would score positive on HBA1c at a cut-point of 6.5% (48 mmol/mol), 3 in 4 being missed.
Algorithm approaches
Regarding aim 2 of the study, we investigated seven algorithm approaches to the diagnosis of diabetes. The algorithm proposed by van Winkel [20], namely a fasting glucose >100mg/dl followed by a conventional OGTT for patients positive in step 1, gave 100% specificity and PPV and very good NPV (96.7%) suggesting a potentially useful approach. Its main limitation was the sensitivity of only 59.7% meaning 4 in 10 people with diabetes would potentially be missed. A more convenient screening algorithm “Mitchell (b)” consisting of (HBA1c >5.8% then OGTT) offered almost identical accuracy as the van Winkel proposal, but without requiring an initially fasting sample. “Mitchell (b)” would necessitate OGTT in only 25% of patients. The van Winkel algorithm would require a fasting sample on everyone, but OGTT in only 20% of patients [20]. The only approaches that offered good or better clinical utility were an initial HBA1c at a lower threshold (≥5.9 or ≥5.7) followed by conventional diabetes testing with the addition of IFG and OGTT. For maximum accuracy, this protocol requires patients initially screening positive to have IFG and OGTT as well as reinterpretation of HBA1c ≥6.5. At a HBA1c cut-off of ≥5.9, this protocol achieves 97.7% overall accuracy, but only requires OGTT and fasting glucose in 25% of the sample, and at a cut-off of ≥5.7 this protocol achieves 98.5% overall accuracy and only requires OGTT and fasting glucose in 32.7% of the sample as opposed to 100% testing of all three measures to achieve 100% accuracy. The latter strategy achieves 80.6% sensitivity and 100% specificity.
Predictive accuracy of biochemical variables for detecting diabetes (2010 Diabetes definition)
Single metabolic tests
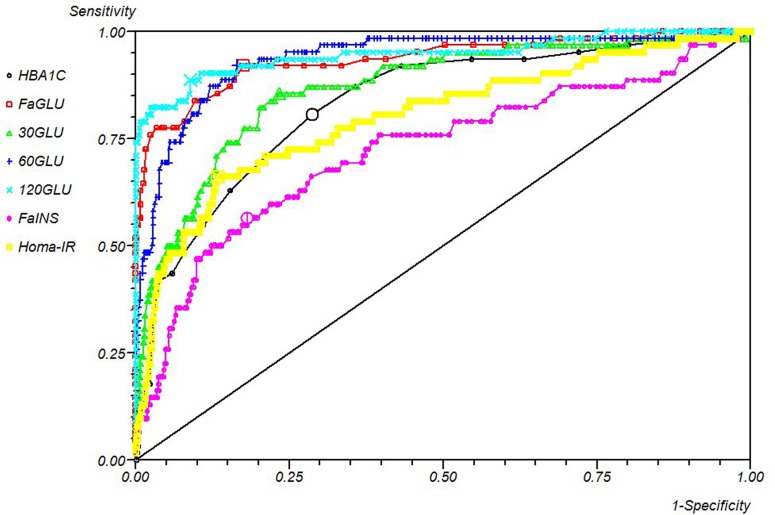
The performance of metabolic biochemical markers against a close adaption of the 2010 definition of diabetes is shown in Table 5 and Fig 4. All tests were disappointing in their ability to diagnose diabetes when used alone. OGTT (at ≥145 mg/dl) had 50% PPV and HBA1c only 31.5%. As above, all tests performed better in a rule-out capacity that excludes those without a diagnosis of diabetes with minimal false negatives. All tests had NPVs above 90%, but the highest values were those related to the OGTT, fasting glucose with HBA1c. When combined with their occurrence in patients without diabetes, the optimal screening test was an adapted OGTT. Judging the overall performance by fraction/overall correct: OGTT was the optimal single test.
Table 5. Optimal metabolic measure vs new definition of diabetes.
| Symptom | Sensitivity (95% CI) | Specificity(95% CI) | PPV(95% CI) | NPV(95% CI) | Clinical Utility (+)(95% CI) | Clinical Utility (-)(95% CI) | Overall Correct | AUC(95% CI) | Optimal / Preselected Cut-Off ≥ | LR+(95% CI) | LR-(95% CI) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Single Metabolic Tests | |||||||||||
| HBA1C | 77.8% (68.7–86.8) |
80.9% (78.0–83.8) |
31.5% (23.7–39.3) |
97.0% (95.6–98.4) |
Very Poor 0.245 (0.240–0.250) |
Good 0.785 (0.784–0.785) |
80.58 | 0.885 (0.841–0.930) |
5.8 | 4.07 (3.37–4.92) |
0.27 (0.18–0.41) |
| Fasting Glucose | 77.8% (68.7–86.8) |
82.8% (80.1–85.6) |
33.9% (25.5–42.4) |
97.1% (95.7–98.4) |
Very Poor 0.263 (0.258–0.269) |
Good 0.804 (0.804–0.805) |
82.33 | 0.849 (0.791–0.907) |
98 mg/dl | 4.53 (3.72–5.53) |
0.27 (0.18–0.40) |
| OGTT 30min | 72.5% (62.7 = 82.3) |
80.2% (77.3–83.2) |
29.4% (21.9–37.0) |
96.2% (94.7–97.8) |
Very Poor 0.213 (0.208–0.219) |
Good 0.772 (0.772–0.773) |
79.44 | 0.814 (0.759–0.870) |
179 mg/dl | 3.67 (3.00–4.48) |
0.34 (0.24–0.49) |
| OGTT 60min | 80.0% (71.2–88.8) |
84.1% (81.4–86.8) |
36.4% (27.5–45.2) |
97.4% (96.1–98.6) |
Very Poor 0.291 (0.285–0.297) |
Excellent 0.819 (0.818–0.819) |
83.65 | 0.869 (0.819–0.919) |
181 mg/dl | 5.02 (4.10–6.15) |
0.24 (0.15–0.37) |
| OGTT 120min | 75.0% (65.5–84.5) |
91.6% (89.6–93.7) |
50.4% (37.7–63.1) |
97.0% (95.7–98.3) |
Poor 0.378 (0.371–0.386) |
Excellent 0.888 (0.888–0.889) |
89.91 | 0.870 (0.816–0.923) |
145 mg/dl | 8.94 (6.79–1.77) |
0.27 (0.19–0.40) |
| Fasting Insulin | 51.9% (41.0–62.7) |
82.4% (79.6–85.2) |
25.0% (17.5–32.5) |
93.8% (91.9–95.7) |
Very Poor 0.130 (0.125–0.135) |
Good 0.773 (0.773–0.774) |
79.32 | 0.685 (0.614–0.755) |
14.7 | 2.95 (2.27–3.84) |
0.58 (0.46–0.73) |
| HOMA-IR | 60.5% (49.8–71.1) |
82.7% (79.9–85.5) |
28.3% (20.4–36.2) |
94.9% (93.2–96.6) |
Very Poor 0.171 (0.166–0.177) |
Good 0.785 (0.784–0.785) |
80.45 | 0.748 (0.683–0.813) |
3.38 | 3.50 (2.76–4.44) |
0.48 (0.36–0.63) |
| Apriori Thresholds | |||||||||||
| HBA1C ≥6.5 | 44.4% (33.6–55.3) |
100.0% (100–100) |
100.0% (100–100) |
94.1% (92.4–95.8) |
Poor 0.444 (0.432–0.457) |
Excellent 0.941 (0.941–0.941) |
94.36 | 0.722 (0.667–0.776) |
≥6.5 | N/A | 0.56 (0.46–0.68) |
| Fasting Glucose >125mg/dl | 37.0% (26.5–47.6) |
100.0% (100–100) |
100.0% (100–100) |
93.4% (91.6–95.1) |
Poor 0.370 (0.357–0.384) |
Excellent 0.934 (0.933–0.934) |
93.61 | 0.685 (0.632–0.738) |
>125mg/dl | N/A | 0.63 (0.53–0.74) |
| OGTT 120min ≥199mg/dl | 56.8% (46.0–67.6) |
100.0% (100–100) |
100.0% (100–100) |
95.3% (93.8–96.8) |
Fair 0.568 (0.558–0.578) |
Excellent 0.953 (0.953–0.954) |
95.61 | 0.784 (0.730–0.838) |
≥199mg/dl | N/A | 0.43 (0.34–0.55) |
| Algorithm Approaches | |||||||||||
| Van Winkel (FG then OGTT) | 45.7% (34.8–56.5) |
100.0% (100–100) |
100.0% (100–100) |
94.2% (92.6–95.9) |
Poor 0.457 (0.445–0.469) |
Excellent 0.942 (0.942–0.942) |
94.49 | 0.728 (0.717–0.826) |
As per algorithm | N/A | 0.54 (0.44–0.66) |
| Mitchell(a) (HBA1c > FG AND OGTT) | 54.3% (43.5–65.2) |
100.0% (100–100) |
100.0% (100–100) |
95.1% (93.6–96.6) |
Fair 0.543 (0.533–0.554) |
Excellent 0.951 (0.951–0.951) |
95.36 | 0.772 (0.717–0.826) |
As per algorithm | N/A | 0.46 (0.36–0.58) |
| Mitchell(b) (HBA1c > OGTT) | 42.0% (31.2–52.7) |
100.0% (100–100) |
100.0% (100–100) |
93.8% (92.1–95.6) |
Poor 0.420 (0.407–0.433) |
Excellent 0.938 (0.938–0.939) |
94.11 | 0.710 (0.656–0.764) |
As per algorithm | N/A | 0.58 (0.48–0.70) |
| Mitchell(c) (HBA1c> FG) | 25.9% (16.4–35.5) |
100.0% (100–100) |
100.0% (100–100) |
92.3% (90.4–94.2) |
Very Poor 0.259 (0.245–0.274) |
Excellent 0.923 (0.923–0.923) |
92.48 | 0.629 (0.582–0.678) |
As per algorithm | N/A | 0.74 (0.65–1.18) |
| Mitchell(d)(HBA1c > FG or OGTT) | 34.6% (24.2–44.9) |
100.0% (100–100) |
100.0% (100–100) |
93.1% (91.3–94.9) |
Very Poor 0.346 (0.332–0.360) |
Excellent 0.931 (0.931–0.931) |
93.36 | 0.568 (0.530–0.605) |
As per algorithm | N/A | 0.65 (0.56–0.77) |
| Mitchell(e)(HBA1c5.8>ALL) | 77.8% (68.7–86.8) |
100.0% (100–100) |
100.0% (100–100) |
97.6% (96.4–98.7) |
Good 0.778 (0.772–0.783) |
Excellent 0.976 (0.975–0.976) |
97.74 | 0.889 (0.843–0.934) |
As per algorithm | N/A | 0.22 (0.15–0.33) |
| Mitchell(f)(HBA1c5.7 >ALL) | 85.2% (77.4–92.9) |
100.0% (100–100) |
100.0% (100–100) |
97.6% (96.4–98.7) |
Excellent 0.852 (0.848–0.855) |
Excellent 0.984 (0.983–0.984) |
98.50 | 0.925 (0.887 to 0.965) |
As per algorithm | N/A | 0.15 (0.09–0.25) |
Footnote: AUC- Area under receiver operator characteristic curve; PPV–Positive predictive value; NPV—Negative predictive value; UI = Clinical utility index. The positive clinical utility index (UI+ = sensitivity x PPV) measures rule-in value and the negative clinical utility index (UI- = specificity x NPV) measures rule-out value. The following qualitative grades of diagnostic accuracy have been applied to the clinical utility index were > = 0.81: excellent, > = 0.64: good and > = 0.49: fair <0.49 = poor.OGTT–oral glucose tolerance test. Algorithm approaches are as follows: Van Winkel (FG >100mg/dl then OGTT?199mg/dl); Mitchell(a) (HBA1c >5.8 then IFG AND OGTT); Mitchell(b) (HBA1c >5.8 then OGTT); Mitchell(c) (HBA1c >5.8 then IFG); Mitchell(d) (HBA1c >5.8 then IFG or OGTT); Mitchell(e) (HBA1c ?5.9 then IFG and OGTT and HBA1c) and Mitchell(f) (HBA1c >5.7 then IFG and OGTT and HBA1c)
Fig 4. ROC curve of metabolic measures vs new definition of diabetes.
Single metabolic tests (a priori thresholds)
Using conventional cut-offs in HBA1c by definition produced a correct confirmation of diabetes. In addition, all produced excellent rule-out statistics with few false negatives, suitable for use as an initial screening step. However, only 2hr OGTT can be seriously considered for case-finding newly defined diabetes in those taking antipsychotic medication. Here, although an OGTT >199 would define diabetes (100% PPV), such a result would only occur in 57% of people with diabetes. HBA1c was unsatisfactory as a method of confirming newly redefined diabetes, as only 4 out of 10 patients with diabetes would score positive on HBA1c at a cut-point of 6.5% (48 mmol/mol).
Algorithm approaches
We investigated the same seven algorithm approaches to the diagnosis of newly defined diabetes. The most accurate approach was an initial HBA1c followed by conventional testing. The main question is what cut-off on HBA1c is optimal. At a cut-off of ≥5.9 this protocol achieves 97.7% overall accuracy, and only requires OGTT and fasting glucose in 25% of the sample, and at a cut-off of ≥5.7, this protocol achieves 98.5% overall accuracy, and only requires OGTT and fasting glucose in 32.7% of the sample as opposed to 100% testing of all three measures to achieve 100% accuracy. Its only limitation was a slight loss in sensitivity to 85.2%. As the cut-off of ≥5.7 is also now recommended for prediabetes, this is the one we would recommend in SMI.
Discussion
Diabetes is increasingly recognised as important in patients with SMI. Observational studies have reported a clear association between patients with established severe mental illness and diabetes [8, 44, 45]. The risk appears particularly severe in those maintained on antipsychotic drugs, notably most atypical antipsychotics although risk is elevated in those older conventional antipsychotics [46, 47]. A series of meta-analyses have documented that the rate of diabetes is high in those with chronic schizophrenia (12.8%; N = 9; n = 2142; 39.3±9.4 yrs), bipolar disorder (9.0%; N = 4; n = 1118; 42.1±8.5 yrs) and depression (7.6%; N = 6; n = 2827; 47.1±7.6 yrs) [48, 49, 50]. Pre-diabetes defined by impaired fasting glucose > 100mg/dl was also high in schizophrenia (24.2%) bipolar disorder (17.3%) and depression (17.6%). In short, all those with SMI appear to have a high risk of pre-diabetes and diabetes. This is particularly the case for those maintained on long-term atypical antipsychotic medication [44]. There is therefore a great need to identify and treat glucose dysregulation in patients on typical and non-atypical antipsychotics. Unfortunately it is also clear that the implementation of screening for diabetes and metabolic components is inconsistent [51, 52]. Even after the launch of many national guidelines on physical healthcare monitoring, blood glucose is only tested in about half of patients under psychiatric care [43]. Rates of metabolic surveillance appear to be significantly lower for patients with SMI than for patients with known diabetes [53, 54]. Indeed biochemical tests are often inadequately collected in patients with SMI. This may be particularly the case for patients in prison, in long-stay facilities, those seen in primary care and also those seen at home and in general medical hospital settings [55]. Yet, non-invasive clinical tests are often more frequently offered. A recent meta-analysis showed that 75% of patients with SMI on antipsychotic medication received assessment of body weight. Whilst 75% is still less than ideal, many clinicians consider measurement of clinical variables as practical and measurable in busy settings but would consider measurement of biochemical variables impractical.
This is the first study to examine whether clinical variables can be used to identify patients at high risk of diabetes in mental health settings. None of the clinical variables: gender, age, mental health global assessment of function (GAF), mental health clinicians’ global impression (CGI), duration of severe mental illness, height, weight, BMI, waist/hip ratio were completely satisfactory and none could be used instead of conventional biochemical testing. Where biochemical testing is considered impractical or inconvenient (or perhaps when facilities are not available) BMI or waist/hip ratio could be used as an approximate initial screen in order to rule-out those at low risk. A BMI less than 30 would correctly identify non-diabetic patients with SMI with about 92.5% accuracy (NPV), that is, with a 7.5% false negative rate. BMI could not be used to confirm the presence of diabetes because of its low PPV (21.3%). Given the high rates of not just diabetes, but also pre-diabetes in SMI, there may be some merit in screening for pre-diabetes and diabetes combined. If the BMI was used to identify not just diabetes but pre-diabetes and diabetes, then the PPV would increase from 21% to 65.2% but NPV would fall to from 92.5% to 58.5%. Thus performance of the optimal clinical variable (BMI) would remain unsatisfactory even if pre-diabetes was the target. This means that basing judgments about diabetes (or pre-diabetes) upon single clinical factors cannot be recommended.
The next question we addressed is whether a combination of clinical variables can be used to identify patients at high risk of diabetes in mental health settings. Previous studies appear to suggest that combination models may improve upon the accuracy offered by individual clinical variables alone. Based on the published literature regarding the performance of clinical models in the general population (Table 1) we chose to examine the Leicester Practice Risk Score (LPRS) and the Topics Diabetes Risk Score (TDRS) in patients with SMI. In large scale population studies the LPRS achieved an area under the curve 0.72 and a Youden score of 0.239.30 The TDRS achieved an area under the curve of 0.77 and a Youden score of 0.408 [31]. In this study, we found very similar results. Here the LPRS achieved an area under the curve 0.756 and a Youden score of 0.383. The TDRS achieved an area under the curve of 0.765 and Youden score of 0.388. Thus the performance of these models was almost identical to their own validation cohorts and indeed the recommended cut points were the same on ROC curve testing. Yet in practical terms, neither could be used to reliably confirm diabetes. A negative TDRS (a score of <8) was potentially useful in that the TDRS had 95.4% negative predictive value (yielding 5% false negatives) but the TDRS would only be negative (under threshold) in 67% of non-diabetic SMI patients. Further when looking for at risk patients (with either pre-diabetes or diabetes) their performance was further reduced. Overall then the clinical prediction models appear to have little if any advantage over the individual clinical variables used alone.
The prompt detection of diabetes is a priority in patients with SMI but we find little to support the routine use of clinical variables in order to accurately identify those with diabetes (or indeed at risk with prediabetes). It is possible that clinical risk factors of importance were not measured in this study and the future risk profiling may prove beneficial. For example several models incorporated diet and fitness, not measured in this study. In this study age, BMI, waist/hip ratio and illness duration were associated with diabetes and could be incorporated informally by clinicians concerned about risk of diabetes in order to focus advice or resources. However, no clinical variables were a satisfactory proxy for a diagnosis of diabetes and as such we recommend conventional biochemical testing for patients with SMI where diabetes or prediabetes is a potential concern.
Regarding biochemical predictors, we found that a single application of HBA1c and other markers of glucose regulation should be used with caution in patients with SMI taking antipsychotics. Repeat testing (after several weeks) using the HBA1c would likely improve accuracy but this has not been formally studied even in the general population [56]. Since this study was conducted before the introduction of 2010 guidelines we could not precisely replicate the 2010 recommendations. Nevertheless results of this large prospective observation study demonstrate that in settings using the older definition of diabetes, the two-hour OGTT is the optimal single test at a cut point of 199 mg/dL, but this is not perfect, as reliance on this one test would miss 25.8% of diabetic cases. At an a-priori cut of 199 mg/dL (7.0mol/L), there was excellent rule-out ability, but a reduced performance for case-finding diabetes, simply because one in four people with diabetes have a normal OGTT, but abnormal fasting glucose. However, reliance on IFG (at a cut point of >125 mg/dL) would miss 51.6% of people with diabetes and reliance on HBA1c (at a cut point of ≥6.5% (48 mmol/mol)) would miss 72.6% of people with diabetes. This means that the gold standard of a fasting glucose and an OGTT cannot be replaced by a single test if optimal accuracy is required. Regarding metabolic tests for the newly proposed definition of diabetes, the same limitations apply. Conventional OGTT, IFG and HBA1c miss 43.2%, 67% and 55.6% people with diabetes respectively when used alone. HBA1c is sometimes proposed as a single one-off test of diabetes in some centres. We have shown this is not recommended at the conventional cut-point of ≥6.5% (48 mmol/mol) in this population due to its poor sensitivity. Only about 4 in 10 patients with diabetes score at 6.5% (48 mmol/mol) or above. In clinical practice, this would result in the majority of people with diabetes patients being missed if this were the only test used. HbA1c is generally less sensitive than IFG and OGTT in diagnosing diabetes in those with mild disease [8].
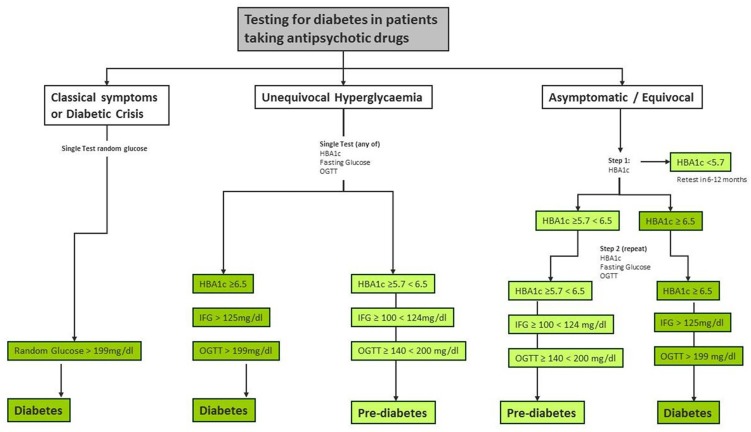
We found that HBA1c can be used as an initial screening step in a diagnostic algorithm (Fig 5). An algorithm consisting of HBA1c ≥5.7% followed by both FG and OGTT was the optimal test that did not require all patients to have HBA1c, OGTT and FG. It is important to note that modification of the cut-point to 5.7% improves sensitivity from 44.4% to up to 85%. A higher cut-point of ≥5.9% could be chosen, but at the penalty of loss in sensitivity. At ≥5.7% only one in three people would need to have fasting and challenge test. Therefore, in psychiatric settings, for patients with SMI taking antipsychotics we recommend a modification of the cut-point to ≥5.7% as defined by the newly defined World Health Organisation/American Diabetes Association standard [57] when looking for diabetes. We believe these results are generalisable to most organizations treating patients with antipsychotic medication. An HBA1c cut-point of ≥5.7% is identical to the one found using ROC analyses of the US NHANES data (≥5.7%) as the best combination of sensitivity (39%) and specificity (91%) to identify pre-diabetes [58]. HBA1c ≥5.7% was subsequently adopted internationally as the threshold to diagnose prediabetes. Several previous studies measured HBA1c in patients with SMI taking antipsychotics. Krein et al (2006) found mean HbA1c to be lower among those with versus those without SMI, although testing was not systematic in this study [59]. Brown et al (2011) found that diabetic patients with SMI had lower HbA1c levels than those without SMI [60]. In this context, second-generation antipsychotics appear to adversely influence HBA1c levels [61].
Fig 5. Optimal protocol for testing diabetes in SMI.
Recommendations for application of a diagnostic test for diabetes
Taking results together we suggest the following approach. All patients with SMI taking antipsychotic medications at initiation of the drug should be tested using HBA1c ≥5.7%. If negative, the test should be repeated again in 6 months. If positive, proceed to step 2, immediately (but certainly within 2 weeks) obtain and use HBA1c and fasting glucose and OGTT at conventional cuts-offs. If testing is done immediately, HBA1c does not need to be repeated, as those scoring ≥6.5% (48 mmol/mol) are already apparent. OGTT and FG can be obtained at the same time, minimizing patient burden. Repeat testing with more than a single test is in accordance with national recommendations for the diagnosis of diabetes in asymptomatic patients [3, 48]. These recommend repeat testing for all patients with an abnormal initial test, except for those in a hyperglycemic crisis or classic symptoms of hyperglycemia and a random plasma glucose ≥200 mg/dL. They also recommend that it is preferable that the same test be repeated (at a later date) for confirmation. The value of repeat testing at two points in time on the same patients has not yet been studied in patients taking antipsychotics. In our opinion, all patients taking antipsychotics should be routinely tested for diabetes and prediabetes due to the high risk and adverse consequences. Currently, recommendations for routine testing for diabetes in asymptomatic, undiagnosed adults include adults of any age with BMI ≥25 kg/m2 and one or more of the known risk factors for diabetes [53]. We suggest that patients with SMI taking antipsychotic medication is added to the list of known diabetic risk factors.
It is important that once diabetes is detected, that timely and appropriate treatment is given. In the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study, 38% of those with detected diabetes at baseline were left untreated [62]. Mitchell et al (2010) reviewed eleven studies that compared the quality of diabetes care in patients with and without mental illness in routine clinical settings and found significant disparities [63]. Mai et al (2012) studied quality of diabetes care in 139,208 people with mental illness and 294,180 matched controls from Western Australia [64]. Patients had lower rates of screenings (HbA1c, blood lipids), but increased risks of hospitalization for diabetes complications including diabetes-related mortality.
However, it is important to note some considerations when interpreting our results. We did not have prospective data on how pre-diabetes or diabetes might change in this sample. We did not have data on repeat testing which could be examined as a possible diagnostic strategy. One important factor is that we did not have data on end organ dysfunction which is an important and adverse outcome from diabetes. Future research should seek to ascertain this information.
In conclusion, patients with SMI taking antipsychotics are at significantly increased risk of diabetes and, therefore, clinicians must be vigilant for symptoms of diabetes, diabetic risk factors and also screen at regular intervals (we recommend annually). In order to best identify newly redefined diabetes, we recommend a simple biochemical algorithm as follows: step 1. HBA1c ≥5.7%; if negative, test again in 6 months, but if step 1 is positive, proceed to conventional testing (HBA1c and fasting glucose and OGTT at conventional cuts-offs). Patients with diabetes should be referred to an appropriate specialist and at the same time have a review by mental health specialist to clarify which risk factors, including prescription of potentially hazardous antipsychotic medication can be addressed. Such recommendations may be improved by improved integrated and collaborative care between physical and mental healthcare facilities.
Supporting information
(XLS)
Data Availability
All relevant data is within the paper and its Supporting Information files.
Funding Statement
This research was supported by an EFSD/Lilly grant.
References
- 1.Danaei G, Finucane MM, Lu Y, Singh GM, Cowan MJ, Paciorek CJ, et al. Global Burden of Metabolic Risk Factors of Chronic Diseases Collaborating Group (Blood Glucose). National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis oaf health examination surveys and epidemiological studies with 370 country-years and 2·7 million participants. Lancet. 2011;378(9785):31–40. 10.1016/S0140-6736(11)60679-X [DOI] [PubMed] [Google Scholar]
- 2.International Diabetes Federation. IDF Diabetes Atlas, 8th edn Brussels, Belgium: International Diabetes Federation, 2017. http://www.diabetesatlas.org [Google Scholar]
- 3.American Diabetes Association Standards of Medical Care in Diabetes—2010 Diabetes Care. 2010; 33(Supplement_1): S11–S61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Diabetes Association. 2. Classification and Diagnosis of Diabetes. Diabetes Care. 2016;39Suppl 1:S13–22. [DOI] [PubMed] [Google Scholar]
- 5.Selvin E, Steffes MW, Zhu H et al. Glycated hemoglobin, diabetes, and cardiovascular risk in nondiabetic adults.N Engl J Med 2010;362:800–811 10.1056/NEJMoa0908359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang X, Gregg EW, Williamson DF et al. A1C level and future risk of diabetes: a systematic review. Diabetes Care 2010;33:1665–167 10.2337/dc09-1939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van’t Riet E, Alssema M, Rijkelijkhuizen JM et al. Relationship between A1c and glucose levels in the general Dutch population. Diabetes Care 2010; 33: 61–66. 10.2337/dc09-0677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Karnchanasorn R1, Huang J2, Ou HY3, Feng W2, Chuang LM4, Chiu KC2, et al. Comparison of the Current Diagnostic Criterion of HbA1c with Fasting and 2-Hour Plasma Glucose Concentration.J Diabetes Res. 2016:6195494 10.1155/2016/6195494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostafa SA, Davies MJ, Webb D, Gray LJ, Srinivasan BT, Jarvis J, et al. The potential impact of using glycated haemoglobin as the preferred diagnostic tool for detecting Type 2 diabetes mellitus.Diabet Med. 2010;27(7):762–9. 10.1111/j.1464-5491.2010.03015.x [DOI] [PubMed] [Google Scholar]
- 10.Cohen D., Stolk R.P., Grobbee D.E., Gispen-de Wied C.C. Hyperglycemia and diabetes in patients with schizophrenia or schizoaffective disorders.Diabetes care 2006; 29 (4), 786–791. [DOI] [PubMed] [Google Scholar]
- 11.De Hert M, Dekker J.M., Wood D., Kahl K.G., Holt R.I.G., Möller H.-J. Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC) Eur Psychiatry. 2009;24(6):412–24. 10.1016/j.eurpsy.2009.01.005 [DOI] [PubMed] [Google Scholar]
- 12.Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry 2006;18:183–194. [DOI] [PubMed] [Google Scholar]
- 13.Galling B1, Roldán A2, Nielsen RE3, Nielsen J4, Gerhard T5, Carbon M1, et al. Type 2 Diabetes Mellitus in Youth Exposed to Antipsychotics: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2016. March;73(3):247–59. 10.1001/jamapsychiatry.2015.2923 [DOI] [PubMed] [Google Scholar]
- 14.De Hert M, Hanssens L, Wampers M, et al. Prevalence and incidence rates of metabolic abnormalities and diabetes in a prospective study of patients treated with second-generation antipsychotics. Schizophr Bull 2007;33:560. [Google Scholar]
- 15.Mitchell AJ, Vancampfort D, De Herdt A, Yu W, De Hert M. Is the prevalence of metabolic syndrome and metabolic abnormalities increased in early schizophrenia? A comparative meta-analysis of first episode, untreated and treated patients.Schizophr Bull. 2012. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Winkel R, De Hert M, Wampers M, et al. Major changes in glucose metabolism, including new onset diabetes, within 3 months after initiation or switch to atypical antipsychotic medication in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2008; 69:472–479. [DOI] [PubMed] [Google Scholar]
- 17.Roerig JL, Steffen KJ, Mitchell JE. Atypical antipsychotic-induced weight gain: insights into mechanisms of action. CNS Drugs. 2011. 1;25(12):1035–59. [DOI] [PubMed] [Google Scholar]
- 18.Correll CU, Kane JM, Manu P. Identification of high-risk coronary heart disease patients receiving atypical antipsychotics: Single low-density lipoprotein cholesterol threshold or complex national standard? J Clin Psychiatry 2008; 69:578–583. [DOI] [PubMed] [Google Scholar]
- 19.De Hert M, Van Eyck D, Hanssens L, Peuskens H, Thys E, Wampers M, et al. Oral glucose tolerance tests in treated patients with schizophrenia. Data to support an adaptation of the proposed guidelines for monitoring of patients on second generation antipsychotics? Eur Psychiatry. 2006;21(4):224–6. [DOI] [PubMed] [Google Scholar]
- 20.van Winkel R, De Hert M, Van Eyck D, Hanssens L, Wampers M, Scheen A, et al. Screening for diabetes and other metabolic abnormalities in patients with schizophrenia and schizoaffective disorder: evaluation of incidence and screening methods. J Clin Psychiatry. 2006; 67(10):1493–500. [DOI] [PubMed] [Google Scholar]
- 21.Manu P, Correll CU, van Winkel R, Wampers M, DeHert M. Prediabetes in patients treated with antipsychotic drugs. J Clin Psychiatry. 2012; 73(4):460–6. 10.4088/JCP.10m06822 [DOI] [PubMed] [Google Scholar]
- 22.Agarwal SK. Prediabetes in a schizophrenia population. European Psychiatry 2012, 27 (suppl 1)P-1198 [Google Scholar]
- 23.Hanssens L, De Hert M, Van Eyck D, Wampers M, Peuskens J, Scheen A. Usefulness of Glycated haemoglobin (HbA1c) to screen for diabetes in patients with schizophrenia Schizophrenia Research 2006;. 85, iss. 1–3: 296–297. [DOI] [PubMed] [Google Scholar]
- 24.Paulweber B, Valensi P, Lindstrom J, Lalic NM, Greaves CJ, McKee M, et al. A European evidence-based guideline for the prevention of type 2 diabetes.HormMetab Res 2010;42(suppl 1):S3–36. [DOI] [PubMed] [Google Scholar]
- 25.Alberti KG, Zimmet P, Shaw J. International Diabetes Federation: a consensus on type 2 diabetes prevention. Diabet Med 2007;24:451–63 [DOI] [PubMed] [Google Scholar]
- 26.Schwarz PE, Li J, Lindstrom J, Tuomilehto J. Tools for predicting the risk of type 2 diabetes in daily practice. 2009. HormMetab Res 41: 86–97 [DOI] [PubMed] [Google Scholar]
- 27.Collins GS, Mallett S, Omar O, Yu LM. Developing risk prediction models for type 2 diabetes: a systematic review of methodology and reporting. BMC Med. 2011;9:103 10.1186/1741-7015-9-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buijsse B, Simmons RK, Griffin SJ, Schulze MB. Risk assessment tools for identifying individuals at risk of developing type 2 diabetes. Epidemiol Rev 2011;33:46–62 10.1093/epirev/mxq019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noble D, Mathur R, Dent T, Meads C, Greenhalgh T. Risk models and scores for type 2 diabetes: systematic review. BMJ 2011;343:d7163 10.1136/bmj.d7163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abbasi A, Peelen LM, Corpeleijn E, et al. Prediction models for risk of developing type 2 diabetes: systematic literature search and independent external validation study BMJ Open. 2012; 345: e5900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brown N, Critchley N, Bogowicz P, et al. Risk scores based on self-reported or available clinical data to detect undiagnosed Type 2 Diabetes: A systematic review Diabetes Research and Clinical Practice 2012 in press. [DOI] [PubMed]
- 32.Heikes KE, Eddy DM, Arondekar B, Schlessinger L. Diabetes Risk Calculator: a simple tool for detecting undiagnosed diabetes and pre-diabetes. Diabetes Care. 2008;31(5):1040–1045 [DOI] [PubMed] [Google Scholar]
- 33.Gray LJ, Taub NA, Khunti K, Gardiner E, Hiles S, Webb DR, et al. The Leicester Risk Assessment score for detecting undiagnosed Type 2 diabetes and impaired glucose regulation for use in a multiethnicUK setting. Diabet Med. 2010;27(8):887–95. 10.1111/j.1464-5491.2010.03037.x [DOI] [PubMed] [Google Scholar]
- 34.Heianza Y, Arase Y, Saito K, Hsieh SD, Tsuji H, Kodama S, et al. Development of a Screening Score for Undiagnosed Diabetes and Its Application in Estimating Absolute Risk of Future Type 2 Diabetes in Japan: Toranomon Hospital Health Management Center Study 10 (TOPICS 10). J ClinEndocrinolMetab.2013 [DOI] [PubMed] [Google Scholar]
- 35.Lindström J, Absetz P, Hemio K et al. Reducing the risk of type 2 diabetes with nutrition and physical activity—efficacy and implementation of lifestyle interventions in Finland. Public Health Nutr 2010;13(6A):993–9 10.1017/S1368980010000960 [DOI] [PubMed] [Google Scholar]
- 36.Aleksandra Gilis-Januszewska A, Szybinski Z, Kissimova-Skarbek K et al. Prevention of type 2 diabetes by lifestyle intervention in primary health care setting in Poland: Diabetes in Europe Prevention using Lifestyle, physical Activity and Nutritional intervention (DE-PLAN) project. British Journal of Diabetes & Vascular Disease 2011;11:4 198–203 [Google Scholar]
- 37.Costa B, Barrio F, Cabré JJ, Piñol JL, Cos X, Solé C, et al. ; DE-PLAN-CAT Research Group. Delaying progression to type 2 diabetes among high-risk Spanish individuals is feasible in real-life primary healthcare settings using intensive lifestyle intervention. Diabetologia. 2012;55(5):1319–28. 10.1007/s00125-012-2492-6 [DOI] [PubMed] [Google Scholar]
- 38.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and and beta-cell function from fasting plsma glucose and insulin concentration in man. Diabetologia 1985;28:412–419. [DOI] [PubMed] [Google Scholar]
- 39.Aas IH. Guidelines for rating Global Assessment of Functioning (GAF). Arch Gen Psychiatry 2011; 20;10:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rabinowitz J, Mehnert A, Eerdekens M. To what extent do the PANSS and CGI-S overlap? J ClinPsychopharmacol 2006;26:303–307. [DOI] [PubMed] [Google Scholar]
- 41.Mitchell AJ. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int J Geriatr Psychiatry. 2008; 23(11): 1191–1202 10.1002/gps.2053 [DOI] [PubMed] [Google Scholar]
- 42.Mitchell AJ. A meta-analysis of the accuracy of the mini-mental state examination in the detection of dementia and mild cognitive impairment. J Psychiatr Res. 2009;43(4):411–31. 10.1016/j.jpsychires.2008.04.014 [DOI] [PubMed] [Google Scholar]
- 43.Mitchell AJ. Sensitivity × PPV is a recognized test called the clinical utility index (CUI+). Eur J Epidemiol. 2011;26(3):251–2 10.1007/s10654-011-9561-x [DOI] [PubMed] [Google Scholar]
- 44.Gianfrancesco F, Pesa J, Wang RH, et al. Assessment of anti psychotic-related risk of diabetes mellitus in a Medicaid psychosis population: Sensitivity to study design. American Journal Of Health-System Pharmacy 2006; Volume: 63 Issue: 5 Pages: 431–441 [DOI] [PubMed] [Google Scholar]
- 45.Smith M; Hopkins D; Peveler RC, et al. First- V. second-generation antipsychotics and risk for diabetes in schizophrenia: systematic review and meta-analysis. British Journal Of Psychiatry 2008; 192 Issue: 6 Pages: 406–411 10.1192/bjp.bp.107.037184 [DOI] [PubMed] [Google Scholar]
- 46.Ramaswamy K, Masand PS, Nasrallah HA. Do certain atypical antipsychotics increase the risk of diabetes? A critical review of 17 pharmacoepidemiologic studies. Ann Clin Psychiatry 2006;18:183–194. [DOI] [PubMed] [Google Scholar]
- 47.Rajkumar AP1, Horsdal HT1, Wimberley T1, Cohen D1, Mors O1, Børglum AD1, et al. Endogenous and Antipsychotic-Related Risks for Diabetes Mellitus in Young People with Schizophrenia: A Danish Population-Based Cohort Study. Am J Psychiatry. 2017. 1;174(7):686–694 10.1176/appi.ajp.2016.16040442 [DOI] [PubMed] [Google Scholar]
- 48.Mitchell AJ, Vancampfort D, Sweers K, van Winkel R, Yu W, De Hert M. Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders—a systematic review and meta-analysis. Schizophr Bull. 2013;39(2):306–18. 10.1093/schbul/sbr148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vancampfort D, Vansteelandt K, Correll CU, Mitchell AJ, De Herdt A, Sienaert P, et al. Metabolic syndrome and metabolic abnormalities in bipolar disorder: A meta-analysis of prevalence rates and moderators. Am J psychiatry 2013; 10.1176/appi.ajp.2012.12050620 [DOI] [PubMed] [Google Scholar]
- 50.Vancampfort D, Correll CU, Wampers M, Sienaert P, Mitchell AJ, De Herdt A, et al. Metabolic syndrome and metabolic abnormalities in patients with major depressive disorder: a meta-analysis of prevalences and moderating variables. Psychol Med. 2014;44(10):2017–28. 10.1017/S0033291713002778 [DOI] [PubMed] [Google Scholar]
- 51.De Hert M, Vancampfort D, Correll CU, Mercken V, Peuskens J, Sweers K, et al. Guidelines for screening and monitoring of cardiometabolic risk in schizophrenia: systematic evaluation. Br J Psychiatry. 2011;199(2):99–105. 10.1192/bjp.bp.110.084665 [DOI] [PubMed] [Google Scholar]
- 52.Mitchell AJ, Delaffon V, Vancampfort D, Correll CU, De Hert M. Guideline concordant monitoring of metabolic risk in people treated with antipsychotic medication: systematic review and meta-analysis of screening practices. Psychol Med. 2012;42(1):125–47. 10.1017/S003329171100105X [DOI] [PubMed] [Google Scholar]
- 53.Hardy S, Hinks P, Gray R. Screening for cardiovascular risk in patients with severe mental illness in primary care: A comparison with patients with diabetes. J Ment Health. 2013;22(1):42–50. 10.3109/09638237.2012.759194 [DOI] [PubMed] [Google Scholar]
- 54.Mitchell AJ, Hardy S. Surveillance for metabolic risk factors in patients with severe mental illness vs diabetes: National Comparison of Screening Practices. Psychiatric Services 2013. in press [DOI] [PubMed] [Google Scholar]
- 55.Osborn DP, Baio G, Walters K, Petersen I, Limburg H, Raine R, et al. Inequalities in the provision of cardiovascular screening to people with severe mental illnesses in primary care: cohort study in the United Kingdom THIN Primary Care Database 2000–2007. Schizophr Res. 2011;129(2–3):104–10. 10.1016/j.schres.2011.04.003 [DOI] [PubMed] [Google Scholar]
- 56.Owora AH1.Commentary: Diagnostic Validity and Clinical Utility of HbA1c Tests for Type 2 Diabetes Mellitus .Curr Diabetes Rev. 2018;14(2):196–199. 10.2174/1573399812666161129154559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.American Diabetes Association.Standards of medical care in diabetes—2012.Diabetes Care. 2012;35Suppl 1:S11–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.American Diabetes A. Standards of medical care in diabetes 2011. Diabetes Care 2011; 34 (suppl 1): S11–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Krein SL, Bingham CR, McCarthy JF, Mitchinson A, Payes J, Valenstein M. Diabetes treatment among VA patients with comorbid serious mental illness. Psychiatr Serv. 2006;57:1016Y1021. [DOI] [PubMed] [Google Scholar]
- 60.Brown CH, Medoff D, Dickerson FB, Kreyenbuhl JA, Goldberg RW, Fang L, et al. Long-term glucose control among type 2 diabetes patients with and without serious mental illness. J NervMent Dis. 2011;;199(11):899–902. [DOI] [PubMed] [Google Scholar]
- 61.Castilla-Puentes R. Effects of psychotropics on Glycated hemoglobin (HbA1c) in a cohort of bipolar patients. Bipolar Disord. 2007;9(7):772–8. [DOI] [PubMed] [Google Scholar]
- 62.Nasrallah HA, Meyer JM, Goff DC, et al. Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006;86:15–22. [DOI] [PubMed] [Google Scholar]
- 63.Mitchell AJ, Malone D, Doebbeling CC. Quality of medical care for people with and without comorbid mental illness and substance misuse: systematic review of comparative studies. Br J Psychiatry. 2009;194(6):491–9. 10.1192/bjp.bp.107.045732 [DOI] [PubMed] [Google Scholar]
- 64.Mai Q, D'Arcy C, Holman J, Sanfilippo FM, Emery JD, Preen DB. Mental illness related disparities in diabetes prevalence, quality of care and outcomes: a population-based longitudinal study. BMC Medicine 2011;9:118 10.1186/1741-7015-9-118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aekplakorn W, Bunnag P, Woodward M, Sritara P, Cheepudomwit S, Yamwong S, et al. : A risk score for predicting incident diabetes in the Thai population. Diabetes Care 2006;29:1872–1877. [DOI] [PubMed] [Google Scholar]
- 66.Balkau B, Lange C, Fezeu L, Tichet J, de Lauzon-Guillain B, Czernichow S, et al. Predicting diabetes: clinical, biological, and genetic approaches: data from the Epidemiological Study on the Insulin Resistance Syndrome (DESIR). Diabetes Care 2008;31:2056–2061. 10.2337/dc08-0368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gao WG, Qiao Q, Pitkäniemi J, Wild S, Magliano D, Shaw J, et al. Risk prediction models for the development of diabetes in Mauritian Indians. Diabet Med 2009;16:996–1002. [DOI] [PubMed] [Google Scholar]
- 68.Kahn HS, Cheng YJ, Thompson TJ, Imperatore G, Gregg EW. Two risk-scoring systems for predicting incident diabetes mellitus in U.S. adults age 45 to 64 years. Ann Intern Med 2009;150:741–751 [DOI] [PubMed] [Google Scholar]
- 69.Lindstrom J, Tuomilehto J. The diabetes risk score: a practical tool to predict type 2 diabetes risk. Diabetes Care 2003;26:725–31. [DOI] [PubMed] [Google Scholar]
- 70.Robinson CA, Agarwal G, Nerenberg K. Validating the CAN-RISK prognostic model for assessing diabetes risk in Canada’s multi-ethnic population. Chronic Dis Inj Can 2011;32:19–31 [PubMed] [Google Scholar]
- 71.Schulze M, Hoffman K, Boeing H, Linseisen J, Rohrmann S, Mohlig M, et al. An accurate risk score based on anthropometric, dietary, and lifestyle factors to predict the development of type 2 diabetes. Diabetes Care 2007;30:510–5. [DOI] [PubMed] [Google Scholar]
- 72.Al-Lawati JA, Tuomilehto J. Diabetes risk score in Oman: a tool to identify prevalent type 2 diabetes among Arabs of the Middle East. Diabetes Res ClinPract 2007;77:438–44. [DOI] [PubMed] [Google Scholar]
- 73.Baan CA, Ruige JB, Stolk RP, Witteman JCM, Dekker JM, Heine RJ, et al. Performance of a predictive model to identify undiagnosed diabetes in a health care setting. Diabetes Care 1999;22:213–219. [DOI] [PubMed] [Google Scholar]
- 74.Bang H, Edwards A, Bomback A, Ballantyne C, Brillon D, Callahan M, et al. Development and validation of a patient self-assessment score for diabetes risk. Ann Intern Med 2009;151:775–83. 10.7326/0003-4819-151-11-200912010-00005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gao WG, Dong YH, Pang ZC, Nan HR, Wang SJ, Ren J, et al. : A simple Chinese risk score for undiagnosed diabetes. Diabet Med 2010;27:274–281. 10.1111/j.1464-5491.2010.02943.x [DOI] [PubMed] [Google Scholar]
- 76.Glümer C, Carstensen B, Sabdbaek A, Lauritzen T, Jørgensen T, Borch-Johnsen K: A Danish diabetes risk score for targeted screening. Diabetes Care 2004;27:727–733. [DOI] [PubMed] [Google Scholar]
- 77.Pires de Sousa AG, Pereira AC, Marquezine GF, Marques do Nascimento-Neto R, Freitas SN, Nicolato RLdC, et al. Derivation and external validation of a simple prediction model for the diagnosis of type 2 diabetes mellitus in the Brazilian urban population. Eur J Epidemiol 2009;24:101–109. 10.1007/s10654-009-9314-2 [DOI] [PubMed] [Google Scholar]
- 78.Ramachandran A, Snehalatha C, Vijay V, Wareham N, Colagiuri S. Derivation and validation of diabetes risk score for urban Asian Indians. Diabetes Res ClinPract 2005;70:63–70. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data is within the paper and its Supporting Information files.