Abstract
The first synthesis of a pentacyclic ambiguine (ambiguine P) is reported. The synthesis takes advantage of sequential alkylations of an indole core to rapidly construct the pentacyclic framework of the natural product. Key to the success of the synthesis was the use of a Nicholas reaction to alkylate at C2, crafting a fused seven-membered ring that is characteristic of the pentacyclic ambiguines, as well as the use of an amidedirected functionalization at C12 to set a requisite quaternary center. A versatile late-stage intermediate was prepared that may be applicable to the synthesis of the other pentacyclic ambiguines.
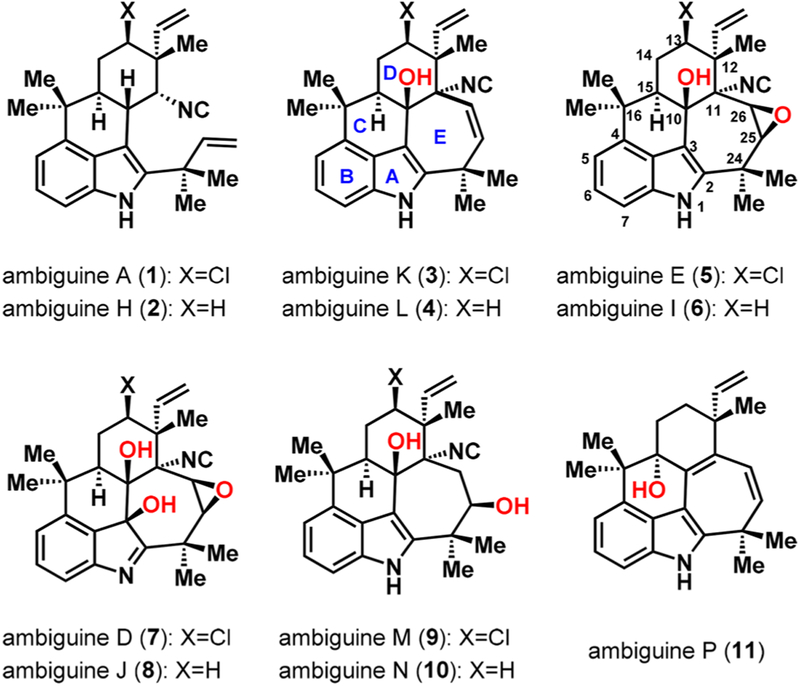
The ambiguine natural products,1 selected examples of which are shown in Figure 1, are a subset of a larger family of indole secondary metabolites known as the hapalindoles.2 With the exception of four ambiguines, including A (1) and H (2), which are tetracyclic, the ambiguines possess a fused pentacyclic scaffold featuring a characteristic seven-membered ring moiety. Since the first reported isolation of ambiguine congeners in 1992 from Fischerella ambigua,1a there have been continued efforts aimed at their total synthesis3–5 given their intriguing structures. In addition, some members of the hapalindole family, such as the fischerindoles6 and welwitindolinones,6b,7 exert a broad spectrum of biological activities, including antimicrobial, antifungal, insecticidal, and anticancer activity as well as phytotoxicity.1a,c,2a,c,d,6b,8 Therefore, there has also been interest in exploring the function of the ambiguines in a biological context. While preliminary bioactivity studies on the ambiguines have been conducted,1c–f comprehensive studies have yet to be undertaken. It is our anticipation that structurally related derivatives of the ambiguines, which may be accessed en route to their chemical preparation, may also possess interesting activity. As such, a total synthesis of the pentacyclic ambiguines could unveil their biological potential as well as set the stage for an understanding of their biosynthesis, especially the poorly understood late-stage oxidations of the fused seven-membered ring.9a–c
Figure 1.
Selected ambiguine natural products.
No syntheses exist for the pentacyclic ambiguines (3–11) despite numerous reported attempts.3 On the other hand, successful syntheses of tetracyclic ambiguine H (2) were disclosed by the Baran group in 20074 and the Maji group in 2018.5 Presumably, the seven-membered E ring that is resident in the pentacyclic ambiguines poses an added synthetic challenge. Herein we report a strategy for the preparation of the pentacyclic ambiguines that has culminated in the total synthesis of (−)-ambiguine P (11).
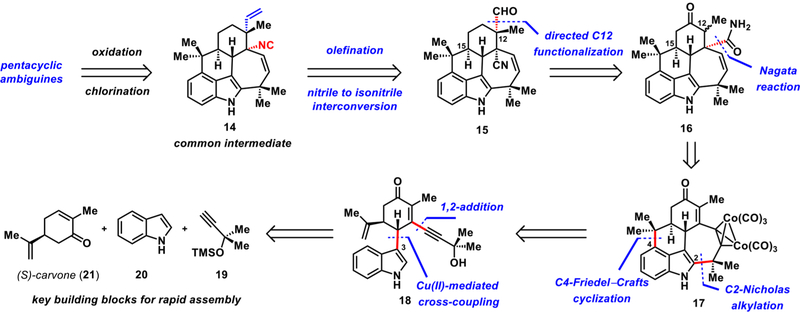
In our retrosynthesis (Scheme 1), we envisioned that ambiguine P and related pentacyclic congeners could arise from common intermediate 14 via late-stage oxidation and/or chlorination. Pentacyclic isocyanide 14 could be accessed from 15, which in the forward sense would require olefination of the formyl group and conversion of the angular nitrile group to an isonitrile group. The C12 quaternary center in 15 would be installed using the primary amide of 16 as a directing group. To access the pentacyclic core of 16, we envisioned sequential indole C3, C2, and C4 functionalizations, which would ultimately allow the use of readily available starting materials (see 16 ⇒ 19 + 20 + 21). Notably, to forge the seven-membered ring of the pentacyclic framework, a cobaltmediated Nicholas alkylation at C2 of 18 would be employed, followed by Friedel–Crafts cyclization at C4 to build the C ring.
Scheme 1.
Retrosynthesis
The key synthetic challenge that had to be considered in preparing the pentacyclic ambiguine natural products lay in the diastereoselective α-functionalization at C12 (see the structure of 5 in Figure 1 for numbering). The stereochemical outcome of this α-functionalization on related systems10 is dictated by the stereochemistry at C15 leading to the cis-disposed stereodiad, whereas the trans-disposed grouping is required in our case (see the relative stereochemistry between C12 and C15 in 5). In our approach, we take advantage of a directed α-functionalization using the amide group in 16 to overcome this challenge.11
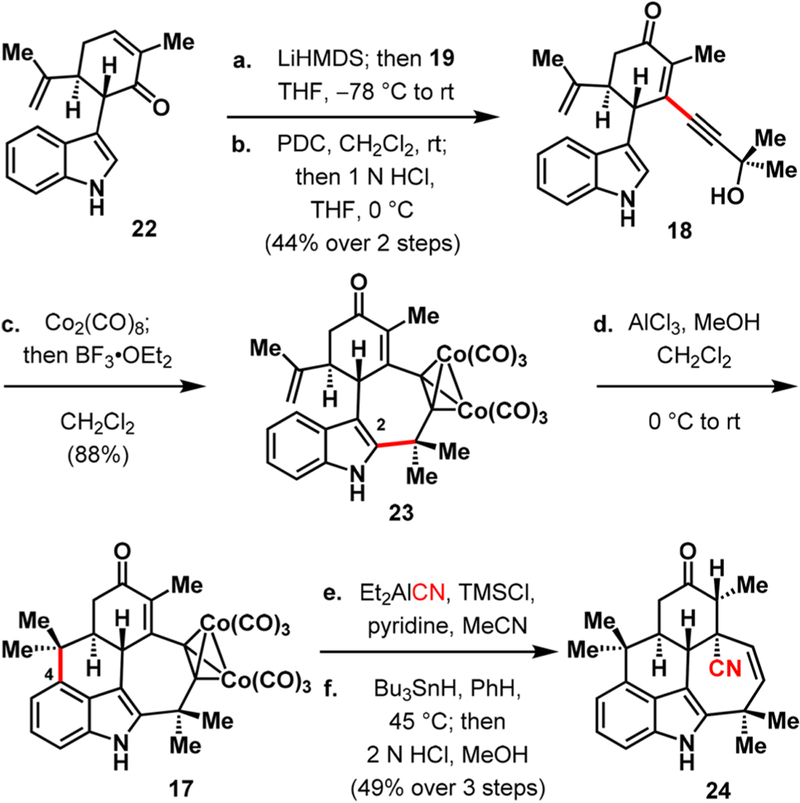
Our synthesis commenced with the elegant Cu(II)-mediated oxidative coupling of indole (20) with (S)-carvone (21) to afford C3-functionalized indole 22 (Scheme 2), following the precedent of Baran.10 Subsequent 1,2-addition of propargylic alcohol 19 to the enone carbonyl of 22 and Babler–Dauben oxidative transposition12 afforded enone 18 in 44% yield over the two steps. Cyclization to forge the seven-membered ring that is characteristic of the pentacyclic ambiguines was accomplished in excellent yield through an intramolecular Nicholas reaction13 wherein the indole C2 site preferentially reacted over C3 and C4. While site-selective Friedel–Crafts alkylation at C4 of the indole nucleus is often challenging because of competition with C2 and C3,14 with the seven-membered E ring in place in 23, we found that the combination of 15 equiv each of aluminum chloride and methanol successfully furnished the C ring in the desired pentacycle 17. Conjugate addition of cyanide to the enone moiety using Nagata’s reagent15 installed a nitrile group that would later serve both as a directing group and a surrogate for the isonitrile moiety. Concomitant trapping of the enolate that resulted from conjugate addition of the cyanide with trimethylsilyl chloride (TMSCl) accomplished in situ protection of the carbonyl group. At this stage, reductive removal of the dicobalt group using tributyltin hydride16 yielded pentacycle 24 in 49% yield over three steps.
Scheme 2.
Construction of the Pentacyclic Core
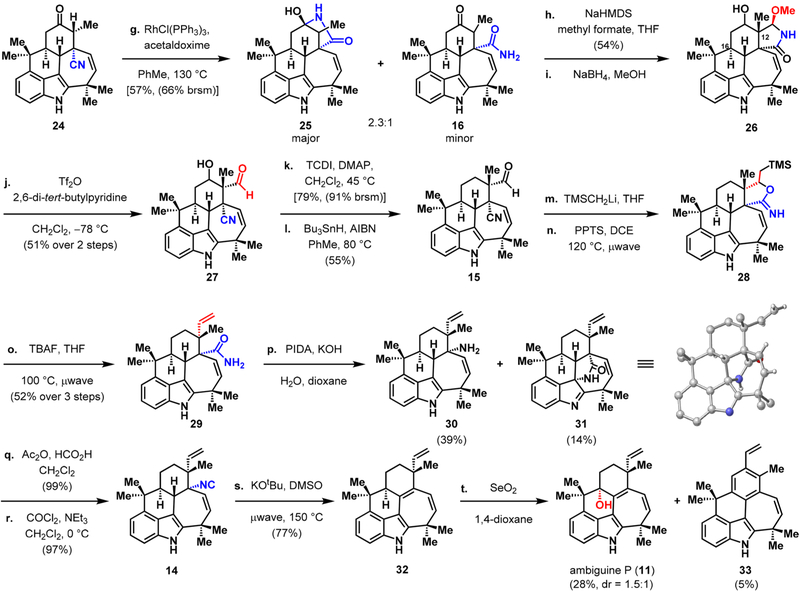
With access to the key pentacycle 24 in gram quantities over a seven-step sequence from carvone, we sought to address the challenge of setting the C12 quaternary center. Not surprisingly, direct α-vinylation at C1217 yielded the transdisposed vinyl group (with respect to C15) in accord with Fürst–Plattner selectivity.18 To overcome this undesired diastereoselectivity, we sought to employ the nitrile group as a directing group. Thus, Rh(I)-catalyzed nitrile hydration19 gave a mixture of 25 and 16 (Scheme 3). Treatment of this mixture with methyl formate under basic conditions afforded the hemiaminal ether in 54% yield, setting the C12 quaternary center with complete selectivity for the desired stereochemistry. Reduction of the ketone group with sodium borohydride generated alcohol 26.20
Scheme 3.
Setting the Quaternary Center at C12 and Completion of Ambiguine P
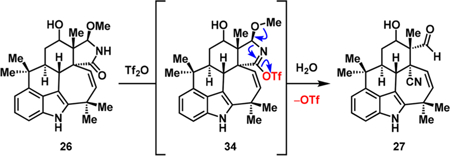
Ring opening of hemiaminal ether 26 to give β-cyano aldehyde 27 occurred in 51% yield using triflic anhydride.21 Presumably, the triflate is ejected upon aqueous workup, which leads to opening of the hemiaminal ether ring.22 At this stage, transforming 27 by Barton–McCombie deoxygenation23 of the corresponding thiocarbamate intermediate gave 15 in 43% yield over two steps. While exploring the olefination and nitrile hydration sequence, we discovered that these two transformations could be achieved in a single step. In the event, treatment of 15 with TMSCH2Li produced the secondary alcohol, which engaged the nitrile group in the presence of pyridinium p-toluenesulfonate (PPTS)24 under microwave irradiation. Subsequent cleavage of the TMS group with tetrabutylammonium fluoride (TBAF) yielded the primary amide and the vinyl group, resulting in an effective marriage of a Peterson-type olefination25 and nitrile hydration. At this stage, it was necessary to convert the primary amide at C11 to an isonitrile, a functional group that is present in the majority of ambiguine natural products. Hypervalent iodine-mediated Hofmann rearrangement26 of amide 29 progressed in 39% yield to give the desired tertiary amine 30 along with indolenine 31, which presumably arose from competing aziridination of the indole C2–C3 double bond27 followed by ring opening. Subjecting the intermediate tertiary amine (30) to a formylation–dehydration sequence yielded pentacyclic isonitrile 14. Sequential elimination of the isonitrile and allylic oxidation using selenium dioxide gave ambiguine P (11) as a mixture of diastereomers (1.5:1 ratio in favor of 11).
In summary, the first total synthesis of a pentacyclic ambiguine has been achieved in 20 steps from C3-functionalized indole 22, which is readily available in multigram quantities in a single step from commercial materials.10 Overall, our approach to the pentacyclic ambiguines highlights rapid construction of the pentacyclic skeleton through sequential alkylative functionalizations of indole using robust C–C bond-forming reactions as well as a novel application of an amide group to accomplish a stereoselective C12 functionalization. With ready access to pentacyclic isonitrile 14, current efforts are being directed toward its late-stage derivatization to access other pentacyclic members of the ambiguine family that retain the isonitrile group.
Supplementary Material
ACKNOWLEDGMENTS
We are grateful to the NIH NIGMS (R01 086374) for support of this work. R.E.J. thanks the NIH for fellowship support (F31GM123603). We are indebted to Prof. David Sherman (University of Michigan) and Vikram Shende (University of Michigan) for helpful discussions and efforts toward the biosynthesis of the pentacyclic ambiguines as well as Dr. Svitlana Kulyk (Theravance BioPharma) for her mentorship and support during the initial exploration of this work.
Footnotes
Supporting Information
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/jacs.8b13388.
Experimental details and spectroscopic data (PDF)
Crystallographic data for 31 (CIF)
Crystallographic data for S3 (CIF)
The authors declare no competing financial interest.
REFERENCES
- (1).(a) Smitka TA; Bonjouklian R; Doolin L; Jones ND; Deeter JB; Yoshida WY; Prinsep MR; Moore RE; Patterson GML Ambiguine isonitriles, fungicidal hapalindole-type alkaloids from three genera of blue-green algae belonging to the Stigonemataceae. J. Org. Chem 1992, 57, 857. [Google Scholar]; (b) Huber U; Moore RE; Patterson GML Isolation of a nitrile-containing indole alkaloid from the terrestrial blue-green Alga Hapalosiphon delicatulus. J. Nat. Prod 1998, 61, 1304. [DOI] [PubMed] [Google Scholar]; (c) Raveh A; Carmeli S Antimicrobial Ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J. Nat. Prod 2007, 70, 196. [DOI] [PubMed] [Google Scholar]; (d) Mo S; Krunic A; Chlipala G; Orjala J Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod 2009, 72, 894. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Mo S; Krunic A; Santarsiero BD; Franzblau SG; Orjala J Hapalindole-related alkaloids from the cultured cyanobacterium Fischerella ambigua. Phytochemistry 2010, 71, 2116. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Walton K; Gantar M; Gibbs PDL; Schmale MC; Berry JP Indole alkaloids from Fischerella inhibit vertebrate development in the zebrafish (Danio rerio) embryo model. Toxins 2014, 6, 3568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).(a) Moore RE; Cheuk C; Patterson GML Hapalindoles: new alkaloids from the blue-green alga Hapalosiphon fontinalis. J. Am. Chem. Soc 1984, 106, 6456. [Google Scholar]; (b) Moore RE; Cheuk C; Yang XQG; Patterson GML; Bonjouklian R; Smitka TA; Mynderse JS; Foster RS; Jones ND; Swartzendruber JK; Deeter JB Hapalindoles, antibacterial and antimycotic alkaloids from the cyanophyte Hapalosiphon fontinalis. J. Org. Chem 1987, 52, 1036. [Google Scholar]; (c) Klein D; Daloze D; Braekman JC; Hoffmann L; Demoulin V New hapalindoles from the cyanophyte Hapalosiphon laingii. J. Nat. Prod 1995, 58, 1781. [Google Scholar]; (d) Becher PG; Keller S; Jung G; Sussmuth RD; Juttner F Insecticidal activity of 12-epi-hapalindole J isonitrile. Phytochemistry 2007, 68, 2493. [DOI] [PubMed] [Google Scholar]; (e) Kim H; Lantvit D; Hwang CH; Kroll DJ; Swanson SM; Franzblau SG; Orjala J Indole alkaloids from two cultured cyanobacteria, Westiellopsis sp. and Fischerella muscicola. Bioorg. Med. Chem 2012, 20, 5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).(a) Chandra A; Viswanathan R; Johnston JN Synthesis of the ABC-and D-ring systems of the indole alkaloid ambiguine. Org. Lett 2007, 9, 5027. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Rafferty RJ; Williams RM Synthetic studies on the ambiguine family of alkaloids: construction of the ABCD ring system. Tetrahedron Lett. 2011, 52, 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Rafferty RJ; Williams RM Formal Synthesis of Hapalindole O and Synthetic Efforts towards Hapalindole K and Ambiguine A. Heterocycles 2012, 86, 219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).(a) Baran PS; Maimone TJ; Richter JM Total synthesis of marine natural products without using protecting groups. Nature 2007, 446, 404. [DOI] [PubMed] [Google Scholar]; (b) Maimone TJ; Ishihara Y; Baran PS Scalable total syntheses of (–)-hapalindole U and (+)-ambiguine H. Tetrahedron 2015, 71, 3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Sahu S; Das B; Maji MS Stereodivergent total synthesis of hapalindoles, fisherindoles, hapalonamide H, and ambiguine H alkaloids by developing a biomimetic, redox-neutral, cascade Prinstype cyclization. Org. Lett 2018, 20, 6485. [DOI] [PubMed] [Google Scholar]
- (6).(a) Park A; Moore RE; Patterson GML Fischerindole L, a new isonitrile from the terrestrial blue-green alga Fischerella muscicola. Tetrahedron Lett 1992, 33, 3257. [Google Scholar]; (b) Stratmann K; Moore RE; Bonjouklian R; Deeter JB; Patterson GML; Shaffer S; Smith CD; Smitka TA Welwitindolinones, unusual alkaloids from the blue-green algae Hapalosiphon welwitschii and Westiella intricata. Relationship to fischerindoles and hapalindoles. J. Am. Chem. Soc 1994, 116, 9935. [Google Scholar]; (c) Kim H; Krunic A; Lantvit D; Shen Q; Kroll DJ; Swanson SM; Orjala J Nitrile-containing fischerindoles from the cultured cyanobacterium Fischerella sp. Tetrahedron 2012, 68, 3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jimenez JI; Huber U; Moore RE; Patterson GML Oxidized welwitindolinones from terrestrial Fischerella spp. J. Nat. Prod 1999, 62, 569. [DOI] [PubMed] [Google Scholar]
- (8).(a) Doan NT; Rickards RW; Rothschild JM; Smith GD Allelopathic actions of the alkaloid 12-epi-hapalindole E isonitrile and calothrixin A from cyanobacteria of the genera Fischerella and Calothrix. J. Appl. Phycol 2000, 12, 409. [Google Scholar]; (b) Doan NT; Stewart PR; Smith GD Inhibition of bacterial RNA polymerase by the cyanobacterial metabolites 12-epi-hapalindole E isonitrile and calothrixin A. FEMS Microbiol. Lett 2001, 196, 135. [DOI] [PubMed] [Google Scholar]; (c) Asthana R; Srivastava A; Singh A; Singh S; Nath G; Srivastava R; Srivastava B; Deepali. Identification of an antimicrobial entity from the cyanobacterium Fischerella sp. isolated from bark of Azadirachta indica (Neem) tree. J. Appl. Phycol 2006, 18, 33. [Google Scholar]; (d) Smith CD; Zilfou JT; Stratmann K; Patterson GM; Moore RE Welwitindolinone analogues that reverse P-glycoprotein-mediated multiple drug resistance. Mol. Pharmacol 1995, 47, 241. [PubMed] [Google Scholar]; (e) Zhang X; Smith CD Microtubule effects of welwistatin, a cyanobacterial indolinone that circumvents multiple drug resistance. Mol. Pharmacol 1996, 49, 288. [PubMed] [Google Scholar]
- (9).(a) Hillwig ML; Zhu Q; Liu X Biosynthesis of ambiguine indole alkaloids in cyanobacterium Fischerella ambigua. ACS Chem. Biol 2014, 9, 372. [DOI] [PubMed] [Google Scholar]; (b) Li S; Lowell AN; Yu F; Raveh A; Newmister SA; Bair N; Schaub JM; Williams RM; Sherman DH Hapalindole/ambiguine biogenesis is mediated by a cope rearrangement, C–C bond-forming cascade. J. Am. Chem. Soc 2015, 137, 15366. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Liu X; Hillwig ML; Koharudin LMI; Gronenborn AM Unified biogenesis of ambiguine, fischerindole, hapalindole and welwitindolinone: identification of a monogeranylated indolenine as a cryptic common biosynthetic intermediate by an unusual magnesium-dependent aromatic prenyltransferase. Chem. Commun 2016, 52, 1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).(a) Baran PS; Richter JM Direct coupling of indoles with carbonyl compounds: short, enantioselective, gram-scale synthetic entry into the hapalindole and fischerindole alkaloid families. J. Am. Chem. Soc 2004, 126, 7450. [DOI] [PubMed] [Google Scholar]; (b) Richter JM; Ishihara Y; Masuda T; Whitefield BW; Llamas T; Pohjakallio A; Baran PS Enantiospecific total synthesis of the hapalindoles, fischerindoles, and welwitindolinones via a redox economic approach. J. Am. Chem. Soc 2008, 130, 17938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).For a previous example of a directed functionalization in the synthesis of the hapalindoles, see:; Fukuyama T; Chen X Stereocontrolled synthesis of (–)-hapalindole G. J. Am. Chem. Soc 1994, 116, 3125. [Google Scholar]
- (12).(a) Babler JH; Coghlan MJ A facile method for the bishomologation of ketones to α,β-unsaturated aldehydes: application to the synthesis of the cyclohexanoid components of the boll weevil sex attractant. Synth. Commun 1976, 6, 469. [Google Scholar]; (b) Dauben WG; Michno DM Direct oxidation of tertiary allylic alcohols. A simple and effective method for alkylative carbonyl transposition. J. Org. Chem 1977, 42, 682. [Google Scholar]
- (13).(a) Djurdjevic S; Yang F; Green JR Intramolecular Nicholas reactions in the synthesis of dibenzocycloheptanes. Synthesis of allocolchicine NSC 51046 and analogues and the formal synthesis of (–)-allocolchicine. J. Org. Chem 2010, 75, 8241. [DOI] [PubMed] [Google Scholar]; (b) Nicholas KM; Pettit R On the stability of α-(alkynyl)dicobalt hexacatbonyl carbonium ions. J. Organomet. Chem 1972, 44, C21. [Google Scholar]; (c) Schreiber SL; Sammakia T; Crowe WE Lewis acid mediated version of the Nicholas reaction: synthesis of syn-alkylated products and cobaltcomplexed cycloalkynes. J. Am. Chem. Soc 1986, 108, 3128. [Google Scholar]; (d) Ni R; Mitsuda N; Kashiwagi T; Igawa K; Tomooka K Heteroatom-embedded medium-sized cycloalkynes: concise synthesis, structural analysis, and reactions. Angew. Chem., Int. Ed 2015, 54, 1190. [DOI] [PubMed] [Google Scholar]
- (14).For previous attempts at Friedel–Crafts reactions at indole C4 of related systems, see ref 3c and:; (a) Chandra A; Johnston JN Total synthesis of the chlorine-containing hapalindoles K, A, and G. Angew. Chem., Int. Ed 2011, 50, 7641. [DOI] [PMC free article] [PubMed] [Google Scholar]; For a report of a purported C4 cyclization, see:; (b) Bonjouklian R; Moore RE; Patterson GML Acid-catalyzed reactions of hapalindoles. J. Org. Chem 1988, 53, 5866. [Google Scholar]; For a discussion of the structural characterization error made in ref 14b, see:; (c) Vaillancourt V; Albizati KF Synthesis and absolute configuration of (+)-hapalindole Q. J. Am. Chem. Soc 1993, 115, 3499. [Google Scholar]
- (15).Nagata W; Yoshioka M Diethylaluminum cyanide. Org. Synth 1972, 52, 90. [Google Scholar]
- (16).Hosokawa S; Isobe M Reductive decomplexation of biscobalthexacarbonyl acetylenes into olefins. Tetrahedron Lett. 1998, 39, 2609. [Google Scholar]
- (17).See the Supporting Information for the experimental procedures and characterization data.
- (18).Fürst A; Plattner PA Über Steroide und Sexualhormone. 160. Mitteilung. 2α, 3α-und 2β, 3β-Oxido-chlolestane; Konfiguration der 2-Oxy-cholestane. Helv. Chim. Acta 1949, 32, 275. [DOI] [PubMed] [Google Scholar]
- (19).Lee J; Kim M; Chang S; Lee HY Anhydrous hydration of nitriles to amides using aldoximes as the water source. Org. Lett 2009, 11, 5598. [DOI] [PubMed] [Google Scholar]
- (20).For mechanistic proposals regarding the transformation of 16 to 26, see page S10 in the Supporting Information.
- (21).For activation of amides using Tf2O, see:; (a) Charette AB; Chua P A new mild method for the cleavage of the amide bond: conversion of secondary and tertiary amides to esters. Synlett 1998, 1998, 163. [Google Scholar]; (b) Charette AB; Grenon M Spectroscopic studies of the electrophilic activation of amides with triflic anhydride and pyridine. Can. J. Chem 2001, 79, 1694. [Google Scholar]
-
(22).This transformation is hypothesized to proceed as follows:
- (23).Barton DHR; McCombie SW A new method for deoxygenation of secondary alcohols. J. Chem. Soc., Perkin Trans. 1 1975, 1 (0), 1574. [Google Scholar]
- (24).Xu S; Ciufolini MA Formal synthesis of (±)-tetrodotoxin via the oxidative amidation of a phenol: on the structure of the Sato lactone. Org. Lett 2015, 17, 2424. [DOI] [PubMed] [Google Scholar]
- (25).(a) Peterson DJ Carbonyl olefination reaction using silylsubstituted organometallic compounds. J. Org. Chem 1968, 33, 780. [Google Scholar]; (b) Ager DJ The Peterson reaction. Synthesis 1984, 1984, 384. [Google Scholar]
- (26).Zagulyaeva AA; Banek CT; Yusubov MS; Zhdankin VV Hofmann rearrangement of carboxamides mediated by hypervalent iodine species generated in situ from iodobenzene and oxone: reaction scope and limitations. Org. Lett 2010, 12, 4644. [DOI] [PubMed] [Google Scholar]
- (27).Kumar PR Addition of phthalimidonitrene to substituted indoles. Heterocycles 1987, 26, 1257. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.