Abstract
Background:
Surveys of patients with cardiovascular disease have suggested that “home-time”—being alive and out of any healthcare institution—is a prioritized outcome. This novel measure has not been studied among patients with heart failure (HF).
Objectives:
This study sought to characterize home-time following hospitalization for HF and to assess its relationship with patient characteristics and traditionally reported clinical outcomes.
Methods:
Using Get With The Guidelines-Heart Failure registry data, patients with a HF hospitalization and discharged alive between 2011 to 2014 and age ≥65 years were identified. Using Medicare claims, post-discharge home-time over 30-day and 1-year follow-up was calculated for each patient as the number of days alive and spent outside of a hospital, skilled nursing facility (SNF), or rehabilitation facility.
Results:
Among 59,736 patients, 57,992 (97.1%) and 42,153 (70.6%) had complete follow-up for home-time calculation through 30 days and 1 year, respectively. Mean (standard deviation) home-time was 21.6 (11.7) days at 30 days and 243.9 (137.6) days at 1 year. Contributions to reduced home-time varied by follow-up period, with days spent in SNF being the largest contributor though 30 days and death being the largest contributor through 1 year. Over 1 year, 2,044 (4.8%) patients had no home-time following index hospitalization discharge, while 8,194 (19.4%) had 365 days of home-time. In regression models, several conditions were associated with substantially reduced home-time, including pulmonary disease, renal insufficiency, and dementia. Through 1-year, home-time was highly correlated with time-to-event endpoints of death (r=0.94) and the composite of death or HF readmission (r=0.73).
Conclusions:
Home-time, which can be readily calculated from administrative claims data, is substantially reduced for many patients following hospitalization for HF and is highly correlated with traditional time-to-event mortality and hospitalization outcomes. Home-time represents a novel, easily measured, patient-centered endpoint that may reflect effectiveness of interventions in future HF studies.
Keywords: patient-centered, outcomes, heart failure, hospitalization, post-discharge
CONDENSED ABSTRACT
“Home-time”—being alive and out of any healthcare institution—is a prioritized outcome among patients with cardiovascular disease, but has not been studied among heart failure (HF) patients. Using administrative claims data, post-discharge home-time was readily calculated for the vast majority of patients hospitalized for HF. Substantial reductions in home-time were seen throughout intervals of follow-up, with nursing facilities the largest contributor through 30 days and death the largest contributor through 1 year. Home-time was associated with several patient characteristics and closely correlated with time-to-event mortality and hospitalization outcomes. A home-time endpoint may be complementary to traditionally reported HF outcomes, for purposes of patient-centered care, health outcomes research, and clinical trials.
Graphical Abstract
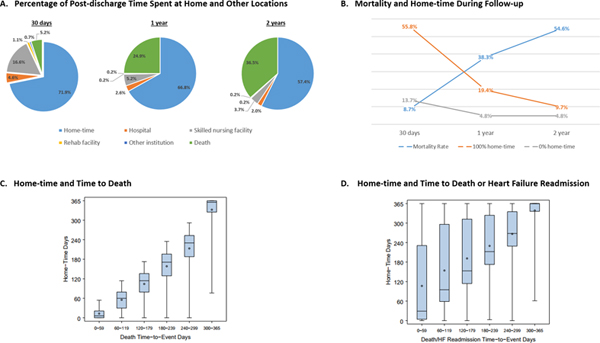
(A) Among patients hospitalized for HF, the mean proportion of post-discharge time spent at home decreases with duration of follow-up. At 30 days follow-up, most days away from home were attributed to SNF stays; at 1 year follow-up, most days away from home were due to death. (B) Post-discharge mortality rate increased with increasing duration of follow-up and the proportion of patients who spent all post-discharge time at home and no post-discharge time at home declined with longer follow-up. (C and D) Home-time is closely correlated with traditional time-to-event mortality and hospitalization outcomes. Specifically, home-time is strongly and positively correlated with the length of time patients survive free from their first event. Box plots of home-time by time-to-event secondary outcomes for mortality (C) and the composite of death or HF readmission (D) are shown. These figures present the distribution of home-time by month in which each secondary outcome occurred. Edges of box denote quartilel and quartile 3 values. Line within the box denotes median value. Circle denotes mean value. Whiskers extend to minimum and maximum values. Only patients with complete follow-up were included. As an example of box-plot interpretation, among patients who died between (and including) day 180 and day 239 after discharge, home-time ranged from 0 days to 235 days and median (Q1, Q3) home-time was 171 (129, 196) days. As an additional example, among patients who died or experienced their first post-discharge HF hospitalization between (and including) day 120 and day 179 after discharge, home-time ranged from 0 days to 362 days and median (Q1, Q3) home-time was 153 (114, 312) days. HF, heart failure; SNF, skilled nursing facility
Heart failure (HF) represents 1–2% of all hospital admissions and is the most common reason for hospitalization among patients age 65 and older.(1,2) Despite available therapy and quality improvement initiatives, HF hospitalization remains associated with high rates of mortality and readmission, as well as significant post-discharge impairment in patient quality-of-life (QOL).(3,4) Effective patient-centered communication regarding downstream clinical course remains difficult, with the majority of HF patients underestimating their risk of adverse outcomes.(5–7) Further, despite increasing attention towards incorporating patient reported outcomes (PROs) within clinical trials and regulatory decision making, there remains an unmet need for easily calculated and objective patient-centered measures as complements to conventional PROs.(8–10)
The concept of “home-time,” defined as the time a patient spends alive and out of a healthcare institution, has been studied in ischemic stroke populations where it was shown to be a robust and easily measured patient-centered outcome.(11,12) This endpoint has not been previously studied in HF populations, but may hold several key advantages. Description of home-time may be more easily understood by HF patients and may better communicate associated morbidity and mortality, including the burden of repeated hospitalizations and time spent in rehabilitation and nursing facilities. Moreover, from a clinical trial perspective, home-time could represent a novel trial endpoint that intrinsically reflects traditionally reported mortality and hospitalization events, but with a patient-centered dimension and potential added sensitivity to detect changes in downstream clinical course. In this context, we evaluated the distribution of home-time among patients hospitalized for HF and assessed correlations between home-time, patient characteristics, and traditional HF outcome measures using a national HF registry linked to Medicare claims.
METHODS
Data Source
This study utilized data from the Get With The Guidelines Heart Failure (GWTG-HF) registry, which is an ongoing observational, national, quality improvement program initiated in 2005 by the American Heart Association. Details of the design and objectives of this program have been previously described.(13,14) The registry includes patients hospitalized with a primary diagnosis of new or worsening HF or patients who develop significant HF symptoms during hospitalization such that HF was the primary diagnosis. Trained personnel at participating centers use an internet-based patient management tool (Quintiles Real-World and Late Research, Cambridge, Massachusetts) to collect patient-level information on consecutive HF patients admitted to the hospital. Collected data include patient demographics, socioeconomic status, medical history, medications, laboratory data, in-hospital treatment, in-hospital outcomes, discharge medications, discharge status, and post-discharge follow-up.
All participating centers are required to obtain institutional review board approval for the GWTG-HF protocol and follow local regulatory and privacy guidelines. Given the primary role of data collection is for quality improvement purpose, all participating centers are granted a waiver of informed consent under the Common Rule. Quintiles (Durham, NC, USA) serves as the data collection coordination center for American Heart Association GWTG programs. The Duke Clinical Research Institute serves as the data analysis center.
Participants aged 65 years and older with fee-for-service Medicare coverage were linked to Medicare data using a previously validated technique.(15) In brief, patients were robustly linked using a combination of index hospitalization dates, date of birth, sex, and location of index hospitalization. For this study, we used inpatient claims data, skilled nursing facility (SNF) claims, and the beneficiary summary file for the period from January 1, 2011 to December 31, 2014. The claims files have information about institutional stays, including service dates and associated diagnoses and procedures. The beneficiary summary file has information about mortality and program enrollment. For linked patients, the Medicare data enabled us to get more information about their index hospitalization, as well as information about post-discharge institutional stays, death, and data censoring during follow-up.
Study Design and Outcomes
The present study included GWTG-HF participants age 65 years and older who were hospitalized between January 2011 and December 2014 and discharged alive. Aside from age, which was pre-specified to allow Medicare claims linkage, there were no other formal exclusion criteria. The primary outcome of interest for this analysis was post-discharge home-time. Consistent with prior work, home-time was calculated from Medicare claims data as the number of days alive and spent outside of a hospital (i.e., not admitted to the hospital), SNF, or inpatient rehabilitation facility during a specified post-discharge time interval.(11,12) Any part of a day spent in one of these facilities counted against home-time. Hospital days from the index HF hospitalization were not included in home-time calculations. Within the Centers for Medicare and Medicaid Services (CMS) data, the CMS beneficiary summary file was used to identify days alive over a defined follow-up period. Both the inpatient claims file and the SNF claims file were used to identify time spent in a facility.
Secondary outcomes of interest included death, the composite of death or all-cause readmission, and the composite of death or HF readmission. These time-to-event outcomes were compared to and correlated with the home-time outcome. Death was determined by the presence of a death date in the CMS beneficiary summary file. All-cause readmission was determined by looking in the CMS inpatient claims files for any post-discharge admission to an acute care hospital. Heart failure readmissions were the subset of these admissions having a primary diagnosis of heart failure (ICD-9-CM diagnosis code 428.×, 402.×1, 404.×1, 404.×3). The time period for ascertainment of home-time and evaluation of secondary outcomes extended 2 years from index hospitalization discharge. The Duke University Health System Institutional Review Board approved this study using the GWTG-HF and Medicare claims data.
Statistical Analysis
Baseline patient characteristics were reported as percentages for categorical variables and as means with standard deviation or median with interquartile range for continuous variables. We summarized home-time for different follow-up intervals, including 30 days, 1 year and 2 years post-discharge. The distribution of home time was summarized using mean, standard deviation, median, and quartiles. The percentage of patients with no home-time (i.e., 0 days) and complete home-time (i.e., alive and at home for all days in a given interval) was calculated. In addition, the percentage of patients for whom home-time was not calculable due to censoring was determined for each interval. Censoring occurred either because a patient enrolled in a Medicare managed care plan during the follow-up interval or the follow-up interval extended beyond the latest date of Medicare data availability (i.e., December 31, 2014). Patients with censored data were labeled as having “incomplete” follow-up for the particular follow-up interval. Patients for whom home-time was calculable were counted as having “complete” follow-up for that interval.
For each follow-up interval, the correlation between home-time and time-to-event days for each secondary outcome (among patients for whom home-time was calculable) was determined. For patients not experiencing the event, their time-to-event was set at the length of the specific interval. The relationship between home-time and the secondary outcomes was also shown graphically using box plots. These figures present the distribution of home-time by month in which each secondary outcome occurred.
Finally, for the 1-year and 2-year follow-up intervals, median regression models were used to estimate the association between baseline patient characteristics and median home-time. There was insufficient variability in home-time at 30 days to produce meaningful estimates from the regression model. Models were first used within the overall population for a given interval, and then in analyses stratified by baseline left ventricular ejection fraction (EF). For these follow-up intervals, median regression was robust to the distribution of home-time, which had substantial numbers of patients at both the lower bound (i.e., 0 days) and the upper bound (i.e., length of the interval).
Parameter estimates from models were reported as days and reflect changes in the median home-time associated with changes in patient characteristics. Patient level characteristics included in the model were selected based on clinical knowledge and derived from the GWTG- HF case report form or, if not collected in GWTG-HF, from claims in the year prior to index admission. Variables derived from the GWTG-HF case report form included age, race, gender, admission variables (systolic blood pressure, heart rate, creatinine, EF), and comorbidities (anemia, atrial fibrillation, chronic renal insufficiency [serum creatinine >2.0 mg/dL], coronary artery disease, chronic obstructive pulmonary disease, diabetes, hypertension, hyperlipidemia, and pacemaker/implantable cardioverter defibrillator). Variables derived from claims included dementia, disability, liver disease, major psychiatric disorder, and protein-calorie malnutrition.(16) All statistical tests were 2 sided, with P<0.05 considered statistically significant. All analyses were performed using SAS software, version 9.4 (SAS Institute, Cary, NC, USA).
RESULTS
Baseline Characteristics
Overall, 59,736 patients were included in this study. Baseline patient characteristics are displayed in Table 1. Mean age was 80.7 years, median EF was 50%, and 84.4% of patients were white. Comorbidities, such as atrial fibrillation, diabetes, anemia, and chronic renal insufficiency were common. The most frequent disposition at time of discharge was home with self-care (43.7%), with an additional 25.6% of patients discharged home with home healthcare, 21.6% discharged to a SNF, and 4.1% discharged to inpatient hospice care.
Table 1.
Baseline characteristics of the study population
| Overall (N=59,736) | |
|---|---|
| Demographics | |
| Age (years), Mean (SD) | 80.7 (8.5) |
| Gender, Male | 27,273 (45.7%) |
| Race | |
| White | 50,403 (84.4%) |
| African American | 6,365 (10.7%) |
| Other/Unknown | 2,968 (5.0%) |
| Year of index hospitalization | |
| 2011 | 12,942 (21.7%) |
| 2012 | 15,039 (25.2%) |
| 2013 | 15,550 (26.0%) |
| 2014 | 16,205 (27.1%) |
| Comorbidities | |
| Anemia | 12,789 (21.4%) |
| Atrial Fibrillation | 25,897 (43.4%) |
| Chronic renal insufficiency* | 13,708 (22.9%) |
| Coronary artery disease | 31,268 (52.3%) |
| COPD | 18,617 (31.2%) |
| Diabetes mellitus | 24,681 (41.3%) |
| Hypertension | 48,727 (81.6%) |
| Hyperlipidemia | 32,829 (55.0%) |
| Pacemaker or ICD implanted | 16,876 (28.3%) |
| Index hospitalization diagnoses (claims-based) | |
| Dementia | 3,900 (6.5%) |
| Disability | 2,382 (4.0%) |
| Liver disease | 850 (1.4%) |
| Major psychiatric disorder | 1,227 (2.1%) |
| Protein-calorie malnutrition | 2,990 (5.0%) |
| Vital sign and laboratory findings at admission | |
| Systolic blood pressure (mmHg), Median (IQR) | 140 (122, 159) |
| Diastolic blood pressure (mmHg), Median (IQR) | 73 (63, 84) |
| Heart rate (bpm), Median (IQR) | 80 (70, 94) |
| Ejection fraction (%), Median (IQR) | 50 (30, 58) |
| Serum creatinine (mg/dL), Median (IQR) | 1.3 (1.0, 1.8) |
| B-type Natriuretic Peptide (pg/mL), Median | 785 (404, 1531) |
| (IQR) | |
| Hemoglobin (g/dL), Median (IQR) | 11.6 (10.2, 12.9) |
| Discharge location | |
| Home, self-care | 26,132 (43.7%) |
| Transfer to SNF | 12,891 (21.6%) |
| Transfer to other healthcare institution | 2,966 (5.0%) |
| Home, home health | 15,287 (25.6%) |
| Hospice | 2,460 (4.1%) |
| Discharge medication | |
| ACE/ARB | 31,726 (53.1%) |
| Aldosterone antagonist | 9,831 (16.5%) |
| Beta blocker | 47,086 (78.8%) |
| Digoxin | 5,536 (9.3%) |
| Diuretic | 32,838 (55.0%) |
Data presented as n (%) unless otherwise noted.
Defined per GWTG-HF case report form as chronic elevation in serum creatinine >2.0 mg/dL.
ACE, angiotensin-converting enzyme inhibitor; ARB, angiotensin II receptor blocker; COPD, chronic obstructive pulmonary disease; GWTG-HF, Get With The Guidelines Heart Failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; SD, standard deviation; SNF, skilled nursing facility
Distribution of Home-Time
From the total study population, home-time was calculated through 30 days (N=57,992; 97.1%), 1 year (N=42,153; 70.6%), and 2 years (N=26,621; 44.6%) for those with complete data on post-discharge status and location of care (Figure 1). Rates of death at each follow-up interval were 8.7%, 38.3%, and 54.6%, respectively. The distribution of home-time by duration of post-discharge follow-up is presented in Table 2. Through 1-year follow-up, mean (standard deviation) home-time was 243.9 (137.6) days and median (quartile 1, quartile 3) home-time was 322 (107, 361) days. At 30-day follow-up, most days away from home were due to SNF stays (mean 5.0 days), with lesser contributions from death and repeat hospitalization. At 1 year, the greatest contributor to reduced home-time was death (mean 90.8 days), followed by SNF (mean 18.9 days) and repeat hospitalization (mean 9.6 days). Overall, through 1 year, 2,044 (4.8%) patients had no home-time following index hospitalization discharge, while 8,194 (19.4%) had 365 days of home-time.
Figure 1.

Selection of GWTG-HF patients for home-time analysis. Home-time was assessed at 30 days, 1 year, and 2 year follow-up among patients with complete follow-up data. GWTG-HF, Get With The Guidelines Heart Failure Registry; HF, heart failure.
Table 2.
Home-Time and Time Spent in Other Locations By Duration of Post-discharge Follow-up
| 30 days | 1 year | 2 years | |
|---|---|---|---|
| Follow-up status | |||
| Incomplete, n (%) | 1,744 (2.9%) | 17,583 (29.4%) | 33,115 (55.4%) |
| Complete, n (%) | 57,992 (97.1%) | 42,153 (70.6%) | 26,621 (44.6%) |
| 0% home-time during follow-up | 7,952 (13.7%) | 2,044 (4.8%) | 1,267 (4.8%) |
| 100% home-time during follow-up | 32,352 (55.8%) | 8,194 (19.4%) | 2,576 (9.7%) |
| Died during interval, n (%) | 5,034 (8.7%) | 16,141 (38.3%) | 14,546 (54.6%) |
| Reporting days during follow-up | |||
| Home time, median (IQR) days | 30.0 (12.0, 30.0) | 322.0 (107.0, 361.0) | 496.0 (105.0, 711.0) |
| Home time, mean (SD) days | 21.6 (11.7) | 243.9 (137.6) | 418.9 (288.2) |
| Hospital, mean (SD) days | 1.4 (3.4) | 9.6 (14.1) | 14.8 (19.7) |
| SNF, mean (SD) days | 5.0 (9.9) | 18.9 (42.1) | 27.2 (63.4) |
| Rehab, mean (SD) days | 0.3 (2.1) | 0.9 (4.2) | 1.3 (5.4) |
| Other institutional, mean (SD) days | 0.2 (2.1) | 0.9 (6.3) | 1.3 (8.0) |
| Death, mean (SD) days | 1.5 (5.7) | 90.8 (132.7) | 266.5 (288.6) |
| Reporting proportion of follow-up | |||
| Home time, median (IQR) proportion | 1.000 (0.400, 1.000) | 0.882 (0.293, 0.989) | 0.679 (0.144, 0.974) |
| Home time, mean (SD) proportion | 0.719 (0.390) | 0.668 (0.377) | 0.574 (0.395) |
| Hospital, mean (SD) proportion | 0.046 (0.113) | 0.026 (0.039) | 0.020 (0.027) |
| SNF, mean (SD) proportion | 0.166 (0.328) | 0.052 (0.115) | 0.037 (0.087) |
| Rehab, mean (SD) proportion | 0.011 (0.070) | 0.002 (0.011) | 0.002 (0.007) |
| Other institutional, mean (SD) proportion | 0.007 (0.069) | 0.002 (0.017) | 0.002 (0.011) |
| Death, mean (SD) proportion | 0.052 (0.189) | 0.249 (0.364) | 0.365 (0.395) |
IQR, interquartile range; SD, standard deviation; SNF, skilled nursing facility
Home-Time and Time-to-Event Endpoints
The degree of correlation between home-time and the composite of death or all-cause readmission was moderate at all follow-up time points. The Central Illustration presents box plots displaying distributions of home-time by timing of death and timing of the composite of death or HF readmission over the course of the 1-year follow-up.
Patient Characteristics and Home-Time
In regression models, several conditions were associated with substantially reduced home-time at 1- and 2-years, including anemia, chronic renal insufficiency, chronic obstructive pulmonary disease, diabetes, dementia, and malnutrition (Table 4). Higher systolic blood pressure, lower heart rate, and higher ejection fraction were associated with more home-time.
Table 4.
Associations Between Patient Characteristics and Changes in Home-Time
| Change in Days of Home-Time (95% CI)* | ||
|---|---|---|
| 1 year | 2 years | |
| Demographics | ||
| Age (per 5 years increase) | −13.8 (−14.8, −12.8) | −61.6 (−65.0, −58.1) |
| Gender, male | 3.0 (0.7, 5.3) | −4.4 (−16.3, 7.6) |
| Race, African American | −2.3 (−5.7, 1.0) | 2.9 (−11.2, 17.0) |
| Race, Other/unknown | 4.2 (0.0, 8.4) | 28.5 (10.9, 46.1) |
| Baseline Clinical Factors | ||
| Systolic blood pressure (per 10 mmHg increase) | 4.8 (4.4, 5.2) | 19.6 (18.0, 21.1) |
| Heart rate (per 10 bpm increase) | −2.7 (−3.3, −2.1) | −8.9 (−11.6, −6.2) |
| Serum creatinine (per 1 mg/dL increase) | −0.2 (−0.4, 0.0) | −0.5 (−1.0, 0.0) |
| Ejection fraction (per 10% increase) | 1.5 (0.7, 2.3) | 10.3 (7.1, 13.5) |
| Comorbidities | ||
| Anemia | −19.7 (−24.5, −14.9) | −66.2 (−81.6, −50.8) |
| Atrial fibrillation | −4.8 (−7.3, −2.3) | −10.1 (−21.1, 0.8) |
| Chronic renal insufficiency | −31.3 (−35.8, −26.8) | −109.4 (−124.2, −94.7) |
| Coronary artery disease | −4.2 (−6.9, −1.6) | −25.7 (−35.9, −15.4) |
| COPD | −18.6 (−21.8, −15.4) | −73.5 (−86.2, −60.8) |
| Diabetes mellitus | −11.3 (−13.9, −8.7) | −44.1 (−54.9, −33.3) |
| Hypertension | 7.9 (4.6, 11.2) | 30.0 (14.6, 45.5) |
| Hyperlipidemia | 9.8 (7.4, 12.2) | 34.4 (24.0, 44.7) |
| Pacemaker or ICD implanted | 3.9 (1.1, 6.7) | 3.1 (−9.1, 15.3) |
| Index hospitalization diagnoses (claims-based) | ||
| Dementia | −77.7 (−90.8, −64.6) | −138.9 (−159.5, −118.4) |
| Disability | −52.1 (−66.1, −38.1) | −141.5 (−181.4, −101.6) |
| Liver disease | −50.0 (−71.3, −28.7) | −177.8 (−233.4, −122.2) |
| Major psychiatric disorder | −31.5 (−43.8, −19.1) | −54.8 (−90.8, −18.7) |
| Protein-calorie malnutrition | −141.2 (−159.4, −123.0) | −217.4 (−238.3, −196.4) |
Data presented as change in median home-time
Defined per GWTG-HF case report form as chronic elevation in serum creatinine >2.0 mg/dL.
COPD, chronic obstructive pulmonary disease; GWTG-HF, Get With The Guidelines Heart Failure; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; SD, standard deviation; SNF, skilled nursing facility
Home-time and Baseline Ejection Fraction
Data for home-time over 1-year follow-up by baseline EF are presented in Supplementary Table 1. There was no significant difference in total post-discharge home-time by EF group, with mean home-time ranging from 240.5 days among patient with reduced EF to 249.5 days among patients with borderline EF 40–49% (P=0.12). In contrast, the relative contributions of different care facilities to time spent away from home differed by EF. Specifically, preserved EF patients tended to spend the most time in SNF and rehabilitation facilities and reduced EF patients tended to spend the least time. In addition, the mortality rate at 1 year (40.6%) and the contribution of death to reduced home-time was greatest among patients with reduced EF, as compared to other EF groups.
The association between select patient characteristics and home-time varied by baseline EF (Supplementary Table 2). For example, increasing age and male gender were associated with greater reductions in home-time among patients with reduced EF (P for interaction ≤0.04). Chronic renal insufficiency was associated with progressively larger reductions in home-time across EF groups, with the greatest reduction among patients with reduced EF (P for interaction <0.001). Index hospitalization diagnoses, such as dementia, disability, and malnutrition, were associated with significant and severe reductions in home-time, irrespective of EF (P for interaction ≥0.45).
DISCUSSION
In this large contemporary cohort of older patients hospitalized for HF, home-time was readily determined for >97% and >70% of patients at respective 30-day and 1-year follow-up. Substantial reductions in home-time were seen early and late after HF hospitalization, with only 56% and 19% of patients achieving 100% home-time through 30 days and 1 year post-discharge, respectively. Relative contributions to reduced home-time varied by follow-up duration, with days spent in SNF being the largest contributor at 30-days and death being the largest contributor at 1- and 2-years. Similarly, relative contributions to reduced home-time at 1 year varied by baseline EF, with reduced EF patients having the largest contribution from death and preserved EF patients having the largest contribution from SNF. In regression models, several comorbidities and patient characteristics were associated with substantially reduced home-time, with the nature of many associations differing by EF. In comparisons of the home-time endpoint with traditional time-to-event post-discharge outcomes, home-time showed a high degree of correlation with time free from death and the composite of death or HF hospitalization at 1- and 2-year follow-up. Collectively, these findings support consideration of home-time as a continuous patient-centered outcome that can be effectively derived from administrative claims data for patients hospitalized with HF.
To our knowledge, we present the first characterization of post-discharge home-time in the HF population. Prior research using administrative claims from New Zealand has reported days alive and out of the hospital following HF hospitalization.(17) In contrast, our data are directly relevant to United States (US) care practices and capture the substantial contribution of SNF and other facilities to post-hospitalization care. Prior US registry-based work has described hospital disposition among patients hospitalized for HF, but has not accounted for duration of post-discharge time spent in each care location. For instance, an analysis from the ADHERE (Acute Decompensated Heart Failure) registry of HF patients enrolled between the years 2001 and 2005 found nearly 80% of patients were discharged home with self- or additional care and only 18% of patients discharged to any kind of rehabilitation institution, including SNF, rehabilitation facility, or short- or long-term care hospital.(18) A subsequent GWTG-HF analysis of patients discharged home versus SNF between 2005 and 2006 found 76% and 24% of the study cohort discharged home and to SNF, respectively.(19) While the present analysis was based on a more recent GWTG-HF cohort and showed roughly similar proportional use of SNF at discharge (22%), <70% of patients were discharged home. Although differences in construction of the various study cohorts could potentially explain a proportional decline in patients discharged home, differences could be partially accounted for by increased utilization of non-SNF care institutions over time. For example, <2% of patients were discharged to hospice care in ADHERE, compared to >4% in the current study.(18)
Compared to traditionally reported post-discharge HF outcomes, use of the home-time endpoint may offer distinct advantages. From the patient perspective, description of home-time may be more easily understood by HF patients, families, and clinicians. Likewise, home-time may better communicate and represent the risk of mortality and morbidity associated with HF, including the burden of repeated hospitalizations, hospitalization complexity and length of stay, and time spent in rehabilitation and nursing facilities. In recent years, clinical research has increasingly incorporated a focus on patient-centered care with particular focus on measures that reflect outcomes important to patients.(8) Similarly, the US Food and Drug Administration has issued guidance statements on the use of PROs for evaluating treatment effects and product labeling.(10) Despite this added emphasis, given their inherently subjective nature and the availability of multiple grading instruments, many relevant PROs for HF patients (e.g., dyspnea) remain difficult to study.(20,21) In this context, home-time may represent an effective complement to PROs in the execution of patient-centered HF research. Although time at home does not necessarily equate to freedom from symptoms and disability and situations could exist where patients report improved QOL despite less home-time, the present study supports home-time as a feasible and objective patient-centered measure that further contextualizes the patient experience.(22)
As an additional advantage, home-time may better reflect the burden of HF care on the healthcare system by being inclusive of the significant number of days attributed to facilities other than hospitals. In the US, cost containment efforts for HF care have heavily focused on prevention of 30-day hospital readmission, spurred by financial penalties from the CMS for hospitals with higher-than-expected readmission rates. However, use of 30-day readmission as a HF quality metric has been heavily scrutinized, with recent evidence of comparable quality of care and clinical outcomes among hospitals with high versus low risk-adjusted readmission rates.(23–25) Accordingly, alternative measures that account for the duration within a care setting, such as total inpatient hospital days during a 30-day period, have been proposed as potential better reflections of overall resource use and healthcare quality.(26) Although not inclusive of index hospitalization length of stay, the present analysis of post-discharge home-time builds on this theme, accounting for the length of time patients receive various types of care rather than simply whether or not they receive it. Thus, while not completely evaluated or studied as a quality metric, home-time may better gauge overall health resource use as compared to the hospital readmission rate, with the ability to capture both the time-dependent and location-dependent elements of post-discharge care.
From a clinical trial perspective, home-time could represent a novel trial endpoint that intrinsically reflects traditionally used mortality and hospitalization events, but from a patient-centered perspective and with the potential of added sensitivity to detect meaningful differences in downstream clinical course. Despite numerous investigational studies, phase III clinical trials among patients hospitalized with HF have been consistently neutral or negative.(27,28) Potential reasons for the persistent lack of positive trial results are well documented and likely multifactorial, but increasing attention has been focused on trial design elements as a means of optimizing trial performance.(27–30) Prior phase III hospitalized HF studies with post-discharge primary endpoints have been exclusively time-to-event trials focused on mortality and HF hospitalization(31–33). Two trials prospectively evaluated days alive and out of the hospital as an exploratory endpoint, but uptake as a primary study endpoint remains to be seen.(32,34) While contemporary time-to-event trials may represent an important methodological advance, potential concerns arise regarding the sensitivity of this approach to detect clinically meaningful benefits.(35,36) For example, recurrent HF hospitalizations are generally not counted, and a patient with a brief hospitalization early post-discharge and subsequent benign course may be weighted as “worse” than a patient initially hospitalized slightly later but with repeated complicated hospitalizations. Thus, for purposes of comparing study groups in a HF randomized trial, home-time may more fully capture the net clinical benefit of an intervention. This potential added sensitivity may be particularly advantageous in trials of moderate size and follow-up duration, where the number of fatal or hospitalization events may be limited. This will require prospective testing with HF clinical trials integrating home-time as an endpoint.
Limitations
Limitations of this study should be noted. First, this study was an observational analysis of registry and administrative claims data among hospitals voluntarily participating in the GWTG-HF program and these findings may not be generalizable to hospitals with differing care practices, patient case mix, or resource availability. Second, this study was restricted to Medicare Beneficiaries in the United States ≥65 years of age. It is unclear if the current home-time results would apply to younger or other HF populations and this study does not address the feasibility of the home-time calculation among patient for whom administrative claims data are not available. Third, due to the nature of claims data, more specific granularity on the exact meaning of home-time for different patients was not possible. For example, we were unable to distinguish if home-time meant a patient was living in their own home, living with a family member or friend, living in an assisted-living center, or living at home with home health services. Fourth, this study was not designed to determine the degree to which HF patients value home-time. Further study is needed to determine the self-reported importance of home-time to HF patients, as compared to other measures. Fifth, the prospective use of a home-time endpoint could be limited by the requirement for complete data over an entire interval and by delays in data availability. As seen in the current analysis, home-time for a given interval may not be calculable until a period sometime after the interval of interest. Lastly, prior data suggest patient socioeconomic status (SES) and geographic location may impact HF outcomes, treatment, and discharge disposition, and thus it is plausible these factors could lead to important differences in home-time.(37,38) Future work is encouraged to examine the role of social, geographic, and health system factors on home-time following hospitalization for HF.
CONCLUSIONS
In this cohort of older patients hospitalized for HF, many patients spent substantial post-discharge time away from home. Home-time was associated with several patient characteristics and was closely correlated with post-discharge mortality and hospitalization outcomes. Home-time can be readily derived from administrative claims data and may be complementary to traditionally reported HF outcomes, for purposes of patient-centered care, health outcomes research, and clinical trials.
Supplementary Material
Table 3.
Correlation Between Home-Time and Traditional Time-to-Event Endpoints
| 30 days | 1 year | 2 years | |
|---|---|---|---|
| Death | .404 | .935 | .969 |
| Death or heart failure readmission | .380 | .732 | .740 |
| Death or all-cause readmission | .464 | .585 | .549 |
Data reflect Pearson correlation coefficients between event-free survival time and home-time. Data only represent patients with complete follow-up (i.e., patients for whom home-time could be calculated).
PERSPECTIVES.
Competency in Medical Knowledge:
Home-time, defined as days alive and out of any healthcare institution, is readily calculated among older patients hospitalized with HF using administrative claims data. Many HF patients have reduced home-time throughout intervals of post-discharge follow-up, with nursing facilities the largest contributor at 30 days and death the largest contributor at 1 year.
Competency in Medical Knowledge:
Contributions to reduced home-time at 1 year varied by baseline EF, with reduced EF patients tending to have the most days accounted for by death and preserved EF patients tending to have the most days spent in SNF.
Competency in Medical Knowledge:
Among patients hospitalized for HF, post-discharge home-time is associated with several patient characteristics and closely correlated with traditional time-to-event mortality and hospitalization outcomes.
Translational Outlook:
Compared to traditionally reported post-discharge mortality and rehospitalization outcomes, home-time may offer distinct advantages, such as facilitating easier communication of risk and clinical course to patients, better capturing the patient experience in the healthcare system, and potential added sensitivity to detect net clinical benefits of an intervention. Prospective testing of home-time as a patient-centered endpoint complementary to traditionally reported HF outcomes is warranted.
Acknowledgments
FUNDING SOURCES
This study was funded by Novartis Pharmaceuticals Corporation (East Hanover, NJ, USA).
Dr. Greene receives research support from the National Heart Lung and Blood Institute T32 postdoctoral training grant (T32HL069749-14), a Heart Failure Society of America/ Emergency Medicine Foundation Acute Heart Failure Young Investigator Award funded by Novartis, and Novartis. Dr. O’Brien has received research support from Bristol-Myers Squibb, GlaxoSmithKline, Pfizer, Janssen Pharmaceuticals, and Novartis. Dr. Mentz receives research support and honoraria from Novartis. Dr. Chang is an employee of Novartis Pharmaceuticals Corporation and owns stock in Novartis AG. Mr. Turner is an employee of Novartis Pharmaceuticals Corporation. Dr. Curtis receives research support from NIH, PCORI, Novartis, GlaxoSmithKline, Gilead, Boston Scientific, and St. Jude. Dr. Fonarow receives research support from NIH, and is a consultant for Amgen, Medtronic, Novartis, and St. Jude Medical. Dr. Hammill receives research support from Novartis, GlaxoSmithKline, Abbott, Boston Scientific, and St. Jude.
ABBREVIATIONS
- CMS
Centers for Medicare and Medicaid Services
- EF
Ejection fraction
- GWTG-HF
Get With The Guidelines Heart Failure Registry
- HF
Heart failure
- PRO
Patient reported outcome
- SNF
Skilled nursing facility
Footnotes
DISCLOSURES
All other authors report no disclosures.
REFERENCES
- 1.Benjamin EJ, Blaha MJ, Chiuve SE et al. Heart Disease and Stroke Statistics-2017 Update: A Report From the American Heart Association. Circulation 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blecker S, Paul M, Taksler G, Ogedegbe G, Katz S. Heart failure-associated hospitalizations in the United States. J Am Coll Cardiol 2013;61:1259–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Krumholz HM, Normand SL, Wang Y. Trends in hospitalizations and outcomes for acute cardiovascular disease and stroke, 1999–2011. Circulation 2014;130:966–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Allen LA, Gheorghiade M, Reid KJ et al. Identifying patients hospitalized with heart failure at risk for unfavorable future quality of life. Circ Cardiovasc Qual Outcomes 2011;4:389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yancy CW, Jessup M, Bozkurt B et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013;62:e147–239. [DOI] [PubMed] [Google Scholar]
- 6.Institute of Medicine Committee on Quality of Health Care in America: Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press, 2001. [Google Scholar]
- 7.Allen LA, Yager JE, Funk MJ et al. Discordance between patient-predicted and model-predicted life expectancy among ambulatory patients with heart failure. JAMA 2008;299:2533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hernandez AF, Fleurence RL, Rothman RL. The ADAPTABLE Trial and PCORnet: Shining Light on a New Research Paradigm. Ann Intern Med 2015;163:635–6. [DOI] [PubMed] [Google Scholar]
- 9.Jones WS, Roe MT, Antman EM et al. The Changing Landscape of Randomized Clinical Trials in Cardiovascular Disease. J Am Coll Cardiol 2016;68:1898–1907. [DOI] [PubMed] [Google Scholar]
- 10.Administraton FaD. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonarow GC, Liang L, Thomas L et al. Assessment of Home-Time After Acute Ischemic Stroke in Medicare Beneficiaries. Stroke 2016;47:836–42. [DOI] [PubMed] [Google Scholar]
- 12.O’Brien EC, Xian Y, Xu H et al. Hospital Variation in Home-Time After Acute Ischemic Stroke: Insights From the PROSPER Study (Patient-Centered Research Into Outcomes Stroke Patients Prefer and Effectiveness Research). Stroke 2016;47:2627–33. [DOI] [PubMed] [Google Scholar]
- 13.Smaha LA, American Heart A. The American Heart Association Get With The Guidelines program. Am Heart J 2004;148:S46–8. [DOI] [PubMed] [Google Scholar]
- 14.Hong Y, LaBresh KA. Overview of the American Heart Association “Get with the Guidelines” programs: coronary heart disease, stroke, and heart failure. Crit Pathw Cardiol 2006;5:179–86. [DOI] [PubMed] [Google Scholar]
- 15.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krumholz HM, Wang Y, Mattera JA et al. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation 2006;113:1693–701. [DOI] [PubMed] [Google Scholar]
- 17.Wasywich CA, Gamble GD, Whalley GA, Doughty RN. Understanding changing patterns of survival and hospitalization for heart failure over two decades in New Zealand: utility of ‘days alive and out of hospital’ from epidemiological data. Eur J Heart Fail 2010;12:462–8. [DOI] [PubMed] [Google Scholar]
- 18.Hauptman PJ, Goodlin SJ, Lopatin M, Costanzo MR, Fonarow GC, Yancy CW. Characteristics of patients hospitalized with acute decompensated heart failure who are referred for hospice care. Arch Intern Med 2007;167:1990–7. [DOI] [PubMed] [Google Scholar]
- 19.Allen LA, Hernandez AF, Peterson ED et al. Discharge to a skilled nursing facility and subsequent clinical outcomes among older patients hospitalized for heart failure. Circ Heart Fail 2011;4:293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gheorghiade M, Ruschitzka F. Beyond dyspnoea as an endpoint in acute heart failure trials. Eur Heart J 2011;32:1442–5. [DOI] [PubMed] [Google Scholar]
- 21.Mebazaa A, Pang PS, Tavares M et al. The impact of early standard therapy on dyspnoea in patients with acute heart failure: the URGENT-dyspnoea study. Eur Heart J 2010;31:832–41. [DOI] [PubMed] [Google Scholar]
- 22.Estep JD, Starling RC, Horstmanshof DA et al. Risk Assessment and Comparative Effectiveness of Left Ventricular Assist Device and Medical Management in Ambulatory Heart Failure Patients: Results From the ROADMAP Study. J Am Coll Cardiol 2015;66:1747–61. [DOI] [PubMed] [Google Scholar]
- 23.Vaduganathan M, Bonow RO, Gheorghiade M. Thirty-day readmissions: the clock is ticking. JAMA 2013;309:345–6. [DOI] [PubMed] [Google Scholar]
- 24.Joynt KE, Jha AK. Thirty-day readmissions--truth and consequences. N Engl J Med 2012;366:1366–9. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A, Golwala H, Xu H et al. Association of 30-Day Readmission Metric for Heart Failure Under the Hospital Readmissions Reduction Program With Quality of Care and Outcomes. JACC Heart Fail 2016;4:935–946. [DOI] [PubMed] [Google Scholar]
- 26.Kociol RD, Liang L, Hernandez AF et al. Are we targeting the right metric for heart failure? Comparison of hospital 30-day readmission rates and total episode of care inpatient days. Am Heart J 2013;165:987–994 e1. [DOI] [PubMed] [Google Scholar]
- 27.Vaduganathan M, Greene SJ, Ambrosy AP, Gheorghiade M, Butler J. The disconnect between phase II and phase III trials of drugs for heart failure. Nat Rev Cardiol 2013;10:85–97. [DOI] [PubMed] [Google Scholar]
- 28.Ambrosy AP, Butler J, Gheorghiade M. Clinical trials in acute heart failure: beginning of the end or end of the beginning? Eur J Heart Fail 2017. [DOI] [PubMed] [Google Scholar]
- 29.Greene SJ, Gheorghiade M. Matching mechanism of death with mechanism of action: considerations for drug development for hospitalized heart failure. J Am Coll Cardiol 2014;64:1599–601. [DOI] [PubMed] [Google Scholar]
- 30.Greene SJ, Gheorghiade M. Same protocol, different continents, different patients: should we continue to conduct global heart failure trials? Eur J Heart Fail 2015;17:875–8. [DOI] [PubMed] [Google Scholar]
- 31.Gheorghiade M, Konstam MA, Burnett JC Jr, et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: the EVEREST Clinical Status Trials. JAMA 2007;297:1332–43. [DOI] [PubMed] [Google Scholar]
- 32.O’Connor CM, Starling RC, Hernandez AF et al. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med 2011;365:32–43. [DOI] [PubMed] [Google Scholar]
- 33.Gheorghiade M, Bohm M, Greene SJ et al. Effect of aliskiren on postdischarge mortality and heart failure readmissions among patients hospitalized for heart failure: the ASTRONAUT randomized trial. JAMA 2013;309:1125–35. [DOI] [PubMed] [Google Scholar]
- 34.Teerlink JR, Cotter G, Davison BA et al. Serelaxin, recombinant human relaxin-2, for treatment of acute heart failure (RELAX-AHF): a randomised, placebo-controlled trial. Lancet 2013;381:29–39. [DOI] [PubMed] [Google Scholar]
- 35.Packer M Development and Evolution of a Hierarchical Clinical Composite End Point for the Evaluation of Drugs and Devices for Acute and Chronic Heart Failure: A 20-Year Perspective. Circulation 2016;134:1664–1678. [DOI] [PubMed] [Google Scholar]
- 36.Felker GM, Maisel AS. A global rank end point for clinical trials in acute heart failure. Circ Heart Fail 2010;3:643–6. [DOI] [PubMed] [Google Scholar]
- 37.Hawkins NM, Jhund PS, McMurray JJ, Capewell S. Heart failure and socioeconomic status: accumulating evidence of inequality. Eur J Heart Fail 2012;14:138–46. [DOI] [PubMed] [Google Scholar]
- 38.Akintoye E, Briasoulis A, Egbe A et al. Regional Variation in Mortality, Length of Stay, Cost, and Discharge Disposition Among Patients Admitted for Heart Failure in the United States. Am J Cardiol 2017;120:817–824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


