Abstract
Objective:
To determine whether N-acetylcysteine rinse was safe and could improve thickened secretions and dry mouth during and after radiotherapy.
Patients and Methods:
We designed a prospective, pilot, double-blind, placebo-controlled randomized clinical trial (Alliance MC13C2). Adult patients (age ≥18 years) were enrolled if they underwent chemoradiotherapy (≥60 Gy). Patients initiated testing rinse within 3 days of starting radiotherapy. With swish-and-spit, they received 10% N-acetylcysteine (2,500 mg daily) or placebo rinse solution 5 times daily during radiotherapy and 2 weeks postradiotherapy. Primary aim was to evaluate N-acetylcysteine in improvement of saliva viscosity with Groningen Radiotherapy-Induced Xerostomia questionnaire. Secondary aims included xerostomia improvement by the same questionnaire and European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Head and Neck-35 Questions survey and adverse event profiles. Type I error rate was 20%.
Results:
Thirty-two patients undergoing chemoradiotherapy were enrolled. Baseline characteristics were balanced for placebo (n=17) and N-acetylcysteine (n=15). N-acetylcysteine was better for improving sticky saliva (area under curve, P=.12). Scores of multiple secondary end points favored N-acetylcysteine, including sticky saliva daytime (P=.04), daytime and total xerostomia (both P=.02), pain (P=. 18), and trouble with social eating (P=.15). Repeated measures models confirmed findings. Taste was a major dissatisifer for N-acetylcysteine rinse; however, both testing rinses were safe and well tolerated overall.
Conclusion:
Our pilot data showed that N-acetylcysteine rinse was safe and provided strong signal of potential efficacy for improving thickened saliva and xerostomia by patient-reported outcome. A confirmatory phase 3 trial is required.
Keywords: cancer, chemotherapy, oncology, radiation
Introduction
Combined chemotherapy and radiotherapy (RT) provide substantial improvement in disease outcomes, including locoregional control and overall survival, for patients with head and neck cancer in definitive and adjuvant settings.1, 2 However, patients often have treatment-related acute toxicities involving the oral cavity and oropharynx that are morbid and diminish quality of life.3 The important symptoms include mucositis, xerostomia, and particularly thickened salivary secretions. Thickened saliva often leads to difficulties with swallowing and respiration during the final weeks of RT.4
As a mucolytic agent, N-acetylcysteine is an aminothiol and precursor to glutathione, thereby protecting against RT-induced free radical damage. It has been shown to inhibit local inflammatory and fibrotic responses by decreasing the levels of interleukin (IL)-1β, IL-8, tumor necrosis factor-α, and nuclear factor κ-light-chain enhancer of activated B cells (NF-κB) and by transforming growth factor-β in animals and in vitro models.5–10 N-acetylcysteine disassociates disulfide bonds and reduces carbohydrate cross-linking and has been shown to decrease salivary mucous viscosity in cystic fibrosis patients.10–12 Additionally, it may increase hydration to the epithelial mucous membrane and lead to less xerostomia. A large number of randomized trials have supported its safety and efficacy and established the role of N-acetylcysteine for patients without cancer who have chronic obstructive pulmonary disease (COPD), acute lung injury, and pulmonary fibrosis conditions.13–18
Previously, a phase 2 study evaluated RK-0202, an acetylcysteine in a polymer matrix-based rinse, in 110 patients who underwent RT alone for head and neck cancer.19 Compared with the placebo group, patients who received 10% acetylcysteine solutions had significantly decreased rates of oral mucositis, feeding tube placement, and narcotic use. However, effects for improving thickened saliva and xerostomia were not evaluated. Using patient-reported outcome (PRO) tools, we now report results of a randomized, placebo-controlled, double-blind trial in evaluating the safety and efficacy of N-acetylcysteine on salivary function of patients with head and neck cancer who underwent concurrent chemoradiotherapy (CRT).
Methods and Materials
The present study was approved by the institutional review board at each participating medical center, and all patients provided informed consent. This pilot-based clinical trial was endorsed and sponsored by the Alliance for Clinical Trials in Oncology (Alliance MC13C2).
We designed a prospective, randomized, double-blind pilot study to determine the safety and efficacy of N-acetylcysteine for reducing salivary viscosity for 34 patients undergoing CRT for head and neck cancer. The inclusion criteria were age 18 years or older; histologic confirmation of the cancer; receipt of concurrent CRT to a minimum dose of 60 Gy in 30 fractions; Eastern Cooperative Oncology Group performance status 0, 1, or 2; ability to initiate the investigational agent 3 days or less after RT started; negative pregnancy test (if applicable); and ability to complete questionnaires. The exclusion criteria were severe comorbid illness, immunocompromised status, and myocardial infarction of 6 months or less; active congestive heart failure; receipt of induction chemotherapy; previous head and neck RT; use of amifostine during CRT; dry mouth or mucositis of grade 2 or more prior to CRT; and active connective tissue disease. Baking soda rinses were allowed but not for 2 hours following the rinse. Oral topical analgesics were allowed at the discretion of the treating provider. There was no restriction on opioid or over-the-counter pain medications.
The primary aim of this trial was to determine the effectiveness of N-acetylcysteine in improving saliva viscosity as measured by the Groningen Radiation-Induced Xerostomia (GRIX) questionnaire. The secondary aims were evaluation of whether N-acetylcysteine could improve other GRIX subscales and PRO based on European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck-35 Questions (EORTC QLQ H&N35), adverse event profile by Common Terminology Criteria for Adverse Events version 4.0 (Supplemental Table 1), patient adherence to the regimen, and long-term effects of N-acetylcysteine vs placebo. Patients were randomly assigned with a 1:1 ratio utilizing the method of Pocock and Simon20 and stratified by adjuvant vs definitive therapy and by disease site. A 10% N-acetylcysteine rinse (100 mg/mL; 5 mL per rinse) and a placebo (sodium chloride) rinse were prepared in a mucoadherent delivery diluent (a well-tolerated oral rinse approved by the US Food and Drug Administration that includes sodium hyaluronate, polyvinylpyrrolidone, glycyrrhetinic acid, aloe vera extract, propylene glycol, and water). Each patient was instructed to orally gargle for 60 seconds, swish, and spit the 5-mL testing solution 5 times daily during their RT treatments and for 2 weeks after the end of chemoradiotherapy. The treating clinicians and the patients were not told which testing solution they received in this double-blind study. The total daily N-acetylcysteine dose was 2,500 mg.
This trial incorporated PRO surveys. The GRIX questionnaire21 is a 14-item survey with 4 subscales for evaluating xerostomia and sticky saliva in both day and night. It has been validated for consistency and test-retest reliability and was significantly correlated with physician-rated salivary and xerostomia functions. The EORTC QLQ H&N35 survey22, 23 complemented the breadth of patients’ other experience during head and neck RT, including patient-oriented questions regarding pain, swallowing, feeding tube use, mucositis, social functioning, and weight loss. It is one of the most commonly used surveys in modern RT clinical trials.
Salivary viscosities were evaluated with the GRIX questionnaire at baseline, weekly during RT, and at 14, 45, and 90 days after RT completion. Head and neck-specific quality of life was measured with EORTC QLQ H&N35 at baseline, every 21 days during RT, and at 14, 45, and 90 days after RT completion. If patients discontinued the rinse, they were still monitored per protocol. Patients who had unacceptable adverse events were excluded from the study immediately, as were patients who elected to discontinue the study. The questionnaires at 45 and 90 days post CRT were completed through prepaid, preaddressed postal mailings.
Statistical Methods
The primary end point was area under the curve (AUC) of the GRIX sticky saliva total score, which was calculated for each patient from baseline to 2 weeks after RT. The AUC values were compared between the 2 arms using either t test or Wilcoxon nonparametric test. AUC values were tested for normality with use of the Shapiro-Wilk test. If the normality assumption was rejected at a 0.20 level (20%), Wilcoxon tests would be used to compare the AUC values between arm A and arm B. If the normality assumption was not rejected, an F test would be used to compare the variances of the AUC values between the 2 arms. This test also would be done with use of a 0.20 level. If the variances were significantly different, an unequal variance t test would be used to compare the AUC values between the arms. Otherwise, an equal variance t test was performed. These Wilcoxon and t tests were 2-sided with a type 1 error rate of 0.20. Linear regression modeling and repeated measures analyses were used to describe changes from baseline to 14 days post RT. Separate models were fit for changes from baseline to last treatment cycle to include additional patients who did not have data for end of RT. GRIX models were adjusted for age, sex, treatment intent (definitive vs adjuvant), primary tumor site, chemotherapy, and mean dose to the salivary glands. The EORTC QLQ H&N35 models were adjusted only for treatment intent. Because of the smaller sample sizes, adjustment for more variables would have overfit the models.
Secondary analyses included comparisons of each of the other GRIX subscales and the EORTC QLQ H&N35 scores between the 2 arms. The other subscales were included. Each secondary end point was compared in the same manner as the primary end point. The AUCs were compared with use of t test (equal variance or unequal variance) or Wilcoxon nonparametric test. Analyses of linear regression and repeated measures were done to adjust for confounding factors.
Based on the empirical rule and a 2-sided, 2-sample equal variance t test with a 20% type 1 error rate,24 a sample size of 15 patients per arm would provide 80% power to detect a large effect size of 0.8 times the standard deviation difference in AUC between the study arms. The study allowed for an increase in the sample size by up to 15% to account for ineligible patients and cancelations, for a total of 36 patients. Eventually, accruement totaled 34 patients (94%).
Results
The study was activated on April 25, 2014. We completed accrual and all patient-related follow-up activities on February 19, 2016. Thirty-four patients from 3 hospitals were screened, and 2 patients were excluded because of ineligibility (Figure 1). Seventeen and 15 eligible patients were in the placebo and N-acetylcysteine arms. The baseline patient, tumor, and RT characteristics were balanced between the 2 arms (Table 1). The mean doses to the oral cavity, bilateral parotid, and bilateral submandibular glands did not statistically differ between arms (all P>.32). Eight patients and 5 patients in the placebo and N-acetylcysteine arms completed all study activities per protocol (P=.73). However, most participants could be evaluated for the primary end point (28/32, 88%).
Figure 1.
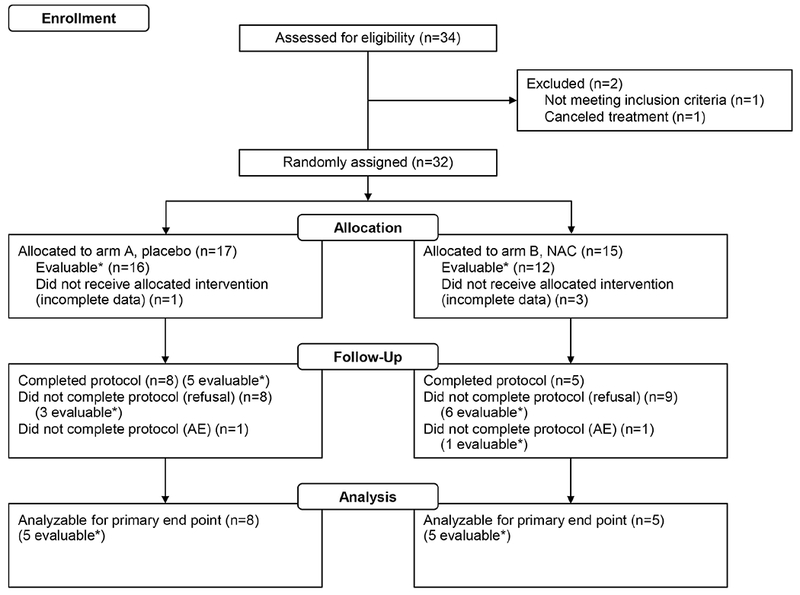
Consolidated Standards of Reporting Trials (CONSORT) Flow Diagram for the MC13C2 (Alliance for Clinical Trials in Oncology) Trial. This randomized trial evaluated placebo rinse (arm A) vs NAC rinse (arm B) for thickened secretions and mucositis during head and neck chemoradiotherapy. The primary end point is the area under the curve for Groningen Radiation-Induced Xerostomia sticky saliva total score. Asterisk indicates evaluable for primary end point. AE = adverse events; NAC = N-acetylcysteine.
Table 1.
Baseline Patient, Tumor, and Treatment Characteristics
| Trial Arm |
||||
|---|---|---|---|---|
| N-Acetylcysteine | Statistical | |||
| Characteristicsa | Placebo (n=17) | (n=15) | P Value | Testb |
| Age, median (range), y | 56.0 (24.0-69.0) | 63.0 (30.0-73.0) | .051 | 1 |
| Age ≥50 y | 14 (82) | 14 (93) | .60 | 2 |
| Sex | ||||
| Male | 11 (65) | 11 (73) | ||
| Female | 6 (35) | 4 (27) | .71 | 2 |
| Race/ethnicityc | ||||
| White | 14 (82) | 15 (100) | .40 | 2 |
| Nonwhite | 3 (18) | 0 (0) | ||
| Chemotherapy | .32 | 2 | ||
| Cisplatin | 16 (94) | 12(80) | ||
| Cetuximab | 1 (6) | 3 (20) | ||
| Dose to salivary glands, meand | >.99 | 2 | ||
| ≥26 Gy | 12 (75) | 9 (69) | ||
| <26 Gy | 4 (25) | 4 (31) | ||
| Missing data | 1 | 2 | ||
| Tumor status | .82 | 3 | ||
| Resected with no residual | 5 (29) | 6 (40) | ||
| Resected with known residual | 4 (24) | 3 (20) | ||
| Unresected | 8 (47) | 6 (40) | ||
| Primary tumor site | .75 | 3 | ||
| Oropharynx | 12 (71) | 10 (67) | ||
| Oral cavity | 4 (24) | 3 (20) | ||
| Supraglottic larynx | 0 (0) | 1 (7) | ||
| Nasopharynx | 1 (6) | 1 (7) | ||
| Treatment intent | .50 | 2 | ||
| Definitive | 8 (47) | 9 (60) | ||
| Adjuvant | 9 (53) | 6 (40) | ||
Values are presented as number (percentage) of patients unless stated otherwise.
1 indicates Kruskal-Wallis test; 2, Fisher exact test; 3, χ2 test.
Race/ethnicity was assessed by the intake form, and patient’s response was voluntary.
Mean radiotherapy dose to total salivary glands (bilateral).
The primary aim’s measurement of GRIX sticky saliva total score by AUC (P=.12) was less than the protocol prespecified requirement of 0.20; as a result, the end point was considered met (N-acetylcysteine was better than placebo for improving thickened saliva and mucositis) (Table 2). A number of secondary end points were also met, with promising results favoring N-acetylcysteine by both GRIX and EORTC QLQ H&N35 surveys (Tables 2 and 3). Patients using N-acetylcysteine had significantly lower (ie, better) AUC values for GRIX sticky saliva daytime, xerostomia daytime, and xerostomia total scores. At week 2, GRIX sticky saliva daytime scores were significantly lower for the N-acetylcysteine arm (P<.05). At week 3, GRIX sticky saliva daytime, xerostomia daytime, and xerostomia total scores were significantly improved for patients who received N-acetylcysteine (Figure 2). By EORTC QLQ H&N35 survey, swallowing scores were better in the N-acetylcysteine arm than placebo (P=.17), which met the protocol’s cutoff of 0.20 (Supplemental Figure 1). At 3 months, all EORTC QLQ H&N35 scores for pain, dry mouth, trouble with social eating, and pain medication use had P values less than .20 and were better for patients using N-acetylcysteine (Table 3).
Table 2.
Groningen Radiation-Induced Xerostomia Questionnaire Baseline and AUC Scores for Patients Receiving Placebo vs NAC
| Scoresa |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total AUC During RT | |||||||||
| Baseline (Cycle 0) |
(All Cycles) |
Post RT at 3 mo |
|||||||
| Characteristics | Placebo | NAC | P Valueb | Placebo | NAC | P Valueb,c | Placebo | NAC | P Valueb |
| Sticky saliva | |||||||||
| Daytime | 11.1 | 6.5 | .38 | 45.2 | 26.1 | .04 | 50.0 | 35.2 | .40 |
| Nighttime | 8.9 | 5.6 | .87 | 34.5 | 29.1 | .56 | 55.6 | 22.2 | .24 |
| Total | 10.2 | 6.1 | .55 | 40.9 | 27.2 | .12 | 51.9 | 30.9 | .29 |
| Xerostomia | |||||||||
| Daytime | 15.9 | 13.9 | .58 | 44.1 | 27.3 | .02 | 46.3 | 29.6 | .59 |
| Nighttime | 29.6 | 20.4 | .44 | 39.8 | 31.3 | .22 | 36.1 | 27.8 | .76 |
| Total | 20.5 | 16.0 | .40 | 43.8 | 28.5 | .02 | 42.2 | 28.9 | .65 |
Abbreviations: AUC = area under the curve; NAC = N-acetylcysteine; RT = radiotherapy.
Lower scores indicate better quality of life.
Calculated with equal variance t testing.
Bold indicates statistical significance.
Table 3.
EORTC QLQ H&N35 Baseline and AUC Scores for Patients Receiving Placebo vs NAC
| EORTC QLQ H&N35 Scorea |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Total AUC During RT | |||||||||
| Baseline AUC (Cycle 0) |
(All Cycles) |
AUC at 3 mo Post RT |
|||||||
| Characteristics | Placebo | NAC | P Valueb | Placebo | NAC | P Valueb | Placebo | NAC | P Valueb |
| Pain | 17.8 | 12.2 | .46 | 32.4 | 32.0 | .95 | 33.3 | 14.6 | .18 |
| Swallowing | 13.7 | 11.1 | .35 | 34.7 | 22.3 | .17 | 27.8 | 8.3 | .23 |
| Teeth problems | 6.7 | 15.4 | .27 | 6.6 | 17.2 | .11 | 5.6 | 8.3 | .88 |
| Opening mouth | 13.3 | 15.4 | .96 | 30.5 | 25.8 | .56 | 16.7 | 16.7 | >.99 |
| Dry mouth | 28.9 | 17.9 | .17 | 44.3 | 48.6 | .66 | 66.7 | 33.3 | .11 |
| Sticky saliva | 13.3 | 10.3 | .46 | 46.3 | 37.9 | .48 | 38.9 | 33.3 | .84 |
| Taste problems | 4.4 | 1.3 | .21 | 27.8 | 29.3 | .78 | 22.2 | 29.2 | .82 |
| Coughing | 24.4 | 20.5 | .53 | 30.9 | 36.7 | .38 | 27.8 | 33.3 | .65 |
| Speech problems | 5.9 | 12.0 | .24 | 17.7 | 22.1 | .52 | 24.1 | 8.3 | .29 |
| Feeling ill | 4.4 | 5.1 | .91 | 23.9 | 24.4 | .96 | 11.1 | 0.0 | .54 |
| Trouble with social contact | 1.8 | 3.6 | .85 | 7.3 | 12.4 | .28 | 11.1 | 1.7 | .69 |
| Trouble with social eating | 8.3 | 8.3 | .82 | 31.8 | 26.7 | .48 | 27.8 | 6.3 | .15 |
| Less sexuality | 10.0 | 19.4 | .12 | 23.9 | 29.9 | .62 | 8.3 | 16.7 | >.99 |
| Use of pain medications | 28.9 | 25.6 | .65 | 31.8 | 47.4 | .18 | 22.2 | 0.0 | .10 |
| Nutritional supplements | 28.9 | 23.1 | .80 | 35.3 | 36.0 | .96 | 13.3 | 16.7 | .66 |
| Feeding tube | 13.3 | 2.6 | .61 | 21.0 | 17.0 | .82 | 33.3 | 0.0 | .28 |
| Weight loss | 28.9 | 25.6 | >.99 | 48.5 | 34.0 | .28 | 27.8 | 16.7 | .91 |
Abbreviations: AUC = area under the curve; EORTC QLQ H&N35 = European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck-35 Questions; NAC = N-acetylcysteine; RT = radiotherapy.
Lower scores indicate better quality of life.
Calculated by equal variance t testing.
Figure 2.
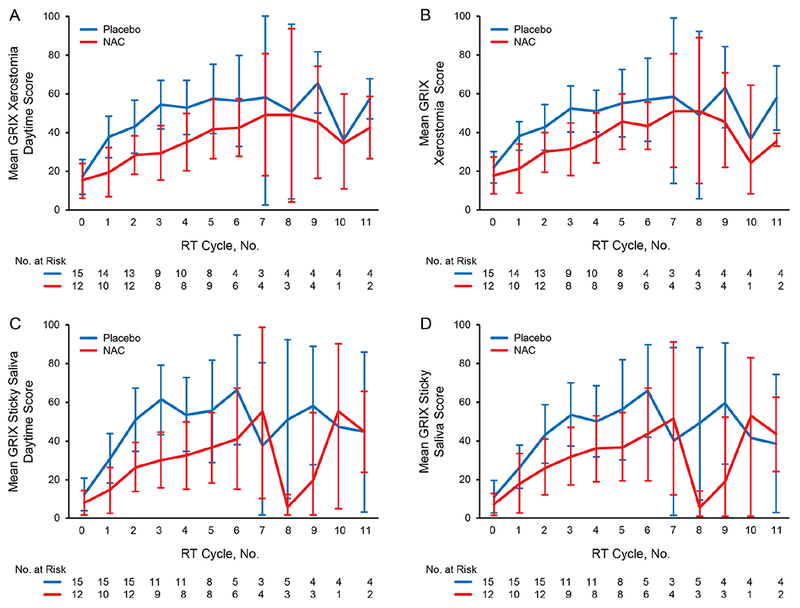
GRIX Xerostomia and Sticky Saliva Scores by Trial Arms. A, GRIX xerostomia daytime score (AUC score for first 3 cycles; P=.02). B, GRIX xerostomia total score (P=. 02). C, GRIX sticky saliva daytime score (P=.04). D, GRIX sticky saliva total score (P=.12). For cycle numbers, 0 indicates baseline, 1-8 indicate weekly measurements during RT, and 9-11 indicate 14, 45, and 90 days post RT, respectively. Arm A = placebo rinse; Arm B = NAC rinse; AUC = area under the curve; GRIX = Groningen Radiation-Induced Xerostomia; NAC = N-acetylcysteine; RT= radiotherapy.
No significant differences in adverse events occurred between the 2 arms. The N-acetylcysteine arm had slightly less nausea and oral pain. Fewer grade 3 events occurred for patients receiving N-acetylcysteine (3/15, 21%) than placebo (8/17, 47%), yet the maximum grade distribution for any adverse event did not statistically differ between the 2 arms (P=.38). There were no grade 4 or 5 toxicities. The grade 3 events were: increased creatinine (1 vs 0), dysphagia (1 vs 1), nausea (3 vs 0), oral pain (4 vs 2), and lung infection (1 vs 0) for patients receiving the placebo and N-acetylcysteine arms, respectively. One patient in each arm had an adverse event and was taken off study as a result.
A linear regression model showed that benefits from N-acetylcysteine were consistent when changes were considered from baseline to end of RT and 14 days post RT (Supplemental Table 2). Repeated measures models using all reported scores showed significantly better scores (P value cutoff, .20) for patients taking N-acetylcysteine for GRIX scores of xerostomia daytime and total and sticky saliva daytime, nighttime, and total and for EORTC QLQ H&N35 swallowing, opening mouth, sticky saliva, and weight loss (Supplemental Table 3).
Discussion
We report the potential clinical benefits of an N-acetylcysteine rinse in decreased salivary mucous viscosity and xerostomia for patients undergoing head and neck CRT. In this pilot trial, applications of patient-reported outcome surveys were successful for end-point evaluations. Both N-acetylcysteine and placebo regimens were well tolerated.
Chemically, N-acetylcysteine disassociates disulfide bonds because of its action by the free sulfhydryl group. It is also a precursor to glutathione and may have antioxidant properties.25 It reduces carbon-carbon linking and decreases mucous viscosity and tension as a result. N-acetylcysteine can aid in secretory functions because mucous glands produce mucins that are cysteine-rich and disulfide-bond linked.26 Additionally, experts have hypothesized that N-acetylcysteine can reduce fluid absorption and lead to increased epithelial hydration by the salivary glands.27
A recent Cochrane review rated the evidence for a number of pharmacological agents including amifostine, pilocarpine,28, 29 palifermin, and bethanechol in preventing xerostomia associated with head and neck CRT from insufficient to low and very low quality.30 There is a large and unmet need for this group of patients. In our present trial, a 10% concentration of N-acetylcysteine was used, which was shown to be effective in a previous trial using RK-0202, a similar compound.19 While that study showed a reduction of severe (grade 3-4) oral mucositis for patients treated with RT for head and neck cancer, patient-reported outcome tools were not utilized. The rates of grade 3 or 4 mucositis were 64% and 92% for patients receiving RK-0202 and placebo (P=.005).19 The safety of N-acetylcysteine has been demonstrated with daily doses as high as 2,800 mg31; a 2,500-mg dose was used in our trial (not ingested).
N-acetylcysteine, an over-the-counter product in the United States and an antidote for acetaminophen overdose, is well known for its safety profile. It was evaluated in a number of clinical trials and was shown to be beneficial for patients with COPD,13, 18 idiopathic pulmonary fibrosis,14 acute bronchitis,16 acute lung injury,17 and cystic fibrosis.31 On the basis of PROs, our study showed promising results that N-acetylcysteine may also benefit patients with RT-related mucositis toxicities, including thickened secretions and xerostomia. When systemically ingested, N-acetylcysteine can cause nausea, vomiting, drowsiness, chest tightness, and bronchoconstriction. With an oral rinse-and-spit application, our trial results showed that the N-acetylcysteine rinse was safe to combine with CRT in a group of patients with head and neck cancer who may already have substantial symptom burden from their planned treatments.
However, N-acetylcysteine does have an unpleasant odor similar to rotten eggs, which makes it less popular for general use. It is a major dissatisifer for the patients. In our study, 8 (47%) and 9 (60%) patients in the placebo and N-acetylcysteine arms refused further treatment after CRT was completed (P=.73). Open sores, mucositis, and changes in salivary quality are already major components of RT-related oral toxicities, which can make the N-acetylcysteine preparation even more difficult to tolerate. In the present trial, the bothering odor was partly mitigated by our choice of mucoadherent delivery diluent (commercially available), which was used in both the N-acetylcysteine and placebo arms to be impartial. The compliances for both rinses were comparable. The diluent solution was alcohol-free and did not produce such additional sensations as stinging and burning. The mucoadherent (coating) properties of the delivery solution also increased the contact time for N-acetylcysteine along the mucosal surfaces which likely contributed to the positive salivary effects that we saw in this trial.
Finally, of importance, careful selection and incorporation of relevant PRO tools21–23 are critical for the success of this trial, along with other studies in symptom control trials for cancer patients.32 Recently, the use of PROs for symptom intervention was shown to even increase overall survival in a randomized trial of 766 patients.33 In our trial, with use of PRO, both primary and multiple secondary end points were positive. Collectively, these outcomes showed the potential abilities of N-acetylcysteine to alleviate a patient’s negative experience associated with thickened saliva and xerostomia, and this outcome certainly deserves a closer look with a more definitive trial in the future.24
Limitations and Future Work
The N-acetylcysteine trial was adequately powered and we completed the accrual of all patients. However, as the EORTC QLQ H&N35 survey was collected every 3 weeks during RT, it seemed to appear less sensitive than the GRIX, which was a weekly tool (as designed). Adherence to the weekly GRIX has been reported at 86%,21 and certainly our trial achieved that adherence level during CRT. The surveys at 45 and 90 days post RT were designed as mail-in questionnaires and returned on a voluntary basis by patients, which resulted in lower adherence and response rates. However, this did not seem to affect the main results of this trial. The 5-time daily use of the testing rinse was inconvenient for the patients. Yet, our pilot experience suggested that it could be accomplished with adequate encouragement clinically. Exploration of a mucoadherent delivery diluent for use in masking the unpleasant taste of N-acetylcysteine may be useful. Currently, because our initial signals are excellent that the N-acetylcysteine rinse can be beneficial in the long term, the testing period of the mucolytic rinse can be extended beyond 2 weeks after the end of CRT or until patients believe it is no longer necessary. Some of our PRO end points, including pain and dry mouth, were still statistically significant at 3 months after CRT. Therefore, a longer follow-up period will be necessary for the next study. These characteristics will be important to consider in the future study design for a phase 3 clinical trial.
Conclusions
Through a placebo-controlled and double-blind trial, our pilot data showed that an N-acetylcysteine rinse was safe and provided strong signals of potential efficacy for improving thickened saliva and xerostomia symptoms in a group of patients with head and neck cancer who underwent CRT. A confirmatory study is needed to validate these findings.
Supplementary Material
Acknowledgments
We are grateful to the clinical and research staff and the patients who assisted with and participated in this trial. They are from Mayo Clinic in Rochester, Minnesota (18 patients), Mayo Clinic in Phoenix, Arizona (13 patients), and Altru Cancer Center in Grand Forks, North Dakota (3 patients). Sunstar Americas, Inc, (Schaumburg, IL) provided the mucoadherent delivery diluent solutions that were used for both arms.
Financial support and disclosure: All authors declare no conflict of interest in relation to this work. Research reported in this publication was supported by the NCI Community Oncology Research Program Cancer Prevention and Symptom Intervention Pilot Project Fund and the National Cancer Institute (NCI) of the National Institutes of Health under Award Number U10CA189823 (to the Alliance for Clinical Trials in Oncology NCI Community Oncology Research Program Grant) and U10CA180790. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Role of the funding source: The funding sources had no role in any aspect of this research project.
Abbreviations
- AUC
area under the curve
- COPD
chronic obstructive pulmonary disease
- CRT
chemoradiotherapy
- EORTC QLQ H&N35
European Organization for Research and Treatment of Cancer Quality of Life Questionnaire Head and Neck-35 Questions
- GRIX
Groningen Radiation-Induced Xerostomia
- IL
interleukin
- NF-κB
nuclear factor κ-light-chain enhancer of activated B cells
- PRO
patient-reported outcome
- RT
radiotherapy
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Co-first authors who contributed to the manuscript equally.
Trial Registration: clinicaltrials.gov Identifier:
References
- 1.Adelstein DJ, Li Y, Adams GL, et al. An intergroup phase III comparison of standard radiation therapy and two schedules of concurrent chemoradiotherapy in patients with unresectable squamous cell head and neck cancer. J. Clin. Oncol 2003;21:92–98. [DOI] [PubMed] [Google Scholar]
- 2.Denis F, Garaud P, Bardet E, et al. Final results of the 94-01 French Head and Neck Oncology and Radiotherapy Group randomized trial comparing radiotherapy alone with concomitant radiochemotherapy in advanced-stage oropharynx carcinoma. J. Clin. Oncol 2004;22:69–76. [DOI] [PubMed] [Google Scholar]
- 3.O’Neill M, Heron DE, Flickinger JC, Smith R, Ferris RL, Gibson M. Posttreatment quality-of-life assessment in patients with head and neck cancer treated with intensity-modulated radiation therapy. Am. J. Clin. Oncol 2011;34:478–482. [DOI] [PubMed] [Google Scholar]
- 4.Jaguar GC, Prado JD, Campanhã D, Alves FA. Clinical features and preventive therapies of radiation-induced xerostomia in head and neck cancer patient: a literature review. Applied Cancer Research. 2017;37:31. [Google Scholar]
- 5.Aihara M, Dobashi K, Akiyama M, Naruse I, Nakazawa T, Mori M. Effects of N-acetylcysteine and ambroxol on the production of IL-12 and IL-10 in human alveolar macrophages. Respiration. 2000;67:662–671. [DOI] [PubMed] [Google Scholar]
- 6.Cu A, Ye Q, Sarria R, Nakamura S, Guzman J, Costabel U. N-acetylcysteine inhibits TNF-alpha, sTNFR, and TGF-beta1 release by alveolar macrophages in idiopathic pulmonary fibrosis in vitro. Sarcoidosis. Vasc. Diffuse Lung Dis 2009;26:147–154. [PubMed] [Google Scholar]
- 7.Gosset P, Wallaert B, Tonnel AB, Fourneau C. Thiol regulation of the production of TNF-alpha, IL-6 and IL-8 by human alveolar macrophages. Eur. Respir. J 1999;14:98–105. [DOI] [PubMed] [Google Scholar]
- 8.Li YQ, Zhang ZX, Xu YJ, et al. N-acetyl-L-cysteine and pyrrolidine dithiocarbamate inhibited nuclear factor-kappaB activation in alveolar macrophages by different mechanisms. Acta Pharmacol. Sin 2006;27:339–346. [DOI] [PubMed] [Google Scholar]
- 9.Moon C, Lee YJ, Park HJ, Chong YH, Kang JL. N-acetylcysteine inhibits RhoA and promotes apoptotic cell clearance during intense lung inflammation. Am. J. Respir. Crit. Care Med 2010;181:374–387. [DOI] [PubMed] [Google Scholar]
- 10.Tirouvanziam R, Conrad CK, Bottiglieri T, Herzenberg LA, Moss RB, Herzenberg LA. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. U. S. A 2006;103:4628–4633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stephan U, Bowing B, Goering U, et al. Acetylcysteine in the oral mucolytic treatment of cystic fibrosis. Eur. J. Respir. Dis. Suppl 1980;111:127–131. [PubMed] [Google Scholar]
- 12.Suk JS, Boylan NJ, Trehan K, et al. N-acetylcysteine enhances cystic fibrosis sputum penetration and airway gene transfer by highly compacted DNA nanoparticles. Mol. Ther 2011;19:1981–1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Decramer M, Rutten-van Molken M, Dekhuijzen PN, et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): a randomised placebo-controlled trial. Lancet. 2005;365:1552–1560. [DOI] [PubMed] [Google Scholar]
- 14.Demedts M, Behr J, Buhl R, et al. High-dose acetylcysteine in idiopathic pulmonary fibrosis. N. Engl. J. Med 2005;353:2229–2242. [DOI] [PubMed] [Google Scholar]
- 15.Edwards GF, Steel AE, Scott JK, Jordan JW. S-carboxymethylcysteine in the fluidification of sputum and treatment of chronic airway obstruction. Chest. 1976;70:506–513. [DOI] [PubMed] [Google Scholar]
- 16.Poole P, Black PN, Cates CJ. Mucolytic agents for chronic bronchitis or chronic obstructive pulmonary disease. Cochrane Database Syst. Rev 2012:CD001287. [DOI] [PubMed] [Google Scholar]
- 17.Suter PM, Domenighetti G, Schaller MD, Laverriere MC, Ritz R, Perret C. N-acetylcysteine enhances recovery from acute lung injury in man. A randomized, double-blind, placebo-controlled clinical study. Chest. 1994;105:190–194. [DOI] [PubMed] [Google Scholar]
- 18.Zheng JP, Kang J, Huang SG, et al. Effect of carbocisteine on acute exacerbation of chronic obstructive pulmonary disease (PEACE Study): a randomised placebo-controlled study. Lancet. 2008;371:2013–2018. [DOI] [PubMed] [Google Scholar]
- 19.Chambers MS, Welsh DV, Scrimger RA, et al. RK-0202 for radiation-induced oral mucositis. J. Clin. Oncol 2006;24:5523–5523. [Google Scholar]
- 20.Pocock SJ, Simon R. Sequential treatment assignment with balancing for prognostic factors in the controlled clinical trial. Biometrics. 1975;31:103–115. [PubMed] [Google Scholar]
- 21.Beetz I, Burlage FR, Bijl HP, et al. The Groningen Radiotherapy-Induced Xerostomia questionnaire: development and validation of a new questionnaire. Radiother. Oncol 2010;97:127–131. [DOI] [PubMed] [Google Scholar]
- 22.Singer S, Wollbruck D, Wulke C, et al. Validation of the EORTC QLQ-C30 and EORTC QLQ-H&N35 in patients with laryngeal cancer after surgery. Head Neck. 2009;31:64–76. [DOI] [PubMed] [Google Scholar]
- 23.Bjordal K, Ahlner-Elmqvist M, Tollesson E, et al. Development of a European Organization for Research and Treatment of Cancer (EORTC) questionnaire module to be used in quality of life assessments in head and neck cancer patients. EORTC Quality of Life Study Group. Acta Oncol. 1994;33:879–885. [DOI] [PubMed] [Google Scholar]
- 24.Sloan JA, Vargas-Chanes D, Kamath CC, et al. Detecting worms, ducks and elephants: a simple approach for defining clinically relevant effects in quality-of-life measures. J Cancer Integr Med. 2003;1:41–47. [Google Scholar]
- 25.Allaveisi F, Hashemi B, Mortazavi SM. Radioprotective effect of N-acetyl-L-cysteine free radical scavenger on compressive mechanical properties of the gamma sterilized cortical bone of bovine femur. Cell Tissue Bank. 2015;16:97–108. [DOI] [PubMed] [Google Scholar]
- 26.Yuta A, Baraniuk JN. Therapeutic approaches to mucus hypersecretion. Curr. Allergy Asthma Rep 2005;5:243–251. [DOI] [PubMed] [Google Scholar]
- 27.Ishibashi Y, Takayama G, Inouye Y, Taniguchi A. Carbocisteine normalizes the viscous property of mucus through regulation of fucosylated and sialylated sugar chain on airway mucins. Eur. J. Pharmacol 2010;641:226–228. [DOI] [PubMed] [Google Scholar]
- 28.Warde P, O’Sullivan B, Aslanidis J, et al. A Phase III placebo-controlled trial of oral pilocarpine in patients undergoing radiotherapy for head-and-neck cancer. Int. J. Radiat. Oncol. Biol. Phys 2002;54:9–13. [DOI] [PubMed] [Google Scholar]
- 29.Fisher J, Scott C, Scarantino CW, et al. Phase III quality-of-life study results: impact on patients’ quality of life to reducing xerostomia after radiotherapy for head-and-neck cancer--RTOG 97-09. Int. J. Radiat. Oncol. Biol. Phys 2003;56:832–836. [DOI] [PubMed] [Google Scholar]
- 30.Riley P, Glenny AM, Hua F, Worthington HV. Pharmacological interventions for preventing dry mouth and salivary gland dysfunction following radiotherapy. Cochrane Database Syst. Rev 2017;7:CD012744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauletbaev N, Fischer P, Aulbach B, et al. A phase II study on safety and efficacy of high-dose N-acetylcysteine in patients with cystic fibrosis. Eur. J. Med. Res 2009;14:352–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Loprinzi CL, Barton DL, Jatoi A, et al. Symptom control trials: a 20-year experience. J. Support. Oncol 2007;5:119–125, 128. [PubMed] [Google Scholar]
- 33.Basch E, Deal AM, Dueck AC, et al. Overall Survival Results of a Trial Assessing Patient-Reported Outcomes for Symptom Monitoring During Routine Cancer Treatment. JAMA 2017;318:197–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


