Abstract
Background:
Chronic kidney disease (CKD) occurs commonly among HIV-infected persons. Statins may delay CKD onset and progression via their cholesterol-lowering and pleiotropic effects.
Methods:
Among 850 HIV-infected men from the Multicenter AIDS Cohort Study with stored urine samples (2009–2011), we evaluated cross-sectional associations of statin use with urine biomarkers of kidney damage (albumin-to-creatinine ratio [ACR], alpha-1-microglobulin, interleukin-18, kidney injury molecule-1, and procollagen type III N-terminal propeptide) using multivariable linear regression. We evaluated the longitudinal associations of statin use with annual change in estimated glomerular filtration rate by creatinine (eGFR) using linear mixed models, and with incident proteinuria and incident CKD (eGFR<60 ml/min/1.73m2) using Cox proportional hazards regression. We employed inverse probability weighting to address potential confounding related to statin use.
Results:
Statin users comprised 30% of participants. In adjusted analyses, each year of cumulative statin use was associated with 4.0% higher baseline ACR levels (P=0.05), but there was no association with baseline levels of other urine biomarkers. Statin use had no overall association with annual eGFR decline. Among participants with baseline proteinuria, statin use was modestly associated with slower annual eGFR decline compared non-use (adjusted difference: 1.33 ml/min/1.73m2 per year; 95% CI: −0.07, 2.70). Statin use was not associated with risk of incident proteinuria or incident CKD.
Conclusion:
Statin use was associated with higher baseline ACR, but not with biomarkers of tubulointerstitial injury. Statin use was associated with modestly slower eGFR decline only among participants with baseline proteinuria. Although these findings may be susceptible to confounding by indication, they suggest a limited effect of statins on CKD risk among HIV-infected men.
Keywords: statins, HIV, chronic kidney disease, albuminuria, urine biomarkers
Introduction
In the era of combination antiretroviral therapy, chronic kidney disease (CKD) has become an important comorbidity of human immunodeficiency virus (HIV) infection.1,2 HIV-infected persons are at higher risk of developing CKD and progressing to end-stage kidney disease compared to the general population.3-5 In addition, HIV-infected persons with CKD are at increased risk of cardiovascular disease and all-cause mortality.6 Despite the significant burden of kidney disease in the HIV-infected population, treatment options remain limited.
Statins lower low-density lipoprotein cholesterol levels and have additional pleotropic, anti-inflammatory effects.7 Atherosclerosis and inflammation contribute to the development and progression of kidney disease, suggesting statins may have a potential nephroprotective role.8-10 In the general population, statins have a proven cardiovascular benefit, but their effects on kidney outcomes are less clear.11,12 In the HIV-infected population, there is a high prevalence of dyslipidemia, and HIV infection is associated with chronic immune activation and vascular inflammation that persist despite sustained virologic suppression on antiretroviral therapy.13,14 Several studies show that surrogate measures for immune activation and inflammation are associated with reduced kidney function in HIV-infected persons, and also decrease with statin use.15-27 However, there are few studies in the HIV-infected population that have investigated whether or not statin use improves kidney health.28
Serum creatinine is the standard filtration marker used to assess kidney function, but it lacks sensitivity for detecting early reductions in kidney function in HIV-infected individuals, and does not adequately capture changes to kidney tubular health.4,29 Urine albumin-to-creatinine ratio (ACR), alpha-1-microglobulin (α1m), interleukin-18 (IL-18), kidney injury molecule-1 (KIM-1), and procollagen type III N-terminal propeptide (PIIINP) are promising biomarkers reflecting varying nephron regions and pathology. Urine albumin reflects glomerular injury, whereas α1m is a freely filtered, low-molecular-weight protein that reflects proximal tubular dysfunction when detected in the urine.30 IL-18 is a proinflammatory cytokine released by proximal tubular cells in response to injury or inflammation, while KIM-1 is upregulated and overexpressed by dedifferentiated proximal tubular cells after injury.31,32 Urine PIIINP reflects the presence type III collagen deposits in the renal interstitium during fibrosis, and higher urine PIIINP concentrations correlate with increased severity of fibrosis on biopsy.33,34 We have shown that HIV-infected persons have significantly higher urine ACR, α1m, IL-18, KIM-1, and PIIINP levels compared to uninfected individuals.35-37 In the Women’s Interagency HIV Study, we demonstrated that higher urine ACR, α1m, IL-18, and KIM-1 were each associated with faster kidney function decline.38,39 To our knowledge, the relationships between statin use and novel urine biomarkers of kidney damage have not been studied.
In this study, we investigated cross-sectional associations of statin use with urine biomarkers of kidney damage and longitudinal associations of statin use with kidney function in a large cohort of HIV-infected men enrolled in the Multicenter AIDS Cohort Study (MACS). We hypothesized that statin use would be associated with lower levels of kidney damage as measured by urine biomarkers, slower kidney function decline as measured by estimated glomerular filtration rate (eGFR) using serum creatinine, and decreased incidence of proteinuria and CKD.
Methods
Study Population
The MACS is an ongoing cohort study designed to understand the natural history and epidemiology of HIV infection among men. MACS enrolled 7,352 HIV-infected and -uninfected men of similar backgrounds between 1984–1985, 1987–1990, 2001–2003, and after 2010 from four sites: Baltimore, MD/ Washington D.C.; Chicago, IL; Los Angeles, CA; and Pittsburgh, PA.40 MACS participants attend semi-annual visits that include standardized interviews, physical examinations, laboratory testing, and collection of biological specimens.
The MACS Kidney Study was designed as a nested cohort study to investigate the onset and progression of kidney disease among HIV-infected men using stored urine and serum samples. The study consists of 883 HIV-infected men with urine samples collected between October 1, 2009 and September 30, 2011; we define the baseline visit in this study as the time of urine specimen collection. In the present analysis, we excluded participants without data on medication use (n=34). In the longitudinal analysis, we also excluded participants with less than two follow-up serum creatinine measurements (n=21).
The institutional review boards of participating institutions approved the study protocol, and informed consent was obtained from all study participants. This study was also approved by the University of California, San Francisco, and San Francisco Veterans Affairs Medical Center committees on human research.
Exposure
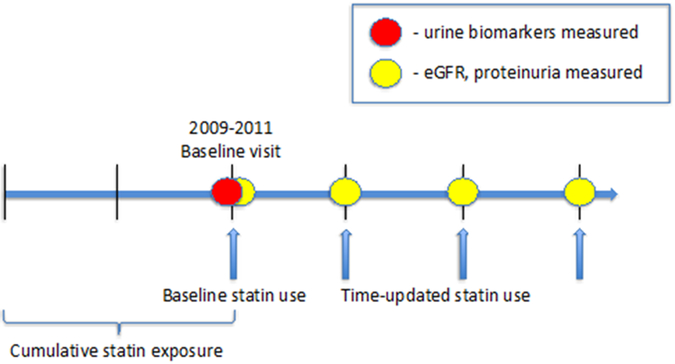
At semi-annual study visits, medication use was ascertained through standardized in-person interviews. For cross-sectional analyses, we evaluated statin use at the baseline visit and cumulative statin exposure, which we defined as the sum of the duration of statin use prior to and including the baseline visit. We designated cumulative statin exposure as our primary predictor because we theorized that any physiological effect would accrue over time. For longitudinal analyses, we evaluated time-updated statin exposure, which we defined as current statin use reported at any visit starting from the baseline visit (Figure 1).
Figure 1. Study design of statin use association with eGFR, proteinuria, and urine biomarkers in HIV-infected men in MACS.
Cumulative statin exposure captures duration of statin use prior to the baseline visit between 2009–2011. Urine biomarkers and eGFR were first measured at the baseline visit, and eGFR measures were repeated approximately every six months thereafter. Time-updated statin use captures statin exposure reported at each follow-up visit.
Outcomes
The outcomes for the cross-sectional analyses included baseline eGFR and five urine biomarkers of kidney injury: ACR, α1m, IL-18, KIM-1, and PIIINP. We calculated eGFR using the CKD-EPI (CKD Epidemiology Collaboration) creatinine equation.41 Serum creatinine was measured semi-annually using the modified Jaffe method, traceable to isotope dilution mass spectrometry. All urine biomarkers were measured at the Cincinnati Children’s Hospital Medical Center Biomarker Laboratory. Urine specimens were refrigerated immediately after collection and centrifuged at 5,000×g to remove cellular debris. The supernatant was aliquoted into 1-mL vials and then stored at −80°C without being thawed until urine biomarker measurements were undertaken. All laboratory personnel were blinded to participants’ clinical information. Details regarding the selected commercial assays and inter-assay coefficients of variation are shown in Supplemental Table 1.
The outcomes for the longitudinal analyses included annual change in eGFR, annual percentage change in urine protein-to-creatinine ratio, incident proteinuria, and incident CKD. We defined incident proteinuria as two consecutive measurements of urine protein-to-creatinine ratio >200 mg/g among persons with urine protein-to-creatinine ratio <200 mg/g at the baseline visit. We defined incident CKD as the combination of reaching an eGFR <60 ml/min/1.73m2 and declining ≥30%, among persons with eGFR ≥60 ml/min/1.73m2 at the baseline visit.
Covariates
Covariates included demographic characteristics, traditional risk factors for kidney disease, and HIV-specific risk factors. Traditional kidney disease risk factors included diabetes (defined as fasting glucose ≥ 126 mg/dL, hemoglobin A1c ≥ 6.5%, or self-reported history of diabetes and diabetes medication use), smoking (current, past, or never), systolic and diastolic blood pressures, hypertension (defined as systolic blood pressure > 140 mmHg, diastolic blood pressure > 90 mmHg, or self-reported history of hypertension and antihypertensive medication use), angiotensin-converting enzyme inhibitor (ACEi) or angiotensin II receptor blocker (ARB) use, cardiovascular disease, body mass index, waist circumference, and serum albumin. HIV-related risk factors included current and nadir CD4+ cell count, current and peak HIV-1 RNA, history of AIDS, men who have sex with men status, heroin use, history of intravenous drug use, history of hepatitis C virus (HCV) and highly active antiretroviral therapy (HAART) use, and tenofovir disoproxil fumarate (TDF) use. The definition of HAART was guided by the DHHS/Kaiser Panel [DHHS/Kaiser Nov. 2014] guidelines and defined as three or more antiretroviral drugs consisting of one or more protease inhibitors or one non-nucleoside reverse transcriptase inhibitor or a nucleoside reverse transcriptase inhibitor or an integrase inhibitor or an entry inhibitor.42
Statistical Analyses
We used inverse probability weights to account for potential differences between statin users and non-users due to confounding by indication; men with worse CKD and CVD profiles were more likely to receive statin therapy. Specifically, stabilized inverse probability of treatment weights (IPTW) were calculated for each participant by modeling statin use as a function of demographic and clinical characteristics (as listed above in the Covariates section). In addition, we used stabilized inverse probability of censoring weights (IPCW) to account for potential bias due to loss of follow-up by modeling each participant’s probability of having a non-missing outcome at follow-up. We used time-fixed weights for cross-sectional analyses and time-varying weights for longitudinal analyses. For longitudinal analyses, we calculated the final weights as the product of the stabilized treatment weights and the stabilized censoring weights. These weights were then applied to subsequent models evaluating the associations of statin use with kidney outcomes.43
We next compared baseline demographic and clinical characteristics by statin use (user vs. non-user) with chi-square and Kruskal-Wallis tests for categorical and continuous variables, respectively. We estimated cross-sectional associations of statin use with baseline kidney outcomes using two linear regression models: (1) adjusted for demographic characteristics only; and (2) adjusted for demographics, traditional kidney disease risk factors, and HIV-related factors. The demographic adjusted models controlled for age and race/ethnicity. For analyses of urine biomarker outcomes, we adjusted additionally for urine creatinine to account for urine tonicity. We chose to apply inverse probability weights to our multivariable linear regression models so that the resulting estimates would be doubly robust.44
In the longitudinal analyses, we used multivariable linear mixed models to evaluate the association of time-updated statin use with annual changes in eGFR and annual percentage changes in urine protein-to-creatinine ratio using time-varying inverse probability weights (calculated as described above). We used extended Cox models with time-varying inverse probability weights to evaluate associations of time-updated statin use with incident CKD and proteinuria. We examined time-varying hazard ratios, but found no interval in which statin use was associated with an increased risk of proteinuria or CKD. Tests of the proportional hazards assumption were non-significant for both proteinuria (P=0.47) and CKD (P=0.45).
Because of racial differences in rates of kidney function decline, we tested for interactions of statin use by race with each kidney outcome.45 Additionally, we stratified the analyses of annual change in eGFR and annual percentage change in urine protein-to-creatinine ratio by baseline proteinuria status in order to evaluate whether statin associations differed by presence of proteinuria. Since diabetes was more prevalent among statin users, we excluded participants with a diagnosis of diabetes as a sensitivity analysis for each longitudinal outcome.
Results
Study Population by Statin Use
Among the 850 HIV-infected men included in this study, 252 (30%) were statin users at the baseline visit. The median duration of statin use at the baseline visit was 6.4 years (interquartile range [IQR]: 3.1–8.7). We first compared baseline characteristics by statin use. Before reweighting the data, we found that statin users were on average older, more likely to be white, had lower prevalence of smoking, higher prevalence of diabetes mellitus and hypertension, more likely to be antihypertensive users and ACEi/ARB users, and lower LDL levels (Table 1). Statin users were also more likely to be HAART users, less likely to be TDF users, have a higher CD4 count, be virally suppressed, and have lower prevalence of HCV infection. Across all study participants, there was no dolutegravir use, and only 1.3% (11/850) used cobicistat during the follow-up period. After reweighting the sample using IPTW, differences in baseline characteristics between statin users and non-users were substantially attenuated. With the exception of higher diabetes mellitus prevalence and lower TDF use among statin users, there were no remaining statistically significant differences in age, race, traditional kidney disease risk factors, or HIV-related risk factors (Table 1).
Table 1:
Baseline characteristics of HIV-infected men in MACS stratified by statin use before and after inverse probability weighting
| Unweighted Study Sample | Weighted Study Sample * | |||||
|---|---|---|---|---|---|---|
| Parameter | Statin user, n=252 | Non-user, n=598 | P-value | Statin user | Non-user | P-value |
| Age, y | 56 (51-61) | 50 (44-55) | <0.01 | 53 (47, 57) | 51 (46, 57) | 0.37 |
| Race: | <0.01 | 0.21 | ||||
| Black | 39 (15%) | 228 (38%) | 33% | 32% | ||
| White | 206 (82%) | 308 (52%) | 62% | 60% | ||
| Other | 7 (3%) | 62 (10%) | 5% | 9% | ||
| Current Smoking | 47 (19%) | 207 (35%) | <0.01 | 32% | 31% | 0.29 |
| Diabetes mellitus | 48 (24%) | 57 (12%) | <0.01 | 23% | 16% | 0.04 |
| Systolic BP, mm Hg | 126 (116-136) | 125 (116-135) | 0.33 | 124 (115, 132) | 126 (117, 136) | 0.11 |
| Diastolic BP, mm Hg | 77 (71-84) | 78 (71-84) | 0.77 | 79 (71, 84) | 78 (72, 84) | 0.96 |
| Hypertension | 157 (67%) | 212 (39%) | <0.01 | 48% | 47% | 0.85 |
| Antihypertensive use | 144 (57%) | 159 (27%) | <0.01 | 40% | 36% | 0.30 |
| ACEi/ARB use | 99 (39%) | 96 (16%) | <0.01 | 27% | 22% | 0.08 |
| LDL, mg/dL | 100 (80-123) | 111 (88-135) | <0.01 | 110 (93, 134) | 109 (88, 134) | 0.62 |
| HDL, mg/dL | 47 (39-55) | 45 (38-55) | 0.21 | 48 (39, 58) | 45 (37, 54) | 0.17 |
| TG, mg/dL | 171 (114-236) | 130 (86-189) | <0.01 | 146 (109, 232) | 137 (94, 204) | 0.52 |
| BMI, kg/m2 | 27 (24-30) | 26 (23-30) | 0.09 | 26 (23, 30) | 26 (24, 30) | 0.23 |
| Waist Circ, cm | 97 (90-104) | 92 (85-100) | <0.01 | 92 (87, 101) | 93 (87, 102) | 0.42 |
| HAART use | 227 (90%) | 493 (83%) | <0.01 | 87% | 85% | 0.56 |
| NRTI use | 224 (89%) | 502 (84%) | 0.05 | 89% | 85% | 0.17 |
| NNRTI use | 141 (56%) | 259 (43%) | <0.01 | 48% | 48% | 0.85 |
| PI use | 119 (47%) | 263 (44%) | 0.37 | 42% | 43% | 0.79 |
| TDF use | 148 (59%) | 413 (70%) | <0.01 | 58% | 67% | 0.02 |
| Current CD4 | 591 (469-764) | 562 (375-734) | <0.01 | 572 (434, 758) | 577 (383, 762) | 0.79 |
| Nadir CD4 | 286 (167-397) | 291 (187-419) | 0.44 | 286 (160, 401) | 292 (180, 432) | 0.37 |
| History of AIDS | 46 (18%) | 72 (12%) | 0.02 | 11% | 13% | 0.34 |
| Current HIV RNA < 80 | 224 (90%) | 454 (76%) | <0.01 | 82% | 81% | 0.13 |
| Peak HIV RNA > 10K | 206 (86%) | 466 (82%) | 0.32 | 82% | 82% | 0.20 |
| MSM status | 244 (97%) | 554 (93%) | 0.02 | 95% | 93% | 0.33 |
| History of IVDU | 32 (13%) | 106 (18%) | 0.07 | 13% | 17% | 0.10 |
| Hepatitis C | 9 (4%) | 78 (13%) | <0.01 | 8% | 10% | 0.42 |
| Serum albumin, mg/dL | 4.6 (4.4-4.8) | 4.5 (4.3-4.7) | <0.01 | 4.6 (4.4, 4.7) | 4.5 (4.3, 4.7) | 0.37 |
Data are presented as median (interquartile range) or n (percent).
Abbreviations: ACEi/ARB, angiotensin-converting enzyme inhibitor/angiotensin receptor blocker; BMI, body mass index; HAART, highly active antiretroviral therapy; HDL, high density lipoprotein cholesterol; IVDU, intravenous drug use; LDL, low density lipoprotein cholesterol; MSM, men who have sex with men; NRTI, nucleoside reverse transcriptase inhibitor; NNRTI, nonnucleoside reverse transcriptase inhibitor; PI, protease inhibitor; TDF, tenofovir disoproxil fumarate; TG, triglycerides.
Estimates shown after applying inverse probability of treatment weights
Cross-Sectional Association of Statin Use with Kidney Damage and Function
In analyses that were reweighted to account for confounding by indication, statin users had somewhat higher levels of baseline ACR, but a similar prevalence of ACR > 30 mg/g, compared with non-users (Table 2). Statin users and non-users had no statistically significant differences in levels of urine α1m, IL-18, KIM-1, PIIINP, or baseline eGFR. In a sensitivity analysis excluding participants with diabetes mellitus, statin use remained associated with somewhat higher ACR (median 6.1 vs 5.4 mg/g, P<0.01), while differences in other urine biomarkers were small and did not reach statistical significance.
Table 2:
Summary of baseline levels of urine biomarkers and eGFR in HIV-infected men in MACS, stratified by statin use*
| Biomarker | Statin user | Non-user | P-value |
|---|---|---|---|
| ACR, mg/g | 7.4 (3.8, 18.5) | 6.0 (3.5, 15.6) | <0.01 |
| ACR > 30, mg/g | 19% | 18% | 0.62 |
| α1m, mg/dL | 1.3 (0.7, 3.0) | 1.5 (0.7, 3.3) | 0.90 |
| Detectable α1m | 82% | 84% | 0.56 |
| IL-18, pg/mL | 32 (16, 58) | 38 (17, 66) | 0.25 |
| KIM-1, pg/mL | 604 (301, 1095) | 645 (347, 1164) | 0.17 |
| PIIINP, ng/mL | 6.6 (3.5, 12.0) | 6.8 (3.9, 13.7) | 0.95 |
| eGFR, ml/min/1.73m2 | 90 (72, 101) | 91 (77, 103) | 0.14 |
Data are presented as Median (IQR) or numbers (percent).
Abbreviations: ACR, albumin-to-creatinine ratio; α1m, α-1-microglobulin; eGFR, estimated glomerular filtration rate; IL-18, interleukin 18; KIM-1, kidney injury molecule-1; PIIINP = pro-collagen type III N-terminal pro-peptide.
Estimates shown after applying inverse probability of treatment weights.
After multivariable adjustment for traditional and HIV-related kidney disease risk factors, baseline statin use was associated with 30.8% higher levels of ACR compared to no use, but the difference did not reach statistical significance (95% confidence interval [CI]: −6.9 to 83.7, P=0.12) (Table 3). Each year of cumulative statin exposure was associated with 4.0% higher baseline ACR levels (95% CI: −0.02 to 8.2, P=0.05). In contrast, cumulative statin exposure showed no statistically significant associations with levels of urine α1m, IL-18, KIM-1, PIIINP, or eGFR.
Table 3:
Association of statin use with percent differences in baseline urine biomarker levels in HIV-infected men in MACS
| Biomarker | Exposure | Unadjusted % difference (95%CI) |
Demographic adjusted % difference (95%CI)* |
Multivariable adjusted % difference (95%CI)** |
|---|---|---|---|---|
| ACR | Baseline statin use (yes vs. no) | 30.2 (−22.7, 119.3), p=0.32 | 27.7 (−22.7, 110.9), p=0.34 | 30.8 (−6.9, 83.7), p=0.12 |
| Cumulative statin exposure (/y) | 5.3 (0.85, 9.9), p=0.02 | 5.8 (1.50, 10.2), p<0.01 | 4.0 (−0.02, 8.2), p=0.05 | |
| α1m | Baseline statin use (yes vs. no) | −11.2 (−26.7, 7.7), p=0.23 | −12.3 (−27.5, 6.1), p=0.18 | −1.69 (−16.9, 16.3), p=0.84 |
| Cumulative statin exposure (/y) | −1.02 (−4.1, 2.1), p=0.52 | −1.43 (−4.5, 1.73), p=0.37 | −1.26 (−4.0, 1.52), p=0.37 | |
| IL-18 | Baseline statin use (yes vs. no) | −12.8 (−36.5, 19.6), p=0.40 | −12.1 (−34.7, 18.5), p=0.40 | −0.63 (−23.7, 29.4), p=0.96 |
| Cumulative statin exposure (/y) | −4.0 (−8.7, 0.88), p=0.11 | −2.4 (−7.2, 2.7), p=0.35 | 0.20 (−4.4, 5.0), p=0.93 | |
| KIM-1 | Baseline statin use (yes vs. no) | −15.1 (−34.1, 9.5), p=0.21 | −16.0 (−34.1, 7.1), p=0.16 | −6.0 (−23.0, 14.6), p=0.54 |
| Cumulative statin exposure (/y) | −0.16 (−2.9, 2.7), p=0.91 | −1.33 (−4.2, 1.65), p=0.38 | 0.53 (−2.2, 3.3), p=0.71 | |
| PIIINP | Baseline statin use (yes vs. no) | −9.3 (−29.0, 15.9), p=0.44 | −10.4 (−29.7, 14.3), p=0.38 | −3.2 (−19.1, 16.0), p=0.73 |
| Cumulative statin exposure (/y) | −1.84 (−4.5, 0.93), p=0.19 | −1.99 (−4.9, 0.99), p=0.19 | −1.54 (−3.8, 0.82), p=0.20 |
Abbreviations: ACR, albumin-to-creatinine ratio; α1m, α-1-microglobulin; eGFR, estimated glomerular filtration rate; IL-18, interleukin 18; KIM-1, kidney injury molecule-1; PIIINP = pro-collagen type III N-terminal pro-peptide.
Note: All estimates are from models that include time-fixed inverse probability of treatment weights. Models adjust for urine creatinine for all outcomes except for ACR.
Adjusted for age and race/ethnicity.
Adjusted for age, race/ethnicity, diabetes mellitus, smoking status, systolic and diastolic blood pressure, hypertension, cardiovascular disease, body mass index, waist circumference, HAART use, current and nadir CD4, current and peak HIV RNA, history of AIDS, heroin use, hepatitis C infection, serum albumin, ACEi/ARB use, TDF use, statin use, MSM status, and history of IVDU.
We then tested interactions of statin use with race on urine ACR levels. After multivariable adjustment for demographics, traditional kidney disease, and HIV-related risk factors, baseline statin use was associated with 133.7% higher ACR levels in black participants (P=0.03) and –0.73% lower ACR levels in white participants (P=0.97), with the test for baseline statin by race interaction statistically significant (P=0.05). In addition, each year of cumulative statin exposure was associated with 21.1% higher baseline ACR levels in black participants (P<0.01) and only 1.8% higher baseline ACR levels in white participants (P=0.42), with the test for cumulative statin exposure by race interaction also statistically significant (P<0.01).
Longitudinal Association of Statin Use with Annual Change in eGFR, Incident Proteinuria, and Incident CKD
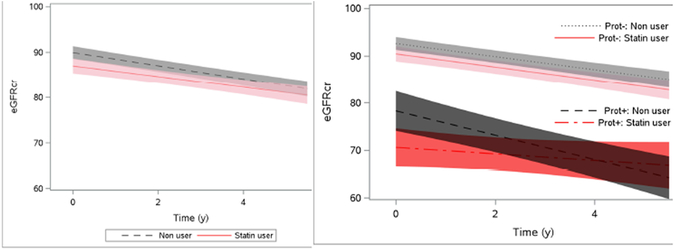
Over a median follow-up time of 4.7 years (IQR: 4.3–5.3), participants had on average 9.2 eGFR follow-up measurements. We observed statistically significant annual eGFR declines in both statin users (−1.18, 95% CI: −1.62 to −0.74 ml/min/1.73m2 per year) and non-users (−1.46, 95% CI: −1.77 to −1.16 ml/min/1.73m2 per year) (Figure 2). In multivariable adjusted analyses, there was no significant difference between statin users and non-users in the rate of annual eGFR decline (adjusted difference between statin user vs. non-user: 0.29, 95% CI: −0.31 to 0.90 ml/min/1.73m2 per year) (Table 4).
Figure 2. Association of statin use with eGFR trajectory in HIV-infected men in MACS.
Panel A shows eGFR over time from model controlling for time-updated statin use with time-updated inverse probability of treatment and censoring weights. Shaded bands denote 95% confidence interval bands. Panel B shows eGFR over time from model controlling for baseline proteinuria and time-updated statin use with time-updated inverse probability of treatment and censoring weights. Red lines denote statin users and black lines denote non-users. Solid lines denote subjects with baseline proteinuria (Prot+), and broken lines denote subjects without baseline proteinuria (Prot-). Shaded bands denote 95% confidence interval bands.
Table 4:
Association of time-updated statin use with annual change in eGFR, annual change in urine protein-to-creatinine ratio, incident proteinuria, and incident CKD in HIV-infected men in MACS
| Statin user | Non-user | Statin user vs. non-user* | |
|---|---|---|---|
| Outcome | Estimate (95% CI) | Estimate (95% CI) | |
| Annual change in eGFR, ml/min/1.73m2 per year | Estimated difference (95% CI) | ||
| Overall: | −1.18 (−1.62, −0.74) | −1.46 (−1.77, −1.16) | 0.29 (−0.31, 0.90) |
| No baseline proteinuria: | −1.39 (−1.83, −0.96) | −1.38 (−1.68, −1.08) | 0.12 (−0.49, 0.72) |
| Baseline proteinuria: | −0.68 (−1.76, 0.39) | −2.60 (−3.50, −1.56) | 1.33 (−0.07, 2.70) |
| Statin use by baseline proteinuria interaction: | P=0.10 | ||
| Annual percentage change in urine protein-to-creatinine ratio, % | Estimated % difference (95% CI) | ||
| Overall: | 1.1 (−0.8, 2.9) | 0.3 (−1.1, 1.8) | −1.1 (3.6, 1.6) |
| No baseline proteinuria: | 2.1 (0.5, 3.7) | 2.2 (1.0, 3.5) | −2.2 (−4.4, 0.15) |
| Baseline proteinuria: | −2.1 (−7.1, 3.2) | −6.5 (−10.8, −2.1) | 2.8 (−4.0, 10.0) |
| Statin use by baseline proteinuria interaction: | P=0.16 | ||
| HR (95% CI) | |||
| Incident CKD, % | 12.6 (9.3, 16.0) | 10.8 (9.0, 12.5) | 1.04 (0.52, 2.10) |
| Incident Proteinuria, % | 14.5 (10.8, 18.4) | 14.8 (12.8, 16.6) | 0.82 (0.45, 1.51) |
Abbreviations: CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; HR, hazard ratio.
Note: All estimates are from models that include time-varying inverse probability of treatment and censoring weights.
Adjusted for age, race/ethnicity, diabetes mellitus, smoking status, systolic and diastolic blood pressure, hypertension, cardiovascular disease, body mass index, waist circumference, HAART use, current and nadir CD4, current and peak HIV RNA, history of AIDS, heroin use, hepatitis C infection, serum albumin, ACEi/ARB use, TDF use, statin use, MSM status, and history of IVDU.
In both statin users and non-users, baseline eGFR was substantially lower among participants with baseline proteinuria compared to those without baseline proteinuria, and longitudinal eGFR declined significantly in all groups except for statin users with baseline proteinuria (Figure 2, Table 4). Among participants without baseline proteinuria, the rate of annual eGFR decline did not vary by statin use (statin users vs. non-users: −1.39 vs. −1.38; adjusted difference: 0.12, 95% CI: −0.49 to 0.72 ml/min/1.73m2 per year). Conversely, among participants with baseline proteinuria, statin users had modestly slower rates of annual eGFR decline compared to non-users (statin users vs. non-users: −0.68 vs. −2.60; adjusted difference: 1.33, 95% CI: −0.07, 2.70 ml/min/1.73m2 per year). The test for statin use by baseline proteinuria interaction for annual change in eGFR in multivariable adjusted analysis resulted in P=0.10.
The median baseline urine protein-to-creatinine ratio in statin users was 110 mg/g (IQR: 72 to 160) and 96 mg/g (IQR: 70 to 167) in non-statin users (P=0.01). The annual percentage change in urine protein-to-creatinine ratio did not vary by statin use or baseline proteinuria status (Supplemental Figure 1, Table 4). The test for statin use by baseline proteinuria interaction for annual change in urine protein-to-creatinine ratio in multivariable adjusted analysis resulted in P=0.16.
Among participants in our study who developed CKD, the median eGFR at baseline and at the time of incident CKD were 82.9 (IQR: 75.8, 90.5) and 52.6 (iQR: 46.1, 56.2) ml/min/1.73m2, respectively. The overall 5-year incidence of CKD appeared to be similar in statin users and non-users (12.6% vs. 10.8%), and in multivariable adjusted analysis this difference was not statistically significant (HR=1.04, 95% CI: 0.52, 2.10) (Supplemental Figure 2).
17.8% (151/850) of participants had proteinuria at baseline. The overall 5-year incidence of proteinuria appeared to be similar in statin users and non-users (14.5% vs. 14.8%). In multivariable adjusted analysis, there was no significant difference in the risk of incident proteinuria (hazard ratio [HR]=0.82, 95% CI: 0.45, 1.51).
Race did not modify the associations of statin use with annual change in eGFR (P=0.26), annual change in urine protein-to-creatinine ratio (P=0.94), incident proteinuria (P=0.66), and incident CKD (P=0.14). In sensitivity analyses excluding participants with diabetes mellitus, there were no significant differences between statin users and non-users for eGFR decline, change in urine protein-to-creatinine ratio, incident CKD, or incident proteinuria.
Discussion
In this ambulatory cohort of HIV-infected men, we evaluated associations of statin use with baseline urine biomarkers of kidney damage, longitudinal changes in eGFR and urine protein-to-creatinine ratio, and incident CKD and proteinuria. In multivariable adjusted cross-sectional analyses, cumulative statin exposure was associated with higher baseline ACR levels, but the association appeared to be limited to black participants. We found no statistically significant cross-sectional associations between statin use and biomarkers of kidney tubular health. In multivariable adjusted longitudinal analyses, we found that statin use was associated with a modestly slower annual eGFR decline compared to no statin use among participants with baseline proteinuria, but not among participants without baseline proteinuria. There were no statistically significant associations between statin use and change in urine protein-to-creatinine ratio, incident CKD, or incident proteinuria. To our knowledge, this is the first study to evaluate the association of statin use with multiple indicators of kidney damage and function in the HIV population.
Multiple trials in the general population have evaluated the effect of statins on kidney outcomes, but overall the findings remain unclear. A meta-analysis of 57 RCTs found statin use was associated with mild reductions in proteinuria and eGFR decline compared to placebo, but not a reduction in kidney failure events.12 The SATURN-HIV trial, which included 147 HIV-infected individuals with normal kidney function on stable antiretroviral therapy and either increased markers of inflammation or immune activation, found that rosuvastatin 10mg daily was associated with slower creatinine-based eGFR decline and decreased serum cystatin C compared to placebo over 24 weeks.28 Decreases in cystatin C in the rosuvastatin group also correlated with decreases in markers reflecting T-cell activation and endothelial activation. In addition, rosuvastatin use was associated with a reduction in levels of lipoprotein-associated phospholipase A2, a marker specific for vascular inflammation, but not with markers of systemic inflammation.22 In our study, statin use was associated with modestly slower annual eGFR decline only among participants with baseline proteinuria. Urine protein excretion is a measure of kidney disease, and is also thought to be a surrogate for systemic endothelial dysfunction and inflammation.46,47 Our findings suggest that statin use in HIV-infected persons with prevalent kidney damage may improve long-term kidney function. The REPRIEVE trial aims to examine the effects of statin use on measures of kidney function in HIV-infected persons on antiretroviral therapy, but the trial will not be completed for several years.48
To our knowledge, our study is the first to investigate the association of statin use with albuminuria in the HIV population. We observed a small association of cumulative statin exposure with higher baseline ACR levels, which was predominantly seen in black participants. However, the difference in ACR levels do not appear to be clinically significant because the majority of urine albumin levels in both statin users and non-users were <30 mg/g. In addition, we found no significant longitudinal association between statin use and change in urine protein-to-creatinine ratio or risk of incident proteinuria. Contrary to our findings, a meta-analysis investigating the effect of statins on albuminuria in the general population found that statins may reduce albuminuria.49 The cross-sectional association of higher baseline ACR levels among statin users may reflect greater atherosclerotic disease burden in statin users compared to non-users in our study that were not completely accounted for by our extensive statistical adjustments. The higher baseline ACR levels among African American statin users in our study may also be explained by statin-mediated effects on apolipoprotein 1 (APOL1). APOL1 is a protein component of high density lipoprotein (HDL) cholesterol, and APOL1 genetic variants confer an increased risk of CKD, associate with proteinuria, and are prevalent among people of African descent.50-52 Whether or not statins modify the effects of APOL1 risk variants on CKD risk warrants further investigation.
No prior study to our knowledge has evaluated the association of statin use with urine biomarkers of kidney tubular health. α1m is a marker of proximal tubular dysfunction; IL-18 and KIM-1 are markers of proximal tubular injury; and PIIINP is a marker of renal interstitial fibrosis. The null cross-sectional associations of statin use with α1m, IL-18, KIM-1, and PIIINP levels are consistent with the overall null longitudinal associations of statin use with kidney function. Alternatively, the pleotropic effects of statins on HIV-associated inflammation may be more pronounced in the glomerulus compared to the tubulo-interstitium.
The strengths of our study include the use of a multicenter, racially and ethnically diverse cohort representative of HIV-infected men, evaluation of long-term cumulative statin as an exposure, average follow-up period of 5 years with frequently measured serum creatinine, the use of novel measures of kidney tubule damage, and the ability to adjust for multiple traditional and HIV-related risk factors for kidney disease. Our study also has several limitations. First, because urine biomarkers of kidney damage were measured once, we were unable to determine if the associations of statin use with higher ACR levels persisted longitudinally. Second, statin use was determined by semi-annual questionnaire, which may be subject to exposure misclassification. Third, we were unable to determine the type and intensity of statin therapy, which precluded evaluation of treatment effect heterogeneity of different statins on kidney measures. Fourth, there may be residual confounding by indication despite extensive statistical adjustments, although this would likely bias statin associations to appear more harmful given the higher prevalence of traditional kidney disease risk factors in the statin group. Fifth, the longitudinal analyses include participants with varying durations of statin use, which may have led to inadequate assessment of potential time-varying and cumulative statin effects on kidney outcomes; adjustment for intermediate confounding variables that may function as mediators; and potential for selection bias if statins have early protective effects that lead to an enrichment of susceptible participants in the statin group. Finally, our results may not be generalizable to HIV-infected women.
In summary, we found that statin use was cross-sectionally associated with higher ACR levels, but not with other biomarkers of kidney damage. Statin use was associated with modestly slower longitudinal eGFR decline only among participants with baseline proteinuria, and there were no associations with incident proteinuria or incident CKD. The association between statin use and higher baseline ACR among African-Americans warrants further study, as does the association with slower eGFR declines among participants with proteinuria. Overall, these findings suggest at most a limited effect of statins on CKD risk among HIV-infected men.
Supplementary Material
Supplemental Figure 1. Association of statin use with urine protein-to-creatinine ratio trajectory in HIV-infected men in MACS Panel A shows urine protein-to-creatinine ratio over time from model controlling for time-updated statin use with time-updated inverse probability of treatment and censoring weights. Shaded bands denote 95% confidence interval bands. Panel B shows urine protein-to-creatinine ratio over time from model controlling for baseline proteinuria and time-updated statin use with time-updated inverse probability of treatment and censoring weights. Red lines denote statin users and black lines denote non-users. Solid lines denote subjects with baseline proteinuria (Prot+), and broken lines denote subjects without baseline proteinuria (Prot-). Shaded bands denote 95% confidence interval bands.
Supplemental Figure 2. Association of statin use with incident proteinuria and incident CKD in HIV-infected men in MACS, excluding participants with baseline proteinuria Panel A shows estimated cumulative incidence of proteinuria from model controlling for time-updated statin use with time-updated inverse probability of treatment and censoring weights. Panel B shows estimated cumulative incidence of CKD from model controlling for baseline proteinuria and time-updated statin use with time-updated inverse probability of treatment and censoring weights.
Acknowledgements
Funding Sources MACS Kidney Study is funded by grant R01 AG034853–01A2 (PI, Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. Data in this manuscript were collected by the Multicenter AIDS Cohort Study (MACS) with centers at Baltimore (U01-AI35042): The Johns Hopkins University Bloomberg School of Public Health: Joseph B. Margolick (PI), Jay Bream, Todd Brown, Barbara Crain, Adrian Dobs, Richard Elion, Richard Elion, Michelle Estrella, Lisette Johnson-Hill, Sean Leng, Anne Monroe, Cynthia Munro, Michael W. Plankey, Wendy Post, Ned Sacktor, Jennifer Schrack, Chloe Thio; Chicago (U01-AI35039): Feinberg School of Medicine, Northwestern University, and Cook County Bureau of Health Services: Steven M. Wolinsky (PI), John P. Phair, Sheila Badri, Dana Gabuzda, Frank J. Palella, Jr., Sudhir Penugonda, Susheel Reddy, Matthew Stephens, Linda Teplin; Los Angeles (U01-AI35040): University of California, UCLA Schools of Public Health and Medicine: Roger Detels (PI), Otoniel Martínez-Maza (Co-P I), Aaron Aronow, Peter Anton, Robert Bolan, Elizabeth Breen, Anthony Butch, Shehnaz Hussain, Beth Jamieson, Eric N. Miller, John Oishi, Harry Vinters, Dorothy Wiley, Mallory Witt, Otto Yang, Stephen Young, Zuo Feng Zhang; Pittsburgh (U01-AI35041): University of Pittsburgh, Graduate School of Public Health: Charles R. Rinaldo (PI), Lawrence A. Kingsley (Co-PI), James T. Becker, Phalguni Gupta, Kenneth Ho, Susan Koletar, Jeremy J. Martinson, John W. Mellors, Anthony J. Silvestre, Ronald D. Stall; Data Coordinating Center (UM1-AI35043): The Johns Hopkins University Bloomberg School of Public Health: Lisa P. Jacobson (PI), Gypsyamber D’Souza (Co-PI), Alison, Abraham, Keri Althoff, Jennifer Deal, Priya Duggal, Sabina Haberlen, Alvaro Muoz, Derek Ng, Janet Schollenberger, Eric C. Seaberg, Sol Su, Pamela Surkan. Institute of Allergy and Infectious Diseases: Robin E. Huebner; National Cancer Institute: Geraldina Dominguez. The MACS is funded primarily by the National Institute of Allergy and Infectious Diseases (NIAID), with additional co-funding from the National Cancer Institute (NCI), the National Institute on Drug Abuse (NIDA), and the National Institute of Mental Health (NIMH). Targeted supplemental funding for specific projects was also provided by the National Heart, Lung, and Blood Institute (NHLBI), and the National Institute on Deafness and Communication Disorders (NIDCD). MACS data collection is also supported by UL1-TR001079 (JHU ICTR) from the National Center for Advancing Translational Sciences (NCATS) a component of the National Institutes of Health (NIH), and NIH Roadmap for Medical Research. The contents of this publication are solely the responsibility of the authors and do not represent the official views of the National Institutes of Health (NIH), Johns Hopkins ICTR, or NCATS. The funders of this study had no role in study design; collection, analysis or interpretation of data; manuscript preparation; or the decision to submit the report for publication. The MACS website is located at http://www.statepi.jhsph.edu/macs/macs.html.
Conflicts of Interest and Source of Funding: The MACS Kidney Study is funded by grant R01 AG034853–01A2 (PI, Shlipak), which was administered by the Northern California Institute for Research and Education, and with resources of the Veterans Affairs Medical Center, San Francisco, California. M.G.S. and M.M.E. report receiving honoraria from Gilead Sciences.
References
- 1.Palella FJ, Baker RK, Moorman AC, et al. Mortality in the highly active antiretroviral therapy era: changing causes of death and disease in the HIV outpatient study. J Acquir Immune Defic Syndr 1999. 2006;43(1):27–34. doi: 10.1097/01.qai.0000233310.90484.16 [DOI] [PubMed] [Google Scholar]
- 2.Fernando SK, Finkelstein FO, Moore BA, Weissman S. Prevalence of chronic kidney disease in an urban HIV infected population. Am J Med Sci. 2008;335(2):89–94. doi: 10.1097/MAJ.0b013e31812e6b34 [DOI] [PubMed] [Google Scholar]
- 3.Eggers PW, Kimmel PL. Is there an epidemic of HIV Infection in the US ESRD program? J Am Soc Nephrol JASN. 2004;15(9):2477–2485. doi: 10.1097/01.ASN.0000138546.53152.A7 [DOI] [PubMed] [Google Scholar]
- 4.Odden MC, Scherzer R, Bacchetti P, et al. Cystatin C level as a marker of kidney function in human immunodeficiency virus infection: the FRAM study. Arch Intern Med. 2007;167(20):2213–2219. doi: 10.1001/archinte.167.20.2213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Estrella MM, Parekh RS, Astor BC, et al. Chronic Kidney Disease and Estimates of Kidney Function in HIV Infection: A Cross-Sectional Study in the Multicenter AIDS Cohort Study: JAIDS J Acquir Immune Defic Syndr. 2011;57(5):380–386. doi: 10.1097/QAI.0b013e318222f461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Choi AI, Li Y, Deeks SG, Grunfeld C, Volberding PA, Shlipak MG. Association between kidney function and albuminuria with cardiovascular events in HIV-infected persons. Circulation. 2010;121(5):651–658. doi: 10.1161/CIRCULATIONAHA.109.898585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davignon J Beneficial cardiovascular pleiotropic effects of statins. Circulation. 2004;109(23 Suppl 1):III39–43. doi: 10.1161/01.CIR.0000131517.20177.5a [DOI] [PubMed] [Google Scholar]
- 8.Muntner P, Coresh J, Smith JC, Eckfeldt J, Klag MJ. Plasma lipids and risk of developing renal dysfunction: the atherosclerosis risk in communities study. Kidney Int. 2000;58(1):293–301. doi: 10.1046/j.1523-1755.2000.00165.x [DOI] [PubMed] [Google Scholar]
- 9.Schaeffner ES, Kurth T, Curhan GC, et al. Cholesterol and the risk of renal dysfunction in apparently healthy men. J Am Soc Nephrol JASN. 2003;14(8):2084–2091. [DOI] [PubMed] [Google Scholar]
- 10.Hiramoto JS, Katz R, Peralta CA, et al. Inflammation and coagulation markers and kidney function decline: the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis Off J Natl Kidney Found. 2012;60(2):225–232. doi: 10.1053/j.ajkd.2012.02.335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanguankeo A, Upala S, Cheungpasitporn W, Ungprasert P, Knight EL. Effects of Statins on Renal Outcome in Chronic Kidney Disease Patients: A Systematic Review and Meta-Analysis. PLOS ONE. 2015;10(7):e0132970. doi: 10.1371/journal.pone.0132970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Su X, Zhang L, Lv J, et al. Effect of Statins on Kidney Disease Outcomes: A Systematic Review and Meta-analysis. Am J Kidney Dis. February 2016. doi: 10.1053/j.ajkd.2016.01.016 [DOI] [PubMed] [Google Scholar]
- 13.Hsue PY, Deeks SG, Hunt PW. Immunologic Basis of Cardiovascular Disease in HIV-Infected Adults. J Infect Dis. 2012;205(Suppl 3):S375–S382. doi: 10.1093/infdis/jis200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deeks SG, Lewin SR, Havlir DV. The End of AIDS: HIV Infection as a Chronic Disease. Lancet. 2013;382(9903):1525–1533. doi: 10.1016/S0140-6736(13)61809-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neuhaus J, Jacobs DR, Baker JV, et al. Markers of inflammation, coagulation, and renal function are elevated in adults with HIV infection. J Infect Dis. 2010;201(12):1788–1795. doi: 10.1086/652749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Riddler SA, Li X, Otvos J, et al. Antiretroviral therapy is associated with an atherogenic lipoprotein phenotype among HIV-1-infected men in the Multicenter AIDS Cohort Study. J Acquir Immune Defic Syndr 1999. 2008;48(3):281–288. doi: 10.1097/QAI.0b013e31817bbbf0 [DOI] [PubMed] [Google Scholar]
- 17.Fichtenbaum CJ, Yeh T-M, Evans SR, Aberg JA. Treatment with pravastatin and fenofibrate improves atherogenic lipid profiles but not inflammatory markers in ACTG 5087. J Clin Lipidol. 2010;4(4):279–287. doi: 10.1016/j.jacl.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aslangul E, Fellahi S, Assoumou LK, Bastard J-P, Capeau J, Costagliola D. High-sensitivity C-reactive protein levels fall during statin therapy in HIV-infected patients receiving ritonavir-boosted protease inhibitors. AIDS Lond Engl. 2011;25(8):1128–1131. doi: 10.1097/QAD.0b013e328346be29 [DOI] [PubMed] [Google Scholar]
- 19.Ganesan A, Crum-Cianflone N, Higgins J, et al. High dose atorvastatin decreases cellular markers of immune activation without affecting HIV-1 RNA levels: results of a double-blind randomized placebo controlled clinical trial. J Infect Dis. 2011;203(6):756–764. doi: 10.1093/infdis/jiq115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calza L, Trapani F, Bartoletti M, et al. Statin therapy decreases serum levels of high-sensitivity C-reactive protein and tumor necrosis factor-α in HIV-infected patients treated with ritonavir-boosted protease inhibitors. HIV Clin Trials. 2012;13(3):153–161. doi: 10.1310/hct1303-153 [DOI] [PubMed] [Google Scholar]
- 21.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;58(4):588–595. doi: 10.1093/cid/cit748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eckard AR, Jiang Y, Debanne SM, Funderburg NT, McComsey GA. Effect of 24 weeks of statin therapy on systemic and vascular inflammation in HIV-infected subjects receiving antiretroviral therapy. J Infect Dis. 2014;209(8):1156–1164. doi: 10.1093/infdis/jiu012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gupta S, Kitch D, Tierney C, et al. Markers of renal disease and function are associated with systemic inflammation in HIV infection: Renal and inflammatory markers in HIV infection. HIV Med. 2015;16(10):591–598. doi: 10.1111/hiv.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr 1999. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weijma RGM, Vos ERA, Ten Oever J, et al. The Effect of Rosuvastatin on Markers of Immune Activation in Treatment-Naive Human Immunodeficiency Virus-Patients. Open Forum Infect Dis. 2016;3(1):ofv201. doi: 10.1093/ofid/ofv201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Longenecker CT, Sattar A, Gilkeson R, McComsey GA. Rosuvastatin slows progression of subclinical atherosclerosis in patients with treated HIV infection. AIDS Lond Engl. 2016;30(14):2195–2203. doi: 10.1097/QAD.0000000000001167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Toribio M, Fitch KV, Sanchez L, et al. Effects of pitavastatin and pravastatin on markers of immune activation and arterial inflammation in HIV. AIDS Lond Engl. 2017;31(6):797–806. doi: 10.1097/QAD.0000000000001427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Longenecker CT, Hileman CO, Funderburg NT, McComsey GA. Rosuvastatin preserves renal function and lowers cystatin C in HIV-infected subjects on antiretroviral therapy: the SATURN-HIV trial. Clin Infect Dis Off Publ Infect Dis Soc Am. 2014;59(8):1148–1156. doi: 10.1093/cid/ciu523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Driver TH, Scherzer R, Peralta CA, et al. Comparisons of creatinine and cystatin C for detection of kidney disease and prediction of all-cause mortality in HIV-infected women. AIDS Lond Engl. 2013;27(14):2291–2299. doi: 10.1097/QAD.0b013e328362e874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weber MH, Verwiebe R. Alpha 1-microglobulin (protein HC): features of a promising indicator of proximal tubular dysfunction. Eur J Clin Chem Clin Biochem J Forum Eur Clin Chem Soc. 1992;30(10):683–691. [PubMed] [Google Scholar]
- 31.Parikh CR, Jani A, Melnikov VY, Faubel S, Edelstein CL. Urinary interleukin-18 is a marker of human acute tubular necrosis. Am J Kidney Dis Off J Natl Kidney Found. 2004;43(3):405–414. [DOI] [PubMed] [Google Scholar]
- 32.Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. Kidney Injury Molecule-1 (KIM-1): a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237–244. doi: 10.1046/j.1523-1755.2002.00433.x [DOI] [PubMed] [Google Scholar]
- 33.Ghoul BE, Squalli T, Servais A, et al. Urinary procollagen III aminoterminal propeptide (PIIINP): a fibrotest for the nephrologist. Clin J Am Soc Nephrol CJASN. 2010;5(2):205–210. doi: 10.2215/CJN.06610909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Soylemezoglu O, Wild G, Dalley AJ, et al. Urinary and serum type III collagen: markers of renal fibrosis. Nephrol Dial Transplant Off Publ Eur Dial Transpl Assoc - Eur Ren Assoc. 1997;12(9):1883–1889. [DOI] [PubMed] [Google Scholar]
- 35.Jotwani V, Scherzer R, Abraham A, et al. Does HIV infection promote early kidney injury in women? Antivir Ther. 2013;19(1):79–87. doi: 10.3851/IMP2677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jotwani V, Scherzer R, Estrella MM, et al. HIV Infection, Tenofovir, and Urine α1-Microglobulin: A Cross-sectional Analysis in the Multicenter AIDS Cohort Study. Am J Kidney Dis Off J Natl Kidney Found. 2016;68(4):571–581. doi: 10.1053/j.ajkd.2016.03.430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jotwani V, Scherzer R, Estrella MM, et al. Association of HIV infection with biomarkers of kidney injury and fibrosis in the Multicenter AIDS Cohort Study. Antivir Ther. January 2017. doi: 10.3851/IMP3124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shlipak MG, Scherzer R, Abraham A, et al. Urinary Markers of Kidney Injury and Kidney Function Decline in HIV-Infected Women: JAIDS J Acquir Immune Defic Syndr. 2012;61(5):565–573. doi: 10.1097/QAI.0b013e3182737706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jotwani V, Scherzer R, Abraham A, et al. Association of urine α1-microglobulin with kidney function decline and mortality in HIV-infected women. Clin J Am Soc Nephrol CJASN. 2015;10(1):63–73. doi: 10.2215/CJN.03220314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaslow RA, Ostrow DG, Phair JP, Detels R, Polk BF, Rinaldo CR. The multicenter aids cohort study: Rationale, organization, and selected characteristics of the participants. Am J Epidemiol. 1987;126(2):310–318. doi: 10.1093/aje/126.2.310 [DOI] [PubMed] [Google Scholar]
- 41.Inker LA, Schmid CH, Tighiouart H, et al. Estimating Glomerular Filtration Rate from Serum Creatinine and Cystatin C. N Engl J Med. 2012;367(1):20–29. doi: 10.1056/NEJMoa1114248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.DHHS/Henry J. Kaiser Family Foundation Panel on Clinical Practices for the Treatment of HIV infection. Guidelines for the use of antiretroviral agents in HIV-infected adults and adolescents. November 2014. https://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultAndAdolescentGL.pdf.
- 43.Robins JM, Finkelstein DM. Correcting for noncompliance and dependent censoring in an AIDS Clinical Trial with inverse probability of censoring weighted (IPCW) log-rank tests. Biometrics. 2000;56(3):779–788. [DOI] [PubMed] [Google Scholar]
- 44.Funk MJ, Westreich D, Wiesen C, Stürmer T, Brookhart MA, Davidian M. Doubly Robust Estimation of Causal Effects. Am J Epidemiol. 2011;173(7):761–767. doi: 10.1093/aje/kwq439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Peralta CA, Katz R, DeBoer I, et al. Racial and Ethnic Differences in Kidney Function Decline among Persons without Chronic Kidney Disease. J Am Soc Nephrol JASN. 2011;22(7):1327–1334. doi: 10.1681/ASN.2010090960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage. The Steno hypothesis. Diabetologia. 1989;32(4):219–226. [DOI] [PubMed] [Google Scholar]
- 47.Gupta J, Mitra N, Kanetsky PA, et al. Association between albuminuria, kidney function, and inflammatory biomarker profile in CKD in CRIC. Clin J Am Soc Nephrol CJASN. 2012;7(12):1938–1946. doi: 10.2215/CJN.03500412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert JM, Fitch KV, Grinspoon SK. HIV-related cardiovascular disease, statins, and the REPRIEVE trial. Top Antivir Med. 2015;23(4):146–149. [PMC free article] [PubMed] [Google Scholar]
- 49.Douglas K, O’Malley PG, Jackson JL. Meta-Analysis: The Effect of Statins on Albuminuria. Ann Intern Med. 2006;145(2):117–124. [DOI] [PubMed] [Google Scholar]
- 50.Genovese G, Friedman DJ, Ross MD, et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science. 2010;329(5993):841–845. doi: 10.1126/science.1193032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jotwani V, Shlipak MG, Scherzer R, et al. APOL1 Genotype and Glomerular and Tubular Kidney Injury in Women With HIV. Am J Kidney Dis Off J Natl Kidney Found. 2015;65(6):889–898. doi: 10.1053/j.ajkd.2015.02.329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen TK, Tin A, Peralta CA, et al. APOL1 Risk Variants, Incident Proteinuria, and Subsequent eGFR Decline in Blacks with Hypertension-Attributed CKD. Clin J Am Soc Nephrol CJASN. 2017;12(11):1771–1777. doi: 10.2215/CJN.01180117 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Association of statin use with urine protein-to-creatinine ratio trajectory in HIV-infected men in MACS Panel A shows urine protein-to-creatinine ratio over time from model controlling for time-updated statin use with time-updated inverse probability of treatment and censoring weights. Shaded bands denote 95% confidence interval bands. Panel B shows urine protein-to-creatinine ratio over time from model controlling for baseline proteinuria and time-updated statin use with time-updated inverse probability of treatment and censoring weights. Red lines denote statin users and black lines denote non-users. Solid lines denote subjects with baseline proteinuria (Prot+), and broken lines denote subjects without baseline proteinuria (Prot-). Shaded bands denote 95% confidence interval bands.
Supplemental Figure 2. Association of statin use with incident proteinuria and incident CKD in HIV-infected men in MACS, excluding participants with baseline proteinuria Panel A shows estimated cumulative incidence of proteinuria from model controlling for time-updated statin use with time-updated inverse probability of treatment and censoring weights. Panel B shows estimated cumulative incidence of CKD from model controlling for baseline proteinuria and time-updated statin use with time-updated inverse probability of treatment and censoring weights.