Abstract
The locus coeruleus-norepinephrine (LC-NE) system is involved in many brain functions and neurological disorders. In this review we discuss how LC-NE signaling affects the activity of cortical and subcortical sensory neurons, and how it influences perception-driven behaviors associated with mammalian somatosensory, visual, auditory, and olfactory systems. We summarize the consistent as well as seemingly inconsistent findings across brain areas and sensory modalities and propose a framework to understand these phenomena from the perspective of adrenergic receptor expression, dose-dependent physiology and excitation-inhibition balance. We also discuss potential future research directions in this field.
Keywords: locus coeruleus, norepinephrine, sensory processing, perception, signal-to-noise ratio, dose-dependent response, E/I balance
1. Introduction
The locus coeruleus (LC) is a small nucleus situated in the pons of the brainstem. Neurons in the LC broadly innervate the brain and release the neuromodulator norepinephrine (NE, also known as noradrenaline, NA) at their terminal fields. Being the first neuromodulatory circuit characterized anatomically and neurochemically, the LC-NE system has long been recognized as critical in mediating a wide spectrum of brain functions ranging from sleep-wake transitions and arousal to higher-order processes such as attention and learning. Clinically, this modulatory system is implicated in attention-, stress- and anxiety-related disorders including attention-deficit hyperactivity disorder (ADHD) and post-traumatic stress disorder (PTSD).
Decades of research has made tremendous progress toward revealing LC-NE functions, and the field abounds with excellent reviews on the molecular compositions, signaling pathways, anatomy, physiology, behavioral correlates, and clinical relevance of this system (Foote et al., 1983; Foote and Morrison, 1987; Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005; Bouret and Sara, 2005; Sara, 2009; Sara and Bouret, 2012; Schwarz and Luo, 2015; Aston-Jones and Waterhouse, 2016; Waterhouse and Navarra, 2018). However, our knowledge of the fundamental neurobiology underlying how the LC-NE system affects the activity of downstream neurons and modulates behavioral states and cognition is still quite incomplete. It has been suggested that the LC-NE system plays key roles in sensory signal processing to facilitate downstream processes such as decision making and motor response (Foote et al., 1983; Berridge and Waterhouse, 2003; Waterhouse and Navarra, 2018). The adaptive gain theory proposes that the LC is more involved in higher brain functions in such a way that LC phasic activity acts as an attentional filter to selectively promote behaviors relevant to the current task, and its tonic activity promotes disengagement from the current task and exploration of alternative behaviors (Aston-Jones and Cohen, 2005). Somewhat complementing this theory, it has also been suggested that LC phasic activity reorganizes the functional network of downstream neurons to allow rapid behavior adaptation and cognitive shifts (Bouret and Sara, 2005; Sara, 2009). It is likely that LC executes these functions by interacting with both the bottom-up stream that directly conveys sensory information and the top-down control signals (Sara and Bouret, 2012).
Here we propose that understanding how LC-NE modulates sensory processing and perception offers a stepping stone toward unraveling its roles in higher cognitive functions and potentially provides insight into the abnormalities underlying the diseased states. The rationale goes as follows. First, LC extensively innervates sensory cortical and subcortical structures (Morrison and Foote, 1986; Simpson et al., 1997), and single LC neurons collaterally project to multiple relay stations along the ascending sensory pathway (Simpson et al., 1997). Such anatomical organization strongly suggests that the LC nucleus can profoundly affect the transmission of sensory information. Second, attention involves selectively processing the relevant sensory cues while filtering out the competing, irrelevant information. Modulation of sensory processing is a key feature of attentional modulation (Thiele and Bellgrove, 2018), and a large body of literature has demonstrated enhanced sensory responses to relevant cues and reduced responses to irrelevant ones (e.g., Spitzer et al., 1988; Reynolds et al., 2000; Martinez-Trujillo and Treue, 2004). Attentional modulation of neuronal spiking is also accompanied by changes in inter-neuronal correlation and oscillation in sensory areas (e.g., Steinmetz et al., 2000; Fries et al., 2001; Cohen and Maunsell, 2009). Finally, the prefrontal cortex, which plays an essential role in attentional modulation, is the major source of cortical input to LC (Arnsten and Goldman-Rakic, 1984; Jodo et al., 1998). Thus, LC is likely to be an important hub that broadcasts the command signals from prefrontal cortex to other brain areas.
Despite the importance of LC-NE modulation of sensory processing and perception, few review articles focus on this topic (Berridge and Waterhouse, 2003; Hurley et al., 2004; Waterhouse and Navarra, 2018). Here we discuss how LC-NE signaling affects the activity of cortical and subcortical sensory neurons, and how LC-NE influences perception-driven behaviors associated with mammalian somatosensory, visual, auditory, and olfactory systems. In doing so we attempt to bridge the analysis presented here with existing theories in the field.
Previous lesion studies have provided valuable insights into LC-NE functions (reviewed in Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005). However, chronic lesions may induce compensatory, plasticity changes such that the mechanisms underlying behavioral changes are difficult to define (Acheson et al., 1980; Harik et al., 1981; Chiodo et al., 1983; Harik, 1984; Valentini et al., 2004; Aston-Jones and Cohen, 2005). Here we focus on reviewing studies that employed acute manipulations such as local pharmacological, electrical, chemogenetic or optogenetic perturbations that target the LC nucleus or NE content in downstream brain areas (Fig. 1). We point out that transient manipulations may produce indirect ‘off-target’ effects that could lead to misinterpretation or overestimation of the effects (e.g., Otchy et al., 2015).
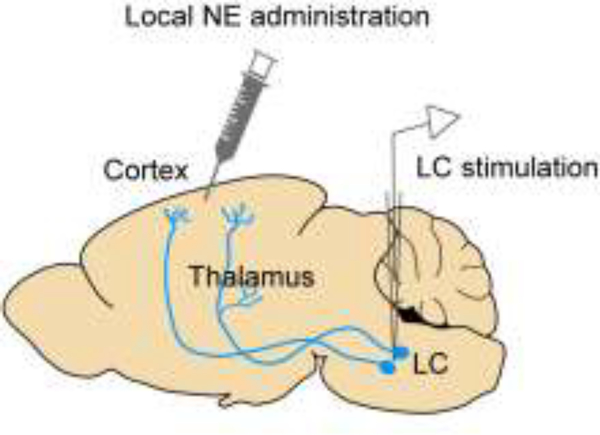
Figure 1.
Perturbing LC-NE in vivo. Manipulations are performed mainly by local pharmacological administration in the target area, or by direct LC stimulation. As illustrated here, LC stimulation may affect brain areas (e.g., sensory thalamus) that are upstream to the targeted region of interest (e.g., sensory cortex), and influence the latter both directly and indirectly.
This review aims not to enumerate observations reported in the literature, but rather to summarize the consistent as well as mixed findings across brain areas and sensory modalities. We try to synthesize the available information from the literature and to provide potential explanations to unify these findings under a proposed framework of LC-NE functions. By doing so, we hope to help identify future research directions and promote the scientific endeavors in this exciting and fast-progressing field.
2. A brief overview of adrenergic receptors and NE synaptic effects
There are three main types of adrenergic receptors (AR) in the brain: α1, α2 and β, with several subtypes in each family. α2 ARs have the highest affinity to NE. Presynaptic α2 AR functions as an autoreceptor. α2 ARs are linked to the Gi protein and inhibit the production of cyclic adenosine monophosphate (cAMP). Activating α2 may increase K+ conductance and inhibit Ca2+ channels. α1 ARs have a lower affinity to NE, and activate the Gq pathway to promote phospholipase C (PLC), protein kinase C (PKC) and Ca2+ release, and to decrease K+ conductance. β ARs have the lowest affinity to NE. They activate adenylate cyclase via the Gs pathway. Activating β ARs may decrease K+ conductance, increase cAMP, enhance hyperpolarization-activated currents and Ca2+ currents (Ramos and Arnsten, 2007; Marzo et al., 2009).
The intracellular mechanisms of NE-mediated effects have been mainly examined in vitro (e.g., McCormick and Prince, 1988; Nicoll et al., 1990; McCormick, 1992a, 1992b). NE can produce both excitatory and inhibitory effects on neuronal activity. The inhibitory hyperpolarization effect is mainly mediated by α2 ARs, due to an increase in K+ conductance and a decrease in Ca2+ currents. NE may cause a small hyperpolarization and block the slow afterhyperpolarization (AHP) through β ARs. Activation β ARs can also depolarize neurons by decreasing K+ conductance or activating adenylate cyclase. The primary excitatory effect of NE is a slow depolarization via α1-mediated decrease of K+ currents. Depending on NE concentration, brain regions, cortical layers and AR types, NE mediates diverse effects of glutamatergic and GABAergic signaling (Salgado et al., 2016).
3. LC-NE modulation of sensory neuron activity
3.1. Somatosensory system
In the somatosensory cortex of rats and cats, most studies generally agree that LC-NE activation facilitates the representation of sensory signals by inhibiting spontaneous activity more than sensory-evoked responses, thus effectively enhancing the signal-to-noise ratio (SNR) at the population level (Fig. 2A, B). Specifically, local NE administration (Waterhouse and Woodward, 1980; Waterhouse et al., 1980, 1981; Armstrong-James and Fox, 1983; Warren and Dykes, 1996; Castro-Alamancos and Gulati, 2014) or LC stimulation (Lecas, 2001; Devilbiss and Waterhouse, 2004) inhibits both spontaneous activity and periphery stimuli-evoked responses of the majority of somatosensory cortex neurons (50–80% of sampled population), while a smaller population show increased firing rate (10–40%). LC-NE also potentiates sensory- or artificially-evoked inhibitory responses (Waterhouse and Woodward, 1980; Waterhouse et al., 1980). If the evoked activity has a phasic-tonic temporal profile, NE tends to differentially enhance the initial transient phasic component and inhibit the following long-lasting tonic component (Waterhouse and Woodward, 1980; Warren and Dykes, 1996; Waterhouse et al., 1998; Lecas, 2004). In addition, LC-NE activation has been shown to enhance the fidelity of stimulus representation by reducing response latency and jitter (Devilbiss and Waterhouse, 2004; Lecas, 2001, 2004), and making previously unresponsive neurons fire action potentials in the presence of sensory stimuli (sensory gating, Waterhouse et al., 1988; Devilbiss and Waterhouse, 2004, 2011; Vazey et al., 2018). Vazey and colleagues further showed that phasic, but not tonic LC activation facilitates cortical representation of sensory inputs (Vazey et al., 2018), consistent with the idea that LC tonic and phasic activity patterns serve different functions (Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005; Bouret and Sara, 2005).
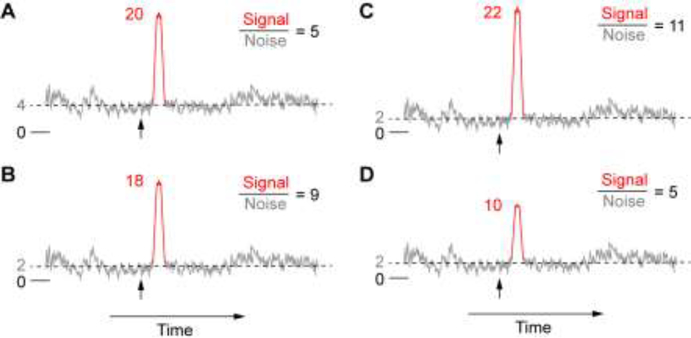
Figure 2.
Schematic of LC-NE modulation of the signal-to-noise ratio (SNR). A. In the control condition, sensory-evoked response (signal, red) is 20 and spontaneous activity (noise, grey) is 4 in arbitrary unit, and SNR is 5. Vertical arrow indicates stimulus onset. B-D: Example scenarios illustrating how LC-NE changes SNR. B. SNR may increase when LC-NE suppresses spontaneous activity to a greater degree (50%) than evoked response (10%). C. SNR may increase when LC-NE suppresses spontaneous activity and facilitates evoked response. D. SNR may remain the same when LC-NE suppresses spontaneous activity and evoked response to a similar degree (50%).
LC-NE modulatory effects vary across different layers of the somatosensory cortex. The general consensus is that inhibition dominates all cortical layers (Waterhouse and Woodward, 1980; Armstrong-James and Fox, 1983; Devilbiss and Waterhouse, 2004), and facilitation is restricted mainly to layer (L) 5 and 6 (Waterhouse and Woodward 1980; Warren and Dykes 1996; Waterhouse et al., 1998; Devilbiss and Waterhouse, 2011). However, an overwhelming facilitation in superficial layers and suppression in L4 of cats have been reported (Warren and Dykes, 1996). We think that the documented layer-specific, dose-dependent effects could help understand such differences: facilitation occurs during iontophoresis of low concentrations of NE ([NE], Armstrong-James and Fox, 1983; Warren and Dykes, 1996), and increasing [NE] either switches the facilitating effect to inhibition, or further potentiates the existing inhibitory action. Armstrong-James and Fox also demonstrated that about 30% of deeper layer neurons can be excited by low [NE] (applying small iontophoretic currents) which readily inhibits superficial layers, and higher [NE] suppresses the majority of neurons located in superficial as well as deeper layers (Armstrong-James and Fox, 1983). In light of these findings, most studies that reported a predominantly inhibitory effect employed high [NE] of 0.5–1.0 M for iontophoresis (e.g., Waterhouse and Woodward, 1980; Waterhouse et al., 1980, 1981;). In comparison, facilitation occurs during 0.1 M [NE] administration (e.g., Armstrong-James and Fox, 1983).
Fewer studies have investigated the role of LC-NE in modulating subcortical regions of the somatosensory pathway. Limited data reveal that local NE microdialysis inhibits both spontaneous activity and whisker-evoked neuronal spiking in the whisker-responsive intermediate layers of the superior colliculus (Bezdudnaya and Castro-Alamancos, 2014). In the whisker-representing ventral posteromedial nucleus (VPM) of the thalamus, LC-NE inhibits spontaneous activity of most neurons (Hirata et al., 2006), but the primary effect on sensory response is a facilitation (Devilbiss and Waterhouse, 2004, 2011; Devilbiss et al., 2006; Hirata et al., 2006): the net effect is an SNR enhancement (e.g., Fig. 2C), similar to the situation in the cortex. A recent work (Rodenkirch et al., 2019) reported that LC stimulation improves thalamic information transmission in both anesthetized and awake rats, and provided evidence to suggest that this is likely due to LC-NE modulation of the interactions between VPM and the reticular nucleus. By systematically varying LC stimulation parameters, Devilbiss and colleagues found that both the firing rate of individual VPM neurons and their pairwise correlation change non-monotonically with stimulation frequency, despite significant heterogeneity (Devilbiss and Waterhouse, 2004; Devilbiss et al., 2006). They also showed that LC tonic and phasic activation mediate diverse modulatory effects at single-cell, pairwise and ensemble levels in both somatosensory thalamus and cortex (Devilbiss and Waterhouse, 2011). LC phasic stimulation preferentially enhances stronger sensory inputs, and produces larger changes in functional connectivity compared with tonic stimulation. Interestingly, when LC is activated by stress-related corticotropin-releasing factor, spontaneous activity is enhanced and evoked response suppressed (Devilbiss et al., 2012), suggesting that abnormally activated LC-NE signaling likely engages different pathways and impairs information processing.
3.2. Visual system
LC-NE actions have been characterized in various stages along the visual pathway. Similar to the findings in the somatosensory cortex, NE iontophoresis decreases spontaneous activity in the visual cortex of cats and rats (typically >80% of recorded cells, Kolta et al., 1987; Kolta and Reader, 1989; Ego-Stengel et al., 2002). Other studies found a less pronounced, but still dominant inhibitory effect (~40–50% of the population, Olpe et al., 1980; Sato et al., 1989). Most groups reported that the majority of visual cortex neurons exhibit reduced evoked responses upon LC-NE activation (50–80% of the population, Videen et al., 1984; Kolta et al., 1987; Kolta and Reader, 1989; Sato et al., 1989; McLean and Waterhouse, 1994; Ego-Stengel et al., 2002), with a few exceptions showing equal subpopulations that exhibit increased, decreased and unaffected responses (Kasamatsu and Heggelund, 1982), or even a predominant facilitation (Waterhouse et al., 1990). Different from the somatosensory cortex, most work on the visual cortex reported an insignificant change of SNR at the population level (e.g., Fig. 2D), with comparable proportions of cells showing an increase or decrease (Videen et al., 1984; Sato et al., 1989; Ego-Stengel et al., 2002). However, a few groups identified a dominant SNR increase (50–60% of sampled population, Kasamatsu and Heggelund, 1982; Waterhouse et al., 1990). In addition, local NE administration sharpens velocity tuning but not orientation tuning of visual cortex neurons (McLean and Waterhouse, 1994; Ego-Stengel et al., 2002).
Both dose-dependent modulation and layer-specific effects, similar to those in the somatosensory cortex, have been reported in the visual cortex. For example, increasing LC-NE activation intensity potentiates its inhibitory action (Olpe et al., 1980; McLean and Waterhouse, 1994). Suppression dominates superficial layers, and facilitation is more pronounced in L5 and L6 (Sato et al., 1989).
In subcortical visual areas, NE suppresses both spontaneous and evoked activity in the visual layers of the superior colliculus of rats and hamsters (60–80% inhibition vs. 10–25% facilitation at the population level), with no obvious change in SNR (Sato and Kayama, 1983; Zhang et al., 1999). In the lateral geniculate nucleus (LGN), however, most neurons (>80%) exhibit enhanced spontaneous activity as well as elevated evoked responses in the presence of local administration of low [NE] (0.1 M) or LC stimulation (Nakai and Takaori, 1974; Rogawski and Aghajanian, 1980a, 1980b; Kayama et al., 1982; Holdefer and Jacobs, 1994). In comparison, the predominant inhibitory effects in most of the visual cortex and superior colliculus studies are under a much higher [NE] (0.4–0.5 M). Therefore, the discussed dose-dependent NE modulation may also account for the differential modulatory effects in different visual areas. However, two studies (Sato and Kayama, 1983; Ego-Stengel et al., 2002) using low, comparable iontophoresis parameters still found an overwhelming suppression, which suggests that region-specific expression of adrenergic receptors also plays a role.
3.3. Auditory system
The main effect of LC-NE activation on the auditory system is still inhibitory. One pioneering work (Foote et al., 1975) reported that the spontaneous and evoked activity of all cells recorded in the primary auditory cortex of awake squirrel monkeys are inhibited during NE iontophoresis. Qualitatively similar suppressive actions were observed in the cochlear nucleus of bats and cats (Chikamori et al., 1980; Kossl and Vater, 1989). In contrast, a series of studies by Edeline and colleagues identified the inhibitory effect in a smaller fraction of auditory cortex neurons in rats (Manunta and Edeline, 1997, 1999; <50% compared with 70–80% in previous work). Given the dose-dependent NE modulation discussed above, the observations made by Edeline and colleagues may be due to the lower [NE] administered in their experiments (0.1 M vs. 0.2–0.5 M in other studies). Other factors such as animal states (i.e., awake vs. anesthetized, and depth of anesthesia) may also be considered, because anesthesia restricts facilitation to a smaller population of cells while expanding the suppressive effect to a larger population (Kössl and Vater, 1989; Manunta and Edeline, 1997, 1999). Regardless, in most studies inhibition dominates over excitation (<20% of the population). Yet, most groups did not find an overall significant change of SNR (except for Foote et al., 1975; Kössl and Vater, 1989).
In contrast, repetitive pairing of tones with brief LC stimulation in a temporally precise manner produces somewhat different changes in the auditory cortex (Edeline et al., 2011; Martins and Froemke, 2015). An overall facilitating effect on frequency tuning (elevated tuning curve) was observed after pairing LC stimulation with tones of a particular frequency, but neuronal responses to the paired frequency vary: strong LC stimulation (100 Hz) during pairing yields comparable subpopulations exhibiting increased or decreased response (Edeline et al., 2011), whereas moderate LC stimulation (20 Hz) produces a more ubiquitous facilitating effect (Martins and Froemke, 2015). On the other hand, pairing tones with brief local NE administration exerts a pronounced inhibitory effect on auditory responses, with maximal reduction of the firing rate at the paired frequency (Manunta and Edeline, 2004). Different results obtained by LC stimulation and NE iontophoresis suggest that these two perturbation methods may involve different pathways/mechanisms to affect auditory cortex activity (e.g., NE in auditory cortex alone vs. NE in auditory thalamus + cortex).
3.4. Olfactory system
LC modulation of olfactory processing (mostly in the olfactory bulb, OB) has been previously reviewed (Linster et al., 2011). Here, we briefly discuss recent work and relate the findings to other sensory modalities. Despite its heterogeneous modulatory effects, LC stimulation exerts stronger suppression of spontaneous activity of both olfactory sensory neurons and mitral/tufted cells (MT) than odor-evoked responses in rats and mice (Jiang et al., 1996; Eckmeier and Shea, 2014; Manella et al., 2017), a recurring phenomenon in other sensory modalities (e.g., Foote et al., 1975; Waterhouse and Woodward, 1980). In addition, Manella and colleagues showed that NE infusion inhibits a larger fraction of cells in the OB than LC stimulation (Manella et al., 2017), further indicating that direct LC stimulation may involve additional pathways to modulate downstream areas compared with local NE infusion. Intriguingly, a non-monotonic relationship between the overall inhibitory effect and [NE] (or LC stimulation frequency) again emerges. Extremely low and high [NE] (or LC stimulation) produces stronger bulbar inhibition. The sensory gating effect has also been reported in the OB, as activating LC increases MT response to peri-threshold epithelial stimulation, but not to supra-threshold intensities (Jiang et al., 1996). In addition, pairing olfactory cues with LC stimulation significantly reduces OB response to the paired odor (Shea et al., 2008). Just as the studies on the auditory system, these findings collectively suggest that prolonged, temporally aligned coincident occurrence of LC-NE activation and sensory input induces different plasticity mechanisms to modulate neuronal activity.
We are only able to identify one study that examined LC-NE effects on the olfactory pathway outside of the olfactory bulb. In the rat piriform cortex, Bouret and Sara reported that LC stimulation facilitates cortical responses to odor, and has both sensory gating effect and differential tonic-phasic modulations (Bouret and Sara, 2002).
To summarize, LC-NE activation appears to exert a ubiquitous, possibly layer-specific inhibition on sensory cortices. Cortical representation of sensory inputs can be enhanced by different mechanisms such as sensory gating, suppression of spontaneous activity, and reduction of response latency and jitter. On the other hand, LC-NE-mediated facilitation is more pronounced in subcortical sensory neurons. For both cortical and subcortical regions, the dose-dependent modulation of LC-NE activation on neuronal response typically follows a non-monotonic relationship.
4. LC-NE modulation of perception-driven behavior
Given that LC-NE affects neuronal activity from single-cell to population levels across multiple sensory modalities, it is natural to expect that behavioral effects would ensue. For example, if LC-NE specifically facilitates neuronal responses to weak stimuli, it would enhance an animal’s ability to perceive peri-threshold sensory inputs. However, to our knowledge only a few studies directly tested LC-NE effects on perception-driven behaviors, and even fewer attempted to link the modulation of neuronal responses to behavioral effects.
In the somatosensory system, rats were trained to perform a Go/NoGo tactile discrimination task, where the Go stimulus is an 8 Hz whisker deflection, and NoGo stimulus is 4 or 6 Hz (Rodenkirch et al., 2019). Optogenetic LC stimulation significantly improves rats’ perceptual sensitivity d’. Interestingly, LC stimulation produces a larger improvement when the NoGo stimulus is more perceptually similar to the Go stimulus (NoGo vs. Go, 6 vs. 8 Hz, compared with 4 vs. 8 Hz). Behavioral enhancement was abolished by locally blocking NE in the VPM during LC stimulation, in agreement with the electrophysiological findings that LC-NE actions on somatosensory thalamus facilitates information transmission.
In studies involving the auditory system, LC activation facilitates operant perceptual learning. One study trained rats to associate tones of a particular frequency (target) with food reward while ignoring other frequencies (distractors). Pairing LC stimulation with a weak target tone significantly enhances stimulus detection, with no behavioral changes to distractor tones (Martins and Froemke, 2015). Such behavioral improvement is consistent with the electrophysiological evidence showing that this pairing paradigm facilitates auditory cortex response to the target tone. When distractor tones were changed to within ½ octave from the target frequency (perceptually similar), task performance was initially impaired during LC pairing, but eventually recovered and rose above the control level over the course of many hours. This observation also agrees with the initial-broadening and later-sharpening temporal profile of the tuning curve. During reversal learning where the contingencies of target and distractor tones were switched, pairing LC stimulation with the new target (previously distractor) tone significantly reduced the amount of time needed to acquire the switching (Martins and Froemke, 2015; Glennon et al., 2018).
LC-NE modulation of olfaction has been reviewed before (Linster et al., 2011; Linster and Escanilla, 2018). The behavioral data generally agree with electrophysiological studies that bulbar NE facilitates olfaction. In a series of experiments (Escanilla et al., 2010), rats were initially presented with an odorless control substance, followed by a novel odor A. More time spent exploring the space where odor A was delivered indicates spontaneous ‘detection’. Subsequent presentations of the same odor induced habituation, i.e., less investigation time. After multiple trials with odor A, a second novel odor B was introduced. More time spent exploring odor B than the last trial of odor A indicates spontaneous ‘discrimination’. Compared with control animals, rats with bulbar NE infusion showed signs of increased investigation during trials where low concentrations of odor A or B was first presented. These behavioral findings strongly indicate that bulbar NE signaling improves perceptual sensitivity, consistent with the electrophysiological evidence that NE enhances SNR in the OB (e.g., Manella et al., 2017).
Interestingly, rats trained to associate reward with olfactory cues have lower detection thresholds than in spontaneous detection, and blocking bulbar NE impairs operant detection performance as well as the ability to discriminate perceptually similar odors (Doucette et al., 2007; Escanilla et al., 2012). Importantly, these results indicate that the LC-NE circuit is engaged in motivated perceptual behavior and acts to improve sensitivity in early sensory processing stations to facilitate olfaction.
5. A proposed framework to understand LC-NE modulatory effects
LC-NE modulatory effects appear to vary systematically across brain regions and cortical layers. Specifically, NE-mediated facilitation occurs more frequently in sensory thalamus than the associated cortical regions, and neurons in deeper cortical layers exhibit more pronounced facilitating effects than those in superficial layers. Here, we attempt to explain these region- and layer-specific effects from the perspective of adrenergic receptor (AR) expression and physiology.
From a regional standpoint, the expression of different ARs appears to have a strong correlation with the specific modulatory effects. There is a relatively abundant expression of α1 and β ARs and a reduced α2 expression in sensory cortices. In contrast, sensory thalamic regions show a relatively low expression level of β AR, and possibly sparser expressions of α1 and α2 ARs (Young and Kuhar, 1980; Rainbow et al., 1984; McCune et al., 1993; Nicholas et al., 1993; Pieribone et al., 1994; Scheinin et al., 1994; Allen Brain Atlas; Table 1). As discussed earlier, α1 ARs are mainly excitatory, while β ARs can mediate both excitatory and inhibitory postsynaptic effects. Previous work also suggests that α1 and β ARs may enhance GABAergic signaling (Papay et al., 2006; Ramos and Arnsten, 2007; Salgado et al., 2016). Based on these lines of evidence, we hypothesize that α1- and/or β-mediated inhibition underlies the predominantly inhibitory NE actions on cortical activity compared with thalamus.
Table 1.
Qualitative assessment of AR expression levels across sensory thalamic and cortical regions and cortical layers, based on collated data from Young and Kuhar, 1980; Rainbow et al., 1984; McCune et al., 1993; Nicholas et al., 1993; Pieribone et al., 1994; Scheinin et al., 1994 and the Allen Brain Mouse Atlas for α1a, α1d, α2a, α2b, α2c and β1 ARs. Expression level: L-Low, M-Moderate, H-High. Right: Example sagittal sections of ISH for α1d, α2b and β1 ARs from Allen Brain Mouse Atlas (Experiments 69236807, 80525494, 80472045), illustrating differential AR expression levels in sensory cortical and thalamic regions.7
| α1 | α2 | β | |
|---|---|---|---|
| Somatosensory Cortex | |||
| L2/3 | M-H | L | H |
| L4 | L-M | L | L |
| L5 | M-H | L | M |
| L6 | L-M | L | L |
| Visual Cortex | |||
| L2/3 | M | L | H |
| L4 | L-M | L | L |
| L5 | M | L | M |
| L6 | L | L | M |
| Auditory Cortex | |||
| L2/3 | L-M | L | H |
| L4 | L | L | L |
| L5 | M-H | L | M |
| L6 | M | L | M |
| VPM | L | L | L-M |
| LGN | L | L | L-M |
Expression level: L - Low, M – Moderate, H – High.
Within sensory cortices, β ARs are more abundant in superficial layers than L4, L5 and L6 (Nicholas et al., 1993; Pieribone et al., 1994; Allen Brain Atlas; Table 1). Such layer-specific AR distribution also likely contributes to the layer-specific NE modulation (i.e., predominant inhibition in superficial layers and more pronounced facilitation in deeper layers). To further support this notion, lower expressions of α1 and/or β ARs are associated with both region-specific (thalamus) and layer-specific (deeper layers) facilitating effects. To quantitatively and causally link receptor expression patterns to NE modulatory effects, one needs to assess AR expression levels and their dose-dependent physiological responses in a cell type-specific manner (such as patch-seq, Cadwell et al., 2016).
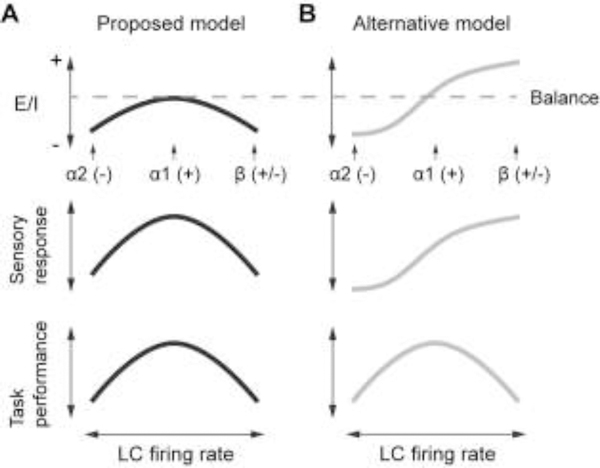
The excitation-inhibition (E/I) balance can profoundly affect single-cell and circuit-level activity, neural computation, and animal behavior (e.g., Wehr and Zador, 2003; Higley and Contreras, 2006; Haider and McCormick, 2009; Yizhar et al., 2011; Yang et al., 2012). Based on receptor affinity (α2 > α1 > β, Arnsten, 2000), increasing LC activity will progressively activate α2, α1 and β ARs, which will differently affect E/I balance (Fig. 3). Here, we propose that different states of E/I balance due to the activation ratios of different ARs are important for the dose-dependent modulatory effects (as in Armstrong-James and Fox, 1983; McLean and Waterhouse, 1994), and underlie how LC modulates sensory responses. We further propose that at least for perceptual-related behaviors, LC modulation of sensory responses plays an important role in the modulation of behavior (e.g., Aston-Jones et al., 1999; Aston-Jones and Cohen, 2005; Fig. 3): Low LC activity mainly activates the inhibitory α2 ARs, and overwhelming inhibition reduces sensory responses and impairs task performance. Increasing LC activity will activate excitatory α1 and then β ARs. Intermediate LC activity leads to balanced E/I, enhanced sensory response and optimal behavior. Too high LC activity may again cause overwhelming inhibition and therefore impairs behavior (Fig. 3A). Existing literature appears to be in line with this model, showing that the relationship between LC activity/NE concentration and sensory response follows an inverted- U shaped curve (e.g., Devilbiss and Waterhouse, 2004; Devilbiss et al., 2006; Manella et al., 2017), and likewise between LC activity/NE concentration and task performance (e.g., Rajkowski et al., 1994; Usher et al., 1999). More recent work involving LC manipulations further supports a causal relationship by demonstrating that activating LC with DREADDs (Designer Receptor Exclusively Activated by Designer Drugs) facilitates the emergence from anesthesia (Vazey and Aston-Jones, 2014). LC stimulation enhances perceptual task performance (Rodenkirch et al., 2019), and more intense activation may induce abnormal behavior (Carter et al., 2010). Alternatively, too high LC activity may cause overwhelming excitation and amplify neuronal responses to noise, again leading to a reduction in performance (Fig. 3B). To fully test this theory requires establishing causal links between AR expression levels and neuronal responses (discussed earlier), and between neuronal responses and animal behavior. The later will be discussed in the following section.
Figure 3.
A proposed framework to understand LC-NE modulation of sensory processing and behavior. A. Increasing LC activity will progressively activate α2, α1 and β ARs, based on their affinities to NE (α2 > α1 > β). Because different ARs mediate different physiological effects (+: excitatory, −: inhibitory), increasing LC activity may non-monotonically change E/I balance, sensory response and perceptual-related behavior. B. An alternative model: LC activity will monotonically change E/I balance and sensory response. In this scenario, during high LC activity, performance is impaired because the circuit is more prone to noise.
6. Current challenges and future directions
Over the past decades, much progress has been made to understand LC-NE functions in intact animal models under anesthesia, during wakefulness, or even in behaving conditions. Nevertheless, very few studies attempt to directly link the modulatory effects at cellular and circuit levels to behavior. As a result, our knowledge of the fundamental neurobiology underlying how the LC-NE system modulates the activity of downstream neurons to affect behavioral states and cognitive processes remains incomplete. Here, we propose that simultaneous measurement and perturbation of LC-NE signaling combined with recording of downstream neuronal activity during well-controlled behavior is needed to substantially advance our understanding of this modulatory system.
6.1. Improved methods to monitor NE signals
Recording the spiking activity of LC neurons has remained the main approach to monitor LC-NE signaling and to infer NE release (e.g., Devilbiss et al., 2006). However, NE content at terminal fields may not scale linearly, or even monotonically with the firing rate of LC neurons (e.g., Florin-Lechner et al., 1996). We thus need new methods to monitor and quantify NE release in behaving animals. Recent development in optical imaging methods to monitor axonal activity or neuromodulator content with sub-second temporal resolution provides such a possibility (Muller et al., 2014; Reimer et al., 2016; Patriarchi et al., 2018; Dunn et al., 2018; Jing et al., 2018; Sun et al., 2018; Feng et al., 2019). Muller and colleagues developed cell-based neurotransmitter fluorescent engineered reporters (CNiFERs) that use the specificity of G protein-coupled receptors (GPCRs) to discriminate dopamine and NE with high sensitivity (nanomolar concentration). CNiFERs are clonal cell lines that express a specific GPCR that triggers an increase in intracellular calcium concentration, which is read out by a calcium sensor. This approach requires the injection of exogenous cells into the target brain region to measure local neuromodulator release. A series of more recent work leveraged the design principle of inserting a circularly permuted green fluorescent protein (cpGFP) into the GPCR to develop sensors for neurotransmitters and neuromodulators. Ligand binding induces conformational changes in the GPCR, causing the fluorescence intensity of cpGFP to change. Using this strategy, Jing and colleagues devised GACh, an acetylcholine (ACh) sensor, based on the human muscarinic ACh receptor subtype 3 (M3R); Sun and colleagues developed GRABda, a dopamine sensor based on the human dopamine receptor subtype 2 (D2R); and Patriarchi and colleagues introduced dLight1, another dopamine indicator, based on the human dopamine receptor subtype 1 (D1R). More recently, Feng and colleagues designed a family of NE indicators GRABne, again based on the same principle. In addition, Dunn and colleagues leveraged the concept of fluorescent false neurotransmitter (FFN), and developed FFN270, a small molecule as an optical tracer of NE neurotransmission. FFN270 is designed as a fluorescent substrate of NE transporter as well as the neuronal vesicular monoamine transporter (VMAT2), and thus can be taken up into synaptic vesicles in NE axonal varicosities and measures synaptic release by de-staining during exocytosis. With the continuing efforts to develop NE sensors, we expect to see more studies using them to measure NE release directly with high spatiotemporal precision in behaving animals.
6.2. Perturbing the LC-NE circuit during behaviors that mobilize this modulatory system
Most of the previous work on LC-NE modulation of sensory processing and perception activated this circuit artificially (LC stimulation or exogenous NE application). While such approaches provide valuable insights into the fundamental neurobiology, it is now time to identify appropriate behavior paradigms that mobilize this modulatory circuit, so that we may investigate LC-NE functions in biologically relevant behavioral conditions. Importantly, in gain- and loss-of-function experiments to test causal relationships, it is crucial to fine-tune perturbation parameters to constrain the effects within physiological limits, and to closely mimic the naturally occurring and behaviorally relevant neuronal activity.
6.3. Better understanding of the modulation of downstream neurons
Thus far, most in vivo work only has assessed how LC-NE affects the spiking activity of downstream neurons. While the intracellular mechanisms of LC-NE modulation have been studied in vitro (e.g., Waterhouse et al., 1982; McCormick and Prince, 1988; Dodt et al., 1991; McCormick, 1992c; Favero et al., 2012), limited in vivo work has been done to uncover the modulation of subthreshold membrane potential dynamics that underlie neuronal excitability and spiking. Local blockade of NE in the barrel cortex changes desynchronized membrane potential fluctuations to up-down states (Constantinople and Bruno, 2011), and blocking NE in the visual cortex abolishes the locomotion-associated depolarization (Polack et al., 2013). These results are consistent with observations that LC spiking is tightly linked to fluctuations of cortical/behavioral states at multiple scales (Hirata and Castro-Alamancos, 2011; Eschenko et al., 2012; Castro-Alamancos and Gulati, 2014; Fazlali et al., 2016). However, it is unclear how to link these intracellular findings to the predominant LC-NE inhibitory actions on neuronal spiking. With the emergence of new perturbation techniques such as optogenetics and chemogenetics, which allow us to manipulate genetically-defined LC-NE neurons in a cell-type, temporally precise and possibly pathway-specific manner, it is time to address these questions and gain deeper insight into how the moment-by-moment fluctuations of LC-NE activity correlate/modulate membrane potential and spiking of sensory neurons in awake, behaving animals. Importantly, these electrophysiology and perturbation techniques will enable us to test the proposed theory by linking the changes in E/I balance at the cellular level (Okun and Lampl, 2008) and at the circuit level (Shew et al., 2009; Yang et al., 2012) to behavior.
6.4. Improved understanding of the LC-pupil relationship
Primarily in human research, pupil diameter has been often used to index LC activity (e.g., Beatty, 1982; Aston-Jones and Cohen, 2005). Not until recently had we begun to rigorously test the correlative or even casual relationship between LC activity and pupil dilation (Murphy et al., 2014; Joshi et al., 2016; Reimer et al., 2016; Liu et al., 2017). Pupil diameter has also been found to co-fluctuate with brain states, task performance and sensory neuron activity (Reimer et al., 2014; McGinley et al., 2015a, 2015b; Vinck et al., 2015; Lee and Margolis, 2016; Schriver et al., 2018). A better understanding of the LC-pupil relationship may open up new avenues to study LC functions.
6.5. Coping with heterogeneity
Mounting evidence suggests that LC is composed of a heterogeneous population of NE-expressing neurons that project to distinct brain regions and co-release other neurochemicals (Berridge and Waterhouse, 2003; Robertson et al., 2013; Chandler et al., 2014; Hickey et al., 2014; Schwarz and Luo, 2015; Kebschull et al., 2016; Kempadoo et al., 2016; Uematsu et al., 2017; Plummer et al., 2017; Totah et al., 2018; Chen et al., 2018; Breton-Provencher and Sur, 2019). It remains challenging to identify the distinct subpopulations, their upstream and downstream circuits and specific roles in modulating brain functions and behavior (as in Uematsu et al., 2017; Yackle et al., 2017; Sciolino et al., 2018; Breton-Provencher and Sur, 2019). Fortunately, with the advance of genetic fate mapping (Robertson et al., 2013; Plummer et al., 2017; Chen et al., 2018), pathway-specific tracing (Schwarz et al., 2015; Tervo et al., 2016) and perturbation techniques (Sciolino et al., 2016; McCall et al., 2017; Uematsu et al., 2017), we are now able to address these questions that were intractable just 10 years ago.
6.6. Functional implications
LC neurons preferentially innervate multiple relay stations along the ascending sensory pathway (Simpson et al., 1997), and NE actions on the upstream sensory areas likely impact the downstream dynamics in a feed-forward manner (e.g., Hirata and Castro-Alamancos, 2011). In future research, it is crucial to dissect the direct modulatory actions on the area of interest from the indirect effects inherited from NE modulation of the upstream relay stations (as in Rodenkirch et al., 2019). For example, if we were to stimulate LC and assess how it affects sensory cortex activity, it would be important to quantify how much of the observed cortical effect is due to LC modulation of the sensory thalamus (e.g., LGN to V1, and VPM to S1, Fig. 1). This feature of collateral innervation may as well underlie the different modulatory effects reported in sensory cortex during LC stimulation and local NE administration.
We are still far from obtaining a comprehensive picture of how LC-NE signaling affects sensory information transformation and perceptual behavior. For instance, what is the functional importance for this system to enhance thalamic response and suppress cortical activity? How is sensory information transformed across cortical layers when LC-NE differentially modulates these layers, and what is the impact of such region- and layer-specific modulation on the readout by the downstream brain areas?
6.7. A unified understanding of LC-NE functions
Although this review focuses on LC-NE modulation of sensory processing, it should be pointed out that this modulatory circuit is involved in many other cognitive functions. For example, LC tonic activity has been linked to states of arousal/attention; LC phasic response is thought to be task-specific (e.g., Aston-Jones and Bloom, 1981; Aston-Jones et al., 1994; Rajkowski et al., 1994; Usher et al., 1999; Bouret and Richmond, 2015), and is associated with goal-directed behavioral processes (Bouret and Richmond, 2008; Kalwani et al., 2014). Deeper insights into LC-NE modulation of basic perceptual processes will help elucidate how this neuromodulatory system affects the activity of downstream neurons to modulate perception, and will pave the way toward a unified framework that encompasses LC-NE modulation of other (possibly higher-order) brain functions, behavior, and neurological disorders.
Highlights.
The locus coeruleus-norepinephrine (LC-NE) system mediates important brain functions.
We review studies on LC-NE modulation of sensory response and perceptual behavior.
We suggest a mechanism to unify mixed findings in the literature.
We propose a framework to understand LC-NE functions.
We discuss current limitations and future research directions.
Acknowledgements
We thank B.A. Bari, D.H. O’Connor and the anonymous reviewers for comments on the manuscript. H.Y. is supported by UCR startup funds and Klingenstein-Simons Fellowship Awards in Neuroscience. Y.Z. is supported by the National Institute of Mental Health (R01MH104227 and R01MH109475).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Acheson A, Zigmond M, Stricker E (1980) Compensatory increase in tyrosine hydroxylase activity in rat brain after intraventricular injections of 6-hydroxydopamine. Science (80- ) 207:537–540. [DOI] [PubMed] [Google Scholar]
- Armstrong-James M, Fox K (1983) Effects of ionophoresed noradrenaline on the spontaneous activity of neurones in rat primary somatosensory cortex. J Physiol 335:427–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT (2000) Through the Looking Glass: Differential Noradenergic Modulation of Prefrontal Cortical Function. Neural Plast 7:133–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AFT, Goldman-Rakic PS (1984) Selective prefrontal cortical projections to the region of the locus coeruleus and raphe nuclei in the rhesus monkey. Brain Res 306:9–18. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE (1981) Activity of norepinephrine-containing locus coeruleus neurons in behaving rats anticipates fluctuations in the sleep-waking cycle. J Neurosci 1:876–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD (2005) An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci 28:403–450. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Cohen J (1999) Role of locus coeruleus in attention and behavioral flexibility. Biol Psychiatry 46:1309–1320. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T (1994) Locus Coeruleus Neurons in Monkey Are Selectively Activated by Attended Cues in a Vigilance Task. J Neurosci 14:4467–4480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Waterhouse B (2016) Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res 1645:75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beatty J (1982) Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychol Bull 91:276–292. [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD (2003) The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev 42:33–84. [DOI] [PubMed] [Google Scholar]
- Bezdudnaya T, Castro-Alamancos MA (2014) Neuromodulation of Whisking Related Neural Activity in Superior Colliculus. J Neurosci 34:7683–7695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ (2008) Relation of Locus Coeruleus Neurons in Monkeys to Pavlovian and Operant Behaviors. J Neurophysiol 101:898–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Richmond BJ (2015) Sensitivity of Locus Ceruleus Neurons to Reward Value for Goal-Directed Actions. J Neurosci 35:4005–4014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2002) Locus coeruleus activation modulates firing rate and temporal organization of odour-induced single-cell responses in rat piriform cortex. Eur J Neurosci 16:2371–2382. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ (2005) Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci 28:574–582. [DOI] [PubMed] [Google Scholar]
- Breton-Provencher V, Sur M (2019) Active control of arousal by a locus coeruleus GABAergic circuit. Nat Neurosci 22:218–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadwell CR, Palasantza A, Jiang X, Berens P, Deng Q, Yilmaz M, Reimer J, Shen S, Bethge M, Tolias KF, Sandberg R, Tolias AS (2016) Electrophysiological, transcriptomic and morphologic profiling of single neurons using Patch-seq. Nat Biotechnol 34:199–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter ME, Yizhar O, Chikahisa S, Nguyen H, Adamantidis A, Nishino S, Deisseroth K, de Lecea L (2010) Tuning arousal with optogenetic modulation of locus coeruleus neurons. Nat Neurosci 13:1526–1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Gulati T (2014) Neuromodulators Produce Distinct Activated States in Neocortex. J Neurosci 34:12353–12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler DJ, Gao W-J, Waterhouse BD (2014) Heterogeneous organization of the locus coeruleus projections to prefrontal and motor cortices. Proc Natl Acad Sci U S A 111:6816–6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Das M, Oyarzabal EA, Cheng Q, Plummer NW, Smith KG, Jones GK, Malawsky D, Yakel JL, Shih YYI, Jensen P (2018) Genetic identification of a population of noradrenergic neurons implicated in attenuation of stress-related responses. Mol Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chikamori Y, Sasa M, Fujimoto S, Takaori S, Matsuoka I (1980) Locus coeruleus-induced inhibition of dorsal cochlear nucleus neurons in comparison with lateral vestibular nucleus neurons. Brain Res 194:53–63. [DOI] [PubMed] [Google Scholar]
- Chiodo LA, Acheson AL, Zigmond MJ, Stricker EM (1983) Subtotal destruction of central noradrenergic projections increases the firing rate of locus coeruleus cells. Brain Res 264:123–126. [DOI] [PubMed] [Google Scholar]
- Cohen MR, Maunsell JHR (2009) Attention improves performance primarily by reducing interneuronal correlations. Nat Neurosci 12:1594–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantinople CM, Bruno RM (2011) Effects and mechanisms of wakefulness on local cortical networks. Neuron 69:1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD (2006) Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci 26:9860–9872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD (2004) The effects of tonic locus ceruleus output on sensory-evoked responses of ventral posterior medial thalamic and barrel field cortical neurons in the awake rat. J Neurosci 24:10773–10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD (2011) Phasic and tonic patterns of locus coeruleus output differentially modulate sensory network function in the awake rat. J Neurophysiol 105:69–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devilbiss DM, Waterhouse BD, Berridge CW, Valentino R (2012) Corticotropin-releasing factor acting at the locus coeruleus disrupts thalamic and cortical sensory-evoked responses. Neuropsychopharmacology 37:2020–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodt HU, Pawelzik H, Zieglgansberger W (1991) Actions of noradrenaline on neocortical neurons in vitro. Brain Res 545:307–311. [DOI] [PubMed] [Google Scholar]
- Doucette W, Milder J, Restrepo D (2007) Adrenergic modulation of olfactory bulb circuitry affects odor discrimination. Learn & Mem 14:539–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn M, Henke A, Clark S, Kovalyova Y, Kempadoo KA, Karpowicz RJ, Kandel ER, Sulzer D, Sames D (2018) Designing a norepinephrine optical tracer for imaging individual noradrenergic synapses and their activity in vivo. Nat Commun 9:2838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckmeier D, Shea SD (2014) Noradrenergic Plasticity of Olfactory Sensory Neuron Inputs to the Main Olfactory Bulb. J Neurosci 34:15234–15243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edeline JM, Manunta Y, Hennevin E (2011) Induction of selective plasticity in the frequency tuning of auditory cortex and auditory thalamus neurons by locus coeruleus stimulation. Hear Res 274:75–84. [DOI] [PubMed] [Google Scholar]
- Ego-Stengel V, Bringuier V, Shulz DE (2002) Noradrenergic modulation of functional selectivity in the cat visual cortex: An in vivo extracellular and intracellular study. Neuroscience 111:275–289. [DOI] [PubMed] [Google Scholar]
- Escanilla O, Alperin S, Youssef M, Ennis M, Linster C (2012) Noradrenergic but not cholinergic modulation of olfactory bulb during processing of near threshold concentration stimuli. Behav Neurosci 126:720–728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escanilla O, Arrellanos A, Karnow A, Ennis M, Linster C (2010) Noradrenergic modulation of behavioral odor detection and discrimination thresholds in the olfactory bulb. Eur J Neurosci 32:458–468. [DOI] [PubMed] [Google Scholar]
- Eschenko O, Magri C, Panzeri S, Sara SJ (2012) Noradrenergic neurons of the locus coeruleus are phase locked to cortical up-down states during sleep. Cereb Cortex 22:426–435. [DOI] [PubMed] [Google Scholar]
- Favero M, Varghese G, Castro-Alamancos MA (2012) The state of somatosensory cortex during neuromodulation. J Neurophysiol 108:1010–1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fazlali Z, Ranjbar-Slamloo Y, Adibi M, Arabzadeh E (2016) Correlation between Locus Coeruleus Activity and Cortical State : Implications for Sensory Coding in Rat Barrel Cortex. Front Neural Circuits 10:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J, Zhang C, Lischinsky JE, Jing M, Zhou J, Wang H, Zhang Y, Dong A, Wu Z, Wu H, Chen W, Zhang P, Zou J, Hires SA, Zhu JJ, Cui G, Lin D, Du J, Li Y (2019) A Genetically Encoded Fluorescent Sensor for Rapid and Specific In Vivo Detection of Norepinephrine. Neuron:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Florin-Lechner SM, Druhan JP, Aston-Jones G, Valentino RJ (1996) Enhanced norepinephrine release in prefrontal cortex with burst stimulation of the locus coeruleus. Brain Res 742:89–97. [DOI] [PubMed] [Google Scholar]
- Foote SL, Bloom FE, Aston-Jones G (1983) Nucleus locus ceruleus: new evidence of anatomical and physiological specificity. Physiol Rev 63:844–914. [DOI] [PubMed] [Google Scholar]
- Foote SL, Freedman R, Oliver AP (1975) Effects of putative neurotransmitters on neuronal activity in monkey auditory cortex. Brain Res 86:229–242. [DOI] [PubMed] [Google Scholar]
- Foote SL, Morrison JH (1987) Extrathalamic Modulation of Cortical Function. Annu Rev Neurosci 10:67–95. [DOI] [PubMed] [Google Scholar]
- Fries P, Reynolds JH, Rorie AE, Desimone R (2001) Modulation of oscillatory neuronal synchronization by selective visual attention. Science 291:1560–1563. [DOI] [PubMed] [Google Scholar]
- Glennon E, Carcea I, Martins ARO, Multani J, Shehu I, Svirsky MA, Froemke RC (2018) Locus coeruleus activation accelerates perceptual learning. Brain Res. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haider B, McCormick DA (2009) Rapid Neocortical Dynamics: Cellular and Network Mechanisms. Neuron 62:171–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik S (1984) Locus ceruleus lesion by local 6-hydroxydopamine infusion causes marked and specific destruction of noradrenergic neurons, long-term depletion of norepinephrine and the enzymes that synthesize it, and enhanced dopaminergic mechanisms in the ipsilateral ce. J Neurosci 4:699–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harik S, Duckrow R, LaManna J, Rosenthal M, Sharma V, Banerjee S (1981) Cerebral compensation for chronic noradrenergic denervation induced by locus ceruleus lesion: recovery of receptor binding, isoproterenol-induced adenylate cyclase activity, and oxidative metabolism. J Neurosci 1:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey L, Li Y, Fyson SJ, Watson TC, Perrins R, Hewinson J, Teschemacher a. G, Furue H, Lumb BM Pickering a. E (2014) Optoactivation of Locus Ceruleus Neurons Evokes Bidirectional Changes in Thermal Nociception in Rats. J Neurosci 34:4148–4160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higley MJ, Contreras D (2006) Balanced excitation and inhibition determine spike timing during frequency adaptation. J Neurosci 26:448–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Aguilar J, Castro-Alamancos MA (2006) Noradrenergic activation amplifies bottom-up and top-down signal-to-noise ratios in sensory thalamus. J Neurosci 26:4426–4436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirata A, Castro-Alamancos MA (2011) Effects of cortical activation on sensory responses in barrel cortex. J Neurophysiol 105:1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdefer RN, Jacobs BL (1994) Phasic stimulation of the locus coeruleus: effects on activity in the lateral geniculate nucleus. Exp Brain Res 79:444–452. [DOI] [PubMed] [Google Scholar]
- Hurley LM, Devilbiss DM, Waterhouse BD (2004) A matter of focus: monoaminergic modulation of stimulus coding in mammalian sensory networks. Curr Opin Neurobiol 14:488–495. [DOI] [PubMed] [Google Scholar]
- Jiang M, Griff ER, Ennis M, Zimmer LA, Shipley MT (1996) Activation of locus coeruleus enhances the responses of olfactory bulb mitral cells to weak olfactory nerve input. J Neurosci 16:6319–6329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jodo E, Chiang C, Aston-Jones G (1998) Potent excitatory influence of prefrontal cortex activity on noradrenergic locus coeruleus neurons. Neuroscience 83:63–79. [DOI] [PubMed] [Google Scholar]
- Jing M et al. (2018) A genetically encoded fluorescent acetylcholine indicator for in vitro and in vivo studies. Nat Biotechnol 36:726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshi S, Li Y, Kalwani RM, Gold JI (2016) Relationships between Pupil Diameter and Neuronal Activity in the Locus Coeruleus, Colliculi, and Cingulate Cortex. Neuron 89:221–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwani RM, Joshi S, Gold JI (2014) Phasic Activation of Individual Neurons in the Locus Ceruleus/Subceruleus Complex of Monkeys Reflects Rewarded Decisions to Go But Not Stop. J Neurosci 34:13656–13669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kasamatsu T, Heggelund P (1982) Single cell responses in cat visual cortex to visual stimulation during iontophoresis of noradrenaline. Exp brain Res 45:317–327. [DOI] [PubMed] [Google Scholar]
- Kayama Y, Negi T, Sugitani M, Iwama K (1982) Effects of locus coeruleus stimulation on neuronal activities of dorsal lateral geniculate nucleus and perigeniculate reticular nucleus of the rat. Neuroscience 7:655–666. [DOI] [PubMed] [Google Scholar]
- Kebschull JM, Garcia da Silva P, Reid AP, Peikon ID, Albeanu DF, Zador AM (2016) High-Throughput Mapping of Single-Neuron Projections by Sequencing of Barcoded RNA. Neuron 91:975–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempadoo KA, Mosharov EV., Choi SJ, Sulzer D, Kandel ER (2016) Dopamine release from the locus coeruleus to the dorsal hippocampus promotes spatial learning and memory. Proc Natl Acad Sci 113:14835–14840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolta a, Reader T a (1989) Modulatory effects of catecholamines on neurons of the rat visual cortex: single-cell iontophoretic studies. Can J Physiol Pharmacol 67:615–623. [DOI] [PubMed] [Google Scholar]
- Kolta A, Diop L, Reader TA (1987) Noradrenergic effects on rat visual cortex: Single-cell microiontophoretic studies of alpha-2 adrenergic receptors. Life Sci 41:281–289. [DOI] [PubMed] [Google Scholar]
- Kossl M, Vater M (1989) Noradrenaline enhances temporal auditory contrast and neuronal timing precision in the cochlear nucleus of the mustached bat. J Neurosci 9:4169–4178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecas JC (2001) Noradrenergic modulation of tactile responses in rat cortex. Current source-density and unit analyses. Comptes Rendus I’Academie des Sci - Ser III 324:33–44. [DOI] [PubMed] [Google Scholar]
- Lecas JC (2004) Locus coeruleus activation shortens synaptic drive while decreasing spike latency and jitter in sensorimotor cortex. Implications for neuronal integration. Eur J Neurosci 19:2519–2530. [DOI] [PubMed] [Google Scholar]
- Lee CR, Margolis DJ (2016) Pupil Dynamics Reflect Behavioral Choice and Learning in a Go/NoGo Tactile Decision-Making Task in Mice. Front Behav Neurosci 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linster C, Nai Q, Ennis M (2011) Nonlinear effects of noradrenergic modulation of olfactory bulb function in adult rodents. J Neurophysiol 105:1432–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Rodenkirch C, Moskowitz N, Schriver B, Wang Q (2017) Dynamic Lateralization of Pupil Dilation Evoked by Locus Coeruleus Activation Results from Sympathetic, Not Parasympathetic, Contributions. Cell Rep 20:3099–3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manella LC, Petersen N, Linster C (2017) Stimulation of the locus coeruleus modulates signal-to-noise ratio in the olfactory bulb. J Neurosci 37:2026–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manunta Y, Edeline J-M (1997) Effects of Noradrenaline on Frequency Tuning of Rat Auditory Cortex Neurons. Eur J Neurosci 9:833–847. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline J-M (2004) Noradrenergic Induction of Selective Plasticity in the Frequency Tuning of Auditory Cortex Neurons. J Neurophysiol 92:1445–1463. [DOI] [PubMed] [Google Scholar]
- Manunta Y, Edeline JM (1999) Effects of noradrenaline on frequency tuning of auditory cortex neurons during wakefulness and slow-wave sleep. Eur J Neurosci 11:2134–2150. [DOI] [PubMed] [Google Scholar]
- Martinez-Trujillo JC, Treue S (2004) Feature-Based Attention Increases the Selectivity of Population Responses in Primate Visual Cortex. Curr Biol 14:744–751. [DOI] [PubMed] [Google Scholar]
- Martins ARO, Froemke RC (2015) Coordinated forms of noradrenergic plasticity in the locus coeruleus and primary auditory cortex. Nat Neurosci 18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marzo A, Bai J, Otani S (2009) Neuroplasticity regulation by noradrenaline in mammalian brain. Curr Neuropharmacol 7:286–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall JG, Siuda ER, Bhatti DL, Lawson LA, McElligott ZA, Stuber GD, Bruchas MR (2017) Locus coeruleus to basolateral amygdala noradrenergic projections promote anxiety-like behavior. Elife 6:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA (1992a) Cellular mechanisms underlying cholinergic and noradrenergic modulation of neuronal firing mode in the cat and guinea pig dorsal lateral geniculate nucleus. J Neurosci 12:278–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA, Prince D (1988) Noradrenergic modulation of firing pattern in guinea pig and cat thalamic neurons, in vitro. J Neurophysiol 59:978–996. [DOI] [PubMed] [Google Scholar]
- McCormick DA (1992b) Neurotransmitter actions in the thalamus and cerebral cortex. [Review] [75 refs]. J Clin Neurophysiol 9:212–223. [DOI] [PubMed] [Google Scholar]
- McCormick DA (1992c) Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Prog Neurobiol 39:337–388. [DOI] [PubMed] [Google Scholar]
- McCune SK, Voigt MM, Hill JM (1993) Expression of multiple alpha adrenergic receptor subtype messenger RNAs in the adult rat brain. Neuroscience 57:143–151. [DOI] [PubMed] [Google Scholar]
- McGinley MJ, David SV., McCormick DA (2015a) Cortical Membrane Potential Signature of Optimal States for Sensory Signal Detection. Neuron 87:179–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinley MJ, Vinck M, Reimer J, Batista-Brito R, Zagha E, Cadwell CR, Tolias AS, Cardin JA, McCormick DA (2015b) Waking State: Rapid Variations Modulate Neural and Behavioral Responses. Neuron 87:1143–1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean J, Waterhouse BD (1994) Noradrenergic modulation of cat area 17 neuronal responses to moving visual stimuli. Brain Res 667:83–97. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Foote SL (1986) Noradrenergic and serotoninergic innervation of cortical, thalamic, and tectal visual structures in old and new world monkeys. J Comp Neurol 243:117–138. [DOI] [PubMed] [Google Scholar]
- Muller A, Joseph V, Slesinger PA, Kleinfeld D (2014) Cell-based reporters reveal in vivo dynamics of dopamine and norepinephrine release in murine cortex. Nat Methods 11:1245–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy PR, O’Connell RG, O’Sullivan M, Robertson IH, Balsters JH (2014) Pupil diameter covaries with BOLD activity in human locus coeruleus. Hum Brain Mapp 35:4140–4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakai Y, Takaori S (1974) Influence of norepinephrine-containing neurons derived from the locus coeruleus on lateral geniculate neuronal activities of cats. Brain Res 71:47–60. [DOI] [PubMed] [Google Scholar]
- Nicholas AP, Pieribone V, Hokfelt T (1993) Distributions of mRNAs for alpha-2 adrenergic receptor subtypes in rat brain: an in situ hybridization study. J Comp Neurol 328:575–594. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Malenka RC, Kauer JA (1990) Functional comparison of neurotransmitter receptor subtypes in mammalian central nervous system. Physiol Rev 70:513–565. [DOI] [PubMed] [Google Scholar]
- Okun M, Lampl I (2008) Instantaneous correlation of excitation and inhibition during ongoing and sensory-evoked activities. Nat Neurosci 11:535–537. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Glatt A, Laszlo J, Schellenberg A (1980) Some electrophysiological and pharmacological properties of the cortical, noradrenergic projection of the locus coeruleus in the rat. Brain Res 186:9–19. [DOI] [PubMed] [Google Scholar]
- Otchy TM, Wolff SBE, Rhee JY, Pehlevan C, Kawai R, Kempf A, Gobes SMH, Ölveczky BP (2015) Acute off-target effects of neural circuit manipulations. Nature 528:358–363. [DOI] [PubMed] [Google Scholar]
- Papay R, Gaivin R, Jha A, Mccune DF, Mcgrath JC, Rodrigo MC, Simpson PC, Doze VA, Perez DM (2006) Localization of the mouse α1A-adrenergic receptor (AR) in the brain: α1AAR is expressed in neurons, GABAergic interneurons, and NG2 oligodendrocyte progenitors. J Comp Neurol 497:209–222. [DOI] [PubMed] [Google Scholar]
- Patriarchi T, Cho JR, Merten K, Howe MW, Marley A, Xiong W-H, Folk RW, Broussard GJ, Liang R, Jang MJ, Zhong H, Dombeck D, von Zastrow M, Nimmerjahn A, Gradinaru V, Williams JT, Tian L (2018) Ultrafast neuronal imaging of dopamine dynamics with designed genetically encoded sensors. Science 360:eaat4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pieribone VA, Nicholas AP, Dagerlind A, Hokfelt T (1994) Distribution of alpha 1 adrenoceptors in rat brain revealed by in situ hybridization experiments utilizing subtype-specific probes. J Neurosci 14:4252–4268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plummer NW, Scappini EL, Smith KG, Tucker CJ, Jensen P (2017) Two Subpopulations of Noradrenergic Neurons in the Locus Coeruleus Complex Distinguished by Expression of the Dorsal Neural Tube Marker Pax7. Front Neuroanat 11:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polack P-O, Friedman J, Golshani P (2013) Cellular mechanisms of brain state-dependent gain modulation in visual cortex. Nat Neurosci 16:1331–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rainbow TC, Parsons B, Wolfe BB (1984) Quantitative autoradiography of beta 1- and beta 2-adrenergic receptors in rat brain. Proc Natl Acad Sci U S A 81:1585–1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowski J, Kubiak P, Aston-Jones G (1994) Locus coeruleus activity in monkey: Phasic and tonic changes are associated with altered vigilance. Brain Res Bull 35:607–616. [DOI] [PubMed] [Google Scholar]
- Ramos BP, Arnsten AFT (2007) Adrenergic pharmacology and cognition: Focus on the prefrontal cortex. Pharmacol Ther 113:523–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, Froudarakis E, Cadwell CR, Yatsenko D, Denfield GH, Tolias AS (2014) Pupil Fluctuations Track Fast Switching of Cortical States during Quiet Wakefulness. Neuron 84:355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimer J, McGinley MJ, Liu Y, Rodenkirch C, Wang Q, McCormick DA, Tolias AS (2016) Pupil fluctuations track rapid changes in adrenergic and cholinergic activity in cortex. Nat Commun 7:13289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynolds JH, Pasternak T, Desimone R (2000) Attention Increases Sensitivity of V4 Neurons. Neuron 26:703–714. [DOI] [PubMed] [Google Scholar]
- Robertson SD, Plummer NW, de Marchena J, Jensen P (2013) Developmental origins of central norepinephrine neuron diversity. Nat Neurosci 16:1016–1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodenkirch C, Liu Y, Schriver BJ, Wang Q (2019) Locus coeruleus activation enhances thalamic feature selectivity via norepinephrine regulation of intrathalamic circuit dynamics. Nat Neurosci 22:120–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK (1980a) Modulation of lateral geniculate neurone excitability by noradrenaline microiontophoresis or locus coeruleus stimulation. Nature 287:731–734. [DOI] [PubMed] [Google Scholar]
- Rogawski MA, Aghajanian GK (1980b) Activation of lateral geniculate neurons by norepinephrine: Mediation by an α-adrenergic receptor. Brain Res 182:345–359. [DOI] [PubMed] [Google Scholar]
- Salgado H, Trevino M, Atzori M (2016) Layer- and area-specific actions of norepinephrine on cortical synaptic transmission. Brain Res 1641:163–176. [DOI] [PubMed] [Google Scholar]
- Sara SJ (2009) The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci 10:211–223. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Bouret S (2012) Orienting and Reorienting: The Locus Coeruleus Mediates Cognition through Arousal. Neuron 76:130–141. [DOI] [PubMed] [Google Scholar]
- Sato H, Fox K, Daw NW (1989) Effect of electrical stimulation of locus coeruleus on the activity of neurons in the cat visual cortex. J Neurophysiol 62:946–958. [DOI] [PubMed] [Google Scholar]
- Sato H, Kayama Y (1983) Effects of noradrenaline applied iontophoretically on rat superior collicular neurons. Brain Res Bull 10:453–457. [DOI] [PubMed] [Google Scholar]
- Scheinin M, Lomasney JW, Hayden-Hixson DM, Schambra UB, Caron MG, Lefkowitz RJ, Fremeau RT (1994) Distribution of α2-adrenergic receptor subtype gene expression in rat brain. Mol Brain Res 21:133–149. [DOI] [PubMed] [Google Scholar]
- Schriver BJ, Bagdasarov S, Wang Q (2018) Pupil-linked arousal modulates behavior in rats performing a whisker deflection direction discrimination task. J Neurophysiol 120:1655–1670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz LA, Luo L (2015) Organization of the locus coeruleus-norepinephrine system. Curr Biol 25:R1051–R1056. [DOI] [PubMed] [Google Scholar]
- Schwarz LA, Miyamichi K, Gao XJ, Beier KT, Weissbourd B, DeLoach KE, Ren J, Ibanes S, Malenka RC, Kremer EJ, Luo L (2015) Viral-genetic tracing of the input-output organization of a central noradrenaline circuit. Nature 524:88–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Plummer NW, Chen YW, Alexander GM, Robertson SD, Dudek SM, McElligott ZA, Jensen P (2016) Recombinase-Dependent Mouse Lines for Chemogenetic Activation of Genetically Defined Cell Types. Cell Rep 15:2563–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciolino NR, Mazzone CM, Plummer NW, Amin J, Smith KG, Mcgee CA, Yang CX, Krashes MJ, Kravitz AV, Bruchas MR, Cushman JD, Gui G, Jensen P (2018) A locus coeruleus to lateral hypothalamus circuit for suppression of feeding. Soc. Neurosci Abstract 598.05 [Google Scholar]
- Shea SD, Katz LC, Mooney R (2008) Noradrenergic Induction of Odor-Specific Neural Habituation and Olfactory Memories. J Neurosci 28:10711–10719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shew WL, Yang H, Petermann T, Roy R, Plenz D (2009) Neuronal avalanches imply maximum dynamic range in cortical networks at criticality. J Neurosci 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson KL, Altman DW, Wang L, Kirifides ML, Lin RCS, Waterhouse BD (1997) Lateralization and functional organization of the locus coeruleus projection to the trigeminal somatosensory pathway in rat. J Comp Neurol 385:135–147. [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J (1988) Increased attention enhances both behavioral and neuronal performance. Science (80- ) 240:338–340. [DOI] [PubMed] [Google Scholar]
- Steinmetz PN, Roy a, Fitzgerald PJ, Hsiao SS, Johnson KO, Niebur E (2000) Attention modulates synchronized neuronal firing in primate somatosensory cortex. Nature 404:187–190. [DOI] [PubMed] [Google Scholar]
- Sun F et al. (2018) A Genetically Encoded Fluorescent Sensor Enables Rapid and Specific Detection of Dopamine in Flies, Fish, and Mice. Cell 174:481–496.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tervo DGR, Hwang BY, Viswanathan S, Gaj T, Lavzin M, Ritola KD, Lindo S, Michael S, Kuleshova E, Ojala D, Huang CC, Gerfen CR, Schiller J, Dudman JT, Hantman AW, Looger LL, Schaffer DV., Karpova AY (2016) A Designer AAV Variant Permits Efficient Retrograde Access to Projection Neurons. Neuron 92:372–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiele A, Bellgrove MA (2018) Neuromodulation of Attention. Neuron 97:769–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Totah NK, Neves RM, Panzeri S, Logothetis NK, Eschenko O (2018) The Locus Coeruleus Is a Complex and Differentiated Neuromodulatory System. Neuron 99:1055–1068.e6. [DOI] [PubMed] [Google Scholar]
- Uematsu A, Tan BZ, Ycu EA, Cuevas JS, Koivumaa J, Junyent F, Kremer EJ, Witten IB, Deisseroth K, Johansen JP (2017) Modular organization of the brainstem noradrenaline system coordinates opposing learning states. Nat Neurosci 20:1602–1611. [DOI] [PubMed] [Google Scholar]
- Usher M, Cohen JD, Servan-Schreiber D, Rajkowski J, Aston-Jones G (1999) The role of locus coeruleus in the regulation of cognitive performance. Science 283:549–554. [DOI] [PubMed] [Google Scholar]
- Valentini V, Frau R, Di Chiara G (2004) Noradrenaline transporter blockers raise extracellular dopamine in medial prefrontal but not parietal and occipital cortex: Differences with mianserin and clozapine. J Neurochem 88:917–927. [DOI] [PubMed] [Google Scholar]
- Vazey EM, Aston-Jones G (2014) Designer receptor manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc Natl Acad Sci U S A 111:3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazey EM, Moorman DE, Aston-Jones G (2018) Phasic locus coeruleus activity regulates cortical encoding of salience information. Proc Natl Acad Sci 115:E9439–E9448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Videen T, Daw N, Rader R (1984) The effect of norepinephrine on visual cortical neurons in kittens and adult cats. J Neurosci 4:1607–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinck M, Batista-Brito R, Knoblich U, Cardin JA (2015) Arousal and Locomotion Make Distinct Contributions to Cortical Activity Patterns and Visual Encoding. Neuron 86:740–754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren R a Dykes RW (1996) Transient and long-lasting effects of iontophoretically administered norepinephrine on somatosensory cortical neurons in halothane-anesthetized cats. Can J Physiol Pharmacol 74:38–57. [PubMed] [Google Scholar]
- Waterhouse BD, Ausim Azizi S, Burne RA, Woodward DJ (1990) Modulation of rat cortical area 17 neuronal responses to moving visual stimuli during norepinephrine and serotonin microiontophoresis. Brain Res 514:276–292. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1980) Noradrenergic modulation of somatosensory cortical neuronal responses to lontophoretically applied putative neurotransmitters. Exp Neurol 69:30–49. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1981) Alpha-receptor-mediated facilitation of somatosensory cortical neuronal responses to excitatory synaptic inputs and iontophoretically applied acetylcholine. Neuropharmacology 20:907–920. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Woodward DJ (1998) Phasic activation of the locus coeruleus enhances responses of primary sensory cortical neurons to peripheral receptive field stimulation. Brain Res 790:33–44. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Moises HC, Yeh HH, Woodward DJ (1982) Norepinephrine enhancement of inhibitory synaptic mechanisms in cerebellum and cerebral cortex: mediation by beta adrenergic receptors. J Pharmacol Exp Ther 221:495–506. [PubMed] [Google Scholar]
- Waterhouse BD, Navarra RL (2018) The locus coeruleus-norepinephrine system and sensory signal processing: A historical review and current perspectives. Brain Res:1–15. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Sessler FM, Jung-Tung C, Woodward DJ, Azizi SA, Moises HC (1988) New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull 21:425–432. [DOI] [PubMed] [Google Scholar]
- Waterhouse BD, Woodward DJ (1980) Interaction of norepinephrine with cerebrocortical activity evoked by stimulation of somatosensory afferent pathways in the rat. Exp Neurol 67:11–34. [DOI] [PubMed] [Google Scholar]
- Wehr M, Zador AM (2003) Balanced inhibition underlies tuning and sharpens spike timing in auditory cortex. Nature 426:442–446. [DOI] [PubMed] [Google Scholar]
- Yackle K, Schwarz LA, Kam K, Sorokin JM, Huguenard JR, Feldman JL, Luo L, Krasnow MA (2017) Breathing control center neurons that promote arousal in mice. Science 355:1411–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Shew WL, Roy R, Plenz D (2012) Maximal Variability of Phase Synchrony in Cortical Networks with Neuronal Avalanches. J Neurosci 32:1061–1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yizhar O, Fenno LE, Prigge M, Schneider F, Davidson TJ, O’Shea DJ, Sohal VS, Goshen I, Finkelstein J, Paz JT, Stehfest K, Fudim R, Ramakrishnan C, Huguenard JR, Hegemann P, Deisseroth K (2011) Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 477:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young WS, Kuhar MJ (1980) Noradrenergic alpha 1 and alpha 2 receptors: light microscopic autoradiographic localization. Proc Natl Acad Sci U S A 77:1696–1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Mooney RD, Rhoades RW (1999) Effects of norepinephrine on the activity of visual neurons in the superior colliculus of the hamster. Vis Neurosci 16:541–555. [DOI] [PubMed] [Google Scholar]