Abstract
The striatum mediates a broad range of cognitive and motor functions. Within the striatum, recently discovered tyrosine hydroxylase expressing interneurons (THINs) provide a source of intrastriatal synaptic connectivity that is critical for regulating striatal activity, yet the role of THIN’s in behavior remains unknown. Given the important role of the striatum in reward-based behaviors, we investigated whether loss of striatal THINs would impact instrumental behavior in mice. We selectively ablated striatal THINs in TH-Cre mice using chemogenetic techniques, and then tested THIN-lesioned or control mice on three reward-based striatal-dependent instrumental tests: 1) progressive ratio test; 2) choice test following selective-satiety induced outcome devaluation; 3) outcome reinstatement test. Both striatal-THIN-lesioned and control mice acquired an instrumental response for flavored food pellets, and their behavior did not differ in the progressive ratio test, suggesting intact effort to obtain rewards. However, striatal THIN lesions markedly impaired choice performance following selective-satiety induced outcome devaluation. Unlike control mice, THIN-lesioned mice did not adjust their choice of actions following a change in outcome value. In the outcome reinstatement test THIN-lesioned and control mice showed response invigoration by outcome presentation, suggesting the incentive properties of outcomes were not disrupted by THIN lesions. Overall, we found that striatal THIN lesions selectively impaired goal-directed behavior, while preserving motoric and appetitive behaviors. These findings are the first to describe a function of striatal THINs in reward-based behavior, and further illustrate the important role for intrastriatal interneuronal connectivity in behavioral functions ascribed to the striatum more generally.
Keywords: basal ganglia, devaluation, reward, interneuron, THIN
Graphical Abstract:

Tyrosine Hydroxylase Interneurons (THIN)’s are a recently discovered class of interneurons that form local inhibitory circuits within the striatum. We found that loss of striatal THIN’s impaired mice’s ability to adjust their actions to changes in outcome value but preserved effortful responding and the motivating effects of rewarding outcomes more generally. These results suggest a specific role for striatal THIN’s in goal-directed learning and action control.
The striatum mediates a broad range of motor and cognitive functions. As the primary input nucleus of the basal ganglia, the striatum receives extensive cortical and thalamic afferents, as well as midbrain neuromodulatory input. Approximately 95% of striatal neurons are spiny projection neurons (SPNs), with the remainder consisting of interneurons (Graveland & Difiglia, 1985; Gerfen & Surmeier, 2011). Recently, the diversity of known cell types comprising striatal interneurons has expanded, and with the discovery of these new classes of interneurons come new questions regarding their functions (Tepper et al., 2010; Assous & Tepper, 2018; Munoz-Manchado et al., 2018). Tyrosine-hydroxylase expressing interneurons (THINs) are a class of interneurons identified based on their unique electrophysiological characteristics and expression of TH (although they are not dopaminergic (Xenias et al., 2015)). THINs receive excitatory input from parafascicular thalamus and cortex, are GABA-ergic, and inhibit SPNs (Ibanez-Sandoval et al., 2010; Xenias et al., 2015). THINs also mediate thalamic inhibition of another class of striatal interneuron, the low-threshold spiking (LTS) interneuron (Assous et al., 2017). THINs are located in dorsal and ventral striatum, in predominately matrix and Mu-opioid receptor domains in ventral striatum (Unal et al., 2011). THINs are also responsive to dopamine and acetylcholine, via activation of D1/D5 and nicotinic receptors, respectively (Ibanez-Sandoval et al., 2015; Assous & Tepper, 2018).
The function of striatal THINs in behavior is largely unknown. Striatal THIN lesions impair pre-pulse inhibition of the startle reflex (Assous et al., 2017), suggesting an important role in attention and behavioral inhibition. We examined striatal THIN’s role in reward-based learning and decision making, given the striatum’s well-described functions in these domains (Balleine et al., 2009; Peak et al., 2018; Sharpe et al., 2018). Specifically, we examined instrumental behavior and tests of effortful responding using i) the progressive ratio test, ii) a choice test following selective-satiety induced outcome devaluation, and iii) an outcome reinstatement test. These tests depend on activity within the dorsal and/or ventral striatum (Salamone & Correa, 2002; Yin et al., 2005; Shiflett et al., 2010; Hart et al., 2014). We chemogenetically ablated striatal THINs by injecting a viral-mediated Cre-dependent diphtheria toxin in TH-Cre transgenic mice. We have previously shown that this method effectively ablates THINs from the striatum (Assous et al., 2017).
Materials and Methods
Subjects and Housing
Subjects were 34 adult male (4–6 month) Bacterial Artificial Chromosome (BAC) transgenic TH–Cre [Tg(TH–Cre)12Gsat; Gene Expression Nervous System Atlas (GENSAT)] mice originally sourced from the Mutant Mouse Resource and Research Center (MMRRC) UC Davis. Offspring were genotyped and housed in standard mouse containers with bedding and ad lib water. During behavioral testing, mice were individually housed and placed on food restriction. Mice were weighed daily during food restriction. They received approximately 5 g of standard chow each day after testing for that day was completed. Mice were weighed daily, and their daily food ration adjusted to maintain their body weight at 90–95% of their free-feeding body weight. All procedures complied with Rutgers Institutional Animal Care and Use Committee protocols.
Surgical Procedures
The surgical methods have been previously described in detail (Assous et al., 2017). Briefly, we used stereotaxic surgical procedures to intracranially inject virus targeting the striatum in TH-Cre mice. Mice were anesthetized with isofluorane (1.5–2.5% delivered with O2) and the surgery took place in an isolation hood. Bupivacaine was used as a local anesthetic at the surgery site. To ablate cre-expressing THINs, we injected an adeno-associated virus containing the neurotoxin DT-A (AAV5-EF1a-DIO-DT-A-mCherry, UNC vector core). These mice also received a second virus encoding YFP (AAV5-EF1a-DIO-ChR2-eYFP). The second virus was used to identify the extent of the THIN lesion. Any spared THINs would be identified as YFP positive. The coordinates for injection were: AP:0.5 mm, ML:1.8 mm, DV:2.75 mm (330 nl) and 3.3 mm (440 nl) and AP:1.3 mm, ML: 1.0 mm, DV: 3.9 (160 nl). Virus was delivered via a glass pipette. The same volume was used for both viruses. The control group consisted of TH-Cre mice in which we injected an adeno-associated virus containing the fluorescent protein mCherry (AAV5-EF1a-DIO-mCherry or AAV5-hSyn-DIO-hM4Di-mcherry, UNC vector core). Mice were given acetaminophen (100 mg/kg body weight, once daily oral) for one week after surgery and monitored for signs of distress. We tested mice 4–6 weeks following surgery, which we have previously shown sufficiently allows the virus to transfect and ablate striatal THINs (Assous et al., 2017).
Instrumental Training Procedures
Mice were tested in eight identical operant conditioning chambers (Med Associates, St. Albans VT, USA). Each operant conditioning chamber measured 15.9 cm x 14.0 cm x 12.7 cm (w/h/d) and was constructed of stainless steel and clear plastic walls and had a stainless-steel grid floor. A food cup was centered on one wall with retractable levers situated to the left and right of the food cup. Responses on one lever caused delivery of a single 20-mg grain-based chocolate flavored food pellet (Bio-serv, Frenchtown NJ USA) into the food cup from a dispenser mounted outside the operant conditioning chamber. A 28 V light was located on the opposite wall from the food cup and illuminated the operant conditioning chamber during behavioral procedures. Each operant conditioning chamber was housed in a sound-attenuating shell and equipped with a ventilation fan that was activated during behavioral procedures. Control over the operant conditioning chambers was enabled by a PC operating through an interface. Operant conditioning chamber operation and data collection were carried out with Med Associates proprietary software (Med-PC).
Instrumental training commenced after one week of food restriction. Mice were habituated to the operant conditioning chamber in one 15-min session, which was followed the next day by a 20-min session in which food pellets were dispensed on a random-time 60 sec schedule. The levers were withdrawn during this phase. Mice then underwent daily instrumental training sessions. During each session, a single lever was inserted into the operant conditioning chamber and responses on the lever delivered a food pellet. For the first 2 sessions each response resulted in pellet delivery. For the remaining 8 sessions, outcomes were delivered according to a variable-ratio schedule, which required, on average, 7.5 responses to issue pellet delivery.
Progressive Ratio Test.
In the progressive ratio test, a single lever was inserted in the chamber. Responses on the lever earned a pellet, with the response requirement to earn the pellet increasing by 4 with each successive reward delivery (1, 4, 8, 12…). The breakpoint was defined by no reward delivery for 10 minutes. The session terminated after 90 minutes.
Selective satiety outcome devaluation and extinction test.
Outcome devaluation took place in a standard mouse enclosure identical to the home cage. An empty glass dish was placed within the enclosure. Mice were habituated to the enclosure and dish for 3 days alternating before or after their instrumental training sessions. During the satiety procedure, 10 grams of food pellets (either the instrumental outcome, or an alternative flavored-pellet) were placed within the glass dish. Mice remained in the enclosure for one hour, after which they were given a 10-min extinction test. In the extinction test the lever was inserted into the chamber and mice could make free responses; however, no pellets were delivered. Different types of pellets (instrumental outcome or alternative) were presented on different test sessions and the order was randomized across treatment groups.
We conducted a second experiment in a separate cohort of mice to confirm the results of the first outcome devaluation test. In this experiment we trained mice on two separate responses (left or right lever press) for two types of outcomes (chocolate or grain pellets). Instrumental training was otherwise identical to the first experiment. Mice underwent outcome devaluation in an identical manner to the first experiment. During the choice test both levers were available, and responses on the two levers were classified as “valued” or “devalued” depending on the type of pellet that was fed to the mice prior to the test. The choice test was conducted twice, with the fed pellet type alternating between tests. Mice received 3 days of instrumental training in between tests.
Outcome reinstatement test.
Following the devaluation test, mice received an additional 3 days of instrumental training, and then underwent the outcome reinstatement test. In this test mice had access to the lever in extinction for 10 min, after which a single pellet was delivered non-contingently. The pellet was either the same type as the previously trained instrumental outcome (match) or was the alternative outcome (mismatch). A total of four pellets were delivered, one pellet every 6 minutes. Two of the pellets were matched, and two mismatched, with the order of presentation pseudorandomized.
Histological Procedures
Mice were deeply anesthetized with ketamine (100 mg/kg), diluted in saline and perfused with 10 ml of ice-cold artificial cerebrospinal fluid (pH 7.2–7.4) followed by 90–100 ml of solution containing 4% paraformaldehyde, 15% picric acid dissolved in phosphate buffer. After post-fixation overnight, brains were sliced at 40 µM on a Vibratome and mounted in Vectashield (Vector Labs, Burlingame, CA) on slides. Slides were viewed on an epifluorescence microscope at 10X and 20X objective. Location of virus injection was based on tissue damage.
Statistical Procedures
Lever press response rates were subject to analysis of variance (ANOVA) and two-tailed Student’s t-tests. Lever press rates from the outcome devaluation and reinstatement tests were normalized as a percent of total responses the animal made during the test. Normalization controlled for variations in response rate, as our primary interest was to identify the proportion of responses animals allocated to the two levers. Lesion status (THIN lesion or sham) was the between-subjects factor. For instrumental training, training block (the average of two training sessions) was a within-subjects factor. For the outcome devaluation test, outcome value (valued or devalued) was a within-subjects factor. For the outcome reinstatement test, lever-outcome pairing (match or mismatch) was the within-subjects factor. The significance level was set to 0.05. For significant t-test comparisons, Cohen’s effect size (d) are reported. Bonferroni correction was used for multiple comparisons. All statistical analyses were performed using IBM SPSS statistics v 25 (IBM).
Results
Histology
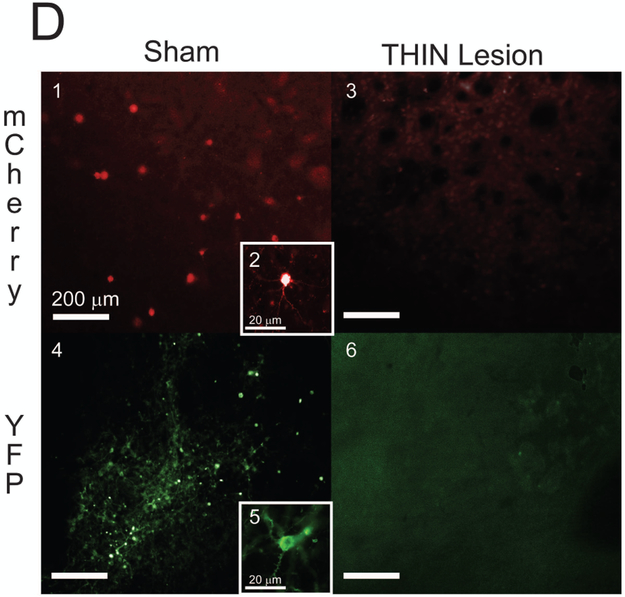
Figure 1B depicts virus injection placement in the dorsal striatum at sites along the anterior-posterior axis. Injections span the anterior-posterior axis of the striatum and were present primarily in dorsal striatum. Viral transfection was assessed by examining expression of the fluorophore mCherry. Among sham-treated animals, we found transfected cells residing within the dorsomedial, dorsolateral and ventral striatum. The greatest overlap of expression was in the dorsomedial striatum, with significant expression also found in the lateral striatum and the anterior ventral striatum (Figure 1C). Importantly, we detected no mCherry expression in midbrain dopaminergic regions. In mice injected with virus containing DT-A, we found virtually no mCherry expression. Mice that received DT-A were transfected with a second cre-dependent virus containing eYFP. This marker identifies THINs spared from the DT-A lesion. The advantage of using a cre-dependent fluorescent tag over TH immunohistochemistry is that the cre-dependent signal labels THIN interneurons without labeling dopaminergic afferents containing TH. Also, this method provides a strong signal for identifying THINs that ordinarily are difficult to detect with immunochemistry owing to their low level of TH expression. We found virtually no eYFP expression in the striatum in TH-Cre mice treated with DT-A (Figure 1D). These data suggest a significant loss of THIN’s in the dorsomedial, dorsolateral and ventral striatum in animals transfected with the virus containing DT-A.
Figure 1.
Striatal THIN lesions (A) TH-cre transgenic mice received intrastriatal viral injections containing the toxin DT-A. (B) Injection locations were identified in histological sections based on tissue damage. Numbers indicate location relative to bregma in mm. (C) The spread of viral transfection as estimated by mCherry expression in sham animals. Shaded areas indicate regions where mCherry expression was detected. Areas of overlap across animals are indicated by darker shading. (D) Examples of transfected THINs from a sham-lesioned animal expressing AAV5-EF1a-DIOmCherry (1-2) and a THIN-lesioned animal (3). A second cre-dependent marker eYFP used to identify THINs was virally expressed in striatum. We found eYFP expression in sham animals (4-5) and no expression in THIN-lesioned animals (6). Photomicrographs were taken at 10X or 40x (insets) objective from the dorsal striatum. Schematics are adapted from Paxinos & Franklin (2008).
Progressive ratio test
Instrumental training proceeded normally for both control and THIN-lesioned mice. We observed a significant increase in response rate over the course of training (ANOVA F13,168 = 29.40, P < 0.001), but no effect of lesion or interaction involving lesion and session on response rate (Figure 2A). In the progressive ratio test, mice earned pellets according to a schedule with increasing response requirements. Most mice earned pellets for the entirety of the 90-min session. There were no differences between treatment and control mice in the number of pellets earned, the breakpoint, or the total responses made during the test (Figure 2B-D).
Figure 2.
Instrumental acquisition and progressive ratio responding in THIN-lesioned and control mice. (A) During instrumental training both control and THIN-lesioned mice increased their response rate over the course of training, with no difference between groups. (B-D) During the progressive ratio test, no difference between groups was observed in either the breakpoint, responses made, and outcomes earned. (E) Performance in the outcome devaluation test. During the devaluation test, THIN-lesioned mice did not change their response rate following satiety on the instrumental outcome, unlike control mice. CRF=continuous reinforcement; VR=variable ratio. * = significant at P = 0.001. Error bars indicate +/− 1 SEM.
Outcome devaluation test
All mice consumed pellets during the satiety procedure, and there was no effect of THIN lesion on the number of pellets consumed. Responses in the two tests were normalized as a percent of total responding. Analysis of these data revealed a significant main effect of outcome type (instrumental or alternative outcome) (F1,16 = 6.46, P = 0.022) and a marginally significant outcome type by lesion interaction (F1,16 = 4.39, P = 0.052) (Figure 2E). Sham-lesioned mice made a significantly lower proportion of responses on the lever following pre-feeding of the instrumental outcome compared to responses made after pre-feeding the alternative outcome (paired t-test, t8 = 5.61, P < 0.001, d = 3.76). In contrast, TH-lesioned mice responded similarly in the two sessions (paired t-test, P = 0.81). These data indicate that, unlike the behavior of sham mice, instrumental responses of THIN-lesioned mice are insensitive to changes in outcome value.
Reinstatement Test
In both sham and THIN-lesioned animals, pellet delivery significantly invigorated responding relative to the response rate in the last minute of extinction. Moreover, responses following outcome delivery were significantly greater for the instrumental “matched” outcome compared to the alternative “mismatched” outcome. Responses were tallied for the two minutes following pellet delivery, and these values were normalized as a percent of total responses. Analysis of these data revealed a significant main effect of outcome type (match or mismatch) (ANOVA F1,18 = 21.19 P < 0.001) and no significant interaction (P = 0.43) (Figure 3A). Both sham and THIN-lesioned mice made significantly more responses following delivery of the matched relative to the mismatched outcome (paired t-test: sham t8 = 3.68 P = 0.006, d = 2.45; TH-lesioned t10 = 2.81 P = 0.02 d = 2.60).
Figure 3.
Outcome reinstatement in THIN-lesioned and control mice. (A) Non-contingent delivery of the instrumental outcome invigorated the instrumental action (“match” responses) to a greater degree than responses caused by delivery of an alternative outcome (“mismatch” responses) in both control and THlesioned mice. (B-C) Outcome delivery more strongly invigorated responding in THIN-lesioned mice compared to control mice. Mean response rates for the six minutes following outcome delivery were divided into 3 2-min blocks. THIN-lesioned mice showed greater responding in the 3rd block compared to control mice. * = significant at P = 0.02; ** = significant at P = 0.006; # = P =0.036. Error bars indicate +/− 1 SEM.
Interestingly, THIN-lesioned mice sustained their responses following outcome delivery to a greater degree than sham mice. The 6-min interval between pellet deliveries was divided into 3 2-min blocks. Responses in the last block (minutes 4–6) were significantly greater in THIN-lesioned mice compared to shams (t18 = 2.27, P = 0.036 d = 1.07) (Figure 3B-C).
Replication of outcome devaluation test
We ran an additional cohort of animals with the aim of replicating the effect of the THIN lesion on outcome sensitivity. We additionally tested whether having THIN-lesioned mice learn two action-outcome associations would spare outcome sensitivity. All mice acquired instrumental responses for two outcomes, and we found no difference in response rates for the different outcomes, nor any difference between groups. As we observed previously, THIN-lesioned mice’s actions did not change following selective satiety of the instrumental outcome (Figure 4A). We found a significant lesion x outcome value interaction on the proportion of responses made during the choice test (ANOVA F1,14 = 14.34, P = 0.002). A significantly greater portion of control mice’s responses were toward the valued action compared to the devalued action (paired t-test, t7 = 4.75, P =0.002 d = 3.35), whereas TH-lesioned mice showed no difference in their allocation of actions (P=0.31). We found a pattern of mCherry expression that was similar to the first cohort, suggesting that the viral transfection and THIN lesion was localized to the striatum (Figure 4B).
Figure 4.
The lack of sensitivity to changes in outcome value was observed in a second cohort of THINlesioned mice that were trained on an instrumental paradigm with two actions leading to two distinct outcomes. (A) Following selective satiety sham-lesioned mice showed a significant difference between valued and devalued actions, whereas THIN-lesioned mice did not. (B) The spread of viral transfection in sagittal sections as estimated by mCherry expression in sham animals. Shaded areas indicate regions where mCherry expression was detected. Areas of overlap across animals are indicated by darker shading. Values indicate position relative to bregma (in mm). (C) The relationship between goal-directed behavior and viral injection location in THIN-lesioned animals. The GD score reflects the ratio of an animal’s valued response rate relative to their overall response rate. The X axis is the anterior-posterior position of the virus injection relative to bregma (in mm). We found a significant positive correlation between anterior-posterior position and goal-directed behavior (Pearson correlation coefficient = 0.608, P = 0.03), suggesting posterior-located virus injection was more effective at disrupting goal-directed behavior. * = significant at P = 0.002. Error bars indicate +/− 1 SEM. Schematics are adapted from Paxinos & Franklin (2008) Tyrosine Hydroxylase Interneurons (THIN)’s are a recently discovered class of interneurons that form local inhibitory circuits within the striatum. We found that loss of striatal THIN’s impaired mice’s ability to adjust their actions to changes in outcome value but preserved effortful responding and the motivating effects of rewarding outcomes more generally. These results suggest a specific role for striatal THIN’s in goal-directed learning and action control.
We additionally examined whether THIN lesions exhibited any regional specificity in their effects on goal-directed behavior, given previous findings of different striatal sub-regions mediating goal-directed versus habitual responding (Yin et al., 2005). For all THIN-lesioned animals we calculated a goal-directed score, which was the ratio of the difference in valued and devalued response rates over the sum of those response rates. These scores were regressed against the anterior-posterior or medial-lateral virus injection locations. We found a significant positive correlation between the anterior-posterior virus injection location and goal-directed responding (R2 = 0.37; Pearson correlation coefficient = 0.608, P = 0.036 (Figure 4C). That is, posterior-located THIN lesions disrupted goal-directed behavior whereas THIN lesions in the anterior striatum did not. We found no significant relationship between medial-lateral injection location and goal-directed behavior (R2 = 0.06; Pearson correlation coefficient = 0.25, P = 0.49).
Discussion
We found that loss of striatal THINs causes a significant and selective impairment in goal-directed behavior in mice. Our findings revealed no general impairments caused by THIN lesions in motor behavior. THIN-lesioned mice could perform instrumental responses at similar rates to control animals. THIN lesions had no impact on appetite generally. We observed no difference in the amount or pattern of food consumption during the satiety procedure, suggesting intact appetite. THIN lesions did not reduce the motivation to obtain food in the progressive ratio test. Overall, the lack of deficit in motor ability, appetite and effort indicate that the impairments we observed in the outcome devaluation test are due to a selective function of THINs in cognitive/motivational operations mediated by the striatum. Although THINs represent a relatively small number of striatal neurons, they have an outsized impact on striatal activity. The selective loss of goal-directed responding suggests an important function of these neurons in enabling the striatum to learn, organize, and/or select appropriate actions based on the current value of instrumental incentives.
THIN-lesioned mice were incapable of adjusting their instrumental actions to a change in outcome value. This behavioral flexibility relies on an animal forming an association between its actions and the outcomes or consequences of those actions (Adams & Dickinson, 1981; Dickinson & Balleine, 1994; Balleine & Ostlund, 2007). Action-outcome associations enable behavioral flexibility under changing environmental conditions, whereas, inflexible (or, habitual) behavior is evident when an animal’s actions do not reflect changes in outcome value. Considerable evidence supports the notion that the dorsomedial striatum is necessary for action-outcome learning, whereas the dorsolateral striatum is necessary for habit learning (Yin et al., 2005; Shiflett et al., 2010; Shan et al., 2014; O’Hare et al., 2016). However, more recent evidence suggests that medial and lateral striatum may be collectively recruited during learning (Kupferschmidt et al., 2017; Malvaez et al., 2018). We found viral expression along the anterior-posterior axis in both medial, lateral, and ventral striatum; therefore, we are unable to determine whether the effects of THIN ablation on goal-directed behavior is specific to a particular striatal sub-region. Whether the intra- and extra-striatal connectivity of THINs differs in any significant way across striatal sub-regions, as may be the case for GABA-ergic fast-spiking interneurons and LTS interneurons (Fino et al., 2018), is not known. We did find a significant positive correlation between anterior-posterior infusion location and goal-directed behavior, suggesting that loss of posterior-located THINs had a greater disruptive effect on goal-directed behavior. This is consistent with Yin et al.’s (2005) finding that posterior DMS lesions disrupted goal-directed behavior, whereas anterior lesions had no effect. Future studies that target discrete striatal sub-regions will address this issue. We found no evidence of viral transfection within the midbrain dopaminergic regions. Based on these data we are confident the effects of viral injection were to ablate THINs within the striatum.
The precise role for THINs in goal-directed learning and/or performance remains to be determined. THINs receive strong excitatory input from the parafascicular nucleus of the thalamus (Assous et al., 2017). The role of the thalamostriatal pathway in goal-directed learning is still under investigation. Some studies limit the role of this pathway to integration of new information into existing representations, such as when action-outcome contingencies require updating, but not necessarily of initial action-outcome learning (Bradfield et al., 2013; Bradfield & Balleine, 2017; Yamanaka et al., 2018). Nevertheless, while disruption of the thalamostriatal pathway may be insufficient to disrupt new action-outcome learning, it is possible that thalamic input together with other afferent input to THINs (e.g., from cortex) is necessary for initial action-outcome learning.
Another possibility is that THIN’s role in goal-directed behavior operates through its modulation of dopamine input to the striatum. Midbrain dopamine activity incorporates value and the sensory features of predicted outcomes (Sadacca et al., 2016; Takahashi et al., 2017), and as such, dopamine neuron activity may be essential for adjusting actions to changes in outcome value. Dopamine increases the excitability of striatal THINs, which themselves act to inhibit striatal SPN’s (Ibanez-Sandoval et al., 2010; Ibanez-Sandoval et al., 2015). This feedforward inhibition may have an important function in selecting striatal cell ensembles representing appropriate actions and inhibiting inappropriate actions (Roux & Buzsaki, 2015; Burke et al., 2017; Markowitz et al., 2018). Alternatively, THINs may provide a similar action selection function based on their interactions with other striatal interneurons, including striatal cholinergic interneurons (English et al., 2012; Lee et al., 2017; Yamanaka et al., 2018).
Loss of THINs enhanced reinstatement by outcome delivery. One explanation for these findings is that THIN lesions may have influenced incentive learning. During selective satiety, animals update the incentive value of a particular outcome by associating the sensory features of the outcome (taste, smell etc.) with its current incentive value, a process known as incentive learning (Balleine & Dickinson, 1991; Balleine & Dickinson, 1998). Failure to adjust actions following selective-satiety devaluation in THIN mice could therefore reflect impaired incentive learning. According to this account, altered incentive processes could also influence behavior in the outcome reinstatement test. In this test, both THIN-lesioned and control mice showed an invigorating effect on instrumental responding by outcome presentation, with THIN-lesioned mice showing a larger and more sustained number of instrumental responses following pellet delivery compared to controls. If THIN lesions cause mice to inflate the incentive properties of an instrumental outcome, it may augment an instrumental response during reinstatement, as well as prevent the reduction in incentive value during selective satiety. Future tests that specifically examine incentive learning in THIN-lesioned mice are needed to address this possibility.
During the outcome reinstatement test, both THIN-lesioned and control mice showed a selective invigoration of the instrumental response when presented with the instrumental outcome, as compared to an alternative outcome not associated with the instrumental response. This selective invigoration may argue for preserved action-outcome associations in these mice. However, selectivity in this test could be preserved if the instrumental outcome serves as a stimulus or cue for the instrumental response (de Wit et al., 2007). THIN-lesioned animals could have formed stimulus-response (S-R) associations during instrumental learning with the outcome serving as the stimulus and the instrumental action as the response. Indeed, if THIN lesions abolished action-outcome learning, then these mice are more likely to rely on S-R associations to guide their behavior.
Our findings indicate an important function of THINs in goal-directed behavior. These results, along with other recent reports (O’Hare et al., 2017; Martiros et al., 2018), highlight the importance of striatal GABAergic interneurons in mediation of reward-based behavior and functions typically ascribed to the striatum more generally.
Acknowledgements
This work was funded by National Institutes of Health R01NS034865–20 to JMT and TK, and by the Rutgers University Initiative for Multidisciplinary Research Teams to MWS, JK, and TK.
Abbreviations
- THIN
tyrosine hydroxylase Interneuron
- SPN
Spiny Projection Neuron
- LTS
Low Threshold Spiking
Footnotes
Conflict of Interest Statement
The authors declare no conflicts of interest.
Data Accessibility Statement
All data will be made available upon request to MWS
References
- Adams CD & Dickinson A (1981) Instrumental responding following reinforcer devaluation. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology, 33, 109–121. [Google Scholar]
- Assous M, Kaminer J, Shah F, Garg A, Koos T & Tepper JM (2017) Differential processing of thalamic information via distinct striatal interneuron circuits. Nat Commun, 8, 15860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assous M & Tepper JM (2018) Excitatory extrinsic afferents to striatal interneurons and interactions with striatal microcircuitry. Eur J Neurosci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balleine B & Dickinson A (1991) Instrumental Performance Following Reinforcer Devaluation Depends on Incentive Learning. Quarterly Journal of Experimental Psychology Section B-Comparative and Physiological Psychology, 43, 279–296. [PubMed] [Google Scholar]
- Balleine BW & Dickinson A (1998) The role of incentive learning in instrumental outcome revaluation by sensory-specific satiety. Anim Learn Behav, 26, 46–59. [Google Scholar]
- Balleine BW, Liljeholm M & Ostlund SB (2009) The integrative function of the basal ganglia in instrumental conditioning. Behav Brain Res, 199, 43–52. [DOI] [PubMed] [Google Scholar]
- Balleine BW & Ostlund SB (2007) Still at the choice-point: action selection and initiation in instrumental conditioning. Ann N Y Acad Sci, 1104, 147–171. [DOI] [PubMed] [Google Scholar]
- Bradfield LA & Balleine BW (2017) Thalamic Control of Dorsomedial Striatum Regulates Internal State to Guide Goal-Directed Action Selection. J Neurosci, 37, 3721–3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield LA, Bertran-Gonzalez J, Chieng B & Balleine BW (2013) The thalamostriatal pathway and cholinergic control of goal-directed action: interlacing new with existing learning in the striatum. Neuron, 79, 153–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke DA, Rotstein HG & Alvarez VA (2017) Striatal Local Circuitry: A New Framework for Lateral Inhibition. Neuron, 96, 267–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit S, Niry D, Wariyar R, Aitken MR & Dickinson A (2007) Stimulus-outcome interactions during instrumental discrimination learning by rats and humans. J Exp Psychol Anim Behav Process, 33, 1–11. [DOI] [PubMed] [Google Scholar]
- Dickinson A & Balleine B (1994) Motivational control of goal-directed action. Anim Learn Behav, 22, 1–18. [Google Scholar]
- English DF, Ibanez-Sandoval O, Stark E, Tecuapetla F, Buzsaki G, Deisseroth K, Tepper JM & Koos T (2012) GABAergic circuits mediate the reinforcement-related signals of striatal cholinergic interneurons. Nat Neurosci, 15, 123–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fino E, Vandecasteele M, Perez S, Saudou F & Venance L (2018) Region-specific and state-dependent action of striatal GABAergic interneurons. Nat Commun, 9, 3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen CR & Surmeier DJ (2011) Modulation of striatal projection systems by dopamine. Annu Rev Neurosci, 34, 441–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graveland GA & Difiglia M (1985) The frequency and distribution of medium-sized neurons with indented nuclei in the primate and rodent neostriatum. Brain Research, 327, 307–311. [DOI] [PubMed] [Google Scholar]
- Hart G, Leung BK & Balleine BW (2014) Dorsal and ventral streams: the distinct role of striatal subregions in the acquisition and performance of goal-directed actions. Neurobiol Learn Mem, 108, 104–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Tecuapetla F, Unal B, Shah F, Koos T & Tepper JM (2010) Electrophysiological and morphological characteristics and synaptic connectivity of tyrosine hydroxylase-expressing neurons in adult mouse striatum. J Neurosci, 30, 6999–7016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez-Sandoval O, Xenias HS, Tepper JM & Koos T (2015) Dopaminergic and cholinergic modulation of striatal tyrosine hydroxylase interneurons. Neuropharmacology, 95, 468–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupferschmidt DA, Juczewski K, Cui G, Johnson KA & Lovinger DM (2017) Parallel, but Dissociable, Processing in Discrete Corticostriatal Inputs Encodes Skill Learning. Neuron, 96, 476–489.e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Holley SM, Shobe JL, Chong NC, Cepeda C, Levine MS & Masmanidis SC (2017) Parvalbumin Interneurons Modulate Striatal Output and Enhance Performance during Associative Learning. Neuron, 93, 1451–1463.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malvaez M, Greenfield VY, Matheos DP, Angelillis NA, Murphy MD, Kennedy PJ, Wood MA & Wassum KM (2018) Habits Are Negatively Regulated by Histone Deacetylase 3 in the Dorsal Striatum. Biol Psychiatry, 84, 383–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markowitz JE, Gillis WF, Beron CC, Neufeld SQ, Robertson K, Bhagat ND, Peterson RE, Peterson E, Hyun M, Linderman SW, Sabatini BL & Datta SR (2018) The Striatum Organizes 3D Behavior via Moment-to-Moment Action Selection. Cell, 174, 44–58.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martiros N, Burgess AA & Graybiel AM (2018) Inversely Active Striatal Projection Neurons and Interneurons Selectively Delimit Useful Behavioral Sequences. Curr Biol, 28, 560–573.e565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz-Manchado AB, Bengtsson Gonzales C, Zeisel A, Munguba H, Bekkouche B, Skene NG, Lonnerberg P, Ryge J, Harris KD, Linnarsson S & Hjerling-Leffler J (2018) Diversity of Interneurons in the Dorsal Striatum Revealed by Single-Cell RNA Sequencing and PatchSeq. Cell reports, 24, 2179–2190.e2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare JK, Ade KK, Sukharnikova T, Van Hooser SD, Palmeri ML, Yin HH & Calakos N (2016) Pathway-Specific Striatal Substrates for Habitual Behavior. Neuron, 89, 472–479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Hare JK, Li H, Kim N, Gaidis E, Ade K, Beck J, Yin H & Calakos N (2017) Striatal fast-spiking interneurons selectively modulate circuit output and are required for habitual behavior. eLife, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G & Franklin KBJ (2008) The Mouse Brain in Stereotaxic Coordinates. Elsevier. [Google Scholar]
- Peak J, Hart G & Balleine BW (2018) From learning to action: the integration of dorsal striatal input and output pathways in instrumental conditioning. Eur J Neurosci. [DOI] [PubMed] [Google Scholar]
- Roux L & Buzsaki G (2015) Tasks for inhibitory interneurons in intact brain circuits. Neuropharmacology, 88, 10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadacca BF, Jones JL & Schoenbaum G (2016) Midbrain dopamine neurons compute inferred and cached value prediction errors in a common framework. eLife, 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD & Correa M (2002) Motivational views of reinforcement: implications for understanding the behavioral functions of nucleus accumbens dopamine. Behavioural Brain Research, 137, 3–25. [DOI] [PubMed] [Google Scholar]
- Shan Q, Ge M, Christie MJ & Balleine BW (2014) The Acquisition of Goal-Directed Actions Generates Opposing Plasticity in Direct and Indirect Pathways in Dorsomedial Striatum. The Journal of Neuroscience, 34, 9196–9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpe MJ, Stalnaker T, Schuck NW, Killcross S, Schoenbaum G & Niv Y (2018) An Integrated Model of Action Selection: Distinct Modes of Cortical Control of Striatal Decision Making. Annu Rev Psychol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiflett MW, Brown RA & Balleine BW (2010) Acquisition and performance of goal-directed instrumental actions depends on ERK signaling in distinct regions of dorsal striatum in rats. J Neurosci, 30, 2951–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi YK, Batchelor HM, Liu B, Khanna A, Morales M & Schoenbaum G (2017) Dopamine Neurons Respond to Errors in the Prediction of Sensory Features of Expected Rewards. Neuron, 95, 1395–1405.e1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepper JM, Tecuapetla F, Koos T & Ibanez-Sandoval O (2010) Heterogeneity and diversity of striatal GABAergic interneurons. Front Neuroanat, 4, 150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unal B, Ibanez-Sandoval O, Shah F, Abercrombie ED & Tepper JM (2011) Distribution of tyrosine hydroxylase-expressing interneurons with respect to anatomical organization of the neostriatum. Front Syst Neurosci, 5, 41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xenias HS, Ibanez-Sandoval O, Koos T & Tepper JM (2015) Are striatal tyrosine hydroxylase interneurons dopaminergic? J Neurosci, 35, 6584–6599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka K, Hori Y, Minamimoto T, Yamada H, Matsumoto N, Enomoto K, Aosaki T, Graybiel AM & Kimura M (2018) Roles of centromedian parafascicular nuclei of thalamus and cholinergic interneurons in the dorsal striatum in associative learning of environmental events. Journal of neural transmission (Vienna, Austria : 1996), 125, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin HH, Ostlund SB, Knowlton BJ & Balleine BW (2005) The role of the dorsomedial striatum in instrumental conditioning. Eur J Neurosci, 22, 513–523. [DOI] [PubMed] [Google Scholar]