Abstract
BACKGROUND:
Asthma disproportionately affects low-income and minority adults. In an era of electronic records and internet-based digital devices, it is unknown whether portals, for patient-provider communication, can improve asthma outcomes.
OBJECTIVE:
To estimate the effect on asthma outcomes of an intervention using home visits by community health workers plus training in patient portals compared to usual care and portal training only.
METHODS:
301 predominantly African American and Hispanic/Latino adults with uncontrolled asthma, were recruited from primary care and asthma-specialty practices serving low income urban neighborhoods, directed to internet access, and given portal training. Half were randomized to home visits over six months by community health workers to facilitate competency in portal use and promote care coordination.
RESULTS:
170 (56%) patients used the portal independently. Rates of portal activity did not differ between randomized groups. Asthma control and asthma-related quality of life improved in both groups over one year. Differences in improvements over time were greater for the home visit group for all outcomes, but reached conventional levels of statistical significance only for yearly hospitalization rate (−0.53, 95% CI= −1.08 to −0.024). Poor neighborhoods and living conditions plus limited internet access were barriers for patients to complete the protocol and for community health workers to make home visits.
CONCLUSION:
For low-income adults with uncontrolled asthma, portal access and community health workers produced small incremental benefits. Home visits with emphasis on self-management education might be necessary to facilitate patient-clinician communication and to improve outcomes for asthma.
Keywords: asthma, patient portal, community health worker, electronic health record, information technology, health disparities
CAPSULE SUMMARY:
For low-income adults with uncontrolled asthma, communication-focused interventions may improve outcomes, but patients were unable to capitalize on portal use. Home visits by community health workers assisting with care coordination and portal use produced small incremental benefits in asthma outcomes.
INTRODUCTION
Asthma disproportionately affects low-income and minority adults, particularly African and Puerto Rican Americans; 65% of adult patients are women.1-4 Compared with children, adults are more likely to die from asthma and experience additional health problems like hypertension, diabetes, and obesity, which increase the complexity of managing asthma.3
According to the Institute of Medicine, improving access to care and patient-provider communication, are critical for eliminating health disparities.5 The 2009 Health Information Technology for Economic and Clinical Health Act authorized the Centers for Medicare & Medicaid Services to reimburse eligible professionals and hospitals that became “meaningful users” of certified electronic health record (EHR) technology. Certification requires a patient portal, a web-based patient-access platform for patient-clinician and patient-practice communication.
In theory, a portal allows patients to access their medical record, review test results, make appointments, request refills, and message with providers on a secure platform. However, portals are not widely used and are less available to low-income and minority patients.6-8 Limited availability may exacerbate disparities in health care access as medical practices increasingly use communication technology.7,9-12
Community Health Workers (CHWs) provided home visits to patients with chronic disease in order to improve self-management. They can provide insight into social and economic barriers to access and communication. Patients widely trust these workers who share cultural, economic, and linguistic characteristics, and understanding of the community.13-16 Bryant-Stephens and colleagues reported that asthma education and household environmental intervention delivered by CHWs reduced asthma triggers, increased caregiver asthma knowledge, and reduced symptoms and urgent care in disadvantaged children with asthma.17 However, CHW-led home visit interventions have not resulted in improved outcomes in all populations.18 Krieger and colleagues16 found in-home asthma self-management support and environmental interventions for low-income adults by CHWs improved asthma control and quality of life for asthmatic adults but not unscheduled health care use. The investigators recommended confirmation of these findings and the value of wider implementation of this approach. Other randomized interventions using CHWs have shown limited success.19,20 Building on this prior work, we tested the benefits to low-income, predominantly African American and Hispanic/Latino, inner-city adults with uncontrolled asthma, of using the portal with and without home visits by CHWs.21
METHODS
Design
The study has been previously described.21 Briefly, we planned to enroll 300 adults, randomized 1:1 into two groups. The controls received usual care plus access to and training in the use of a web-based patient portal (PT). The intervention group (PT+ HV) received in addition the periodic assistance of CHWs via home visits. CHWs were to promote care coordination and facilitate the use of the patient portal. The intervention was to take place over 6 months with 4 home visits in the first 6 months, the last 6 months being observational. Data collection was to occur quarterly over the year. Patients were followed and measured for several asthma outcomes with the hypothesis that home visits by a community health worker would improve asthma outcomes.
A randomized trial was chosen because studies involving health behavior where the complex influences on behavior may not be completely understood, require careful balancing. We did not plan a placebo or usual care arm because the study sponsor, The Patient-Centered Outcomes Research Institute (PCORI), requested a demonstration of comparative effectiveness with an active control, here an introduction to and training in the use of the patient portal. Additionally, our prior experience demonstrated that patients were reluctant to enroll and remain active participants in an experimental intervention that did not offer some direct benefits. Thus, all patients received portal training. In addition, our study design purposely avoided data collection for, and analysis of, cost effectiveness because the Federal statute that founded PCORI expressly prohibits the comparison of costs of alternative treatments or interventions. Although the CHWs and data collectors were aware of the treatment assignment, the Principal Investigator and Co-Principal Investigator were blinded to the allocation until the close of recruitment.
Sites and Participants
Participating sites included outpatient practices of family medicine, general internal medicine (2), pulmonary medicine (2), and allergy-immunology of an academic medical center; and a primary care practice serving mainly Spanish-speaking patients. Patients, 18 years or older, qualified for inclusion if they lived in a Philadelphia neighborhood where at least 20% of households have incomes below the federal poverty level, had a doctor’s diagnosis of asthma, currently were prescribed an inhaled corticosteroid-containing medication; required prednisone, an ED visit, or hospitalization for asthma in the past year; had not previously signed on to a portal more than 3 times; and had received care in a participating clinic. Excluded were individuals with severe psychiatric or cognitive problems that would impair understanding and following this protocol.
Recruitment
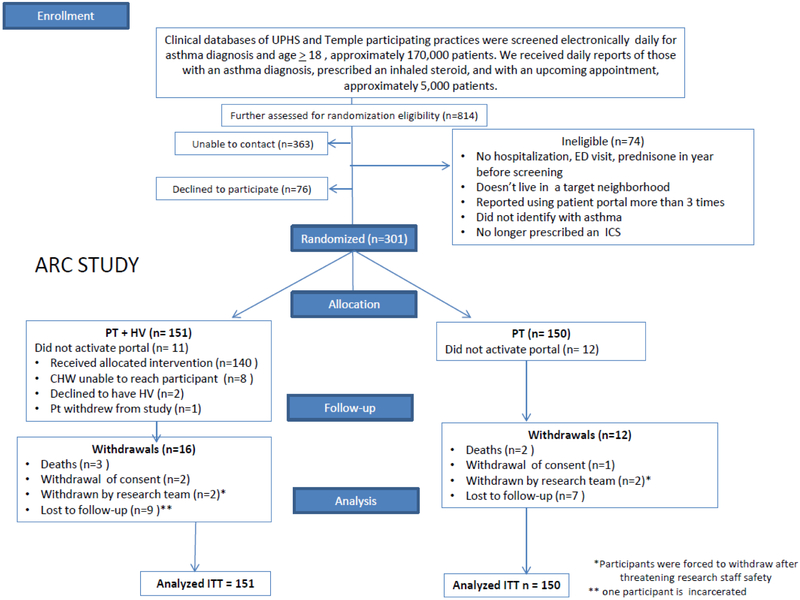
At staff meetings of participating sites, we described our protocol to clinicians. Through the electronic medical record, we received lists of potentially eligible patients, approximately 5000 adults (See Figure 1) for further screening. We then sent “opt-out” letters to clinicians for permission to contact their patients. If clinicians did not respond to two letters or gave permission, we mailed letters to patients asking to contact them for screening. If patients gave permission, or did not respond, we telephoned the potential participant or approached them at a clinic visit to explain the study and ask for permission to screen further. Reasons for declining included inability to commit to the study timeline, disinterest in research participation, and patient illness.
Figure 1.
Participant flow diagram (CONSORT).
Internet access
We were unable to find a company or companies that would donate tablets and providing these was too costly. Technical consultants worried that installation and maintenance of broadband might be difficult in old homes. We decided to rely on patients’ current internet access as the basis for portal use. We insured internet access for participants at clinic locations, and suggested that participants use the nearest library or hot spot (recognizing low income communities have fewer), and/or via smartphone apps. Despite the availability of the portal by smartphone app and that cell phones are more commonly owned than computers, the patient portal does not allow texting for communication with the medical team.
Portal Training (PT), conducted by CHWs, consisted of seven tasks: locate a laboratory test result, check an upcoming doctor’s appointment, schedule an appointment with their clinician, locate medication lists, find their immunization record, determine how to request a prescription refill, and send a secure message.22 Portal training was scheduled within two weeks of enrollment. Initially, we planned two teaching sessions: PT1 to demonstrate how to login and accomplish the seven tasks and PT2, the second session, to review and test patients’ recall. We found it was most convenient for patients to accomplish PT1 and PT2 in the same session. All sites used the same EHR patient portal, EPIC MyChart.23
Home Visits (HVs), designed and piloted
Informed by patient focus groups24 and piloting studies,17,22,25 home visits were intended to link the patient to the medical practice, empowering patients to communicate with their clinicians via the portal, reconcile medications, facilitate appointment scheduling,21 and ultimately improving asthma control.26 Home visits were conducted by CHWs, high school graduates who had lived in low income neighborhoods, were familiar with asthma, and had at least 3 years’ work experience. The four home visits were planned to occur at approximately 2-4 weeks, 4-7 weeks, 6-11 weeks and 23-27 weeks of enrollment.
Each home visit had two parts: 1) care coordination and promotion of portal use and 2) improving familiarity with health information technology.21 For care coordination the patient completed a needs assessment, identified goals for asthma management with the CHW, drafted an asthma care plan for review/revision/approval by the patient’s asthma clinician, and advised a visit to discuss the plan with the clinician. The scheduling was done if possible through the portal. CHWs asked patients to produce their medications, to show where emergency phone numbers and medications and instructions (action plan) are kept, explained the difference between controller and rescue medications and the proper use of inhalers and encouraged patients to communicate, if possible via the portal, with the clinician regarding exposures to tobacco smoke, pollutants, and potentially relevant allergens. CHWs referred patients to community resources as feasible, e.g. smoking cessation programs and housing opportunities.
For the second part of each visit, CHWs reviewed the portal and how to access its educational materials. They demonstrated use of the internet to obtain health and weather information, conduct a “Google” search, and use email.
At visit end the participant and CHW completed a brief report to the clinician. At quarterly phone calls planned for the remaining 6 months, information on portal training was reviewed along with information from the prior home visits.
Outcomes
The outcomes recommended by the Asthma Outcomes Workshop,27 were selected to be meaningful to patients: asthma control as the primary outcome.28-31 Secondary outcomes included asthma-related quality of life, 32-34 and the yearly rate of ED visits, hospitalizations, and prednisone “bursts” (a new or increased prednisone prescription).
Both asthma control and asthma quality of life were determined with the use of well-accepted and validated patient-centered instruments.30-33,35 The asthma control instrument also has published standards for translating scale differences into clinical improvements and changes.30-33,35 We also planned to calculate the predicted number of patients who achieved asthma control (Asthma Control Questionnaire (ACQ) score ≤1.531) at 12 months compared to 0 months and those who had a significant improvement in asthma control (decrease in ACQ of at least 0.5) over the 12 months. (Appendix, Appendix Table 3).
Hospitalizations, including ICU admissions, ED visits, urgent medical visits (scheduled < 24 hours in advance), prednisone bursts, and other medical visits were obtained by self-report because not all such events occurred within our health systems. Medical records were examined for validation of these events as possible.36 Over time we determined these endpoints by patient reports of adverse events since the last interview.
Data collection was to occur quarterly at months 0, 3, 6, 9, and 12 by a research coordinator who did not conduct the home visits. Patients were asked if there had been a hospitalization, ED visit, urgent visit in the last month and if they thought such an event was asthma-related.
Randomization and allocation concealment
Randomization was stratified by clinical site. To maintain allocation concealment, we implemented randomly permuted blocks with varying block sizes (2 to 4) by means of opaque envelopes prepared by one of the biostatisticians.
Enrollment and baseline data collection
In English or Spanish, as preferred by the participant, a CHW read/spoke the IRB-approved consent with the patient reviewing the printed document and all communications. After patient consent, the CHW unsealed the randomization envelope. Baseline questionnaires assessed socio-demographics, asthma severity, health literacy, and comorbidities. The first and last visits were in person. For other collection times, we offered a phone call, email, or visit to minimize the burden of participation. Responses were captured in a secure, encrypted, web-based database: REDCap (Research Electronic Data Capture).21,37
Statistical Analysis
The primary analyses were as randomized (intent-to-treat) with the assumption that any dropout visits were missing completely at random. Longitudinal models were used to estimate changes in treatment effects between months 0 and 12 in the primary and secondary outcomes. Irregular actual data collection times, required the use of time as a continuous measure and longitudinal data analysis models using splines. To examine the sensitivity to dropout, in addition to the randomized treatment assignment and the pre-planned stratification variable (clinical site), the covariates that might be related to dropout and the primary outcome were added to the longitudinal model. (See Appendix).
Latent-class analysis of baseline variables provided the basis both for adjustment for loss to follow-up and the identification of heterogeneity of intervention effects across patient subgroups. (See Appendix)
Predicted values from statistical models estimated individual levels of asthma control at 0 and 12 months. (Few patients were observed at exactly 12-month post randomization.) We compared changes in individual predictions of outcome at 0 months and 12 months. For asthma control, we further counted the number of predictions that improved by at least 0.5, the minimally important difference for asthma control (See Appendix).31,38
We estimated that approximately 300 patients, 150 in each group would provide 82% to 87% power to detect a minimally important clinical difference in asthma control between groups over time.30,38 The study is registered at ClinicalTrials.gov (). This protocol was approved by the University of Pennsylvania Institutional Review Board. All participants gave informed consent.
RESULTS
From June 2014 through June 2017, 301 patients were enrolled and randomized (Table 1, Figure 1). More than half reported hospitalizations for asthma and 83% at least one ED visit in the year before randomization. Past or present smokers numbered 170 (57%); 28% were current smokers. Comorbidities were prevalent: hypertension (58%), diabetes (32%), and obesity (BMI> 30) (70%). Ninety-three participants (31%) had less than adequate literacy as judged by the Short Test of Functional Health Literacy in Adults.39 Of the 49 who scored 0, 20 stated they could not read, 13 reported visual impairment and 16 declined to complete the test. One-hundred ninety (63%) were enrolled in Medicaid. Twenty-one reported having no housing at least once in the past 6 months, and 49 had some days without telephone or electricity. Half of participants (53%) owned a computer (Table 2). Except for income, rates of missing values were negligible.
Table 1.
Baseline characteristics of 301 adults with moderate to severe asthma, assigned randomly 1:1 to PT + HV or PT, expressed as frequency (percent) for discrete and mean (±SD) for continuous variables.
| Characteristic | PT + HV N=151 |
PT N=150 |
Total N= 301 |
|---|---|---|---|
| Socio-demographics | |||
| Age (years) | 50 ± 14 | 48 ± 12 | 49 ± 13 |
| Range | 18-88 | 21-78 | 18-88 |
| Sex | |||
| Female | 136 (90.1%) | 134 (89.3%) | 270 (89.7%) |
| Race/Ethnicity | |||
| Non-Hispanic Black/African-American | 116 (76.8%) | 111 (74.0%) | 227 (75.4%) |
| Hispanic or Latino | 31 (20.5%) | 35 (23.3%) | 66 (21.9%) |
| Non-Hispanic White | 1 (0.7%) | 3 (2.0%) | 4 (1.3%) |
| Other | 3 (2.0%) | 1 (0.7%) | 4 (1.3%) |
| Household income | |||
| Less than $30,000 | 119 (79%) | 115 (77%) | 234 (78%) |
| $30,000 - $49,999 | 12 (7.9%) | 7 (4.7%) | 19 (6.3%) |
| $50,000 - $99,999 | 7 (4.6%) | 4 (2.7%) | 11 (3.7%) |
| $100,000 or over | 0 (0%) | 2 (1.3%) | 2 (0.7%) |
| Decline to answer | 13 (8.6%) | 22 (14.7%) | 35 (11.6%) |
| Insurance type | |||
| Medicaid | 90 (59.6%) | 100 (66.7%) | 190 (63.1%) |
| Medicare only | 21 (13.9%) | 8 (5.3%) | 29 (9.6%) |
| Self-pay | 2 (1.3%) | 2 (1.3%) | 4 (1.3%) |
| Commercial with or without Medicare | 38 (25.2%) | 40 (26.7%) | 78 (25.9%) |
| Education attainment (highest level achieved) | |||
| 8th grade or less | 12 (7.9%) | 10 (6.7%) | 22 (7.3%) |
| Some high school | 38 (25.2%) | 29 (19.3%) | 67 (22.3%) |
| High school graduate or G.E.D. | 50 (33.1%) | 61 (40.7%) | 111 (36.9%) |
| Some college or trade school | 34 (22.5%) | 32 (21.3%) | 66 (21.9%) |
| College graduate | 17 (11.3%) | 18 (12.0%) | 35 (11.6%) |
| Literacy | |||
| S-TOFHLA* | 25 ± 14 | 25 ± 13 | 25 ± 13 |
| Minimum, maximum | 0, 36 | 0, 36 | 0, 36 |
| Asthma Numeracy (ANQ)† | 1.2 ± 1.2 | 1.2 ± 1.3 | 1.2 ± 1.2 |
| Minimum, maximum | 0. 4 | 0, 4 | 0, 4 |
| eHEALS‡ owns a cell phone | 25.4 ± 7.7 | 24.8 ± 8.7 | 25.1 ± 8.2 |
| Minimum, maximum | 8, 40 | 8, 40 | 8, 40 |
| Asthma severity at baseline | |||
| # with ≥ 1 hospitalization for asthma in past year | 77 (51.0%) | 74 (49.3%) | 151 (50.2%) |
| # with ≥ 1 ED visit for asthma in past year | 125 (82.8%) | 126 (84.0%) | 251 (83.4%) |
| FEV-1 (percent predicted)§ | 70 ± 22 | 69 ± 23 | 69 ± 23 |
| Minimum, maximum | 11, 118 | 16, 117 | 11, 118 |
| Asthma control (ACQ)∥ | 2.4 ± 1.1 | 2.4 ± 1.2 | 2.4 ± 1.2 |
| Minimum, maximum | 0.1, 5.8 | 0.0, 6.0 | 0.0, 6.0 |
| Asthma-related quality of life (AQLQ)¶ | 3.7 ± 1.4 | 3.6 ± 1.4 | 3.6 ± 1.4 |
| Minimum, maximum | 1, 6.7 | 1, 6.9 | 1, 6.9 |
| Co-morbidities | |||
| Hypertension | 87 (57.6%) | 88 (58.7%) | 175 (58.1%) |
| Diabetes | 50 (33.1%) | 46 (30.7%) | 96 (31.9%) |
| Body Mass Index (BMI) | 35.2 ± 9.6 | 35.4 ± 9.7 | 35.3 ± 9.6 |
| Ever smoked in the past | 80 (53.0%) | 87 (58.0%) | 167 (55.5%) |
| Current smoker | 38 (25.2%) | 46 (30.7%) | 84 (27.9%) |
| Depressive symptoms (CES-D) ** | 83 (55.0%) | 85 (56.7%) | 168 (55.8%) |
S-TOFHLA = Short Test of Functional Health Literacy in Adults39 is a test of reading comprehension. It can be administered in English and Spanish. The score is the number correct 0-26 and a score>23 is adequate reading comprehension
ANQ= Asthma Numeracy Questionnaire is a numeracy scale.42 It is a 4-item questionnaire of numerical concepts (arithmetic, percentage) with the score the number of items correct 0 - 4
eHEALS= eHealth Literacy Scale43 has 8 items graded on a 5-point Likert scale so the score ranges from 8 – 40 with higher score indicating higher electronic literacy.
Note: N=203. 98 patients did not have baseline spirometry because spirometer equipment was not available to the Community Health Worker at the start of the study. For that reason, FEV1 was not used in analysis.
ACQ= Asthma Control Questionnaire (ACQ) has 6 items each scored from 0-6 with lower score indicating better control.29-31
AQLQ = is 15 item Mini Asthma-related Quality of Life Questionnaire.32-34,44 It is scored on a 1 to 7 scale with higher scores indicating better asthma-related quality of life. The AQLQ is a useful indicator of asthma-related quality of life in low-income adults.45
For both ACQ and AQLQ a within-person change of at least 0.5 points is considered clinically meaningful.31,34
Center for Epidemiologic Studies Depression Scale (CES-D) has 20 items with 4-point Likert scale. The score ranges from 0 to 60 with scores >=16 suggestive of depressive symptoms or general distress.46
Table 2.
Baseline characteristics of patients’ homes, exposures to and perceptions of information technology in 301 adults with moderate to severe asthma, assigned randomly 1:1 to PT + HV or PT, expressed as frequency (percent) for discrete and mean (±SD) for continuous variables.
| Characteristic | PT N=150 |
PT + HV N=151 |
Total N=301 |
|---|---|---|---|
| Home | |||
| Type of Housing | |||
| Single family detached home | 6 (4.0%) | 3 (2.0%) | 9 (3.0%) |
| Row house | 104 (69.3%) | 103 (68.2%) | 207 (68.8%) |
| Apartment | 34 (22.7%) | 41 (27.2%) | 75 (24.9%) |
| Shelter | 1 (0.7%) | 0 (0%) | 1 (0.3%) |
| Other | 5 (3.3%) | 4 (2.6%) | 9 (3.0%) |
| Housing Status | |||
| Rent | 96 (64.0%) | 101 (66.9%) | 197 (65.4%) |
| Own | 46 (30.7%) | 45 (29.8%) | 91 (30.2%) |
| Other | 8 (5.3%) | 5 (3.3%) | 13 (4.3%) |
| Number of occupants | 3.1 ± 2.1 | 3.0 ± 2.8 | 3.1 ± 2.4 |
| minimum, maximum | 0, 11 | 1, 29 | 0, 29 |
| Number of bedrooms | 2.5 ± 0.9 | 2.7 ± 1.1 | 2.6 ± 1.0 |
| minimum, maximum | 1, 5 | 1, 9 | 1, 9 |
| Without housing in last 6 months | 14 (9%) | 7 (5%) | 21 (7%) |
| Without utilities in last 6 months | 29 (19%) | 20 (13%) | 49 (16%) |
| Information technology | |||
| Own a computer at home | 76 (50.7%) | 82 (54.3%) | 158 (52.5%) |
| Access to computer outside of home | 85 (56.7%) | 84 (56.0%) | 169 (56.1%) |
| Use a computer for internet | 94 (62.7%) | 92 (60.9%) | 186 (61.8%) |
| Active Email account | 103 (68.7%) | 102 (67.5%) | 205 (68.1%) |
| Smart phone | 104 (69.3%) | 101 (66.9%) | 205 (68.1%) |
| Heard of patient portal | 70 (46.7%) | 66 (43.7%) | 136 (45.2%) |
Data collection
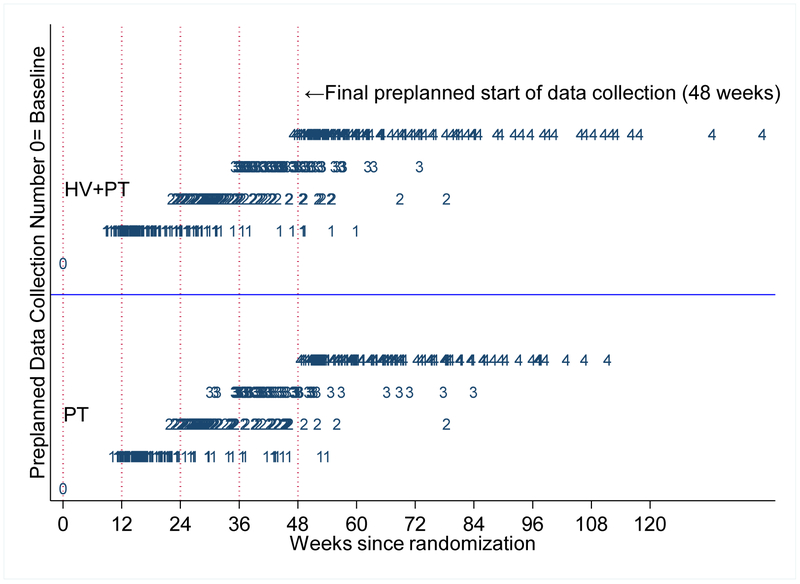
72% of patients had at least 4 of the 5 possible data collections. The percentage did not differ by treatment assignment. However, timing of data collection was irregular (See Figure 2A). Adverse events were mostly asthma-related (Table 3). Although there was irregularity in visit timing, few patients were terminated or lost to follow-up. Mean time to the final data collection ranged from 0 (no data collected after baseline) to 33 months with median time 13.2 months for the PT + HV and 12.9 months for the PT group.
Figure 2. Irregular home visits and data collection.
A. Time from randomization to data collection by treatment group. All patients had first data collection at time of randomization (week 0), and following collections were to begin at weeks 12, 24, 36 and 48, corresponding to 3, 6, 9 and 12 months (vertical dotted lines). Numerals 1, 2, 3 and 4 indicate the collection number and the timing, in weeks, of those collections. PT=portal training, PT+HV=home visit plus portal training.
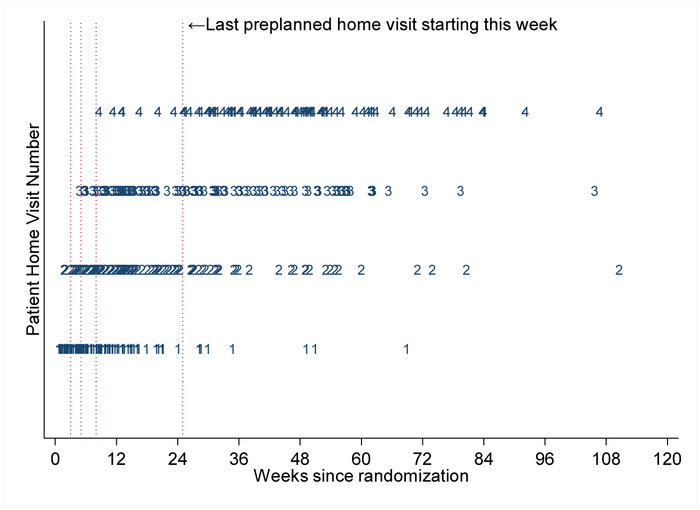
B. Time from randomization to home visits. Anticipated home visit times at start of study were at 3, 5, 8 and 25 weeks, with ranges of a few weeks before and after (vertical dotted lines). Numerals 1, 2, 3 and 4 indicate the visit number, and the weeks on the x-axis correspond to the actual visit times in weeks from randomization. A patient could have fewer than 4 home visits. If the last visit was also the second visit, then the patient would have only a "1" and a "2" in this figure; "3"' and "4" would be missing.
Table 3.
Number and percent of patients with hospitalizations(s), ED visits, and Urgent Office visits for any cause and for asthma during study participation.
| Treatment | ED Visits | Hospitalizations | Urgent visits | Total patients | |||
|---|---|---|---|---|---|---|---|
| No (%) pts (all cause) |
No (%) pts (asthma) |
No (%) pts (all cause) |
No (%) pts (asthma) |
No (%) pts (all cause) |
No (%) pts (asthma) |
||
| PT | 43 (29%) | 20 (13%) | 32 (21%) | 30 (20% | 18 (12%) | 17 (11%) | 150 |
| PT + HV | 37 (24%) | 26 (17%) | 24 (16%) | 21 (14%) | 14 (9%) | 11 (7%) | 151 |
Portal Use
Fifty-six percent of patients used the portal at least once outside of the times in which they received training; rates of use over time did not differ between randomization groups. 58% in the PT+HV and 55% in the PT group used the portal at least once.
Home visits
Community health workers (CHWs) made 501 home visits. Of the 151 participants assigned to home visits (PT + HV),140 (93%) had at least one visit. All four visits were completed by 103 (68%) patients (See Figure 2b, Appendix Table 6). Home visits occurred at irregular intervals owing to challenges of contacting patients (See Appendix).
Portal Training and Home Visit (PT + HV) versus Portal Training Only (PT) Groups
Eleven percent (16/151) of patients in the home visitor group (PT + HV) and 8% (12/150) in the portal only group (PT) could not be followed until the end of the study (Figure 1).
The five asthma outcomes at baseline and estimated at 12 months appear in Table 4. The mean asthma control at baseline (2.4) was worse than the threshold for adequate control (1.5). Quality of life was low compared to other populations (mean at baseline =3.6.; minimum 1, maximum 6.9). Patients reported frequent exacerbations, ED visits, and hospitalizations in the year before enrollment. Improvements over time all favored the HV intervention, but only for hospitalizations did improvement reach conventional levels of statistical significance.
Table 4.
Asthma outcomes at baseline and changes over time by treatment assignment in 301 adults with uncontrolled asthma. Intention to treat (as randomized) analysis.
| Characteristic | Baseline | Expected Value at 12 months* |
Change in expected values at 12 months† from baseline (95% Confidence Interval†) |
Difference between groups (95% CI†) |
|||
|---|---|---|---|---|---|---|---|
| PT+HV | PT | PT+HV | PT | PT + HV N=151 |
PT N=150 |
||
| Asthma Control | 2.42 (2.25,2.63) |
2.39 (2.19,2.58) |
1.96 (1.69,2.22) |
2.11 (1.86,2.38) |
−0.48 (−0.76, −0.22) |
−0.27 (−0.52, −0.01) |
−0.21 (−0.56, 0.15) |
| Asthma-related quality of life | 3.68 (3.43,3.91) |
3.64 (3.41,3.87) |
4.40 (4.04,4.76) |
4.05 (3.73,4.36) |
+0.72 (0.40, 1.08) |
+0.41 (0.10, 0.71) |
+0.31 (−0.13, 0.79) |
| Emergency room visits (rate per year)‡ | 3.64 (2.46,5.09) |
3.16 (2.43,4.17) |
0.35 (0.15,0.68) |
0.48 (0.24,0.82) |
−3.28 (−4.73, −2.10) |
−2.69 (−3.67, −1.88) |
−0.60 (−2.21, 0.97) |
| Hospitalizations (rate per year)‡ | 1.36 (1.00,1.72) |
1.17 (0.91,1.48) |
0.17 (0.04,0.37) |
0.50 (0.26,0.82) |
−1.19 (−1.56, −0.80) |
−0.66 (−1.02, −0.30) |
−0.53 (−1.08, −0.024) |
| Prednisone bursts (Rate per year)‡ | 0.42 (0.34,0.50) |
0.35 (0.27,0.43) |
0.21 (0.12,0.30) |
0.23 (0.15,0.38) |
−0.21 (−0.32, −0.09) |
−0.12 (−0.21, −0.02) |
−0.09 (−0.24, 0.06) |
Confidence intervals are based on percentile bounds from 999 bootstrap replications with resampling at the patient level stratified by intervention group. In a few instances the bootstrap replication could not produce an estimate. Hospitalization −3 replicates out of 999.
Outcomes were estimated as expected values at 12 months based on longitudinal analysis with time modeled with splines to ensure that comparisons between groups were as of the same time from baseline.
For emergency room visits, hospitalizations, and prednisone bursts, baselines were retrieved from patient recall from interview back for 12 months. From randomization date onward, these values were estimated from patient reported adverse events by actual over time. These two different measures of rates are therefore not strictly comparable over time within patient groups. They are, however, comparable for between group comparisons because all patients were evaluated in the same manner. The shaded areas in the table highlight the affected baseline measures.
The predicted number of patients who achieved asthma control (ACQ score ≤ 1.531) was 42 (27.8%) in PT + HV compared with 35 (23.3%) in PT) at 12 months compared to 0 months) was greater among the home-visit-assigned (PT + HV) patients (Appendix, Appendix Table 3). Our results also predicted that 37 (25%) persons assigned to the home visit group (PT + HV) had a clinically important degree of improvement (0.5 points of Asthma Control score), while none in the portal only group had such a predicted improvement (Appendix, Appendix Table 2).
Modeling to adjust for baseline covariates that could explain the limited degree of loss to follow-up uncovered no changes in estimates in asthma control. Investigations of potential subgroups of patients with greater or less potential for benefit from home visits proved to be negative (Appendix).
DISCUSSION
In this longitudinal two-arm randomized controlled trial of 301 low income, inner-city adults with uncontrolled asthma, we compared (1) the effect of introducing patients to, and training them in an electronic health-record-based patient portal to enhance patient-clinician communication, versus (2) an intervention that added to this portal training a series of regular home visits by specially trained CHWs. By design, a no-intervention condition was absent, the funding agency supports comparative effectiveness research, comparison of at least two interventions. While both groups improved in asthma outcomes, we could not estimate what would have happened with only usual care. Improvements in outcomes over time were observed for both intervention groups. While greater for the home visit group, these improvements reached conventional levels of statistical significance over the portal-only group only for yearly hospitalization rate (−0.53, 95% CI= −1.08 to −0.024). Thus, our longitudinal findings offer only limited evidence that a patient-portal intervention alone might improve asthma outcomes. Despite our expectation that the addition of home visits would improve outcomes over and above that shown for the active controls, we also found only limited evidence to support that hypothesis. These findings reflect the need for formal study designs, including randomization, to estimate the benefits of new patient care initiatives such as home visits or communication technologies, at least for patients suffering from chronic diseases.
Our need to rely on self-report and patient recall to elicit outcomes of hospitalization and emergency department visits led to potentially problematic estimates of changes in utilization within group over time for hospitalizations, emergency department visits and prednisone bursts. Nevertheless, our randomized design still permitted between-group estimates of differences in these outcomes because all patients used the same data collection questions, for reporting outcomes. Future designs should consider alternatives to patient recall to ascertain health care utilization outcomes.
Several factors could have contributed to the lack of large benefit over time. First, contacting patients for both home visits and data collection represented a formidable challenge. Poverty in some neighborhoods was extreme, with two of the 20 zipcodes having median household income below the first US percentile.40 The study required that CHWs drive and walk regularly in blighted, sometimes violent neighborhoods with abandoned homes just to maintain contact with patients, conduct home visits, and collect data. Yet, here is where asthma patients live and require disease management. Owing to challenges in making repeated contacts, for some patients we had to evaluate outcomes over several more months than the originally designed and more concentrated 12-month period. The irregularity of visits might have dampened the impact of home visits (Figure 2B). Patients were unlikely to have home computers and were most able to embrace information technology using cell phones and texting, but the patient portal does not permit texting.
Second, the study’s time horizon restricted the maximum number of home visits that were possible. Increasing the dose (number and intensity) of home visits might have revealed a critical level of contact necessary for patient improvements. Longer studies are advisable. They will require added resources to support the intensive tasks of maintaining ongoing patient contact over longer periods.
Third, we found during home visits that patients experienced many exposures that not only might interfere with their ability to communicate with their clinical team but also might undermine their ability to adhere to their disease management.41 CHWs found some participants living as tenants in poorly maintained structures, sometimes having poor ventilation, water leaks, and structural inadequacies. Many homes had strong odors of tobacco smoke. Rodents and cockroaches were common. Other stressors from financial hardships included poor heating, electricity shut-offs, and crowding. Under these living conditions, patients are constantly exposed to health risks that enhanced patient-clinician communication cannot resolve. Although Centers for Medicare & Medicaid Services champions patient portals as a means of patient-clinician communications, improvements in care will require not only patient access to the internet but also ability to act on the information that is received.
Fourth, many patients lacked social support.24 With limited numbers of visits, CHWs could not fill that gap. The study protocol did not include asthma education but only care coordination. Future home-visit-based interventions should add asthma self-management education about reducing environmental triggers including exposure to tobacco smoke.
Fifth, the EPIC MyChart patient portal is general, designed for all patients and conditions and currently only in English. Even with the support of a CHW at home, chronically ill asthma patients, who have comorbidities such as obesity and heart disease, might need more comprehensive communication tools that offer real-time contact with the clinical team. MyChart, as an example of current portal technology, requires patient initiation and regular contact but only through text or email. Although text messaging might support ongoing communication, even that limited communication modality might be a costly part of patients’ telecommunication plans, discouraging use of a patient portal app.
Our project does not consider other potentially necessary requirements for effective home visitor programs. We did not consider geographic diversity, for example; our cohort lived entirely in the inner-city. We do not know if home visits are practical in rural environments with long travel times. We did not attempt having CHWs provide visits by telemedicine, an approach that might be more feasible or even essential in rural settings. Our country is ethnically diverse and the resources of our study did not allow us to consider many other subpopulations with respect to portal use and home visits: other languages, other cultures, other exposures.
In conclusion, we found some, but only limited evidence that regular home visits added to typical forms of patient portal tools might help patients to improve their asthma control. Future research perhaps should combine an intervention targeting patients both at home and in the clinic that places clinicians more directly in communication with the home visitor. It might also be important to investigate effects among more affluent patient groups, for whom home resources and environments might enable patients to communicate with the clinical team more effectively from home. Because of the barriers from poverty and poor living environment, health care interventions, even those that venture beyond the clinic to the home and community, might be inadequate without additional commitments addressing social factors such as housing, and safer neighborhoods.
KEY MESSAGES.
Low-income adults with uncontrolled asthma, frequently have comorbidities and may benefit from communication-based interventions.
Although patients with training can use patient portals, access is often limited.
Asthma patients welcome home visits but short-term benefits are small.
Acknowledgments
FUNDING SOURCE: Research reported in this manuscript was funded through a Patient-Centered Outcomes Research Institute (PCORI) Award (AS 1307-05218) as a response to its funding announcement “Treatment Options for African Americans and Hispanic/Latinos with Uncontrolled Asthma.” Dr. Apter, Dr. Morales, Dr. Klusaritz; Ms. Perez and Ms. Han also have received support from National Heart Lung & Blood Institute/ NIH for R18 HL116285.
ABBREVIATIONS
- ACQ
Asthma Control Questionnaire
- ANQ
Asthma Numeracy Questionnaire
- AQLQ
Asthma-related Quality of Life Questionnaire
- CES-D
Center of Epidemiologic Studies Depression Scale
- CHW
Community health worker
- eHEALS
eHealth Literacy Scale
- EHR
Electronic health record
- HV
Home visits
- PCORI
Patient-Centered Outcomes Research Institute
- PT
Portal training
- PT + HV
Portal Training plus Home Visits
- PT1
Portal Training, Session 1
- PT2
Portal Training, Session 2
- REDCap
Research Electronic Data Capture
- S-TOFHLA
Short Test of Functional Health Literacy in Adults
Appendix
This appendix outlines additional details of methods and some ancillary results to enhance reproducibility.
Part 1: Data sources and project oversight
The IRB-approved consent and all communications were read/spoken in English or Spanish as preferred by the potential participant. Data collection was accomplished by a community health worker, called a Data Collector, who did not act as the home visitor if the patient was so assigned. This choice of Data Collector reduced any pressure on the patient to answer in a way to please the researcher CHW. Baseline questionnaires assessed socio-demographics, asthma severity, health literacy, and comorbidities (Table 1). The first and last data collection visits were in person. For the other data collection times, we offered a phone or email or in-person visit to minimize the burden of and effect on participation in a study. The Data Collector entered responses either directly into a secure, encrypted, web-based database REDCap (Research Electronic Data Capture) or on paper for transfer to REDCap later.1 Data Collectors used tablets (iPads) equipped with Wi-Fi but internet access was difficult at times and CHWs noted many patients were not comfortable with having their information entered onto an iPad.
To be able to reach patients for visits and appointment, we collected their contact information and that of three persons. For some of the patients, we also were able to read the electronic record to determine when patients had appointments and to meet them there. Additionally, we wrote letters, contacted by phone and text, visited homes, and left postcards.
There were no substantive changes to the original study protocol. Small changes helped patients to adhere to the protocol. For example, if it were easier for the patient, we went to their home for enrollment. For some patients the first portal training session and the second were given on the same day for those for whom a single training session was more convenient. An external Data Safety Monitoring Board reviewed progress and data every six months and monitored adverse events and serious adverse events (unexpected emergency department (ED) visits and hospitalizations). Because patients had moderate or severe asthma and often comorbidities, we expected ED visits and hospitalizations to occur but hypothesized the frequency of occurrence would change as a result to the interventions.
Part 2: Data management
REDCap allows data attribution and audit capabilities, integrity checks, real-time validation, data storage and backup, and export functions.1 Whenever any response of the patient required elaboration, the interviewer entered comments into this database. At weekly meetings, the community health workers, project manager, the staff who entered data into REDCap, the project statisticians, and the principal investigators discussed data collection problems. The principal investigator then reviewed all reported adverse events. These meetings then led to the development of project rules for interpreting any of the questions that presented difficulties for patients to answer. A set of 30 tables stored in the REDCap database contain data from interviews and phone assessments of patients over time (Appendix Table 1).
Appendix Table 1. Summary of data collection.
Quarterly data collection was to occur at months 3, 6, 9, and 12.
| Measure | At enrollment (Baseline) |
Quarterly | 12 months or final Visit |
|---|---|---|---|
| Outcomes | |||
| Asthma control* | X | X | X |
| Asthma-related quality of life† | X | X | X |
| ED visits for asthma or any cause | X | X | X |
| FEV1‡ | X | X | |
| Prednisone bursts§ | X | X | X |
| Hospitalizations for asthma or any cause | X | X | X |
| Mediators (intermediate end points) | |||
| Appointments kept with asthma doctor | X | X | X |
| Use of patient portal | X | X | X |
| Inhaler Adherence Scale∥ | X | X | X |
| Covariates for identifying subgroups | |||
| Socio-demographics | X | ||
| Computer at home | X | ||
| Convenience of internet access | X | ||
| Educational attainment | X | ||
| Health literacy: S-TOFHLA¶, ANQ**, eHEALS†† | X | ||
| Comorbidities | X | ||
| Depressive thoughts‡‡ | X | ||
| Primary language | X |
ED= emergency department
Asthma control, the primary outcome, was assessed with the Asthma Control Questionnaire (ACQ). The ACQ has 6 items each scored from 0-6 with lower score indicating better control.2-4
Asthma-related quality of life was measured with the 15-item Mini Asthma-related Quality of Life Questionnaire (AQLQ).5-8 Items are scored on a 1 to 7 scale with higher scores indicating better asthma-related quality of life. The overall AQLQ score is the mean of scores of the individual items. The AQLQ is a useful indicator of asthma-related quality of life in low-income adults.9
For both ACQ and AQLQ a within-person change of at least 0.5 points is considered clinically meaningful.4,5
FEV1 could be collected for only 203 patients owing to non-availability of spirometry equipment at the start of the study. For that reason, it was not used in analysis.
Prednisone bursts are defined as a new prescription or an increase in dose of prednisone.
The Inhaler Adherence Scale asks participant’s report of non-adherence.10,11 It is a 6-item scale with each item scored 1 (non-adherence) or 0 (adherence. The range is 0-6, with 0 being optimal adherence.
S-TOFHLA= Short Test of Functional Health Literacy in Adults,12
ANQ = Asthma Numeracy Questionnaire13
eHEALS= eHealth Literacy Scale14
Center for Epidemiologic Studies Depression Scale (CES-D) has 20 items with 4-point Likert scale. The score ranges from 0 to 60 with scores >=16 suggestive of depressive symptoms or general distress.15
Part 3: Analytical and statistical approaches—Statistical Analysis Plan
The primary analysis was “as randomized” (intent-to-treat), with the assumption that any dropout visits were missing completely at random (MCAR). The estimand of interest was the difference between the home-visitor versus portal-training-only groups in the change in outcomes over time. The within-group change in the portal-only group allowed measurement of portal alone, the active control. Subtracting any change over time in the portal plus home visit group from the portal group allowed estimates of the additional effect of home visits. Additional details appear below.
(A). Modeling approach
Flexible modeling of time
Irregular data collection times (see results) required models that used outcome time as a continuous measure. For that reason, analysis models implemented spline-based longitudinal models with marginal splines to estimate expected values of outcomes at 0 months and 12 months and outcome changes between 0 and 12 months. We used all actual data collection times; we did not artificially categorize data collection times to equate to the preplanned 0, 12, 24, 36, and 48 weeks from randomization. We did not plan nor implement “windows” of time. We did not consider any visit or data collection time to be within or outside of a window. The contrast (estimator) of interest was the difference between the treatment groups in the changes in expected values from 0 months to 12 months. Examples in the statistical literature support this approach.16 All confidence bounds were estimated using 999 bootstrap resamples (percentile-based). By using 999 samples, percentile confidence bounds are from the 25th and 975th order statistics without interpolation.
Model form
We used both marginal and mixed effects models, with the marginal model being the primary approach owing to convergence problems with generalized linear mixed effects models (noted below).17 By including splines for time and group-by-time interaction terms, these models could produce the expected values at 0 and 12 months and predicted values of interest. For the primary outcome of asthma control, the skewed distribution of outcomes necessitated that we use log gamma models. Log Poisson models were used for ED visits, hospitalizations, and prednisone bursts. Asthma related quality of life used a Gaussian model. The treating clinic was a stratifying factor in all models to account for the stratified randomization.
Alternative approaches for reporting asthma control
Differences between intervention groups in expected values at given follow up times, although statistically appropriate for comparing groups, lacks an immediate connection to the manner in which clinicians might want to interpret results. For that reason, and as a sensitivity analysis to the primary outcomes, we pre-planned two methods for translating findings into clinically useful metrics for the primary outcome – asthma control. The metrics were (1) the fraction of patients who achieved asthma control (a level below 1.5 on the Juniper asthma control scale, and (2) the fraction that achieved a minimally important improvement. For estimates of the improvements at the patient level on the asthma control (Juniper) scale, the minimally important difference is 0.5.4,18
We avoided dichotomizing data, an ad hoc approach, for these sets of estimates but instead used mixed effects linear models and predictions to report the change in the fraction of patients who achieved adequate asthma control as of 12 months. Once we estimated each patient’s predictions at baseline and at 12 months, we dichotomized these model-based predicted values to estimate the fraction of patients who achieved asthma control at baseline and at 12 months in each group and the number of predictions that improved by at least 0.5. This approach to reporting is not the primary analysis, however, but represents an alternative representation of intervention effectiveness.
For two reasons, we could not and should not use raw data to report individual asthma control. First, as we report in the main text, patients were measured at times that departed from the pre-specified start times and goals for data collection. Most of these departures reflected delays in obtaining interviews. Patients were difficult to locate and schedule, in part because of their many illnesses and their often poor living conditions and communication options. For that reason, actual 12-month measures of outcomes, e.g., asthma control, were routinely not observed. Rather, observed (measured) asthma control occurred at various times around 12 months from the date of randomization for the individual. Times of the final measurement in some cases differed substantially from 12 months. We therefore had to adopt a method of estimating measures of asthma control as of a common date – 12 months from randomization – to account for this variation in the timing of data collection. Second, as with many health measures based on patient responses to an interview instrument, asthma control is measured with error. The observed asthma control is but one estimate of the patien's asthma symptoms on a particular day out of several days that represent actual, steady-state symptoms. For both of these reasons, an observed measure will not accurately reflect the actual level of control.
Mixed effects models that include random effects to represent individual patient departures from average levels of outcomes, such as asthma control, have long been used to address these issues of measurement error. The resulting "predictions" of individual levels of outcomes are in theory better, less biased, estimates of individual-level outcomes that are the raw observations because they account for measurement error as well as variation across individual patients.19 In this application, we implemented longitudinal models with two different types of random effects. Random intercepts reflect the individual departure of outcomes (asthma control) at baseline, in our case at randomization (month 0). Random slopes reflect the departure of individual level trajectories from the average trajectory of change in outcomes over time. With these two types of random effects, we could predict each patien's level of outcome at two common times: the date of randomization and 12 months later. We used these predictions, and their differences, of asthma control to estimate the number of patients who were in control (score <1.5) and who had achieved a clinically important reduction in score (decrease of 0.5), which reflects an improvement in asthma control.
We used restricted maximum likelihood (REML) as implemented in the program "mixed" in Stata version 15.1 to fit a linear mixed effects model. Our attempts to fit generalized linear mixed models, such as a log gamma model with log link and gamma error, all failed to achieve convergence. Thus, this linear model did not fully reflect the skewed nature of the data but represented the best alternative.
In our application, defining and implementing random slopes became more complex because of our use of splines to estimate the changes over time in outcomes. The time line of observation was divided into segments, with each segment allowing us to have a different slope (or trajectory) of the outcome over time. We began with a model that had the same set of 5 line segments for time as did the primary model. We then added all of the baseline covariates (as in the primary analysis) in order to explain as much as possible the individual patient-level variation in outcome. To this model, we added a random intercept for each patient and a single random slope to reflect overall departure of the individual over time from the average trajectory not explained by these observed covariates. The model converged. We then added a second random slope to represent an additional element of individual patient departure from average as the follow-up time progressed. This model converged in 10 iterations. Models with additional random slopes to reflect the additional time segments of the spline model would not converge. These presentations are meant only to translate our overall results (reported in the main text) into terms that might be clinically more meaningful.
These predictions we feel are sensitive to the form of the mixed effects models and might be unstable because of the need to fit more than one random effect for slope/trajectory for an analysis based on relatively few patients. To obtain these potentially important clinical estimates in future studies, investigators should consider (a) recruiting more patients, (b) measuring patients more frequently to obtain more stable individual estimates, and (c) making every attempt to measure each patient at the same time interval throughout the study. This last goal might be unrealistic for some patient populations and will likely require analytic approaches that reflect reality.
Appendix Table 2. Improvement over 12 months of individual-level asthma control by intervention group. Number and percentage of patients who improved by at least 0.5 in asthma control.
| Improved by at least 0.5 points |
Portal Only n(%) |
Portal + Home Visitor n(%) |
Total n(%) |
|---|---|---|---|
| No | 150 (100%) | 114 (75.5%) | 264 (88%) |
| Yes | 0 (0%) | 37 (24.5%) | 37 (12.3%) |
| 150 | 151 |
Appendix Table 3. Control level of 1.5 or better at baseline and 12 months by intervention group.
| Controlled Asthma |
Portal Only n(%) |
Portal + Home Visitor n(%) |
Total n(%) |
|
|---|---|---|---|---|
| Baseline | No | 130 | 136 | 266 |
| Yes | 20 (13.3%) | 15 (9.9%) | 35 (11.6%) | |
| At 12 months | No | 115 | 109 | 224 |
| Yes | 35 (23.3%) | 42 (27.8%) | 77 (25.6%) | |
| ALL | 150 | 151 |
(B). Heterogeneity of treatment effect (effect modification)—latent class modeling to identify subgroups.
Effect modifiers are baseline variables or groups of factors, gleaned from the literature, hypothesized to affect the intervention-outcomes relationships.20,21 Recent literature on heterogeneity of treatment effect suggests that one-by-one testing or estimation of candidate effect modifiers leads to underpowered contrasts, excessive reliance on p-value testing, inadequate pre-specification of candidates and their rationale, and no attention to multiple comparisons.22 To address these criticisms, we grouped candidate measures into several clearly pre-specified themes from which profiles or latent classes are derived. We implemented a latent class analysis for each group of candidate measures to distinguish profiles of common responses to the set of variables.22,23 Then the degree of effect modification of the association of the intervention (home visitors) and the key outcome (asthma control) was estimated for each candidate modifier group by testing interactions between the time*by*intervention contrast and the modifier For those candidate effect modifiers that approached conventional levels of statistical significance, we estimated the intervention effect and 95% confidence intervals for each level of the effect modifier. Our working hypothesis was that patients with comorbidities and with limited computer literacy might (a) not benefit from portal education at all, and (b) might have additional benefit with the home visitor intervention. Candidate measures appear in Appendix Table 4.
Appendix Table 4. Relevant candidate patient subgroups, effect modifiers, for targeting the initiative and their data elements.
| Relevant subgroup | Data Elements |
|---|---|
| (1) Primary language* | Primary language is English, Spanish |
| (2) Primary care vs specialty practices | Internal medicine or family medicine versus allergy-immunology or pulmonary |
| (3) Age | 18-39 years, 40-49 years, ≥ 50 years |
| (4) Skills that would support use of portal and asthma self-management | Numeracy† Literacy-reading comprehension† Education Computer literacy (Electronic Health Literacy Scale† Inhaler technique‡ |
| (5) Social Community barriers | Food or clothing inadequacy MOS Social Support§ Exposure to Violence∥ |
| (6) Trust of patient portal | Patient portal preserves privacy¶ |
| (7) Depression and chronic disease load | Depression** Diabetes Hypertension High cholesterol Obesity Cancer Current Smoker |
| (8) Asthma severity | Hospitalizations ED visits Intubation Years taking ICS Prednisone (Days/week) |
| (9) Home environment | Crowding at home (number of rooms, number of people at home) Been without housing in last 6 months Utilities shut off in the last 6 months Times moved in last 12 months Exposure to second hand smoke |
Primary language was self-reported: English, Spanish.
Three literacy measures were used: Asthma Numeracy Questionnaire for numeracy,13 the Short Test of Functional Health Literacy in Adults12 for reading comprehension, and an item from the Electronic Health Literacy Scale: “I feel confident in using information from the internet to make health decisions.”14
Inhaler technique consisted of items from the Expert Panel Report24 and manufacturers’ instructions that were common to the type of inhaler, metered dose or dry powder inhaler. Inhaler technique was measured using a 7-point scale for a metered dose inhaler and a 6-point scale for a dry powder inhaler, testing the patient using the inhaler used for the patient’s inhaled steroid.
Social Support was measured with MOS Social Support Study.25
Privacy concerns with the patient portal assessed using the Portal Use Baseline Survey (“I am concerned about looking up my personal health information on the internet” with a Likert 5-point response.)
Depression as measured by the Center for Epidemiologic Studies Depression Scale, a validated 20-item scale.15
Estimating latent classes – for missing covariate data and for effect modification
The self-reported candidate measures were, a priori, grouped into nine categories (Appendix Table 4). Latent class analysis was implemented for each group of candidate measures, which will distinguish profiles of common responses to the set of variables.23 Latent class regression or finite mixture modeling is concerned with deriving information about a categorical latent variable from observed multivariate response patterns. The method takes advantage of the full data likelihood; therefore, subjects with missing elements contribute to the classification model unless all elements are missing. Full information maximum likelihood, the underlying algorithm, functions with missing data with comparable effectiveness as multiple imputation. The amount of missing data in baseline covariates was small, except for self-reported income. Thus, this approach for identifying latent classes also served to adjust for missing data with missing at random assumptions. Latent classes thus served several functions in our analysis: as a basis for defining effect modifiers and as a method of dealing with the small amount of missing covariate data.
Data analysis was performed using Mplus version 7.4, which uses an efficient estimation-maximization algorithm for maximum likelihood estimation.27 The number of latent classes was determined through examination of fit indices and in relation to clinical interpretation of results. Specifically, the Lo-Mendell-Rubin Adjusted Likelihood Ratio Test of model28 offered a formal comparison of a model with its reduced form. Model estimation yields predicted probabilities of class membership for each individual, which was used to assign an individual to a class or group associated with the highest predicted probability of class membership. The indicator representing the class membership became the effect modifier for further investigation. Then, the degree of effect modification of the association of the intervention (home visitors) and the key outcome (asthma control) was estimated for each candidate modifier group by testing interactions between the group indicator and the randomization indicator, and time. These preliminary analyses were based on Wald tests for 3-way interactions of time (fit with marginal splines), intervention (home visitor versus patient portal only), and the candidate effect modifier. Further investigations used separate regressions (of intervention*time interactions) for each level of the potential effect modifier, while controlling for the baseline covariates based on latent classes of factors (see description of latent classes). We used a common p-value for testing interactions of p<0.1. Our working hypothesis was that patients with comorbidities and with limited computer literacy could face additional challenges in the use of the patient portal. They also might need a more intensive (in frequency and in home education) home visitor program than the 4-visit program our intervention offered.
Age, primary language, type of clinical site, and trust of the medical system each consisted of one variable and latent class analysis was not applied. The model for home environment did not produce distinct groups (i.e., the one-class model yielded the best model fit) and individual variables were selected to represent the theme (crowding at home and exposure to second hand smoke). The model for skills that support use of the portal and asthma self-management yielded 4 groups which were labeled: moderate education level with high literacy; high education, literacy, and inhaler technique; low education and literacy with high inhaler technique; moderate education level with moderate literacy. The model for social/community barriers yielded 4 groups which were reduced to 3 groups for parsimony and due to small cell sizes. The groups were labeled: high barriers with low social support; low barriers with high social support; and low barriers with moderate social support. The model for depression and chronic disease load yielded 3 groups which were labeled: healthy; depressed; and chronic comorbidity. Two groups were identified for baseline asthma severity (least severe and most severe).
With these latent classes defined, we were able to represent all relevant baseline covariates more efficiently than if we were to model outcome as a function of treatment assignment (home visitor versus portal only) and each baseline factor. Therefore, in models that required adjustment for baseline factors (apart from modeling effect modification), we used the set of latent classes described in this Appendix. This approach allowed us to use all observations, because the latent classes accounted for the small number of missing values. The initial analysis suggested that, of the nine candidate subgroups, Spanish as the primary language and trust in the internet and the patient portal for clinical information were possible effect modifiers, but these subgroups did not prove to have different benefits for the portal plus home visit intervention.
Part 4: Sensitivity analyses
(A). Sensitivity analysis #1. Selection bias from loss to follow-up – covariate adjustment
In keeping with current recommendations,29 we included in our longitudinal analysis model (described previously), in addition to the randomized treatment assignment and the pre-planned stratification variable (clinical site), the covariates that might be related to dropout and the primary outcome. Missing covariate data were infrequent, but some patients were missing an element of a questionnaire, or responded “did not know”. Details on how we used latent class models to resolve the small amount of data missing on baseline factor appear in Appendix Part 3 (B). We used marginal models adjusted for baseline covariates as combined using latent class models. The estimates are qualitatively similar to those in the main analysis, with somewhat larger estimates of the degree of improvement over time in the home visitor group and somewhat smaller estimates of improvement in the portal-only group. Differences between groups in changes over time (−0.26) remained not significant at conventional levels.
Appendix Table 5. Asthma control outcomes at baseline, expected values at 12 months, and changes over time by treatment assignment in 301 adults with uncontrolled asthma.
| Treatment Group |
baseline | Expected Value at 12 months | Difference from baseline to 12 months |
|---|---|---|---|
| Portal + Home Visit | 2.45 | 1.94 | −0.51(−0.79,−0.24) |
| Portal | 2.39 | 2.14 | −0.25(−0.51,+0.02) |
| Difference | −0.26(−0.66, +0.10) |
Note: Intention to treat (as randomized) analysis with adjustment for baseline covariates as sensitivity analysis for dropout.
(B). Sensitivity analysis #2. Irregular visit times
The sensitivity analyses we have described (#1) make assumptions that the dropout and visit times are not related to the values of outcomes, i.e., that dropout is at random and that visit times are ignorable. Our dropout was limited, but irregular interview times were common. The study protocol assumed that data collection would occur at baseline and then at months 3, 6, 9, and 12 (Figure 2A). The challenges of contacting patients, making appointments, and then interviewing the patients meant that the interviewers needed to phone repeatedly, leave messages, contact relatives, and in some cases drive by the patient’s home to locate the patient. This process could take days, weeks, and sometimes months with this difficult-to-reach population. Across the 301 patients, there were 1232 data collection visits: 625 in the portal group and 607 in the portal plus home visit. Mean time to the final data collection ranged from 0 months (no data collection after baseline) to 33 months. The median time to the final data collection interview did not differ between treatment groups: 13.2 months for the home visitor (PT+HV) group and 12.9 months for the control (portal only) patients. Mean times to the last data collection differed by only 0.12 months. Twenty-five percent of patients had total follow-up times in excess of 16 months, and 10% of patients required more than 20 months of follow-up, compared to the predefined goal of having all data collections completed at 12 months. These findings reflect the months of effort needed to find and interview a substantial portion of the patients in our sample. The rate, or intensity of the data collection visits, did not differ between the two groups (incidence rate ratio= 0.95, 95% CI= 0.87 to 1.03) (Figure 2A).
Typically, reports of longitudinal studies use time as a categorical factor. When all patients are measured at the same time, this analytic approach is entirely appropriate. As with many studies, and in particular as with studies that involve mostly disadvantaged patients who also have underlying comorbidities that limit their mobility, contact and meeting availability with research personnel for the preplanned data collection times, this difficulty presents challenges. The preplanned times were 0, 12, 24, 36, and 48 weeks from randomization. Because patients could not be contacted and interviewed at these times due to the previously mentioned challenges, measurement times differed from, but mostly followed, the preplanned times (Figures 2a and 2b, Appendix Table 6). With irregular data collection times, we therefore used the following method of analysis. (1) For analysis, we used all data whenever obtained. (2) Unlike many studies, we modeled time as a continuous measure rather than as a categorical factor. (3) With continuous time, we allowed for non-linear trajectories by use of splines. This approach we implemented in standard statistical software packages. (4) As noted previously, we estimated expected values, and individual predicted values, as of baseline at the pre-planned 12-month time. The contrast of interest in estimating the effects of the intervention versus control becomes a difference between groups of the within-group differences over time from 0 months to 12 months. This approach used all available data, distinguished, for example, between a final measurement at 11 months versus another at 14 months, and permitted standard methods of covariate adjustment for covariate imbalance arising out of the loss to follow up at any time during the study. Alternative, ad hoc approaches can induce bias. Moving actual times to pre-planned time induces measurement error in time. Approaches that use a patient’s final measurement, even if it occurs before the pre-specified end date, as the final measurement is simply another version of last observation carried forward (LOCF) approaches, which have been thoroughly discredited in numerous analyses, reports, and editorials. Examples in the statistical literature support this suggested approach.16
Appendix Table 6. Anticipated and Actual times of home visits in weeks after randomization among the 151patients assigned to the treatment intervention and home visits.*.
| Visit Number | Number of Patients |
Anticipated Timing (weeks) |
Median Time (weeks) |
Minimum | Maximum |
|---|---|---|---|---|---|
| 1 | 140 | 2-4 | 5.1 | 0.6 | 69 |
| 2 | 134 | 4-6 | 13.1 | 1.7 | 110 |
| 3 | 124 | 6-11 | 25.4 | 4.7 | 106 |
| 4 | 103 | 23-27 | 42.1 | 8.6 | 107 |
140 patients at least 1 home visit, 134 had at least 2 visits, 124 had at least 3 visits, and 103 had all 4 home visits.
Appendix Table 7. Frequency of data collections over time by intervention group.
| Number of Data collections |
Portal only N(col%) |
Portal + Home Visits N(col%) |
Total |
|---|---|---|---|
| 1 | 4(3%) | 10(6%) | 14(5%) |
| 2 | 9(6%) | 8(5%) | 17 (6%) |
| 3 | 24(16%) | 29(19%) | 53(18%) |
| 4 | 34(23%) | 26(17%) | 60(20%) |
| 5 | 79(53%) | 78(52%) | 157(52%) |
| Total | 150 | 151 | 301 |
Note: 72% of patients had at least 4 of the 5 specified data collections. P-value for association assuming ordered categories p=0.48.
Programs and software
Analyses were performed in SAS v9.4 (SAS Institute Cary NC), Stata v 15.0 and 15.1 (College Station Texas), and M Plus v7.0 (Los Angeles CA).
REFERENCES
- 1.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–7. [DOI] [PubMed] [Google Scholar]
- 3.Juniper EF, O'Byrne PM, Ferrie PJ, King DR, Roberts JN. Measuring asthma control. Clinic questionnaire or daily diary? Am J Respir Crit Care Med 2000;162:1330–4. [DOI] [PubMed] [Google Scholar]
- 4.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006;100:616–21. [DOI] [PubMed] [Google Scholar]
- 5.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81–7. [DOI] [PubMed] [Google Scholar]
- 6.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32–8. [DOI] [PubMed] [Google Scholar]
- 7.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115:1265–70. [DOI] [PubMed] [Google Scholar]
- 8.Apter AJ, Garcia LA, Boyd RC, Wang X, Bogen DK, Ten Have T. Exposure to community violence is associated with asthma hospitalizations and emergency department visits. J Allergy Clin Immunol 2010;126:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leidy KN, Chan KS, Coughlin C. Is the asthma quality of life questionnaire a useful measure for low-income asthmatics? Am J Respir Crit Care Med 1998;158:1082–90. [DOI] [PubMed] [Google Scholar]
- 10.Morisky DE, Green LW, Levine DM. Concurrent and predictive validity of a self-reported measure of medication adherence. Med Care 1986;24:67–74. [DOI] [PubMed] [Google Scholar]
- 11.Dolce JJ, Crisp C, Manzella B, Richards JM, Hardin JM, Bailey WC. Medication adherence patterns in chronic obstructive pulmonary disease. Chest 1991;99:837–41. [DOI] [PubMed] [Google Scholar]
- 12.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999;38:33–42. [DOI] [PubMed] [Google Scholar]
- 13.Apter AJ, Cheng J, Small D, Bennet IM, Albert C, Fein DG, et al. Asthma Numeracy Skill and Health Literacy. J Asthma 2006;43:705–10. [DOI] [PubMed] [Google Scholar]
- 14.Norman CD, Skinner HA. eHEALS: The eHealth Literacy Scale. J Med Internet Res 2006;8:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radloff LS. The CES-D Scale: a self-report depresion scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]
- 16.Howe LD, Tilling K, Matijasevich A, Petherick ES, Santos AC, Fairley L, et al. Linear spline multilevel models for summarising childhood growth trajectories: A guide to their application using examples from five birth cohorts. Stat Methods Med Res 2016;25:1854–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzmaurice GM, Laird NM, J.H. W Applied Longitudinal Analysis, 2nd Edition. Hoboken, NJ: John Wiley & Sons; 2011. [Google Scholar]
- 18.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553–8. [DOI] [PubMed] [Google Scholar]
- 19.Efron B, Morris C. Data analysis using Stein's estimator and its geralizations. J American Statistical Association 1975;70:311–9. [Google Scholar]
- 20.Holmbeck GN. Toward terminological, conceptual, and statistical clarity in the study of mediators and moderators: examples from the child-clinical and pediatric psychology literatures. J Consulting and Clinical Psychology 1997;65:599–610. [DOI] [PubMed] [Google Scholar]
- 21.VanderWeele TJ. Explanation in Causal Inference: Methods for Mediation and Interaction. Oxford: Oxford University Press; 2015. [Google Scholar]
- 22.Alosh M, Huque MF, Bretz F, D'Agostino RB Sr., Tutorial on statistical considerations on subgroup analysis in confirmatory clinical trials. Stat Med 2017;36:1334–60. [DOI] [PubMed] [Google Scholar]
- 23.McCutcheon AL. Latent Class Analysis. Beverly Hill, CA: Sage University Press Series on Quantiative Applications in the Social Sciences; 1987. [Google Scholar]
- 24.Expert Panel Report 3: Guidelines for the diagnosis and management of asthma Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; 2007. [Google Scholar]
- 25.Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. [DOI] [PubMed] [Google Scholar]
- 26.Wright RJ, Mitchell H, Visness CM, Cohen S, Stout J, Evans R, et al. Community violence and asthma morbidity: the inner-city asthma study. Am J Public Health 2004;94:625–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm with discussion. J R Stat Soc B 1977;39:1–38. [Google Scholar]
- 28.Lo Y, Mendell NR, Rubin DB. Testing the number of components in a normal mixture. Biometrika 2001;88:767–78. [Google Scholar]
- 29.Council NR. The Prevention and Treatment of Missing Data in Clinical Trials Panel on Handling Missing Data in Clinical Trials. Committee on National Statistics, Division of Behavioral and Social Sciences and Education. Washington, DC: The National Academies Press; 2010:106. [Google Scholar]
Footnotes
Potential Conflicts of Interest: Dr. Apter is a consultant for UpToDate and an associate editor of J Allergy Clin Immunol. K. Morales owns stock in Altria Group, Inc. British American Tobacco PLC, and Phillip Morris International Inc. The other authors have nothing to report.
REGISTRATION: The study is registered at ClinicalTrials.gov: .
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Asthma and African Americans. 2017. (Accessed 12/27/2017, at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=15.)
- 2.Office of Minority Health. Asthma and Hispanic Americans. Department of Health and Human Services, 2012. (Accessed January 24, 2014, 2013, at http://minorityhealth.hhs.gov/templates/browse.aspx?lvl=3&lvlid=532; http://minorityhealth.hhs.gov/templates/content.aspx?lvl=3&lvlID=532&ID=6173.) [Google Scholar]
- 3.Akinbami LJ, Moorman JE, Bailey C, Zahran HS, King ME, Johnson CA, et al. Trends in asthma prevalence, health care use, and mortality in the United States, 2001–2010. NCHS Data Brief 2012:1–8. [PubMed] [Google Scholar]
- 4.Asthma and Hispanic Americans. Office of Minority Health, 2017. (Accessed 12/27/2017, at https://minorityhealth.hhs.gov/omh/browse.aspx?lvl=4&lvlid=60.) [Google Scholar]
- 5.Smedley BD, Stith AY, Nelson AR. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. Washington, D.C.: Institute of Medicine. The National Academies Press; 2003. [PubMed] [Google Scholar]
- 6.Seidman J, Wolf MS. The role of health literacy in health information technology Innovations in Health Literacy Research. Washington, DC: The National Academies Press; 2011:23–32. [Google Scholar]
- 7.Goel MS, Brown TL, Williams A, Hasnain-Wynia R, Thompson JA, Baker DW. Disparities in enrollment and use of an electronic patient portal. J Gen Intern Med 2011;26:1112–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sarkar U, Karter AJ, Liu JY, Adler NE, Nguyen R, Lopez A, et al. The literacy divide: health literacy and the use of an internet-based patient portal in an integrated health system-results from the diabetes study of northern California (DISTANCE). J Health Commun 2010;15 Suppl 2:183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goel MS, Brown TL, Williams A, Cooper AJ, Hasnain-Wynia R, Baker DW. Patient reported barriers to enrolling in a patient portal. J Am Med Inform Assoc 2011;18 Suppl 1:i8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lyles CR, Fruchterman J, Youdelman M, Schillinger D. Legal, Practical, and Ethical Considerations for Making Online Patient Portals Accessible for All. Am J Public Health 2017:e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldzweig CL, Orshansky G, Paige NM, Towfigh AA, Haggstrom DA, Miake-Lye I, et al. Electronic patient portals: evidence on health outcomes, satisfaction, efficiency, and attitudes: a systematic review. Ann Intern Med 2013;159:677–87. [DOI] [PubMed] [Google Scholar]
- 12.Tieu L, Schillinger D, Sarkar U, et al. Online patient websites for electronic health record access among vulnerable populations: portals to nowhere? J Am Med Inform Assoc 2017;24:e47–e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah M, Kaselitz E, Heisler M. The role of community health workers in diabetes: update on current literature. Curr Diab Rep 2013;13:163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh P, Chokshi DA. Community health workers--a local solution to a global problem. N Engl J Med 2013;369:894–6. [DOI] [PubMed] [Google Scholar]
- 15.Postma J, Karr C, Kieckhefer G. Community health workers and environmental interventions for children with asthma: a systematic review. J Asthma 2009;46:564–76. [DOI] [PubMed] [Google Scholar]
- 16.Krieger J, Song L, Philby M. Community health worker home visits for adults with uncontrolled asthma: the HomeBASE Trial randomized clinical trial. JAMA Intern Med 2015;175:109–17. [DOI] [PubMed] [Google Scholar]
- 17.Bryant-Stephens T, Kurian C, Guo R, Zhao H. Impact of a household environmental intervention delivered by lay health workers on asthma symptom control in urban, disadvantaged children with asthma. Am J Public Health 2009;99 Suppl 3:S657–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrasquillo O, Lebron C, Alonzo Y, Li H, Chang A, Kenya S. Effect of a Community Health Worker Intervention Among Latinos With Poorly Controlled Type 2 Diabetes: The Miami Healthy Heart Initiative Randomized Clinical Trial. JAMA Intern Med 2017;177:948–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kangovi S, Mitra N, Norton L,Harte R, Zhao X, Carter T, et al. Effect of Community Health Worker Support on Clinical Outcomes of Low-Income Patients Across Primary Care Facilities: A Randomized Clinical Trial. JAMA Intern Med 2018;178:1635–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nelson K, Taylor L, Silverman J, Kiefer M, Hebert P, Lessler D, et al. Randomized Controlled Trial of a Community Health Worker Self-Management Support Intervention Among Low-Income Adults With Diabetes, Seattle, Washington, 2010–2014. Prev Chronic Dis 2017;14:E15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Apter AJ, Bryant-Stephens T, Morales KH, Wan F, Hardy S, Reed-Wells S, et al. Using IT to improve access, communication, and asthma in African American and Hispanic/Latino Adults: Rationale, design, and methods of a randomized controlled trial. Contemp Clin Trials 2015;44:119–28. [DOI] [PubMed] [Google Scholar]
- 22.Black H, Gonzalez R, Priolo C, Schapira MM, Sonnad SS, Hanson CW 3rd, et al. True "meaningful use": technology meets both patient and provider needs. The American journal of managed care 2015;21:e329–37. [PubMed] [Google Scholar]
- 23.EPIC. (Accessed 8/18/18, 2018, at https://www.epic.com/software#PatientEngagement.)
- 24.Black HL, Priolo C, Akinyemi D, Gonzalez R, Jackson DS, Garcia L, et al. Clearing clinical barriers: enhancing social support using a patient navigator for asthma care. J Asthma 2010;47:913–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Apter AJ, Wan F, Reisine S, Bogen D, Rand C, Bender B, et al. Feasibility, acceptability and preliminary effectiveness of patient advocates for improving asthma outcomes in adults. J Asthma 2013;50:850–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krieger JW, Philby ML, Brooks MZ. Better home visits for asthma lessons learned from the Seattle-King County Asthma Program. Am J Prev Med 2011. ;41 :S48–51. [DOI] [PubMed] [Google Scholar]
- 27.Busse WW, Morgan WJ, Taggart V, Togias A. Asthma outcomes workshop: overview. J Allergy Clin Immunol 2012;129:S1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Juniper EF, O'Byrne PM, Roberts JN. Measuring asthma control in group studies: do we need airway caliber and rescue beta2-agonist use? Respir Med 2001;95:319–23. [DOI] [PubMed] [Google Scholar]
- 29.Juniper EF, O'Byrne PM, Ferrie PJ, King DR, Roberts JN. Measuring asthma control. Clinic questionnaire or daily diary? Am J Respir Crit Care Med 2000;162:1330–4. [DOI] [PubMed] [Google Scholar]
- 30.Juniper EF, O'Byrne PM, Guyatt GH, Ferrie PJ, King DR. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999;14:902–7. [DOI] [PubMed] [Google Scholar]
- 31.Juniper EF, Bousquet J, Abetz L, Bateman ED. Identifying 'well-controlled' and 'not well-controlled' asthma using the Asthma Control Questionnaire. Respir Med 2006;100:616–21. [DOI] [PubMed] [Google Scholar]
- 32.Juniper EF, Guyatt GH, Cox FM, Ferrie PJ, King DR. Development and validation of the Mini Asthma Quality of Life Questionnaire. Eur Respir J 1999;14:32–8. [DOI] [PubMed] [Google Scholar]
- 33.Juniper EF, Buist AS, Cox FM, Ferrie PJ, King DR. Validation of a standardized version of the Asthma Quality of Life Questionnaire. Chest 1999;115:1265–70. [DOI] [PubMed] [Google Scholar]
- 34.Juniper EF, Guyatt GH, Willan A, Griffith LE. Determining a minimal important change in a disease-specific Quality of Life Questionnaire. J Clin Epidemiol 1994;47:81–7. [DOI] [PubMed] [Google Scholar]
- 35.Zvarova J New approaches to health promotion and informatics education using Internet in the Czech Republic. Rocz Akad Med Bialymst 2005;50:138–41. [PubMed] [Google Scholar]
- 36.Standarization of Spirometry, 1994 Update. Am J Respir Crit Care Med 1995;152:1107–36. [DOI] [PubMed] [Google Scholar]
- 37.Research Electronic Data Capture. 2015. (Accessed 3/26/2015, at http://project-redcap.org/.)
- 38.Juniper EF, Svensson K, Mork AC, Stahl E. Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respir Med 2005;99:553–8. [DOI] [PubMed] [Google Scholar]
- 39.Baker DW, Williams MV, Parker RM, Gazmararian JA, Nurss J. Development of a brief test to measure functional health literacy. Patient Educ Couns 1999;38:33–42. [DOI] [PubMed] [Google Scholar]
- 40.Zip Code Characteristics: Mean and Median Household Income. https://www.psc.isr.umich.edu/dis/census/Features/tract2zip/. Last accessed 09/07/2018 . In: University of Michigan IfSR, Population Studies Center, ed.2018. [Google Scholar]
- 41.Bryant-Stephens T, Reed-Wells S, Canales M, Perez L, Rogers M, Localio AR, et al. Home visits are needed to address asthma health disparities in adults. J Allergy Clin Immunol 2016;138:1526–30. [DOI] [PubMed] [Google Scholar]
- 42.Apter AJ, Cheng J, Small D, Bennett IM, Albert C, Fein DG, et al. Asthma Numeracy Skill and Health Literacy. J Asthma 2006;43:705–10. [DOI] [PubMed] [Google Scholar]
- 43.Norman CD, Skinner HA. eHEALS: The eHealth Literacy Scale. J Med Internet Res 2006;8:e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Apter AJ, Garcia LA, Boyd RC, Wang X, Bogen DK, Ten Have T. Exposure to community violence is associated with asthma hospitalizations and emergency department visits. J Allergy Clin Immunol 2010;126:552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leidy KN, Chan KS, Coughlin C. Is the asthma quality of life questionnaire a useful measure for low-income asthmatics? Am J Respir Crit Care Med 1998; 158:1082–90. [DOI] [PubMed] [Google Scholar]
- 46.Radloff LS. The CES-D Scale: A self-report depression scale for research in the general population. Applied Psychological Measurement 1977;1:385–401. [Google Scholar]