Abstract
Nicotine-craving progressively increases, or incubates, over abstinence following extended access self-administration. While not yet examined for nicotine, the incubation of cocaine-seeking is accompanied by changes in synaptic plasticity in the nucleus accumbens. Here, we determined whether such changes also accompany enhanced nicotine-seeking following extended access self-administration and abstinence, and whether exercise, a potential intervention for nicotine addiction, may exert its efficacy by normalizing these changes. Given that in humans, tobacco/nicotine use begins during adolescence, we used an adolescent-onset model. Nicotine-seeking was assessed in male rats following extended access nicotine or saline self-administration (23-hr/day, 10 days) and 10 days of abstinence, conditions known to induce the incubation of nicotine-seeking, using a within-session extinction/cue-induced reinstatement procedure. A subset of rats had 2-hr/day access to a running wheel during abstinence. Ultrastructural alterations of synapses in the nucleus accumbens core and shell were examined using electron microscopy. Nicotine-seeking was elevated following extended access self-administration and abstinence (in sedentary group), and levels of seeking were associated with an increase in the density of asymmetric (excitatory) and symmetric (inhibitory) synapses onto dendrites in the core, as well as longer asymmetric synapses onto spines, a marker of synaptic potentiation, in both the core and shell. Exercise normalized each of these changes; however, in the shell, exercise and nicotine similarly increased synapse length. Together, these findings indicate an association between nicotine-seeking and synaptic plasticity in the nucleus accumbens, particularly the core, and indicate that the efficacy of exercise to reduce nicotine-seeking may be mediated by reversing these adaptations.
Keywords: Electron Microscopy, Incubation Effect, Relapse, Self-Administration, Wheel-Running
Graphical Abstract
Nicotine-seeking was enhanced (in sedentary controls) following extended access self-administration and abstinence and levels of seeking were associated with an increased density of asymmetric and symmetric synapses onto dendrites in the core and longer asymmetric synapses onto spines in both the core and shell. Exercise normalized each of these changes, indicating that the efficacy of exercise to reduce nicotine-seeking may be mediated by reversing these adaptations.
Introduction
An important goal of addiction research is to reduce or eliminate drug craving during abstinence. Drug craving precipitates relapse, and results from both humans and animal models show that drug craving/seeking increases over a period of abstinence, a phenomenon termed the “incubation effect” (Venniro et al., 2016). This occurs following extended access drug self-administration and abstinence for numerous drugs of abuse, including nicotine (Abdolahi et al., 2010; Funk et al., 2016; Bedi et al., 2011; Pickens et al., 2011). The neurobiological mechanisms underlying the incubation of nicotine-seeking are unknown but may be due to long-term alterations in the structure and organization of the nucleus accumbens (NAc), an area implicated in the reinforcing effects of nicotine, nicotine-seeking, and the incubation effect (Balfour, 2015; Funk et al., 2016). Findings with other drugs of abuse, such as cocaine, also indicate that the incubation effect is associated with adaptations in the morphology of the medium spiny neurons in the NAc (Christian et al., 2017; Ferrario et al., 2005; Loweth et al., 2014; Wolf & Tseng, 2012). Similar changes are also likely to underlie the incubation of nicotine-seeking given that nicotine administration induces marked changes in the morphology of dendritic spines in the NAc (Brown & Kolb, 2001; Ehlinger et al., 2016; McDonald et al., 2005; 2007). Additionally, Gipson et al. (2013b) showed that dendritic spine head diameter, a marker of synaptic potentiation, was increased in the NAc core following short access nicotine self-administration (2-hr/day) and 14-days of extinction training, and was further increased in response to nicotine-associated cues. It is not yet known whether similar changes are associated with the incubation of nicotine-seeking following extended access nicotine self-administration. This is critical since structural modifications are often less robust or in a different direction following non-contingent versus self-administered drug exposure, and following short versus extended access self-administration (Ferrario et al., 2005; McCutcheon et al., 2011; Robinson & Kolb, 2004). Thus, one goal of this study was to characterize changes in synaptic plasticity associated with nicotine-seeking following extended access nicotine self-administration (23-hr/day) and 10-days of abstinence, conditions known to induce the incubation of nicotine-seeking (Abdolahi et al., 2010; Funk et al., 2016).
Another objective of the present study was to examine the effects of exercise, a potential intervention for nicotine addiction, on markers associated with the incubation of nicotine-seeking. Exercise has been reported to reduce craving and smoking in humans (Abrantes et al., 2018; Taylor et al., 2007; Roberts et al., 2012; Haasova et al., 2013), and we demonstrated that when animals are allowed to exercise during abstinence from nicotine self-administration they show markedly reduced levels of subsequent nicotine-seeking (Lynch et al., 2017; Sanchez et al., 2013; 2014). Exercise has also been reported to impact dendrite length and spine density within numerous brain regions, including the NAc (Eadie et al., 2005; Eddy & Green, 2017; Redila & Christie, 2006; Ruegsegger et al., 2017; Stranahan et al., 2007). Although the effect of exercise on drug-induced synaptic plasticity is not known, these results suggest that it may prevent the incubation effect by altering the synaptic plasticity that occurs during abstinence.
We addressed each of these goals using an adolescent-onset model of nicotine self-administration given that, in humans, tobacco/nicotine use typically begins during adolescence (U.S. Department of Health and Human Services, 2014). The effects of exercise on structural plasticity also appear to be more robust in adolescents as compared to adults (Eddy & Green, 2017). Nicotine-seeking was assessed under a within–session extinction/cue-induced reinstatement procedure in rats that had 2-hr/day access to a locked (sedentary) or unlocked (exercise) running wheel during abstinence, a treatment that has been shown to block abstinence-induced increases in nicotine-seeking (Lynch et al., 2017; Sanchez et al., 2013; 2014). To assess changes in synaptic organization and connectivity associated with nicotine-seeking and its modulation by exercise, we performed an ultrastructural examination of asymmetric (excitatory) and symmetric (inhibitory) synapses and their postsynaptic profiles (i.e., location on dendritic shafts or spines) within the NAc core and shell using electron microscopy (Harris & Weinberg, 2012). We focused on changes in the density and length of synapses based on longstanding evidence showing that the lengthening of the synapse, and thus the postsynaptic density (PSD), is associated with a proportional increase in the probability of neurotransmitter release, the number of docked synaptic vesicles and postsynaptic receptors, and the size of spine heads—all of which are markers of synaptic strength (Bourne & Harris, 2011; Harris & Stevens, 1989; Meyer et al., 2014; Mulholland & Chandler, 2007; Santuy et al., 2017; Yasumatsu et al., 2008). Based on findings with nicotine and cocaine (Alcantara et al., 2011; Ferrario et al., 2005; Gipson et al., 2013b), we hypothesized that increased nicotine-seeking following abstinence would be associated with markers predictive of synaptic potentiation (e.g., increase in the length and/or density of excitatory synapses), and that exercise during abstinence would reverse these adaptations.
Materials and Methods
Animals
Adolescent male Sprague-Dawley (Charles River Laboratories, Portage, ME, USA) rats (N = 14) were used in this experiment as a follow-up to our previous study in males which demonstrated the efficacy of exercise in attenuating nicotine-seeking (Sanchez et al., 2013). While exercise also reduces nicotine-seeking in females, these effects are more variable (Sanchez et al., 2013). Rats arrived on postnatal day 22 and were singly housed in self-administration chambers (ENV-008CT, Med Associates, St. Albans, VT, USA) under a 12-hour light/dark cycle (lights on 0700) with ad libitum access to food and water. During habituation, rats were briefly food pre-trained to lever press for sucrose pellets under a fixed ratio 1 (FR1) schedule to ensure rapid acquisition of nicotine self-administration during the narrow window of adolescence (Sanchez et al., 2013). Training sessions continued daily until rats obtained 50 or more pellets on 2 consecutive days. On postnatal day 28, rats were implanted with a chronic indwelling catheter into the right jugular vein to allow intravenous nicotine (n = 8) or saline (n = 6) self-administration, as described previously (Sanchez et al., 2013). Effort was made to limit the number of animals used in these experiments to that necessary for histological analyses (n=3–4 group with 45 pictures/region/rat and 7914 total synapses analyzed). The health of the animals was monitored daily and they were weighed every Monday, Wednesday, and Friday. All procedures were in accordance with NIH guidelines and approved by the University of Virginia Animal Care and Use Committee.
Drugs
Nicotine bitartrate (Sigma-Aldrich) was dissolved in 0.9% sterile saline (pH 7.4) and passed through a microfilter. A low dose (5 μg/kg/infusions) of nicotine was selected based on previous work demonstrating that this dose engenders rapid rates of acquisition, high levels of self-administration under extended access conditions, and robust levels of subsequent nicotine-seeking following 10 days of abstinence (Sanchez et al., 2013; 2014). The dose is expressed as the free base weight and infusions were delivered at a rate of 0.1 ml/sec based on the animal’s weight. Nicotine solution was stored at 4 °C but was available at room temperature during self-administration sessions.
Behavioral Paradigm
The same methods used previously to examine the effects of exercise on nicotine-seeking were used here (Sanchez et al., 2013; also see Fig 1). Briefly, nicotine or saline self-administration training (5 days) began on postnatal day 30 under a FR1 schedule with a maximum of 20 infusions/day. A 10-day extended access self-administration phase began on postnatal day 35 during which animals were able to respond for an unlimited number of infusions under a FR1 schedule during 23-hour sessions. A 10-day abstinence period began after the last self-administration session wherein rats were housed in a polycarbonate cage with either a locked (saline, n = 3; nicotine, n = 4) or unlocked wheel attachment (saline, n = 3; nicotine, n = 4). Each day, the experimenter removed a metal gate separating the polycarbonate cage from the wheel and rats were permitted to move freely between their cage and the wheel for 2 hours. Revolutions within the unlocked wheel were recorded daily. In the locked wheel condition, rats were free to enter the wheel, however, the wheel did not rotate, and therefore they could not run. After the last exercise session, rats were moved back to their self-administration boxes to re-habituate to the self-administration chamber overnight. The following morning, nicotine-seeking was tested under a within-session extinction and cue-induced reinstatement procedure consisting of a minimum of 5, 1-hour extinction sessions followed by a 1-hour cue-induced reinstatement session. Each of the extinction sessions began with the introduction of the active-lever into the chamber, and responses on this lever were counted, but had no programmed consequence. Extinction was defined as fewer than 15 responses in the last session, and in this study all rats met the acquisition extinction criterion within 5 sessions. A 1-hour reinstatement session began following the last extinction session with the introduction of the active-lever and a 5-sec presentation of the cues formerly associated with a nicotine infusion (i.e., a light above the formerly active-lever and the sound of the pump). Each subsequent response on the active-lever during the reinstatement session resulted in the presentation of these cues, however no infusions were delivered.
Figure 1.
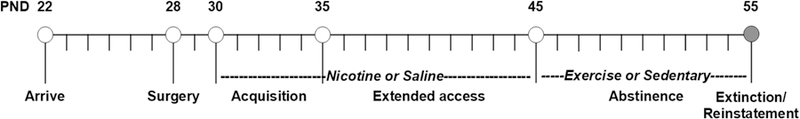
Summary of the experimental time-line as a function of postnatal day (PND). Rats arrived at PND 22, and following a 6-day habituation period, underwent jugular catheterization surgery on PND 28. A 5-day self-administration training period began following recovery on PND 30 wherein rats had fixed ratio 1 access to 20 nicotine or saline infusions/day. A 10-day extended access period began on PND 35 wherein rats had access to an unlimited number of infusions for 23-hours/day. During the 10-day abstinence period that followed, rats had 2-hour/day access to an unlocked (exercise) or locked (sedentary) running wheel (PND 45–54). After the last exercise/sedentary session, rats were returned to their self-administration boxes. Nicotine-seeking was assessed the next day on PND 55 using a within-session extinction/cue-induced reinstatement procedure.
Tissue Preparation for Electron Microscopy
Coronal brain slices were prepared using a standard protocol as described previously (Erisir & Harris, 2003). Immediately following the end of the reinstatement session, rats were deeply anesthetized with a lethal dose of sodium pentobarbital, and transcardially perfused with Tyrode’s solution until the vasculature was clear (< 2 minutes) and then with a 4% paraformaldehyde with 0.5% gluteraldehyde fixative solution in 0.1 M phosphate buffer (PB, pH 7.4) for 20–30 minutes. Brains were removed and placed in the same fixative solution overnight at 4 °C. The following day, 60 μm coronal sections including the NAc were cut on a vibratome and stored in 0.1% sodium azide in PB at 4 °C. Sections were washed in 0.1 M PB and then postfixed in 1% osmium tetroxide in 0.1 M PB for 1 hour. Sections were subsequently washed with 0.1 M PB three times (3 minutes/wash) followed by 50% ethanol for 3 minutes and then stained with 4% uranyl acetate for 1 hour. The sections were then serially dehydrated in 70% ethanol for 1 minute, 90% ethanol for 5 minutes, 100% ethanol for 5 minutes twice, and acetone for 2 minutes 3 times. The last acetone wash was replaced with a 1:1 dilution of EPON in acetone in which the sections remained overnight. The following day the EPON-acetone mixture was replaced with EPON for 2–4 hours. Tissue was then sandwiched in between two aclar sheets, and placed in an oven at 60 °C overnight for polymerization. Camera lucida was used to trace landmarks of the tissue for localization of the NAc core and shell regions. These regions were then cut from the flat embedded tissue and placed in a capsule BEEM that was subsequently filled with EPON and allowed to polymerize in the 60 °C oven for 3 days. Blocks were then trimmed so that a trapezoidal 1×2 mm strip of tissue, which was perpendicular to shell and core borders and contained the ventral half of the anterior commissure, was prepared for ultra-thin sectioning. This approach was taken in order to ensure that the same region of the NAc was analyzed in each brain. Using a diamond knife, 70 nm sections of tissue were cut and collected on grids for visualization with a Joel 1010 transmission electron microscope (TEM). Images were captured using a 16-Mpixel CCD camera (SIA).
Quantitative Analysis of Electron Micrographs
For each rat, a single section of one hemisphere was used to analyze the NAc core and shell regions. Sections were selected at approximately 1.92 mm Bregma based on a standard neuroanatomical atlas (Paxinos & Watson, 2006). From each region (core and shell), 3 areas were selected parallel to the midline of the brain in the middle of the shell region and 3 corresponding areas were selected in the core region (green and blue boxes in Fig 2, respectively). Areas in the core were selected so that they were adjacent to areas that contained the anterior commissure. Within each of these 3 areas, 15 non-overlapping pictures were taken of neuropil at 10,000 times magnification with a 16M-pixel camera (2.75 nm pixel size resolution), for a total of 45 pictures from each core and shell/rat.
Figure 2.
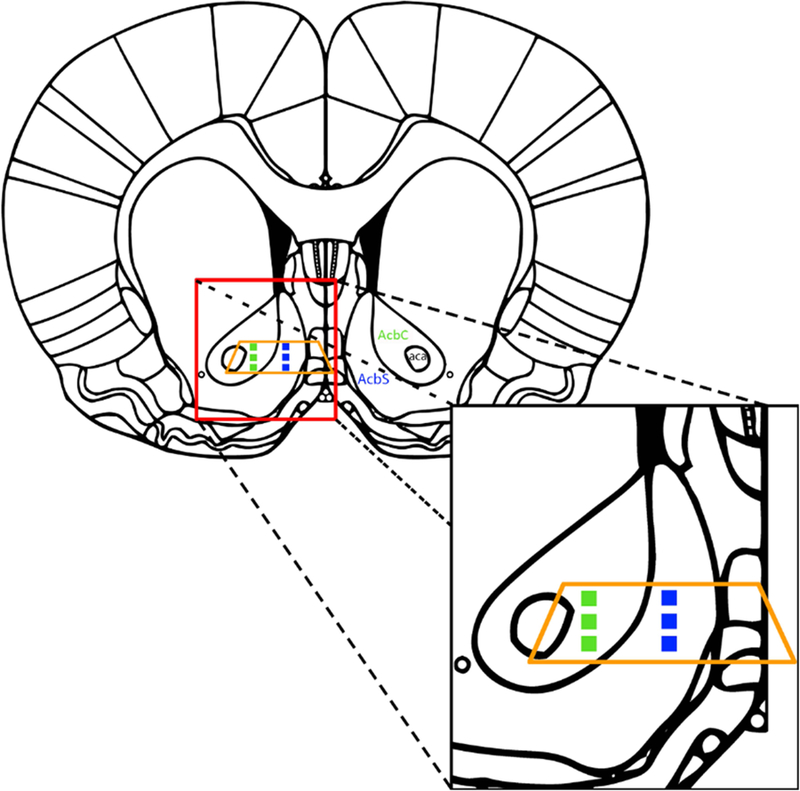
Schematic of areas within the nucleus accumbens core (AcbC; green squares) and shell (AcbS; blue squares) analyzed at approximately Bregma 1.92 mm. The orange trapezoid represents the area from each coronal slice that used to make ultrathin sections. Modified from Paxinos and Watson 2006.
Each photograph was examined by an experimenter blinded to the condition at a final resolution of 363 pixels/μm using Image Pro Plus 4.0 software (Media Cybernetics, Silver Spring, MD, USA). Synapses were defined by the presence of at least 3 synaptic vesicles in the presynaptic terminal and the parallel alignment of the pre- and post-synaptic membranes creating a synaptic cleft. Synapses were identified as either asymmetric (highlighted in blue in Fig 3A) or symmetric (highlighted in green with black arrow in Fig 3A) based on the presence or absence of a postsynaptic density, respectively. Postsynaptic profiles were classified as spines if mitochondria and microtubules were absent (red arrows in Fig 3B and C). The presence of a spine apparatus (black arrow in Fig 3B and C) was also used for classifying postsynaptic profiles as a spine, but was not necessary. Other postsynaptic profiles were also identified as (1) dendritic shafts (dendrites) if microtubules and mitochondria were present (star in Fig 3B and D) (2) cell bodies by the presence of endoplasmic reticulum, Golgi apparatus, or a nucleus or (3) an axon terminal if vesicles were present. On each photograph, the length of each synapse was measured along the parallel-aligned plasma membranes (i.e., darkly-stained, electron-dense structure comprised of the presynaptic density and PSD) and was classified based on the type of synapse (i.e., symmetric or asymmetric) and the type of the postsynaptic profile (e.g., spine, dendrite, etc.). The area of myelinated axons (highlighted in orange in Fig 3A), blood vessels and cell bodies were also determined. Total area of each photograph, excluding myelinated axons, blood vessels and cell bodies, was then determined. The number of synapses per area (NA) was calculated and used to determine the volumetric density of synapses (NV=NA/ mean synapse length), a reliable estimate of the number of synapses contained within a unit volume (Colonnier & Beaulieu, 1985; DeFelipe et al., 1999). These values were then averaged across the 15 photographs within each of the 3 areas and brain regions. However, since no significant overall or interactive effects were observed for area within either the core or the shell, the data are presented collapsed across each of the 3 areas.
Figure 3.
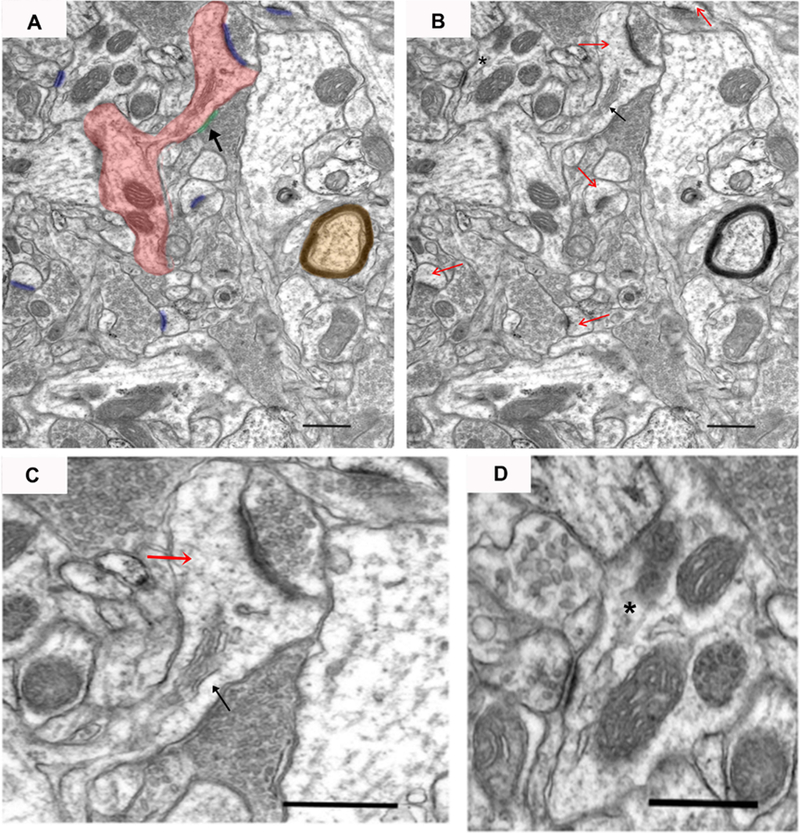
An example electron micrograph of neuropil from which synapses were measured. In panel A, a dendritic spine that connects back to the dendritic shaft (dendrite) is highlighted in red, asymmetric synapses are highlighted in blue, symmetric synapse is highlighted in green with black arrow, and a myelinated axon is highlighted in orange. In panel B, red arrows mark dendritic spines, the black arrow marks a spine apparatus, and asterisk marks a postsynaptic dendrite. Panel C shows an enlarged image of a dendritic spine (red arrow) with asymmetric and symmetric synapses. Panel D shows an enlarged image of an asymmetric synapse onto a dendrite (asterisk) with a clearly defined synaptic cleft. Scale bar represents 0.5 μm.
Statistical Analyses
Behavioral data are presented as the mean ± SEM. Self-administration and wheel running data were analyzed using linear mixed effects models with group, session, and group by session as fixed factors and with subject as a random effect. A similar analysis was used to compare active-lever responses during the last extinction session versus the reinstatement session between the four groups. Univariate ANOVA was used to analyze group differences in active-lever responding during extinction and, as a measure of total nicotine-seeking, active-lever responding during both extinction and reinstatement. Post-hoc comparison to controls (saline sedentary and nicotine sedentary) were made using Dunnett’s one-tailed (for predicted differences in extinction responding) t-tests (Nesil et al., 2018; Ohlsson et al., 2014).
Linear mixed effects models were also used to examine group differences in the volumetric density of synapses in the core and shell with separate models used for each type of synapse and their postsynaptic profile (i.e., asymmetric or symmetric onto dendrites or spines). Given that no significant overall and interactive effects of area were observed, it was removed as a fixed factor (to simplify the model), but considered along with subject as a random effect. Post-hoc comparison to sedentary controls (saline and nicotine) were made using Dunnett’s two-tailed (symmetric synapses) or one-tailed (for predicted differences in asymmetric synapses) t-tests. The association between synaptic density and nicotine-seeking was assessed by calculating the Pearson Correlation Coefficient using average volumetric density across the three areas within the core or shell and total active-lever responses during extinction and reinstatement for each animal. Associations were assessed across all four groups when there were no significant group differences with regard to slope of the associations (i.e., non-significant interaction of group by response; asymmetric synapses onto dendrites), and within each of the four groups separately when there were significant group differences (i.e., symmetric synapses onto dendrites).
Group differences in the distribution of synapse lengths, sorted into 0.04 µm bins up to 1.0 µm, were determined using the chi-square test for goodness of fit, calculated with the chisq.test() function within the stats package in R (R Core Team, 2018). Post-hoc comparisons to controls (saline sedentary and nicotine sedentary) were made using Dunnett’s two-tailed t-tests. Statistical analyses were performed using SPSS (24) and R (3.5.0). Alpha was set at 0.05.
Results
Behavioral Findings
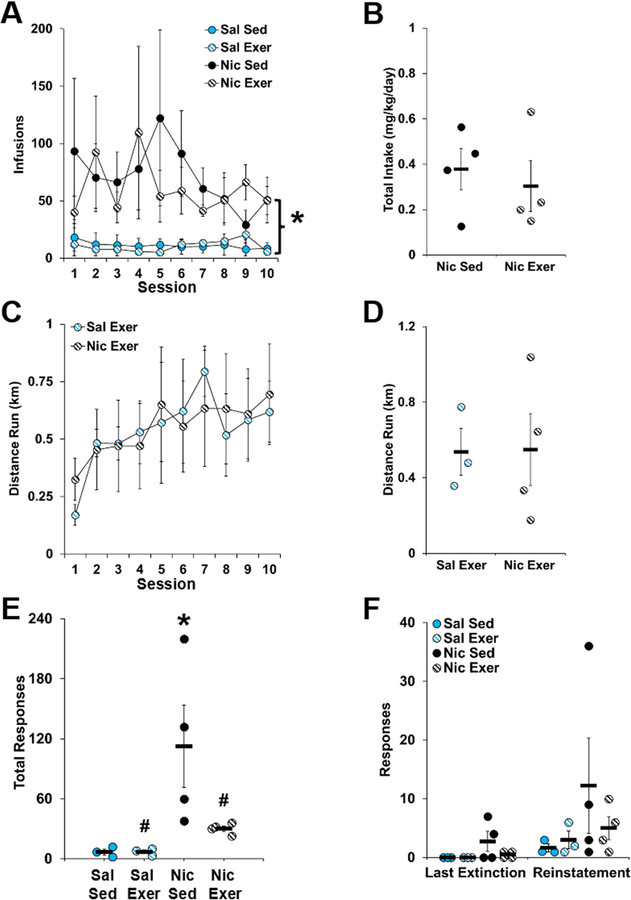
During extended access, animals that self-administered nicotine obtained significantly more infusions than those that self-administered saline with an overall effect of drug (F1,12 = 12.89, P = 0.004; Fig 4A), but no effect of day or interaction of day by group (all P > 0.80). In addition, prior to exercise or sedentary exposure, no differences were observed for intake between the two nicotine groups (Fig 4B). During abstinence, the average daily distance run was similar between the saline and nicotine exercise groups (Fig 4C and D). Thus, prior to extinction/reinstatement testing, levels of nicotine intake and exercise were similar between the groups.
Figure 4.
Behavioral data (mean ± SEM) for extended access self-administration (A and B), distance run during abstinence (C and D), and active-lever responses during extinction (E) and reinstatement testing (F). In panel A, the number of infusions obtained is plotted for each of the groups and for each of the sessions during the 10-day extended access self-administration period. An asterisk indicate a significant difference between the saline and nicotine groups. In panel B, average daily nicotine intake is plotted for each animal in the nicotine sedentary and nicotine exercise groups. In panel C, the daily distance run in kilometers (km) is plotted for each exercise session during the 10-day abstinence period. In panel D, the average daily distance run is plotted for each animal in the saline exercise and nicotine exercise groups. The active-lever responses made during all extinction sessions (Panel E) and during the last extinction session versus the reinstatement test session (Panel F) is plotted for each animal in each of the groups. In panel E, an asterisk indicates a significant difference from the saline sedentary group, and a number signs indicates a significant difference from the nicotine sedentary group. n = 3 (saline, sedentary and exercise) or 4 (nicotine, sedentary and exercise). Exer, exercise; Nic, nicotine; Sal, saline; Sed, sedentary.
As predicted, active-lever responses during extinction were highest in the nicotine sedentary group, and were decreased to saline control levels in the nicotine exercise group (group, F3,10= 4.531, P = 0.03; Fig 4E). Post-hoc comparisons to the saline sedentary group revealed a significant difference for the nicotine sedentary group only (P = 0.02); whereas, comparison to the nicotine sedentary group revealed a significant difference for both groups (nicotine exercise P = 0.04; saline exercise P = 0.02). A similar pattern of differences was observed for active-lever responses during reinstatement; but, the overall and interactive effects of group were not significant (Fig 4F). An analysis of total drug-seeking, which includes both extinction and reinstatement responding however, revealed a significant effect of group (group, F3,10 = 4.00, P = 0.04), with post-hoc comparisons to saline sedentary controls revealing a significant difference for the nicotine sedentary group only (P = 0.02), and comparison to the nicotine sedentary group revealing a significant difference for both groups (nicotine exercise P = 0.05; saline exercise P = 0.02; data not shown). Thus, nicotine-seeking during extinction was increased following extended access nicotine self-administration and abstinence, and this increase was blocked to saline control levels by exercise during abstinence.
Volumetric Density of Synapses by Type within the NAc Core and Shell
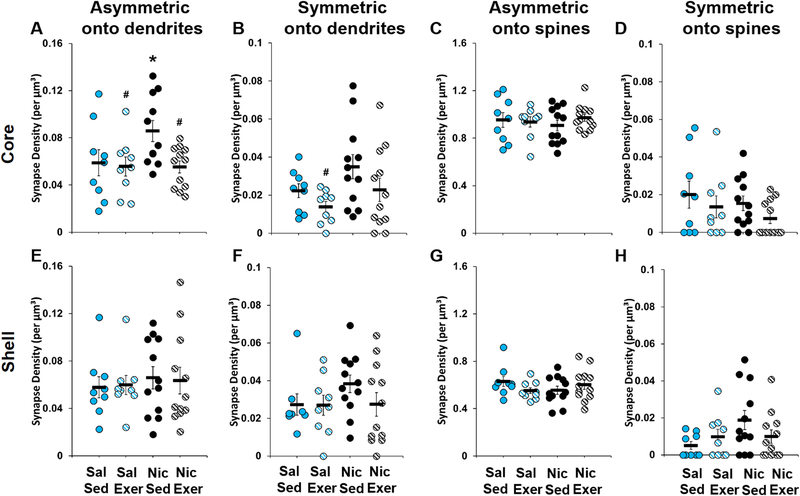
Across groups, the most common type of synapses observed were asymmetric synapses onto dendritic spines for both the core (88% of total) and shell (83% of total) with the second most common being asymmetric synapses onto dendrites (7% and 10% of total core and shell, respectively). Symmetric synapses were more commonly observed on dendrites (3% and 5% of total core and shell, respectively) than on spines (1.2% and 1.5% of total core and shell, respectively). Synapses onto cell bodies and axons were rare and made up < 1% of total synapses in the core and shell regions and were not included in any further analyses. The total volumetric density of all synapse types was not affected by group in either the core (saline sedentary, 1.06 ± 0.01; saline exercise, 1.02 ± 0.05; nicotine sedentary, 1.02 ± 0.06; nicotine exercise, 1.05 ± 0.04) or shell (saline sedentary, 0.71 ± 0.05; saline exercise, 0.65 ± 0.03; nicotine sedentary, 0.67 ± 0.04; nicotine exercise, 0.75 ± 0.05).
In the NAc core, the volumetric density of asymmetric synapses onto dendrites was highest in the nicotine sedentary group, and decreased to saline control levels in the nicotine exercise group (group, F3,8 = 4.497, P = 0.04; Fig 5A). Post-hoc comparisons to the saline sedentary group revealed a significant difference for the nicotine sedentary group only (P = 0.03); whereas, comparison to the nicotine sedentary group revealed a significant difference for both the nicotine exercise (P = 0.008) and the saline exercise groups (P = 0.02). A similar, but more variable, pattern of differences was observed for symmetric synapses onto dendrites (group, F3,10 = 3.716, P = 0.05; Fig 5B), with post-hoc comparison to nicotine sedentary controls revealing a significant difference for the saline exercise group (P = 0.009), but only trends for a difference for the saline sedentary (P = 0.06) and nicotine exercise (P = 0.08) groups. The volumetric density of asymmetric and symmetric synapses onto spines were not significantly different between groups (Fig 5C-D). There were also no differences within the shell region for volumetric density of any synapse type measured (Fig 5E-H).
Figure 5.
Volumetric density (mean ± SEM) of asymmetric synapses onto dendrites (A and E), symmetric synapses onto dendrites (B and F), asymmetric synapses onto spines (C and G), and symmetric synapses onto spines (D and H) within the core (A-D) and shell (E-H). Data are based on average densities for each of the three regions within the core and shell for each of the rats in the saline (n = 3) and nicotine (n = 4) groups (data points are shown for each rat for each of the three regions). An asterisk indicates a significant difference from the saline sedentary group (Panel A), and a number signs indicates a significant difference from the nicotine sedentary group (Panels A and B). Exer, exercise; Nic, nicotine; Sal, saline; Sed, sedentary.
Notably, the volumetric density of asymmetric synapses onto dendrites in the core significantly correlated with active-lever responses during extinction (r12 = 0.89; P = 2.20e-05; Fig 6A), and reinstatement (r12 = 0.74; P = 0.003; data not shown). A similar association was observed for symmetric synapses onto dendrites in the core, but in this case, the positive association was observed only in the nicotine sedentary group (r2 = 0.99; P = 0.01; Fig 6B), and was non-significant in the nicotine exercise (P = 0.48) and saline sedentary (P = 0.83) and saline exercise groups (P = 0.55). Thus, nicotine-seeking following abstinence was associated with increased volumetric density of asymmetric and symmetric synapses onto dendrites in the core, but not shell, and this effect was blocked by exercise during abstinence.
Figure 6.
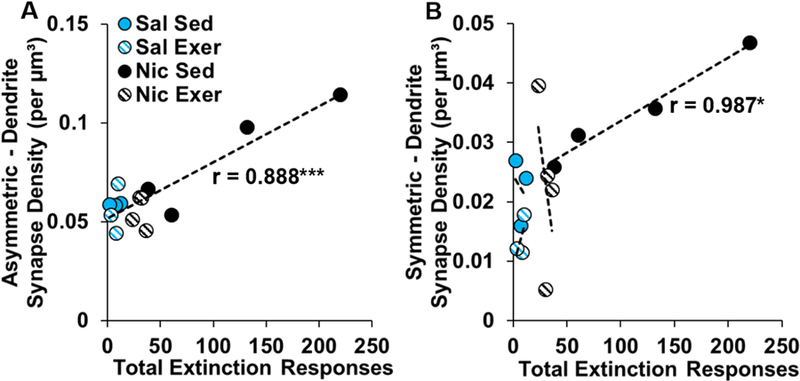
Association between total active-lever responses during extinction and the density of asymmetric (A) and symettric (B) synpases onto dendrites averaged over the three regions of the nucleus accumbens core for each subject. n = 3 (saline, sedentary and exercise) or 4 (nicotine, sedentary and exercise). Exer, exercise; Nic, nicotine; Sal, saline; Sed, sedentary.
Length Distribution of Asymmetric Synapses onto Spines in the NAc Core and Shell
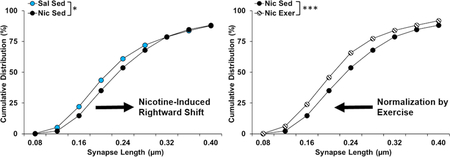
As mentioned above, asymmetric synapses onto spines were the most commonly observed type of synapse. Although no significant differences were observed for their density in either the core or the shell, marked differences were observed for the distributions of lengths of these types of synapses within both regions. Specifically, in the core, there was a rightward shift in the distribution of lengths of asymmetric synapses onto spines in the nicotine sedentary group as compared to all other groups (group, χ2(69) = 123.78, P = 5.77e-05; Fig 7A). Subsequent pairwise comparisons to the saline sedentary group revealed a difference in the distribution of synapse lengths for the nicotine sedentary group (χ2(23) = 43.571, P = 0.02; Fig 7B), which was normalized by exercise during abstinence (P = 0.97; Fig 7D). In contrast, comparisons to the nicotine sedentary group revealed significant differences for both the nicotine exercise (χ2(23) = 70.981, P = 1.71e-06 ; Fig 7C) and the saline exercise groups (χ2(23) = 44.718, P = 0.009; Fig 7A). Thus, the abstinence-induced increase in nicotine-seeking, as observed in the sedentary group, was associated with longer asymmetric synapses onto spines, and these effects were normalized to saline control levels by exercise during abstinence.
Figure 7.
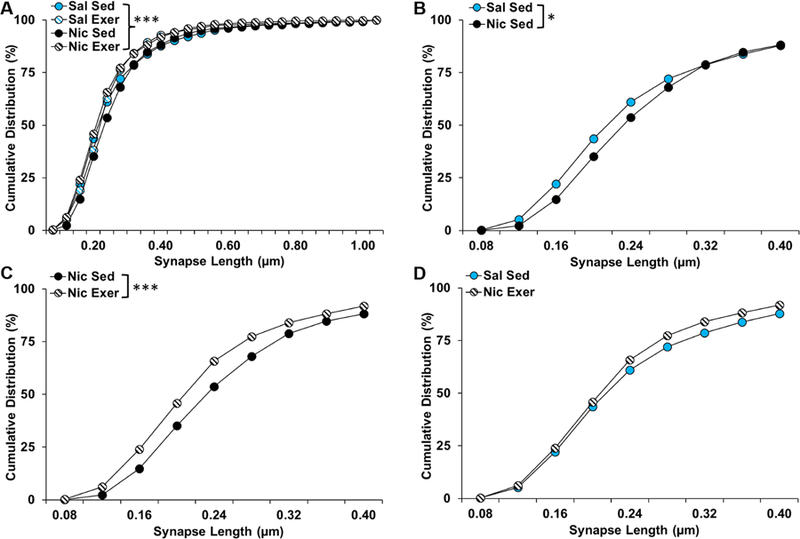
Cumulative distribution (% of total) of asymmetric synapses onto spines by length in nucleus accumbens core for each of the groups up to 1.00 µm (A), and for a focused comparison of the saline and nicotine sedentary groups (B), the nicotine sedentary and exercise groups (C), and the saline sedentary and nicotine exercise groups up to to 0.40 µm. Asterisks indicate significant differences at a p < 0.05 (*) or 0.001 level (***). Exer, exercise; Nic, nicotine; Sal, saline; Sed, sedentary.
Similar group differences were observed for the distribution of lengths of asymmetric synapses onto spines in the shell region (χ2(69) = 107.53, P = 0.002; Fig 8A), with post-hoc comparisons to the saline sedentary group revealing a significant rightward shift in the distribution of lengths in the nicotine sedentary group (χ2(23) = 43.148, P = 0.02; Fig 8B), which was normalized by exercise during abstinence (P = 1.50; Fig 8D). Interestingly, a similar, though non-significant, rightward shift was observed in the saline exercise group (P = 0.06; Fig 8A). Additionally, while post-hoc comparisons to the nicotine sedentary group revealed a significant difference for the nicotine exercise group (χ2(23) = 51.294, P = 0.001; Fig 8C), this comparison was not significant for the saline exercise group (P = 0.68) indicating that both exercise and nicotine similarly affected the distribution of synapse lengths in the shell.
Figure 8.
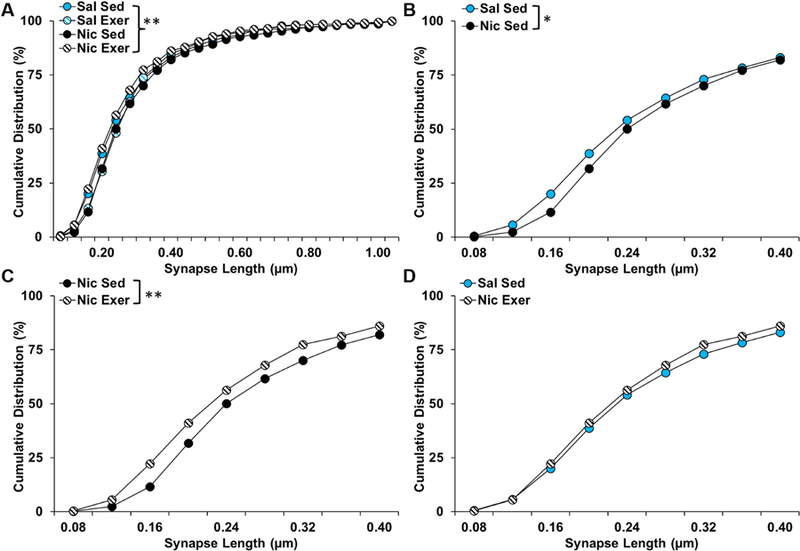
Cumulative distribution (% of total) of asymmetric synapses onto spines by length in nucleus accumbens shell for each of the groups up to 1.00 µm (A), and for a focused comparison of the saline and nicotine sedentary groups (B), the nicotine sedentary and exercise groups (C), and the saline sedentary and nicotine exercise groups up to to 0.40 µm. Asterisks indicate significant differences at a p < 0.05 (*) or 0.01 level (**). Exer, exercise; Nic, nicotine; Sal, saline; Sed, sedentary.
Discussion
The purpose of these experiments was to characterize ultrastructural synaptic plasticity in the NAc associated with nicotine-seeking following extended access nicotine self-administration and abstinence, and to determine whether exercise during abstinence may exert its efficacy by normalizing these synaptic changes. Consistent with our hypothesis, abstinence from extended access nicotine self-administration was associated with high levels of nicotine-seeking and an increase in the density of asymmetric synapses in the NAc core. We also observed an increase in the density of symmetric synapses, and for both types, the increased density was specific to synapses onto dendrites, not spines, and for the NAc core, not shell. The density of both asymmetric and symmetric synapses onto dendrites in the core also positively correlated with nicotine-seeking. Although no difference was observed for the density of the most frequently observed synapse type, asymmetric onto spines, we did find marked differences in the distribution of their lengths. Differences were observed in both the core and the shell, and for both regions, heightened nicotine-seeking, as observed in the sedentary nicotine group, was associated with a rightward shift in the distribution of synapse lengths. Importantly, exercise during abstinence blocked increases in nicotine-seeking, and normalized each of the associated changes in synaptic plasticity to saline control levels. However, while exercise alone was without effect in the core, it produced similar effects as nicotine in the shell. Together, these findings indicate an association between the enhancement of nicotine-seeking following extended access self-administration and abstinence and synaptic plasticity in the NAc, particularly in the core, and indicate that the efficacy of exercise to reduce nicotine-seeking may be mediated by reversing these adaptations.
Many previous studies have demonstrated that nicotine exposure and nicotine-seeking is associated with adaptations in the morphology and density of spines in the NAc (Brown & Kolb, 2001; McDonald et al., 2005; 2007; Gipson et al., 2013b), but this study is the first to examine changes associated with nicotine-seeking at the level of the synapse. As predicted, enhanced nicotine-seeking was associated with an increase in the density of asymmetric synapses, and their densities also positively correlated with levels of nicotine-seeking. The fact that these synapses were on dendrites, however, is a relatively novel finding that may reflect the migration of asymmetric synapses from spines to the dendrite as the result of spine retraction (Alcantara et al., 2011; Ovtscharoff et al., 2008). The fact that there were no differences in the density of asymmetric synapses onto spines is consistent with previous findings showing that while nicotine-seeking following short access self-administration and extinction training was associated with an increase in spine head diameter, there were no differences in the density of spine heads in the core (Gipson et al., 2013b).
We also observed an increase in the density of symmetric synapses onto dendrites, and these synapses were also positively associated with levels of nicotine-seeking. This is a novel finding since few studies, even with other drugs of abuse, have reported changes in symmetric synapses suggesting that the effect may be specific to nicotine-seeking. Like asymmetric synapses, this increase was specific to symmetric synapses onto dendrites. These synapses are likely dopaminergic inputs from the midbrain, which modulate the excitatory drive originating from asymmetrical contacts, presumably glutamatergic inputs from the prefrontal cortex, on the same dendritic spines (Koya et al., 2009; Robinson & Kolb, 2004; Sesack & Pickel, 1990; Smith & Bolam, 1990). That each of these changes were observed in the core, and not shell, is consistent with numerous studies indicating a preferential role for the core over the shell in drug-seeking following extended access self-administration (Ferrario et al., 2005; Fischer et al., 2013; Guillem et al., 2014). While these findings suggest that synapses involved in integrating glutamatergic and dopaminergic signaling in the NAc core become re-organized with the development of the incubation of nicotine-seeking, further research using vesicular glutamate transporter or tyrosine hydroxylase antibodies is needed to directly examine the source of the terminals forming the asymmetric and symmetric synapses. Additionally, due to the 3D nature of synapses and their highly variable sizes and shapes, the use of 3D reconstruction methods is necessary in the future to more reliably address the changes in synapse density observed here.
Further support for the idea that changes represent an increase in glutamatergic synaptic plasticity is provided by our findings showing that enhanced nicotine-seeking, as observed in the nicotine sedentary group, was associated with the elongation of asymmetric synapses onto spines. This effect may be analogous to the increase in spine head size reported by Gibson et al. (2013b) as these two measures are directly correlated (i.e., larger spines contain longer synapses; Harris & Sevens, 1989), and is likely associated with an increase in α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor surface expression (Baude et al., 1995; Kharazia & Weinberg, 1999; Takumi et al., 1999). Indeed, along with abstinence-induced enlargement of spine head diameter following extinction training during abstinence, Gipson et al. (2013b) observed evidence of increased surface expression of calcium permeable AMPA receptors. Findings with cocaine further show that the incubation effect is associated with the insertion of highly conductive calcium permeable AMPA receptors in the postsynaptic membrane of NAc neurons, and preventing this insertion, prevents its development (Conrad et al., 2008; Loweth et al., 2014). Taken together, these results suggest that an increase in the length of synapses not only represents an increase in structural and functional plasticity, but also may underlie heightened nicotine-seeking following extended access nicotine self-administration and abstinence.
One limitation to our study is that changes were not compared to earlier time-points during abstinence leaving open the possibility that changes are due to effects of nicotine and/or extinction/reinstatement testing, and not necessarily reflective of incubated nicotine-seeking. It is possible that some of our changes reflect rapid, transient changes resulting from cue-induced reinstatement testing (Gibson et al., 2013a; b; Shen et al., 2011). In fact, Gibson et al. (2013b) showed that following exposure to nicotine-associated cues, spine head diameter showed a rapid, but transient increase. These types of changes are thought to occur through a Hebbian form of long-term potentiation (LTP) and happen within seconds or minutes following activity-dependent neuronal activation (Fauth & Tetzlaff, 2016). However, some of the changes observed here, particularly the elongation of synapses, were likely mediated by nicotine self-administration and abstinence given that we obtained the tissue immediately after the test session and these changes require several hours or days to occur (Lisman, 2017). The elongation of synapses is likely slower because the processes needed for the integration of additional AMPARs, namely the growth of presynaptic boutons, the enlargement of the PSD, and protein synthesis (Lisman, 2017), take time. This late stage of LTP can be described as ‘neoHebbian’, as in addition to the Hebbian requirement of synchronized activation of pre- and postsynaptic neurons, a third factor marking the salience, novelty, or reward of the stimuli and signaled through neuromodulators, such as dopamine, is also involved (Gerstner et al., 2018; Lisman, 2017). Indeed, dopamine has been reported to promote dendritic protein synthesis in the CA1 hippocampal region as well as the enlargement of dendritic spines in the NAc when paired with pre- and postsynaptic spikes (Smith et al., 2005; Yagishita et al., 2014). While this idea is also consistent with our finding of increased symmetric synapses onto dendrites, future research is needed to directly examine this possibility.
Another limitation of the present study is that presentation of nicotine-associated cues did not significantly reinstate nicotine-seeking in our model. This finding is likely attributable to the small sample size used for behavioral testing, especially considering that effects were examined in adolescent/young adult rats which are known to respond at lower levels than adults in response to drug-associated cues (Anker & Carroll, 2010). However, it is important to note that robust and consistent nicotine-seeking was observed in the absence of cues, during extinction. Additionally, total nicotine-seeking, which includes cue-induced reinstatement responding, was highest in the nicotine sedentary group.
Another goal of this study was to determine if exercise, a behavioral intervention that has been shown to decrease nicotine-seeking in this adolescent-onset model (Sanchez et al., 2013), also affects structural plasticity in the NAc. As expected, exercise during abstinence attenuated subsequent nicotine-seeking. Interestingly, this behavioral normalization was accompanied by normalized volumetric density of asymmetric and symmetric synapses onto dendrites, further supporting the notion that these synapses are important for nicotine-seeking. In addition, exercise during abstinence normalized synapse length in both the core and shell. However, our observation that nicotine and exercise alone similarly increased synapse length in the shell suggests that the efficacy of exercise to reduce nicotine-seeking likely does not rely on synaptic plasticity in the NAc shell. Taken together, these results indicate that the effectiveness of exercise may be associated with its ability to reverse increases in the number of excitatory and inhibitory synapses onto dendrites and size of excitatory synapses onto spines within the NAc core following extended access self-administration and abstinence.
The mechanism through which exercise might impact abstinence-induced synaptic plasticity is unknown. It is possible that effects are mediated through either, or both, glutamatergic and dopaminergic systems (Lynch et al., 2013; Greenwood et al., 2011; Grigsby et al., 2018; Guezennec et al., 1998; Real et al., 2010; Robison et al., 2018). Exercise has been shown to modulate glutamatergic signaling in cortical regions, and microdialysis studies have demonstrated that during exercise, extracellular glutamate and dopamine levels are increased in the striatum (Meeusen et al., 1997) and NAc (Wilson & Marsden, 1995). Given that exercise has been shown to be rewarding and to affect dopaminergic signaling (Iversen, 1993; Belke, 1997; Lett et al., 2000; 2001; Belke & Wagner, 2005; Brené et al., 2007; Greenwood et al., 2011), it is possible that exercise could block nicotine-induced changes in glutamatergic and dopaminergic signaling that lead to synaptic plasticity. Exercise also promotes stable changes in gene expression through epigenetic regulation of chromatin (Gomez-Pinilla et al., 2011), and these effects are believed to underlie the persistent beneficial effects of exercise as an intervention for cocaine-seeking since the protection can persist well beyond the period of exercise (Beiter et al., 2016 ). Whether this type of mechanism also occurs with nicotine is not yet known.
In summary, nicotine-seeking following extended access self-administration and abstinence is associated with an increase in the volumetric density of asymmetric and symmetric synapses onto dendrites as well as the length of asymmetric synapses onto spines in the NAc. In addition, the effectiveness of exercise in reducing nicotine-seeking may involve its ability to prevent the plasticity of glutamatergic and dopaminergic connectivity within the NAc core following extended access self-administration and abstinence. Future studies should aim to elucidate the identity and location of neurons that project to the NAc involved in both abstinence-induced plasticity and the efficacy of exercise. These findings describe novel synaptic plasticity associated with enhanced nicotine-seeking and point to a potential mechanism for the effectiveness of exercise in treating nicotine addiction. Future research is necessary to determine if similar mechanisms underlie the efficacy of exercise to reduce nicotine-seeking in females, particularly considering that relapse vulnerability is enhanced in females versus males.
Acknowledgements
This work was supported by grants from NIDA RO1 grants DA024716 and DA039093 (W.J.L.), Virginia Foundation for Healthy Youth 8520667 and 8520893 (D.H.B.), NIH training grant T32 HD007323 (V.S.), and NIDA F31 DA033087 (V.S.).
Abbreviations
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid
- FR
fixed ratio
- LTP
long-term potentiation
- NAc
nucleus accumbens
- PSD
postsynaptic density
Footnotes
Conflict of Interest Statement
The authors declare no competing financial, or other, conflicts of interests.
Data accessibility
Data can be obtained by contacting the corresponding author.
References
- Abdolahi A, Acosta G, Breslin FJ, Hemby SE & Lynch WJ (2010) Incubation of nicotine seeking is associated with enhanced protein kinase A‐regulated signaling of dopamine‐ and cAMP‐regulated phosphoprotein of 32 kDa in the insular cortex. Eur. J. Neurosci, 31, 733–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abrantes AM, Farris SG, Minami H, Strong DR, Riebe D & Brown RA (2018) Acute effects of aerobic exercise on affect and smoking craving in the weeks before and after a cessation attempt. Nicotine Tob. Res, 20, 575–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara AA, Lim HY, Floyd CE, Garces J, Mendenhall JM, Lyons CL & Berlanga ML (2011) Cocaine- and morphine-induced synaptic plasticity in the nucleus accumbens. Synapse, 65, 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anker JJ & Carroll ME (2010) Reinstatement of cocaine seeking induced by drugs, cues, and stress in adolescent and adult rats. Psychopharmacology, 208, 211–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balfour DJ (2015) The role of mesoaccumbens dopamine in nicotine dependence. Curr. Top. Behav. Neurosci, 24, 55–98. [DOI] [PubMed] [Google Scholar]
- Baude A, Nusser Z, Molnar E & McIlhinney R (1995) High-resolution immunogold localization of AMPA type glutamate receptor subunits at synaptic and non-synaptic sites in rat hippocampus. Neuroscience, 69, 1031–1055. [DOI] [PubMed] [Google Scholar]
- Bedi G, Preston KL, Epstein DH, Heishman SJ, Marrone GF, Shaham Y & de Wit H (2011) Incubation of cue-induced cigarette craving during abstinence in human smokers. Biol. Psychiatry, 69, 708–711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beiter RM, Peterson AB, Abel J & Lynch WJ (2016) Exercise during early, but not late abstinence, attenuates subsequent relapse vulnerability in a rat model. Transl. Psychiatry, 6, e792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW (1997) Running and responding reinforced by the opportunity to run: effect of reinforcer duration. J. Exp. Anal. Behav, 67, 337–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belke TW & Wagner JP (2005) The reinforcing property and the rewarding aftereffect of wheel running in rats: a combination of two paradigms. Behav. Processes, 68, 165–172. [DOI] [PubMed] [Google Scholar]
- Bourne JN & Harris KM (2011) Coordination of size and number of excitatory and inhibitory synapses results in a balanced structural plasticity along mature hippocampal CA1 dendrites during LTP. Hippocampus, 21, 354–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brené S, Bjørnebekk A, Aberg E, Mathé AA, Olson L & Werme M (2007) Running is rewarding and antidepressive. Physiol. Behav, 92, 136–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RW & Kolb B (2001) Nicotine sensitization increases dendritic length and spine density in the nucleus accumbens and cingulate cortex. Brain Res, 899, 94–100. [DOI] [PubMed] [Google Scholar]
- Christian DT, Wang X, Chen EL, Sehgal LK, Ghassemlou MN, Miao JJ, Estepanian D, Araghi CH, Stutzmann GE & Wolf ME (2017) Dynamic alterations of rat nucleus accumbens dendritic spines over 2 months of abstinence from extended-access cocaine self-administration. Neuropsychopharmacology, 42, 748–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colonnier M & Beaulieu C (1985) An empirical assessment of stereological formulae applied to the counting of synaptic disks in the cerebral cortex. J. Comp. Neurol, 231, 175–179. [DOI] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng LJ, Shaham Y, Marinelli M & Wolf ME (2008) Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature, 454, 118–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeFelipe J, Marco P, Busturia I & Merchán-Pérez A (1999) Estimation of the number of synapses in the cerebral cortex: methodological considerations. Cereb. Cortex, 9, 722–732. [DOI] [PubMed] [Google Scholar]
- Eadie BD, Redila VA & Christie BR (2005) Voluntary exercise alters the cytoarchitecture of the adult dentate gyrus by increasing cellular proliferation, dendritic complexity, and spine density. J. Comp. Neurol, 486, 39–47. [DOI] [PubMed] [Google Scholar]
- Eddy MC & Green JT (2017) Running wheel exercise reduces renewal of extinguished instrumental behavior and alters medial prefrontal cortex neurons in adolescent, but not adult, rats. Behav. Neurosci, 131, 460–469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehlinger DG, Bergstrom HC, Burke JC, Fernandez GM, McDonald CG & Smith RF (2016) Adolescent nicotine-induced dendrite remodeling in the nucleus accumbens is rapid, persistent, and D1-dopamine receptor dependent. Brain Struct. Funct, 221, 133–45. [DOI] [PubMed] [Google Scholar]
- Erisir A & Harris JL (2003) Decline of the critical period of visual plasticity is concurrent with the reduction of NR2B subunit of the synaptic NMDA receptor in layer 4. J. Neurosci, 23, 5208–5218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fauth M & Tetzlaff C (2016) Opposing effects of neuronal activity on structural plasticity. Front. Neuroanat, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B & Robinson TE (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol. Psychiatry, 58, 751–759. [DOI] [PubMed] [Google Scholar]
- Fischer KD, Houston AC & Rebec GV (2013) Role of the major glutamate transporter GLT1 in nucleus accumbens core versus shell in cue-induced cocaine-seeking behavior. J. Neurosci, 33, 9319–27.23719800 [Google Scholar]
- Funk D, Coen K, Tamadon S, Hope BT, Shaham Y & Lê AD (2016) Role of central amygdala neuronal ensembles in incubation of nicotine craving. J. Neurosci, 36, 8612–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner W, Lehmann M, Liakoni V, Corneil D & Brea J (2018) Eligibility traces and plasticity on behavioral time scales: experimental support of neoHebbian three-factor learning rules. Front. Neural Circuits, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Kupchik YM, Shen H, Reissner KJ, Thomas CA & Kalivas PW (2013a) Relapse induced by cues predicting cocaine depends on rapid, transient synaptic potentiation. Neuron, 77, 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gipson CD, Reissner KJ, Kupchik YM, Smith ACW, Stankeviciute N, Hensley-Simon ME & Kalivas PW (2013b) Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. PNAS, 110, 9124–9129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, Zhuang Y, Feng J, Ying Z & Fan G (2011) Exercise impacts brain-derived neurotrophic factor plasticity by engaging mechanisms of epigenetic regulation. Eur. J. Neurosci, 33, 383–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood BN, Foley TE, Le TV, Strong PV, Loughridge AB, Day HEW & Fleshner M (2011) Long-term voluntary wheel running is rewarding and produces plasticity in the mesolimbic reward pathway. Behav. Brain Res, 217, 354–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigsby KB, Kovarik CM, Rottinghaus GE & Booth FW (2018) High and low nightly running behavior associates with nucleus accumbens N-Methyl-d-aspartate receptor (NMDAR) NR1 subunit expression and NMDAR functional differences. Neurosci. Lett, 671, 50–55. [DOI] [PubMed] [Google Scholar]
- Guezennec CY, Abdelmalki A, Serrurier B, Merino D, Bigard X, Berthelot M, Pierard C & Peres M (1998) Effects of prolonged exercise on brain ammonia and amino acids. Int. J. Sports Med, 19, 323–327. [DOI] [PubMed] [Google Scholar]
- Guillem K, Ahmed SH & Peoples LL (2014) Escalation of cocaine intake and incubation of cocaine seeking are correlated with dissociable neuronal processes in different accumbens subregions. Biol. Psychiatry, 76, 31–9. [DOI] [PubMed] [Google Scholar]
- Haasova M, Warren FC, Ussher M, Janse Van Rensburg K, Faulkner G, Cropley M, Byron-Daniel J, Everson-Hock ES, Oh H & Taylor AH (2013) The acute effects of physical activity on cigarette cravings: systematic review and meta‐analysis with individual participant data. Addiction, 108, 26–37. [DOI] [PubMed] [Google Scholar]
- Harris KM & Stevens JK (1989) Dendritic spines of CA 1 pyramidal cells in the rat hippocampus: serial electron microscopy with reference to their biophysical characteristics. J. Neurosci, 9, 2982–2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris KM & Weinberg RJ (2012) Ultrastructure of synapses in the mammalian brain. Cold Spring Harb. Perspect. Biol, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iversen IH (1993) Techniques for establishing schedules with wheel running as reinforcement in rats. J. Exp. Anal. Behav, 60, 219–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharazia VN & Weinberg RJ (1999) Immunogold localization of AMPA and NMDA receptors in somatic sensory cortex of albino rat. J. Comp. Neurol, 412, 292–302. [DOI] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT & Shaham Y (2009) Role of the ventral medical prefrontal cortex in incubation of cocaine craving. Neuropharmacology, 56, 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lett BT, Grant VL, Byrne MJ & Koh MT (2000) Pairings of a distinctive chamber with the aftereffect of wheel running produce conditioned place preference. Appetite, 34, 87–94. [DOI] [PubMed] [Google Scholar]
- Lett BT, Grant VL & Koh MT (2001) Naloxone attenuates the conditioned place preference induced by wheel running in rats. Physiol. Behav, 72, 355–358. [DOI] [PubMed] [Google Scholar]
- Lisman J (2017) Glutamatergic synapses are structurally and biochemically complex because of multiple plasticity processes: long-term potentiation, long-term depression, short-term potentiation and scaling. Phil. Trans. R. Soc. B, 372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY & Wolf ME (2014) Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacology, 76, 287–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Peterson AB, Sanchez V, Abel J & Smith MA (2013) Exercise as a novel treatment for drug addiction: a neurobiological and stage-dependent hypothesis. Neurosci. Biobehav. Rev, 37, 1622–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch WJ, Tan L, Narmeen S, Beiter R & Brunzell DH (2017) Exercise or saccharin during abstinence block estrus-induced increases in nicotine-seeking. Physiol. Behav, 17, 30377–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCutcheon JE, Wang X, Tseng KY, Wolf ME & Marinelli M (2011) Calcium-permeable AMPA receptors are present in nucleus accumbens synapses after prolonged withdrawal from cocaine self-administration but not experimenter-administered cocaine. J. Neurosci, 31, 5737–5743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CG, Dailey VK, Bergstrom HC, Wheeler TL, Eppolito AK, Smith LN & Smith RF (2005) Periadolescent nicotine administration produces enduring changes in dendritic morphology of medium spiny neurons from nucleus accumbens. Neurosci. Lett, 385, 163–167. [DOI] [PubMed] [Google Scholar]
- McDonald CG, Eppolito AK, Brielmaier JM, Smith LN, Bergstrom HC, Lawhead MR & Smith RF (2007) Evidence for elevated nicotine-induced structural plasticity in nucleus accumbens of adolescent rats. Brain Res, 1151, 211–218. [DOI] [PubMed] [Google Scholar]
- Meeusen R, Smolders I, Sarre S, de Meirleir K, Keizer H, Serneels M, Ebinger G & Michotte Y (1997) Endurance training effects on neurotransmitter release in rat striatum: an in vivo microdialysis study. Acta. Physiol. Scand, 159, 335–341. [DOI] [PubMed] [Google Scholar]
- Meyer D, Bonhoeffer T & Scheuss V (2014) Balance and stability of synaptic structures during synaptic plasticity. Neuron, 82, 430–43. [DOI] [PubMed] [Google Scholar]
- Mulholland PJ & Chandler LJ (2007) The thorny side of addiction: adaptive plasticity and dendritic spines. Sci. World J, 7, 9–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nesil T, Narmeen S, Bakhti-Suroosh A & Lynch WJ (2018) Effect of menthol on nicotine intake and relapse vulnerability in a rat model of concurrent intravenous menthol/nicotine self-administration. Psychopharmacology, [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohlsson C, Engdahl C, Fak F, Andersson A, Windahl SH, Farman HH, Moverare-Skrtic S, Islander U & Sjogren K (2014) Probiotics protect mice from ovariectomy-induced cortical bone loss. PLoS One, 9, e92368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ovtscharoff W, Segal M, Goldin M, Helmeke C, Kreher U, Greenberger V, Herzog A, Michaelis B & Braun K (2008) Electron microscopic 3D-reconstruction of dendritic spines in cultured hippocampal neurons undergoing synaptic plasticity. Dev. Neurobiol, 68, 870–6. [DOI] [PubMed] [Google Scholar]
- Paxinos G & Watson C (2006) The rat brain in stereotaxic coordinates: hard cover edition [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT & Shaham Y (2011) Neurobiology of the incubation of drug craving. Trends Neurosci, 34, 411–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Core Team (2018) R: a language and environment for statistical computing R Foundation for Statistical Computing, Vienna, Austria: URL https://www.R-project.org/ [Google Scholar]
- Real CC, Ferreira AF, Hernandes MS, Britto LR & Pires RS (2010) Exercise-induced plasticity of AMPA-type glutamate receptor subunits in the rat brain. Brain Res, 1363, 63–71. [DOI] [PubMed] [Google Scholar]
- Redila VA & Christie BR (2006) Exercise-induced changes in dendritic structure and complexity in the adult hippocampal dentate gyrus. Neuroscience, 137, 1299–1307. [DOI] [PubMed] [Google Scholar]
- Roberts V, Maddison R, Simpson C, Bullen C & Prapavessis H (2012) The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect, and smoking behaviour: systematic review update and meta-analysis. Psychopharmacology, 222, 1–15. [DOI] [PubMed] [Google Scholar]
- Robinson TE & Kolb B (2004) Structural plasticity associated with exposure to drugs of abuse. Neuropharmacology, 47, 33–46. [DOI] [PubMed] [Google Scholar]
- Robison LS, Swenson S, Hamilton J & Thanos PK (2018) Exercise reduces dopamine D1R and increases D2R in rats: implications for addiction. Med. Sci. Sports Exerc, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Ruegsegger GN, Toedebusch RG, Childs TE, Grigsby KB & Booth FW (2017) Loss of Cdk5 function in the nucleus accumbens decreases wheel running and may mediate age-related declines in voluntary physical activity. J. Physiol, 595, 363–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH & Lynch WJ (2013) Effect of wheel-running during abstinence on subsequent nicotine-seeking in rats. Psychopharmacology, 227, 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez V, Moore CF, Brunzell DH & Lynch WJ (2014) Sex differences in the effect of wheel running on subsequent nicotine-seeking in a rat adolescent-onset self-administration model. Psychopharmacology, 231, 1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santuy A, Rodríguez J, DeFelipe J & Merchán-Pérez A (2017) Study of the size and shape of synapses in the juvenile rat somatosensory cortex with 3D electron microscopy. eNeuro, 5, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR & Pickel VM (1990) In the rat medial nucleus accumbens, hippocampal and catecholamingeric terminals converge on spiny neurons and are in apposition to each other. Brain Research, 527, 266–279. [DOI] [PubMed] [Google Scholar]
- Shen H, Moussawi K, Zhou W, Toda S & Kalivas PW (2011) Heroin relapse requires long-term potentiation-like plasticity mediated by NMDA2b-containing receptors. PNAS, 108, 19407–19412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AD & Bolam JP (1990) The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci, 13, 259–265. [DOI] [PubMed] [Google Scholar]
- Smith WB, Starck SR, Roberts RW & Schuman EM (2005) Dopaminergic stimulation of local protein synthesis enhances surface expression of GluR1 and synaptic transmission in hippocampal neurons. Neuron, 45, 765–779. [DOI] [PubMed] [Google Scholar]
- Stranahan AM, Khalil D & Gould E (2007) Running induces widespread structural alterations in the hippocampus and entorhinal cortex. Hippocampus, 17, 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takumi Y, Ramírez-León V, Laake P & Rinvik E (1999) Different modes of expression of AMPA and NMDA receptors in hippocampal synapses. Nature, 2, 618–624. [DOI] [PubMed] [Google Scholar]
- Taylor AH, Ussher MH & Faulkner G (2007) The acute effects of exercise on cigarette cravings, withdrawal symptoms, affect and smoking behaviour: a systematic review. Addiction, 102, 534–543. [DOI] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General Atlanta: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention, National Center for Chronic Disease Prevention and Health Promotion, Office on Smoking and Health, 2014. [Google Scholar]
- Venniro M, Caprioli D & Shaham Y (2016) Animal models of drug relapse and craving: from drug priming-induced reinstatement to incubation of craving after voluntary abstinence. Prog. Brain Res, 224, 25–52. [DOI] [PubMed] [Google Scholar]
- Wilson WM & Marsden CA (1995) Extracellular dopamine in the nucleus accumbens of the rat during treadmill running. Acta. Physiol. Scand, 155, 465–466. [DOI] [PubMed] [Google Scholar]
- Wolf ME & Tseng KY (2012) Calcium-permeable AMPA receptors in the VTA and nucleus accumbens after cocaine exposure: when, how, and why? Front. Mol. Neurosci, 5, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagishita S, Hayashi-Takagi A, Ellis-Davies GC, Urakubo H, Ishii S & Kasai H (2014) A critical time window for dopamine actions on the structural plasticity of dendritic spines. Science, 345, 1616–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasumatsu N, Matsuzaki M, Miyazaki T, Noguchi J & Kasai H (2008) Principles of long-term dynamics of dendritic spines. J. Neurosci, 28, 13592–13608. [DOI] [PMC free article] [PubMed] [Google Scholar]