Abstract
Background.
Timely postoperative radiation therapy (RT) within 50 days of surgery for head and neck cancers provides a survival advantage.
Methods.
Using the NCDB, we performed a propensity score-matched analysis comparing patients undergoing open or endoscopic surgery for squamous cell carcinoma (SCC) of the nasal cavity and paranasal sinuses from 2010–2015.
Results.
Among 168 pairs, patients undergoing endoscopic surgery had shorter time to surgery (24.2 vs. 36.7 days, p<0.001) and shorter postoperative time to RT (PTTR, 51.2 vs. 58.4 days, p=0.020). On multivariable linear regression, endoscopic surgery predicted shorter PTTR (β=−7.6, p=0.013). Using the Kaplan-Meier method, patients in the longest PTTR quartile had decreased overall survival (Q1 vs. Q4, 3-year OS 76.5% vs. 53.3%, p=0.007), a durable finding when adjusted for covariates (Q1 vs. Q4, HR 0.50, p=0.008).
Conclusions.
Patients undergoing endoscopic surgery for sinonasal SCC experience shorter PTTR. Shorter PTTR is associated with extended OS.
Keywords: National Cancer Database, overall survival, postoperative time to radiation therapy, sinonasal squamous cell carcinoma, time to surgery
Introduction
Malignant sinonasal tumors constitute 3% of head and neck malignancies.1 These lesions most commonly arise from the nasal cavity, followed by the maxillary and ethmoid sinuses.2 Squamous cell carcinoma (SCC) is the most common histology and carries a poor prognosis with a 5-year overall survival ranging from 30.2% to 59.5%.3–6 The traditional surgical management of advanced sinonasal tumors with skull base invasion involves open surgical resection via a craniofacial approach, as it offers excellent access and visualization for optimal complete resection.7–10 Open surgery requires a facial incision and for those undergoing craniofacial resection, a craniotomy. Although these techniques are effective and in many cases the only appropriate approach, they are associated with significant rates of wound infections and central nervous system complications.9–11 As a result, for the properly selected candidate, an endoscopic approach to sinonasal tumor resection has become an increasingly attractive alternative to open surgery, with the literature demonstrating reduced complications and comparable rates of complete resection.12–19
Recent studies have reported that delays in treatment for head and neck cancers20 are associated with clinical-to-pathologic upstaging and inferior survival.21,22 One recent study demonstrated that postoperative time to radiation therapy (RT) of at least 50 days was associated with inferior overall survival.23 However, within the context of sinonasal cancers, no studies have been performed specifically investigating the timing of postoperative treatment. Furthermore, the relationship between surgical approach and postoperative time to RT (PTTR) has not been studied. In the present study, we sought to assess differences in radiation treatment timing between patients undergoing open surgery and those undergoing endoscopic surgery, as well as associations with treatment timing with long-term survival. We hypothesized that given the decreased time required for postoperative healing and the decreased risk of adverse events following endoscopic surgery, patients undergoing endoscopic approaches experience shorter PTTR. In addition, we hypothesize that shorter PTTR is associated with extended overall survival.
Methods
Data Source
The National Cancer Database (NCDB) is a nationwide clinical oncology database jointly sponsored by the American College of Surgeons and the American Cancer Society. The NCDB contains hospital registry data on malignancies, treatments, and outcomes from more than 1,500 Commission on Cancer-accredited facilities, representing more than 70% of newly diagnosed cancer cases in the United States and including more than 34 million historical records since 1985.24 The data used in the study are derived from a de-identified NCDB file. The ACS and CoC have not verified and are not responsible for the statistical methodology employed, or the conclusions drawn from these data. Additionally, this study was exempt from institutional approval, as all data analyzed were from the NCDB with no identifying information; accordingly, no written informed consent was required.
Patient Population
For the present study, we searched the NCDB for all patients recorded from 2010 to 2015 with head and neck cancers, and selected tumors originating from the nasal cavity and paranasal sinuses. The following International Classification of Diseases for Oncology, 3rd edition (ICD-O-3) histology codes were used to confirm patients with sinonasal SCC: 8070, 8071, 8072, 8074, 8075, 8083. The following ICD-O-3 topological codes were used to confirm patients with SCC of the nasal cavity and paranasal sinuses: C300, C310, C311. Patients treated without a primary surgical approach or postoperative RT were excluded. Patients treated by non-open, non-endoscopic surgical approaches or unknown surgical approaches were excluded. Patients with unknown clinical staging or distant metastatic disease at diagnosis were excluded. Patients were not excluded on the basis of missing pathologic staging. Patients with missing outcomes, such as length of hospital stay following surgery, unplanned 30-day readmissions, and overall survival (OS) status, were also excluded. Patients were grouped into two cohorts, one treated with open surgery and one treated with endoscopic surgery; a propensity score match was then performed to arrive at two balanced cohorts for analysis.
Study Outcomes and Variable Definitions
The primary outcome of the study was PTTR, measured as days elapsed between surgery and RT. Patients were also grouped based upon PTTR quartile to examine the general effect of PTTR duration rather than smaller day-by-day changes. Sociodemographic, disease, treatment, and follow-up data were extracted for each patient from the NCDB. Patient comorbidity was assessed using the Charlson comorbidity index, reported in the NCDB as the Charlson-Deyo score, with three possible truncated values of 0, 1, or more than 1.25,26 Additional time points recorded included time from diagnosis to surgery (TTS), time from diagnosis to RT (TTR), and time from surgery to discharge (LOS). Additional outcomes of interest included unanticipated 30-day readmission and OS using the time from diagnosis to death or last follow-up.
Statistical Analysis
A propensity score match was performed to arrive at two balanced cohorts of patients treated with open or endoscopic surgery. To perform the propensity score match, we created a logistic regression model where the response variable was surgical approach. All variables listed in Table 1 were included in the model. The match was made using the predicted values on the logit scale from the final model using the MatchIt 3.0.2 package.27 Propensity scores within one unit of each other on the logit scale were considered a match, and the ratio of open to endoscopic patients was fixed at 1:1. To measure covariate balance between the two groups, we computed the standardized difference for each variable before and after matching.28 The following formulas were used to calculate standardized differences: 100(X2 – X1)/[(S22 + S12)/2]1/2, X2 and X1 are sample means in the endoscopic and open groups, respectively, and S22 + S12 are the corresponding sample variances for continuous variables; 100(P2-P1)/[(P2(1-P2)+P1(1-P1))/2]1/2, P1 and P2 are the sample proportion in the endoscopic and open groups, respectively, for categorical variables. Standardized differences less than 10.0 in absolute value were considered balanced.28
Table 1.
Summary Patient Characteristics
| All Patients | Matched Patients | |||||
|---|---|---|---|---|---|---|
| Characteristic | Open | Endoscopic | Std Diff† | Open | Endoscopic | Std Diff† |
| N | 663 | 168 | 168 | 168 | ||
| Age (Years) | 62.7±12.4 | 62.7±12.8 | 0.00 | 63.3±11.5 | 62.7±12.8 | −4.93 |
| Sex | ||||||
| Male | 434 (65.5%) | 105 (62.5%) | −6.25 | 111 (66.1%) | 105 (62.5%) | −7.52 |
| Female | 229 (34.5%) | 63 (37.5%) | 6.25 | 57 (33.9%) | 63 (37.5%) | 7.52 |
| Race | ||||||
| White | 557 (84.0%) | 142 (84.5%) | 1.37 | 144 (85.7%) | 142 (84.5%) | −3.37 |
| Black | 73 (11.0%) | 22 (13.1%) | 6.45 | 21 (12.5%) | 22 (13.1%) | 1.80 |
| Other | 31 (4.7%) | 3 (1.8%) | −16.41 | 3 (1.8%) | 3 (1.8%) | 0.00 |
| Unknown | 2 (0.3%) | 1 (0.6%) | 4.48 | 0 (0.0%) | 1 (0.6%) | 10.99 |
| Hispanic Ethnicity | ||||||
| Non-Hispanic | 608 (91.7%) | 157 (93.5%) | 6.88 | 159 (94.6%) | 157 (93.5%) | −4.65 |
| Hispanic | 40 (6.0%) | 8 (4.8%) | −5.31 | 6 (3.6%) | 8 (4.8%) | 5.99 |
| Unknown | 15 (2.3%) | 3 (1.8%) | −3.53 | 3 (1.8%) | 3 (1.8%) | 0.00 |
| Insurance Status | ||||||
| Private Insurance | 260 (39.2%) | 67 (39.9%) | 1.43 | 70 (41.7%) | 67 (39.9%) | −3.66 |
| Medicare | 288 (43.4%) | 78 (46.4%) | 6.03 | 79 (47.0%) | 78 (46.4%) | −1.20 |
| Medicaid | 67 (10.1%) | 16 (9.5%) | −2.02 | 18 (10.7%) | 16 (9.5%) | −3.98 |
| Other Government | 13 (2.0%) | 3 (1.8%) | −1.46 | 1 (0.6%) | 3 (1.8%) | 11.04 |
| Uninsured | 29 (4.4%) | 4 (2.4%) | −11.05 | 0 (0.0%) | 4 (2.4%) | 22.18 |
| Unknown | 6 (0.9%) | 0 (0.0%) | −13.48 | 0 (0.0%) | 0 (0.0%) | 0.00 |
| ZIP-Code Level Income ($) | ||||||
| < 38,000 | 149 (22.5%) | 29 (17.3%) | −13.05 | 24 (14.3%) | 29 (17.3%) | 8.23 |
| 38,000–47,999 | 149 (22.5%) | 43 (25.6%) | 7.26 | 47 (28.0%) | 43 (25.6%) | −5.42 |
| 48,000–62,999 | 180 (27.1%) | 50 (29.8%) | 5.99 | 46 (27.4%) | 50 (29.8%) | 5.31 |
| ≥ 63,000 | 183 (27.6%) | 45 (26.8%) | −1.80 | 49 (29.2%) | 45 (26.8%) | −5.35 |
| Unknown | 2 (0.3%) | 1 (0.6%) | 4.48 | 2 (1.2%) | 1 (0.6%) | −6.36 |
| CDCC | ||||||
| 0 | 480 (72.4%) | 127 (75.6%) | 7.30 | 128 (76.2%) | 127 (75.6%) | −1.40 |
| 1 | 148 (22.3%) | 28 (16.7%) | −14.17 | 25 (14.9%) | 28 (16.7%) | 4.94 |
| 2 | 27 (4.1%) | 9 (5.4%) | 6.11 | 12 (7.1%) | 9 (5.4%) | −7.03 |
| ≥ 3 | 8 (1.2%) | 4 (2.4%) | 9.04 | 3 (1.8%) | 4 (2.4%) | 4.19 |
| Facility Type | ||||||
| Academic/Research | 442 (66.7%) | 99 (58.9%) | −16.19 | 100 (59.5%) | 99 (58.9%) | −1.22 |
| Comprehensive Community | 109 (16.4%) | 44 (26.2%) | 24.11 | 48 (28.6%) | 44 (26.2%) | −5.38 |
| Community | 19 (2.9%) | 9 (5.4%) | 12.56 | 4 (2.4%) | 9 (5.4%) | 15.54 |
| Integrated Network | 71 (10.7%) | 11 (6.5%) | −15.02 | 14 (8.3%) | 11 (6.5%) | −6.88 |
| Unknown | 22 (3.3%) | 5 (3.0%) | −1.72 | 2 (1.2%) | 5 (3.0%) | 12.58 |
| Transition Between Facilities for Treatment | ||||||
| No | 231 (34.8%) | 71 (42.3%) | 15.46 | 77 (45.8%) | 71 (42.3%) | −7.05 |
| Yes | 432 (65.2%) | 97 (57.7%) | −15.46 | 91 (54.2%) | 97 (57.7%) | 7.05 |
| Cancer Primary Site | ||||||
| Sinuses | 344 (51.9%) | 68 (40.5%) | −23.02 | 70 (41.7%) | 68 (40.5%) | −2.44 |
| Nasal Cavity | 319 (48.1%) | 100 (59.5%) | 23.02 | 98 (58.3%) | 100 (59.5%) | 2.44 |
| Clinical T Classification | ||||||
| 1 | 103 (15.5%) | 34 (20.2%) | 12.30 | 33 (19.6%) | 34 (20.2%) | 1.50 |
| 2 | 115 (17.3%) | 28 (16.7%) | −1.60 | 23 (13.7%) | 28 (16.7%) | 8.36 |
| 3 | 144 (21.7%) | 35 (20.8%) | −2.20 | 34 (20.2%) | 35 (20.8%) | 1.49 |
| 4 | 301 (45.4%) | 71 (42.3%) | −6.25 | 78 (46.4%) | 71 (42.3%) | −8.26 |
| Clinical N Classification | ||||||
| 0 | 544 (82.1%) | 150 (89.3%) | 20.68 | 149 (88.7%) | 150 (89.3%) | 1.92 |
| 1 | 52 (7.8%) | 6 (3.6%) | −18.19 | 9 (5.4%) | 6 (3.6%) | −8.69 |
| 2 | 67 (10.1%) | 12 (7.1%) | −10.72 | 10 (6.0%) | 12 (7.1%) | 4.45 |
Values are presented as mean ± standard deviation or number (%).
Abbreviations: N, Number; ZIP, Zone Improvement Plan; CDCC, Charlson/Deyo Comorbidity Condition; T, Tumor; N, Node.
Standardized difference = 100(X2 – X1)/[(S22 + S12)/2]1/2, X2 and X1 are sample means in the endoscopic and open groups, respectively, and S22 + S12 are the corresponding sample variances for continuous variables. Standardized difference = 100(P2-P1)/[(P2(1-P2)+P1(1-P1))/2]1/2, P2 and P2 are the sample proportion in the endoscopic and open groups, respectively, for categorical variables. Standardized differences less than 10 in absolute value are considered to be balanced.
After establishing the matched cohorts, quantitative outcomes were compared using the Wilcoxon rank-sum test for non-parametric comparisons and qualitative outcomes were compared using Pearson’s chi-squared tests. Kaplan-Meier analysis was performed to estimate TTS, LOS, TTR, and PTTR, and log-rank tests were used to compare distributions between patients receiving open surgery and those undergoing endoscopic surgery. Kaplan-Meier analysis was also performed to estimate OS with log-rank tests comparing survival distributions. Multivariable linear regression was used to identify independent predictors of PTTR, and Cox proportional hazards regression was used to identify independent predictors of OS. Backward stepwise regression was used to arrive at the final model, with p<0.20 as the stopping criterion. All values of p<0.05 were considered statistically significant. All data were analyzed using R version 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria)29 and graphs were produced using GraphPad Prism 7.30
Results
Patient Characteristics
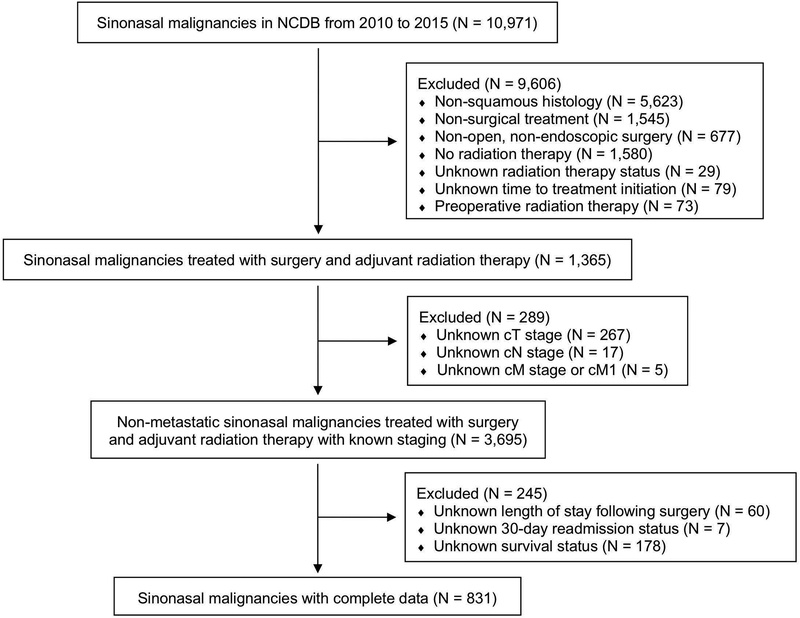
A total of 831 patients met inclusion criteria. A Consolidated Standards of Reporting Trials diagram detailing the complete process for establishing our final cohort is shown in Figure 1. Among these patients, 663 underwent open surgery and 168 underwent endoscopic surgery. Prior to the propensity score match, several patient characteristics were unbalanced, with endoscopic patients more often having nasal cavity tumors (59.5% vs. 48.1%, standardized difference 23.02), T1 tumors (20.2% vs. 15.5%, standardized difference 12.30), and N0 disease (89.3% vs. 82.1%, standardized difference 20.68) compared to patients undergoing open surgery (Table 1). Following the propensity score match, we arrived at our final cohorts with 168 patients in each. Nearly all variables were found to be balanced, with only rare characteristics being unbalanced due to small sample size: unknown race, other government insurance, no insurance, treatment at community facilities, and treatment at unknown facilities.
Figure 1. Consolidated Standards of Reporting Trials Diagram Detailing the Study Inclusion Criteria.
NCDB, National Cancer Database; N, number; cT, tumor classification; cN, node classification; cM, metastasis classification; cM1, positive metastasis classification
Timing of Patient Treatments and Outcomes
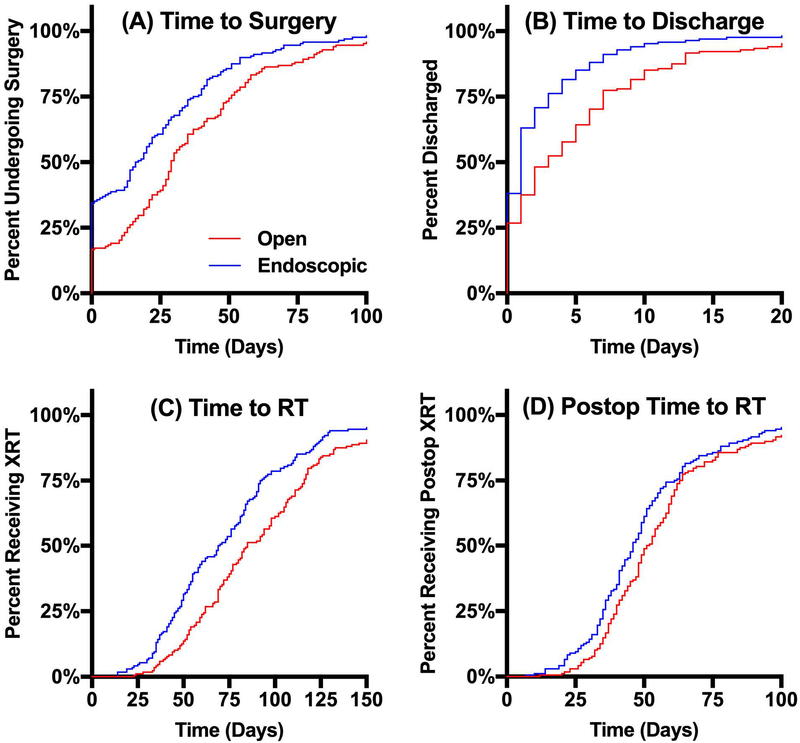
The two matched cohorts were compared to assess for differences in treatment timing associated with surgical approach (Table 2). Patients who underwent endoscopic surgery were found to have significantly shorter median TTS (16.5 [IQR 0.0–38.5] vs. 29.0 [13.3–51.0] days, p<0.001). Following surgery, patients who underwent endoscopic surgery had significantly shorter median LOS (1.0 [0.0–3.0] vs. 3.0 [0.0–7.0] days, p<0.001). Patients who underwent endoscopic surgery had significantly shorter TTR (69.5 [46.3–92.8] vs. 85.0 [62.0–116.0] days, p<0.001), as well as PTTR (46.0 [35.0–61.8] vs. 51.5 [40.0–63.8], p=0.010). No significant difference in 30-day unplanned readmission rate was observed between endoscopic and open surgery (2.4% vs. 3.6%, p=0.748). The Kaplan-Meier method was also employed to visually compare the distributions of TTS, LOS, TTR, and PTTR as shown in Figure 2.
Table 2.
Treatment Timing and Outcomes
| Characteristic | Open | Endoscopic | p-value |
|---|---|---|---|
| Time from Diagnosis to Surgery (days) | 29.0 [13.3–51.0] | 16.5 [0.0–38.5] | <0.001* |
| Time from Surgery to Discharge (days) | 3.0 [0.0–7.0] | 1.0 [0.0–3.0] | <0.001* |
| Time from Diagnosis to RT (days) | 85.0 [62.0–116.0] | 69.5 [46.3–92.8] | <0.001* |
| Time from Surgery to RT (days) | 51.5 [40.0–63.8] | 46.0 [35.0–61.8] | 0.010* |
| 30-Day Unplanned Readmission | 6 (3.6%) | 4 (2.4%) | 0.748 |
Values are presented as median [interquartile range] or number (%). P-values calculated using the Wilcoxon rank-sum test or Pearson’s chi-squared test comparing characteristics between groups.
Abbreviations: RT, Radiation Therapy.
Statistically significant: p≤0.05
Figure 2. Kaplan-Meier Curves Estimating Times to Events.
Kaplan-Meier estimates of time to events subset by surgical approach. (A) Median time to surgery did not have a statistically significant association between endoscopic surgery and open surgery (16.5 vs. 29.0 days, p<0.001). (B-D) Median time to discharge (1.0 vs. 3.0 days, p<0.001), median time to RT (69.5 vs. 85.0 days, p<0.001), and median postoperative time to RT (46.0 vs. 51.5 days, p=0.010) were all significantly shorter for patients undergoing endoscopic surgery compared to those undergoing open surgery.
The results of our multivariable linear regression to model PTTR are reported in Table 3. The lowest median ZIP-code level income was associated with significantly longer PTTR on multivariable regression (>$63,000 vs. <$38,000, β = −12.7, 95% Confidence Interval [CI] −20.7 to −4.8, p=0.002). Patients treated at comprehensive community centers had significantly shorter PTTR compared to those treated at academic facilities on multivariable regression (β = −10.3, 95% CI −17.6 to −2.9, p=0.006). Patients with decreased comorbidity load had shorter PTTR (CDCC 1 vs. 0, β = −8.3, 95% CI −16.5 to −0.2, p=0.046) while more severe comorbidity load had no statistically significant difference in PTTR (CDCC ≥ 3 vs. 0, β = 15.1, 95% CI −0.7 to 30.8, p=0.061) on multivariable regression. Nodal classification had an inconsistent association with PTTR, as cN1 patients had shorter PTTR (β = −10.4, 95% CI −20.7 to −0.1, p=0.021) but cN2 patients had longer PTTR (β = 10.8, 95% CI 1.6 to 19.9, p=0.016). Patients who underwent endoscopic surgery had significantly shorter PTTR compared to those who underwent open surgery (β = −3.6, 95% CI −6.5 to −0.7, p=0.016).
Table 3.
Multivariable Linear Regression Model Predicting Postoperative Time to RT:
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Characteristic | β (95% CI) | p-value | β (95% CI) | p-value |
| Age | 0.2 (−0.1 to 0.4) | 0.151 | 0.2 (−0.1 to 0.5) | 0.101 |
| Sex | ||||
| Male | [ref] | - | - | |
| Female | 1.0 (−2.1 to 4.2) | 0.529 | - | - |
| Race | ||||
| White | [ref] | - | - | |
| Black | 8.1 (−8.2 to 24.3) | 0.330 | - | - |
| Other | −4.2 (−26.2 to 17.8) | 0.708 | - | - |
| Unknown | −3.9 (−45.9 to 38.1) | 0.857 | - | - |
| Hispanic Ethnicity | ||||
| Non-Hispanic | [ref] | - | - | |
| Hispanic | 0.7 (−11.8 to 13.2) | 0.911 | - | - |
| Unknown | 6.2 (−9.7 to 22.1) | 0.445 | - | - |
| Insurance Status | ||||
| Private Insurance | [ref] | - | - | |
| Medicare | 5.2 (−3.6 to 14.1) | 0.246 | - | - |
| Medicaid | 4.8 (−5.2 to 15.8) | 0.395 | - | - |
| Other Government | 2.2 (−20.9 to 25.2) | 0.854 | - | - |
| Uninsured | −16.3 (−39.4 to 6.7) | 0.164 | - | - |
| ZIP-Code Level Income ($) | ||||
| < 38,000 | [ref] | [ref] | ||
| 38,000–47,999 | −12.6 (−20.7 to −4.6) | 0.002* | −12.4 (−20.4 to −4.3) | 0.003* |
| 48,000–62,999 | −13.4 (−21.4 to −5.5) | 0.001* | −13.4 (−21.4 to −5.4) | 0.001* |
| ≥ 63,000 | −13.8 (−21.8 to −5.8) | <0.001* | −12.7 (−20.7 to −4.8) | 0.002* |
| Unknown | 54.0 (28.8 to 79.2) | <0.001* | 52.9 (27.6 to 78.1) | <0.001* |
| CDCC | ||||
| 0 | [ref] | [ref] | ||
| 1 | −6.9 (−15.2 to 1.5) | 0.107 | −8.3 (−16.5 to −0.2) | 0.046* |
| 2 | −0.6 (−11.3 to 10.1) | 0.910 | −1.4 (−12.0 to 9.1) | 0.788 |
| ≥ 3 | 11.5 (−4.6 to 27.7) | 0.161 | 15.1 (−0.7 to 30.8) | 0.061 |
| Facility Type | ||||
| Academic/Research | [ref] | [ref] | ||
| Comprehensive Community | −7.8 (−15.1 to −0.5) | 0.037* | −10.3 (−17.6 to −2.9) | 0.006* |
| Community | 9.7 (−3.5 to 22.9) | 0.149 | 7.4 (−5.8 to 20.6) | 0.270 |
| Integrated Network | 6.0 (−4.3 to 16.3) | 0.253 | 4.9 (−5.4 to 15.2) | 0.351 |
| Unknown | −5.7 (−22.8 to 11.4) | 0.514 | −0.1 (−18.1 to 17.8) | 0.987 |
| Transition Between Facilities for Treatment | ||||
| No | [ref] | - | - | |
| Yes | 0.8 (−2.3 to 3.8) | 0.623 | - | - |
| Cancer Primary Site | ||||
| Sinuses | [ref] | - | - | |
| Nasal Cavity | −2.2 (−5.3 to 0.8) | 0.153 | - | - |
| Clinical T Classification | ||||
| 1 | [ref] | - | - | |
| 2 | −2.1 (−8.5 to 4.3) | 0.523 | - | - |
| 3 | 1.3 (−4.5 to 7.0) | 0.668 | - | - |
| 4 | 3.0 (−1.6 to 7.6) | 0.196 | - | - |
| Clinical N Classification | ||||
| 0 | [ref] | [ref] | ||
| 1 | −8.1 (−18.5 to 2.2) | 0.124 | −10.4 (−20.7 to −0.1) | 0.021* |
| 2 | 8.5 (−0.8 to 17.7) | 0.072 | 10.8 (1.6 to 19.9) | 0.016* |
| Surgical Approach | ||||
| Open | [ref] | [ref] | ||
| Endoscopic | −3.6 (−6.6 to −0.6) | 0.020* | −3.6 (−6.5 to −0.7) | 0.016* |
β, Beta Coefficient; ZIP, Zone Improvement Plan; CDCC, Charlson/Deyo Comorbidity Condition
Statistically significant: p≤0.05.
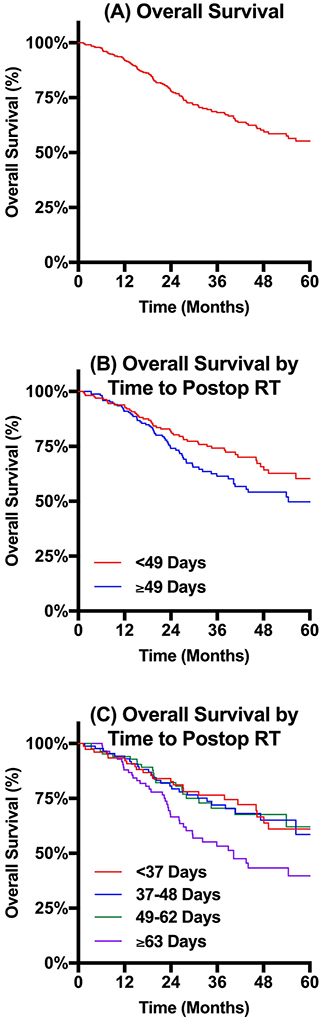
Kaplan-Meier curves estimate OS for all patients and comparing OS distributions between subsets of patients defined by PTTR are reported in Figure 3. 3-year OS for the entire cohort was 68.2%. When patients were subset by PTTR relative to the median PTTR of 49 days, patients with PTTR less than 49 days had significantly extended OS compared to those with PTTR of at least 49 days (74.2% vs. 61.4%, p=0.043). When patients were further subset by PTTR quartile, there was no statistically significant difference observed at 3-year OS between the lowest three quartiles (76.5% vs. 72.0% vs. 70.5%). In contrast, the quartile of patients with the longest PTTR of at least 63 days had significantly shorter 3-year OS (53.3%, p=0.007).
Figure 3. Kaplan-Meier Curves Estimating Overall Survival.
Kaplan-Meier estimates of overall survival. (A) Overall survival for the complete cohort had a 68.2% 3-year OS. (B) Overall survival subset by postoperative time to RT relative to the median (49 days) revealed significantly longer survival for patients waiting less than the median compared to those waiting at least the median (3-year OS 74.2% vs. 61.4%, p=0.043). Overall survival subset by quartile of postoperative time to RT revealed no significant differences in OS between the first three quartiles (3-year OS 76.5% vs. 72.0% vs. 70.5%), but significantly shortened OS for the quartile with the longest wait (3-year OS 53.3%, p=0.007).
Cox proportional hazards regression modeling OS adjusting for covariates is reported in Table 4. Increasing T classification was found to be prognostic of inferior OS (HR 2.02, 95% CI 1.20–3.63, p=0.008). Finally, shorter PTTR was associated with significantly extended OS; compared to the longest PTTR quartile (Q4, ≥63 days), Q3 (49–62 days, HR 0.57, 95% CI 0.33–0.95, p=0.032), Q2 (37–48 days, HR 0.51, 95% CI 0.31–0.84, p=0.007), and Q1 (<37 days, HR 0.50, 95% CI 0.29–0.83, p=0.008) all predicted extended OS.
Table 4.
Cox Proportional Hazards Regression Model Predicting Overall Survival:
| Univariable | Multivariable | |||
|---|---|---|---|---|
| Characteristic | HR (95% CI) | p-value | HR (95% CI) | p-value |
| Age | 1.01 (0.99–1.03) | 0.204 | - | - |
| Sex | ||||
| Male | [ref] | - | - | |
| Female | 0.81 (0.54–1.18) | 0.277 | - | - |
| Race | ||||
| White | [ref] | - | - | |
| Black | 1.13 (0.64–1.86) | 0.656 | - | - |
| Other | 1.25 (0.21–3.97) | 0.759 | - | - |
| Hispanic Ethnicity | ||||
| Non-Hispanic | [ref] | - | - | |
| Hispanic | 1.31 (0.51–2.74) | 0.538 | - | - |
| Unknown | 1.04 (0.17–3.29) | 0.953 | - | - |
| Insurance Status | ||||
| Private Insurance | [ref] | - | - | |
| Medicare | 1.22 (0.82–1.82) | 0.325 | - | - |
| Medicaid | 0.89 (0.43–1.84) | 0.754 | - | - |
| Other Government | 0.83 (0.11–6.06) | 0.852 | - | - |
| Uninsured | 0.59 (0.08–4.27) | 0.566 | - | - |
| ZIP-Code Level Income ($) | ||||
| < 38,000 | [ref] | - | - | |
| 38,000–47,999 | 0.88 (0.48–1.62) | 0.690 | - | - |
| 48,000–62,999 | 1.05 (0.59–1.88) | 0.867 | - | - |
| ≥ 63,000 | 1.10 (0.61–1.99) | 0.745 | - | - |
| Unknown | 2.12 (0.49–9.17) | 0.363 | - | - |
| CDCC | ||||
| 0 | [ref] | - | - | |
| 1 | 1.01 (0.58–1.65) | 0.984 | - | - |
| 2 | 1.40 (0.65–2.64) | 0.360 | - | - |
| ≥ 3 | 2.33 (0.71–5.60) | 0.144 | - | - |
| Facility Type | ||||
| Academic/Research | [ref] | - | - | |
| Comprehensive Community | 0.75 (0.48–1.17) | 0.197 | - | - |
| Community | 1.55 (0.62–3.83) | 0.377 | - | - |
| Integrated Network | 0.61 (0.27–1.41) | 0.220 | - | - |
| Unknown | 0.27 (0.04–1.93) | 0.098 | - | - |
| Transition Between Facilities for Treatment | ||||
| No | [ref] | - | - | |
| Yes | 0.88 (0.61–1.28) | 0.509 | - | - |
| Cancer Primary Site | ||||
| Sinuses | [ref] | - | - | |
| Nasal Cavity | 0.82 (0.56–1.19) | 0.283 | - | - |
| Clinical T Classification | ||||
| 1 | [ref] | [ref] | ||
| 2 | 1.02 (0.48–2.13) | 0.948 | 1.04 (0.49–2.16) | 0.926 |
| 3 | 1.39 (0.72–2.72) | 0.323 | 1.33 (0.69–2.61) | 0.394 |
| 4 | 2.09 (1.24–3.75) | 0.005* | 2.02 (1.20–3.63) | 0.008* |
| Clinical N Classification | ||||
| 0 | [ref] | - | - | |
| 1 | 1.26 (0.44–2.79) | 0.626 | - | - |
| 2 | 1.39 (0.65–2.60) | 0.369 | - | - |
| Surgical Approach | ||||
| Open | [ref] | [ref] | ||
| Endoscopic | 1.33 (0.92–1.94) | 0.133 | 1.41 (0.96–2.08) | 0.079 |
| Time from Diagnosis to Surgery (days) | 1.00 (0.99–1.00) | 0.575 | - | - |
| Time from Surgery to Discharge (days) | 1.00 (0.98–1.01) | 0.907 | - | - |
| Time from Surgery to RT (days) | 1.01 (1.00–1.02) | <0.001* | - | - |
| Q4 (≥63 days) | [ref] | [ref] | ||
| Q3 (49–62 days) | 0.51 (0.30–0.85) | 0.010* | 0.57 (0.33–0.95) | 0.032* |
| Q2 (37–48 days) | 0.53 (0.32–0.86) | 0.010* | 0.51 (0.31–0.84) | 0.007* |
| Q1 (<37 days) | 0.49 (0.29–0.81) | 0.006* | 0.50 (0.29–0.83) | 0.008* |
| Margins | ||||
| Negative | [ref] | - | - | |
| Positive | 1.50 (0.98–2.28) | 0.059 | - | - |
| Unknown | 1.15 (0.69–1.86) | 0.579 | - | - |
| 30-Day Unplanned Readmission | ||||
| No | [ref] | - | - | |
| Yes | 0.69 (0.17–1.84) | 0.504 | - | - |
β, Beta Coefficient; ZIP, Zone Improvement Plan; CDCC, Charlson/Deyo Comorbidity Condition
Statistically significant: p≤0.05.
Discussion
Given the established link between treatment delays and inferior outcomes for patients with head and neck cancer,20–22 it is critical to understand the relationship between surgical approach and timing of treatments. Sometimes open surgical resection is the only approach suitable for a sinonasal tumor; however, when there are options available between an open and endoscopic approach, it is imperative to understand the nuances between approaches with respect to treatment outcomes. In the present study, after performing a propensity-score match to arrive at comparable cohorts of patients with sinonasal tumors, patients who underwent endoscopic surgery were found to have significantly decreased wait times with shorter TTS, total TTR, and PTTR. When modeling PTTR, patients who underwent endoscopic surgery had postoperative waiting times more than one week shorter than those who underwent open surgery. Additionally, patients who had extended PTTR were found to have the worst OS outcomes with the longest PTTR quartile of at least 63 days achieving only 53.3% 3-year OS. Taken together, these data reveal a possible advantage of endoscopic surgery in the appropriately selected patient, in addition to the potential for reduced complications.12–19
Murphy et al. recently used the NCDB to report increases in time to treatment initiation in recent years across patients with cancer of the oral cavity, oropharynx, larynx, and hypopharynx.20 Additionally, follow-up studies have revealed such delays in treatment to be associated with increased mortality,21 with clinical-to-pathologic upstaging likely functioning as the underlying mechanism with increasing delays.22 When examining PTTR using the NCDB, one recent study showed that patients with head and neck cancers waiting at least 50 days for postoperative RT experienced significantly inferior OS.23 Another recent study by Tumati et al. assessed relationships between treatment delays and adverse cancer outcomes like locoregional recurrence and distant metastases.31 Time from biopsy to surgery greater than 50 days was associated with increased distant metastases, while time to radiation therapy greater than 43 days was associated with locoregional recurrence. While no prior studies have specifically investigated patterns of delay in treating sinonasal cancers, it follows that treatment delays are suboptimal and best avoided if possible.32–34
The use of propensity-score matching in this study ensured comparable cohorts of patients treated with either open or endoscopic surgery. After establishing these cohorts, endoscopic patients experienced significantly shorter wait times for treatments, both following diagnosis awaiting surgery and following surgery awaiting adjuvant RT. While not the focus of the present study, the greatest difference in wait time was observed for time to surgery. Patients undergoing open surgery had wait times more than 50% longer than those undergoing endoscopic surgery (36.7 vs. 24.2 days), perhaps suggesting faster preoperative preparation for endoscopic cases. More notably, and as hypothesized, endoscopic patients waited approximately one week less than open patients following surgery for adjuvant RT. Of note, delays in both TTS and PTTR for patients undergoing open surgery resulted in a nearly 20-day delay in total TTR compared to those undergoing endoscopic surgery.
Using multivariable linear regression the endoscopic approach continued to significantly predict shorter PTTR by more than three days. Overall patient complexity, approximated by the CDCC measure of comorbidity status, had an inconsistent relationship with PTTR, as CDCC score of 1 had decreased PTTR, while CDCC greater than 1 had no statistically significant association with PTRR; however, given the likely increased risk of adverse events following surgery as well as delayed healing among patients with greater comorbidity load, it follows that the most ill patients are also the most challenging to optimally manage and can result in delayed adjuvant treatment. Furthermore, patients from the lowest median household income level (<$38,000) consistently had significantly longer PTTR than all other income levels by nearly two weeks. Additionally, certain treatment settings had an association with PTTR with comprehensive community centers having the shortest PTTR compared to academic centers. These findings identifying access to care, income, comorbidity and treatment facilities as potential predictors of PTTR are consistent with findings by prior studies and warrant further investigation.35,36 Of note, sex, race, ethnicity, and insurance status were not associated with PTTR. While there is no method to confirm our hypothesized causative path with endoscopic surgery requiring shorter postoperative healing permitting shorter PTTR, this model demonstrates that endoscopic surgery is a significant factor contributing to shorter PTTR as the only significant variable after adjusting for covariates.
Finally, the relationship between PTTR and OS was assessed using both the Kaplan-Meier method and Cox proportional hazards regression. When the entire cohort of patients was subset by PTTR, longer PTTR was significantly associated with inferior OS. Patients were first subset relative to median PTTR, revealing significantly longer OS for patients with PTTR less than 49 days. When further subset analysis is performed by PTTR quartile, the benefit of earlier treatment becomes less clear. The fourth quartile, with PTTR of at least 63 days, had a significantly lower 3-year OS of 53.3% compared to those with PTTR less than 63 days. Taken together, this relationship between extended PTTR and inferior OS along with the association between endoscopic surgery and decreased PTTR further demonstrate the advantages of endoscopic surgery for sinonasal cancers in the appropriately selected patient.
There are several limitations within the present study that must be considered when interpreting the data. Like all registries, the NCBD inherently carries the potential for selection, information, and recall bias, as well as unmeasured confounding factors. While we used strict inclusion criteria to produce the final cohort for our analyses in order to minimize bias, the generalizability of our findings is uncertain; further analyses using additional population-based data sets or prospective studies could be instrumental in validating our results. In addition, while matched pairs were used for our cohorts, care should be taken in comparisons between groups. For example, advanced T4 tumors are more likely to be represented in the open cohort, as these lesions are less amenable to an endoscopic approach. Similarly, endoscopic surgery is more likely to be performed at specialized tertiary centers which could contribute to differences in treatment timing. Despite the size of the NCDB, power was limited in this analysis due to the small sample of patients undergoing surgery and postoperative RT with complete data. Additionally, the NCDB is limited to only 30-day readmission as a short-term outcome and OS as an endpoint, which precludes further understanding of cancer-specific outcomes. Furthermore, while we found a longer time to both surgery and radiation for the open group, there may be a subtle difference in comorbidities not adequately accounted for using the evenly matched CDCC in our matched groups. This is an inherent flaw in database analysis and should be kept in mind with interpretation of the data. Finally, although a single research hypothesis was tested in the present investigation, multiple comparisons were made to examine this hypothesis and perform additional secondary testing, significantly increasing the potential for false discovery.
Conclusion
Patients undergoing surgical resection for sinonasal squamous cell carcinoma experience significantly decreased delays in treatment when undergoing endoscopic surgery compared to those who undergo open surgery. Both time from diagnosis to surgery and time from surgery to adjuvant radiation therapy are significantly shorter for patients undergoing endoscopic surgery. While the exact time point needed to start radiation therapy after surgery is unclear, postoperative time to radiation therapy is a potentially modifiable risk factor associated with overall survival. In addition to shorter healing times and reduced adverse events, endoscopic surgery for sinonasal tumors, in the appropriately selected patient, offers the additional benefit of significantly reduced wait times that may ultimately extend overall survival.
Footnotes
Financial Disclosures, Conflicts of Interest: This research was funded in part the NIH/NCI Cancer Center Support Grant, P30CA008748.
Presentation: 2019 North American Skull Base Meeting, Orlando, Florida
References
- 1.Osguthorpe JD, Richardson M. Frontal sinus malignancies. Otolaryngol Clin North Am. 2001;34(1):269–281. [DOI] [PubMed] [Google Scholar]
- 2.Kilic S, Shukla PA, Marchiano EJ, et al. Malignant Primary Neoplasms of the Nasal Cavity and Paranasal Sinus. Curr Otorhinolaryngol Rep. 2016;4(4):249–258. [Google Scholar]
- 3.Kilic S, Kilic SS, Baredes S, Liu JK, Eloy JA. Survival, Morbidity, and Quality-of-Life Outcomes for Sinonasal and Ventral Skull Base Malignancies. Otolaryngol Clin North Am. 2017;50(2):467–480. [DOI] [PubMed] [Google Scholar]
- 4.Lee CH, Hur DG, Roh H-J, et al. Survival rates of sinonasal squamous cell carcinoma with the new AJCC staging system. Arch Otolaryngol Head Neck Surg. 2007;133(2):131–134. [DOI] [PubMed] [Google Scholar]
- 5.Ansa B, Goodman M, Ward K, et al. Paranasal sinus squamous cell carcinoma incidence and survival based on Surveillance, Epidemiology, and End Results data, 1973 to 2009. Cancer. 2013;119(14):2602–2610. [DOI] [PubMed] [Google Scholar]
- 6.Sanghvi S, Khan MN, Patel NR, Yeldandi S, Baredes S, Eloy JA. Epidemiology of sinonasal squamous cell carcinoma: a comprehensive analysis of 4994 patients. Laryngoscope. 2014;124(1):76–83. [DOI] [PubMed] [Google Scholar]
- 7.KETCHAM AS, WILKINS RH, VANBUREN JM, SMITH RR. A COMBINED INTRACRANIAL FACIAL APPROACH TO THE PARANASAL SINUSES. Am J Surg. 1963;106:698–703. [DOI] [PubMed] [Google Scholar]
- 8.Abu-Ghanem S, Fliss DM. Surgical approaches to resection of anterior skull base and paranasal sinuses tumors. Balkan Med J. 2013;30(2):136–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Patel SG, Singh B, Polluri A, et al. Craniofacial surgery for malignant skull base tumors: report of an international collaborative study. Cancer. 2003;98(6):1179–1187. [DOI] [PubMed] [Google Scholar]
- 10.Ganly I, Patel SG, Singh B, et al. Craniofacial resection for malignant paranasal sinus tumors: Report of an International Collaborative Study. Head Neck. 2005;27(7):575–584. [DOI] [PubMed] [Google Scholar]
- 11.Gray ST, Lin A, Curry WT, et al. Delayed complications after anterior craniofacial resection of malignant skull base tumors. J Neurol Surg B Skull Base. 2014;75(2):110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naunheim MR, Goyal N, Dedmon MM, et al. An Algorithm for Surgical Approach to the Anterior Skull Base. J Neurol Surg B Skull Base. 2016;77(4):364–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hanna E, DeMonte F, Ibrahim S, Roberts D, Levine N, Kupferman M. Endoscopic resection of sinonasal cancers with and without craniotomy: Oncologic results. Arch Otolaryngol - Head Neck Surg. 2009;135(12):1219–1224. [DOI] [PubMed] [Google Scholar]
- 14.Rawal RB, Farzal Z, Federspiel JJ, Sreenath SB, Thorp BD, Zanation AM. Endoscopic resection of sinonasal malignancy: A systematic review and meta-analysis. Otolaryngol - Head Neck Surg (United States). 2016;155(3):376–386. [DOI] [PubMed] [Google Scholar]
- 15.Eloy JA, Vivero RJ, Hoang K, et al. Comparison of transnasal endoscopic and open craniofacial resection for malignant tumors of the anterior skull base. Laryngoscope. 2009;119(5):834–840. [DOI] [PubMed] [Google Scholar]
- 16.Goffart Y, Jorissen M, Daele J, et al. Minimally invasive endoscopic management of malignant sinonasal tumours. Acta Otorhinolaryngol Belg. 2000;54(2):221–232. [PubMed] [Google Scholar]
- 17.Suh JD, Ramakrishnan VR, Chi JJ, Palmer JN, Chiu AG. Outcomes and complications of endoscopic approaches for malignancies of the paranasal sinuses and anterior skull base. Ann Otol Rhinol Laryngol. 2013;122(1):54–59. [DOI] [PubMed] [Google Scholar]
- 18.Farquhar D, Kim L, Worrall D, et al. Propensity score analysis of endoscopic and open approaches to malignant paranasal and anterior skull base tumor outcomes. Laryngoscope. 2016;126(8):1724–1729. [DOI] [PubMed] [Google Scholar]
- 19.Vergez S, du Mayne MD, Coste A, et al. Multicenter study to assess endoscopic resection of 159 sinonasal adenocarcinomas. Ann Surg Oncol. 2014;21(4):1384–1390. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CT, Galloway TJ, Handorf EA, et al. Increasing time to treatment initiation for head and neck cancer: An analysis of the National Cancer Database. Cancer. 2015;121(8):1204–1213. [DOI] [PubMed] [Google Scholar]
- 21.Murphy CT, Galloway TJ, Handorf EA, et al. Survival Impact of Increasing Time to Treatment Initiation for Patients With Head and Neck Cancer in the United States. J Clin Oncol. 2016;34(2):169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xiao R, Ward MC, Yang K, et al. Increased pathologic upstaging with rising time to treatment initiation for head and neck cancer: A mechanism for increased mortality. Cancer. 2018;124(7):1400–1414. [DOI] [PubMed] [Google Scholar]
- 23.Harris JP, Chen M, Orosco RK, Sirjani D, Divi V, Hara W. Association of survival with shorter time to radiation therapy after surgery for US patients with head and neck cancer. JAMA Otolaryngol - Head Neck Surg. 2018;144(4):349–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bilimoria KY, Stewart AK, Winchester DP, Ko CY. The National Cancer Data Base: a powerful initiative to improve cancer care in the United States. Ann Surg Oncol. 2008;15(3):683–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. [DOI] [PubMed] [Google Scholar]
- 26.Mell LK, Dignam JJ, Salama JK, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol. 2010;28(1):15–20. [DOI] [PubMed] [Google Scholar]
- 27.Ho DE, Imai K, King G, Stuart EA. MatchIt : Nonparametric Preprocessing for Parametric Causal Inference. J Stat Softw. 2011;42(8):1–28. [Google Scholar]
- 28.Rosenbaum PR, Rubin DB. Constructing a control group using multivariate matched sampling methods that incorporate the propensity score. Am Stat. 1985;39(1):33–38. [Google Scholar]
- 29.R Core Team. R: A Language and Environment for Statistical Computing. 2017.
- 30.GraphPad Prism. 2017.
- 31.Tumati V, Hoang L, Sumer BD, et al. Association between treatment delays and oncologic outcome in patients treated with surgery and radiotherapy for head and neck cancer. Head Neck. 2019;41(2):315–321. [DOI] [PubMed] [Google Scholar]
- 32.Brouha XDR, Tromp DM, Hordijk G-J, Winnubst JAM, de Leeuw JRJ. Oral and pharyngeal cancer: analysis of patient delay at different tumor stages. Head Neck. 2005;27(11):939–945. [DOI] [PubMed] [Google Scholar]
- 33.Christophe V, Leroy T, Seillier M, et al. Determinants of patient delay in doctor consultation in head and neck cancers (Protocol DEREDIA). BMJ Open. 2014;4(7):e005286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seoane J, Takkouche B, Varela-Centelles P, Tomás I, Seoane-Romero JM. Impact of delay in diagnosis on survival to head and neck carcinomas: a systematic review with meta-analysis. Clin Otolaryngol. 2012;37(2):99–106. [DOI] [PubMed] [Google Scholar]
- 35.Albano JD, Ward E, Jemal A, et al. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99(18):1384–1394. [DOI] [PubMed] [Google Scholar]
- 36.Shavers VL, Harlan LC, Winn D, Davis WW. Racial/ethnic patterns of care for cancers of the oral cavity, pharynx, larynx, sinuses, and salivary glands. Cancer Metastasis Rev. 2003;22(1):25–38. [DOI] [PubMed] [Google Scholar]