Abstract
False-negative (FN) intraoperative frozen section (FS) results of sentinel lymph nodes (SLN) have been reported to be more common after neoadjuvant chemotherapy (NAC) in the primary surgical setting. We evaluated SLN FS assessment in breast cancer patients treated with NAC to determine the FN rate and the histomorphologic factors associated with FN results. Patients who had FS SLN assessment following NAC from July 2008 to July 2017 were identified. Of the 711 SLN FS cases, 522 were negative, 181 positive, and 8 deferred. The FN rate was 5.4% (28/522). There were no false-positive results. Of the 8 deferred cases, 5 were positive on permanent section and 3 were negative. There was a higher frequency of micrometastasis and isolated tumor cells in FN cases (p <0.001). There was a significant increase in tissue surface area present on permanent section slides compared to FS slides (p <0.001), highlighting the inherent technical limitations of FS and histological under-sampling of tissue which leads to most FN results. The majority (25/28, 89%) of FN cases had metastatic foci identified exclusively on permanent sections and were not due to a true diagnostic interpretation error. FN cases were more frequently ER positive (p <0.001), PR positive (p = 0.001), HER2 negative (p = 0.009) and histologic grade 1 (p = 0.015), which most likely reflects the lower rates of pathologic complete response in these tumors. Despite its limitations, FS is a reliable modality to assess the presence of SLN metastases in NAC treated patients.
Keywords: breast cancer, sentinel lymph nodes, neoadjuvant chemotherapy, intraoperative frozen section, false negative rate
Introduction
Complete pathologic response of nodal metastases is seen in approximately 40% of patients after neoadjuvant chemotherapy (NAC), and can be as high as 60% to 70% in human epidermal growth factor receptor-2 (HER2) positive disease.1 The high rate of nodal downstaging lead to the development of four multi-institutional trials which demonstrated that SLNB reliably stages the axilla in women who are initially node positive and become clinically node negative after NAC provided that more than two sentinel lymph nodes (SLN) are obtained. These results have increased the number of women receiving NAC who are candidates for SLNB.1–5 Unlike earlier studies, the Sentinel Node Biopsy Following Neoadjuvant Chemotherapy (SN FNAC) trial mandated the use of cytokeratin immunohistochemistry (IHC) to detect metastatic tumor cells if the hematoxylin and eosin stained (H&E) slides were negative. Their data showed that the rate of positive non-SLN was high independent of the size of the SLN metastasis, and any size metastasis should be considered significant, in contrast to the non-NAC setting where the size of SLN metastases is not correlated with rate of positive non-SLN.2 As a result, the American Joint Committee on Cancer (AJCC) staging manual states patients treated with NAC who have micrometastases (foci consisting of more than 200 cells and/or greater than 0.2 mm but none greater than 2.0 mm) or isolated tumor cells (ITCs), defined as a focus consisting of less than 200 cells and spanning no more than 0.2 mm, are considered to have residual nodal disease, mandating ALND.6 To avoid the inconvenience, cost and complications of a second surgery, SLN are sent for intraoperative frozen section (FS) consultation to determine the need for ALND.
Having to recognize even a single tumor cell to correctly identify a SLN as positive can be incredibly challenging as the size of a metastasis is directly proportional to its probability of being found on FS.7,8 Meta-analysis shows SLN FS has a mean sensitivity of about 73%, with higher sensitivity for macrometastasis (metastasis greater than 2.0 mm) than micrometastasis (94% versus 40%).9 Evaluation can be even more difficult in the presence of chemotherapy induced stromal and cellular changes, known commonly as treatment effect, such as fibrosis, foamy histiocytic infiltrate, hemosiderin laden macrophages and partial obliteration of tissue architecture which can obscure and/or alter the appearance of epithelial cells.10,11 Errors are the result of either diagnostic misinterpretation, i.e. the diagnosis is missed or made incorrectly, or technical complications, such as tissue folds or poor staining. There are limits in the amount of tissue which can be sampled and evaluated during FS and given the technical quality of FS slides compared to permanent histologic sections, discrepancies between FS and final diagnosis can be expected. This study evaluated the false-negative rate (FNR) of SLN FS in breast cancer patients treated with NAC and determined what, if any, histomorphologic factors are consistently associated with false-negative results.
Methods and Materials
After obtaining institutional review board approval, all breast cancer patients who had intraoperative FS assessment after NAC between July 2008 and July 2017 were identified from a prospectively maintained institutional database. Demographic and clinical data were recorded. Tumor characteristics and results of SLN biopsy at time of FS and on permanent paraffin embedded H&E sections were obtained from pathology reports. Only those SLN which were frozen and reviewed intraoperatively were included. The number of SLN removed for FS and the number of positive SLN were recorded. SLN metastases were categorized by size, defined by the AJCC staging guidelines.6 As previously described, ITCs are SLN metastases spanning ≤ 0.2 mm and consisting of < 200 cells, micrometastases are > 0.2 mm and ≤2.0 mm and/or greater than 200 cells, and macrometastases measure > 2.0 mm. The presence or absence of treatment effect in the SLN tissue was noted. As described by Barrio et al12, treatment effect was defined as fibrosis, histiocytic infiltrate, hemosiderin laden macrophages, and increased vascularity in the lymph node tissue and/or a thickened lymph node capsule. Treatment related changes in the nuclei or cytoplasm of metastatic tumor cells was not included in this definition and the SLN was not recorded as containing treatment effect if epithelial changes were present in the absence of changes in the SLN tissue. Additionally, results of fine needle aspiration (FNA) of an axillary lymph node before NAC were recorded, where applicable.
SLN received for intraoperative FS were sectioned into 2 mm thick slices parallel to the long axis of the lymph node, embedded in Optimal Cooling Temperature (OCT) (Sakura Finetek, Torrance, CA) media and frozen in isopentane cooled with liquid nitrogen. Lymph nodes less than 4 mm in diameter were embedded uncut. The frozen block was carefully aligned and faced to avoid wasting nodal tissue and then stained by routine H&E and immediately interpreted by an attending pathologist. In general, one H&E section per block was evaluated at time of FS. Deeper level sections were requested and evaluated at the discretion of FS pathologist. Following FS, all remaining frozen tissue was thawed, fixed in 10% neutral buffered formalin and embedded in paraffin blocks. For definitive histopathologic examination, one H&E slide was evaluated for each SLN block that had been frozen. Only selected cases with suspicious histologic findings, such as indeterminate epithelioid cells, treatment effect, or crush/processing artifact, had additional deeper level H&E slides and/or IHC for cytokeratin ordered.
Intraoperative FS results were considered false-negative if the FS was reported as negative and the permanent section subsequently showed metastatic carcinoma, of any size. Cases in which the FS interpretation was the same as the final permanent section were considered concordant. When a definitive diagnosis could not be provided at the time of FS, the case was deferred by the reviewing pathologist and the final interpretation was made following permanent histologic section review with or without additional IHC work up. From a pathologic standpoint, when a diagnosis is not provided at time of FS a diagnostic error has not occurred and thus deferred cases were considered concordant with the final pathology result. All FS and permanent H&E slides for false-negative and deferred SLN were re-reviewed by two pathologists (AG and ME).
Beginning in January 2015, our institution digitally scanned all FS and permanent FS control slides for quality assurance purposes. All images were stored in our pathology database. Nineteen false-negative cases, consisting of 83 lymph nodes embedded in 69 tissue blocks, had scanned images available. Nine false-negative cases obtained prior to 2015 were not digitally archived and images were unavailable for analysis. The digital images were analyzed to measure and compare the area of visible tissue available for analysis in both false-negative SLN and concordant SLN slides. The images were digitally annotated to measure the total tissue surface area, which included the sampled lymph node and any attached adipose tissue, and the lymph node surface area alone on both the FS and permanent FS control digitally scanned slides for each block. The difference in the total tissue surface area and lymph node area were calculated and recorded.
Data analysis
The FNR was calculated using the total number of negative FS results as the denominator. Continuous factors were summarized with the median and interquartile range (IQR) and categorical factors were summarized with the frequency and percentage. Tests for association with a false-negative FS result were conducted with the Wilcoxon rank-sum test for continuous factors and Fisher’s exact test for categorical factors. To enable analysis as a continuous variable, ITC were assigned a value of zero for largest SLN metastasis.
Because there were multiple surface area measurements for each of the 19 patients in the subsample with these measures available, correlated data analysis methods were used. Generalized estimating equations models were used to examine the association between the difference in FS and permanent section surface area with respect to a false-negative FS result, using a logit link, and to examine the association between FS versus permanent section and overall surface area, using an identity link.
A p-value < 0.05 was considered statistically significant. All statistical analyses were conducted in R software version 3.5.0 (R Core Development Team, Vienna, Austria).
Results
We identified 711 SLN FS cases following NAC from 702 patients (nine bilateral surgical procedures). Of these, 360 were clinically node negative and 351 were clinically node positive. All patients were female with a median age of 50 years (range 24–82). All patients received systemic chemotherapy; a subset (7%, 51/202) received preoperative endocrine therapy in addition to chemotherapy. The predominant histologic tumor type was invasive ductal carcinoma (83.7%, 595/711), followed by invasive lobular carcinoma (4.4%, 31/711). Additional histologies included micropapillary carcinoma (29 cases), apocrine (26), metaplastic (9), mixed ductal and lobular (11), neuroendocrine (4), pleomorphic lobular carcinoma (4) and other not specified (2). From cases with these additional tumor types, three false-negative results were identified, two from carcinomas with mixed ductal and lobular features and one from a metaplastic carcinoma (Table 1). The median number of SLN removed for FS assessment was 3 (range 1–14). On FS, 181 SLN were positive for metastatic disease, 522 were negative and 8 were deferred. The deferred rate was 1.1% and of those deferred, 5 showed metastatic carcinoma on permanent H&E section (3 of which had treatment effect) and 3 were negative (2 with treatment effect). Of 522 negative cases, 28 were reported to be positive for metastatic disease on permanent section, for an FNR of 5.4%. Following morphologic review of all false-negative SLN FS and permanent section slides, the FS results were confirmed. The sensitivity and specificity were 84.6% and 99.4%, respectively, with an accuracy of 94.9%. Treatment effect was identified in 50% (14/28) of the FS false-negative cases. In 89% (25/28) of false-negative FS cases, the metastatic focus was seen exclusively on permanent sections; SLNs had macrometastases in 5 cases, micrometastases in 11 and ITCs in 9. The macrometastatic foci ranged in size from 2.1 mm to 5 mm. The micrometastatic tumor deposits consisted of foci with >200 cells up to 1 mm in greatest dimension. Of these 25 FS false-negative SLNs, five (20%) nodal metastases (comprised of 3 micrometastases and 2 ITCs) were definitively identified on cytokeratin stained slides only. Three FS cases were misinterpreted as negative at the time of FS analysis, with metastases identified on the FS control on permanent sections; these cases included two micrometastases (0.6 mm and 0.8 mm) and one macrometastasis (2.5 mm). Each of these three false-negative FS SLN showed evidence of treatment effect. The adjusted FNR was 0.6% (3/522). There were no false-positive FS results.
Table 1:
Summary of clinicopathologic features of data set
| Variable | Overall (n = 711) | False-negative FS (n = 28) | Concordant FS (n = 683) | p-value |
|---|---|---|---|---|
| Median Age, years (range) | 50 (24–82) | 49 (27–76) | 50 (24–82) | 0.416 |
| Median number of SLN removed (range) | 3 (1–14) | 2 (1–10) | 3 (1–14) | 0.102 |
| Tumor type | 0.061 | |||
| Ductal | 595 (83.7) | 23 (82.1) | 572 (83.7) | |
| Lobular | 31 (4.4) | 2 (7.1) | 29 (4.2) | |
| Mixed ductal and lobular | 11 (1.5) | 2 (7.1) | 9 (1.3) | |
| Other | 74 (10.4) | 1 (3.6) | 73 (10.7) | |
| Histologic grade | 0.015 | |||
| I/III | 5 (0.7) | 2 (7.1) | 3 (0.4) | |
| II/III | 61 (8.6) | 1 (3.6) | 60 (8.8) | |
| III/III | 626 (88) | 24 (85.7) | 602 (88.1) | |
| NA | 19 (2.7) | 1 (3.6) | 18 (2.6) | |
| ER status | < 0.001 | |||
| Positive | 403 (56.7) | 25 (89.3) | 378 (55.3) | |
| Negative | 302 (42.5) | 3 (10.7) | 299 (43.8) | |
| NA | 6 (0.8) | 0 (0) | 6 (0.9) | |
| PR status | 0.001 | |||
| Positive | 329 (46.3) | 22 (78.6) | 307 (44.9) | |
| Negative | 376 (52.9) | 6 (21.4) | 370 (54.2) | |
| NA | 6 (0.8) | 0 (0) | 6 (0.9) | |
| HER2 status | 0.009 | |||
| Positive | 259 (36.4) | 4 (14.3) | 255 (37.3) | |
| Negative | 437 (61.5) | 24 (85.7) | 431 (60.5) | |
| NA | 15 (2.1) | 0 (0) | 15 (2.2) | |
| LVI | 0.336 | |||
| Negative | 422 (59.4) | 16 (57.1) | 406 (59.4) | |
| Positive | 196 (27.6) | 12 (42.9) | 184 (26.9) | |
| Suspicious/Not confirmed | 21 (3) | 0 (0) | 21 (3.1) | |
| NA | 72 (10.1) | 0 (0) | 72 (10.5) | |
| SLN metastasis size | < 0.001 | |||
| ITC | 12 (5.6) | 9 (32.1) | 3 (1.6) | |
| Micrometastasis | 58 (27.1) | 13 (46.4) | 45 (24.2) | |
| Macrometastasis | 133 (62.1) | 6 (21.4) | 127 (68.3) | |
| NA | 11 (5.1) | 0 (0) | 11 (5.9) | |
Data are expressed as n (%) unless otherwise specified
FS frozen section, NA not available, ER estrogen receptor, PR progesterone receptor, HER2 human epidermal growth factor receptor-2, LVI lymphovascular invasion, SLN sentinel lymph node, ITC isolated tumor cells
Clinicopathologic features of the study cohort are shown in Table 1. False-negative SLN cases were significantly more likely to be estrogen receptor (ER) positive (p <0.001), progesterone receptor (PR) positive (p = 0.001) and HER2 negative (p = 0.009). They were also more frequently histologic grade 1 (p = 0.015). A significant association was identified between the size of metastasis and false-negative FS result, such that the frequency of micrometastases and ITCs were much higher in false-negative cases (p <0.001). No significant association was found between histologic type or presence of lymphovascular invasion (LVI). There was no significant association between false-negative cases and the number of SLN removed for FS (p = 0.102) or with axillary lymph node FNA results (p = 1). Intraoperative analysis was performed by a dedicated breast pathologist in 30% of cases and by a non-breast pathologist in 70% and a false-negative FS result was found in 8 cases and 20 cases, respectively. The sensitivity was comparable for breast pathologists (89%) and non-breast pathologists (86%). The specificity for both breast and non-breast pathologists was 100%. The amount of residual disease at ALND of this study cohort has been previously published.13
The median difference in the total tissue surface area between the permanent H&E slide and FS slide was 75 mm2 (IQR: 29, 136). The median difference in total tissue surface area for concordant SLN slides was 62 mm2 (IQR: 27, 120) and in false-negative SLN slides was 100 mm2 (IQR: 49, 153). The median difference in the lymph node area between the permanent H&E slide and FS slide was 1 mm2 (IQR: −10, 6). The median difference in lymph node area for concordant SLN slides was 0 mm2 (IQR: −8, 7) and for false-negative SLN slides was 3 mm2 (IQR: −17, 4). There was no significant association between difference in permanent and FS slide surface area and false-negative status for either the total surface area (p = 0.287) or lymph node area (p = 0.182). However, a significant association was found between permanent versus FS slides and total tissue surface area, such that the permanent section has, on average, an increase in total surface area of 94.3 mm2 when compared to the FS slide (p <0.001). No association was seen when comparing the lymph node area alone between slide types (p = 0.905).
Discussion
The primary purpose of intraoperative pathology consultation is to guide surgical management. In the case of SLNB, the information provided allows surgeons to either forgo or immediately proceed to ALND in the same surgical setting. FS is not ideal though and can preclude proper evaluation due to either diagnostic error or technical limitations. These issues can be amplified in the setting of NAC where treatment related changes alter the normal appearance of tissues making accurate interpretation challenging. However, in this study we demonstrate a FNR of 5.4%, lower than reported FNRs which range between 6.2% and 18.5% in NAC cases.13,14 We observed a sensitivity of 84.6% which is within the reported range of 74% to 93.8%,13,15 while our results demonstrate a higher accuracy (94.9%) than that reported previously.16 The size of metastases and the technical limitations of FS analysis were the most significant factors associated with false-negative events.
Size of SLN metastatic disease is the most commonly reported factor contributing to FNR in studies of both non-NAC17–23 and NAC13,24 patients with small metastatic deposits more likely to be missed during FS assessment. Akay et al24 evaluated the accuracy of SLNB in both non-NAC and NAC cases and found a more than eight-fold increase in FNR for micrometastatic disease. When only NAC cases were included in the analysis, the size of the metastasis was the only significant factor contributing to false-negative FS result. We also found a significant association between small metastatic foci and higher FNR with 79% (22/28) of false-negative cases consisting of either ITCs or micrometastases. It has been suggested that the use of rapid IHC or one-step nuclei acid amplification (OSNA) could improve accuracy. However, rapid IHC, a technique in which cytokeratin IHC could be done at time of FS, was reported to be the least effective method of detection in SLN FS in patients without NAC having a lower sensitivity (57%) when compared to routine cytologic touch preparation (69%), FS (86%) and permanent section (100%).25 OSNA is an automated molecular assay developed by Sysmex (Kobe, Japan) which uses reverse transcriptase polymerase chain reaction for cytokeratin 19-mRNA to detect metastases. The process is costly and time consuming and discrepant results are reported, including both false-negatives and false-positives due to detection of benign epithelial inclusions.14,26,27 Despite the development of these techniques to detect small metastatic foci, IHC is not commonly performed at our institution in the evaluation of SLNs.
The significance of microscopic nodal metastases in patients treated with NAC is controversial. Hennessy et al28 observed that a pathologic complete response (pCR) in the axillary lymph nodes is associated with an excellent prognosis, even when residual primary tumor is identified in the breast, and that the presence of any amount of residual nodal disease confers worse outcomes. Additionally, Moo et al13 observed that patients with low-volume disease (ITCs and micrometastases) had higher rates of positive non-sentinel lymph nodes at ALND than that seen in the primary setting. The authors conclude that low-volume disease is not indicative of low risk of additional positive axillary lymph nodes and any amount of disease after NAC remains an indication for ALND. However, other series have reported that the presence of ITCs in axillary lymph nodes, even occult tumor cells identified on cytokeratin IHC slides only, did not significantly impact disease free survival or overall survival.29,30 At our Center, cytokeratin IHC is ordered at the discretion of the reviewing pathologist based on review of the H&E sections. In this series, following identification of suspicious atypical cells on the H&E sections, cytokeratin IHC studies were ordered and 5 false-negative SLN had metastatic foci that were able to be definitively identified on these IHC stained slides only. Overall, we found the most consistent factor contributing to the FS FNR was sampling error.
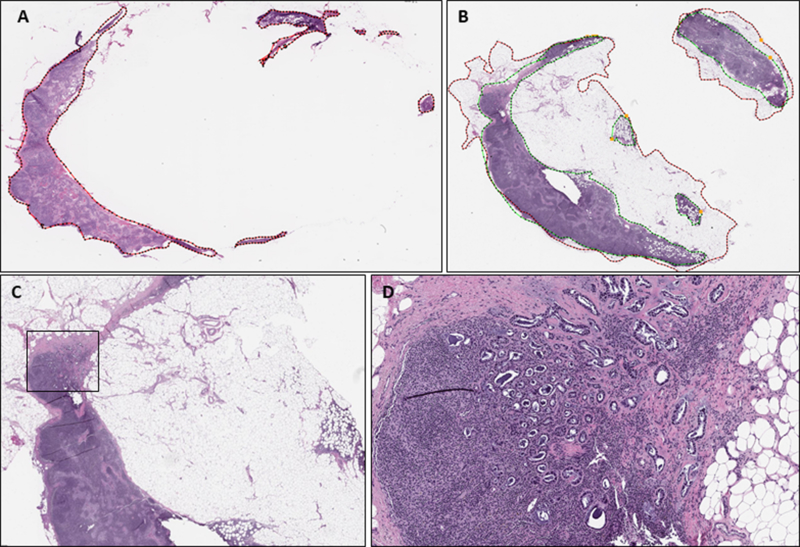
Upon review of FS section and permanent sections, 89% (25/28) of false-negative cases were due to sampling error, i.e. the metastatic focus was present exclusively on permanent section slides (Figure 1). Errors related to sampling of axillary SLN in which sections taken at the time of FS did not cut through the tumor deposit are common, reported in up to 90% of cases.19,31 This can be due to inadequate gross handling of submitted tissues by failing to properly dissect and section the lymph nodes to ensure adequate representation or from superficial histological sampling, which may lead to missing micro- or even macrometastatic disease. Routinely serially sectioning and entirely submitting all lymph node tissue may help in decreasing false-negative results. Espinosa-Bravo et al14 reported an FNR of 18.5% after NAC, and notably in this study only half of the lymph node was submitted for FS analysis. Additionally, fatty tissues are difficult to cut, affecting interpretation of the slides. For example, if there is extensive fatty replacement of the lymph node it may be impossible to obtain a complete or unfolded tissue section at the time of FS. In many instances, this adipose tissue may be visible on permanent formalin-fixed sections only. The technical competence of the pathologist or histotechnologist preparing the histologic section may also contribute to the error rate. Through analysis of the area visible for histologic evaluation on tissue slides we found there was a significant average increase in the total surface area of approximately 94 mm2 on the permanent H&E slide when compared to the FS slide, indicating that the FS slide may not always provide a complete representation of the tissue in the block, limiting the area available for histologic examination by the pathologist and subsequently decreasing the ability to detect malignancy. The rim of adipose tissue surrounding the lymph node capsule and areas of fatty replacement of the lymph node were included in the total surface area measurement as metastatic carcinoma can be present in these areas. Areas of extra-capsular extension involving the surrounding axillary tissue should be examined as closely as the lymph node itself. These findings highlight one of the more common challenges faced by pathologists performing FS. Sams et al32 evaluated FS in all subspecialties and specimen types and found that processing errors, either due to gross sampling and/or histologic sampling, accounted for 54% of all discrepant results. It is our recommendation that the FS pathologist should not hesitate in obtaining additional deeper levels to increase the likelihood of obtaining a complete tissue section. White et al33 also found discrepant results to be caused by specimen sampling however in their series they were almost equally as likely to be due to errors in interpretation.
Figure 1:
Example of histologic sampling error in false-negative sentinel lymph node. A: Whole slide image of frozen section slide with total surface/lymph node area outlined in red (24.9 mm2), B: Whole slide image of permanent frozen section control slide after formalin fixation and paraffin embedding with total lymph node area outlined in green (51 mm2) and total surface area outlined in red (130 mm2), C: Permanent section of false-negative lymph with metastatic focus outlined in black (H&E, 40X), D: Permanent section of false-negative lymph node with metastatic focus not present in original FS slide (H&E, 100X)
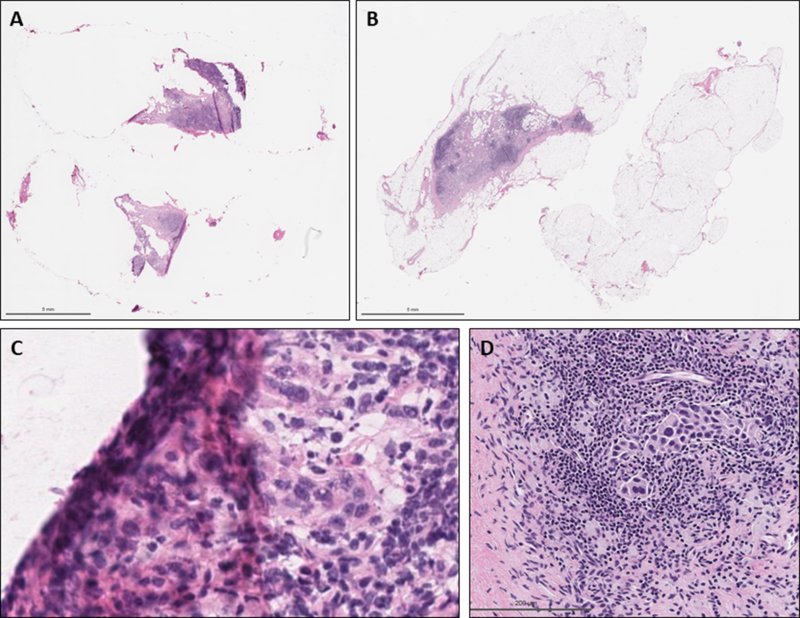
Three out of 28 false-negative cases in this series were due to a diagnostic misinterpretation, causing the SLN to be underdiagnosed as negative on FS (Figure 2). Two cases were micrometastatic, both measuring less than 1 mm, and one was macrometastatic, consisting of scattered clusters of 10 to 20 cells spanning 2.5 mm. All three SLN showed treatment related changes. Shimazu et al15 observed that the sensitivity of FS for SLN macrometastases was lower in NAC patients (76%) than in treatment naïve (92%) and concluded this could be due to the treatment related changes seen in these SLN. In contrast, the presence of treatment effect slightly increased the sensitivity for ITCs and micrometastases in the NAC versus the non-NAC groups (67% vs 31%) by creating an artifactual retraction of the tissue to form a clear border around the single cells and cell nests. This artifact was not identified in the false-negative cases in our data set. Interestingly, Brown et al10 found that SLN without treatment effect were more likely to be false-negative compared with a SLN with those histologic changes. Overall our data showed even numbers of false-negative SLN with treatment effect and without. While it has been suggested that sensitivity of SLN FS varies between 57% and 87% based on the diagnostic acumen of the interpreting pathologist, Holck et al18 still observed false-negative reports when including only cases read by senior pathologists with a special interest in breast pathology and the incidence of false-negative reports remained relatively constant throughout their entire 20-month study period. We show an extremely low rate of misinterpretation of FS with only minimal variations in the sensitivities for FS read by breast pathologists and non-breast pathologists.
Figure 2:
Example of diagnostic interpretation error in false-negative sentinel lymph node. A: Whole slide image of frozen section slide, B: Whole slide image of permanent frozen section slide of false-negative lymph node after formalin fixation and paraffin embedding, C: Frozen section slide showing metastatic focus under tissue fold (H&E, 400X), D: Permanent section slide with metastatic focus (H&E, 200X)
Our data suggest that false-negative results were significantly more likely in tumors which were of low histologic grade, ER and PR positive and HER2 negative. However, these findings most likely reflect the lower probability tumors with these histologic features will achieve a pathologic complete response (pCR). ER negative and HER2 positive breast carcinomas are significantly more likely to show a pCR and nodal downstaging following NAC when compared to ER positive and HER2 negative tumors. Additionally, tumors with high nuclear and histologic grade have also been found to be associated with higher likelihood of pCR.34,35 Breast cancers of low histologic grade characteristically display only mild cytologic atypia. Low grade cytology has been shown to contribute to interpretative error in SLN FS.19 Indeed, Barakat et al31 observed that metastatic deposits were not initially identified on FS in 23% of cases and all were found to be of low nuclear grade. Of note, only five cases in this series were of histologic grade 1. Due to the low number of cases in this subset, the significance of this finding should be interpreted with caution.
Out of 711 SLN FS cases performed over a span of nine years, only 28 were discordant with the final histologic diagnosis, each having been read initially as negative and ultimately found to contain metastatic disease. Eighty-nine percent (25/28) of the false-negative cases were the result of a histologic under-sampling where the metastatic focus was seen exclusively on permanent H&E sections and from a strictly pathologic examination point of view these do not represent a true diagnostic interpretation discrepancy. Careful gross inspection and histological sampling is necessary along with examination of additional deeper levels when warranted to lessen the impact of the technical limitations of FS. Three SLN were underdiagnosed as negative at time of FS analysis and each showed chemotherapy induced changes obscuring the neoplastic cells. When eliminating those cases which were only found to be positive on deeper levels sections our FNR is exceptionally low (0.6%), indicating intraoperative assessment of sentinel lymph nodes is a reliable modality in NAC treated patients.
Footnotes
Disclosure/Conflict of Interest
Peter J. Schüffler is a cofounder of Paige.AI. All other authors declare no conflict of interest.
References
- 1.Boughey JC, Ballman KV, Le-Petross HT, et al. Identification and Resection of Clipped Node Decreases the False-negative Rate of Sentinel Lymph Node Surgery in Patients Presenting With Node-positive Breast Cancer (T0-T4, N1-N2) Who Receive Neoadjuvant Chemotherapy: Results From ACOSOG Z1071 (Alliance). Ann Surg 2016;263(4):802–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boileau JF, Poirier B, Basik M, et al. Sentinel node biopsy after neoadjuvant chemotherapy in biopsy-proven node-positive breast cancer: the SN FNAC study. J Clin Oncol 2015;33(3):258–264. [DOI] [PubMed] [Google Scholar]
- 3.Giuliano AE, Ballman K, McCall L, et al. Locoregional Recurrence After Sentinel Lymph Node Dissection With or Without Axillary Dissection in Patients With Sentinel Lymph Node Metastases: Long-term Follow-up From the American College of Surgeons Oncology Group (Alliance) ACOSOG Z0011 Randomized Trial. Ann Surg 2016;264(3):413–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg 2010;252(3):426–432; discussion 432–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamounas EP, Brown A, Anderson S, et al. Sentinel node biopsy after neoadjuvant chemotherapy in breast cancer: results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol 2005;23(12):2694–2702. [DOI] [PubMed] [Google Scholar]
- 6.Amin M, Edge S, Green F, et al. , ed AJCC Cancer Staging Manual Eighth Edition Chicago, Illinois: 2017. Amin M, ed. [Google Scholar]
- 7.Bleiweiss IJ. Sentinel lymph nodes in breast cancer after 10 years: rethinking basic principles. Lancet Oncol 2006;7(8):686–692. [DOI] [PubMed] [Google Scholar]
- 8.Klepchick PR, Dabbs DJ, Bonaventura M, et al. Selective intraoperative consultation for the evaluation of sentinel lymph nodes in breast cancer. Am J Surg 2004;188(4):429–432. [DOI] [PubMed] [Google Scholar]
- 9.Maguire A, Brogi E. Sentinel Lymph Nodes for Breast Carcinoma: A Paradigm Shift. Arch Pathol Lab Med 2016;140(8):791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown AS, Hunt KK, Shen J, et al. Histologic changes associated with false-negative sentinel lymph nodes after preoperative chemotherapy in patients with confirmed lymph node-positive breast cancer before treatment. Cancer 2010;116(12):2878–2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher ER, Wang J, Bryant J, Fisher B, Mamounas E, Wolmark N. Pathobiology of preoperative chemotherapy: findings from the National Surgical Adjuvant Breast and Bowel (NSABP) protocol B-18. Cancer 2002;95(4):681–695. [DOI] [PubMed] [Google Scholar]
- 12.Barrio AV, Mamtani A, Edelweiss M, et al. How Often Is Treatment Effect Identified in Axillary Nodes with a Pathologic Complete Response After Neoadjuvant Chemotherapy? Ann Surg Oncol 2016;23(11):3475–3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moo TA, Edelweiss M, Hajiyeva S, et al. Is Low-Volume Disease in the Sentinel Node After Neoadjuvant Chemotherapy an Indication for Axillary Dissection? Ann Surg Oncol 2018;25(6):1488–1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Espinosa-Bravo M, Navarro-Cecilia J, Ramos Boyero M, et al. Intraoperative assessment of sentinel lymph node by one-step nucleic acid amplification in breast cancer patients after neoadjuvant treatment reduces the need for a second surgery for axillary lymph node dissection. Breast 2017;31:40–45. [DOI] [PubMed] [Google Scholar]
- 15.Shimazu K, Tamaki Y, Taguchi T, Tsukamoto F, Kasugai T, Noguchi S. Intraoperative frozen section analysis of sentinel lymph node in breast cancer patients treated with neoadjuvant chemotherapy. Ann Surg Oncol 2008;15(6):1717–1722. [DOI] [PubMed] [Google Scholar]
- 16.Han C, Yang L, Zuo W. A mini-review on factors and countermeasures associated with false-negative sentinel lymph node biopsies in breast cancer. Chin J Cancer Res 2016;28(3):370–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fortunato L, Amini M, Farina M, et al. Intraoperative examination of sentinel nodes in breast cancer: is the glass half full or half empty? Ann Surg Oncol 2004;11(11):1005–1010. [DOI] [PubMed] [Google Scholar]
- 18.Holck S, Galatius H, Engel U, Wagner F, Hoffmann J. False-negative frozen section of sentinel lymph node biopsy for breast cancer. Breast 2004;13(1):42–48. [DOI] [PubMed] [Google Scholar]
- 19.Jensen AJ, Naik AM, Pommier RF, Vetto JT, Troxell ML. Factors influencing accuracy of axillary sentinel lymph node frozen section for breast cancer. Am J Surg 2010;199(5):629–635. [DOI] [PubMed] [Google Scholar]
- 20.Qiao G, Cong Y, Zou H, et al. False-negative Frozen Section of Sentinel Lymph Node Biopsy in a Chinese Population with Breast Cancer. Anticancer Res 2016;36(3):1331–1337. [PubMed] [Google Scholar]
- 21.Russo L, Betancourt L, Romero G, et al. Frozen section evaluation of sentinel lymph nodes in breast carcinoma: a retrospective analysis. Ecancermedicalscience 2017;11:774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Turner RR, Hansen NM, Stern SL, Giuliano AE. Intraoperative examination of the sentinel lymph node for breast carcinoma staging. Am J Clin Pathol 1999;112(5):627–634. [DOI] [PubMed] [Google Scholar]
- 23.Wong J, Yong WS, Thike AA, et al. False negative rate for intraoperative sentinel lymph node frozen section in patients with breast cancer: a retrospective analysis of patients in a single Asian institution. J Clin Pathol 2015;68(7):536–540. [DOI] [PubMed] [Google Scholar]
- 24.Akay CL, Albarracin C, Torstenson T, et al. Factors impacting the accuracy of intra-operative evaluation of sentinel lymph nodes in breast cancer. Breast J 2018;24(1):28–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beach RA, Lawson D, Waldrop SM, Cohen C. Rapid immunohistochemistry for cytokeratin in the intraoperative evaluation of sentinel lymph nodes for metastatic breast carcinoma. Appl Immunohistochem Mol Morphol 2003;11(1):45–50. [DOI] [PubMed] [Google Scholar]
- 26.Cserni G Intraoperative analysis of sentinel lymph nodes in breast cancer by one-step nucleic acid amplification. J Clin Pathol 2012;65(3):193–199. [DOI] [PubMed] [Google Scholar]
- 27.Shigematsu H, Ozaki S, Yasui D, et al. Comparison of CK-IHC assay on serial frozen sections, the OSNA assay, and in combination for intraoperative evaluation of SLN metastases in breast cancer. Breast Cancer 2018;25(2):191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol 2005;23(36):9304–9311. [DOI] [PubMed] [Google Scholar]
- 29.Loya A, Guray M, Hennessy BT, et al. Prognostic significance of occult axillary lymph node metastases after chemotherapy-induced pathologic complete response of cytologically proven axillary lymph node metastases from breast cancer. Cancer 2009;115(8):1605–1612. [DOI] [PubMed] [Google Scholar]
- 30.Sakakibara M, Nagashima T, Kadowaki M, et al. Clinical significance of axillary microresiduals after neoadjuvant chemotherapy in breast cancer patients with cytologically proven metastases. Ann Surg Oncol 2009;16(9):2470–2478. [DOI] [PubMed] [Google Scholar]
- 31.Barakat FH, Sulaiman I, Sughayer MA. Reliability of frozen section in breast sentinel lymph node examination. Breast Cancer 2014;21(5):576–582. [DOI] [PubMed] [Google Scholar]
- 32.Sams SB, Wisell JA. Discordance Between Intraoperative Consultation by Frozen Section and Final Diagnosis. Int J Surg Pathol 2017;25(1):41–50. [DOI] [PubMed] [Google Scholar]
- 33.White VA, Trotter MJ. Intraoperative consultation/final diagnosis correlation: relationship to tissue type and pathologic process. Arch Pathol Lab Med 2008;132(1):29–36. [DOI] [PubMed] [Google Scholar]
- 34.Li XB, Krishnamurti U, Bhattarai S, et al. Biomarkers Predicting Pathologic Complete Response to Neoadjuvant Chemotherapy in Breast Cancer. Am J Clin Pathol 2016;145(6):871–878. [DOI] [PubMed] [Google Scholar]
- 35.Rouzier R, Perou CM, Symmans WF, et al. Breast cancer molecular subtypes respond differently to preoperative chemotherapy. Clin Cancer Res 2005;11(16):5678–5685. [DOI] [PubMed] [Google Scholar]