Abstract
The lung represents a unique immune environment. The primary function of the lung is to enable gas exchange by facilitating the transfer of oxygen into and carbon dioxide out of the blood. However, as a direct byproduct of this process the lung is also constantly exposed to particles, allergens, and pathogens alongside air itself. Due to this, the pulmonary immune system exists in a fine balance between quiescence and inflammation, deviations from which can lead to a failure in respiratory function. A rich history exists attempting to define the critical features of lung immunity, and most recently advances in intravital microscopy have enabled the visualization of intercellular immune dynamics in both steady-state and a variety of disease conditions. In this review, we will summarize a variety of approaches to intravital lung imaging as well as how its application has advanced our understanding of normal lung function as well as disease states such as pulmonary metastasis, asthma, and lung injury.
Keywords: Intravital Microscopy, Pulmonary Imaging, Lung Imaging, Pulmonary Metastasis, Platelets, Asthma, Immune Cells, Lung Injury
Overview of Lung Immune System and Disease States
Even during homeostatic conditions the lung is an incredibly dynamic organ. The pulmonary environment is subject to constant motion from a combination of respiration, pulsatile flow of blood through the vasculature, and the beating of the heart itself. The lung additionally contains one of the most intricate vasculature networks in the body. This dense network of capillaries facilitates gas exchange by minimizing the spatial distance between blood and air enabling maximal efficiency of the oxygen gradient through which hemoglobin-rich red blood cells pass as they transit the lung. Beyond respiration, the lung is exposed to the environment and therefore has a large network of immune cells that act to surveil the airways and blood defending against incoming pathogens. This immune network is highly diverse including alveolar macrophages, parenchymal macrophages, patrolling and conventional monocytes, neutrophils, multiple varieties of dendritic cells, mast cells, megakaryocytes, innate lymphocytes, T cells, B cells, NK cells, NKT cells and others. At steady-state many of these populations are quite small in number helping to maintain the lung as a quiescent immune environment. However, in disease conditions, be that allergy, cancer, pathogen exposure, or lung injury, cell numbers and diversity can expand exponentially in a matter of hours to days. Understanding this incredible dynamism has led to creative approaches to visualize and measure the various elements of a pulmonary immune response in real-time.
The history of lung imaging:
The history and specific challenges of lung imaging have been recently reviewed elsewhere[1–3]. We will however cover those aspects in brief here as they are important to understanding differences in the various approaches that have now been developed to visualize cellular behavior in live lungs. The application of microscopy for live lung imaging extends back to the earliest days of both the microscope and lung biology. Marcello Malphigi, a venerated Italian scientist and contemporary of early microscopy pioneer Anton Van Leeuwenhoek, applied the then cutting edge compound microscope to visualize the exterior surface of the frog lung[4,5]. In doing so, he made two of the most important discoveries in lung biology, the existence and structure of both the pulmonary capillaries and the alveolus. Together these form the critical functional unit of the lung providing an interface for oxygen exchange and recirculation to the rest of the organism. Malphigi initially had his sights set on applying his technical acumen to the study of the mammalian lung, however, his efforts were largely met with failure. It would be nearly 300 years before modern science finally achieved this task.
Approaches to Lung Intravital Microscopy (LIVM):
The immune cells of the lung are an exceptionally dynamic entity. In order to accurately observe and characterize the behaviors of such cells, steps must be taken to maximize both spatial and temporal resolution while maintaining the physiology of the organ in as normal a state as possible. The challenges of this in live lung imaging are substantial but well-defined. First and foremost, tissues such as the liver can be exposed and externalized for imaging with relatively minor effort and impact to the animal. The lung however, requires the pressure environment of an intact thoracic cavity to maintain normal respiratory function. While this is a complicating feature, it is readily addressed through the use of mechanical ventilation. Care must be taken to avoid ventilator-induced injury to the lung, however, a number of commercially available small-animal ventilators now exist providing robust control over respiratory volume, rate, and pressure greatly simplifying this process and enabling longterm maintenance of lung function during imaging. With any of the intravital lung imaging techniques, the use of anesthesia is obligatory. Historically, a variety of anesthetics have been utilized but the most common in current use for LIVM are either lsoflurane[6,7] or a combination of Ketamine/Xylazine[8,9]. Vaporized Isoflurane is the general preference for sustained imaging as it enables fine control of the depth of anesthesia. This enables the generation of a deep anesthetic field for the initial surgery whilst tempering this slightly during imaging to minimize some of the negative effects of the drug. Some confounding variables do arise from the necessity of anesthetic use. Isoflurane has been reported to decrease airway resistance and atelectasis in experimental allergic asthma[10]. More pertinent to immune dynamics in the lung, all currently used anesthetics have been reported to suppress inflammatory responses to variable degrees [10–14]. Isoflurane generally displays the least of this activity in comparative studies and is thus preferred in most cases for LIVM.
The most complex challenge in LIVM is that during normal function, the lung is constantly undergoing macroscale tissue movement while the cells and subcellular structures to be visualized are firmly rooted in the microscopic realm. Below we will summarize the pros and cons of the various approaches people have taken to address this issue (summarized in Table 1).
Table 1 :
Summary of Approaches to Intravital Lung Imaging
| Approach | Methodology | Pros/Cons | Well-suited for Immune Study | Animal Models Studied | Key References | |
|---|---|---|---|---|---|---|
| 1 | Bronchus Clamping | Bronchus of one lobe is clamped preventing airflow induced motion |
Pros: a) Minimal motion artifacting b) Relatively simple surgical preparation Cons: a) Imaged lobe is subjected to severe hypoxia b) Only short imaging duration achievable |
No | Rabbit, Cat, Dog, Mouse | 1) H. L. Hall, Am. J. Physiol. 72, 446–457 (1925). 2) R. G. Macgregor, J. Physiol. 80, 65–77 (1933) 3) A. Hasegawa, K. Hayashi, et al., J. Allergy Clin. Immunol. 125, 461–468.e6 (2010). |
| 2 | Prolonged Apnea | Image capture restricted to the expiratory plateau which is prolonged to ~15secs through mechanical ventilation |
Pros: a) Minimized hypoxia compared to clamping b) Relatively minimal surgical trauma Cons: a) Frequency of timelapse and overall Imaging duration very short b) Image stability is generally poor |
No | Rat, Mouse | 1) A. Tabuchi, M. Mertens, et al., J. Appl. Physiol. 104, 338–346 (2008). 2) H. Mitsuoka, N. Unno, et al., J. Surg. Res. 87, 143–151 (1999). 3) H. Mitsuoka, T. Sakurai, et al., Crit. Care Med. 27, 1862–1868 (1999). 4) P. Schneider, T. Foitzik, et al., Microvasc. Res. 62, 421–434 (2001). 5) E. P. Schmidt, Y. Yang, et al., Nat. Med. 18, 1217–1223 (2012). 6) L. Preu, M. Bischoff, et al., Inflammation (2015). 7) N. T. Veith, T. Tschernig, et al., Respir. Res. 15, 85 (2014). 8) T. Tschernig, N. T. Veith, et al., Exp. Toxicol. Pathol. 65, 883–886 (2013). |
| 3 | Glue-based Stabilization | Lung parenchyma is exposed and glue is utilized to affix a small portion ofthe lung to a coverslip for imaging. |
Pros: a) Long duration of highly stable continuous imaging is possible b) Has been applied to the development of a permanently-placed window enabling longitudinal imaging studies Cons: a) In some studies substantial inflammation has been reported shortly after window implantation which may complicate interpretation |
Yes | Mouse | 1) D. Kreisel, R. G. Nava, et al., Proc. Natl. Acad. Sci. U.S.A. 107, 18073–18078 (2010). 2) J. H. Spahn, W. Li, et al., J. Immunol. 194, 4039–4048 (2015). 3) D. Entenberg, S. Voiculescu, et al., Nat. Methods. 113. 3073 (2017) |
| 4 | Suction-based Stabilization | Lung parenchyma is exposed and vacuum-pressure is utilized to stabilize a small portion ofthe lung beneath a coverslip for imaging. |
Pros: a) Long duration of highly stable continuous imaging is possible b) Minimal early inflammation Cons: a) Imaging sessions limited to ~12 hours b) Imaging >6 hours may be confounded by method-induced inflammation |
Yes | Dog, Rat, Mouse | 1) W.W. Wagner, J. Appl. Physiol. 26, 375–377 (1969) 2) D. G. McCormack, S. Mehta, etal, Microvasc. Res. 60, 131–140(2000). 3) N. Funakoshi, M. Onizuka, et al. , Microvasc. Res. 59, 361–367 (2000). 4) H. M. Razavi, L. F.Wang, etal., Am. J. Respir. Crit. Care Med. 170, 227–233 (2004). 5) R. G. Presson, M. B. Brown, etal., Am.J.Pathol.179,75–82 (2011). 6) M. R. Looney, E. E. Thornton, et al., , Nat. Methods 8, 91–96 (2011). 7) E. E. Thornton, M. R. Looney,et al.,,J. Exp. Med.209, 1183–1199 (2012). 8) S. Devi, Y. Wang, etal., , J. Exp. Med. 210, 2321–2336 (2013). 9) G. Ortiz-Muñoz, B. Mallavia, et al., Blood 124, 2625–2634 (2014). 10) M. F. Bennewitz, et al., IntraVital 3, e29748 (2014). 11) A. Caudrillier, B. Mallavia, et al., PLoS One 10, e0133022 (2015). 12) R. N. Hanna, C. Cekic, et al., Science 350, 1–9 (2015). 13) D Entenberg. C. Rodriguez-Tirado, et. al., Intravital 4:31–11 (2015) 14) M.B. Headley, A. Bins, etal.,Nature.531. 513–517.(2016) 15) S.Z. Chong, M. Evrard, et al., J.Exp.Med.213. 2293–2314. (2016) 16) E. Lefrancais, G. Ortiz-Munoz, etal., Nature. 544.105–109. (2017) 16) B.G.Yipp, J.H. Kim, etal., SciImmunol.2 (2017) 17) E. Lefrancais, B. Mallavia, etal.JCI Insight. 3(3) (2018) |
Bronchus Clamping:
Bronchial clamping was one of the earliest approaches taken in modern science to provide a stabilized view of the lung. Experiments of this nature extend back to the early 1930s and were initially applied to larger animals such as rabbits, cats, and dogs[15,16], but much more recently a similar approach was successfully used to interrogate immune responses in the mouse lung[17]. This method involves clamping off the bronchial supply to the lung lobe being imaged, preventing inhalation and exhalation mediated respiratory motion. Oxygenation of the rest of the body is maintained via ventilation of the contralateral lung. Preparations such as this enable stable imaging of the lung for between minutes and an hour. The key drawback to this approach is that the lobe of lung to be imaged is subjected to significant hypoxia dramatically lowering the potential for longterm imaging. Due to this, even at short intervals, interpretation of the data is challenging. Significant contributions to our understanding of pulmonary circulation were made with this technique, however, due to the drawbacks mentioned it has largely been left behind in favor of less invasive modalities.
Prolonged Apnea
During normal respiration a plateau exists during exhalation where for a period of ~300ms the pressure environment of the lung is constant. Through mechanical ventilator-induced apnea (and neuromuscular blockade), this plateau can be extended for as long as 15 seconds[3]. The researcher can then gate their imaging such that data is acquired only during these sustained plateaus. This approach has been used to elegantly profile vascular flow[18] and pulmonary endothelial response to injury[19]. It has additionally been successfully applied to the study of alveolar macrophage phagocytic activity[20] however by and large the short duration of imaging requisite to this approach makes it ill-suited for detailed analysis of immune dynamics.
Glue-Based Stabilization:
This approach pioneered by the Miller group[21,22] involves affixing the uppermost surface of the lung parenchyma to a cover glass using veterinary glue. This approach provides very stable imaging which enabled sustained high-resolution observation of the lung in the normal and inflamed state. The substantial downside to this method is the process of affixing the lung to the cover glass appears to induce an appreciable amount of inflammation on its own[21]. While the exact source of this is not clear, it is likely due to the chemical bonding requisite to the use of glue for stabilization[23]. Very recently, Entenberg et al[6] have developed a surgical approach which implants a permanent window into the ribcage of mice. Following recovery this approach enables lung imaging repeatedly over days to weeks without the need for mechanical ventilation. Interestingly, while the same adhesive was utilized, the inflammatory response was not observed even up to 2 weeks later. It is feasible that an acute inflammatory response exists in this context (as the first timepoint addressed was 1 day post-op) however, clearly this resolved over time. This new method offers the unique potential to visualize the same region of lung over many days allowing for the first true assessment of how lung immune responses mature over their entire natural history.
Vacuum-based Stabilization
Stabilization of the lung using vacuum suction has become the de facto gold standard in intravital lung imaging. At least 4 distinct approaches[7,24,25] have been published in the last 8 years. Revisiting some of the earliest work done in modern science on pulmonary imaging[26], our group[24] and another [25] sought to combine a suction window-based approach utilizing modern machining and 3D printing technology to develop fine-featured windows enabling intimate contact with the lung surface while minimizing stress to the organ itself (Figure 1). Combining this with two-photon imaging provided a stable, high-resolution view of cells of the lung. These methods provide rapid temporal resolution over many hours. We have since refined this technique[7] using a novel intercostal window design to minimize both surgical trauma and surface exposure of the lung during imaging. In combination with optimized anesthesia and intravenous hydration, this has enabled continuous imaging of the pulmonary immune response for up to 12 hours. Importantly, this method is relatively high throughput as the surgery itself takes only 30 minutes to complete enabling high resolution, high integrity, multimouse, experiments to be undertaken on the same day. Some issues do remain and limit this approach. Principally, sustained suction-mediated contact on the lung surface does have the potential to induce local damage and inflammation. Short-term imaging (<2 hours) does not appear to induce neutrophilic inflammation in the imaging field[24]. However, while imaging up to 12 hours is readily attainable, sustained suction for greater than 6 hours generally leads to increased neutrophilia in the local site meaning great care must be taken in interpretation of very long imaging durations (Headley, unpublished observations).
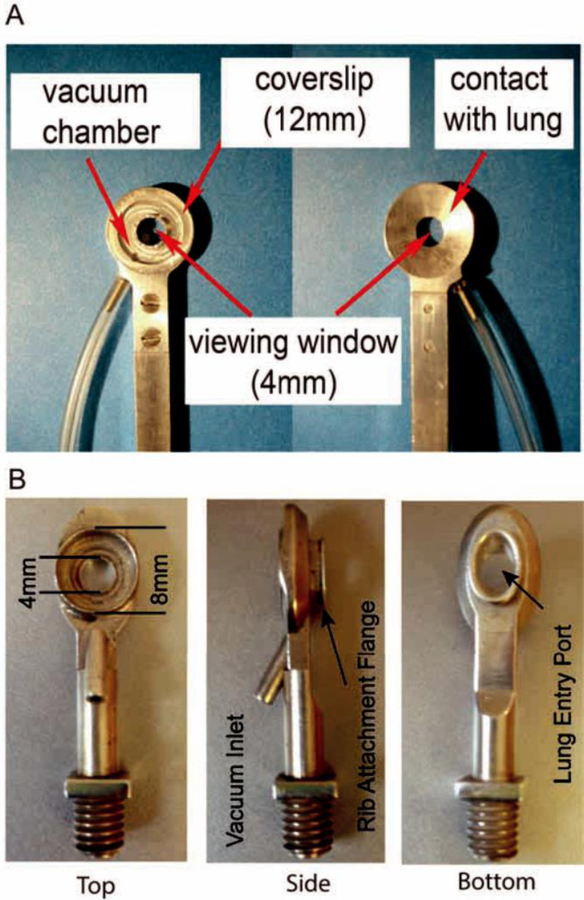
Figure 1. Evolution of The Modern Thoracic Suction Window.
A._Photographic schematic of a thoracic imaging window designed to lay directly over lung, exposed through resection of ribs. Low pressure (20 mmHg) vacuum is applied through inlet to temporarily stabilize lung tissue against the coverslip. Reprinted with permission[24]. B. Revised thoracic imaging window design incorporating an intracostal flange enabling placement directly between two ribs without need for resection. The modified window design allows for increased image stability with reduced surface exposure of the lung. Reprinted with permission[7].
Complementary approaches to LIVM
All of the above intravital imaging approaches suffer from one key drawback. Outer regions of the lung are predominantly composed of alveoli and their associated vasculature. Maximum achievable imaging depth in most tissues (lymph node, liver, skin, brain, etc...) is driven by the absorption of photons by the tissue itself. The refractive index (a numerical measure of how light is propogated through the substance of interest) of most tissues remains essentially constant as you go through the tissue, as such, in most cases, simply increasing the power of the excitation laser can yield greater imaging depth. This method works because the energy of light at a particular wavelength is directly proportional to the number of photons it contains. In this context, absorption is the primary driver of signal loss, the downside is, as you increase laser power you also increase the risk of heat-induced damage within the tissue. The alveoli of the lung make the tissue unique in this regard, as anyone who has looked at a pencil within a clear glass of water can attest, the interface between air and liquid is subject to a dramatic change in refractive index. Alveoli are essentially an air-liquid interface surrounded by vascular endothelium, as such any light entering or returning from these structures is subject to strong scattering properties. As a result, imaging of any sort is only achievable for 50–100um into the tissue, approximately one or two alveolar layers[24]. This effectively restricts any analysis of lung immune responses to those relevant to the outermost regions of the lung with the large airways being essentially inaccessible.
Fortunately, several methodologies have also been developed to interrogate these regions of the lung in the form of explanted tissue. There are three primary approaches allowing imaging of live lung tissue that don’t rely on an intravital preparation and that vary in the preservation of tissue integrity. The most well established is the isolated perfused lung preparation, which involves explantation of the entire lung, connection of the trachea to a mechanical ventilator and continuous perfusion of the vasculature. While this system does not solve the issue of depth directly it does increase the flexibility of where imaging can occur, which with care of microscope placement can yield images at greater depth than intravital imaging allows[27,28]. More recently, it has been shown that explant of entire lobes and full perfusion in media can enable greater imaging depth than intravital methods although normal respiratory pressure and vascular flow are lost[29]. By submerging the entire lobe in media the scattering effect of the air-liquid interface is significantly ameliorated. Lastly, if complete control of not only imaging depth but also relative depth in the lung itself is required, lung slices can be prepared using a precision vibratome[30]. This is currently the only method that enables thorough examination of the entirety of the live lung. However, it sacrifices the normal physiology of the organ and introduces a substantial inflammatory component via tissue damage that must be considered in interpreting any immunologic data generated through this system.
An additional approach to extend imaging of the lungs to greater depths is whole lung/whole lobe imaging in combination with tissue clearing methodologies. While these approaches do not allow study of the living lung and cellular dynamics therein, they do allow for unfettered access to the entirety of the organ and can provide detailed snapshots with superb resolution, which can be a powerful comparator to intravital studies. Tissue clearing involves reducing or removing various elements (most commonly lipids) from the tissue, which absorb and scatter photons, whilst preserving the cellular and molecular structure of the tissue. The unique environment of the lung (as it is rich in collagens and other ECM components that limit light penetration as well as the aforementioned alveolar scatter and lipid-mediated absorption) has rendered a number of common clearing methods less efficient in the lung than other tissues. However, several protocols have recently been published which achieve excellent lung clearing with relative ease. The advantages and disadvantages of these various approaches have been reviewed recently in detail elsewhere [31,32]. However, we will draw special attention to two such protocols that have shown particular success in the lung and can be done simply, inexpensively, and rapidly in order to complement live imaging experiments. The first, Ce3D, renders the lung (in addition to essentially every other mouse tissue) optically clear in approximately 2 days whilst preserving structure and importantly most major fluorescent proteins, enabling simple and direct comparison to intravital studies[33]. The second, uDISCO[34], is an evolution of another successful and popular lung clearing method 3DISCO[35]. The primary feature of this method is an actual substantial reduction in size of the cleared tissue (approaching 65%), while maintaining relatively intact distance relationships between cells and structures. The key advantage to this technique is that whole body clearing and imaging is achievable, enabling visualization of the lung, intact and in proper context within the animal.
Application of LIVM to the study of the Pulmonary Immune System
Intravital imaging methods have now been applied to the study of a variety of lung disease settings. Most notably pulmonary metastasis, lung injury, and asthma have been particularly fruitful areas of interrogation.
Pulmonary Metastasis
Intravital imaging has become a frequent approach to the study of both primary tumorigenesis and metastasis. However, until very recently these methods were not applied to the most frequently targeted metastatic site, the lung[36–38]. However, with the advent of robust lung imaging methodology there has been a veritable boom in the publication of studies examining interaction between tumor cells and the immune response in pulmonary metastasis. Two of the LIVM methods in current use were developed specifically for the purpose of studying pulmonary metastasis[6,8]. In the case of our recently published method utilizing an intercostal imaging window (Figure 1B) [7,39,40], our previous platform was modified specifically to fit the needs of metastasis research, though these modifications have proved fruitful in other areas of research as well. In all these cases, long-term stable imaging has been the primary focus to suit the measured pace of events that characterize metastatic spread. Prior to the development of robust lung intravital imaging a handful of studies utilized explant methodology to explore the questions of cellular dynamics in the lung during metastasis[28,41]. These studies provided important insight into the nature of tumor cell proliferation in the lung, including revealing the potential for intravascular proliferation[28]. However, neither of these studies sought to explore the role played by immune cells in this process.
More recently several studies have defined the early immune dynamics of the pulmonary metastatic environment. It has previously been shown that a population of monocytes variously referred to as Ly6Clow, non-canonical, or patrolling monocytes (defined in mice by robust expression of Cx3CR1 and Nur77[42–44]) which are almost exclusively intravascularly localized, play important roles in the pulmonary vasculature[45,46]. Hanna et. al. hypothesized that the intravascular localization of incoming metastatic cells would place them in a prime position to be influenced by patrolling monocytes, which had not been overtly explored previously[8]. Using a thoracic suction window, confocal microscopy, and an IV injection model of lung metastasis they found patrolling monocytes to be actively recruited to incoming metastases within the first 4 hours, and that even out to 7 days, patrolling monocytes are significantly increased in regions of the lung bearing metastases. Building off this initial imaging based-observation, the researchers demonstrated a novel axis by which patrolling monocytes are recruited to the tumor via CX3CR1-dependent chemotaxis, mice genetically deficient in patrolling monocytes display increased metastatic burden indicating an anti-metastatic role for these cells. Of note, in ex vivo analysis patrolling monocytes proved inefficient at directly killing tumor cells, instead the authors found that the presence of patrolling monocytes enabled recruitment of NK cells to the lung, which appear to mediate the killing of the tumor cells. The model they propose suggests a heretofore unknown relationship between patrolling monocytes as an early responder in metastasis modulating the recruitment and behavior of downstream effector NK cells. Future intravital imaging studies will be required to elucidate whether NK cells and patrolling monocytes engage in direct interaction as part of this process.
A separate but similar set of studies performed in our group took a broader approach to the question of the nature of immune dynamics in the early metastatic environment[7]. Utilizing the intracostal imaging window and 2P microscopy we found that upon entry into the lung, circulating tumor cells release large extracellular microparticles (Figure 2A). These particles are encountered by a diverse array of lung phagocytes, which engage with and ingest them (Figure 2B). This observation of immune uptake by pulmonary myeloid cells through LIVM enabled the design of a high-resolution flow cytometric assay in order to identify and parse the nature of these metastasis-ingesting cells. It was identified that, in tandem, populations of macrophages and dendritic cells encounter and ingest this tumor material. Importantly, patrolling monocytes also represent a large portion of the cells ingesting these particles, consistent with Hanna et al[8]. Intriguingly we found evidence through LIVM of both macrophages and dendritic cells interacting directly with these early metastatic cells. In keeping with other literature[47] macrophages were found to promote survival and growth of the burgeoning metastasis while, surprisingly, dendritic cells were found to be potent inhibitors of metastasis. Considering these two studies together, application of LIVM has led to characterization of an early metastatic niche that consists of both protumoral and anti-tumoral players, the balance of which likely sets the tone for metastatic success. More recently it has been shown that consistent with other metastatic sites a conjugation between tumor cells, endothelial cells, and macrophages termed TMEM exists within the lung[6]. This study made use of a permanently placed thoracic imaging window, which additionally allowed the authors to observe tumor cell behavior and even division over the course of days or even weeks. While the application of this to the study of the natural history of metastasis-immune interactions has just begun, the potential of this approach for in vivo insight is very high.
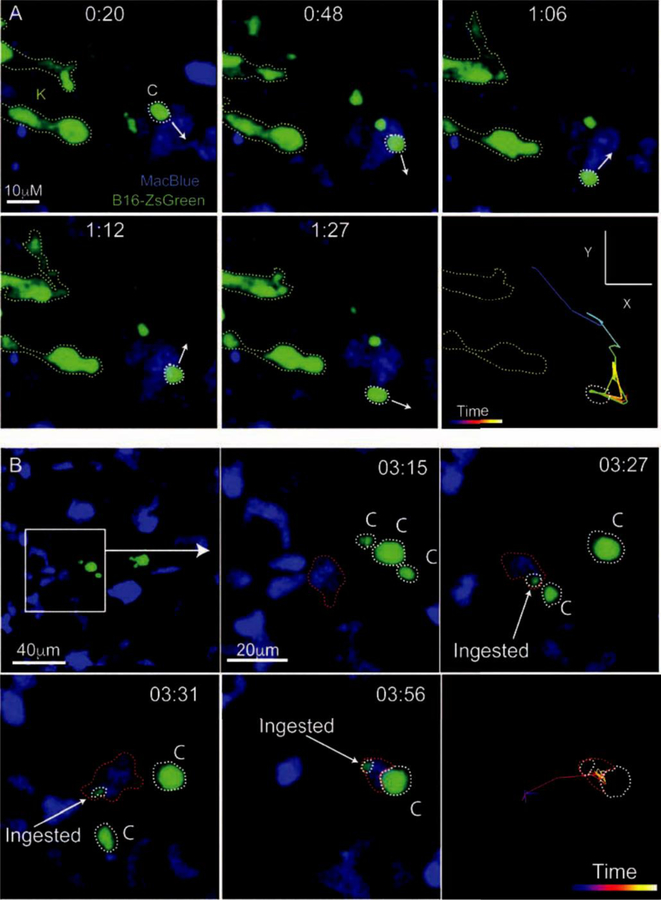
Figure 2. Live Imaging of The Early Pulmonary Metastatic Niche.
A. LIVM imaging of a ZsGreen+ B16F10 melanoma cell (Green, labeled K for karyoplast) releasing microparticles (labeled C for their alternate name cytoplasts) into the pulmonary vasculature of the myeloid reporter mouse MacBlue. In this mouse, monocytes and their progeny as well as neutrophils are labeled with CFP. White arrows show the trajectory of the myeloid cell at each timepoint as it migrates autonomously through the vasculature. The last frame of this series shows the entire path of the monocyte over the imaging time. B. LIVM of tumor microparticle (Green) being encountered and subsequently phagocytosed by a CFP+ myeloid cell (blue) in the pulmonary vasculature between 3 and 4 hours following the entry of the parental tumor cell into the lung. Microparticles are outlined in white and ingesting myeloid cell in red. The final frame of the image shows the path taken by the ingesting myeloid cell throughout the imaging time. Both A and B are reprinted with permission from[7].
Platelet Production and Hematopoietic Progenitors in the Lung
Intravital imaging has proven to be a powerful method for the analysis of platelet production and immune interactions. Initial investigation into the role of platelet and neutrophil conjugates in the pathogenesis of lung injury (summarized below) has led to the important finding that the lung vasculature accounts for a significant portion of all platelet production.[40] LIVM revealed that intravascular megakaryocytes of bone marrow origin release proplatelets in the lung circulation (Figure 3A and B). This finding confirmed previous suspicions that the lung has major role in platelet biogenesis. Using LIVM it was also discovered that the lung contains a large pool of extravascular megakaryocytes that are sessile during imaging, but may have immune-like features that are distinguishing compared to their counterparts in the bone marrow. Importantly, the recognition through LIVM and subsequent exploration of the role played by the lung in platelet biogenesis led to the important discovery that the lung is a repository for various hematopoietic progenitor cells. These cells which reside in the lung at steady-state are capable of repopulating the immune system under the conditions of stem cell deficiency[40].
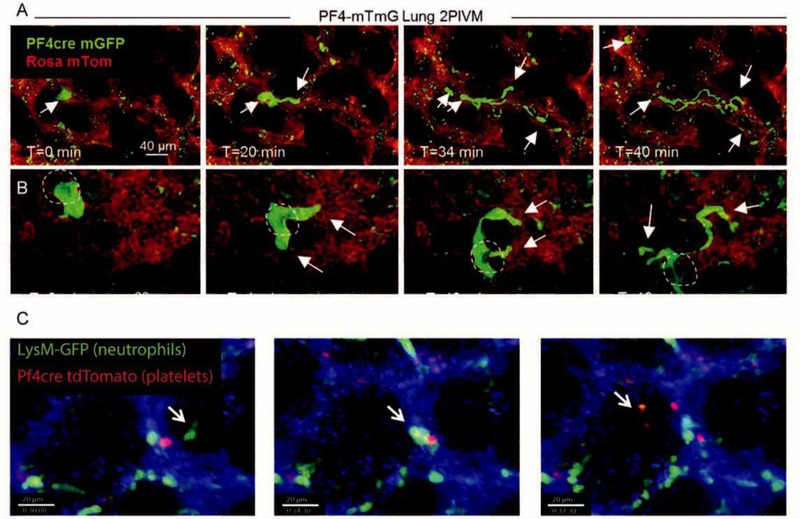
Figure 3. Platelet and Neutrophil Dynamics In The Live Lung.
A and B. Intravital imaging of intrapulmonary megakaryocytes generating proplatelets and releasing them into the lung vasculature. The PF4 promoter (a megakaryocyte and platelet specific marker) was used to drive Cre recombinase expression in membraneTomato membraneGFP (mTmG) reporter mice. When Cre is present in a cell, expression of TdTomato (red) is switched to GFP (green). B. Dark shadow highlights the cell nucleus, establishing its nature as a megakaryocyte as opposed to a conglomeration of platelets. A and B were reprinted with permission from[40]. C. Intravital imaging of mice wherein platelets are labeled in red (PF4-Cre x ROSA-TdTomato) and neutrophils in green (LysM-GFP). Timelapse shows the dynamic formation of a neutrophil-platelet aggregate over the course of 7.5 minutes of imaging. Reprinted with permission from[39].
Airways Inflammatory Disease/Asthma
Asthma is a complex chronic airway disease with various etiologies, including but not limited to allergic states. Some of the earliest efforts in modern LIVM were applied to the study of asthma[30]. However, it was recognized rapidly that the method was ill-suited for this purpose as the achievable imaging depth did not allow access to the large airways, preventing analysis of the anatomical site most relevant to disease development.
Instead, the features of this disease have largely been explored in viable lung slices[30]. It had long been understood that an interaction between Dendritic Cells and T cells to promote antigen-specific adaptive immunity lay at the heart of asthma pathogenesis. Given the airway localized nature of asthmatic disease it was generally thought that allergen sampling and presentation occurred in this same airway localized fashion. However, using lung slice imaging it was found that while DCs accumulate strongly at the airways in conjugation with T cells, few if any of these DCs engaged with the epithelium in a way that would allow for allergen uptake. In contrast, it was found that DCs in the alveolar regions interdigitated with the alveolar epithelium and could be observed to directly sample fluorescently-labeled antigen with subsequent transit to both airway proximal regions as well as the lung-draining lymph node to engage with and activate antigen-specific T cells. In addition to antigen sampling at the alveoli by DCs, viable slice imaging has also recently shown that lung localized monocytes survey the lung vasculature and airways for incoming antigenic material[48,49]. Interestingly, upon antigen acquisition it appears these monocytes may enter a partially differentiated state that allows interaction with T cells but with low productivity, a mechanism that could, under normal conditions, promote tolerance to airway antigens. However, in the context of allergy, these cells proceed to a fully differentiated state where they become highly effective at activating T cells[49].
Neutrophil Dynamics in the Lung Steady-State and In Disease
The lung contains a substantial marginated pool of neutrophils which appear poised for rapid mobilization in the context of disease[50]. Advent of robust intravital imaging methodology has significantly increased our understanding of the function and behavior of these cells and is the area of research where LIVM has been most broadly applied. Some of the first studies examined features of normal neutrophil behavior in mice. Intravital videomicroscopy of dog lungs[51] found that neutrophil passage through the lung occurs at a very slow pace (mean transit time 6.1 min) compared to an estimated of 1.4 seconds for plasma flow in the lung. These studies were performed using adoptively transferred labeled neutrophils, raising some question of the broad applicability of the estimates. However, subsequently, application of LIVM in mice using otherwise unmanipulated fluorescent reporter mice found that neutrophils passage through the capillary beds with a mean speed of 0.9 µm/sec while fluorescent beads (as a gauge of base plasma flow) circulate with a mean speed of 109 µm/sec [24], consistent with the earlier estimates. This data has proved consistent in at least 2 separate studies[21,52]. Intriguingly, through intravital analysis of neutrophils in the lung during CXCR4-inhibition with Plerixafor™ (a drug in use for the treatment of neutropenia) Devi et al. [50], found that this large pool of neutrophils can be induced to demarginate, mobilize, and repopulate into the systemic circulation all suggestive that the lung acts as a critical biorepository for neutrophils.
Essentially, all relevant studies on pulmonary neutrophil dynamics, have found that the presence of an inflammatory stimulus increases the numbers of neutrophils in the lung[9,21,24]. These cells appear poised to rapidly respond to pulmonary insult. Moreover, neutrophils present in the pulmonary vasculature rapidly mobilize upon encounter with bloodborne pathogens, ingest, and move out of the pulmonary microcirculation at increased rates[9] suggesting that this rapid vascular mobilization of pulmonary neutrophils may in fact represent a systemic host-defense mechanism against bloodborne infection. Adding to this, pulmonary neutrophils appear to act as shepherds for intravascular iNKT cells[53]. Following intranasal administration of α-Galcer (a model system for S. pneumoniae infection), neutrophils responded by producing large amounts of CCL17 which in turn enabled the extravasation of iNKT cells into the pulmonary interstitium. This response proved critical to activation of these cells as it put them into direct contact with monocyte-derived dendritic cells capable of presenting antigen to these potent inflammatory cells. Importantly, this same axis appeared functional in the context of S. pneumoniae infection further arguing for the critical role played by neutrophils in pulmonary surveillance[53]. This prominence of neutrophil function in the lung can be a two-edged sword as intravital microscopy has also implicated conjugation of vascular neutrophils and platelets in response to inflammation (Figure 3C) as a critical factor in the pathogenesis of acute lung injury[39].
Circulating Monocytes in Sepsis-induced Lung Injury
In addition to the critical role played by neutrophils during the pathogenesis of lung injury, as discussed above, it has been proposed the Ly6Chi monocytes may be uniquely sequested in the pulmonary vasculature in response to systemic inflammatory signals associated with sepsis (LPS). Their presence there pre-disposing the organ and contributing to pulmonary injury[54]. An elegant set of LIVM studies used a combination of intravascular labeling with an anti-Ly6B.2 and the monocyte-lineage reporter mouse, Cx3cr1-GFP, to clearly distinguish between Ly6Chi (conventional) and Ly6clo (patrolling) monocytes. Using this method, the authors established an axis whereby CXCR4+Ly6Chi monocytes are recruited to the pulmonary vasculature where they marginate and are sequestered in vast numbers in response to intravascular LPS administration. LIVM in combination with CXCR4-inhibition revealed a clear release of monocyte sequestration as monocytes were seen to rapidly de-marginate and increase in velocity following drug treatment, highlighting a parallel dynamic to that of CXCR4+ neutrophils discussed above. Intrigingly, removing CXCR4 expression from Ly6Chi monocytes (using a CXCR4 conditional KO strategy driven by Lyz2-Cre) resulted in not reduced monocyte recruitment to the lung in a lung injury setting, but limited disease in the form of reduced vascular leakage and increased survival[54].
The Future of Intravital Lung Imaging
While the past 10 years have introduced a renaissance in the study of both pulmonary physiology and dynamics of the lung immune system, there is still room for improvement. As stated earlier, the key feature of intravital lung imaging that remains unassailed is the depth of imaging. No current methodology has achieved greater than 100 pm imaging depth in an intravital preparation. Fortunately, this region of the lung is of great interest in diseases such as pulmonary metastasis, lung injury, and basic homeostasis of the lung. However, many diseases such as asthma, lung bacterial and viral infection, and even primary lung cancer frequently target the larger airways and blood vessels. Until methods are developed to access greater depths of the lung through intravital imaging, the true in vivo dynamics of the immune system will largely remain a mystery in these diseases. Several technologies currently on the horizon offer promise. Foremost of this is adaptive optics[55,56]. This technology pairs predictive modeling of the light scattering properties of a substance/tissue with on demand deformable mirrors to allow the researcher to ‘pre-bend’ the path of the excitation laser such that it compensates for the scatter of the substance. This method has now been successfully applied to deep imaging of tissues such as brain and retina[56], but the numerous tandem air-liquid refractive index shifts in the lung represent a unique challenge. Ex vivo approaches have shown some headway[57], however a great deal more study will be required, perhaps coupled with machine learning algorithms[58,59], before we can achieve the imaging depth in the lung needed to explore all relevant pulmonary conditions.
Highlights.
The lung represents a dynamic and challenging organ for intravital imaging.
A variety of creative approaches have been taken to provide stable long-term imaging in live animals, enabling direct visualization of the pulmonary immune response
Pulmonary intravital imaging has yielded critical insights into steady-state lung function and such disease states as pulmonary metastasis, asthma, and lung injury.
Acknowledgments
The work on this review was supported by the Fred Hutchinson Cancer Research Center and the University of California, San Francisco.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Looney MR, Bhattacharya J, Live imaging of the lung, Annu. Rev. Physiol 76 (2014) 431–445. doi: 10.1146/annurev-physiol-021113-170331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Lelkes E, Headley MB, Thornton EE, Looney MR, Krummel MF, The spatiotemporal cellular dynamics of lung immunity, Trends Immunol 35 (2014) 379–386. doi: 10.1016/j.it.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fiole D, Tournier J-N, Intravital microscopy of the lung: minimizing invasiveness, J Biophotonics 9 (2016) 868–878. doi: 10.1002/jbio.201500246. [DOI] [PubMed] [Google Scholar]
- [4].Bartholin T, Malpighi M, van der Aa Pieter., Malpighii Marcelli ... Opera omnia, seu, Thesaurus locupletissimus botanico-medico-anatomicus :viginti quator tractatus complectens et in duos tomos distributus, quorum tractatum seriem videre est dedicatione absolutâ., Apud Petrum vander Aa ..., Lugduni Batavorum :, 1687. doi: 10.5962/bhl.title.566. [DOI] [Google Scholar]
- [5].West JB, Marcello Malpighi and the discovery of the pulmonary capillaries and alveoli, American Physiological Society Bethesda, MD, 2013. doi: 10.1152/ajplung.00016.2013. [DOI] [PubMed] [Google Scholar]
- [6].Entenberg D, Voiculescu S, Guo P, Borriello L, Wang Y, Karagiannis GS, et al. , A permanent window for the murine lung enables high-resolution imaging of cancer metastasis, Nat. Methods 113 (2017) 3073. doi: 10.1038/nmeth.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Headley MB, Bins A, Nip A, Roberts EW, Looney MR, Gerard A, et al. , Visualization of immediate immune responses to pioneer metastatic cells in the lung, Nature 531 (2016) 513–517. doi: 10.1038/nature16985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Hanna RN, Cekic C, Sag D, Tacke R, Thomas GD, Nowyhed H, et al. , Patrolling monocytes control tumor metastasis to the lung, Science 350 (2015) 985–990. doi: 10.1126/science.aac9407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Yipp BG, Kim JH, Lima R, Zbytnuik LD, Petri B, Swanlund N, et al. , The Lung is a Host Defense Niche for Immediate Neutrophil-Mediated Vascular Protection, Sci Immunol 2 (2017) eaam8929. doi: 10.1126/sciimmunol.aam8929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Burburan SM, Silva JD, Abreu SC, Samary CS, Guimaraes IHL, Xisto DG, et al. , Effects of inhalational anaesthetics in experimental allergic asthma, Anaesthesia 69 (2014) 573–582. doi: 10.1111/anae.12593. [DOI] [PubMed] [Google Scholar]
- [11].Li J-T, Wang H, Li W, Wang L-F, Hou L-C, Mu J-L, et al. , Anesthetic Isoflurane Posttreatment Attenuates Experimental Lung Injury by Inhibiting Inflammation and Apoptosis, Mediators of Inflammation 2013 (2013) 1–16. doi: 10.1155/2013/108928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Tighe RM, Birukova A, Yaeger MJ, Reece SW, Gowdy KM, Euthanasia and Lavage Mediated Effects on Bronchoalveolar Measures of Lung Injury and Inflammation, American Journal of Respiratory Cell and Molecular Biology (2018) rcmb.2017–03570C. doi: 10.1165/rcmb.2017-03570C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].do Vale EM, Xavier CC, Nogueira BG, Campos BC, de Aquino PEA, R.0. da Costa, et al. , Antinociceptive and Anti-Inflammatory Effects of Ketamine and the Relationship to Its Antidepressant Action and GSK3 Inhibition, Basic Clin. Pharmacol. Toxicol 119 (2016) 562–573. doi: 10.1111/bcpt.12637. [DOI] [PubMed] [Google Scholar]
- [14].Loix S, De Kock M, Henin P, The anti-inflammatory effects of ketamine: state of the art, Acta Anaesthesiol Belg 62 (2011) 47–58. [PubMed] [Google Scholar]
- [15].Olkon DM, Joannides M, Capillaroscopic appearance of the pulmonary alveoli in the living dog, The Anatomical Record 45 (1930) 121–127. doi: 10.1002/ar.1090450204. [DOI] [Google Scholar]
- [16].Macgregor RG, Examination of the pulmonary circulation with the microscope, J. Physiol. (Lond.) 80 (1933) 65–77. doi: 10.1111/(ISSN)1469-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Hasegawa A, Hayashi K, Kishimoto H, Yang M, Tofukuji S, Suzuki K, et al. , Color-coded real-time cellular imaging of lung T-lymphocyte accumulation and focus formation in a mouse asthma model, J. Allergy Clin. Immunol 125 (2010) 461–468.e6. doi: 10.1016/j.jaci.2009.09.016. [DOI] [PubMed] [Google Scholar]
- [18].Tabuchi A, Mertens M, Kuppe H, Pries AR, Kuebler WM, Intravital microscopy of the murine pulmonary microcirculation, J Appl Physiol 104 (2008) 338–346. doi: 10.1152/japplphysiol.00348.2007. [DOI] [PubMed] [Google Scholar]
- [19].Mitsuoka H, Unno N, Sakurai T, Kaneko H, Suzuki S, Konno H, et al. , Pathophysiological role of endothelins in pulmonary microcirculatory disorders due to intestinal ischemia and reperfusion, J. Surg. Res 87 (1999) 143–151. doi: 10.1006/jsre.1999.5694. [DOI] [PubMed] [Google Scholar]
- [20].Veith NT, Tschernig T, Gutbier B, Witzenrath M, Meier C, Menger M, et al. , Surfactant protein A mediates pulmonary clearance of Staphylococcus aureus, Respir. Res 15 (2014) 85. doi: 10.1186/s12931-014-0085-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Kreisel D, Nava RG, Li W, Zinselmeyer BH, Wang B, Lai J, et al. , In vivo two-photon imaging reveals monocyte-dependent neutrophil extravasation during pulmonary inflammation, Proc. Natl. Acad. Sci. U.S.a 107 (2010) 18073–18078. doi: 10.1073/pnas.1008737107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Spahn JH, Li W, Bribriesco AC, Liu J, Shen H, Ibricevic A, et al. , DAP12 expression in lung macrophages mediates ischemia/reperfusion injury by promoting neutrophil extravasation, J. Immunol 194 (2015) 4039–4048. doi: 10.4049/jimmunol.1401415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Bhattacharya J, Seeing is believing, Nat. Methods 8 (2011) 57–58. doi: 10.1038/nmeth0111-57. [DOI] [PubMed] [Google Scholar]
- [24].Looney MR, Thornton EE, Sen D, Lamm WJ, Glenny RW, Krummel MF, Stabilized imaging of immune surveillance in the mouse lung, Nat. Methods 8 (2011)91–96. doi: 10.1038/nmeth.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Presson RG, Brown MB, Fisher AJ, Sandoval RM, Dunn KW, Lorenz KS, et al. , Two-photon imaging within the murine thorax without respiratory and cardiac motion artifact, Am. J. Pathol 179 (2011) 75–82. doi: 10.1016/j.ajpath.2011.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Wagner WW, Pulmonary microcirculatory observations in vivo under physiological conditions, J Appl Physiol 26 (1969) 375–377. doi: 10.1152/jappl.1969.26.3.375. [DOI] [PubMed] [Google Scholar]
- [27].Kandasamy K, Parthasarathi K, Quantifying single microvessel permeability in isolated blood-perfused rat lung preparation, J Vis Exp (2014) e51552–e51552. doi: 10.3791/51552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Al-Mehdi AB, Tozawa K, Fisher AB, Shientag L, Lee A, Muschel RJ, Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis, Nat. Med 6 (2000) 100–102. doi: 10.1038/71429. [DOI] [PubMed] [Google Scholar]
- [29].van den Bijgaart RJE, Kong N, Maynard C, Plaks V, Ex vivo Live Imaging of Lung Metastasis and Their Microenvironment, J Vis Exp (2016) e53741. doi: 10.3791/53741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Thornton EE, Looney MR, Bose O, Sen D, Sheppard D, Locksley R, et al. , Spatiotemporally separated antigen uptake by alveolar dendritic cells and airway presentation toT cells in the lung, J. Exp. Med 209 (2012) 1183–1199. doi: 10.1084/jem.20112667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Azaripour A, Lagerweij T, Scharfbillig C, Jadczak AE, Willershausen B,Van Noorden CJF, A survey of clearing techniques for 3D imaging of tissues with special reference to connective tissue, Prog Histochem Cytochem 51 (2016) 9–23. doi: 10.1016/j.proghi.2016.04.001. [DOI] [PubMed] [Google Scholar]
- [32].Tainaka K, Kuno A, Kubota SI, Murakami T, Ueda HR, Chemical Principles in Tissue Clearing and Staining Protocols for Whole-Body Cell Profiling, Annu. Rev. Cell Dev. Biol 32 (2016)713–741. doi: 10.1146/annurev-cellbio-111315-125001. [DOI] [PubMed] [Google Scholar]
- [33].Li W, Germain RN, Gerner MY, Multiplex, quantitative cellular analysis in large tissue volumes with clearing-enhanced 3D microscopy (Ce3D), Proc. Natl. Acad. Sci. U.S.a 114 (2017) E7321–E7330. doi: 10.1073/pnas.1708981114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Pan C, Cai R, Quacquarelli FP, Ghasemigharagoz A, Lourbopoulos A, Matryba P, et al. , Shrinkage-mediated imaging of entire organs and organisms using uDISCO, Nat. Methods 13 (2016) 859–867. doi: 10.1038/nmeth.3964. [DOI] [PubMed] [Google Scholar]
- [35].Ertürk A, Becker K, Jährling N, Mauch CP, Hojer CD, Egen JG, et al. , Three-dimensional imaging of solvent-cleared organs using 3DISCO, Nat Protoc 7 (2012) 1983–1995. doi: 10.1038/nprot.2012.119. [DOI] [PubMed] [Google Scholar]
- [36].Sceneay J, Smyth MJ, Möller A, The pre-metastatic niche: finding common ground, Cancer Metastasis Rev 32 (2013) 449–464. doi: 10.1007/s10555-013-9420-1. [DOI] [PubMed] [Google Scholar]
- [37].Perlikos F, Harrington KJ, Syrigos KN, Key molecular mechanisms in lung cancer invasion and metastasis: a comprehensive review, Crit. Rev. Oncol. Hematol 87 (2013) 1–11. doi: 10.1016/j.critrevonc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- [38].Ferlay J, Shin H-R, Bray F, Forman D, Mathers C, Parkin DM, Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008, International Journal of Cancer 127 (2010) 2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- [39].Ortiz-Muñoz G, Mallavia B, Bins A, Headley M, Krummel MF, Looney MR, Aspirin-triggered 15-epi-lipoxin A4 regulates neutrophil-platelet aggregation and attenuates acute lung injury in mice, Blood 124 (2014) 2625–2634. doi: 10.1182/blood-2014-03-562876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lefrangais E, Ortiz-Muñoz G, Caudrillier A, Mallavia B, Liu F, Sayah DM, et al. , The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors, Nature 544 (2017) 105–109. doi: 10.1038/nature21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Mendoza A, Hong S-H, Osborne T, Khan MA, Campbell K, Briggs J, et al. , Modeling metastasis biology and therapy in real time in the mouse lung, J. Clin. Invest 120 (2010) 2979–2988. doi: 10.1172/JCI40252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Hanna RN, Carlin LM, Hubbeling HG, Nackiewicz D, Green AM, Punt JA, et al. , The transcription factor NR4A1 (Nur77) controls bone marrow differentiation and the survival of Ly6C-monocytes, Nat Immunol 12 (2011) 778–785. doi: 10.1038/ni.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Carlin LM, Auffray C, Geissmann F, Measuring Intravascular Migration of Mouse Ly6C lowMonocytes In Vivo Using Intravital Microscopy, John Wiley & Sons, Inc, Hoboken, NJ, USA, 2001. doi: 10.1002/0471142735.im1433s101. [DOI] [PubMed] [Google Scholar]
- [44].Carlin LM, Stamatiades EG, Auffray C, Hanna RN, Glover L, Vizcay-Barrena G, et al. , Nr4a1-dependent Ly6C(low) monocytes monitor endothelial cells and orchestrate their disposal, Cell 153 (2013) 362–375. doi: 10.1016/j.cell.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jakubzick C, Tacke F, Ginhoux F, Wagers AJ, van Rooijen N, Mack M, et al. , Blood monocyte subsets differentially give rise to CD103+ and CD103-pulmonary dendritic cell populations, J. Immunol 180 (2008) 3019–3027. [DOI] [PubMed] [Google Scholar]
- [46].Landsman L, Varol C, Jung S, Distinct differentiation potential of blood monocyte subsets in the lung, J. Immunol 178 (2007) 2000–2007. [DOI] [PubMed] [Google Scholar]
- [47].Qian B-Z, Li J, Zhang H, Kitamura T, Zhang J, Campion LR, et al. , CCL2 recruits inflammatory monocytes to facilitate breast-tumour metastasis, Nature 475 (2011) 222–225. doi: 10.1038/nature10138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Rodero MP, Poupel L, Loyher P-L, Hamon P, Licata F, Pessel C, et al. , Immune surveillance of the lung by migrating tissue monocytes, Elife 4 (2015) e07847. doi: 10.7554/eLife.07847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Sen D, Jones SM, Oswald EM, Pinkard H, Corbin K, Krummel MF, Tracking the Spatial and Functional Gradient of Monocyte-To-Macrophage Differentiation in Inflamed Lung, PLoS ONE 11 (2016) e0165064. doi: 10.1371/journal.pone.0165064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Devi S, Wang Y, Chew WK, Lima R, A-González N, Mattar CNZ, et al. , Neutrophil mobilization via plerixafor-mediated CXCR4 inhibition arises from lung demargination and blockade of neutrophil homing to the bone marrow, J. Exp. Med 210 (2013) 2321–2336. doi: 10.1084/jem.20130056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Lien DC, Wagner WW, Capen RL, Haslett C, Hanson WL, Hofmeister SE, et al. , Physiological neutrophil sequestration in the lung: visual evidence for localization in capillaries, J Appl Physiol 62 (1987) 1236–1243. doi: 10.1152/jappl.1987.62.3.1236. [DOI] [PubMed] [Google Scholar]
- [52].Bennewitz MF, Jimenez MA, Vats R, Tutuncuoglu E, Jonassaint J, Kato GJ, et al. , Lung vaso-occlusion in sickle cell disease mediated by arteriolar neutrophil-platelet microemboli, JCI Insight 2 (2017) e89761. doi: 10.1172/jci.insight.89761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Thanabalasuriar A, Neupane AS, Wang J, Krummel MF, Kubes P, iNKT Cell Emigration out of the Lung Vasculature Requires Neutrophils and Monocyte-Derived Dendritic Cells in Inflammation, Cell Reports 16 (2016) 3260–3272. doi: 10.1016/j.celrep.2016.07.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chong SZ, Evrard M, Devi S, Chen J, Lim JY, See P, et al. , CXCR4 identifies transitional bone marrow premonocytes that replenish the mature monocyte pool for peripheral responses, J. Exp. Med 213 (2016) 2293–2314. doi: 10.1084/jem.20160800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Ji N, Milkie DE, Betzig E, Adaptive optics via pupil segmentation for high-resolution imaging in biological tissues, Nat. Methods 7 (2010) 141–147. doi: 10.1038/nmeth.1411. [DOI] [PubMed] [Google Scholar]
- [56].Ji N, Adaptive optical fluorescence microscopy, Nat. Methods 14 (2017) 374–380. doi: 10.1038/nmeth.4218. [DOI] [PubMed] [Google Scholar]
- [57].Kang S, Kang P, Jeong S, Kwon Y, Yang TD, Hong JH, et al. , High-resolution adaptive optical imaging within thick scattering media using closed-loop accumulation of single scattering, Nat Commun 8 (2017) 2157. doi: 10.1038/s41467-017-02117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].González-Gutierrez C, Santos JD, Martínez-Zarzuela M, Basden AG, Osborn J, Diaz-Pernas FJ, et al. , Comparative Study of Neural Network Frameworks for the Next Generation of Adaptive Optics Systems, Sensors (Basel) 17 (2017) 1263. doi: 10.3390/s17061263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Smith T, Smith N, Detection of cone photoreceptors in adaptive optics retinal images using topographical features and machine learning, in: Cosi, OSA, Washington, D.C, 2014: p. JTu5A.41. doi: 10.1364/COSI.2014.JTu5A.41. [DOI] [Google Scholar]