Summary.
The expression of heparan sulfate endosulfatases (Sulfs) was investigated in the intervertebral disc (IVD) to clarify their role in IVD homeostasis. Sulf-1 and −2 expression was elucidated in normal and degenerated human IVD. Age-related effects on Sulf expression, type II collagen levels, and structural changes were analyzed in IVDs of wild-type (WT) and Sulf-1 knockout (Sulf-1−/−) mice. The effect of recombinant Sulf-1 (100 ng/ml) and Sulf-1 knockdown on heparan sulfate proteoglycan and collagen expression in ATDC5 cells was examined. Finally, the effect of Sulf-1 on transforming growth factor (TGF) β1-induced signaling was evaluated. Results show that Sulf-1 and −2 levels were higher in degenerated human IVDs. In WT mice, Sulf-1 and −2 expression generally declined as the animals aged. In particular, Sulf-1 in the nucleus pulposus was higher compared with Sulf-2 at the age of 1 and 6 months and significantly declined with aging. Sulf-1−/− mice showed more severe IVD pathology than WT mice, with lower type II collagen levels in nucleus pulposus, and degeneration with type I collagen in annulus fibrosus. In vitro, Sulf-1 induced type II collagen expression and significantly increased TGF-β1-induced Smad2/3 phosphorylation in ATDC5 cells. In conclusion, Sulf-1 might play a critical role from development to maintenance of IVD homeostasis by regulating collagen expression.
Keywords: intervertebral disc, Sulf-1, type II collagen, Smad2/3
Introduction
Intervertebral disc (IVD) degeneration is one of the most common causes of lower back pain (Luoma et al., 2000). The risk of IVD degeneration is increased by aging, genetic factors, mechanical stress, and trauma (Jaumard et al., 2011; Vo et al., 2013; 2016), and can result in herniated nucleus pulposus (NP). Although various cytokines and growth factors are known to be involved in IVD degeneration (Ellman et al., 2008), little is known about the role of NP cells in this condition (Sakai et al., 2012). The NP is located centrally within the IVD, and it is populated by clusters of vacuolated notochordal cells and chondrocyte-like cells in rodent IVDs, but notochordal cells in humans decrease rapidly after birth and are undetectable at the age of 4–10 years (Zhao et al., 2007). Two major collagens (types I and II) are found within IVDs; type II collagen is a major constituent of the NP, and type I collagen is found in the annulus fibrosus (AF) (Eyre and Muir, 1976). NP degeneration is associated with a strongly age-dependent reduced expression of type II collagen (Richardson et al., 2007). Although the loss of type II collagen in NP is known to be associated with a loss of function and accelerated age-related changes, the mechanism remains to be investigated.
Heparan sulfate proteoglycans are ubiquitous in basement membrane and are also present in the IVD (Melrose et al., 2008). Heparan sulfate 6–0 endosulfatases (Sulfs) regulate several cell signaling pathways (Dhoot et al., 2001; Viviano, et al., 2004: Ai et al., 2007), including those that modulate cartilage homeostasis such as bone morphogenetic protein (BMP)/Smad and fibroblast growth factor (FGF2)/extracellular signal-regulated kinase signaling (Otsuki et al., 2008; 2010). Furthermore, intra-articular injection of Sulf-1 was shown to prevent cartilage degeneration (Otsuki et al., 2016). Although Sulf-1 might be critical for cartilage homeostasis and has been shown to have therapeutic potential, its expression and role in IVD have not been elucidated.
The purpose of this study was to investigate the effect of aging on the expression of Sulfs and to clarify the role of Sulf-1 in IVD homeostasis with a focus on collagen expression.
Materials and methods
Human samples
Human lumbar IVD samples were collected from four cadaveric donors (age 40–45 years, mean 43.3 years) without any known history of spine disease, and from four donors (age 53–74 years, mean 62.0 years) undergoing spinal surgery (Alvarez-Garcia et al., 2017). Macroscopic assessment of human lumbar IVD was performed according to Thompson grading (Thompson et al., 1990). The normal group was grade II in all four samples and the degenerated group was grade IV in all four samples.
Animal experiments
All experiments were performed in accordance with the guidelines set by the Osaka Medical College Animal Care Committee (No. 26093) and animals were treated based on the 3R principles. Male wild-type (WT) C57BL/6 mice (n = 24) and Sulf-1 knockout mice (Sulf-1−/−) on C57BL/6 background (n = 24) were used; these animals have been described previously (Ai et al., 2007). The male mice were housed in a temperature- and humidity-controlled environment, and euthanized at ages of 1, 6, 12, and 24 months (n = 4–6 animals per time point).
Histological analysis
IVD samples were fixed in 4% paraformaldehyde at 15–25°C for 24 h and then decalcified in 50% TBD-2 (Fisher Scientific, Pittsburgh, PA) at 4°C for 48 h. The tissues were then dehydrated and embedded in paraffin using standard methods prior to the preparation of 5-μm sagittal sections. To evaluate IVD degeneration, sections of human and mouse IVD were stained with safranin O and then evaluated by Masuda score (for NP and AF) (Masuda et al., 2005) and Boos score (for the endplate; EP) (Boos et al., 2002).
Immunohistochemical analysis
Sulf-1 and Sulf-2 expression in human IVD samples and the lumbar 5/6 disc and in C57BL/6 WT mice at the age of 1, 6, 12, and 24 months were determined by immunohistochemistry. The deparaffinized and rehydrated tissue sections were treated with peroxidase for 5 min at 15–25°C. After washing with phosphate-buffered saline (PBS), the sections were blocked with 4% skim milk for 10 min at room temperature. Primary antibodies to Sulf-1 (1:100; Abcam, Cambridge, MA), Sulf-2 (1:50; Abcam), and type II collagen (1:50; Santa Cruz Biotechnology, Inc., Dallas, TX) were applied and incubated overnight at 4°C. The sections were washed again with PBS prior to incubation of the Sulf antibody-treated sections with a biotinylated anti-rabbit IgG secondary antibody (1:100; Vector Laboratories, Burlingame, CA) for 30 min at 4°C, followed by incubation with horseradish peroxidase-streptavidin (1:200; Vector Laboratories). Finally, the sections were washed with PBS and the signals were visualized by incubation with Vectastain ABC-AP alkaline phosphatase (Vector Laboratories) for 30 min. Type II collagen expression was detected by immunofluorescence following incubation with an Alexa Fluor 568-conjugated goat anti-rabbit antibody (1:400; Thermo Fisher Scientific, Rockford, IL) in PBS/0.3% Triton/1% normal goat serum for 1 h at room temperature. Cells showing NP, AP, and EP were counted at ×400 magnification using a 400 × 400-μm grid, and the percentage of Sulf-positive cells in three different areas was counted and density was evaluated (Otsuki et al., 2010).
Picrosirius red staining (PSR-1-IFU; ScyTek Laboratories Inc., Logan UT) was performed according to the manufacturer’s instructions. Briefly, after sections were deparaffinized, Picrosirius Red Solution (ScyTek Laboratoties, Logan, UT) was applied to completely cover tissue sections and incubated for 60 min at 15–25 °C, followed by rinsing twice with acetic acid solution. After dehydration, sections were evaluated by polarized microscopy.
Effect of Sulf-1 on gene expression on ATDC5 cell line
The ATDC5 cell line is derived from mouse teratocarcinoma cells and is characterized as a chondrogenic cell line that undergoes a sequential process analogous to chondrocyte differentiation (Yao and Wang, 2013).
Chondrogenesis was induced in ATDC5 cells for 2 weeks, as described previously (Shukunami et al., 1996). The cells were either treated with 100 ng/ml recombinant Sulf-1 (Abnova, Taipei, Taiwan) or with Sulf-1 siRNA (Ambion, Carlsbad, CA). After 72 hours, effects on type I, II, collagen, heparan sulfate related proteoglycan and glyceraldehyde 3-phosphate dehydrogenase (GAPDH) gene expression was examined by quantitative real-time polymerase chain reaction (qRT-PCR), which was performed in a LightCycler (Roche Diagnostics, Mannheim, Germany) using TaqMan Gene Expression Assay probes (Roche Diagnostics) according to the protocol provided by the manufacturer.
The effect of Sulf-1 on type II collagen protein expression was investigated by immunocytochemistry in ATDC5 cells that were cultured under chondrogenic conditions for two weeks in the presence or absence of recombinant Sulf-1 (100 ng/ml).
Western blotting and densitometry
Total protein (45 μg) prepared from ATDC5 cells was resolved on polyacrylamide gels (Life Technologies, Carlsbad, CA). The separated proteins were transferred to polyvinylidene difluoride membranes (GE Healthcare, Aliso Viejo, CA), which were blocked using 4% skim milk for 1 h at room temperature. Primary antibodies were applied and incubated with the membranes overnight at 4°C. After washing with PBS for 1 h, the appropriate secondary antibodies were applied and incubated for 1 h at room temperature. To elucidate the cell signaling involved in type II collagen expression, Smad cell signaling in response to TGFß was investigated. ATDC5 cells were incubated with 100 ng/ml Sulf-1 for 72 h; the cells were then exposed to transforming growth factor-β1 (Cell Signaling TGF-β1; 10 ng/ml), and the levels of phosphorylated Smad2/3, Smad2/3 and type II collagen were examined at 30 min, 24 h, and 72 h. The primary antibodies employed were raised in rabbits and detected phosphorylated Smad2/3, Smad2/3 (1:1000; Cell Signaling Technology, Danvers, MA), type II collagen (1:200; SANTA CRUZ) and GAPDH (1:10000; Wako Pure Chemical Industries, Osaka, Japan). Immunoreactive bands were visualized using enhanced chemiluminescence (ECL Plus system; GE Healthcare) and the LAS-3000 Mini Chemilumino Analyzer (Fujifilm, Tokyo, Japan). The bands were quantified by densitometry (Multi Gauge; Fujifilm, Tokyo, Japan). Normalization was performed against GAPDH and analyzed using NIH ImageJ software (1.49v).
Statistical analysis
The results of each evaluation were analyzed using the Mann-Whitney U test. They were considered significant when p < 0.05. All statistical analyses were performed using SAS statistical software, version 22 (SAS Institute Inc., Cary, NC). The results are reported as the mean ± standard deviation.
Results
Expression of Sulfs in human IVDs
Four normal and four degenerative human IVDs were analyzed. Safranin O staining indicated a loss of glycosaminoglycan from the degenerated human IVDs (Fig. 1A). Representative Sulf-1 positive and negative cells are shown in black and white arrows, respectively (Fig 1B). Negative control is shown in Figure 1C. Sulf-1 and −2 were detected in around 20% of the cells in normal IVDs. The expression of Sulf-1 and Sulf-2 was increased in all areas of degenerated IVDs, including NP and inner and outer AF (Fig. 1D; *p < 0.05).
Figure 1:
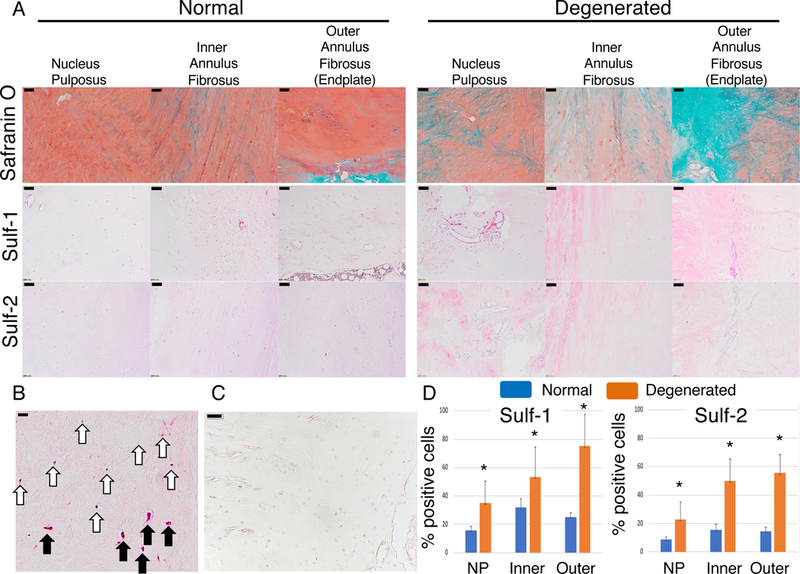
Sulf-1 and -2 expressions in human IVD. (A) Four normal and four degenerative IVDs were stained with safranin O; Sulf-1 and −2 were visualized using immunohistochemistry. Scale bars represent 100 μm. (B) Representative positive and negative cells are shown with black and white arrows, respectively. Scale bars: 50 μm. (C) Negative control is shown. Scale bars: 50 μm. (D) The number of Sulf-positive cells was counted in each of the indicated tissues; *p < 0.05 for the indicated comparison.
Expression of Sulfs in WT mice
In 6 months old mice, Sulf-1 was expressed to a significantly greater extent than Sulf-2 at the NP, AF, and EP, and Sulf-1 expression levels declined with age (Fig. 2A,C; p < 0.05). In contrast, Sulf-2 expression in the mouse IVD was not significantly altered by aging (Fig. 2A, C). Negative control is shown in Figure 2B.
Figure 2:
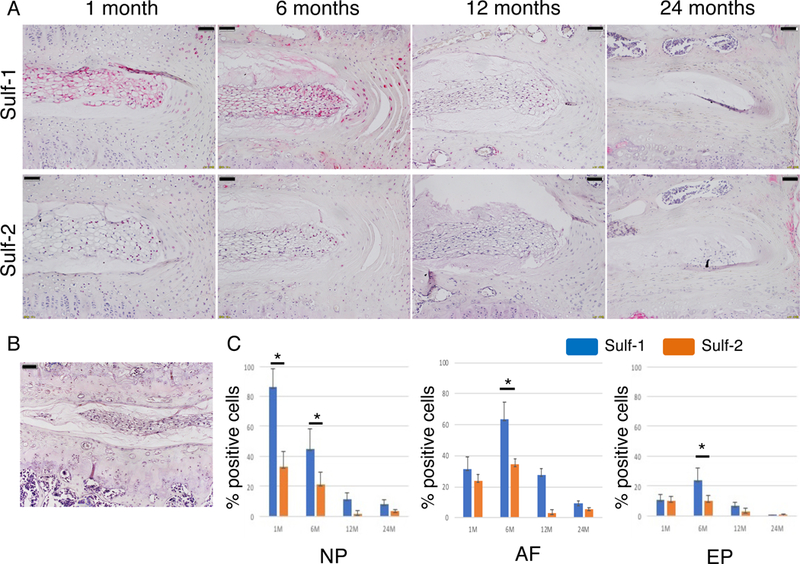
Sulf-1 and Sulf-2 expression in mouse IVDs. (A) IVD samples from WT mice aged 1–24 months were analyzed by immunohistochemistry (scale bars represent 50 μm). (B) Negative control is shown (scale bars represent 50 μm). (C) The number of Sulf-positive cells was counted in each of the indicated tissues; *p < 0.05 for the indicated comparison.
Comparison of IVD degeneration in WT and Sulf-1−/− mice
As Sulf-1 expression showed more profound aging-related changes, we analyzed the consequences of Sulf-1 deletion for spine homeostasis. IVD samples were harvested from WT and Sulf-1−/− mice aged 1, 6, 12, and 24 months to investigate spontaneous changes in spine tissues. Sulf-1−/− mice showed less glycosaminoglycan staining than WT mice, especially in the EP. NP cell numbers were also lower in Sulf-1−/− mice than in WT mice at 1 and 6 months of age. EP bone formation was detected at 24 months in Sulf-1−/− mice, but not in WT mice. The IVD showed more severe pathology in Sulf-1−/− mice than in WT mice (Fig. 3A). Histological scoring revealed that Sulf-1−/− mice showed significant changes in NP and AF at 1 month while differences in EP were significant only by 12 and 24 months as compared with WT mice (Fig. 3B, *p < 0.05).
Figure 3:
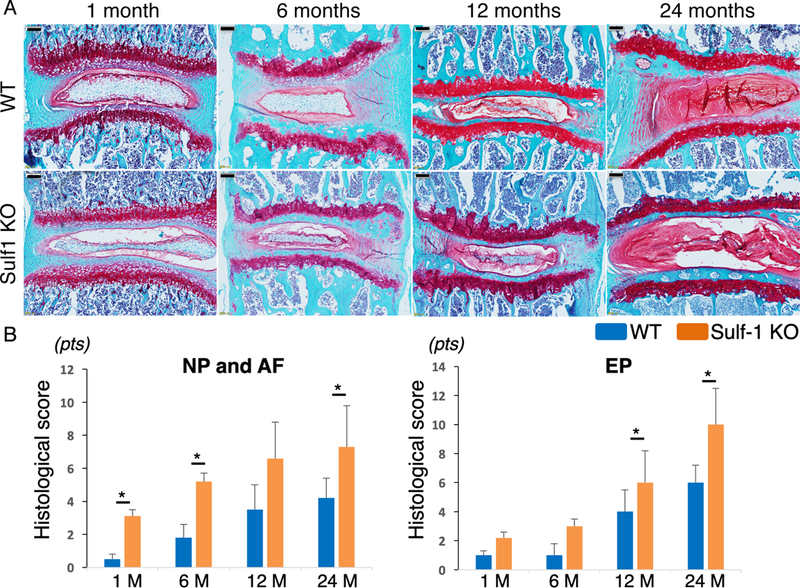
Spontaneous IVD degeneration in Sulf-1−/− mice. (A) IVD samples were harvested from WT and Sulf-1−/− mice at 1, 6, 12, and 24 months of age. Sections were stained with safranin O (n = 4–6 animals per time point). (B) Histological scores for the indicated mice and tissues; *p < 0.05 for the indicated comparisons; scale bars: 100 μm.
Type II collagen expression in IVD
Type II collagen expression in NP, AF, and EP was analyzed by immunofluorescence in 3-month-old mice (Fig. 4A). WT mice showed a significantly higher density of type II collagen expression than Sulf-1−/− mice (Fig. 4B; p = 0.02), although the NP and AF cell numbers did not differ significantly between these animals (Fig. 4B). In the EP, both cell numbers and type II collagen density were significantly greater in WT mice than in Sulf-1−/− mice (Fig. 4A, C; p < 0.01).
Figure 4:
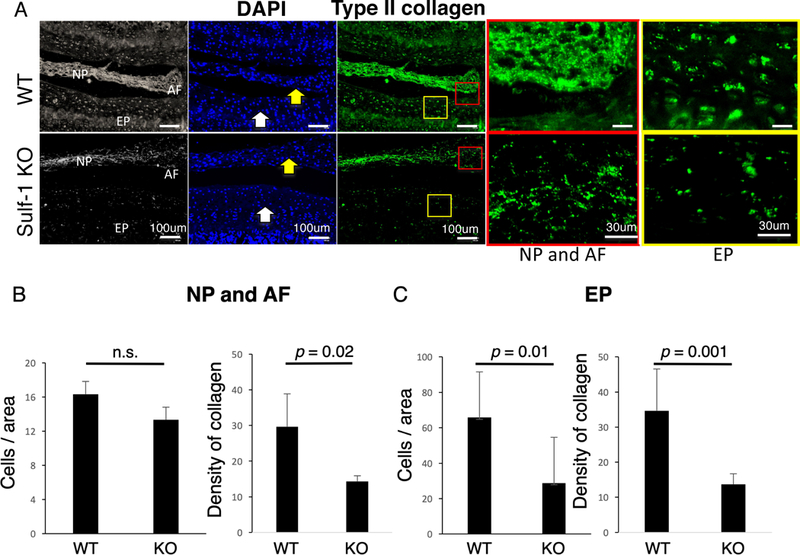
Type II collagen expression in the NP. (A) Anatomical orientation of NP, AF, and EP in mouse IVDs is shown in the left panels. Nuclei are shown with DAPI staining and type II collagen expression in the NP (yellow arrow) and EP (white arrow) was analyzed using immunofluorescence in 3-month old WT and Sulf-1−/− mice. (B) Quantitative analysis of cell numbers and type II collagen density in the indicated animals and tissues. Values represent the mean ± standard deviation of the mean, n = 6 mice per group.
IVD structure and collagen expression with Sulf-1
Regarding the AF extracellular matrix, the collagen structure in Sulf-1 KO was similar to WT mice at 1 month of age. However at the age of 6 months, the collagen structure in Sulf-1 KO was rougher and thinner than in WT mice, especially at the AF and EP (Fig. 5).
Figure 5:
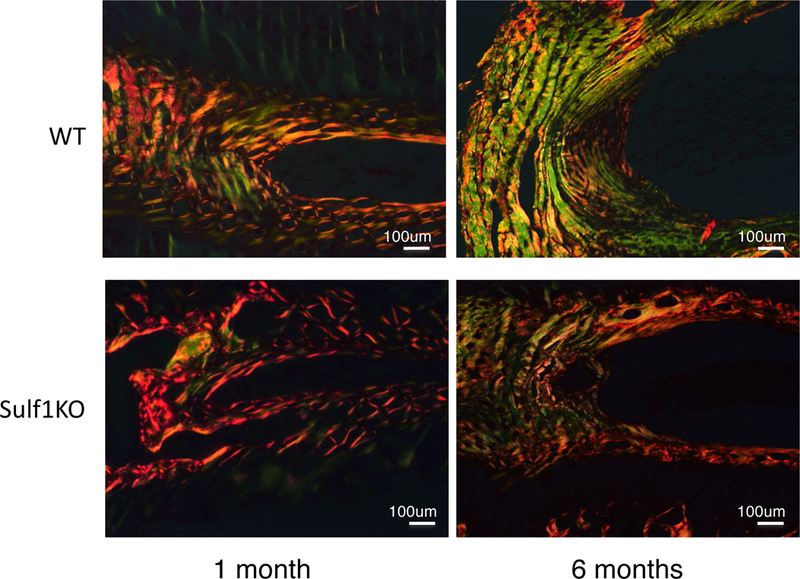
Collagen expression and IVD structure in IVD. Picrosirius red staining was performed on WT and Sulf-1−/− mice at 1 and 6 months of age to elucidate collagen structure with polarized microscopy. Yellow and orange showed type I and green showed type III collagen.
Gene expression following Sulf-1 stimulation and knockdown
Type I and II collagen gene expression in response to addition of recombinant Sulf-1 or Sulf-1 knock down by siRNA was significantly affected, whereas the four types of heparan sulfate proteoglycan were not altered (*p < 0.05, Fig. 6A). Type II collagen was not detected by Immunocytochemistry at 0 weeks, but it was expressed after 2 weeks culture under chondrogenesis conditions. Importantly, ATDC5 cells exposed to Sulf-1 showed higher expression of type II collagen compared to those cultured without Sulf-1 (Fig. 6B).
Figure 6:
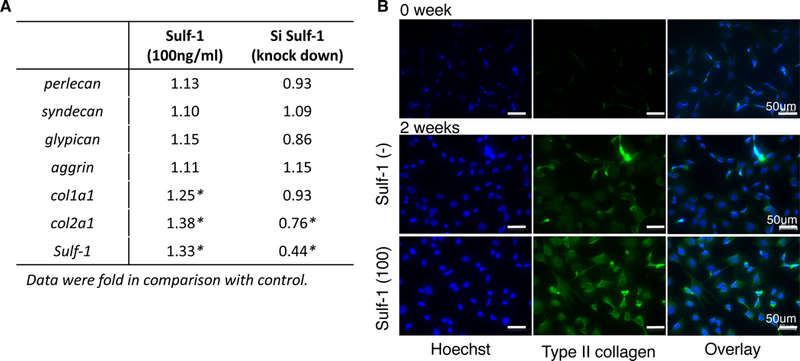
Effect of Sulf-1 on gene expression in ATDC5 cells. (A) Four types of heparan sulfate proteoglycan and collagen expression in response to recombinant Sulf-1 or siSulf-1 were analyzed by real-time PCR (*: p < 0.05). (B) Chondrogenesis was induced in ATDC5 cells and cells were cultured with or without Sulf-1 (100 ng/ml) (scale bars: 50 μm). Immunocytochemistry was performed at baseline (0 weeks) and after 2 weeks for type II collagen and cell nuclei (DAPI).
Sulf-1 regulates the Smad2/3 pathway
To elucidate the mechanism underlying the effect of Sulf-1 on type II collagen expression in ATDC5 cells, we focused on the Smad signaling pathway. Phosphorylation of Smad2/3 in response to TGF-β1 (10 ng/ml) and type II collagen expression was evaluated for up to 72 h (Fig. 7A). We found a significant increase in Smad2/3 phosphorylation 30 min after exposure to Sulf-1 (Fig. 7B; *p < 0.05). In ATDC5 cells exposed to Sulf-1, type II collagen expression was increased 72 h after TGF-β1 stimulation (Fig. 7B; *p < 0.05).
Figure 7:
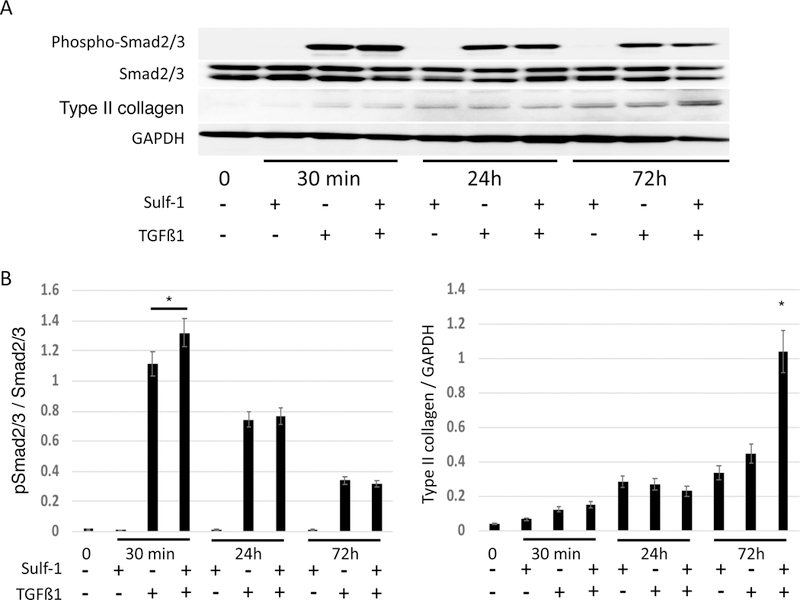
Sulf-1 increases type II collagen expression via Smad2/3 phosphorylation in response to TGF-β1. (A) ATDC5 cells were incubated with or without 100 ng/ml Sulf-1 for 72 h then phosphorylation of Smad2/3 in response to TGF-β1 (10 ng/ml) and type II collagen expression was evaluated for up to 72 h. Densitometric analysis of the immunoblots is shown in panels B; *p < 0.05.
Discussion
IVD degeneration is an irreversible condition, where particularly the NP undergoes a degenerative process characterized by changes in the extracellular matrix, decreased cell viability, and reduced proteoglycan and type II collagen synthesis (Xu et al., 2016). The AF is enclosed by collagen-rich lamellae and situated between two cartilaginous EPs. Extracellular matrix turnover in IVD has been reported to be associated with denaturation of collagen (Navone et al., 2017). The present study is the first to analyze Sulf-1 expression in IVD and to show that Sulf-1 is increased in AF and NP in degenerated human IVD and is reduced with aging in mice. Furthermore, Sulf-1−/− mice showed more severe degenerative changes, especially in the AF and NP, already evident at the age of 1 month. All of these findings suggest that Sulf-1 is critical not only in IVD development but also in IVD homeostasis.
The NP is located at the center of the IVD and manages biomechanical loading of the spine. The changes in the structure of the NP extracellular matrix that occur throughout life may be due to intrinsic events such as notochordal cell loss, mesenchymal cell senescence, loss of vasculature, and calcification of the vertebral EPs, which alter or decrease the synthetic capabilities of the NP cells (Roughley, 2004). Zhao et al. reported that the NP is populated by clusters of vacuolated notochordal cells and by chondrocyte-like NP cells, whereas a degenerating NP is populated by increasingly apoptotic NP cells and possibly fibrochondrocyte-like cells (Zhao et al., 2007). Not only IVD, but also bone formation is quite different between mice and humans because notochordal cells and the growth plate in mice are maintained for a lifetime, whereas those in humans decrease rapidly (Jin et al., 2018). However, mice are used for aging and OA research because of their controllability such as ease of genetic manipulation and costal benefit. Although articular cartilage and the IVD appear to be very different structures morphologically, there are some similarities at the biochemical level (Urban and Roberts, 2003). TGF-β, which counteracts pathological changes in a healthy young joint, shows altered signaling during aging and is a driving force of pathology in osteoarthritic joints (van der Kraan, 2017). Similarly, TGF-β signaling has been recognized to play an essential role in the growth and maintenance of IVD (Jin et al., 2011). The potential use of BMPs has been investigated for the treatment of IVD degeneration because they have the potential to restore IVD homeostasis (Than et al., 2012; Xu et al., 2016). The TGF-β-Smad2/3 pathway is essential for type II collagen expression (Hellingman et al., 2011; Furumatsu et al., 2013; van Caam et al., 2016). In IVD, the Smad2/3 pathway governs the acquisition of the NP cell molecular identity, whereas the Smad1/5/8 pathway controls morphology (Colombier et al., 2016). We have previously described changes in the expression of Sulfs in articular cartilage with aging (Otsuki et al., 2010), which showed a pattern similar to that observed in IVD. Viviano et al. (2004) previously reported that Sulf-1 modulated BMP signaling by releasing surface-bound Noggin. In the present in vivo study, the NP expressed lower levels of type II collagen in Sulf-1−/− mice than in WT mice (Fig. 4), indicating that Sulf-1 might be critical for structural homeostasis in IVD, and it might have some potential as a specific marker of IVD degeneration. In vitro, ATDC5 chondrogenesis in the presence of Sulf-1 protein was associated with greater induction of type II collagen, as compared with cells cultured without Sulf-1 (Fig. 5), and higher levels of TGF-β-Smad2/3 were also detected by western blotting (Fig. 6). Sulf-1 induces type II collagen during cell maturation by stimulating the TGFß-Smad 2/3 signaling pathway. IVD extracellular matrix integrity is dependent on interactions among several types of proteoglycans and related factors (Akeda et al., 2007; Schäfer and Tegeder, 2018). Heparan sulfate proteoglycans such as perlecan, syndecan, agrin, and glypican mainly act at the cell membrane and are critical for regulating cell signaling pathways ( Pacifici et al., 2005; Bishop et al., 2007). Although Sulf-1 was not altered, the gene expression of HSPG, Sulf-1 might affect the cell signaling pathway via HS 6-O endsulfatases.
This study has some limitations. Not only type II collagen, but also type I and other types of collagen and their relationship with Sulf-1 should be researched because AF and EP in Sulf-1 KO were not better aligned compared with WT IVD. Moreover, Sulf-1 regulation of FGF cell signaling pathways might be another critical factor because FGF2 and FGF18 have been implicated in the regulation of articular and IVD cartilage homeostasis. Second, we used ATDC5 cell line, which does nor represent the phenotype of a mature NP cell. Moreover, the in vitro study conditions differ from the in vivo conditions. However, ATDC5 cells, which are a chondrogenic cell line, can be used to evaluate type II collagen expression with cell maturation in response to Sulf-1 expression. Although ATDC5 cells have been used for IVD research (Kato et al., 2010; Zheng et al., 2018), further research is needed for type II collagen expression and related cell signaling pathways in response to Sulf-1 with IVD cells.
Conclusion
Sulf-1 might play a critical role from the development to maintenance of IVD homeostasis by regulating collagen expression.
Acknowledgements
We thank Yoshie Seki, Rintaro Oide, and Merissa Olmer for their expert technical assistance.
Financial support: This work was supported by the Japan Society for the Promotion of Science KAKENHI 25462316 and 15K10498 (Grant-in-Aid for KIBAN C to M.N and S.O) and by NIH AG053747 (for OAG).
Footnotes
Dr. Otsuki had full access to all of the data in the study and takes responsibility for the integrity of these data and the accuracy of the data analyses.
Study design: Otsuki, Neo
Acquisition of data: Otsuki, Alvarez-Garcia, Lotz, Neo
Analysis and interpretation of data: Otsuki, Alvarez-Garcia, Neo
Manuscript preparation: Otsuki, Lotz, Neo
Statistical analysis: Otsuki, Alvarez-Garcia
References
- Ai X, Kitazawa T, Do A-T, Kusche-Gullberg M, Labosky PA, and Emerson CP (2007). SULF1 and SULF2 regulate heparan sulfate-mediated GDNF signaling for esophageal innervation. Development. 134, 3327–3338. [DOI] [PubMed] [Google Scholar]
- Akeda K, An HS, Pichika R, Patel K, Muehleman C, Nakagawa K, Uchida A, and Masuda K (2007). The expression of NG2 proteoglycan in the human intervertebral disc. Spine, 32(3), 306–314. [DOI] [PubMed] [Google Scholar]
- Alvarez-Garcia O, Matsuzaki T, Olmer M, Masuda K, and Lotz MK (2017). Age-related reduction in the expression of FOXO transcription factors and correlations with intervertebral disc degeneration. J.Orthop. Res. 35, 2682–2691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop JR, Schuksz M, and Esko JD (2007). Heparan sulphate proteoglycans fine-tune mammalian physiology. Nature. 446, 1030–1037. [DOI] [PubMed] [Google Scholar]
- Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, and Nerlich AG (2002). Classification of age-related changes in lumbar intervertebral discs. Spine. 27, 2631–2644. [DOI] [PubMed] [Google Scholar]
- Colombier P, Clouet J, Boyer C, Ruel M, Bonin G, Lesoeur J, Camus A, and Guicheux J (2016). TGF-β1 and GDF5 Act Synergistically to Drive the Differentiation of Human Adipose Stromal Cells toward Nucleus Pulposus-like Cells. Stem Cells. 34, 653–667. [DOI] [PubMed] [Google Scholar]
- Dhoot GK, Gustafsson MK, Ai X, Sun W, Standiford DM, and Emerson CP (2001). Regulation of Wnt signaling and embryo patterning by an extracellular sulfatase. Science. 293, 1663–1666. [DOI] [PubMed] [Google Scholar]
- Ellman MB, An HS, Muddasani P, and Im HJ (2008). Biological impact of the fibroblast growth factor family on articular cartilage and intervertebral disc homeostasis. Gene. 420, 82–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyre DR and Muir H (1976). Types I and II collagens in intervertebral disc. Interchanging radial distributions in annulus fibrosus. Biochem J. 157, 267–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furumatsu T, Matsumoto E, Kanazawa T, Fujii M, Lu Z, Kajiki R, and Ozaki T (2013). Tensile strain increases expression of CCN2 and COL2A1 by activating TGF-β-Smad2/3 pathway in chondrocytic cells. J. Biomech. 46, 1508–1515. [DOI] [PubMed] [Google Scholar]
- Hellingman CA, Davidson ENB, Koevoet W, Vitters EL, van den Berg WB, van Osch GJVM, and van der Kraan PM (2011). Smad signaling determines chondrogenic differentiation of bone-marrow-derived mesenchymal stem cells: inhibition of Smad1/5/8P prevents terminal differentiation and calcification. Tissue Eng Part A. 17, 1157–1167. [DOI] [PubMed] [Google Scholar]
- Jaumard NV, Welch WC, and Winkelstein BA (2011). Spinal facet joint biomechanics and mechanotransduction in normal, injury and degenerative conditions. J Biomech Eng. 133, 071010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Shen J, Wang B, Wang M, Shu B, and Chen D (2011). TGF-β signaling plays an essential role in the growth and maintenance of intervertebral disc tissue. FEBS Lett. 585, 1209–1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin L, Balian G, and Li XJ (2018). Animal models for disc degeneration-an update. Histol Histopathol. 33, 543–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Takahashi H, Yoda M, Tohmonda T, Takito J, Fujita N, Hosogane N, Horiuchi K, Kimura T, Okada Y, Saito T, Kawaguchi H, Kikuchi T, Matsumoto M, Toyama Y, Chiba K, (2010). GRIP1 enhances estrogen receptor α-dependent extracellular matrix gene expression in chondrogenic cells. Osteoarthritis Cartilage, 18, 934–941. [DOI] [PubMed] [Google Scholar]
- Luoma K, Riihimäki H, Luukkonen R, Raininko R, Viikari-Juntura E, and Lamminen A (2000). Low back pain in relation to lumbar disc degeneration. Spine. 25(4), 487–492. [DOI] [PubMed] [Google Scholar]
- Masuda K, Aota Y, Muehleman C, Imai Y, Okuma M, Thonar EJ, Andersson GB, and An HS (2005). A novel rabbit model of mild, reproducible disc degeneration by an anulus needle puncture: correlation between the degree of disc injury and radiological and histological appearances of disc degeneration. Spine. 30, 5–14. [DOI] [PubMed] [Google Scholar]
- Melrose J, Hayes AJ, Whitelock JM, and Little CB (2008). Perlecan, the “jack of all trades” proteoglycan of cartilaginous weight‐bearing connective tissues. Bioessays. 30, 457–469. [DOI] [PubMed] [Google Scholar]
- Navone SE, Marfia G, Giannoni A, Beretta M, Guarnaccia L, Gualtierotti R, Nicoli D, Rampini P, and Campanella R (2017). Inflammatory mediators and signalling pathways controlling intervertebral disc degeneration. Histol Histopathol. 32, 523–542. [DOI] [PubMed] [Google Scholar]
- Otsuki S, Hanson SR, Miyaki S, Grogan SP, Kinoshita M, Asahara H, Wong CH, and Lotz MK (2010). Extracellular sulfatases support cartilage homeostasis by regulating BMP and FGF signaling pathways. Proc. Natl. Acad. Sci. USA. 107, 10202–10207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuki S, Murakami T, Okamoto Y, Hoshiyama Y, Oda S, and Neo M (2017). Suppression of cartilage degeneration by intra-articular injection of heparan sulfate 6-O endosulfatase in a mouse osteoarthritis model. Histol Histopathol.32, 725–733. [DOI] [PubMed] [Google Scholar]
- Otsuki S, Taniguchi N, Grogan SP, D’Lima D, Kinoshita M, and Lotz M (2008). Expression of novel extracellular sulfatases Sulf-1 and Sulf-2 in normal and osteoarthritic articular cartilage. Arthritis Res Ther. 10, R61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacifici M, Shimo T, Gentili C, Kirsch T, Freeman TA, Enomoto-Iwamoto M, Iwamoto M, and Koyama E. (2005). Syndecan-3: a cell-surface heparan sulfate proteoglycan important for chondrocyte proliferation and function during limb skeletogenesis. J Bone Miner Metab. 23, 191–199. [DOI] [PubMed] [Google Scholar]
- Richardson SM, Mobasheri A, Freemont AJ, and Hoyland JA (2007). Intervertebral disc biology, degeneration and novel tissue engineering and regenerative medicine therapies. Histol Histopathol. 22, 1033–1041. [DOI] [PubMed] [Google Scholar]
- Roughley PJ (2004). Biology of intervertebral disc aging and degeneration: involvement of the extracellular matrix. Spine. 29, 2691–2699. [DOI] [PubMed] [Google Scholar]
- Sakai D, Nakamura Y, Nakai T, Mishima T, Kato S, Grad S, Alini M, Risbud MV, Chan D, Cheah KS, Yamamura K, Masuda K, Okano H, Ando K, and Mochida J (2012). Exhaustion of nucleus pulposus progenitor cells with ageing and degeneration of the intervertebral disc. Nat Commun. 3, 1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer MKE, and Tegeder I (2018). NG2/CSPG4 and progranulin in the posttraumatic glial scar. Matrix Biol 68–69, 571–588. [DOI] [PubMed] [Google Scholar]
- Shukunami C, Shigeno C, Atsumi T, Ishizeki K, Suzuki F, and Hiraki Y (1996). Chondrogenic differentiation of clonal mouse embryonic cell line ATDC5 in vitro: differentiation-dependent gene expression of parathyroid hormone (PTH)/PTH-related peptide receptor. J. Cell. Biol. 133, 457–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than KD, Rahman SU, Vanaman MJ, Wang AC, Lin CY, Zhang H, La Marca F, and Park P (2012). Bone morphogenetic proteins and degenerative disk disease. Neurosurgery, 70, 996–1002– discussion 1002. [DOI] [PubMed] [Google Scholar]
- Thompson JP, Pearce RH, Schechter MT, Adams ME, Tsang IK, and Bishop PB (1990). Preliminary evaluation of a scheme for grading the gross morphology of the human intervertebral disc. Spine. 15, 411–415. [DOI] [PubMed] [Google Scholar]
- Urban JPG and Roberts S (2003). Degeneration of the intervertebral disc. Arthritis Res Ther. 5, 120–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Caam A, Madej W, Thijssen E, Garcia de Vinuesa A, van den Berg W, Goumans MJ, Ten Dijke P, Blaney Davidson E, and van der Kraan PP (2016). Expression of TGFβ-family signalling components in ageing cartilage: age-related loss of TGFβ and BMP receptors. Osteoarthritis Cartilage. 24, 1235–1245. [DOI] [PubMed] [Google Scholar]
- van der Kraan PM (2017). The changing role of TGFβ in healthy, ageing and osteoarthritic joints. Nature Reviews. Rheumatology, 13, 155–163. [DOI] [PubMed] [Google Scholar]
- Viviano BL, Paine-Saunders S, Gasiunas N, Gallagher J, and Saunders S (2004). Domain-specific modification of heparan sulfate by Qsulf1 modulates the binding of the bone morphogenetic protein antagonist Noggin. J. Bio. Chem. 279, 5604–5611. [DOI] [PubMed] [Google Scholar]
- Vo NV, Hartman RA, Patil PR, Risbud MV, Kletsas D, Iatridis JC, Hoyland JA, Le Maitre CL, Sowa GA, and Kang JD (2016). Molecular mechanisms of biological aging in intervertebral discs. J. Orthop. Res. 34, 1289–1306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo NV, Hartman RA, Yurube T, Jacobs LJ, Sowa GA, and Kang JD (2013). Expression and regulation of metalloproteinases and their inhibitors in intervertebral disc aging and degeneration. Spine J. 13, 331–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, E XQ, Wang NX, Wang MN, Xie HX, Cao YH, Sun LH, Tian J, Chen HJ, and Yan JL (2016). BMP7 enhances the effect of BMSCs on extracellular matrix remodeling in a rabbit model of intervertebral disc degeneration. FEBS J. 283, 1689–1700. [DOI] [PubMed] [Google Scholar]
- Yao Y and Wang Y (2013). ATDC5: an excellent in vitro model cell line for skeletal development. J Cell Biochem. 114, 1223–1229. [DOI] [PubMed] [Google Scholar]
- Zhao CQ, Wang LM, Jiang LS, and Dai LY (2007). The cell biology of intervertebral disc aging and degeneration. Ageing Res Rev. 6, 247–261. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Liu C, Ni L, Liu Z, Mirando AJ, Lin J, Saijilafu Chen D., Hilton MJ, Li B, and Chen J (2018). Cell type-specific effects of Notch signaling activation on intervertebral discs: Implications for intervertebral disc degeneration. J Cell Physiol. 233, 5431–5440. [DOI] [PMC free article] [PubMed] [Google Scholar]


