Abstract
Objective
To compare neuropathological correlates of cognitive impairment between very old and younger individuals from a Brazilian clinicopathological study.
Methods
We assessed the frequency of neuropathological lesions and their association with cognitive impairment (Clinical Dementia Rating scale ≥0.5) in the 80 or over age group compared to younger participants, using logistic regression models adjusted for sex, race and education.
Results
Except for infarcts and siderocalcinosis, all neuropathological lesions were more common in the 80 or over age group (n = 412) compared to 50–79 year olds (n = 677). Very old participants had more than twice the likelihood of having ≥2 neuropathological diagnoses than younger participants (OR = 2.66, 95% CI = 2.03–3.50). Neurofibrillary tangles, infarcts and hyaline arteriolosclerosis were associated with cognitive impairment in the two age groups. Siderocalcinosis was associated with cognitive impairment in the younger participants only, while Lewy body disease was associated with cognitive impairment in the very old only. In addition, we found that the association of infarcts and multiple pathologies with cognitive impairment was attenuated in very old adults (Infarcts: P for interaction = 0.04; and multiple pathologies: P = 0.05). However, the predictive value for the aggregate model with all neuropathological lesions showed similar discrimination in both age groups [Area under Receiver Operating Characteristic curve (AUROC) = 0.778 in younger participants and AUROC = 0.765 in the very old].
Conclusion and relevance
Despite a higher frequency of neuropathological findings in the very old group, as found in studies with high‐income populations, we found attenuation of the effect of infarcts rather than neurofibrillary tangles and plaques as reported previously.
Keywords: aged, 80 and over, Alzheimer’s disease, dementia, neuropathology, vascular dementia, very old
Introduction
Globally, the number of very old people (ie, those aged 80 years or over) increased fourfold from 1980 to 2017, and by 2050 about 425 million people will be older than 80 years 48. The risk of dementia increases exponentially from the age of 65, reaching a prevalence of 17% in individuals aged 75 to 84 years old, and 32% in those aged 85 or more 1. Understanding the neuropathological lesions underlying dementia is an essential step to developing effective diagnostic, prevention and therapeutic strategies against dementia. Because of cultural, logistic and financial constraints, dementia clinics provide most of the participants in clinicopathological studies. Therefore, most of the participants reached end‐stage disease by the time of autopsy. The few community‐based studies that exist tend to attract highly educated, healthy Caucasians and their mean age at death were around 85 years 25, 51. Interestingly, studies comparing the neuropathological correlates of dementia in the very old with the few younger participants available suggest that although very old participants showed a higher prevalence of most neuropathological findings and higher odds of having multiple pathological findings in the brain, the effect of individual neurodegenerative and cerebrovascular changes on cognitive impairment was attenuated 21, 28, 41. This suggests either that other undetected underlying causes contribute to cognitive decline in the very old group, diluting the effect of known neuropathological changes, or that genetic factors conferring longevity and especially longevity free of major diseases may confer neuronal resilience against neurodegenerative and vascular changes in the very old 21, 24, 28, 36, 41. As these differences may represent differences in mechanisms of neurodegeneration and selective neuronal vulnerability in older and younger patients, a better understanding of the differences between neuropathological correlates of dementia between old and very old individuals is warranted.
In 2017, 54% of very old people lived in low‐middle income countries (LMIC), and this proportion is expected to increase to 70% by 2050 48. However, very little is known about the risk factors for dementia and the neuropathological correlates of dementia in the very old living in LMIC. Using a well‐characterized, population‐based clinicopathological study from Brazil that features different race composition, socioeconomic factors, and educational attainment profile compared to available series from high‐income countries (HIC) 43, we demonstrated that even a small a percentage of African descent lower the relative risk to accumulate AD‐related neuritic plaques compared to Caucasian ancestry 42, suggesting that ethnicity may affect the vulnerability to neuropathological changes. Among other results retrieved from the same series, we also demonstrated that the prevalence of vascular dementia is higher in this population than in reported community‐based clinicopathological studies from HIC 45. Here, we sought to investigate the prevalence and impact on cognitive impairment of neuropathological changes in individuals aged 80 and older compared to a group aged from 50 to 79.
Materials and Methods
Participants
We used cases from the Biobank for Aging Studies (BAS) of the University of Sao Paulo, formerly known as the Brain Bank of the Brazilian Aging Brain Study Group 16, which is sourced by the São Paulo Autopsy Service of the University of São Paulo, Brazil. In São Paulo, an autopsy is mandatory in cases where the nontraumatic cause of death is unclear. From 2004 to 2014, we included participants aged 50 years and older with a knowledgeable informant who had had at least weekly contact with the deceased, to provide clinical information. Exclusion criteria for the BB‐BABSG were: (i) brain tissue unsuitable for neuropathological analyses (eg, cerebrospinal fluid pH < 6.5, or major acute brain lesions, such as hemorrhages or tumors); and (ii) inconsistent clinical data provided by the informant. Individuals with monogenic dementia were also excluded.
Neuropathological assessment
Brain tissue was obtained within 24 h of death. One hemisphere was fixed in 4% buffered paraformaldehyde and the other was frozen at −80°C. After fixation, samples from the following 13 regions were selected and embedded in paraffin: middle frontal gyrus, middle and superior temporal gyri, angular gyrus, superior frontal and anterior cingulate gyrus, visual cortex, hippocampal formation at the level of the lateral geniculate body, amygdala, basal ganglia at the level of the anterior commissure, thalamus, midbrain, pons, medulla oblongata and cerebellum. We cut the paraffin blocks into 5 µm thick sections, and stained them in hematoxylin and eosin. In selected sections, we used immunohistochemistry with antibodies against β‐amyloid (4G8, 1:10.000; Signet Pathology Systems, Dedham, Massachusetts), phosphorylated tau (PHF‐1, 1:2.000; donated by Peter Davies, New York, NY, USA), TDP‐43 (1:500, Proteintech, Chicago, IL, USA) and α‐synuclein (EQV‐1, 1:10.000; donated by Kenji Ueda, Tokyo, Japan). We used internationally accepted criteria to diagnose brain pathologies 3, 4, 5, 26, 29.
AD‐neuropathology was evaluated using the Consortium to Establish a Registry for Alzheimer’s disease (CERAD) criteria for neuritic plaques 29, and the Braak and Braak (BB) score for neurofibrillary tangles (NFT) 4. Our assessment of cerebrovascular lesions consisted of gross and microscopic evaluations 45. We registered infarcts by topography, type, stage, size and number. For the current study, inclusion in the group “infarct” was granted to subjects with either one large chronic infarct (>1 cm) or three lacunae (<1 cm) in any of the following strategic areas: thalamus, frontocingular cortex, basal forebrain and caudate, medial temporal area, or angular gyrus 45. The diagnosis of small‐vessel disease included moderate or severe arteriosclerosis/atherosclerosis and lipohyalinosis in three or more cortical regions 18. The group “vascular” consisted of participants meeting criteria for SVD and/or “infarct.” Furthermore, we evaluated the presence of cerebral amyloid angiopathy (CAA) in the meninges, gray matter and white matter as a separate diagnostic group. CAA was considered as present when we observed widespread disease in at least three different cortical areas 18. Finally, we also examined the presence of siderocalcinosis. Siderocalcinosis was defined as vascular mineralization in the middle layer of the walls of small‐ and medium‐sized arteries in the striatum 18, 27, 44 and was classified as present/absent. Hippocampal sclerosis, defined by loss of pyramidal cell and gliosis in the CA1 and subiculum of the hippocampus, was scored as absent or present. The presence of argyrophilic grains (AG) was defined by abundant phosphorylated tau‐positive grains in the hippocampal CA1, pretangles in the hippocampal CA2, and oligodendrocytes with coiled bodies in the hippocampal or temporal white matter 39. Lewy neuropathology was classified using the Braak staging for PD 5. We assessed the presence of transactive response DNA‐binding protein 43 kDA (TDP‐43) in at least the hippocampal formation and the amygdala of 410 participants, since the introduction of immunohistochemistry for this protein in our routine in 2012. Further assessment for TDP‐43 was performed in participants with positivity in the amygdala or hippocampal formation, or suspicious of frontotemporal lobar degeneration. For the current study, TDP‐43 proteinopathy in the amygdala or hippocampal formation was considered enough 7, 31.
Clinicofunctional post‐mortem evaluation
A knowledgeable informant was interviewed to obtain the deceased’s past clinical history using a semi‐structured interview, which was previously validated for post‐mortem use 12, 13. The clinical interview included information about: (i) sociodemographics: age at death, sex, years of education, race (White, Black, Brown, and Asian), frequency of contact between the deceased and the informant; (ii) history of previous medical diagnosis of hypertension, diabetes, coronary artery disease, heart failure, dyslipidemia, stroke, smoking and alcohol use; and (iii) cognitive evaluation using the Clinical Dementia Rating (CDR) 30. The CDR evaluates six domains: memory, orientation, judgment and problem solving, community affairs, home and hobbies, and personal care. Only the informant section was used in this study. The participants were classified into five categories: normal cognition (CDR = 0), questionable dementia (CDR = 0.5), mild dementia (CDR = 1), moderate dementia (CDR = 2) and severe dementia (CDR = 3). The presence of cognitive impairment was considered when the CDR was ≥0.5. We measured the weight and height of the deceased in the supine position, without clothes and shoes, using an electronic scale. We then calculated the body mass index, dividing the weight in kilos by the square of the height in meters.
Statistical analysis
We compared sociodemographic and clinical variables for the participants younger than 80 years old with those aged 80 or older at time of death using the unpaired t‐test for continuous variables and the chi‐square test for categorical ones. We also compared the frequency of neuropathological lesions between participants aged <80 and 80 plus using logistic regression models adjusted for sex, race and education. The association between cognitive impairment (CDR ≥ 0.5) and each neuropathological lesion was investigated in an analysis stratified by age groups (<80 vs. ≥80) using logistic regression models adjusted for sex, race, education and other neuropathological lesions. We performed a sensitivity analysis using dementia (CDR ≥ 1) as the outcome. We investigated the predictive value of the aggregate model with all neuropathological lesions to discriminate participants with from those without cognitive impairment using the area under the receiver operating characteristic (ROC) curve in each age group. We then used the DeLong method to compare the areas under the ROC curves between younger and very old participants 10. In addition, we examined whether the association between neuropathology and cognitive impairment was different between the age groups by including an interaction term between each neuropathological lesion and the two age groups. We further explore if the low educational attainment from our sample was related to differences in the association between pathologies and cognitive impairment in younger and very old participants using interaction terms between age and each neuropathology stratified by low (<10 years) and high level of education (≥10 years). The cutoff of 10 years of formal education was chosen as it represents the 90th percentile in our sample and it selected a group in our sample with low education compared to previous studies 9, 28.
We also investigated whether the association between number of neuropathologies and cognitive impairment varied by age groups, by including an interaction term between number of pathologies (categorized in 0, 1, 2 and 3 or more) and age group (<80 vs. ≥80 yo) in a logistic model adjusted for sex, race and education. Finally, we included an interaction term of age groups with race [white vs. non‐white, excluding Asian (n = 21)] and education (<4 years of education vs. ≥4 years) to investigate whether the association between cognitive impairment and age differed by race and education. We used the software Stata 12 (StataCorp, College Station, TX, USA). The alpha level was set at 0.05 in the two‐tailed tests.
Results
Of the 1089 participants, 677 were younger than 80 years old (range: 50–79 years old), and 412 were aged 80 years or more (range: 80 to 105 years old). Sixty‐nine percent were white, and the mean level of education of the sample was 4.2 ± 3.7 years. Compared to younger participants, participants in the 80 and over age group tended to be female, with fewer years of formal education, lower body mass index, and lower frequency of coronary disease and dyslipidemia. Also, participants who had never smoked or used alcohol were more frequent in the older group. Cognitive impairment was more common among very old participants compared to those aged from 50 to 79 years (Table 1). The majority of informants (80%) had daily contact with the deceased. We examined the associations of arteriolosclerosis with diabetes and hypertension. Diabetes was borderline associated with arteriolosclerosis (OR = 1.43, 95% CI = 0.99–20.8, P = 0.06) using a logistic regression model adjusted for age and sex. Hypertension was not associated with arteriolosclerosis in our sample (OR = 1.02, 95% CI = 0.70–1.47, P = 0.92).
Table 1.
Characteristics of the sample by age groups (n = 1089).
| Age <80 years old n = 677 | Age ≥80 years old n = 412 | P | |
|---|---|---|---|
| Age (years), mean (SD)* | 67.0 (8.9) | 85.7 (4.7) | <0.0001 |
| Male, %† | 54.4 | 38.6 | <0.0001 |
| Race, %† | 0.13 | ||
| White | 69.0 | 70.2 | |
| Black | 9.9 | 12.6 | |
| Brown | 19.4 | 14.8 | |
| Asian | 1.6 | 2.4 | |
| Education (years), mean (SD)* | 4.7 (3.7) | 3.3 (3.4) | <0.0001 |
| CDR ≥0.5, %† | 31.2 | 51.5 | <0.0001 |
| Hypertension, %† | 66.4 | 64.4 | 0.50 |
| Diabetes, %† | 28.9 | 23.9 | 0.07 |
| Coronary artery disease, %† | 27.7 | 19.3 | 0.002 |
| Heart failure, %† | 16.9 | 17.1 | 0.92 |
| Dyslipidemia, %† | 10.3 | 6.6 | 0.04 |
| Stroke, %† | 15.5 | 17.0 | 0.53 |
| BMI (kg/m2) , mean (SD)* | 24.1 (4.9) | 21.8 (4.3) | <0.0001 |
| Smoking, %† | <0.0001 | ||
| Never | 55.2 | 75.2 | |
| Current | 33.0 | 14.6 | |
| Former | 11.8 | 10.2 | |
| Alcohol use, %† | <0.0001 | ||
| Never | 78.7 | 91.4 | |
| Current | 13.1 | 3.2 | |
| Former | 8.2 | 5.4 |
Abbreviations: CDR = Clinical Dementia Rating; BMI = body mass index.
Unpaired t‐test.
Chi‐square test.
Frequency of pathologies by age group
Almost all neuropathological lesions were more frequent in very old participants, except for infarcts (P = 0.09), siderocalcinosis (P = 0.12) and TDP‐43 (P = 0.10) (Table 2). For example, the frequency of AD‐neuropathological lesions was more common in participants ≥80 years old than in younger participants (NFT: OR = 5.03, 95% CI = 3.23–5.57, and neuritic plaque: OR = 4.31, 95% CI = 3.23–5.57). Also, the frequency of multiple pathological diagnoses was higher among the very old (Figure 1). Moreover, being 80 years or older was associated with almost 3 times the odds of meeting the criteria for multiple neuropathological diagnosis compared to younger participants (≥2 neuropathologies: 22.0% vs. 45.1%, OR = 2.66, 95% CI = 2.03–3.50, P < 0.0001; ≥3 neuropathologies: 21.4% vs. 9.2%, OR = 2.62; 95% CI = 1.82–3.76). Most common combinations of pathologies in participants <80 years old were vascular lesions plus siderocalcinosis (n = 24), AD lesions plus siderocalcinosis (n = 19) and AD plus vascular lesions (n = 14); while in the very old we found more AD plus vascular lesions (n = 46), AD lesions and siderocalcinosis (n = 41), and AD lesions and AG (n = 38). Additionally, we described the percentage of most common multiple pathologies within age groups in Figure 2. The distribution of neuropathological diagnoses is presented in Supporting Figure 1. The frequency of multiple diagnoses was also more frequent in the very old compared to younger participants (p < 0.0001).
Table 2.
Frequency of neuropathological lesions by age groups (n = 1089).
| Age <80 yo n = 677 | Age ≥80 yo n = 412 | OR (95% CI)§ | P | |
|---|---|---|---|---|
| BB NFT score, %* | 5.03 (3.85–6.57) | <0.0001 | ||
| 0–II | 79.2 | 39.6 | ||
| III–IV | 14.9 | 41.0 | ||
| V–VI | 5.9 | 19.4 | ||
| CERAD score, %* | 4.31 (3.23–5.57) | <0.0001 | ||
| None/Scarce | 74.7 | 39.1 | ||
| Moderate | 10.6 | 18.7 | ||
| Frequent | 14.6 | 42.2 | ||
| Infarcts, %† | 10.6 | 14.7 | 1.39 (0.95–2.03) | 0.09 |
| Hyaline arteriolosclerosis, %† | 10.7 | 20.9 | 2.00 (1.41–2.84) | <0.0001 |
| CAA, %† | 3.1 | 6.6 | 1.95 (1.07–3.55) | 0.03 |
| Hippocampal sclerosis, %† | 1.2 | 5.3 | 4.41 (1.91–10.21) | 0.001 |
| Siderocalcinosis, %† | 15.9 | 20.0 | 1.30 (0.93–1.80) | 0.12 |
| BB LBD score, %* | 2.50 (1.66–3.76) | <0.0001 | ||
| 0 | 93.1 | 84.2 | ||
| I–III | 3.1 | 6.8 | ||
| IV–VI | 3.8 | 9.0 | ||
| AGD, %† | 12.0 | 23.4 | 2.06 (1.47–2.88) | <0.0001 |
| TDP‐43, %†‡ | 9.8 | 15.7 | 1.68 (0.90–3.15) | 0.10 |
Ordinal logistic regression model, adjusted for sex, race and education.
Logistic regression model adjusted for sex, race and education.
Information available for 410 participants.
Odds ratio for neuropathological lesions in subjects ≥80 years old, compared to those <80.
Abbreviations: BB = Braak & Braak; NFT = neurofibrillary tangles; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; NIA‐Reagan AD likelihood = National Institute of Aging‐Reagan Institute criteria for the neuropathological diagnosis of Alzheimer’s disease; CAA = cerebral amyloid angiopathy; LBD = Lewy Body Disease; AGD = argyrophilic grain disease; TDP‐43 = transactive response DNA‐binding protein 43 kDA.
Figure 1.
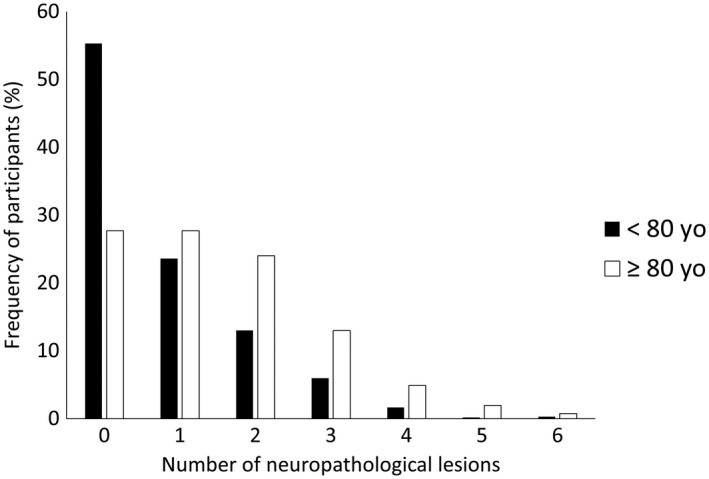
Relative frequency of participants aged under 80 years (black bar) and those aged 80 years or older (white bar) according to the number of neuropathological lesions present in the individual.
Figure 2.
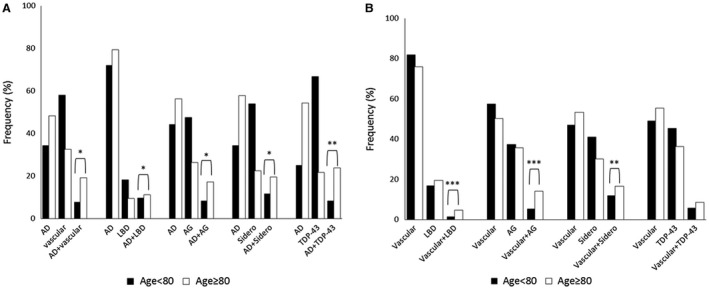
Percentage of single and multiple pathologies within each age group in participants aged under 80 years (black bar) and those aged 80 years or older (white bar). A. Alzheimer’s disease (AD) lesions (Braak & Braak score for neurofibrillary tangles ≥III and CERAD score ≥B) alone and in combination with vascular lesions, Lewy body disease (LBD), argyrophilic grain (AG), siderocalcinosis (sidero) and TDP‐43. B. Vascular lesions (infarcts and small vessel disease) alone and in combination with LBD, AG, siderocalcinosis and TDP‐43. Chi‐square test for the association between each combination of neuropathological lesions and age groups: *P < 0.0001; **P < 0.001; ***P < 0.05.
Neuropathological correlates of dementia in the old and very old groups
The prevalence of each neuropathological lesion by cognitive impairment and age group is shown in Figure 3. We found that BB score, infarcts, hyaline arteriolosclerosis and siderocalcinosis were independently associated with cognitive impairment in participants <80 years old in multivariable models adjusted for sex, race, education and other neuropathological lesions, whereas BB score, infarcts, hyaline arteriolosclerosis, Lewy body disease (LBD) (cortical diffuse, not the other types) and TDP‐43 correlated to cognitive impairment in the very old (Table 3). We found similar findings when we used dementia (CDR ≥ 1) as the outcome (Supporting Table 1). Aggregate models by age group including all neuropathological lesions showed similar discrimination (χ2 = 1.99, P = 0.16). The area under the ROC curve for the aggregate model was 0.778 (95% CI = 0.749–0.806) in younger participants and 0.765 (95% CI = 0.735–0.795) in very old participants (Figure 4).
Figure 3.
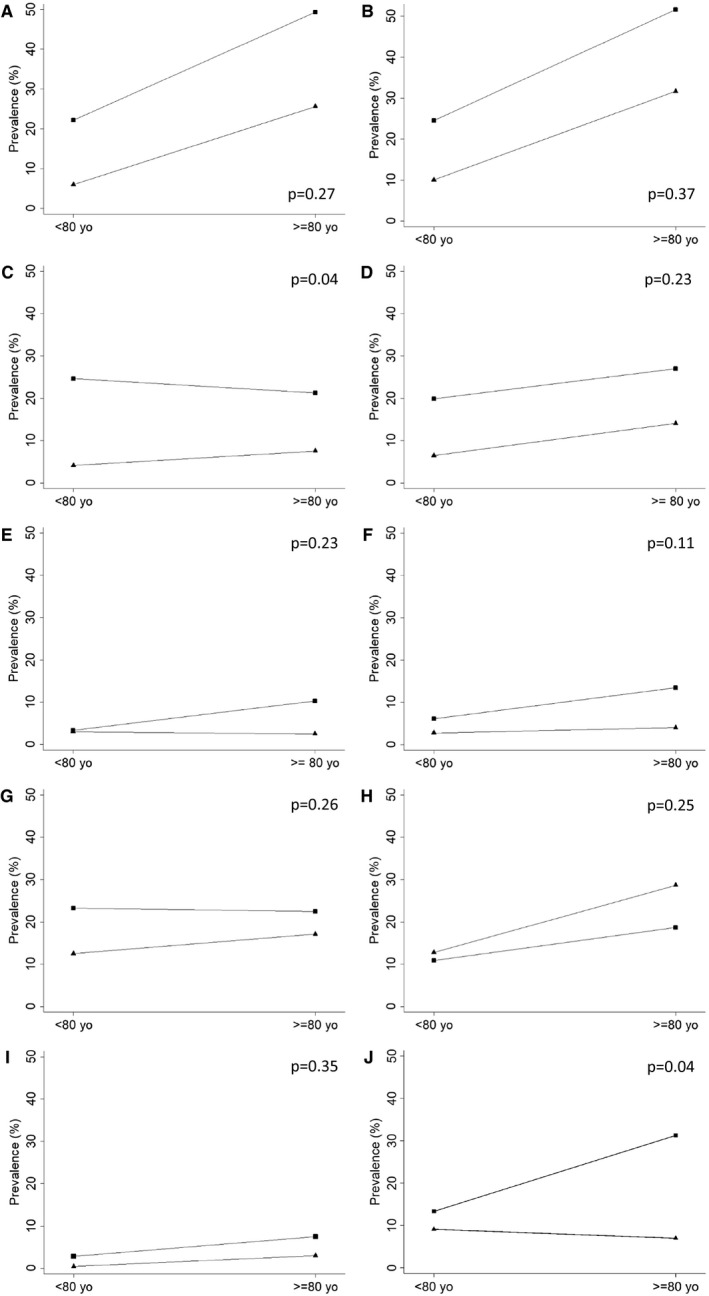
Relative frequency of moderate or severe neuropathological lesion in participants with cognitive impairment (triangle marker) and those without cognitive impairment (square marker), according to age group (<80 yo and ≥80 yo): A. Neurofibrillary tangles (Braak & Braak score ≥ III); B. Neuritic plaques (CERAD ≥ B); C. Infarcts; D. Hyaline arteriolosclerosis; E. Cerebral amyloid angiopathy; F. Lewy body disease (LBD) (Braak & Braak LBD ≥ IV); G. Siderocalcinosis; H. Argyrophilic grain disease; I. Hippocampal sclerosis; J. transactive response DNA‐binding protein 43 kDA (TDP‐43). Information for TDP‐43 was available in 410 participants. P‐values are for the interaction term between each neuropathological lesions and age group.
Table 3.
Odds ratio of cognitive impairment according to the presence of neuropathological lesions, stratified by age groups (n = 1089).
| Age <80 yo (n = 677) | Age ≥80 yo (n = 412) | P‐value for interaction | |
|---|---|---|---|
| OR (95% CI) | OR (95% CI) | with age group† | |
| BB NFT score | 0.27 | ||
| 0–II | 1 (reference) | 1 (reference) | |
| III–IV | 2.79 (1.56–4.97) | 1.50 (0.86–2.63) | |
| V–VI | 18.19 (6.04–54.80) | 4.94 (2.07–11.82) | |
| CERAD score | 0.37 | ||
| None/Scarce | 1 (reference) | 1 (reference) | |
| Moderate | 0.81 (0.43–1.52) | 1.41 (0.76–2.61) | |
| Frequent | 0.74 (0.36–1.52) | 1.10 (0.58–2.09) | |
| Infarcts | 6.92 (3.75–12.76) | 3.26 (1.66–6.41) | 0.04 |
| Hyaline arteriolosclerosis | 2.36 (1.29–4.30) | 1.86 (1.05–3.29) | 0.23 |
| CAA | 0.89 (0.31–2.55) | 2.29 (0.75–6.92) | 0.23 |
| Hippocampal sclerosis | 4.49 (0.78–25.95) | 1.72 (0.57–5.20) | 0.35 |
| Siderocalcinosis | 1.81 (1.10–2.99) | 1.23 (0.70–2.14) | 0.26 |
| BB LBD score | 0.11 | ||
| 0 | 1 (reference) | 1 (reference) | |
| I–III | 1.58 (0.57–4.39) | 0.57 (0.23–1.41) | |
| IV–VI | 1.97 (0.78–4.99) | 3.50 (1.46–8.39) | |
| AGD | 0.56 (0.30–1.04) | 0.73 (0.42–1.25) | 0.25 |
| TDP‐43‡ | 0.95 (0.25–3.63) | 7.76 (1.83–32.9) | 0.04 |
Logistic regression, adjusted for sex, race, education and other neuropathological lesions presented in the table.
P‐value for the interaction term between each neuropathological lesion and the age group.
Information available for 410 participants.
Abbreviations: BB = Braak & Braak; NFT = neurofibrillary tangles; CERAD = Consortium to Establish a Registry for Alzheimer’s Disease; CAA = cerebral amyloid angiopathy; LBD = Lewy Body Disease; AGD = argyrophilic grain disease; TDP‐43 = transactive response DNA‐binding protein 43 kDA.
Figure 4.
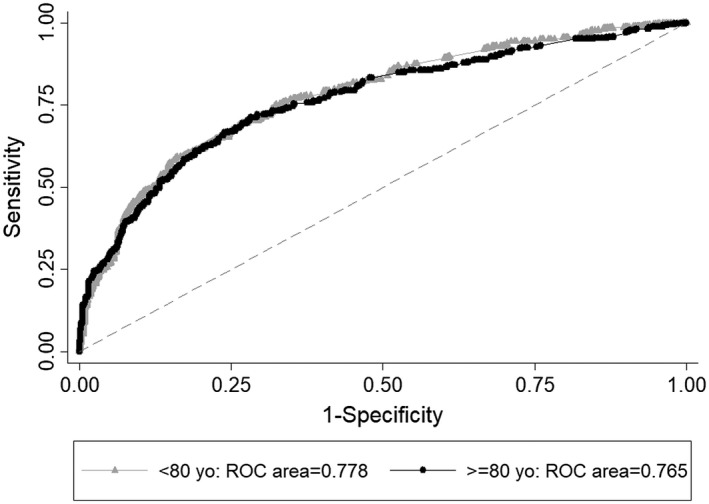
Discrimination of the aggregate model with neuropathological lesions in participants younger than 80 years (gray line with triangle marker) and 80 years and older (black line with circle marker) using the area under the Receiving Operator Curve (ROC). The aggregate model includes neurofibrillary tangles, neuritic plaques, Lewy body disease, infarcts, hyaline arteriolosclerosis, cerebral amyloid angiopathy, siderocalcinosis, hippocampal sclerosis and argyrophilic grain disease.
When we investigated whether the associations between each neuropathological lesion and cognitive impairment were different between the two age groups by adding an interaction term between them, the interaction terms with infarct and TDP‐43 were significant (P = 0.04 for both). Compared to those without infarcts, participants with infarcts had odds of cognitive impairment of almost 7 times in younger participants, while the odds of cognitive impairment was 3 times in the very old. In the subsample of participants with TDP‐43 evaluation, TDP‐43 proteinopathy was associated with almost 8 times the odds of cognitive impairment in the very old, while it was not associated with worse cognition in younger participants (Table 3). The association between TDP‐43 and cognitive impairment in the very old was independent of hippocampal sclerosis presence. After stratifying these analyses by education attainment, we found that the interaction term between age groups and infarcts remained significant only in participants with less than 10 years of formal education (P = 0.005). Stratified analyses by education for the interaction terms between age and other neuropathology failed to show significance.
When we used dementia (CDR ≥ 1) as the outcome, we found significant interaction terms for infarcts and hyaline arteriolosclerosis, suggesting an attenuation between these lesions and dementia in very old participants. The interaction term of TDP‐43 and age group was not significant probably because of the small number of participants with TDP‐43 proteinopathy and dementia (Supporting Table 1). The presence of multiple pathologies was associated with higher odds of cognitive impairment in both age groups (Table 4). However, the odds of cognitive impairment caused by the presence of multiple pathologies were attenuated in the very old compared to younger participants (P‐value for interaction between number of pathologies and age groups = 0.048). Additionally, we did not find any interaction between age groups and race (P = 0.70) or education (P = 0.20).
Table 4.
Odds ratio of cognitive impairment according to the number of moderate or severe neuropathological lesions in the same individual by age group (n = 1089).
| <80 years old | ≥80 years old | ||||||
|---|---|---|---|---|---|---|---|
| Cognitive Impairment | Cognitive Impairment | ||||||
| Number of pathologies | No n = 466 | Yes n = 211 | OR (95% CI) | No n = 200 | Yes n = 212 | OR (95% CI) | P † |
| 0 | 64.5 | 35.2 | 1.00 (reference) | 35.5 | 20.2 | 1.00 (reference) | 0.048 |
| 1 | 25.3 | 20.0 | 1.38 (0.89–2.15) | 36.5 | 19.2 | 0.82 (0.47–1.43) | |
| 2 | 5.9 | 28.6 | 8.57 (5.03–14.59) | 17.5 | 30.3 | 2.70 (1.52–4.79) | |
| 3 or more | 4.3 | 16.2 | 6.19 (3.32–11.50) | 10.5 | 30.3 | 4.57 (2.42–8.65) | |
Logistic regression model adjusted for sex, race and education.
P‐value for the interaction between the number of neuropathological lesions per person and the age group.
Discussion
In this large clinicopathological study from a LMIC, we found that the frequency of most neuropathological lesions was higher in the very old compared to participants in the 50–79 age group. In addition, the odds of multiple pathologies were almost 3 times higher in the very old. Progressive NFT BB stage and meeting the criteria threshold for infarcts and hyaline arteriolosclerosis were associated with higher odds of cognitive impairment in both younger and very old participants. Siderocalcinosis, a vascular mineralization in the middle arterial layers that usually appears in the basal ganglia and thalamus, was associated with cognitive impairment only in those younger than 80 years old, while LBD, the diffuse cortical type only, was associated with cognitive impairment in those older than 80 years. Moreover, we found that the association of cognitive impairment with chronic infarcts and multiple pathologies tended to be weaker in the very old. However, the aggregate model with all neuropathological lesions showed similar discrimination for cognitive impairment in both age groups. Additionally, coronary artery disease, dyslipidemia, current smoking and current alcohol use were more common among younger participants than among older ones. Survival bias may explain this finding as the literature shows that worse cardiovascular profile correlates with a shorter life expectancy 14.
Prevalence of neuropathological lesions and their association with cognitive impairment
We found that most common types of neuropathological lesions associated with cognitive impairment (ie, AD neuropathological changes, hyaline arteriolosclerosis, CAA, hippocampal sclerosis, LBD and AG) were 2 times more common in very old participants. Indeed, Savva et al found a higher frequency of NFT tangles, vascular lesions and LBD among individuals aged 80 years and older 41. In particular, a higher frequency of intermediate to high burden of AD neuropathological changes in participants aged 80+ was previously reported 8, 41.
When we examined the association of neuropathological lesions with cognitive impairment, we found higher odds of impairment with increasing NFT BB stages, chronic infarcts and hyaline arteriolosclerosis in both age groups. Indeed, high NFT BB stages were associated with dementia in several studies with very old participants 15, 28, 37, 46, while neuritic plaque burden has been inconsistently associated with dementia 6, 37, 38, 41, 50. Interestingly, several studies pointed to an attenuation of the association between AD neuropathological changes and dementia in the very old 19, 21, 28, 41. However, we did not confirm this finding in our series, since the interaction terms of NFT and neuritic plaques with age groups were not significant. In fact, the correlation between AD neuropathology and cognitive performance was maintained across age groups according to a study with 390 subjects from a dementia center 33.
Although we and others found that infarcts and small vessel disease (a term encompassing hyaline arteriolosclerosis) were related to cognitive impairment in younger and very old participants 2, 9, 20, 21, 37, 50, it is important to note that we found an attenuation in the association between infarcts and cognitive impairment in very old participants that were not previously described 28, 41. Very old participants with infarcts had more than 3 times the odds of cognitive impairment compared to older participants without infarcts; however the association was much stronger in younger participants with infarcts that showed an odds for cognitive impairment of almost 7 times. In order to explore this novel finding in relation to studies from HIC, we further stratified the analysis by education attainment. Interestingly, the attenuation between cognitive impairment and infarcts among very old participants remained significant only in the group with low educational attainment, and it may explain why our results differ from the other cohorts, which were enriched for individuals with high educational attainment 11, 35. An attenuation of the association between cognitive impairment and infarcts in very old individuals with low educational attainment seems a paradox because a better cognitive reserve is hypothesized to explain the protective effects of education into the risk of cognitive decline. It is possible that these low educated individuals have a genetic composition conferring them protection against many of the conditions that lower life expectancy. Further studies are needed to explore this interesting finding. In addition, TDP‐43 was related to higher odds of cognitive impairment only in very old participants. However, this novel finding needs to be confirmed in future studies with our whole sample and independent samples.
Interestingly, we found that siderocalcinosis was associated with cognitive impairment only in those aged 80 years or under. Siderocalcinosis is an age‐related common neuropathological finding, and previous work demonstrated a weakly association at most to cognitive impairment 49. Further work to understand the role of siderocalcinosis is sought to clarify these findings. On the other hand, we found a correlation between cognition and LBD only in the 80 + group. LBD is found in very old participants, including centenarians, and has been linked to higher risk of dementia 34, 46, 50. It is important to note that advanced LBD lesions were uncommon among younger participants, and the lack of association between these lesions and cognitive impairment may be related to lack of power. The lack of association between AG and cognitive impairment in our community‐based neuropathological study is in line with previous findings 32, 40. In fact, the term disease to define this neuropathological entity featuring abnormal tau deposits as argyrophilic grains, coiled bodies and pretangles, predominantly in the hippocampal formation and other limbic structures is a misnomer. Further studies examining the correlation between AG and cognition in other population‐based autopsy studies are necessary to clarify the role of AG and support a change in nomenclature 21, 22, 41.
When we compared the predictive value of the aggregate model with all evaluated neuropathological lesions in the 80+ and <80 groups, we found very similar discriminative values using the area under the ROC curves. On the other hand, Middleton et al found higher values for the area under the ROC curve for younger participants than for older ones [70–74 years: c‐statistic = 0.93 (95% CI = 0.89–0.96); 75–84 years: 0.91 (0.87–0.95), and ≥85 years: 0.83 (0.80–0.87)] 28. On the other hand, in a recent study with 413 individuals, neuropathological lesions were strongly related to cognitive decline in late life, and this association did not vary by age 22.
Multiple pathologies were frequent in very old participants and were related to cognitive impairment
We also confirmed the higher frequency of multiple pathologies in the very old found in other American and European cohorts, as well as the association between the presence of multiple pathologies and cognitive impairment 21, 23, 24, 46, 50. However, few studies have investigated whether the association between multiple pathologies and cognitive impairment differ among younger and very old adults, using an interaction term between age group and multiple pathologies 21. AD neuropathological changes plus infarcts and/or LBD were both related to dementia in participants aged 90 years or under and in those aged 90 years or older, but the association of mixed Alzheimer’s disease pathology was attenuated in the oldest participants 21. Although we used a different cutoff for age (80 years instead of 90), our findings are in line with those of James et al 21.
Study strength and limitations
Our study should be considered in light of its limitations. Caution is required when interpreting our results and comparing our findings to other studies that evaluated cognitive performance during the participant life course. Clinical information was gathered from a next of kin, rather than in a longitudinal fashion. To minimize biases, we only included cases with a knowledgeable informant who had, at least, weekly contact with the deceased. Indeed, 80% of the informants had daily contact with the deceased. In previous work, we demonstrated that our approach has good accuracy for detecting cognitive impairment 12. In addition, although we collected comprehensive neuropathological data, we did not have TDP‐43 evaluation in our whole sample. Another possible limitation concerns our criteria to assess cerebrovascular lesions of interest. Universally accepted neuropathological criterion for assessing and rating cerebrovascular lesions that may cause cognitive decline is yet to be established, and each center has adopted its own criteria 17, 18, 47. Another potential limitation is that we classified amyloid plaques as diffuse or neuritic based on morphological features seen on β‐amyloid immunohistochemistry, whereas neurofibrillary tangles are classified based on morphological features seen on phospho‐tau immunohistochemistry and were not verified by silver or thioflavin‐S staining. Finally, we did not adjust statistical analyses by apolipoprotein E (APOE) ε4 status since we did not have APOE measurements for the whole sample. On the other hand, our study has several advantages. We were the first to describe the association between neuropathological lesions and old age in a large sample from a LMIC with an admixed race and low level of education (average of 4 years). We used the information on sex, race and education to adjust all the analyses. It is important to note that all neuropathological studies so far have been conducted in developed countries such as the American and European countries, in individuals with high levels of education, and predominantly white 15, 23, 24, limiting the generalizability of the previous findings to developing countries 36. Additionally, the BAS is a community‐based neuropathological study with a large proportion of participants with normal cognition. In contrast, some studies on this topic were based on convenience samples with most participants already having dementia 28, or not representative of the general population 21, 23. The inclusion of a younger age group with preclinical disease is an important strength since individual below 80 years old were underrepresented in previous studies 6, 21, 28.
In conclusion, we found that most neuropathological lesions were more frequent in the very old as in individuals from high‐income countries. However, as opposed to other studies, old age did not seem to modify the association between neuropathological lesions and cognitive impairment, except for infarcts, which appeared to have a weaker association with cognitive impairment in the very old compared to younger participants.
Conflict of Interest
None
Data Availability Statement
Data is available upon request to the corresponding author.
Supporting information
Figure S1. Mosaic plot showing the relationship between neuropathological diagnoses (NP) and age groups (Fisher’s exact test, P < 0.0001). AD = Alzheimer disease; LBD = Lewy body disease; VaD = vascular disease.
Table S1. Odds ratio of dementia (CDR ≥ 1) according to the presence of neuropathological lesions, stratified by age groups (n = 1089).
Acknowledgments
São Paulo Research Foundation (FAPESP) (09/09134‐4, 06/55318‐1), FMUSP/LIM‐22, National Institute of Health (K24AG053435).
References
- 1. Alzheimer's Association (2017) 2017 Alzheimer's disease facts and figures. Alzheimer's Dementia 13:325–373. [Google Scholar]
- 2. Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA (2016) Relation of cerebral vessel disease to Alzheimer's disease dementia and cognitive function in elderly people: a cross‐sectional study. Lancet Neurol 15:934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Braak H, Braak E (1989) Cortical and subcortical argyrophilic grains characterize a disease associated with adult onset dementia. Neuropathol Appl Neurobiol 15:13–26. [DOI] [PubMed] [Google Scholar]
- 4. Braak H, Braak E (1991) Neuropathological staging of Alzheimer‐related changes. Acta Neuropathologica 82:239–259. [DOI] [PubMed] [Google Scholar]
- 5. Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging 24:197–211. [DOI] [PubMed] [Google Scholar]
- 6. Brayne C, Richardson K, Matthews FE, Fleming J, Hunter S, Xuereb JH et al (2009) Neuropathological correlates of dementia in over‐80‐year‐old brain donors from the population‐based Cambridge city over‐75s cohort (CC75C) study. J Alzheimers Dis 18:645–658. [DOI] [PubMed] [Google Scholar]
- 7. Cairns NJ, Bigio EH, Mackenzie IR, Neumann M, Lee VM, Hatanpaa KJ et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114:5–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Corrada MM, Berlau DJ, Kawas CH (2012) A population‐based clinicopathological study in the oldest‐old: the 90+ study. Curr Alzheimer Res 9:709–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Corrada MM, Sonnen JA, Kim RC, Kawas CH (2016) Microinfarcts are common and strongly related to dementia in the oldest‐old: the 90+ study. Alzheimers Dement 12:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. DeLong ER, DeLong DM, Clarke‐Pearson DL (1988) Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics 44:837–845. [PubMed] [Google Scholar]
- 11. Farfel JM, Nitrini R, Suemoto CK, Grinberg LT, Ferretti RE, Leite RE et al (2013) Very low levels of education and cognitive reserve: a clinicopathologic study. Neurology 81:650–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ferretti REdL, Damin AE, Brucki SMD, Morillo LS, Perroco TR, Campora F et al (2010) Post‐Mortem diagnosis of dementia by informant interview. Dementia Neuropsychol 4:138–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ferretti‐Rebustini RE, Balbinotti MA, Jacob‐Filho W, Rebustini F, Suemoto CK, Pasqualucci CA et al (2015) Validity of the Katz Index to assess activities of daily living by informants in neuropathological studies. Rev Esc Enferm USP 49:946–952. [DOI] [PubMed] [Google Scholar]
- 14. Foreman KJ, Marquez N, Dolgert A, Fukutaki K, Fullman N, McGaughey M et al (2018) Forecasting life expectancy, years of life lost, and all‐cause and cause‐specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. Lancet 392:2052–2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gold G, Bouras C, Kovari E, Canuto A, Glaria BG, Malky A, Hof PR, Michel JP, Giannakopoulos P (2000) Clinical validity of Braak neuropathological staging in the oldest‐old. Acta Neuropathol 99:579–582; discussion 83–84. [DOI] [PubMed] [Google Scholar]
- 16. Grinberg LT, Ferretti RE, Farfel JM, Leite R, Pasqualucci CA, Rosemberg S, Nitrini R, Saldiva PH, Filho WJ, Group BABS (2007) Brain bank of the Brazilian aging brain study group—a milestone reached and more than 1,600 collected brains. Cell Tissue Bank 8:151–162. [DOI] [PubMed] [Google Scholar]
- 17. Grinberg LT, Heinsen H (2010) Toward a pathological definition of vascular dementia. J Neurol Sci 299:136–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grinberg LT, Thal DR (2010) Vascular pathology in the aged human brain. Acta Neuropathologica 119:277–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Haroutunian V, Schnaider‐Beeri M, Schmeidler J, Wysocki M, Purohit DP, Perl DP et al (2008) Role of the neuropathology of Alzheimer disease in dementia in the oldest‐old. Arch Neurol 65:1211–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ighodaro ET, Abner EL, Fardo DW, Lin AL, Katsumata Y, Schmitt FA et al (2017) Risk factors and global cognitive status related to brain arteriolosclerosis in elderly individuals. J Cereb Blood Flow Metab 37:201–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. James BD, Bennett DA, Boyle PA, Leurgans S, Schneider JA (2012) Dementia from Alzheimer disease and mixed pathologies in the oldest old. JAMA 307:1798–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jansen WJ, Wilson RS, Visser PJ, Nag S, Schneider JA, James BD et al (2018) Age and the association of dementia‐related pathology with trajectories of cognitive decline. Neurobiol Aging 61:138–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jellinger KA, Attems J (2010) Prevalence and pathology of vascular dementia in the oldest‐old. J Alzheimers Dis 21:1283–1293. [DOI] [PubMed] [Google Scholar]
- 24. Kawas CH, Kim RC, Sonnen JA, Bullain SS, Trieu T, Corrada MM (2015) Multiple pathologies are common and related to dementia in the oldest‐old: the 90+ Study. Neurology 85:535–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kretzschmar H (2009) Brain banking: opportunities, challenges and meaning for the future. Nat Rev Neurosci 10:70–78. England. [DOI] [PubMed] [Google Scholar]
- 26. Mackenzie IR, Neumann M, Bigio EH, Cairns NJ, Alafuzoff I, Kril J et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Martinez J, Montgomery DL, Uzal FA (2012) Vascular mineralization in the brain of horses. J Vet Diagn Invest 24:612–617. [DOI] [PubMed] [Google Scholar]
- 28. Middleton LE, Grinberg LT, Miller B, Kawas C, Yaffe K (2011) Neuropathologic features associated with Alzheimer disease diagnosis: age matters. Neurology 77:1737–1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM et al (1991) The consortium to establish a registry for Alzheimers‐disease (CERAD).2. Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41:479–486. [DOI] [PubMed] [Google Scholar]
- 30. Morris JC (1993) The clinical dementia rating (CDR): current version and scoring rules. Neurology 43:2412–2414. [DOI] [PubMed] [Google Scholar]
- 31. Nascimento C, Suemoto CK, Rodriguez RD, Alho AT, Leite RP, Farfel JM et al (2016) Higher prevalence of TDP‐43 proteinopathy in cognitively normal Asians: a clinicopathological study on a multiethnic sample. Brain Pathol 26:177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Nelson PT, Abner EL, Schmitt FA, Kryscio RJ, Jicha GA, Smith CD et al (2010) Modeling the association between 43 different clinical and pathological variables and the severity of cognitive impairment in a large autopsy cohort of elderly persons. Brain Pathol 20:66–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Nelson PT, Jicha GA, Schmitt FA, Liu H, Davis DG, Mendiondo MS et al (2007) Clinicopathologic correlations in a large Alzheimer disease center autopsy cohort: neuritic plaques and neurofibrillary tangles “do count” when staging disease severity. J Neuropathol Exp Neurol 66:1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Neltner JH, Abner EL, Jicha GA, Schmitt FA, Patel E, Poon LW et al (2016) Brain pathologies in extreme old age. Neurobiol Aging 37:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nitrini R, Bottino CMC, Albala C, Custodio Capunay NS, Ketzoian C, Llibre Rodriguez JJ et al (2009) Prevalence of dementia in Latin America: a collaborative study of population‐based cohorts. Int Psychogeriatrics 21:622–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pierce AL, Kawas CH (2017) Dementia in the oldest old: beyond Alzheimer disease. PLoS Med 14:e1002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robinson JL, Corrada MM, Kovacs GG, Dominique M, Caswell C, Xie SX et al (2018) Non‐Alzheimer's contributions to dementia and cognitive resilience in The 90+ Study. Acta Neuropathol 136:377–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Robinson JL, Geser F, Corrada MM, Berlau DJ, Arnold SE, Lee VM et al (2011) Neocortical and hippocampal amyloid‐beta and tau measures associate with dementia in the oldest‐old. Brain 134(Pt 12):3708–3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rodriguez RD, Suemoto CK, Molina M, Nascimento CF, Leite RE, de Lucena Ferretti‐Rebustini RE et al (2016) Argyrophilic grain disease: demographics, clinical, and neuropathological features from a large autopsy study. J Neuropathol Exp Neurol 75:628–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Sabbagh MN, Sandhu SS, Farlow MR, Vedders L, Shill HA, Caviness JN et al (2009) Correlation of clinical features with argyrophilic grains at autopsy. Alzheimer Dis Assoc Disord 23:229–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Savva GM, Wharton SB, Ince PG, Forster G, Matthews FE, Brayne C (2009) Age, neuropathology, and dementia. N Engl J Med 360:2302–2309. [DOI] [PubMed] [Google Scholar]
- 42. Schlesinger D, Grinberg LT, Alba JG, Naslavsky MS, Licinio L, Farfel JM et al (2013) African ancestry protects against Alzheimer's disease‐related neuropathology. Mol Psychiatry 18:79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Schmidt MI, Duncan BB, e Silva GA, Menezes AM, Monteiro CA, Barreto SM et al (2011) Chronic non‐communicable diseases in Brazil: burden and current challenges. Lancet 377:1949–1961. [DOI] [PubMed] [Google Scholar]
- 44. Slager UT, Wagner JA (1956) The incidence, composition, and pathological significance of intracerebral vascular deposits in the basal ganglia. J Neuropathol Exp Neurol 15:417–431. [DOI] [PubMed] [Google Scholar]
- 45. Suemoto CK, Ferretti‐Rebustini RE, Rodriguez RD, Leite RE, Soterio L, Brucki SM et al (2017) Neuropathological diagnoses and clinical correlates in older adults in Brazil: a cross‐sectional study. PLoS Med 14:e1002267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Tanskanen M, Makela M, Notkola IL, Myllykangas L, Rastas S, Oinas M et al (2017) Population‐based analysis of pathological correlates of dementia in the oldest old. Ann Clin Transl Neurol 4:154–165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Thal DR, Grinberg LT, Attems J (2012) Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 47:816–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. United Nations DoEaSA, Population Division (2017) World population ageing 2017. New York, NY: United Nations DoEaSA, Population Division. [Google Scholar]
- 49. Vinters HV (2001) Aging and the human nervous system. In: Handbook of the Psychology of Aging, Birren JE, Schaie KW (eds), Chapter 6, pp. 135–160. Academic Press: San Diego, London. [Google Scholar]
- 50. White L (2009) Brain lesions at autopsy in older Japanese‐American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu‐Asia aging study. J Alzheimers Dis 18:713–725. [DOI] [PubMed] [Google Scholar]
- 51. Zaccai J, Ince P, Brayne C (2006) Population‐based neuropathological studies of dementia: design, methods and areas of investigation–a systematic review. BMC Neurol 6:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Mosaic plot showing the relationship between neuropathological diagnoses (NP) and age groups (Fisher’s exact test, P < 0.0001). AD = Alzheimer disease; LBD = Lewy body disease; VaD = vascular disease.
Table S1. Odds ratio of dementia (CDR ≥ 1) according to the presence of neuropathological lesions, stratified by age groups (n = 1089).
Data Availability Statement
Data is available upon request to the corresponding author.


