Abstract
Background:
Having a first-degree relative (FDR) with colorectal cancer (CRC) is a significant risk factor for CRC. Counselling for FDRs on CRC risk factors and personalized risk is important to improve knowledge and screening compliance.
Methods:
A three-arm randomized controlled trial compared tailored In-Person and Telephone CRC counselling interventions to Control among FDRs who were not mutation carriers for known hereditary cancer syndromes, but at increased risk based on family history. It was hypothesized that both Telephone and In-Person approaches would increase CRC knowledge, screening adherence, perceived risk accuracy, and psychosocial functioning compared to Control. We anticipated greater satisfaction with the in person approach. CRC knowledge, risk perception, psychosocial functioning, and intention-to-screen were assessed at baseline, 2 weeks, and 2 month follow-up (primary endpoint).
Results:
278 FDRs (Mean=47.4 years, Standard Deviation=11.38) participated. At baseline, participants reported low to moderate CRC knowledge and overestimations of risk. Screening adherence was 73.7%. At 2 months, the In-Person arm and Telephone arm demonstrated improvements in knowledge and perceived risk and were not statistically different from each other. However, when comparing each intervention to Control, knowledge in the In-Person arm was statistically significantly higher, but the difference between Telephone and Control was not. Cancer-related stress reduced over time in all groups. Intervention benefits were maintained at 1 year. Baseline screening intent/adherence were high, and therefore, did not reach statistically significant improvement.
Conclusions:
Tailored In-Person or Telephone formats of providing CRC risk counselling, incorporating behavioral interventions improve knowledge and risk perceptions, with high client satisfaction.
Keywords: Randomized controlled trial, genetic counselling, intervention research, screening, relatives, colorectal cancer
Precis:
First-degree relatives overestimated their risk of developing colorectal cancer. Both the in-person and telephone-based educational/counselling interventions improve colorectal cancer knowledge and risk perceptions and neither were associated with increased distress post-intervention.
Introduction
Colorectal Cancer (CRC) is the forth leading cause of cancer in North America.1, 2 CRC may be preventable if detected in a premalignant stage.3, 4 Five-year survival rates for CRC can significantly increase with early screening, detection, and appropriate management.3, 4
The overall level of CRC screening adherence may be low, both in those at average risk5, 6 and those with a family history of CRC. Family history of CRC is a critical risk factor for developing the disease. Approximately, 5–10% of CRC cases are due to inherited syndromes7 and 25% of CRC cases occur in individuals with at least one first degree relative (FDR) with CRC.7, 8 However, CRC screening rates rarely exceed 50% among FDRs of CRC patients.9
Factors influencing rates of participation in CRC screening include knowledge about the disease and associated screening tests, and psychosocial factors.9, 10 CRC knowledge significantly predicts screening, independent of sociodemographic factors and lower knowledge level is associated with more negative attitudes toward CRC.11, 12 Perceived risk of developing CRC can also affect screening behavior. Elevated perceived risk can cause increased anxiety and cancer worry9, while its underestimation can result in under screening behaviour.13, 14
While improvements in the provision of CRC screening and risk information have occurred, FDRs of CRC probands may still not receive specific information regarding their own CRC risk from a healthcare provider, despite being increased risk.9, 10, 15 Family members are more likely to receive this information if they are at high-risk for CRC or if a genetic mutation has been found in the proband, a group who represent a minority of at-risk families.
Counselling with a behavioral change framework to provide information on risk and the disease may enhance motivation to participate in recommended screening. Telephone and in-person counselling are effective in increasing knowledge of CRC among high-risk FDRs and individuals at average risk.16–19 Tailored approaches improve cancer knowledge and risk perceptions among the general population14 and relatives of cancer patients,20 compared to non-tailored information. However, it is unclear that educational interventions improve screening behaviors,14, 15, 20 with the exception of high-risk individuals. In addition, while brochures have demonstrated some success, they may be less effective than approaches where a counsellor is available to respond to questions and address misinformation or psychosocial issues. Further, for FDRs in underserviced areas, a telephone-based approach may represent a low-intensity option to provide personalized risk and screening information with health behavioral strategies.21–23
Since the onset of our study, Kinney et al. (2014) found that a telephone-based intervention compared to a mailed educational brochure was effective in improving colonoscopy screening rates in “at-risk relatives” of CRC patients.24 They found that more than a third in the telephone group who received a personalized CRC risk assessment and counselling session underwent a colonoscopy within 9 months, compared to 16% of controls.
Given the high incidence and prevalence of CRC, and role of early screening, there continues to be a need to examine methods of risk counselling to improve screening rates among relatives of CRC patients, particularly among those individuals not deemed “high-risk, but are at increased risk, and who require updated knowledge about their potential elevated risk.
Purpose:
The current study was designed to assess the efficacy of tailored In-Person and Telephone-based risk/screening counselling interventions for FDRs of CRC patients in comparison to usual-care on CRC knowledge, perceived risk, intent to adopt a recommended screening regimen and psychosocial functioning.
Methods
Research Design Overview
A three-arm, prospective, randomized unblinded trial was conducted. Participants were randomized into either an In-Person CRC risk/screening counselling, or Telephone version of the same intervention, or Control. FDRs of probands registered in the Ontario Familial Colorectal Cancer Registry (OFCCR) and the Newfoundland Colorectal Cancer Registry (NFCCR) were invited to participate. Once a family history was confirmed informed consent was obtained. FDRs who completed a baseline assessment were randomized to receive the In-Person or Telephone-based CRC Risk and Screening Educational counselling intervention or to usual-care. The study outcomes were assessed through standardized questionnaires before and after the intervention, at 2 weeks, and 2 months (primary endpoint). To assess screening and sustainability of outcomes at 2 months, a 1-year follow-up assessment was also completed. After the 2-month follow-up, participants in the control arm received written information concerning their CRC risk and screening recommendations. The usual-care condition, therefore, became an active control group. The 1-year follow-up assessments were compared among three groups: In-Person, Telephone-based counselling, and written information.
The primary outcomes were CRC knowledge and intent to screen. Secondary outcomes included risk perception, actual screening behavior, psychosocial functioning, and client satisfaction.
We hypothesized that both the Telephone and In-Person interventions would improve CRC knowledge, risk perception and intention-to-screen compared to usual-care. We expected that the In-Person intervention would be associated with greater satisfaction, and those with higher risk perception of CRC would be associated with elevated psychological distress.
The study received ethics approval from the Research Ethic Boards at the University Health Network (UHN), Mount Sinai Hospital, Toronto (REB #04–0729-CE), and Memorial University, NFLD, and was approved by the NIH-funded CFR (ClinicalTrials.gov: NCT00188305).
Participants
Participants were recruited through the OFCCR and NFCCR from 2004–2009. Registry probands had provided permission for contact of their FDRs. Study invitations to participate were mailed to FDRs. The inclusion criteria included: a) having at least one FDR with CRC; b) aged between 25 and 80 years; c) being a member of the OFCCR/NFCCR; d) understanding of English; and e) being low to intermediate risk categories for CRC:25 “Low risk” refers to being at low risk for a hereditary/Familial CRC but still at increased risk of CRC (compared to the general population risk for CRC); “Intermediate risk” refers to being at moderate risk for a hereditary/Familial CRC (but not considered at high risk, referring to being at a high risk for hereditary familial CRC and eligible for genetic testing).25
Individuals with a family history suggestive of hereditary cancer syndromes were excluded and offered genetic testing. Individuals were also excluded if they had a previous diagnosis of CRC or other malignancy, lived more than one hour from the city center, or failed to provide consent.
Recruitment & Randomization
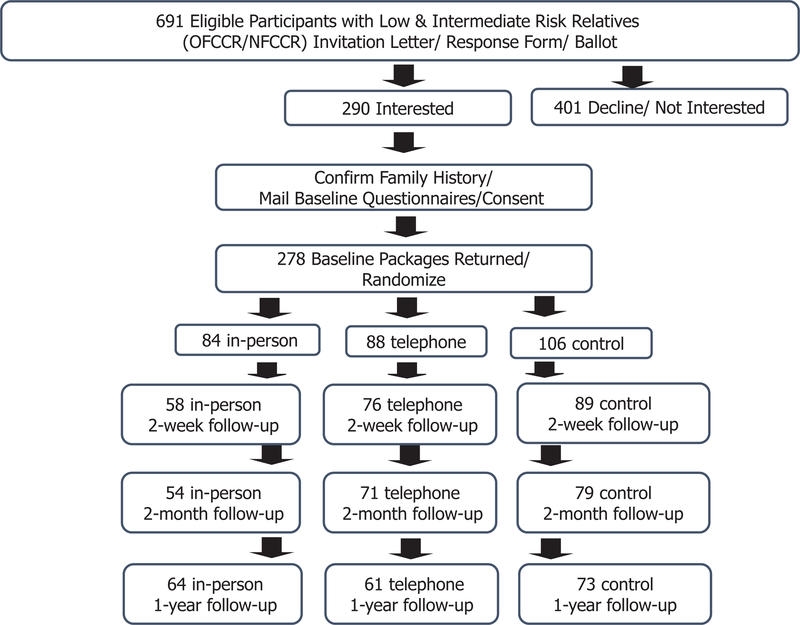
Among 691 individuals identified as FDRs with low to intermediate CRC risk, 290 were interested in the study and 278 provided informed consent (Figure 1). This sample size is sufficient to satisfy the original design of 210 (70 in each intervention arm and 70 in control) that would result in 80% power (alpha=0.05) to detect a difference of 0.49 standard deviations (SD) between groups in the primary outcome - the CRC knowledge at 2-month follow up.
Figure 1 -.
Recruitment Flow Chart
A stratified block randomization method where a set of permuted blocks were generated for a combination of age (<50 vs. 50+) and gender, was used to randomize participants into each of the study groups. Random sequences were generated by the UHN clinical research support unit prior to study onset with randomization lists maintained by an arm-length UHN researcher.
Next, the study coordinator contacted and informed participants of their randomization results. Eighty-four participants were randomized into In-Person, 88 into Telephone, and 106 into Control (Figure 1).
CRC Risk Educational/Counselling Intervention
The manual–based CRC Risk Educational interventions for the In-Person or Telephone group were identical in content. They were developed by a health psychologist (SH) and genetic counsellors (HR; KS; MA) with reviews conducted by clinicians working in CRC, as well as by patients for relevance, accuracy, literacy and comprehension.
The interventions were delivered by a health psychologist and/or genetic counselor with scripts that provided tailored individualized risk information based on their family history. The interventions included: 1) Information on the OFCCR and NFCCR registries; 2) CRC signs and symptoms, the role of polyps in development and risk factors; 3) Review of family history and a personalized CRC risk level; and 4) Screening recommendations.
The Telephone and In-Person interventions were guided by the Health Belief Model (HBM),26 which consisted of four independent predictors: perceived susceptibility of developing illness, perceived severity of the illness, and perceived barriers and benefits to performing the recommended preventive health advice.27 The HBM suggests that an individual’s tendency to take action is increased by having an elevated perceived susceptibility and disease severity, alongside high perceived benefits and low perceived barriers to the screening procedures. An internal (e.g. symptoms) and external stimulus (e.g. recommendations from health professional) are necessary to trigger the decision-making process.
Personalized risk (referred to as a participant’s objective risk in the study) was estimated in comparison to the general population, and based on the OFCCR28, which was also used to generate participants’ CRC screening recommendations.25 For example, a participant with one FDR with CRC > age 35 would be at a low risk for Familial/Hereditary CRC (but still higher than the general population risk) and recommended to have colonoscopy every 5–10 years beginning 10 years younger than the youngest CRC diagnosis, no later than age 40.
Participants were asked about their CRC risk perceptions and about past screening recommendations to address elicited barriers or concerns. Potential barriers included the need to have symptoms prior to screening, time constraint, fear, pain, embarrassment, and uncertainty around screening locations.29 Barriers identified were responded to with knowledge, behavioral interventions, and reassurance, including recommendations for a support person to attend screening if a participant feeling anxious. Pilot-testing was conducted prior to the randomized trial on 5 FDRs.
Procedure
During the initial telephone call participants were asked about their family history of cancer and a personalized CRC risk assessment profile was generated. A family tree outlining the family members with a prior diagnosis of CRC was constructed and reviewed by a genetic counselor. The intervention session (45–60 minutes) was delivered either by Telephone or In-Person. Upon completion, follow-up questionnaires were mailed at 2-week, 2-month, and 1-year post-intervention. If questionnaires were not returned, reminder calls (maximum three times) were made.
The control group received the baseline, 2-week, 2-month and 1-year questionnaires. They also received a mailed letter providing tailored information about CRC, their personal CRC risk, and screening recommendations after the 2-month follow-up.
Baseline Questionnaire
Sociodemographic, medical (including family history), and personal and lifestyle information was collected.
Knowledge Outcomes
The CRC Risk Factor and Screening Knowledge Questionnaire was adapted30 and consisted of 12 true/false questions.
Risk Perception
Perceptions of CRC risk was assessed in various formats, including their risk perception on a scale from 0–100.31
Screening Barriers and Intention-to-Screen
At baseline, participants were asked about their previous screening behaviors and what prompted screening (e.g. doctor recommended). Individuals not previously screened were asked to indicate among ten items as to why they had not been screened (e.g. fear of test).32 These responses were incorporated into the personalized educational session to address potential barriers. Intention-to-screen was measured on a Likert scale, with ratings “4” and “5” indicating an intention-to-screen.
At 2-month and 1-year follow-ups, information on actual screening behaviors was collected. Prior studies supported the use of self-report among FDRs for accurately reflecting screening.33, 34
Psychosocial Functioning
The Impact of Event Scale Revised (IES-R)35 was used to measure cancer-related distress, anchored around the stress of having family history of CRC.
Statistical Analysis
Descriptive analyses were conducted for study variables. Univariate analyses were conducted to compare baseline variables of the three groups using parametric and non-parametric tests according to the normality test. All analyses used an intent-to-treat approach. When handling missing items in an instrument, prorated scores was used if participants had ≤20% of the instrument items missing; otherwise, multiple imputations PROC MI with Markov Chain Monte Carlo (MCMC) and Fully Conditional Specification algorithms were used to estimate the missing continuous and categorical outcome variables, respectively.36 Five datasets were imputed for each outcome of interest to account for the uncertainty of the imputed values estimated.36 The PROC MIANALZE was used to combine the five sets of results of the multivariate analyses to yield parameter estimates of the outcome of interest.
For continuous outcome (knowledge, risk perception, IES-R), a mixed effect model was used to account for repeated-measure within subject and subjects clustered within a particular site. For categorical outcome measures (e.g. intention-to-screen), the generalized estimating equation (GEE) model with unstructured covariance matrix was applied. A general linear model (GLM) was used to access the relationship between baseline perceived risk and psychological distress adjusting for group assignment, and to assess the differences on post-program satisfaction between in-person and telephone groups.
Results
A total of 278 participants were enrolled in the study (Figure 1).
Participant Characteristics
Table 1 highlights characteristics of participants. The mean age was 47.4 (SD=11.4, range 19–80 years); 65% were female. No significant differences were found on any of the demographic characteristics among all groups.
Table 1.
Participant Characteristics
| In-person | Telephone | Control | Total | |||||
|---|---|---|---|---|---|---|---|---|
| N=84 | N=88 | N=106 | N=278 | |||||
| M | SD | M | SD | M | SD | M | SD | |
| Age | 46.05 | 12 | 47.57 | 10.4 | 48.3 | 11.7 | 47.39 | 11.38 |
| n | % | n | % | n | % | n | % | |
| Site | ||||||||
| OFCCR | 66 | 78.6 | 70 | 79.6 | 86 | 81.1 | 222 | 79.9 |
| Gender | ||||||||
| Female | 54 | 64.3 | 50 | 56.8 | 77 | 72.6 | 181 | 65.1 |
| Marital Status | ||||||||
| Married | 67 | 81.7 | 69 | 82.1 | 87 | 82.9 | 223 | 82.3 |
| Education | ||||||||
| University or above | 41 | 54.7 | 41 | 48.8 | 49 | 48.0 | 131 | 50.2 |
| Family Income | ||||||||
| <$50,000 | 11 | 13.1 | 13 | 14.9 | 20 | 20.2 | 44 | 16.3 |
| $50,000 or above | 59 | 70.2 | 62 | 71.3 | 65 | 65.7 | 186 | 68.9 |
| Unknown | 14 | 16.7 | 12 | 13.8 | 14 | 14.1 | 40 | 14.8 |
| Ethnicity (White) | ||||||||
| Anglo-Saxon | 65 | 83.3 | 64 | 79 | 84 | 81.6 | 213 | 81.3 |
| CRC Risk History | ||||||||
| • Ever Discussed CRC Family History with Family Doctor | 68 | 87.2 | 72 | 90 | 94 | 92.2 | 234 | 90 |
| • Ever Had Sigmoidoscopy or Colonoscopy | 58 | 71.6 | 65 | 77.4 | 76 | 72.4 | 199 | 73.7 |
| • Ever Had Cancer Risk Counseling | 5 | 6.8 | 9 | 11.3 | 6 | 6.1 | 20 | 7.9 |
| • Ever Had Genetic Counseling | 5 | 6.3 | 3 | 3.8 | 9 | 8.8 | 17 | 6.5 |
| • Ever Discussed CRC with proband relative | 62 | 78.5 | 57 | 69.5 | 73 | 72.3 | 192 | 73.3 |
OFCCR=Ontario Familial Colorectal Cancer Registry
Baseline Knowledge, Intention-to-Screen, Perceived Risk, & Psychological Functioning
No significant group differences were found regarding risk perception, CRC knowledge, intention-to-screen, or psychological functioning at baseline.
Baseline Knowledge Score Typically, participants identified on average 8.67/12 (72.3%) of the correct answers on the CRC knowledge survey (Supplementary Table S1).
Baseline Intention-to-Screen: Eighty-three percent of participants “agreed or strongly agreed” to intending to undergo CRC screening. For actual screening behaviors, 73.7% completed the recommended screening. Of those who had completed CRC screening, reasons provided included: doctor’s recommendation (61.9%), to decrease cancer worry (58.4%), and to increase chances of a better recovery (52.6%). For those who had not screened, reasons reported included: unpleasant test preparation (32.6%) and lack of time/inconvenience (10.9%). Approximately 84% of participants indicated that they only experienced mild stress about having a relative with CRC.
Baseline Perceived Risk The mean baseline of perceived risk was 43.4% (SE=1.38), higher than the average personalized (actual/objective) risk level for this cohort of FDRs of 15–20%.
Psychosocial Functioning The baseline mean score of the IES-R was 12.12 (SE=0.8), indicating a relatively low level of distress (cutoff=24).
Change in Knowledge, Intention-to-Screen, Perceived Risk, & Psychological Functioning
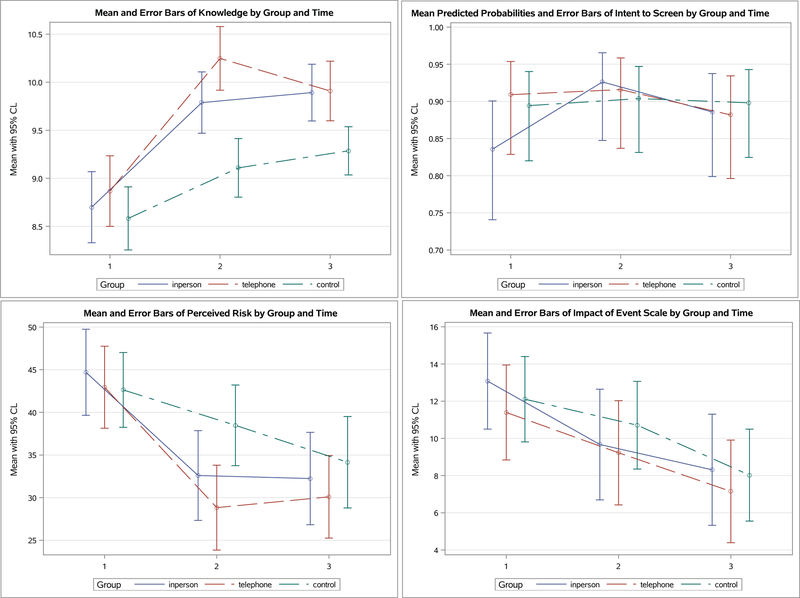
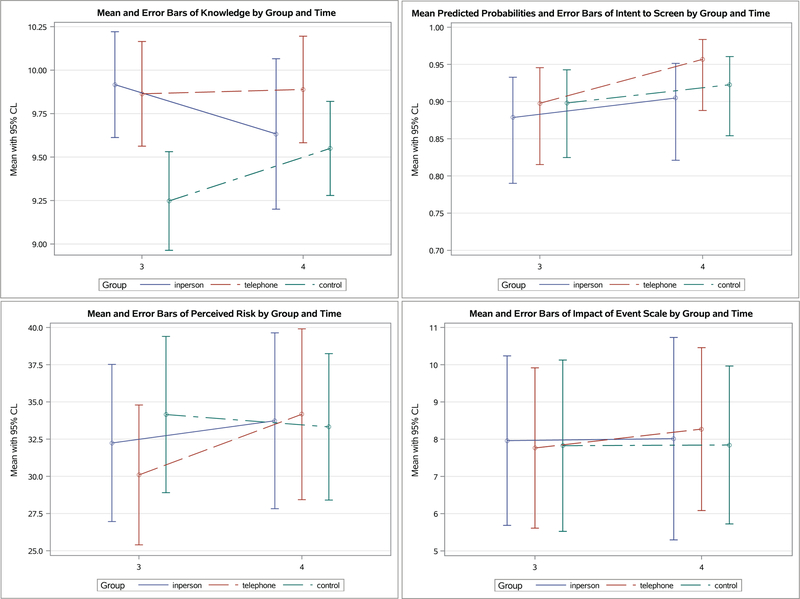
See Figure 2 & 3 for means and standard errors of all outcomes reported by participants and Supplementary Table S2 for the mixed model results which incorporated missing data estimates for all outcome variables.
Figure 2.
Least Squared Means of Study Outcomes by Group and Time at Baseline (Time 1), Two-week (Time 2), and Two-month (Time 3) Follow-up
Figure 3.
Least Squared Means of Study Outcomes by Group and Time at Two-month (Time 3) and One-Year Follow-up (Time 4)
Change in Knowledge Score Participants showed significant increases in knowledge at 2 weeks (p=0.005) (Time effect, S2). When comparing between groups, the two intervention groups were not statistically different from each other (Figure 2). The knowledge score in either In-Person or Telephone arm was higher compared to the Control arm in mixed model analyses (p=0.016 & p=0.020 respectively) (Time × Group effect, S2). At 2 months, the increase in knowledge was significant for the sample (p<0.0001) (Time effect, S2). Again, the In-Person arm and Telephone arm were not statistically different when compared to each other (Figure 2). In mixed model analyses, knowledge score in the In-Person arm remained significantly higher than controls at 2 months (p=0.021) (Time × Group effect, S2), but the difference between Telephone arm and Control was no longer statistically significant. The inclusion of computed missing data estimates in the analyses likely contributed to the above observation.
At 1-year (contrasting to 2 month), compared to In-Person, there was a significant group × time interaction in which Control significantly increased in knowledge after receiving the written material (beta=0.59, 95% CL 0.07 to 1.10, p=0.027, data not shown). No significant changes were found between In-Person and Telephone at 1-year (Figure 3).
Intention-To-Screen showed no significant differences among the three groups over time. At 2 months, the completion rates for appropriate level screening were 63.3%, 69.2%, and 56.7%, respectively. At 1-year, the completion rates were 70.5%, 78.9%, and 76.1%, respectively. At 1-year, there were no significant differences among the three groups. No significant changes were found between In-Person and Telephone over time (Figure 3).
Perceived Risk Both In-Person and Telephone showed significant decreases in perceived risk at 2 weeks compared with Control (p=0.033 & p=0.009 respectively (Time × Group effect, S2). At 2 months, perceived risk showed a significant reduction (p=0.005; Time effect, S2), but there was no significant group difference at 2 month (Figure 2 and S2) nor at 1-year follow up (Figure 3).
Psychological functioning, the cancer-related distress (IES-R), which was mild at baseline, showed a statistically significant reduction at 2 months (p<0.0001; Time effect, S2) for all three groups. There was no significant group difference in all time points (Figure 2 and S2).
Association between baseline perceived risk and cancer-related distress:
A GLM model assessing the association between baseline perceived risk and IES-R total adjusting for group assignment was not significant (p=0.309; data not shown).
Satisfaction
A GLM model assessing participation satisfaction level between In-Person and Telephone at 2-month follow-up was not significant (p=0.264; data not shown).
Discussion
The current randomized controlled trial aimed to compare In-Person and Telephone delivered CRC risk educational/counselling interventions with usual-care to examine changes in knowledge, intention-to-screen, risk perception, and psychological functioning in relatives of CRC patients. Participants at baseline demonstrated an overestimation of their personal risk and knowledge gaps, particularly around myths or barriers related to CRC screening and symptoms. Both intervention formats demonstrated improvements on CRC knowledge and risk perception, compared to Control. Further, participant satisfaction level between the In-Person and Telephone formats was not significantly different. This finding was unexpected as we predicted greater satisfaction with the In-Person format that allows for visual monitoring of cues and emotional reactions believed to facilitate therapeutic encounters. Perhaps the FDRs in the Telephone group welcomed the added benefits of easy access or reduced transport costs associated with Telephone counselling.
At the time of the onset of our study, there was little known about telephone-based cancer risk counselling and its impacts. Genetic counsellors had expressed concerns about telephone-based counselling around its potential contribution to cancer worry or poor comprehension through reduced opportunity for visual assessment of reactions. The telephone-based CRC risk counselling was not associated with increased cancer-related distress, nor inaccurate knowledge. While our findings differ from those conducted in the general population where tailoring of risk information has not consistently resulted in improved screening intent/behavior or knowledge accuracy,14, 20 our results are aligned with a similar study in the USA of FDRs of CRC patients recruited from registries24 using a well-designed telephone intervention. Kinney et al.24 utilized health behavioral theory to guide the design of a multicomponent telephone intervention delivered by a genetic counsellor. Our study similarly was multi-faceted, incorporating personalized risk information with comparators to the general population and behavioral strategies to address barriers. Both studies provided complex information on family history and risk factors, along with print material to support the telephone-based delivery.
FDRs at all levels of risk potentially can benefit from receiving recommendations for screening information and how to manage one’s risk.9 Telephone counselling represents an effective and cost effective way to provide cancer screening recommendations and risk information and may be particularly relevant for outreach to populations in rural settings where there is reduced opportunity to see a genetics specialist, or where limited in-person genetic counselling services are prioritized for individuals deemed “high-risk”. Our study also demonstrated that a trained health care provider can successfully deliver the information. While we used an inter-professional approach in the intervention development (e.g. genetic counselor generated the tailored risk and CRC screening information based on family history) the manualized counselling was delivered by a health psychologist. Health professionals, such as nurses or psychosocial counsellors who work alongside of specialists in the field of colorectal cancer (gastroenterologist; family physician) can provide care and offer health promotional strategies over the phone via a manualized intervention that includes up to date materials and tailored information for patients. Back up support from a specialist (e.g. genetic counselor or colorectal physician) can further address the tailoring of risk and screening information to ensure accuracy in the information provided. Psychoeducation, theories of behavioral change and distress screening are all within the scope of nursing practice for example, and nurses commonly work in primary care. Future studies might consider the training of other health care professionals (e.g. nurses) who work in primary care to deliver tailored CRC risk/screening information to relatives of CRC patients.
Participants demonstrated a mild level of anxiety at baseline, ranging from 11.3–12.4 using IES-R, which limited the potential for the intervention to lower cancer-related distress any further (floor effect). Cancer-related distress in CRC risk populations, interestingly, tends to be lower in general than FDRs from breast cancer families, who often express profound and persistent elevations in their risk perceptions which may impede comprehension of risk/genetic information.37
There were no effects on intention-to-screen or actual screening behavior; however, this finding implies a ceiling effect, as participants at baseline were either engaged in, or demonstrated an intention-to-screen. This finding likely highlights the enabling factor of medical coverage in addressing health screening, as CRC screening costs are covered in Canada. Costs for screening tests can be a barrier to screening uptake.24, 38 The FDRs of CRC patients recruited through a Cancer Registry may also have had greater awareness of screening recommendations through discussions in their families or experience in completing family history questionnaires for the Registry. As such, the generalizability of our findings may be limited in relation to other CRC populations, including the general population.
Group comparisons were no longer significant at one year; however, the benefit of increased knowledge in the In-Person and Telephone CRC counselling interventions was sustained. Our control participants by one year had received personalized written information on their CRC risk and screening recommendations. While these findings may suggest that a printed letter with personalized risk information has benefit, we remain cautious in interpreting our findings. Our study did not have a priori aim to test the impact of a tailored print brochure/letter. Prior studies have shown that tailored or generic print formats have not consistently improved CRC screening intent or behavior in the general population.14 Participating in a study over time, with its repeated questionnaires may have produced a learning effect. Further, ongoing registry contact (or possible related family interactions) may have contributed to increased awareness and comprehension round personal risk and screening needs.
Screening compliance in our study was higher than prior studies,10, 16, 24 but did not reach 100%. While our study interventions aimed to address some known modifiable factors (i.e., knowledge, attitudes or structural barriers for screening) and non-modifiable factors (i.e., demographics),38 given that only 74% of the study participants complied with recommended screening, future studies are needed. Further research can explore which interventions are most ideal for whom, and the role of personal attributes, such as coping style, culture20 or the experiences of cancer in the family.39 These factors were not specifically targeted in our study. Information-oriented interventions may fail to optimally address psychological concerns, such as the intrusion of a CRC test among those with a past history of trauma or who have suffered multiple losses as a result of their family history. For some individuals a more intensive psychological approach may be required so that past history and personal variables are understood and addressed within the larger context on one’s identity and health behavior. Indeed, recommendations to consider a continuum of approaches or a “stepped approach” have been suggested.24 Our team has addressed issues of loss and grief successfully through a group support program for women at risk for breast cancer, who grossly overestimated their cancer risk.40
We also considered the role of perceived risk in relation to screening in our study, but did not find a significant correlation. Given the complexity how risk is interpreted, more research is needed to further examine this area.
With many strengths of the current study, there are a few limitations. Despite our best efforts in recruitment and retention, lost-to-follow up occurred. The intent-to-treat analyses using multiple imputations is a recommended statistical method in dealing with incomplete data36. This approach ensures that every participant is included in the analyses while accounting for the uncertainty of the imputed values. However, this method is not perfect. The imputation makes assumption that the missing data is at random which in reality we cannot be certain. Therefore, the approach using statistical imputation to derive estimates for missing data has its own constraint. Another limitation was the impact of the non-participants who may also be non-compliant with educational interventions. A different design, using population based research would be better suited to address this issue.
Conclusion
In conclusion, FDRs in our study overestimated their risk for developing CRC and demonstrated misperceptions about CRC. Tailored In-Person or Telephone-based formats of providing CRC risk education/counselling incorporating well-established health behavioral interventions demonstrated improvement in knowledge and risk perceptions, as well as client satisfaction. Despite reduced opportunity for visual cues in monitoring reactions to receiving complex and cancer risk information, the telephone-based approach performed well and was not associated with increased distress, with the knowledge increase sustained at one year. Screening intent and adherence were high at baseline, and therefore, did not show improvement. Findings suggest that a less costly, well-tailored telephone-based approach incorporating health-behavioral strategies to address personal barriers can effectively serve individuals with a family history of CRC to learn about their cancer risk and screening needs. Future research is needed to determine how best to implement telephone-based risk counselling into usual care.
Supplementary Material
Acknowledgements:
We would like to acknowledge study participants and funding from the Canadian Institutes of Health Research (CIHR) for a Colorectal Cancer Interdisciplinary Research Team (CRT48321). The first author is a recipient of a CIHR scientist award. This work was supported by the National Cancer Institute (NCI), National Institutes of Health (RFA#CA-96–011) and through cooperative agreements with members of the Colon Cancer Family Registry (CFR) and P.I.s. The content of this manuscript does not necessarily reflect the views or policies of the NCI or any of the collaborating institutions or investigators in the Colon CFR, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government or the Colon CFR.
Footnotes
No conflict of interest to disclose.
References
- 1.Canadian Cancer Statistics Advisory Committee, Canadian Cancer Statistics-A 2018 special report on cancer incidence by stage. Toronto, ON, 2018. [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M. SEER Cancer Statistics Review, 1975–2014, based on November 2016 SEER data submission: SEER, 2018. [Google Scholar]
- 3.Schoen RE, Pinsky PF, Weissfeld JL, et al. Colorectal-Cancer Incidence and Mortality with Screening Flexible Sigmoidoscopy. New England Journal of Medicine. 2012;366: 2345–2357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atkin WS, Edwards R, Kralj-Hans I, et al. Once-only flexible sigmoidoscopy screening in prevention of colorectal cancer: a multicentre randomised controlled trial. Lancet. 2010;375: 1624–1633. [DOI] [PubMed] [Google Scholar]
- 5.James AS, Richardson V, Wang JS, Proctor EK, Colditz GA. Systems intervention to promote colon cancer screening in safety net settings: protocol for a community-based participatory randomized controlled trial. Implement Sci. 2013;8: 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shapiro JA, Klabunde CN, Thompson TD, Nadel MR, Seeff LC, White A. Patterns of colorectal cancer test use, including CT colonography, in the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21: 895–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kanth P, Grimmett J, Champine M, Burt R, Samadder NJ. Hereditary Colorectal Polyposis and Cancer Syndromes: A Primer on Diagnosis and Management. Am J Gastroenterol. 2017;112: 1509–1525. [DOI] [PubMed] [Google Scholar]
- 8.Wells K, Wise PE. Hereditary Colorectal Cancer Syndromes. Surg Clin North Am. 2017;97: 605–625. [DOI] [PubMed] [Google Scholar]
- 9.Lowery JT, Ahnen DJ, Schroy PC 3rd et al. Understanding the contribution of family history to colorectal cancer risk and its clinical implications: A state-of-the-science review. Cancer. 2016;122: 2633–2645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ouakrim DA, Lockett T, Boussioutas A, Hopper JL, Jenkins MA. Screening participation for people at increased risk of colorectal cancer due to family history: a systematic review and meta-analysis. Familial Cancer. 2013;12: 459–472. [DOI] [PubMed] [Google Scholar]
- 11.Christou A, Thompson SCJBPH. Colorectal cancer screening knowledge, attitudes and behavioural intention among Indigenous Western Australians 2012;12: 528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Solbak NM, Xu JY, Vena JE, et al. Patterns and predictors of adherence to colorectal cancer screening recommendations in Alberta’s Tomorrow Project participants stratified by risk. BMC Public Health. 2018;18: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang H, Gies N, Wong C, Sadowski D, Moysey B, Fedorak RN. Patients undergoing colorectal cancer screening underestimate their cancer risk and delay presentation for screening. Can J Gastroenterol. 2012;26: 419–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usher-Smith JA, Silarova B, Sharp SJ, Mills K, Griffin SJ. Effect of interventions incorporating personalised cancer risk information on intentions and behaviour: a systematic review and meta-analysis of randomised controlled trials. Bmj Open. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edwards AGK, Naik G, Ahmed H, et al. Personalised risk communication for informed decision making about taking screening tests. Cochrane Database of Systematic Reviews. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lowery JT, Horick N, Kinney AY, et al. A randomized trial to increase colonoscopy screening in members of high-risk families in the colorectal cancer family registry and cancer genetics network. Cancer Epidemiol Biomarkers Prev. 2014;23: 601–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Manne SL, Coups EJ, Markowitz A, et al. A randomized trial of generic versus tailored interventions to increase colorectal cancer screening among intermediate risk siblings. Ann Behav Med. 2009;37: 207–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rawl SM, Champion VL, Scott LL, et al. A randomized trial of two print interventions to increase colon cancer screening among first-degree relatives. Patient Education and Counseling. 2008;71: 215–227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stoop EM, de Wijkerslooth TR, Bossuyt PM, et al. Face-to-face vs telephone pre-colonoscopy consultation in colorectal cancer screening; a randomised trial. Br J Cancer. 2012;107: 1051–1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Albada A, Ausems MGEM, Bensing JM, van Dulmen S. Tailored information about cancer risk and screening: A systematic review. Patient Education and Counseling. 2009;77: 155–171. [DOI] [PubMed] [Google Scholar]
- 21.Fournier DM, Bazzell AF, Dains JE. Comparing Outcomes of Genetic Counseling Options in Breast and Ovarian Cancer: An Integrative Review. Oncology nursing forum. 2018;45: 96–105. [DOI] [PubMed] [Google Scholar]
- 22.Fenton GL, Smit AK, Freeman L, et al. Development and Evaluation of a Telephone Communication Protocol for the Delivery of Personalized Melanoma Genomic Risk to the General Population. Journal of Genetic Counseling. 2018;27: 370–380. [DOI] [PubMed] [Google Scholar]
- 23.Bradbury AR, Patrick-Miller LJ, Egleston BL, et al. Randomized Noninferiority Trial of Telephone vs In-Person Disclosure of Germline Cancer Genetic Test Results. JNCI: Journal of the National Cancer Institute. 2018: djy015–djy015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kinney AY, Boonyasiriwat W, Walters ST, et al. Telehealth Personalized Cancer Risk Communication to Motivate Colonoscopy in Relatives of Patients With Colorectal Cancer: The Family CARE Randomized Controlled Trial. Journal of Clinical Oncology. 2014;32: 654–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Canadian Cancer Society, Colorectal Cancer (CRC) Risk Triage. Available from URL: https://www.mountsinai.on.ca/care/family-medicine-genetics-program/resources/CRCTriagecard_2760642125.pdf.
- 26.Menon U, Szalacha L. Health Belief Model. In: Donsbach W, editor. The International Encyclopedia of Communication, 2008. [Google Scholar]
- 27.Rosenstock IM. Historical origins of the health belief model. Health Educ Monogr. 1974;2. [DOI] [PubMed] [Google Scholar]
- 28.Cotterchio M, Buchan G, Sutherland H, et al. Ontario Familial Colon Cancer Registry: Methods and first year response rates. American Journal of Epidemiology. 2000;151: S2–S2. [PubMed] [Google Scholar]
- 29.Madlensky L, Esplen MJ, Goel V. Reasons given by relatives of colorectal cancer patients for not undergoing screening. Prev Med. 2004;39: 643–648. [DOI] [PubMed] [Google Scholar]
- 30.Weinrich SP, Weinrich MC, Boyd MD, Johnson E, Frank-Stromborg M. Knowledge of colorectal cancer among older persons. Cancer Nurs. 1992;15: 322–330. [PubMed] [Google Scholar]
- 31.Hopwood P, Howell A, Lalloo F, Evans G. Do women understand the odds? Risk perceptions and recall of risk information in women with a family history of breast cancer. Community Genet. 2003;6: 214–223. [DOI] [PubMed] [Google Scholar]
- 32.Madlensky L, Esplen MJ, Gallinger S, McLaughlin JR, Goel V. Relatives of colorectal cancer patients: factors associated with screening behavior. Am J Prev Med. 2003;25: 187–194. [DOI] [PubMed] [Google Scholar]
- 33.Baier M, Calonge N, Cutter G, et al. Validity of self-reported colorectal cancer screening behavior. Cancer Epidemiol Biomarkers Prev. 2000;9: 229–232. [PubMed] [Google Scholar]
- 34.Lipkus IM, Green LG, Marcus A. Manipulating perceptions of colorectal cancer threat: implications for screening intentions and behaviors. J Health Commun. 2003;8: 213–228. [DOI] [PubMed] [Google Scholar]
- 35.Horowitz M, Wilner N, Alvarez W. Impact of Event Scale: a measure of subjective stress. Psychosomatic medicine. 1979;41: 209–218. [DOI] [PubMed] [Google Scholar]
- 36.Schafer J, Reid N, Cox D, Keiding N, Louis T, Tong H, Isham V. Analysis of Incomplete Multivariate Data. New York: Chapman and Hall/CRC, 1997. [Google Scholar]
- 37.Weinstein ND, Atwood K, Puleo E, Fletcher R, Colditz G, Emmons K. Colon cancer: risk perceptions and risk communication. J Health Commun. 2004;9: 53–65. [DOI] [PubMed] [Google Scholar]
- 38.Gimeno García AZ. Factors influencing colorectal cancer screening participation. Gastroenterology research and practice. 2012;2012: 483417–483417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Esplen MJ, Cappelli M, Wong J, et al. Development and validation of a brief screening instrument for psychosocial risk associated with genetic testing: a pan-Canadian cohort study. Bmj Open. 2013;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Esplen MJ, Leszcz M, Hunter J, et al. A randomized controlled trial of a supportive expressive group intervention for women with a family history of breast cancer. Psychooncology. 2018;27: 2645–2653. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.