Abstract
Hippocampal injury following neonatal hypoxia-ischemia (HI) leads to memory impairments despite therapeutic hypothermia (TH). In the hippocampus, the expression of Calbindin-1 (Calb1), a Ca2+ buffering protein, increases during post-natal development and decreases with aging and neurodegenerative disorders. Since persistent Ca2+ dysregulation after HI may lead to ongoing injury, persistent changes in hippocampal expression of Calb1 may contribute to memory impairments after neonatal HI. We hypothesized that despite TH, neonatal HI persistently decreases Calb1 expression in the hippocampus, a change associated with memory deficits in the mouse. We induced cerebral HI in C57BL6 mice at P10 with right carotid ligation and 45m of hypoxia (FiO2=0.08), followed by normothermia (36°C, NT) or TH (31°C) for 4h with anesthesia-shams as controls. Nissl staining and GFAP immunohistochemistry (IHC) were used to grade brain injury and astrogliosis at P11, P18, and P40 prior to assessment of Calb1 expression by IHC. The subset of mice followed to P40 also performed memory behavior task (Y-Maze) at P22–26. Non-parametric statistics stratified by sex were applied. In both anterior and posterior coronal brain sections, hippocampal calb1 expression doubles between P11 and P40 due to an increase in the cornus ammonis (CA) field (KW p<0.001) and not the dentate gyrus (DG). Neonatal HI produces delayed (P18) and late (P40) deficits in the expression of Calb1 exclusively in the CA field (KW p=0.02) in posterior brain sections. TH did not attenuate Calb1 deficits after HI. Thirty days after HI injury (P40), GFAP scores in the hippocampus (p<0.001, r=−0.47) and CA field (p<0.001, r=−0.39) of posterior brain sections inversely correlated with their respective Calb1 expressions. Although, mice of both sexes demonstrated deficits in Y-maze testing, including ~40% lower SAP and twice as much total impairment compared to sham (KW p<0.001), only in female mice these deficits correlated with the Calb1 expression in the hippocampal CA field (p<0.05) of posterior sections. Although, hippocampal atrophy after neonatal HI also correlated with worse deficits in Y-maze testing, it did not predict Calb1 deficits. Neonatal HI produces long-lasting Calb1 deficit in the hippocampal CA field during development, which TH does not mitigate. Late Calb1 deficit after HI may be the result of persistent astrogliosis and may lead to memory impairment particularly in female mice.
Keywords: Neonatal brain injury, calbindin, memory, astrogliosis
2. INTRODUCTION
Hypoxia-Ischemia (HI) is one of the most common causes of perinatal brain injury.Complete or partial disruption of blood flow to the brain impairs normal brain function by reducing the delivery of oxygen and glucose necessary for aerobic metabolism [1–3]. Along with the basal ganglia and the cortex, the hippocampus is one of the regions with the highest metabolic demands and vulnerability to HI [3,4]. Harmful increase of intracellular Ca2+ concentrations leads to mitochondrial failure and neuronal death [2,4,5]. Ca2+ dysregulation may persist long after the initial HI insult and leads to neuroinflammation and ongoing injury [6].
Neurons use EF-hand type Ca2+ binding proteins (CBPs) to buffer out excess calcium and achieve homeostasis after an insult [2,7,8]. These CBPs – including calbindin (Calb1) – are also involved in many neuronal functions, such as synaptic transmission, cell signaling, release of neuropeptides, gene expression and activation, and cell death [7,9–12]. Inhibition of Ca2+ influx attenuates neuronal cell damage from ischemic injury [8]. Thus, the relative expression of CBPs, or lack thereof, has an important role in neuronal injury in response to an insult such as HI. Although these proteins often work in conjunction with each other, there are distinct differences in their basic functions. Specifically, Calb1, mainly expressed by GABAergic interneurons of the Cornu Ammonis (CA) subfields of the hippocampus and the granular cells of the dentate gyrus [7,11], has the highest affinity to buffer calcium [11], and has been linked to preservation of hippocampal-dependent memory in the mouse [11,12].
Neonatal HI injures the basal ganglia-thalamus and cortical tracks and results in motor impairments, which therapeutic hypothermia (TH) attenuates [13–16]. However, a significant proportion of survivors of neonatal HI brain injury suffers from cognitive and memory impairments, suggesting that TH does not fully protect the hippocampus, an essential brain region for memory consolidation [7,12,13,16]. Persistent disturbance in Calb1 expression within the hippocampus may play a critical role in the memory outcomes following neonatal HI injury. Because, there is a segregation of the rodent hippocampus along the dorsoventral axis, with dorsal hippocampus (anterior in the mouse) functionally linked to spatial learning and memory [17], the study of the relationship between Calb1 and memory outcomes requires stratification by the level of the hippocampal section in the antero-posterior axis. Here, we aimed to study the expression of hippocampal Calb1 following HI and TH and the link with degree of astrogliosis and memory outcomes in a mouse model. We hypothesize that despite TH, neonatal HI persistently impairs Calb1 expression in the hippocampus, a change linked to memory deficits in the mouse.
3. MATERIALS AND METHODS
Mice
Male and female C57BL6 (Charles River Laboratories, Wilmington, MA, USA) pups were used for these experiments, totaling 215 mice. The experimental protocols used in this study were approved by the Institutional Animal Care and Use Committee at Johns Hopkins University School of Medicine, and were carried out with standards of care and housing in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals, US Department of Health and Human Services 85–23, 2011. All efforts were made to minimize the number of mice used and their suffering.
Neonatal HI Injury and TH
Neonatal HI brain injury of postnatal day (P)10 mice was induced using the Rice-Vannucci model adapted for neonatal mice as previously published (Graham, Mccullough, & Murphy, 2004). In short, at P10 mice were anesthetized with inhaled isoflurane (3% for induction and 1% for maintenance) mixture with nitrous oxide prior to the induction of brain HI by unilateral right carotid artery ligation. Mice were returned to their cages for a 1h resting period which was followed by 45 min of hypoxia at FiO2 = 0.08 in an acrylic glass chamber. After HI, mice were randomized to normothermia (NT) at 36°C or TH at 31°C for 4 h, after which mice were returned to their cages. Surgeries lasted between 3 to 5 min. Mice randomly assigned to the sham (S) group received inhaled isoflurane/ nitrous oxide mixture at P10 for 5 min at similar concentrations as described above for the NT/TH groups and then returned to cages. This mouse model of neonatal HI and TH has been validated by T2 MRI techniques, demonstrating sustained neuroprotection against cerebral and cortical atrophy at 8 and 20 d after HI injury at P10 and lack of sustained protection of the hippocampus [18]. This differential regional protection afforded by TH in this model resulted in protection of motor domains and the lack of protection of memory domains of neurodevelopment [19]. After exclusions (see criteria below), we studied the brains in 3 different cohorts of animals – P11 (S, n=9; NT, n=10; TH, n=11), P18 (S, n= 9; NT, n=7; TH, n=14), and P40 (S, n= 37; NT, n=31; TH, n=35) – for immunohistochemistry (IHC). Mice were killed via exposure to 20% (v/v) mixture of isoflurane in propylene glycol via onedrop exposure method [20] prior to being exsanguinated with 0.1M cold PBS (pH 7.4) via cardiac perfusion in the left ventricle. Brains were perfused with 4% paraformaldehyde (PFA) at 4 ml/h, and post-fixed overnight on 4% PFA prior to being cryoprotected in 30% sucrose in PBS until they sank. All brains were then frozen and stored at −80°C prior to being cut on a freezing microtome at 50μm. Terminology: The use of the term “anterior” or “posterior” reflects the position of the coronal brain section in the antero-posterior axis. For all experiments, we evaluated the hippocampal sections located within anterior and posterior coronal sections of the mouse brain. Coronal brain sections were classified as anterior, if the fasciola cinereum was adjacent to the dorsal third ventricle and before the emergence of the dorsal subiculum. Sections were classified as posterior, if the CA3 subfield crossed the level fasciculus retroflexus, but was positioned prior to the emergence of the dentate gyrus granular cell layer in the ventral portion of the coronal section [9]. Based on the description above, the hippocampus located in our anterior coronal brain sections corresponds to the dorsal/ septal hippocampus, while that located in our posterior coronal brain sections corresponds to the transition between the dorsal/ septal and the ventral/temporal hippocampus. Thus, the hippocampus in posterior coronal sections of the brain do not correspond exclusively the ventral hippocampus, but instead it shows an arrangement of dorsal and ventral hippocampus, in the upper and lower areas, respectively. Inclusion criteria: Hippocampus within anterior and posterior coronal brain sections were assessed for severity of injury using Nissl staining and GFAP scoring [9]. Evidence of early (P11) cell death with nuclear changes characterized by pyknosis, karyorrhexis and karyolysis, apoptotic cell bodies, and delayed (P18 and P40) columnar injury of CA1 and CA3 subfields without obliteration in Nissl staining were included in the analysis. GFAP scores of 3 to 7 per subfield or 9 to 21 in whole hippocampus analysis in IHC suggested injury/ astroglyosis and were included in the analysis [9]. Exclusion criteria: Pups demonstrating either minimal or no injury (2 mice at P11,4 mice at P18, and 5 mice at P40) or obliterated hippocampus (2 mice at P11,4 mice at P18, and 9 mice at P40) were not included in the analysis.
Immunohistochemistry for GFAP and Calb1
For IHC, tissue was fixed with 4% PFA and cryoprotected with 15%/30% sucrose gradient as previously described prior to storage at −80° C and cut at 50pm using a freezing microtome [21]. Floating IHC was performed as previously described [21] with whole rabbit antisera anti-Calb1 (Cell Signaling Technology, Inc, Beverly MA; 1:250), or anti-GFAP (DAKO / Agilent Technologies, Santa Clara, CA; 1:1000) followed by goat anti-rabbit antibody (1:200) used as the secondary antibody and DAB. Cresyl-violet (CV) staining was performed to assess injury at P11, P18 and P40 and for volumetric analysis as described ahead. Antibodies. Calb1 (CS13176; RRID:AB_2687400): Rabbit monoclonal antibody raised against a recombinant protein specific to the N-terminus of whole Calb1 protein of human origin detecting product at 28 kDa (2μg/ml). GFAP (DAKO Z0334; RRID:AB_10013382): Rabbit polyclonal antibody raised against GFAP isolated from cow spinal cord with no reported cross reactivity (1μg/ml).
Analysis of Calb1 immunoreactivity (IR) and hippocampal volume
Calb1 IHC and CV stained slides were imaged using a light microscope (Nikon Eclipse E400, Nikon), to produce high resolution photomicrographs and analyzed using ImageJ 1.8.0 software (NIH, Bethesda MD) (Rasband 1997–2018). Calb1 IR analysis - Using the free draw tool, the whole hippocampus and the DG field were outlined separately. All images were converted to 8-bit images and globally calibrated to optical density (OD). The background was removed from the images to measure the Calb1 staining. The background was obtained by drawing 10 circles free of Calb1 staining, 5 in the lacunosum molecular (LM) layer and 5 in the oriens layer (OL). The average OD score from these 10 background circles were subtracted from the whole hippocampus average OD scores to create the adjusted mean OD for the hippocampus. The mean OD of the CA field was determined by the following formula: CA mean OD = [(mean hip OD*hip area) – (mean DG OD*DG area)] / (hip area - DG area). Thus, the Calb1 optical density (OD) used for analysis and correlations was the average of the ODs measured in every pixel contained within the region of interest (whole hippocampus, CA field or DG). Hippocampal volume analysis - we have calculated residual volumes using the hippocampal areas (mm2) obtained from 5 representative 50 μm CV-stained coronal brain sections positioned 600 μm apart in the antero-posterior axis of the brain at P40. Total residual volume was extrapolated using the following formula: Hippocampal volume (mm3) = Σi=1 (n=5) [Si * 0.05] + Σi=1 (n=4) [(Si + Si+i) * 0.6 / 2], where S = area of hippocampal section in mm2, i= section position in the antero-posterior axis, and n= number of sum repeats.
Behavioral Testing
Those mice in the P40 cohort underwent behavioral testing between p22–26 with Y-Maze before harvesting the brain at P40. Testing was conducted in a noise-free room between i and 6 p.m. The order of testing the mice was randomized for each test within the litters. Each litter had a maximum of 6 pups, with up to 2 litters during each behavioral session. Pups were habituated in the experimental room for 30 min prior to the start testing. All scored tasks were recorded using videos which were reviewed and scored manually, by 2 team members blinded to treatment groups (JGH and DS), and electronically using AnyMaze v5.1 (Stoelting Co. Wood Dale, IL, USA). Mice were returned to their cages following testing. Y-Maze. Y-Maze test was used to assess spatial working memory in rodents. Uninjured mice will choose to investigate a new arm before returning to one recently visited. The apparatus was made of acrylic glass forming a “Y” with 3 identical arms positioned in 120° angles. There are two phases to Y-Maze each lasting 5 min; phase 1 assess working memory and phase 2 assess spatial and recognition memory. During both phases, the mice started the test in the center of the maze. During phase 1, the sequence of arm entries was recorded. Spontaneous alterations performance (SAP) assesses working memory as the mice visit 3 consecutive different arms of the maze. Impaired working memory is determined by higher alternate arm returns (AAR, 3 consecutive arm visits in which the first and third arm visit is that same) and same arm returns (SAR, 2 successive visits to the same arm). Phase 2 was performed 3 days after phase 1. During the pre-recorded phase, mice explore the maze with one arm blocked (novel arm) for 5 min. After a 30 min rest period, all arms were open for the mice to explore the maze once more for 5 min,. The percent of time spent and entries into the novel arm was analyzed.
GFAP-derived scoring to assess astrogliosis
Along with CV staining, GFAP IHC was used to assess degree of astrogliosis as a surrogate hippocampal injury in order to exclude those mice demonstrating minimal or no injury. We used a semi-quantitative GFAP-derived scoring system develop in our laboratory [9], for the purpose described above and to establish correlation between persistent astrogliosis and changes in Calb1 expression. The GFAP-derived scoring system takes into account the dispersion of hippocampal IR and the morphological characteristics of astrocytes [22,23] in the CA1 and CA3 subfields and the DG in the hippocampus positioned in anterior and posterior coronal brain sections. Scoring was based on the abundance of GFAP staining and glial scars, astrocyte body size, number and thickness of their branching, and overlap of astrocytic domains at higher magnification [9] Representative high magnification photomicrographs showing GFAP stained astrocytes within the hippocampal CA1 subfield at P40 are shown (Supplemental fig. 1). The presence of astrocytes with small somas and few or many thin branches without domain overlapping granted 1 or 2 points for the subfield, respectively (Supplemental fig. 1A1 and 1A2). The identification of astrocytes with large somas and many thick branches overlapping domains granted 3 points (Supplemental fig. 1A3), while the presence of astrocytes with even larger soma forming 1, 2, or > 3 glial scars granted 4, 5 or 6 points, respectively (Supplemental fig. 1A4). Lastly, diffuse astrocytic activation thru the subfield granted 7 points. The cumulative score was the sum of the scores for each of the three subfields (CA1, CA3, and DG), which varied between 1 and 7. This resulted in a cumulative score between 3 to 21 for the whole hippocampus, and 2 to 14 for the CA field.
Statistics
Multiple groups where analyzed using non-parametric Kruskal-Wallis H one-way ANOVA stratified by sex with post-hoc pair analysis using Dunn-Bonferroni’s test. All results were presented as box and whisker plot, where the box was limited by the 25th and 75th percentiles and the solid line represented the median. Significance was assigned by p-value ≤ 0.05 adjusted for multiple comparisons in all cases. Non-parametric Spearman Rho correlations were applied between Calb-1 expression, hippocampal residual volume, GFAP score and memory outcome. Analysis was performed using IBM SPSS Statistics 24v (IBM Corporation, Armonk, NY).
4. RESULTS
Developmental changes in Calb1 expression in the hippocampus
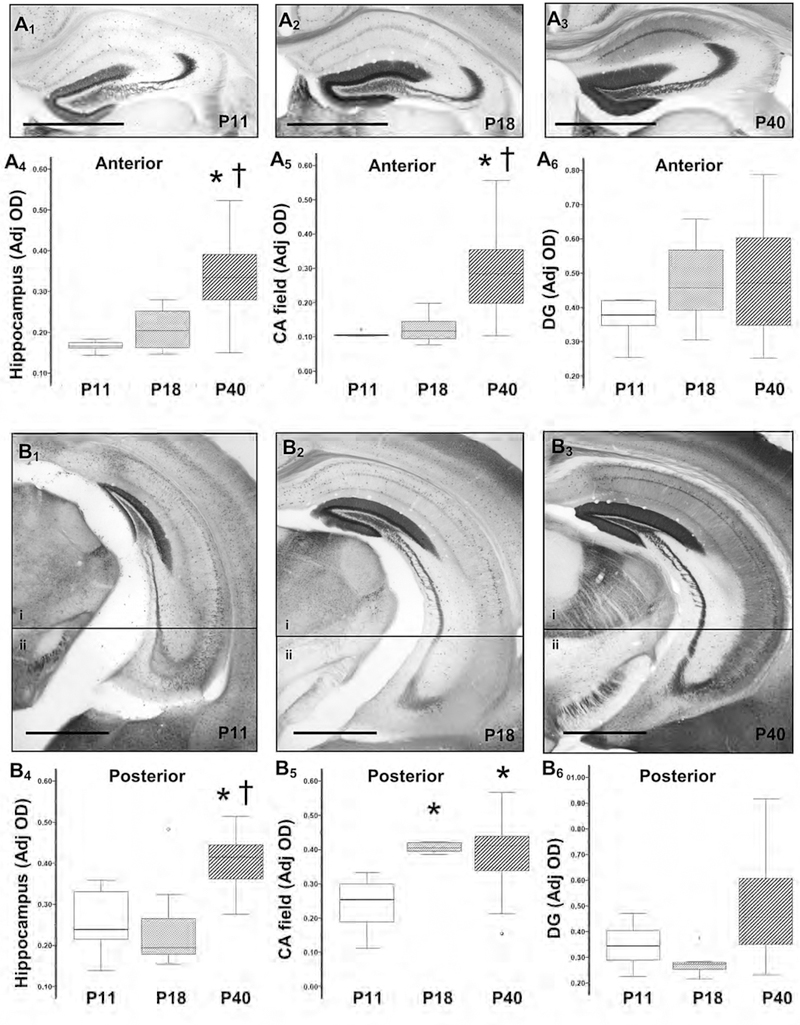
In hippocampal sections located within both, anterior and posterior coronal sections, Calb1 expression increased between P11 and P40. In the hippocampus within anterior coronal sections, Calb1 expression increased from 0.167 a.u. (IQR 1.157 – 0.177) at P11 to 0.204 a.u. (0.161 – 0.263) at P18 and to 0.334 a.u. (0.277 – 0.339) at P40 (Fig 1A1 to 1A4). The expression in the CA field within anterior coronal sections was in average 0.105 a.u (0.103 – 0.110) at P11, 0.117 a.u. (0.936–0.147) at P18 and 0.284 a.u (0.196 – 0.367) at P40 (H 22.7; df 2; KW p<0.001; P11 vs. P40 and P18 vs. P40 adj. p = 0.001, Fig 1A5). Thus, in anterior brain sections, the two-fold increase in hippocampal Calb1 expression between P11 and P40 (H 19.9; df 2; KW p<0.001; P11 vs. P40 adj. p =0.001) was specifically linked to the increase within the CA field (Fig 1A5), and not the DG (Fig 1A6). Similarly, in posterior brain sections, hippocampal Calb1 expression increased from 0.239 a.u. (0.194 – 0.352) at P11 to 0.415 a.u (0.362 – 0.445) at P40 (H 20.4; df 2; KW p<0.001; P11 vs. P40 adj. p=0.002; Fig 1B1 to 1B4). The 75% increase in the hippocampal Calb1 expression between P11 and P40 was dependent on the increase within the CA field of posterior brain sections (H 12.4; df 2; KW p=0.002; P11 vs. P40 adj. p =0.003; Fig 1B5) and not the DG (Fig 1B6). Collectively, the expression of hippocampal Calb1 increased by 54 and 96 % between P11 and P18 (H 29.2; df 2; KW p<0.001; adj. p<0.001) and P18 and P40 (adj. p<0.001), respectively. This changed was linked to the increase in the CA field (H 27.2; df 2; KW p<0.001; P11 vs. P18 and P18 vs. P40 adj. p <0.001; data not shown).
Figure 1. Developmental changes in Calb1 expression in the hippocampus.
Representative Calb1 IHC of hippocampal sections in anterior (A1 to A3) and posterior (B1 to B3) coronal sections at P11 (A1 and B1), P18 (A2 and B2), and P40 (A3 and B3). Bar, 1000 μm. Two photomicrographs (4X) of the hippocampus in the posterior coronal section (i and ii), were used to compose the final figure (B1 to B3). Box and whiskers plot represent mean optical densities (OD) adjusted for background for Calb1 immunoreactivity in anterior and posterior coronal brain sections for the whole hippocampus (A4 and B4, respectively), the CA field (A5 and B5, respectively), and DG (A6 and B6, respectively). Boxes are limited by the 75th and 25th percentiles (interquartile range, IQR) and whiskers are limited by the last data point within 1.5 times the IQR from the median (continuous line inside the box). Analysis by Kruskal-Wallis ANOVA with Dunn-Bonferroni correction; *, adjusted p value <0.05. CA, cornus ammonis; DG, dentate gyrus.
Impaired expression of Calb1 following HI and lack of protection by TH
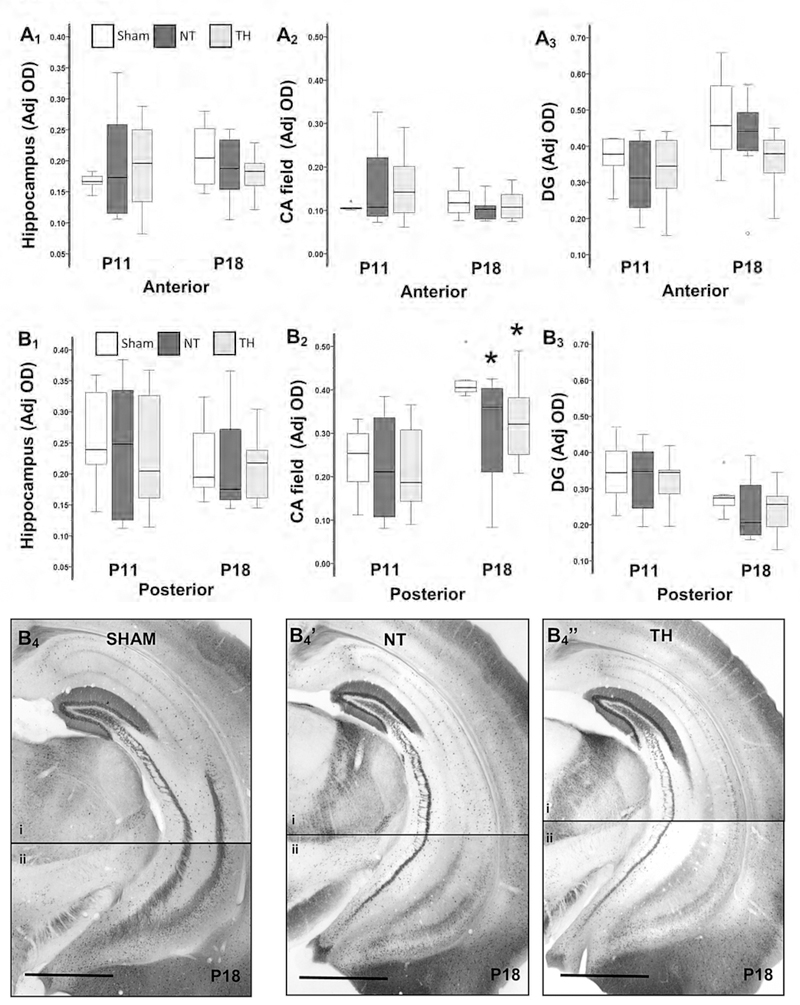
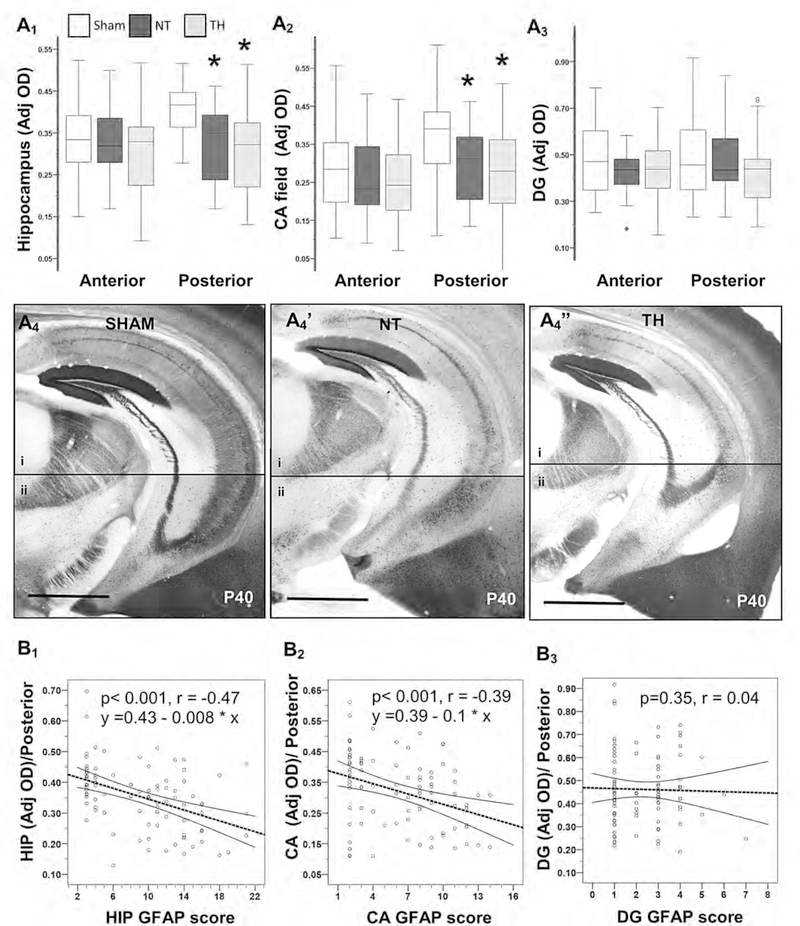
Neonatal HI did not alter the developmental increase in hippocampal Calb1 expression between P11 and P18 in the anterior coronal brain sections (Fig. 2A1 to 2A3). However, deficits in expression of Calb1 are documented in the hippocampus as early as 8 days after injury (P18, Fig. 2B) in posterior brain sections. Specifically, the expression of Calb1 within the CA field was 18% lower 8 days after HI injury (H 7.1; df 2; KW p= 0.02; adj. p = 0.05 vs. sham) and TH did not provide protection (Adj. p = 0.02 vs. sham; Fig 2B2). Impairment in the expression of Calb1 in the hippocampus progressed by P40 (30 days after injury) in posterior brain sections. At P40, hippocampal Calb1 expression in the posterior brain sections of injured mice was 20% lower than the expression in shams (H 22.4; df 2; KW p< 0.001; adj. p ≤ 0.001 vs. sham) and TH did not prevent this deficit (− 24%; adj. p= 0.002 vs. sham, Fig 3A1). The developmental increase in Calb1 expression in the CA field between P11 and P40 (Fig 1B5) was impaired by neonatal HI injury in posterior brain sections (−25%, H 10.8; df 2; KW p= 0.005;. adj. p = 0.03 vs. sham; Fig 3A2). Even those mice treated with TH after HI injury, had 32% lower Calb1 expression in the CA field compared to sham (p=0.008, Fig 3A2). Again, no differences in Cab1 expression were seen in the DG at P40 (Fig 3A3). Calb1 deficits in the hippocampus and CA field were similar in males and females mice in posterior brain sections (Supplemental fig. 2).
Figure 2. Impaired hippocampal expression of Calbi in posterior coronal sections following HI and lack of protection by TH at P18.
Box and whiskers plot represent mean optical densities (OD) adjusted for background for Calb1 immunoreactivity in whole hippocampus (A1 and B1), CA field (A2 and B2), and DG (A3 and B3) in anterior and posterior, respectively. Boxes are limited by the 75th and 25th percentiles (interquartile range, IQR) and whiskers are limited by the last data point within 1.5 times the IQR from the median (continuous line inside the box). Analysis by Kruskal-Wallis ANOVA with Dunn-Bonferroni correction; *, adjusted p-value <0.05. Representative Calb1 IHC section hippocampus within posterior coronal brain sections of sham (B4), NT (B4’), and TH (B4”). Bar, 1000 μm. Two photomicrographs (4X) of the hippocampus (i and ii) within the posterior coronal section were used to compose the final figure (B4). CA, cornus ammonis; DG, Dentate gyrus; NT, normothermia; TH, therapeutic hypothermia.
Figure 3. Persistent Calbi deficits in the hippocampus in posterior coronal sections following HI linked to persistent astrogliosis at P40.
Box and whiskers plot represent mean optical densities (OD) adjusted for background for Calb1 immunoreactivity in whole hippocampus (A1), CA field (A2), and DG (A3) in anterior and posterior coronal sections. Boxes are limited by the 75th and 25th percentiles (interquartile range, IQR) and whiskers are limited by the last data point within 1.5 times the IQR from the median (continuous line inside the box). Analysis by Kruskal-Wallis ANOVA with Dunn-Bonferroni correction; *, adjusted p-value <0.05. Representative Calb1 IHC hippocampal section in posterior brain sections of sham (A4), NT (A4’), and TH (A4”). Bar, 1000 μm. Two photomicrographs (4X) of the hippocampus (i and ii) within posterior coronal sections were used to compose final figure (A4). Calb1 expression in the whole hippocampus (B1), CA field (B2), and DG (B3) of posterior coronal sections were correlated with their respective GFAP score. Spearman correlations were applied. Continuous line represents the predicted linear regression and discontinuous lines represent the projection to the 95% confidence interval. p-value < 0.05 were considered significant. CA, cornus ammonis; DG, Dentate gyrus; GFAP, glial fibrillary acidic protein; HIP, hippocampus; NT, normothermia; Post, posterior; TH, therapeutic hypothermia.
Correlation between persistent astrogliosis and decreased Calb1 expression at P40
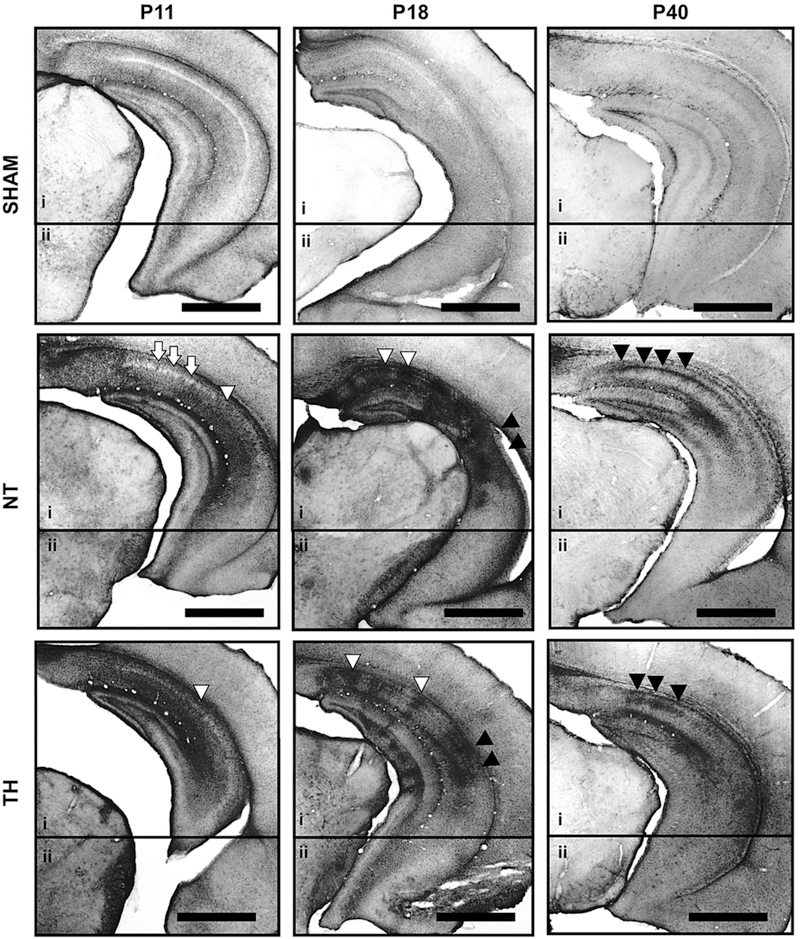
Our previously published GFAP scoring system [9] was applied to grade astrogliosis and correlate with Calb1 expression at P40. Representative photomicrographs showing GFAP staining of the hippocampus within anterior (Supplemental Fig.1B) and the posterior (Fig 4) coronal brain sections at P11, P18 and P40, are shown. The median (IQR) GFAP scores of sham, HI/NT, and HI/TH mice in the hippocampus were in anterior brain sections 3 (3–4), 16 (11–20) and 12 (10–16) [H 68.7; df 2; KW p<0.001], respectively; while in posterior brain sections were 3 (3–4), 14 (10–21) and 12 (10–15) [H 71.5, df 2, KW p<0.001], respectively. Median GFAP scores of sham, HI/NT, and HI/TH mice in the CA field in the anterior sections were 2 (2–3), 12 (8–14), and 9 (7–12) [H 71.6, df 2, KW p<0.001], respectively; while in the posterior sections were 2 (2–3), 11 (7–14), and 9 (8–11) [H 71.4, df 2, KW p<0.001], respectively. The GFAP scores in the hippocampus (Spearman Rho p<0.001, r=−0.47; Fig 3B1) and the CA subfield (Spearman Rho p<0.001, r=−0.39; Fig 3B2) from posterior brain sections inversely correlated with their respective Calb1 expressions at P40. There was no correlation between GFAP score and the Calb1 expression in the DG of posterior sections (Fig 3B2).
Figure 4. Temporal evolution of GFAP immunoreactivity between P11 to P40 in sham and HI-injure mice treated with NT or TH.
Representative GFAP IHC of posterior brain sections containing hippocampi of sham, normothermia (NT), and therapeutic hypothermia (TH). Bar, 1000 μm. Two photomicrographs (4X) of the hippocampus (i and ii) within the posterior coronal section, were used to compose final figure. White arrows, areas of columnar injury or cellular loss within the CA pyramidal cell layer (Py). White arrow heads, glial scars with the CA Py. Black arrow heads, area of diffuse astrogliosis with the CA Py.
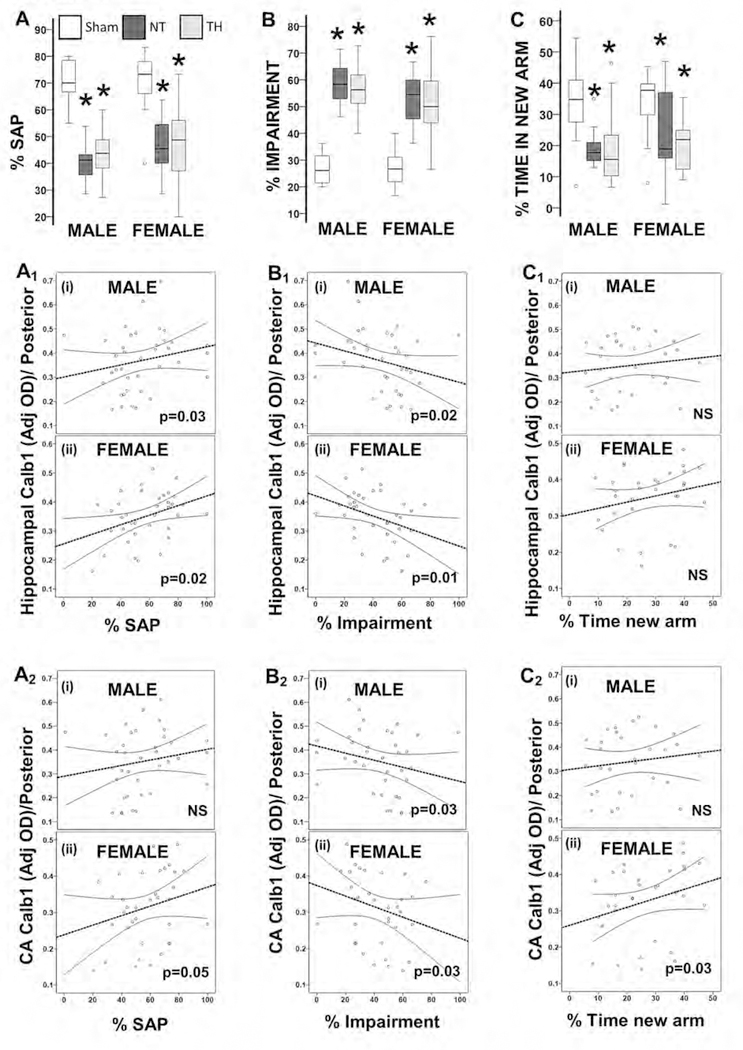
Lower expression of hippocampal Calb1 following neonatal HI and impaired memory
The percent SAP in the Y-maze phase 1 testing was lower in HI-injured male mice [by 40%; H 28.9, df 2, KW p<0.001, adj. p<0.001] and female mice [by 38%; H 19.3, df 2, KW p<0.001, adj. p <0.001] compared to shams (Fig 5A). Accordingly, the percent of AAR was higher in HI-injured male (by 2.6-fold, H 22.6, df 2, KW p<0.001; adj. p<0.001) and female mice (by 1.9-fold, H 23.5, df 2, KW p<0.001, adj. p=0.001) compared to shams (data not shown). These resulted in a 2.2 and 2-fold higher percent of total impairment after HI injury in males and female Hl-injured mice, respectively (KW p<0.001 [both]; Fig 5B) vs. shams. The percent time spent exploring the new arm during phase 2 of the Y-maze was ~50% lower in male (KW p=0.002; adj. p=0.03 vs. sham) and female (KW p=0.02; adj. p= 0.05) injured mice (Fig 5C). TH did not attenuate any of the deficits documented during the Y-maze testing. In general, Calb1 expression in whole hippocampus (Fig 5A1, 5B1 and 5C1) and specifically in the CA field (Fig 5A2, 5B2 and 5C2) within posterior coronal brain sections directly correlated with percent SAP (5A1–2) and time spent exploring the new arm (5C1–2) and inversely correlated with the percent of total impairment (5B1–2). However, the link between Calb1 expression and the performance in the Y-maze was more robust in females than in male mice. Only in female mice Calb1 expression in the CA field of posterior brain sections directly correlated with the percent SAP and time spent exploring the new arm (Fig 5A2 and 5C2). There were no significant correlations between hippocampal Calb1 expression in anterior brain sections and Y-maze performance.
Figure 5. Memory deficits in Y-maze task correlated with Calb1 deficits in the hippocampus of posterior coronal sections following HI at P40.
Box and whiskers plot represent percentage of spontaneous alternation performance (SAP, A), impairment (sum of alternate arm returns and same arm returns, B) and time spend in new arm during Y-maze phase 2 (C), stratified by sex. Boxes are limited by the 75th and 25th percentiles (interquartile range, IQR) and whiskers are limited by the last data point within 1.5 times the IQR from the median (continuous line inside the box). Analysis by Kruskal-Wallis ANOVA with Dunn-Bonferroni correction; *, adjusted p-value <0.05. Calb1 expression in the whole hippocampus (A1, B1 and C1) and CA field (A2, B2 and C2) in posterior coronal sections were correlated with % SAP (A1 and A2), % impairment (B1 and B2), and % time in new arm (C1 and C2) stratified by sex (i, males; ii, females). Spearman correlations were applied. Continuous line represents the predicted linear regression and discontinuous lines represent the projection to the 95% confidence interval. p-value < 0.05 were considered significant. CA, cornus ammonis; NS, nonsignificant; NT, normothermia; TH, therapeutic hypothermia.
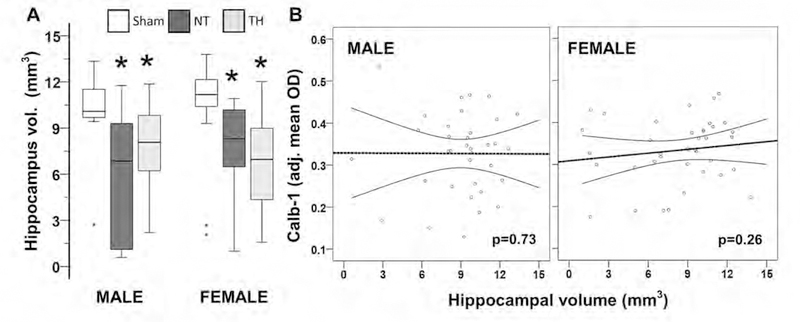
Residual hippocampal volumes, Calb1 expression and memory impairment
Neonatal HI produced a 32% (H 17.5, df 2, KW p<0.001; adj. p<0.001 vs. sham) and 25% (H 16.7, df 2, KW p<0.001; adj. p=0.006 vs. sham) decrease in the hippocampal volume at P40 in male and female mice, respectively. TH did not mitigated hippocampal atrophy (adj. p-value <0.001 [male] and 0.001 [female] vs. sham; Fig 6A). Residual hippocampal volumes did not correlated with hippocampal Calb1 mean OD at P40 (Fig 6B). Nevertheless, residual hippocampal volumes predicted memory outcomes using Y-maze testing. Hippocampal volume directly correlated with percent SAP in the Y-maze phase 1 testing [r=0.45, p=0.02 (males); r=0.47, p=0.01 (females)], percent time spent exploring the new arm during phase 2 of the Y-maze [r=0.39, p=0.03 (female)] and inversely correlated with the percent of total impairment [r=−0.42, p=0.03 (males); r= −0.44, p=0.02 (females)].
Figure 6. Hippocampal atrophy at P40 resulting from neonatal HI at P10 does not correlate with Calb1 deficits.
Box and whiskers plot (A) represent the residual hippocampal volumes in mm3 for of sham, normothermia (NT), and therapeutic hypothermia (TH). Boxes are limited by the 75th and 25th percentiles (interquartile range, IQR) and whiskers are limited by the last data point within 1.5 times the IQR from the median (continuous line inside the box). Analysis by Kruskal-Wallis ANOVA with Dunn-Bonferroni correction; *, adjusted p-value <0.05. Calb1 expression in the whole hippocampus were correlated with residual hippocampal volumes in males and female mice (B). Spearman correlations were applied. Continuous line represents the predicted linear regression and discontinuous lines represent the projection to the 95% confidence interval. p-value < 0.05 were considered significant.
5. DISCUSSION
Here, we show that hippocampal Calb1 expression developmentally increases between P11 and P40 specifically within the CA subfields, and not the DG in the antero-posterior axis of the brain. Neonatal HI produces delayed and late deficits in Calb1 expression exclusively within the CA field of the hippocampus, in posterior, but not in anterior sections of the mouse brain. These persistent deficits in hippocampal Calb1 expression after neonatal HI in posterior sections of the brain are: i) similar in both sexes; ii) inversely correlated with the degree of astrogliosis, iii) not correlated with hippocampal atrophy, and iv) not attenuated by TH. Although, neonatal HI produces deficits in spatial working memory in the Y-maze in both male and female mice, direct correlation with deficits in the expression of Calb1 in the CA subfields is only documented in posterior brain sections and more robustly in female mice at P40. To our knowledge, this is the first report documenting the persistent deficits in the Ca2+ buffering protein Calb1 after neonatal HI, the lack of response to TH, and the correlation with memory deficits.
The developmental increase in hippocampal expression of Calb1 throughout the antero-posterior axis of the brain is specifically linked to the increase within the CA subfields. Our findings are in agreement with the developmental increase of Calb1 described between P6 and P35 in the rat hippocampus [24], as well as between P7 and P22 in other regions of the brain [25]. The increase in Calb1, and other CBPs, is mostly linked to the post-natal maturation of interneurons within the CA subfields and of the mossy fiber system between the DG and the CA3 [9,26–31]. Calb1 plays an important role in protecting the brain against stroke, TBI [32], neurodegenerative disorders [33–37] and aging [38]. In the developing brain massive swifts in intracellular Ca2+ produced by perinatal brain injury or kainic acid administration induces significant hippocampal injury that is attenuated by upregulation of Calb1 expression [39]. Similarly, study of the aging human brain suggests that subtle changes in Ca2+ homeostasis produced by the decrease of CBPs lead to drastic deficits in cognitive function, and increase vulnerability to neurodegeneration [40].
Behavioral, functional, anatomical, and connectivity analysis support the segregation of the hippocampus into anterior, middle and posterior in primates and dorsal, intermediate and ventral in rodents [17,41–45]. While the dorsal hippocampus (positioned anteriorly in rodents and corresponding to the posterior hippocampus in primates) is primarily involved in cognitive functions and retrieval of memories [41,46,47], the ventral hippocampus (mostly positioned posteriorly in rodents and corresponding to the anterior hippocampus in primates) is involved in anxiety and emotions [41]. The function of the intermediate hippocampus is not well defined but appears to overlap the functions of the dorsal and ventral hippocampus and has also a role in spatial learning and memory [41,48]. Identification of the boundaries of the dorsal, intermediate and ventral hippocampus in coronal sections of the mouse brain, as those used for our experiments, is challenging. Based on the anatomical limits used to classified the anterior and posterior coronal brain sections used for our experiments (see methods under “terminology”), we consider that our anterior brain sections indisputably contain sections of the dorsal hippocampus, while our posterior brain sections contain a combination of dorsal and intermediate and less so ventral hippocampus transitioning from the top to bottom of the section, as described by Fanselow and Dong [41]. Here, we show that neonatal HI produces deficits in Calb1 exclusively in the CA subfields of the hippocampus contained in posterior coronal sections, but not in the hippocampus contained in anterior sections of the brain. While in our model the CA subfields are more selectively vulnerable to HI injury than the DG [49], an explanation for the differential expression of Calb1 between hippocampal sections closer to the septal pole (anterior brain sections) and those intermediate between the septal and temporal poles (posterior brain sections), is not obvious. Our analysis of astrogliosis as a surrogate of injury does not suggest differential injury between the hippocampal CA subfields contained in anterior and posterior coronal sections (GFAP scores of 12 and 11, respectively). However, given that the developmental increase in Calb1 in the CA subfields in the anterior brain sections occurs after P18 (Fig 1), while in those in the posterior brain sections occurs before P18; HI injury at P10 most likely would alter the maturational mechanisms leading to Calb1 expression occurring closer to the insult and thus, affecting predominantly the hippocampus in posterior coronal brain sections.
Thirty days after HI, Calb1 deficit within the CA subfields contained in the posterior brain sections directly correlates with worse spatial memory outcomes in our experiments. This link appears to be more robust in female mice compared to male mice and is not dependent on the degree of hippocampal atrophy resulting from HI injury. Although, functionally spatial working memory has been linked to the ventral hippocampus as well [48,50], the strongest evidence [41] suggests that the dorsal and likely intermediate hippocampus (contained within the posterior coronal brain sections) should have the strongest influence in the Y-maze outcomes. However, the lack of correlation between Calb1 expression in the dorsal hippocampal sections (contained within anterior brain sections) and the memory outcomes in our experiments is puzzling. We speculate that the limited variability in Calb1 OD due to the lack of effect of the HI insult compared to sham mice in the most dorsal portions of the hippocampus, does not allow the establishing of a directional correlation with memory outcomes. Alternatively, the intermediate hippocampus may have a more important role in memory outcomes than what previously anticipated.
Decrease in Calb1 and parvalbumin expressing interneurons in the CA subfields of the mouse hippocampus 8d after neonatal HI has been reported recently by our group [9]. Calb1+ interneurons are essential for cognition and memory preservation [51–54]. The GABAergic control of pyramidal cells by hippocampal interneurons allows the preservation of mechanisms of long-term plasticity, a molecular proxy of memory, and thus, GABAergic deficits leads to memory impairments [55]. Evidence between changes in Calb1 and memory outcomes has been extensively described in TBI [56], hippocampal sclerosis [57], and Alzheimer’s disease [58]. Any reduction in the Calb1 expression appears to be critical enough to impair the ability of the hippocampus to function normally [59]. Thus, our current results lead us to speculate that the loss of Calb1 IR is at least in part linked to the decrease of Calb1 + interneurons in the post-natal CA subfields contained in the posterior murine brain. To our knowledge, ours is the first report providing evidence that similar to many other neuropsychiatric and neurodegenerative disorders, neonatal HI may also impair Calb1 expression, but in this case by preventing normal development to proceed.
We evaluated astrogliosis using our semi-quantitative GFAP-derived scoring system recently develop in our laboratory [9]. Here, we report that severity of astrogliosis as determined by the GFAP-derived scoring system, inversely correlated with Calb1 expression in the CA subfields of the posterior brain sections. In this study we are unable to determine, whether persistent astrogliosis directly leads to impaired Calb1 expression in the CA subfields by 30 days after HI, but the severity of HI injury, as suggested by the degree of hippocampal atrophy, does not appear to fully explain Calb1 deficits. Although associations between Calb1 loss and astrogliosis in the hippocampus has been previously described following kainic acid injury [60], ethanol exposure [61], viral infections [62], and even with exposure to radiofrequency [63], the causal mechanism between these two events is still missing and required further studies.
We acknowledge several limitations in our study. We have evaluated the hippocampal sections contained within the anterior and posterior coronal sections of the mouse brain, this allowed us to study well the dorsal hippocampus closest to the septal pole, but not separately the intermediate or the ventral hippocampus, as the hippocampal sections contained in posterior coronal sections are a combination of all of them, as previously published [41]. Thus, the role of the isolated intermediate hippocampus in memory outcomes requires further investigation in association with Calb1 deficits. Additionally, our neonatal HI model produces unilateral injury, and thus compensation from the contralateral (hypoxia-exposed) hemisphere may attenuate the behavioral outcomes. Despite these limitations our laboratory has extensive experience in behavioral testing using this mouse model, which has led to consistent results demonstrating impaired memory outcomes using Y-maze and lack of response to TH [18,19]. Here, we have not analyzed the electrophysiological properties of Calb1+ interneurons within the CA subfields to assess function and alterations of the mechanisms of long-term synaptic plasticity to link Calb1 deficit to memory deficits. Lastly, we acknowledge that we only include mice without obliterated hippocampus due to technical limitations when processing the samples and since HI injury will almost fully eliminate the CA subfields from the analysis.
In conclusion, neonatal HI produces a long-lasting impairment in the increase of Calb1 in the CA subfields of the hippocampus during a critical stage of development and TH does not attenuate the deficit. Late Calb1 deficit correlates with prolonged astrogliosis. Further studies are needed to understand the mechanistic link between deficits in spatial memory and Calb1 expression and the functional role of the intermediate rodent hippocampus.
Supplementary Material
8.1. ACKOWLEDGEMENTS
The authors thank the editorial input from Dr. Frances Northington, the technical support of Mr. Charles Lechner, Armand Sandjeu and Ms. Elizabeth Krisanda and the administrative support of Mrs. Rosie Silva.
8.4. FUNDING SOURCES
The experiments and investigators were funded in parts by grants from the National Institute of Neurological Disorders and Stroke (KO8NS096115 - R.C-V.), the Johns Hopkins University-School of Medicine Clinician Scientist Award (R.C-V.), the Sutland-Pakula Endowment for Neonatal Research (R.C-V.), and by the Doctoral Diversity Program funded by the Health Resource Services Administration (J.G.H).
Footnotes
DISCLOSURE STATEMENT
We confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
STATEMENTS
8.2. STATEMENT OF ETHICS
We confirm that any aspect of the work covered in this manuscript involving experimental animals has been conducted with the ethical approval of all relevant bodies.
APPENDIX
None
SUPPLEMENTARY MATERIAL:
Supplemental figure 1 and supplemental figure 2 submitted separately
9. REFERENCES
- 1.Chen AI, Xiong L-J, Tong YU, Mao M: The neuroprotective roles of BDNF in hypoxic ischemic brain injury. Biomedical Reports 2013;1:167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JM, Grabb MC, Zipfel GJ, Choi DW: Brain tissue responses to ischemia. J Clin Invest 2000;106:723–731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vannucci RC: Hypoxic-ischemic encephalopathy. Am J Perinatol 2000;17:113–120. [DOI] [PubMed] [Google Scholar]
- 4.Novak CM, Ozen M, Burd I: Perinatal Brain Injury: Mechanisms, Prevention, and Outcomes. Clin Perinatol 2018;45:357–375. [DOI] [PubMed] [Google Scholar]
- 5.Mallard C, Tremblay ME, Vexler ZS: Microglia and Neonatal Brain Injury. Neuroscience 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chavez-Valdez R, Flock DL, Martin LJ, Northington FJ: Endoplasmic reticulum pathology and stress response in neurons precede programmed necrosis after neonatal hypoxia-ischemia. Int J Dev Neurosci 2016;48:58–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nikoletopoulou V, Tavernarakis N: Calcium homeostasis in aging neurons. Front Genet 2012;3:200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ouh IO, Kim YM, Gim SA, Koh PO: Focal cerebral ischemic injury decreases calbindin expression in brain tissue and HT22 cells. Lab Anim Res 2013;29:156–161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chavez-Valdez R, Emerson P, Goffigan-Holmes J, Kirkwood A, Martin LJ, Northington FJ: Delayed injury of hippocampal interneurons after neonatal hypoxia-ischemia and therapeutic hypothermia in a murine model. Hippocampus 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Anelli R, Heckman CJ: The calcium binding proteins calbindin, parvalbumin, and calretinin have specific patterns of expression in the gray matter of cat spinal cord. J Neurocytol 2005;34:369–385. [DOI] [PubMed] [Google Scholar]
- 11.Lee YJ, Yan BC, Park JH, Ahn JH, Kim IH, Lee JC, Lee HY, Kim YM, Won MH, Cho JH: Differences of calcium binding proteins immunoreactivities in the young hippocampal CA1 region from the adult following transient ischemic damage. J Neurol Sci 2013;326:40–47. [DOI] [PubMed] [Google Scholar]
- 12.Molinari S, Battini R, Ferrari S, Pozzi L, Killcross AS, Robbins TW, Jouvenceau A, Billard JM, Dutar P, Lamour Y, Baker WA, Cox H, Emson PC: Deficits in memory and hippocampal long-term potentiation in mice with reduced calbindin D28K expression. Proceedings of the National Academy of Sciences of the United States of America 1996;93:8028–8033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azzopardi D, Strohm B, Marlow N, Brocklehurst P, Deierl A, Eddama O, Goodwin J, Halliday HL, Juszczak E, Kapellou O, Levene M, Linsell L, Omar O, Thoresen M, Tusor N, Whitelaw A, Edwards AD, Group TS: Effects of hypothermia for perinatal asphyxia on childhood outcomes. The New England journal of medicine 2014;371:140–149. [DOI] [PubMed] [Google Scholar]
- 14.Derrick M, Drobyshevsky A, Ji X, Tan S: A model of cerebral palsy from fetal hypoxia-ischemia. Stroke 2007;38:731–735. [DOI] [PubMed] [Google Scholar]
- 15.Rocha-Ferreira E, Hristova M: Plasticity in the Neonatal Brain following Hypoxic-Ischaemic Injury. Neural Plast 2016;2016:4901014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shankaran S, Pappas A, McDonald SA, Vohr BR, Hintz SR, Yolton K, Gustafson KE, Leach TM, Green C, Bara R, Petrie Huitema CM, Ehrenkranz RA, Tyson JE, Das A, Hammond J, Peralta-Carcelen M, Evans PW, Heyne RJ, Wilson-Costello DE, Vaucher YE, Bauer CR, Dusick AM, Adams-Chapman I, Goldstein RF, Guillet R, Papile LA, Higgins RD, Eunice Kennedy Shriver NNRN: Childhood outcomes after hypothermia for neonatal encephalopathy. The New England journal of medicine 2012;366:2085–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O’Leary OF, Cryan JF: A ventral view on antidepressant action: roles for adult hippocampal neurogenesis along the dorsoventral axis. Trends in pharmacological sciences 2014;35:675–687. [DOI] [PubMed] [Google Scholar]
- 18.Burnsed JC, Chavez-Valdez R, Hossain MS, Kesavan K, Martin LJ, Zhang J, Northington FJ: Hypoxia-ischemia and therapeutic hypothermia in the neonatal mouse brain--a longitudinal study. PloS one 2015;10:e0118889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Diaz J, Abiola S, Kim N, Avaritt O, Flock D, Yu J, Northington FJ, Chavez-Valdez R: Therapeutic Hypothermia Provides Variable Protection against Behavioral Deficits after Neonatal Hypoxia-Ischemia: A Potential Role for Brain-Derived Neurotrophic Factor. Dev Neurosci 2017;39:257–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Markovic SN, Murasko DM: Anesthesia inhibits interferon-induced natural killer cell cytotoxicity via induction of CD8+ suppressor cells. Cell Immunol 1993;151:474–480. [DOI] [PubMed] [Google Scholar]
- 21.Northington FJ, Koehler RC, Traystman RJ, Martin LJ: Nitric oxide synthase 1 and nitric oxide synthase 3 protein expression is regionally and temporally regulated in fetal brain. Dev Brain Res 1996;95:1–14. [DOI] [PubMed] [Google Scholar]
- 22.Pekny M, Nilsson M: Astrocyte activation and reactive gliosis. Glia 2005;50:427–434. [DOI] [PubMed] [Google Scholar]
- 23.Sofroniew MV, Vinters HV: Astrocytes: biology and pathology. Acta Neuropathol 2010;119:7–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rami A, Lomri N, Brehier A, Thomasset M, Rabie A: Effects of altered thyroid states and undernutrition on the calbindin-D28K (calcium-binding protein) content of the hippocampal formation in the developing rat. Brain Res 1989;485:20–28. [DOI] [PubMed] [Google Scholar]
- 25.Lema Tome CM, Miller R, Bauer C, Smith C, Blackstone K, Leigh A, Busch J, Turner CP: Decline in age-dependent, MK801-induced injury coincides with developmental switch in parvalbumin expression: somatosensory and motor cortex. Dev Psychobiol 2008;50:665–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Salmaso N, Jablonska B, Scafidi J, Vaccarino FM, Gallo V: Neurobiology of premature brain injury. Nature neuroscience 2014;17:341–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abraham H, Veszpremi B, Kravjak A, Kovacs K, Gomori E, Seress L: Ontogeny of calbindin immunoreactivity in the human hippocampal formation with a special emphasis on granule cells of the dentate gyrus. Int J Dev Neurosci 2009;27:115–127. [DOI] [PubMed] [Google Scholar]
- 28.Freund TF, Buzsaki G: Interneurons of the hippocampus. Hippocampus 1996;6:347–470. [DOI] [PubMed] [Google Scholar]
- 29.Gulyas AI, Freund TF: Pyramidal cell dendrites are the primary targets of calbindin D28k-immunoreactive interneurons in the hippocampus. Hippocampus 1996;6:525–534. [DOI] [PubMed] [Google Scholar]
- 30.Gulyas AI, Toth K, Danos P, Freund TF: Subpopulations of GABAergic neurons containing parvalbumin, calbindin D28k, and cholecystokinin in the rat hippocampus. The Journal of comparative neurology 1991;312:371–378. [DOI] [PubMed] [Google Scholar]
- 31.Ludkiewicz B, Wojcik S, Spodnik E, Domaradzka-Pytel B, Klejbor I, Morys J: Cholinergic innervation of parvalbumin- and calbindin-containing neurones in the hippocampus during postnatal development of the rat brain. Folia morphologica 2002;61:89–96. [PubMed] [Google Scholar]
- 32.Dell’Anna E, Geloso MC, Magarelli M, Molinari M: Development of GABA and calcium binding proteins immunoreactivity in the rat hippocampus following neonatal anoxia. Neurosci Lett 1996;211:93–96. [DOI] [PubMed] [Google Scholar]
- 33.Chance SA, Walker M, Crow TJ: Reduced density of calbindin-immunoreactive interneurons in the planum temporale in schizophrenia. Brain Res 2005;1046:32–37. [DOI] [PubMed] [Google Scholar]
- 34.Soto-Ortolaza AI, Behrouz B, Wider C, Vilarino-Guell C, Heckman MG, Aasly JO, Mark Gibson J, Lynch T, Jasinska-Myga B, Krygowska-Wajs A, Opala G, Barcikowska M, Czyzewski K, Uitti RJ, Wszolek ZK, Farrer MJ, Ross OA: Calbindin-1 association and Parkinson’s disease. Eur J Neurol 2010;17:208–211. [DOI] [PubMed] [Google Scholar]
- 35.Kook SY, Jeong H, Kang MJ, Park R, Shin HJ, Han SH, Son SM, Song H, Baik SH, Moon M, Yi EC, Hwang D, Mook-Jung I: Crucial role of calbindin-D28k in the pathogenesis of Alzheimer’s disease mouse model. Cell Death Differ 2014;21:1575–1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iritani S, Niizato K, Emson PC: Relationship of calbindin D28K-immunoreactive cells and neuropathological changes in the hippocampal formation of Alzheimer’s disease. Neuropathology 2001;21:162–167. [DOI] [PubMed] [Google Scholar]
- 37.Stefanits H, Wesseling C, Kovacs GG: Loss of Calbindin immunoreactivity in the dentate gyrus distinguishes Alzheimer’s disease from other neurodegenerative dementias. Neurosci Lett 2014;566:137–141. [DOI] [PubMed] [Google Scholar]
- 38.Moyer JR Jr., Furtak SC, McGann JP, Brown TH: Aging-related changes in calcium-binding proteins in rat perirhinal cortex. Neurobiol Aging 2011;32:1693–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hilton GD, Ndubuizu A, Nunez JL, McCarthy MM: Simultaneous glutamate and GABA(A) receptor agonist administration increases calbindin levels and prevents hippocampal damage induced by either agent alone in a model of perinatal brain injury. Brain Res Dev Brain Res 2005;159:99–111. [DOI] [PubMed] [Google Scholar]
- 40.Bu J, Sathyendra V, Nagykery N, Geula C: Age-related changes in calbindin-D28k, calretinin, and parvalbumin-immunoreactive neurons in the human cerebral cortex. Experimental neurology 2003;182:220–231. [DOI] [PubMed] [Google Scholar]
- 41.Fanselow MS, Dong HW: Are the dorsal and ventral hippocampus functionally distinct structures? Neuron 2010;65:7–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ding SL, Van Hoesen GW: Organization and Detailed Parcellation of Human Hippocampal Head and Body Regions Based on a Combined Analysis of Cyto- and Chemoarchitecture. The Journal of comparative neurology 2015;523:2233–2253. [DOI] [PubMed] [Google Scholar]
- 43.Bannerman DM, Sprengel R, Sanderson DJ, McHugh SB, Rawlins JN, Monyer H, Seeburg PH: Hippocampal synaptic plasticity, spatial memory and anxiety. Nature reviews Neuroscience 2014;15:181–192. [DOI] [PubMed] [Google Scholar]
- 44.Strange BA, Witter MP, Lein ES, Moser EI: Functional organization of the hippocampal longitudinal axis. Nature reviews Neuroscience 2014;15:655–669. [DOI] [PubMed] [Google Scholar]
- 45.Dandolo LC, Schwabe L: Time-dependent memory transformation along the hippocampal anterior-posterior axis. Nat Commun 2018;9:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Duarte IC, Castelhano J, Sales F, Castelo-Branco M: The anterior versus posterior hippocampal oscillations debate in human spatial navigation: evidence from an electrocorticographic case study. Brain Behav 2016;6:e00507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duarte IC, Ferreira C, Marques J, Castelo-Branco M: Anterior/posterior competitive deactivation/activation dichotomy in the human hippocampus as revealed by a 3D navigation task. PloS one 2014;9:e86213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ferbinteanu J, Ray C, McDonald RJ: Both dorsal and ventral hippocampus contribute to spatial learning in Long-Evans rats. Neurosci Lett 2003;345:131–135. [DOI] [PubMed] [Google Scholar]
- 49.Hatanpaa KJ, Raisanen JM, Herndon E, Burns DK, Foong C, Habib AA, White CL 3rd: Hippocampal sclerosis in dementia, epilepsy, and ischemic injury: differential vulnerability of hippocampal subfields. Journal of neuropathology and experimental neurology 2014;73:136–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rudy JW, Matus-Amat P: The ventral hippocampus supports a memory representation of context and contextual fear conditioning: implications for a unitary function of the hippocampus. Behavioral neuroscience 2005;119:154–163. [DOI] [PubMed] [Google Scholar]
- 51.Ahn JH, Chen BH, Yan BC, Park JH, Kang IJ, Lee TK, Cho JH, Shin BN, Lee JC, Jeon YH, Hong S, Lee YJ, Choi SY, Won MH: Effects of longterm scopolamine treatment on cognitive deficits and calcium binding proteins immunoreactivities in the mouse hippocampus. Mol Med Rep 2018;17:293–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Muhia M, Yee BK, Feldon J, Markopoulos F, Knuesel I: Disruption of hippocampus-regulated behavioural and cognitive processes by heterozygous constitutive deletion of SynGAP. The European journal of neuroscience 2010;31:529–543. [DOI] [PubMed] [Google Scholar]
- 53.Hartwich K, Pollak T, Klausberger T: Distinct firing patterns of identified basket and dendrite-targeting interneurons in the prefrontal cortex during hippocampal theta and local spindle oscillations. The Journal of neuroscience : the official journal of the Society for Neuroscience 2009;29:9563–9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Potier B, Krzywkowski P, Lamour Y, Dutar P: Loss of calbindin-immunoreactivity in CA1 hippocampal stratum radiatum and stratum lacunosum-moleculare interneurons in the aged rat. Brain research 1994;661:181–188. [DOI] [PubMed] [Google Scholar]
- 55.Lamsa K, Lau P: Long-term plasticity of hippocampal interneurons during in vivo memory processes. Curr Opin Neurobiol 2018;54:20–27. [DOI] [PubMed] [Google Scholar]
- 56.Sun D, McGinn MJ, Zhou Z, Harvey HB, Bullock MR, Colello RJ: Anatomical integration of newly generated dentate granule neurons following traumatic brain injury in adult rats and its association to cognitive recovery. Experimental neurology 2007;204:264–272. [DOI] [PubMed] [Google Scholar]
- 57.Martinian L, Catarino CB, Thompson P, Sisodiya SM, Thom M: Calbindin D28K expression in relation to granule cell dispersion, mossy fibre sprouting and memory impairment in hippocampal sclerosis: a surgical and post mortem series. Epilepsy research 2012;98:14–24. [DOI] [PubMed] [Google Scholar]
- 58.Odero GL, Oikawa K, Glazner KA, Schapansky J, Grossman D, Thiessen JD, Motnenko A, Ge N, Martin M, Glazner GW, Albensi BC: Evidence for the involvement of calbindin D28k in the presenilin 1 model of Alzheimer’s disease. Neuroscience 2010;169:532–543. [DOI] [PubMed] [Google Scholar]
- 59.Emmanuele V, Garcia-Cazorla A, Huang H-B, Qoku J, Dorado B, Cortes EP, Engelstad K, De Vivo DC, DiMauro S, Bonilla E, Tanji K: Decreased hippocampal expression of calbindin D28K and cognitive impairment in MELAS. Journal of the Neurological Sciences 2012;317:29–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Abad S, Junyent F, Auladell C, Pubill D, Pallas M, Camarasa J, Escubedo E, Camins A: 3,4-Methylenedioxymethamphetamine enhances kainic acid convulsive susceptibility. Progress in neuro-psychopharmacology & biological psychiatry 2014;54:231–242. [DOI] [PubMed] [Google Scholar]
- 61.Satriotomo I, Miki T, Itoh M, Ameno K, Ijiri I, Takeuchi Y: Short-term ethanol exposure alters calbindin D28k and glial fibrillary acidic protein immunoreactivity in hippocampus of mice. Brain research 2000;879:55–64. [DOI] [PubMed] [Google Scholar]
- 62.Verdes JM, de Sant’Ana FJ, Sabalsagaray MJ, Okada K, Calliari A, Morana JA, de Barros CS: Calbindin D28k distribution in neurons and reactive gliosis in cerebellar cortex of natural Rabies virus-infected cattle. J Vet Diagn Invest 2016;28:361–368. [DOI] [PubMed] [Google Scholar]
- 63.Maskey D, Pradhan J, Aryal B, Lee CM, Choi IY, Park KS, Kim SB, Kim HG, Kim MJ: Chronic 835-MHz radiofrequency exposure to mice hippocampus alters the distribution of calbindin and GFAP immunoreactivity. Brain research 2010;1346:237–246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.