Abstract
Purpose
To evaluate the clinical characteristics, histopathology, and treatment outcomes in adult and pediatric patients with nonspecific orbital inflammation (NSOI).
Methods
This retrospective study evaluates 76 patients with NSOI. The patients were categorized in 9 groups according to the site of involvement and histopathology results. These groups included: anterior involvement, dacryoadenitis, myositis, perineural involvement, acute fat involvement, focal mass, orbital apex involvement, diffuse sclerosing form, and multiple tissue involvement. The course of the disease was categorized as acute, subacute, or chronic. The cases with symptom duration of less than 1 week were classified as acute, 1 week to 1 month as subacute, and more than 1 month as chronic.
Results
36 (47.4%) patients were males. The mean age was 41.68 ± 17.62 (6–75) years. The most common signs and symptoms were periorbital pain, periorbital edema, decreased ocular movements or diplopia, and conjunctival injection. The most common group was dacryoadenitis in 29 (38.1%) cases. The most common form of disease was the acute involvement (50% of patients). Most of the patients were treated by oral corticosteroids. Duration of follow-up was 7.17 ± 6.26 months. Recurrence occurred in 9 (11.8%) of patients during the follow-up period.
Conclusions
This study presents a new categorization in which multiple tissue involvements were separated. Some of the NSOI features differ between adults and children. In most patients, treatment especially with corticosteroids, resolves the clinical findings.
Keywords: Idiopathic orbital inflammation, Clinical, Histopathology, Orbital pseudotumor, Pediatric
Introduction
The terms ‘nonspecific orbital inflammation (NSOI)’ and ‘orbital pseudotumor’ were used to explain an orbital nongranulomatous inflammatory condition with unknown etiology and usual spontaneous recovery. This term was first used in 1905 by Birch-Hirchfield.1 This condition is presently assumed to be a benign, space-occupying, and noninfectious lesion, and its diagnosis is made after cautious examinations to exclude other disorders such as orbital tumors and systemic causes of inflammatory mass lesions.2 It accounts for 6%–16% of all orbital lesions and nearly 10% of all orbital tumors and is a common disorder needing orbital biopsy.3, 4, 5, 6, 7, 8 This condition is more common among people with ages between 30 and 60 years, especially middle-aged females.3, 4, 5, 6, 9 Several orbital signs and symptoms are common among these patients.3, 9, 10
Also, according to previous studies, NSOI is much less common among pediatric patients, and some of its presenting signs and symptoms vary from NSOI in adults in that affected children usually suffer from bilateral disease and also more constitutional signs and symptoms.3, 11, 12
Since no previous study has been reported about the clinical characteristics, histopathology, and treatment outcomes in adult and pediatric patients with NSOI in Iran, this study aimed to evaluate these issues.
Methods
This retrospective study evaluates 76 patients with NSOI. The study was done at Farabi Eye Hospital, Tehran, Iran between September 2008 and April 2015. Approval for the study was achieved from the Tehran University of Medical Sciences Research and Ethics Committee, and this research adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Patients with the diagnosis of NSOI [by an oculoplastic surgeon (B.E.)] as a noninfectious, inflammatory, and benign disorder of the orbit without any known local (orbital) or systemic cause were enrolled.4, 9 Suspicious systemic inflammatory patients were evaluated by a rheumatologist, and if proven, they were excluded from the study. Infectious causes were excluded by signs and symptoms and, in some cases, by laboratory data or response to antibiotics. Patients ≤18 years old were categorized as the pediatric group.
The NSOI patients were categorized in 9 groups according to the site of involvement and histopathology results. These groups included: anterior involvement (involvement of lid, conjunctiva, tenon, or sclera), dacryoadenitis (involvement of lacrimal gland), myositis (involvement of one or more of extraocular muscles either unilaterally or bilaterally), perineural involvement, acute fat involvement (as an acute process), focal mass (as a single mass with sharp margins), orbital apex involvement, diffuse sclerosing form (as a fibrosing infiltrative mass with blunt margins), and multiple tissue involvement (more than one of the mentioned sites were involved). In addition, the course of the disease was categorized as acute, subacute, or chronic. The cases with symptom duration of less than 1 week were classified as acute, 1 week to 1 month as subacute, and more than 1 month as chronic. Also, the pathologic findings were used for this classification.
All of the patients underwent complete ocular examination including visual acuity, intraocular pressure, pupil reaction, and relative afferent pupillary defect, extraocular muscle movements, external, slit-lamp, and fundus examinations. Information about the patients’ age, signs and symptoms, concurrent systemic diseases, and disease chronicity were also recorded. Imaging findings of the patients were also collected [either computed tomography (CT) or magnetic resonance imaging (MRI)]. Biopsy was done in patients with atypical findings or those who were unresponsive to treatments. Also, in diffuse or fibrosing involvement cases or recurrent cases, biopsy was done.
Treatment methods were chosen according to several parameters such as severity of clinical or paraclinical findings, response to treatment, patient's age, recurrence, and rheumatologic consults, and the patients were followed up during the treatment and after it (unless the patient him/herself did not follow the treatment or examinations). Type and duration of treatment were registered. The initial treatment was tapering dose of oral steroids and began at 1 mg/kg/day which was administered for two to four months and was tapered slowly. In the situation of recurrence, especially in the setting of pain progression, the treatment was begun with steroids according to the initial protocol, but sometimes the dosage was increased, and the rate of tapering was slowed down. Immunomodulatory therapy (IMT) was administrated for steroid resistant cases. Resection and debulking were used for focal masses, fibrosing dacryoadenitis and other fibrosing cases. Actually, the surgical treatment was used in cases with a long time, non-progressive fibrosing involvement. The main goal of the surgery was volume reduction because other medical treatments were used for decreasing the inflammatory situation.
Statistical analyses were done using SPSS version 22 (SPSS Corp, Armonk, NY). The independent T-test was used for the comparison of quantitative data between groups, and Fisher's exact test was used for qualitative data.
Results
Demographic characteristics
76 NSOI patients were enrolled in this study. 36 (47.4%) of patients were males, and 40 (52.6%) were females. The mean age was 41.68 ± 17.62 (6–75) years (Table 1).
Table 1.
Demographic characteristics and disease features.
| Adult group | Pediatric group | All of the patients | Comparison between adult and pediatric patients (P-value) | |
|---|---|---|---|---|
| Number | 66 | 10 | 76 | |
| Age (years) | 46.00 ± 14.57 | 13.20 ± 4.02 | 41.68 ± 17.62 | |
| Sex | ||||
| Male (number/%) | 30/45.5 | 6/60.0 | 36/47.4 | 0.503 |
| Female (number/%) | 36/54.5 | 4/40.0 | 40/52.6 | |
| Laterality | ||||
| Unilateral (number/%) | 59/89.4 | 8/80.0 | 67/88.2 | 0.337 |
| Bilateral (number/%) | 7/10.6 | 2/20.0 | 9/11.8 | |
| Disease chronicity | ||||
| Acute (number/%) | 33/50.0 | 5/50.0 | 38/50.0 | 0.979 |
| Subacute (number/%) | 8/12.1 | 1/10.0 | 9/11.8 | |
| Chronic (number/%) | 25/37.9 | 4/40.0 | 29/38.2 | |
Unilateral involvement was more common (77 cases, 88.2%). The most common form of disease was the acute involvement (50% of patients). No significant difference was found between adults and pediatrics in the demographic variables (except their ages).
Clinical characteristics
The rate of ophthalmic signs and symptoms on presentation is summarized in Table 2. The most common signs and symptoms were periorbital pain, periorbital edema, decreased ocular movements or diplopia, and conjunctival injection. The number of the patients in each group is shown in Table 3. The most common group was dacryoadenitis in 29 (38.1%) of the cases.
Table 2.
Ophthalmic signs and symptoms at presentation.
| Adult group (Number/%) | Pediatric group (Number/%) | All patients (Number/%) | Comparison between adult and pediatric patients (P-value) | |
|---|---|---|---|---|
| Periorbital edema | 41/62.1 | 7/70.0 | 48/63.2 | 0.737 |
| Ptosis | 17/25.8 | 3/30.0 | 20/26.3 | 0.717 |
| Periorbital pain | 48/72.7 | 6/60.0 | 54/71.1 | 0.462 |
| Decreased ocular movements or diplopia | 25/37.9 | 3/30.0 | 28/36.8 | 0.737 |
| Dystopia | 2/3.0 | 0/0 | 2/2.6 | 0.753 |
| Proptosis | 17/25.8 | 2/20.0 | 19/25.0 | 0.520 |
| Conjunctival injection | 27/40.9 | 1/10.0 | 28/36.8 | 0.082 |
| Chemosis | 10/15.2 | 1/10.0 | 11/14.5 | 0.556 |
| Periorbital hyperemia | 6/9.1 | 0/0 | 6/7.9 | 0.416 |
| Visual loss | 10/15.2 | 0/0 | 10/13.2 | 0.341 |
| Palpable mass | 10/15.2 | 3/30.0 | 13/17.1 | 0.361 |
| Optic disc pallor | 2/3.0 | 0/0 | 2/2.6 | 0.753 |
| Optic disc edema and hyperemia | 3/4.5 | 0/0 | 3/3.9 | 0.651 |
| Uveitis | 1/1.5 | 0/0 | 1/1.3 | 0.868 |
| Choroidal folds | 2/3.0 | 0/0 | 2/2.6 | 0.753 |
Table 3.
Categorization of the site of involvement according to imaging and histopathology results.
| Number (%) of patients | |
|---|---|
| Anterior involvement | 11 (14.4) |
| Dacryoadenitis | 29 (38.1) |
| Myositis | 8 (10.5) |
| Optic nerve involvement | 2 (2.6) |
| Fat involvement | 3 (3.9) |
| Focal mass | 2 (2.6) |
| Orbital apex involvement | 1 (1.3) |
| Diffuse sclerosing form | 6 (7.8) |
| Multi-tissue involvement | 14 (18.4) |
Recurrence occurred in 9 (11.8%) patients. Two of these recurrent cases occurred after successful treatment and remission (in two lacrimal gland involvement cases), and the others occurred during tapering of the treatment (in 1 myositis and 6 dacryoadenitis cases). No significant differences were seen between males and females and among patients with unilateral or bilateral involvement in the recurrence rate (P = 0.568 and 0.288, respectively).
Associations
Concurrent systemic diseases were found in 6 adult patients, including 1 case of flu (as a concurrent trigger), 2 cases of asthma, 2 cases of colitis, and 1 case of severe anemia. Palpebral xanthogranuloma was diagnosed by biopsy in 2 cases. In these cases, the anterior orbital biopsy was reported as xanthogranuloma, and the lacrimal gland biopsy was reported as NSOI. Five adults (7.6%) showed clinical or imaging signs of sinusitis. In the pediatric group, no systemic illness was found, but sinusitis was diagnosed in 1 case (10%).
Misdiagnosis as orbital cellulitis and the treatment with antibiotics as the first step of treatment was found in 1 adult patient (1.5%) and 2 pediatric patients (20%).13
Imaging findings
An initial imaging was done in 73 patients. CT was done for 63 patients (82.9%), MRI for 4 patients (5.3%), and both modalities for 6 patients (7.9%). Imaging findings are summarized in Table 4. The most common site of involvement either in adults or in pediatrics was the lacrimal gland. The most commonly involved extraocular muscle was lateral rectus (15 cases, 68.2%). The other involved muscles were superior (12 cases, 54.5%), inferior (5 cases, 22.7%), and medial (4 cases, 18.2%) recti muscles. Inflammatory involvement of more than one muscle was found in 9 cases (11.8%), 7 of which were associated with multi-tissue involvement in the orbit. Eight cases were categorized as pure myositis. Two of them were bilateral, and the most commonly involved muscle was lateral rectus in 7 patients (87.5%).
Table 4.
The most common locations of inflammatory involvement according to imaging findings (one or more locations may be involved in each patient).
| Adult group (Number/%) | Pediatric group (Number/%) | All patients (Number/%) | Comparison between adult and pediatric patients (P-value) | |
|---|---|---|---|---|
| Lacrimal gland | 34/51.5 | 6/60.0 | 40/52.6 | 0.740 |
| Extraocular muscles | 19/28.8 | 3/30.0 | 22/28.9 | 0.601 |
| Optic nerve | 2/3.0 | 0/0 | 2/2.6 | 0.753 |
| Tenon or sclera | 9/13.6 | 0/0 | 9/11.8 | 0.597 |
Lacrimal gland involvement was diagnosed according to the gland enlargement and tissue molding around the gland. Focal cases were categorized according to their sharp margins. Acute cases were diagnosed regarding increased density of the involved tissue, and fibrosing cases had isodense, blunt margins. Optic nerve involvement was not so common, and we diagnosed it in two cases as an infiltrative mass around the optic nerve.
Biopsy
Biopsy was done in 37 patients (48.7%). In 30 of them, the histopathologic evaluation included cellular infiltrate (inflammatory cells especially lymphocytes, plasma cells, eosinophils, and neutrophils), and the stromal component were consistent with classical orbital pseudotumor findings. The patients were categorized according to the type of cellular infiltrates and amount of fibrosis. Pathologic evaluation revealed no necrosis or granuloma in our NSOI patients. Rare finding included: one case of angiomatous NSOI (around the optic nerve) and two cases of xanthogranuloma and NSOI. No surgical complication or sequel was seen in the patients. Biopsy results were positive in 31 adults and 6 pediatrics, and there was no significant difference between groups according to pathologic findings (P = 0.710).
Treatment and follow-up
Steroid therapy was done via three different routes: oral corticosteroids (68.4%), intravenous steroids (7.9%), and local steroid injection (18.4%). The tapering dose of oral steroids began at 1 mg/kg/day. The IMT included mycophenolate mofetil (n = 1, 1.3%), cyclosporine (n = 1, 1.3%), and rituximab (n = 1, 1.3%). IMT was administrated for one dacryoadenitis, one multiple tissue involvement, and one fibrosing cases. Debulking was used in some cases with focal fibrosing masses (such as a case of bilateral lid involvement), and no other treatments were added. Also, in some cases of non-progressive chronic dacryoadenitis and inappropriate appearance due to mass protrusion, after several months of medical treatment, debulking was used (Table 5).
Table 5.
Frequency of different treatment methods among the patients and the follow-up time of the patients.
| Adult group (Number/%) | Pediatric group (Number/%) | All patients (Number/%) | |
|---|---|---|---|
| Oral corticosteroids | 45/68.2 | 7/70 | 52/68.4 |
| Intravenous steroids | 6/9.1 | 0/0 | 6/7.9 |
| Local steroid injection | 11/16.7 | 3/30 | 14/18.4 |
| Steroid sparing immunomodulatory therapy | 3/4.5 | 0/0 | 3/3.9 |
| Orbital decompression | 1/1.5 | 0/0 | 1/1.3 |
| Resection of the lesion | 3/4.5 | 1/10 | 3/3.9 |
| Debulking of the lesion | 6/9.1 | 4/40 | 10/13.2 |
| Duration of follow-up (months) | 7.19 ± 6.13 | 7.05 ± 7.47 | 7.17 ± 6.26 |
| Recurrence | 7/10.6 | 2/20 | 9/11.8 |
Duration of follow-up was 7.17 ± 6.26 months (range, 0.5–30 months). The information about treatment and follow-up are summarized in Table 5.
Case reports
Case 1
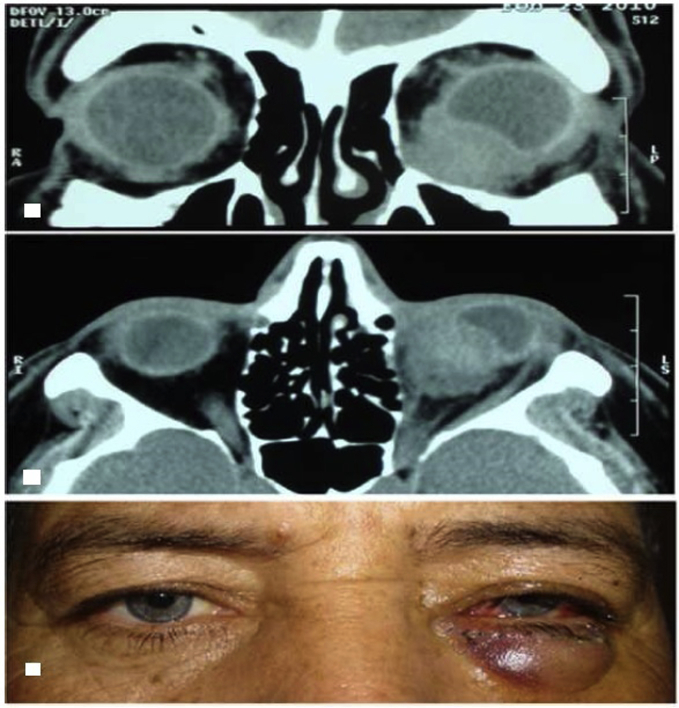
One of the focal mass cases was a 57-year-old male with a history of unilateral painless red eye, decreased visual acuity, and diplopia since 2 weeks earlier. On ophthalmic examination, superior displacement of the globe (hyperophthalmos), mild chemosis, conjunctival injection, and lower lid edema were noted. Orbital CT revealed an infraorbital single focal mass which indented the globe (Fig. 1a, b). Surgical debulking of the lesion was done (Fig. 1c), and on histopathologic examination, fibrosing (sclerosing) orbital pseudotumor was reported.
Fig. 1.
Case number 1. Top. Orbital computed tomography (CT), coronal view demonstrating the indentation of the globe by the focal inflammatory mass. Middle. Orbital CT, axial view. Bottom. The patient's appearance immediately after debulking.
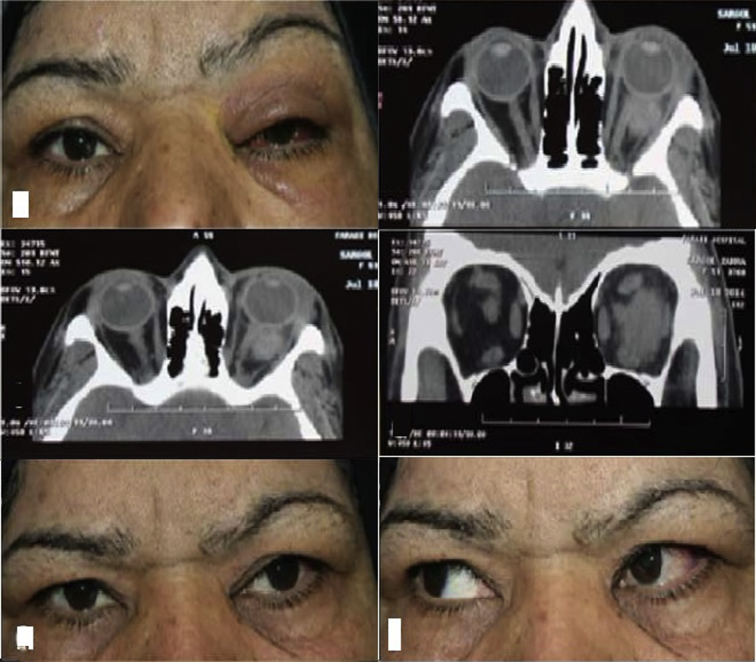
Case 2
The other focal mass case was a 64-year-old female with a history of unilateral painful red eye, decreased visual acuity, and diplopia since one week earlier. On ophthalmic examination, ptosis, mild chemosis, mild upper lid edema, and proptosis were noticed (Fig. 2, top left). Her visual acuity was 6/10 (Snellen chart), and the other eye was 9/10. Orbital CT showed an intraconal mass with relatively sharp margins, temporal to the optic nerve (its inferior margin was infiltrative) (Fig. 2 top right, middle left, and middle right). In this case, regarding its local nature and also because of the difficult, possibly damaging, procedure, we preferred to start medical treatment. Therefore, oral corticosteroid therapy was started at the dose of 1 mg/kg/day and continued for 2 months. The inflammatory lesion responded rapidly and improved (Fig. 2 bottom left and bottom right).
Fig. 2.
Case number 2. Top Left. Patient's appearance before treatment. Top Right, Middle Left, and Middle Right. Computed tomography (CT) (axial and coronal views) of the lesion. Bottom Left and Bottom Right. Patient's appearance after steroid therapy.
Case 3
The other interesting case was the presentation of the disease in a 52-year-old male with a history of periorbital edema since several months earlier, and according to the imaging findings, bilateral painless palpebral masses beneath the skin were diagnosed, which were resected (Fig. 3).
Fig. 3.
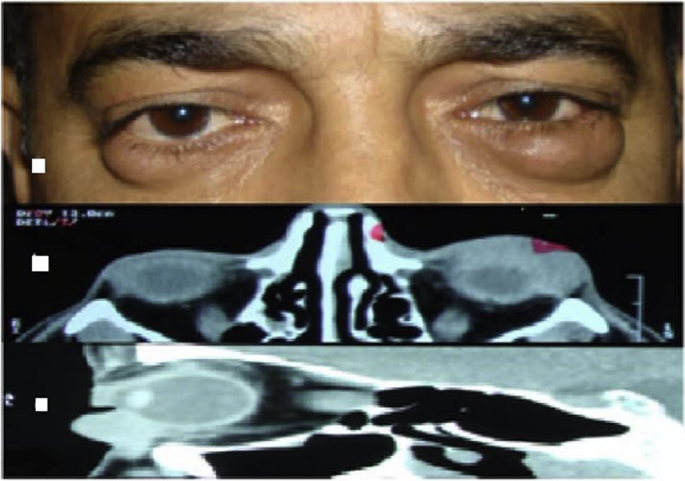
Interesting case. Top. Patient's appearance. Middle. Orbital computed tomography (CT), axial view. Bottom. Orbital CT, Sagittal view.
Discussion
NSOI is a diagnosis of exclusion.14 The diagnosis of NSOI is usually considered according to clinical improvements after systemic steroid therapy, but some other inflammatory and malignant disorders response well to steroid therapy, so histopathologic evaluation is still known as a mainstream of diagnosis.4 The exact etiology of this disorder has not been known, but autoimmune factors, especially with genetic, viral, and environmental triggers, have been suggested.9, 14, 15, 16, 17 Many different classification systems have been suggested, but because of inconstant clinical and pathological characteristics of NSOI, none of them are generally used.15, 18, 19
The study of Spindle et al. found that NSOI is usually unilateral (in 88.2% of patients).4 Bilaterality was reported in 13% of pediatric cases. Bilaterality was seen in 11.8% of this group in our study. However, in contrast with that study, recurrence was not associated with bilateral disease.20 Bilaterality was seen in 10.6% of the adult patients in our study in our cases which was in accordance with other studies. Bilateral involvement is much more common in other inflammatory diseases, like thyroid eye disease and sarcoidosis, and should be ruled out by a rheumatologic workup in any patient presenting bilateral NSOI.9, 14, 21, 22, 23
This NSOI disorder has been reported with no gender or age preference,4 but some recent studies have reported a middle-aged female preponderance.9 In our study, females and males were nearly equal, and the mean age of the patients was about 41 years.
Swamy et al. reported that orbital swelling/mass, proptosis, pain, and extraocular muscles movements restriction were the most common orbital signs and symptoms in adults,4 and in the pediatric group of patients, periorbital edema, ptosis, pain decreased extraocular muscles movements were more common.20 In our study, periorbital edema, periorbital pain, decreased ocular movements or diplopia, and conjunctival injection were the most prevalent findings. NSOI is known as the third most common origin of unilateral proptosis in adults after thyroid eye disease and lymphoproliferative disease.11, 24 In our study, the proptosis was found in 25% of patients.
Concurrent systemic diseases, including flu, asthma, colitis, and severe anemia were diagnosed in 6 adult patients. In the previous studies, several systemic inflammatory or infectious diseases were also reported to have an association with NSOI. Misselwitz et al. described dacryoadenitis with symptomatic retro-orbital and vestibulocochlear involvement as an extrahepatic manifestation of hepatitis C.25 A case of rheumatoid arthritis was reported who developed orbital signs and symptoms due to producing autoantibodies targeting orbital tissues.26 Another association which was found in our study was the diagnosis of palpebral xanthogranuloma in 2 cases. To our knowledge, no previous study has reported this association. In addition, 5 adults diagnosed as sinusitis on the basis of clinical and imaging findings. Some recent studies have proposed a possible relationship between NSOI and sinusitis.27, 28, 29, 30, 31 Yan reported that 17.2% of sinusitis cases were accompanied by orbital pseudotumor.32 Another study showed a close association between the dacryoadenitis subtype of NSOI (especially the IgG4-elevated dacryoadenitis subtype) and sinusitis. A probable description is the adjacent anatomic relationship between each group of paranasal sinuses and the orbital cavity.33
Several imaging modalities are accessible, of them CT and MRI are frequently used in supposed cases of NSOI, and their findings help to classify the NSOI subtypes more accurately.34, 35, 36, 37, 38 The imaging appearance of NSOI on CT and MRI is nonspecific. It is frequently seen as a focal or diffuse involvement that enhances with iodinated contrast or gadolinium.6 According to the previous studies, several orbital structures can be involved either focally or diffusely, and among them orbital fat, lacrimal gland, and the extraocular muscles are the most common sites of involvement.4, 20 In our study, lacrimal gland and extraocular muscles were also commonly involved. Two optic nerve involvement cases were diagnosed in this study, one of which was a fibrosing involvement and the other was an angiomatous pseudotumor and both of them were unresponsive to steroid therapy.
According to the imaging findings in some other studies, unilateral single muscle inflammation with tendon involvement was the most common form of myositic NSOI.17, 39 The most commonly involved muscle was the medial rectus followed by the superior, lateral, and inferior recti muscles.40, 41 But in most of these studies, myositic patients have a tendency to be atypical with involvement of more than one extraocular muscle. In our study, some differences with the previous studies were found. The most common involved extraocular muscles were lateral, superior, inferior, and medial recti muscles, respectively. Involvement of more than one muscle was found in 11.8% of cases.
In previous studies, five patterns of acute inflammatory pseudotumor were reported: anterior and diffuse acute pseudotumors, anterior or diffuse orbital infiltration, lacrimal involvement, posterior or apical involvement, and myositic lesions. Each of these categories was diagnosed according to their related specific signs and symptoms and also the imaging findings.18 In our study, we categorize the patients to nine groups, and we assume that this categorization could help in determination of prognosis, treatment method or follow-up time of the patients.
Biopsy is recommended to confirm the diagnosis of NSOI, except for patients with primary myositis or posteriorly and orbital apex located lesions where there is a noteworthy risk of damage to the optic nerve.15, 20 Some authors believe that biopsy is not considered in the cases that surgery is contraindicated.42 Some investigators recommended biopsy before empiric steroid therapy to avoid delayed or missed diagnosis.43 In another study, about 50% of biopsied inflammatory lacrimal gland specimens were associated with systemic diseases, so biopsy is recommended for isolated inflammation of the lacrimal gland.44 An orbital inflammatory mass biopsy should be considered for lesions which clinical or radiological findings are unspecified or atypical or when presentation or progression diverges from the typical course or when there is a history of neoplasia.45, 46, 47 In our study, biopsy was done in patients with atypical findings or those who were unresponsive to treatments (48.7% of patients). Also in diffuse or fibrosing involvement cases or recurrent cases, biopsy was done. Biopsy was not done in typical cases that were responsive to steroid therapy, unless when they had recurrences or when they did not respond to steroid treatment dramatically. Also, in at-risk cases in which biopsy was considered a damaging procedure, we preferred to begin the medical treatment and followed the patient.
Six diffuse sclerosing involvement cases were evaluated in our study. The ideal treatment method for this group is not obvious, but in this study, an aggressive initial therapy included of steroid pulse therapy, and immunomodulators were used in this regard. But the treatment was challenging, and the final results were not promising as the final visual acuity in the majority of cases (4 cases) was decreased to 2/10 after all the medical or even surgical treatments.
In our study, IMT was administrated for steroid resistant cases (including of one dacryoadenitis, one multiple tissue involvement, and one fibrosing case). All of them were chronic, adult (37.00 ± 1.57 years) and biopsy positive cases.
Duration of follow-up was 7.00 ± 6.45 months in our study, but it should be noted that this period could be limited in some cases, so the recurrence may happen after this time.
A limited number of studies reported the characteristics of pediatric NSOI cases. In one retrospective study of 30 patients which supported our findings, Spindle et al. followed up 9 males and 21 females with pediatric NSOI (age range, 2–18 years) for 19 months. Bilateral involvement was seen in 13%, and constitutional symptoms were reported in 40% of patients. Posttreatment recurrence was reported in 37% of cases. The most common treatment was oral steroids in 24 patients (80%).20 In our study, 10 patients (6 males and 4 females) were enrolled (age range, 6–18 years). Bilateral involvement was seen in 20%, and oral steroids were administered for 70% of patients. They were followed for 7.00 ± 7.52 months, and recurrence occurred in 20% of cases.
The main superiority of our study was a new categorization in which multiple tissue involvements were separated, and also the fat involvement was divided to two acute and fibrosing groups. In this study, we believe that each of these groups had a different prognosis and may need its special treatment and follow-up. Multiple tissue involvement cases usually have poorer prognosis and more difficult treatment course and may need a longer follow-up time. Also in this study, some new interesting cases were presented. But some limitations were also present. The patients were selected from a tertiary referral hospital which could result in selection bias. Some findings were more prevalent than what was expected due to this bias. Furthermore, in this study, we did not evaluate the IgG4 level.
Footnotes
Declaration of interest: The authors report no conflicts of interest.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Kitei D., DiMario F.J. Childhood orbital pseudotumor: case report and literature review. J Child Neurol. 2008;23(4):425–430. doi: 10.1177/0883073807309238. [DOI] [PubMed] [Google Scholar]
- 2.Zborowska B., Ghabrial R., Selva D., McCluskey P. Idiopathic orbital inflammation with extraorbital extension: case series and review. Eye (Lond) 2006;20(1):107–113. doi: 10.1038/sj.eye.6701780. [DOI] [PubMed] [Google Scholar]
- 3.Yan J., Qiu H., Wu Z., Li Y. Idiopathic orbital inflammatory pseudotumor in Chinese children. Orbit. 2006;25(1):1–4. doi: 10.1080/01676830500505608. [DOI] [PubMed] [Google Scholar]
- 4.Swamy B.N., McCluskey P., Nemet A. Idiopathic orbital inflammatory syndrome: clinical features and treatment outcomes. Br J Ophthalmol. 2007;91(12):1667–1670. doi: 10.1136/bjo.2007.124156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shields J.A., Shields C.L., Scartozzi R. Survey of 1264 patients with orbital tumors and simulating lesions: the 2002 Montgomery Lecture, part 1. Ophthalmology. 2004;111(5):997–1008. doi: 10.1016/j.ophtha.2003.01.002. [DOI] [PubMed] [Google Scholar]
- 6.Mendenhall W.M., Lessner A.M. Orbital pseudotumor. Am J Clin Oncol. 2010;33(3):304–306. doi: 10.1097/COC.0b013e3181a07567. [DOI] [PubMed] [Google Scholar]
- 7.Shikishima K., Kawai K., Kitahara K. Pathological evaluation of orbital tumours in Japan: analysis of a large case series and 1379 cases reported in the Japanese literature. Clin Exp Ophthalmol. 2006;34(3):239–244. doi: 10.1111/j.1442-9071.2006.01192.x. [DOI] [PubMed] [Google Scholar]
- 8.Rose G.E., Wright J.E. Exenteration for benign orbital disease. Br J Ophthalmol. 1994;78(1):14–18. doi: 10.1136/bjo.78.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yuen S.J., Rubin P.A. Idiopathic orbital inflammation: ocular mechanisms and clinicopathology. Ophthalmol Clin N Am. 2002;15(1):121–126. doi: 10.1016/s0896-1549(01)00003-7. [DOI] [PubMed] [Google Scholar]
- 10.Espinoza G.M. Orbital inflammatory pseudotumors: etiology, differential diagnosis, and management. Curr Rheumatol Rep. 2010;12(6):443–447. doi: 10.1007/s11926-010-0128-8. [DOI] [PubMed] [Google Scholar]
- 11.Belanger C., Zhang K.S., Reddy A.K., Yen M.T., Yen K.G. Inflammatory disorders of the orbit in childhood: a case series. Am J Ophthalmol. 2010;150(4):460–463. doi: 10.1016/j.ajo.2010.05.012. [DOI] [PubMed] [Google Scholar]
- 12.Berger J.W., Rubin P.A., Jakobiec F.A. Pediatric orbital pseudotumor: case report and review of the literature. Int Ophthalmol Clin. 1996;36(1):161–177. doi: 10.1097/00004397-199603610-00017. [DOI] [PubMed] [Google Scholar]
- 13.Eshraghi B., Keshtcar Jafari A., Akbari M.R., Masoomian B. A case report of orbital pseudotumor with presentation like orbital cellulitis. Iran J Ophthalmol. 2012;24(3):58–61. [Google Scholar]
- 14.Jacobs D., Galetta S. Diagnosis and management of orbital pseudotumor. Curr Opin Ophthalmol. 2002;13(6):347–351. doi: 10.1097/00055735-200212000-00001. [DOI] [PubMed] [Google Scholar]
- 15.Mombaerts I., Goldschmeding R., Schlingemann R.O., Koornneef L. What is orbital pseudotumor? Surv Ophthalmol. 1996;41(1):66–78. doi: 10.1016/s0039-6257(97)81996-0. [DOI] [PubMed] [Google Scholar]
- 16.Kennerdell J.S., Dresner S.C. The nonspecific orbital inflammatory syndromes. Surv Ophthalmol. 1984;29(2):93–103. doi: 10.1016/0039-6257(84)90166-8. [DOI] [PubMed] [Google Scholar]
- 17.Mombaerts I., Koornneef L. Current status in the treatment of orbital myositis. Ophthalmology. 1997;104(3):402–408. doi: 10.1016/s0161-6420(97)30301-7. [DOI] [PubMed] [Google Scholar]
- 18.Rootman J., Nugent R. The classification and management of acute orbital pseudotumors. Ophthalmology. 1982;89(9):1040–1048. doi: 10.1016/s0161-6420(82)34683-7. [DOI] [PubMed] [Google Scholar]
- 19.Fujii H., Fujisada H., Kondo T., Takahashi T., Okada S. Orbital pseudotumor: histopathological classification and treatment. Ophthalmologica. 1985;190(4):230–242. doi: 10.1159/000309523. [DOI] [PubMed] [Google Scholar]
- 20.Spindle J., Tang S.X., Davies B. Pediatric idiopathic orbital inflammation: clinical features of 30 cases. Ophthal Plast Reconstr Surg. 2016;32(4):270–274. doi: 10.1097/IOP.0000000000000494. [DOI] [PubMed] [Google Scholar]
- 21.Harris G.J. Idiopathic orbital inflammation: a pathogenetic construct and treatment strategy: the 2005 ASOPRS Foundation Lecture. Ophthal Plast Reconstr Surg. 2006;22(2):79–86. doi: 10.1097/01.iop.0000203734.52333.93. [DOI] [PubMed] [Google Scholar]
- 22.Ho V.H., Chevez-Barrios P., Jorgensen J.L., Silkiss R.Z., Silkis R.Z., Esmaeli B. Receptor expression in orbital inflammatory syndromes and implications for targeted therapy. Tissue Antigens. 2007;70(2):105–109. doi: 10.1111/j.1399-0039.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 23.Gordon L.K. Orbital inflammatory disease: a diagnostic and therapeutic challenge. Eye (Lond) 2006;20(10):1196–1206. doi: 10.1038/sj.eye.6702383. [DOI] [PubMed] [Google Scholar]
- 24.Weber A.L., Romo L.V., Sabates N.R. Pseudotumor of the orbit. Clinical, pathologic, and radiologic evaluation. Radiol Clin N Am. 1999;37(1):151–168. doi: 10.1016/s0033-8389(05)70084-1. (xi) [DOI] [PubMed] [Google Scholar]
- 25.Misselwitz B., Epprecht J., Mertens J. Orbital pseudotumor as a rare extrahepatic manifestation of hepatitis C infection. Case Rep Gastroenterol. 2016;10(1):108–114. doi: 10.1159/000444011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nakamura A., Miyamura T., Suematsu E. Orbital inflammatory pseudotumor associated with rheumatoid arthritis. Intern Med. 2016;55(11):1503–1504. doi: 10.2169/internalmedicine.55.6408. [DOI] [PubMed] [Google Scholar]
- 27.Fortson J.K., Shapshay S.M., Weiter J.J., Vaughan C.W., Strong M.S. Otolaryngologic manifestations of orbital pseudotumors. Otolaryngol Head Neck Surg. 1980;88(4):342–348. doi: 10.1177/019459988008800405. [DOI] [PubMed] [Google Scholar]
- 28.Eshaghian J., Anderson R.L. Sinus involvement in inflammatory orbital pseudotumor. Arch Ophthalmol. 1981;99(4):627–630. doi: 10.1001/archopht.1981.03930010627007. [DOI] [PubMed] [Google Scholar]
- 29.Heersink B., Rodrigues M.R., Flanagan J.C. Inflammatory pseudotumor of the orbit. Ann Ophthalmol. 1977;9(1):17–22. 25-19. [PubMed] [Google Scholar]
- 30.Leibovitch I., Goldberg R.A., Selva D. Paranasal sinus inflammation and non-specific orbital inflammatory syndrome: an uncommon association. Graefes Arch Clin Exp Ophthalmol. 2006;244(11):1391–1397. doi: 10.1007/s00417-006-0312-8. [DOI] [PubMed] [Google Scholar]
- 31.Mahr M.A., Salomao D.R., Garrity J.A. Inflammatory orbital pseudotumor with extension beyond the orbit. Am J Ophthalmol. 2004;138(3):396–400. doi: 10.1016/j.ajo.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Yan J., Wu Z., Li Y. 36 case idiopathic orbital inflammatory pseudotumor with sinus involvement. Lin Chuang Er Bi Yan Hou Ke Za Zhi. 2002;16(8):410–411. [Article in Chinese] [PubMed] [Google Scholar]
- 33.Li J., Ge X., Ma J.M. Relationship between dacryoadenitis subtype of idiopathic orbital inflammatory pseudotumor and paranasal sinusitis. Int J Ophthalmol. 2016;9(3):444–447. doi: 10.18240/ijo.2016.03.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rootman J., Robertson W., Lapointe J.S. Inflammatory disease. In: Rootman J., editor. Diseases of the Orbit: A Multidisciplinary Approach. JB Lippincott; Philadelphia, PA: 1988. pp. 143–204. [Google Scholar]
- 35.McNab A.A., McKelvie P. IgG4-Related ophthalmic disease. Part II: clinical aspects. Ophthal Plast Reconstr Surg. 2015;31(3):167–178. doi: 10.1097/IOP.0000000000000364. [DOI] [PubMed] [Google Scholar]
- 36.Blodi F.C., Gas J.D. Inflammatory pseudotumour of the orbit. Br J Ophthalmol. 1968;52(2):79–93. doi: 10.1136/bjo.52.2.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blodi F.C., Gas J.D. Inflammatory pseudotumour of the orbit. Trans Am Acad Ophthalmol Otolaryngol. 1967;71(2):303–323. [PubMed] [Google Scholar]
- 38.Yuen S.J., Rubin P.A. Idiopathic orbital inflammation: distribution, clinical features, and treatment outcome. Arch Ophthalmol. 2003;121(4):491–499. doi: 10.1001/archopht.121.4.491. [DOI] [PubMed] [Google Scholar]
- 39.Serop S., Vianna R.N., Claeys M., De Laey J.J. Orbital myositis secondary to systemic lupus erythematosus. Acta Ophthalmol (Copenh) 1994;72(4):520–523. doi: 10.1111/j.1755-3768.1994.tb02807.x. [DOI] [PubMed] [Google Scholar]
- 40.Kau H.C., Kao S.C., Peng C.H., Hsu W.M., Tsai C.C. Methylprednisolone pulse therapy in patient with isolated superior oblique myositis. Eye (Lond) 2006;20(9):1106–1109. doi: 10.1038/sj.eye.6702145. [DOI] [PubMed] [Google Scholar]
- 41.Stidham D.B., Sondhi N., Plager D., Helveston E. Presumed isolated inflammation of the superior oblique muscle in idiopathic orbital myositis. Ophthalmology. 1998;105(12):2216–2219. doi: 10.1016/S0161-6420(98)91218-0. [DOI] [PubMed] [Google Scholar]
- 42.Young S.M., Chan A.S., Jajeh I.A. Clinical features and treatment outcomes of orbital inflammatory disease in Singapore: a 10-year clinicopathologic review. Ophthal Plast Reconstr Surg. 2017;33(3):182–188. doi: 10.1097/IOP.0000000000000690. [DOI] [PubMed] [Google Scholar]
- 43.Mombaerts I., Rose G.E., Garrity J.A. Orbital inflammation: biopsy first. Surv Ophthalmol. 2016;61(5):664–669. doi: 10.1016/j.survophthal.2016.03.002. [DOI] [PubMed] [Google Scholar]
- 44.Mottow L.S., Jakobiec F.A. Idiopathic inflammatory orbital pseudotumor in childhood. I. Clinical characteristics. Arch Ophthalmol. 1978;96(8):1410–1417. doi: 10.1001/archopht.1978.03910060164013. [DOI] [PubMed] [Google Scholar]
- 45.Mombaerts I., Tousseyn T., Van Limbergen E., Demaerel P. Clinically recognizing enlarged extraocular muscles from lymphoid origin. Ophthalmology. 2015;122(1):217–218. doi: 10.1016/j.ophtha.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 46.Rose G.E. A personal view: probability in medicine, levels of (Un)certainty, and the diagnosis of orbital disease (with particular reference to orbital "pseudotumor") Arch Ophthalmol. 2007;125(12):1711–1712. doi: 10.1001/archopht.125.12.1711. [DOI] [PubMed] [Google Scholar]
- 47.Watkins L.M., Carter K.D., Nerad J.A. Ocular adnexal lymphoma of the extraocular muscles: case series from the University of Iowa and review of the literature. Ophthal Plast Reconstr Surg. 2011;27(6):471–476. doi: 10.1097/IOP.0b013e31822e5c1b. [DOI] [PubMed] [Google Scholar]