Abstract
Purpose
To evaluate the optical coherence tomography angiography (OCTA) parameters in patients with diabetic retinopathy following panretinal photocoagulation (PRP).
Methods
Eleven eyes of 6 patients with very severe nonproliferative diabetic retinopathy (NPDR) or early proliferative diabetic retinopathy (PDR) were recruited in this prospective interventional pilot study. All patients underwent OCTA imaging at baseline, and choroidal flow, foveal avascular zone (FAZ), retinal thickness, and vascular density were measured at baseline. Three months after treatment, OCTA was repeated, and the alteration in variables was analyzed.
Results
The FAZ area remained unchanged following treatment, (P = 0.75). Retinal thickness increased along (P = 0.02) with an increase in vascular density in the superficial and deep foveal area and the macular temporal sector (P = 0.007, 0.03 and 0.049, respectively). Choroidal flow surface area was unchanged (P = 0.10).
Conclusion
In this study, foveal vascular density increased and FAZ remained unchanged after PRP for diabetic retinopathy.
Keywords: Diabetic retinopathy, Foveal avascular zone, FAZ, Optical coherence tomography angiography, OCTA, Panretinal photocoagulation
Introduction
Diabetes mellitus (DM) is a chronic disease with an overwhelming impact on public health and a leading cause of blindness worldwide.1 Currently, panretinal photocoagulation (PRP) is considered the standard of care for proliferative diabetic retinopathy. Improved oxygenation to the remaining ischemic retina via destruction of the highly active photoreceptor cells is among the proposed mechanisms for PRP.2 Subsequently, production of vascular endothelial growth factor (VEGF), the key player in neovascularization process, is reduced. The reduction in the level of VEGFs might lead to regression of harmful new vessels.
Alteration of retinal microenvironment following PRP has an impact on retinal vasculature and hemodynamics. However, the reduction in VEGF following PRP may result in vascular occlusion or impairment of retinal blood circulation.3, 4 Alteration of blood-retinal barrier and ocular blood flow after PRP have been documented.5 Additionally, the effects of PRP on choroidal vasculature have been investigated6, 7; however, the results were contradictory, probably due to various imaging and measuring modalities.
With the advent of optical coherence tomography angiography (OCTA), a non-invasive dye-less modality, vascular mapping with high speed and quality is feasible for visualization of vascular system in different retinal and choroidal levels in addition to optic nerve head.8, 9, 10 Several investigations have demonstrated the competence of OCTA in the quantification of microvascular density, capillary dropout, and foveal avascular zone (FAZ) area in diabetic patients.11, 12, 13, 14
The aim of this pilot study is to evaluate the short-term changes in macular vascular density, flow surface area, and FAZ after PRP in patients with very severe nonproliferative diabetic retinopathy (NPDR) and early proliferative diabetic retinopathy (PDR).
Methods
This prospective interventional case series was conducted in Farabi Eye Hospital, a tertiary center in Tehran, Iran. Local Ethics Committee of Tehran University of Medical Sciences approved the protocol, and a written informed consent was obtained from the participants. The study adhered to the tenets of the Declaration of Helsinki.
Consecutive treatment-naive diabetic patients referred to our clinic between July 2017 and December 2017 with very severe NPDR or early PDR who were candidates for PRP treatment were enrolled in this experiment. All patients were recruited by a single physician (F.B.). Patients underwent thorough ophthalmic examination including biomicroscopy, intraocular pressure measurement, dilated fundus exam, and fundus photo. Patients with a previous history of uveitis, uncontrolled glaucoma, fibrovascular traction, extensive neovascularization meeting the definition of high-risk PDR, evidence of vitreomacular traction, clinically significant macular edema, history of previous treatment for diabetic retinopathy including anti-VEGF and laser, and significant media opacity impeding acceptable image quality were excluded. Additionally, the central macular thickness of more than 300 μm in OCT was also a criterion for exclusion in order to omit confounding errors in segmentation and measurements accompanied by macular edema.15
OCTA was performed by an RTVue XR Avanti instrument (version 2015.1.1.98; Optovue, Fremont, CA, USA) using the split-spectrum amplitude-decorrelation angiography (SSADA) algorithm.16 Images were acquired at baseline and 3 months following PRP treatment. A 3 × 3 mm image centered at fovea was obtained consecutively for each eye. To optimize image quality ‘Auto All’ function was utilized and low-quality scans (due to blinking or significant motion artifact) were excluded and repeated until an acceptable quality was achieved.
Automated segmentation with Angio-Vue preset module was utilized for each measurement. In each eye, the measurements were performed in the superficial capillary network [ranging 3 μm from the internal limiting membrane (ILM) with an offset of 15 μm], and deep capillary network (covering from 15 μm of ILM to 71 μm) layer of the retina. The foveal region was outlined as a central circle with a 1 mm diameter and the parafoveal region was delineated as a ring, by 3 mm wide, surrounding the foveal region according to Early Treatment Diabetic retinopathy Study (ETDRS) cirlces.17
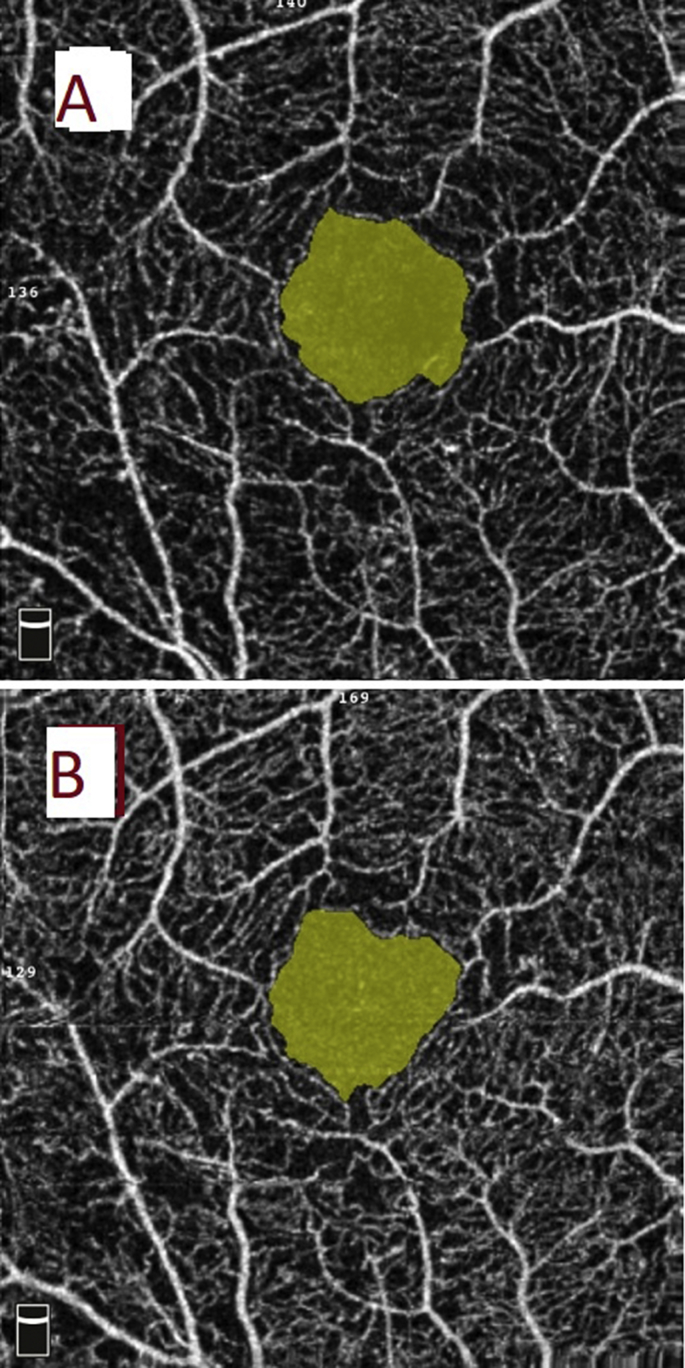
The OCTA commercial software (AngioAnalytics version 2015.1.1.98) calculated vascular density as the percent of the area of interest occupied by retinal vasculature in selected depths. Vascular density along with macular thickness was recorded in 9 macular sectors defined by ETDRS. Flow area was measured at the level choriocapillaris in 3 mm circles. FAZ area was measured in mm2 at the superficial capillary plexus (SCP) automatically by the built-in software.18 A single (K.F.) user manually fine-tuned the plane to maximize the visualization of the retinal capillary bed (Fig. 1).
Fig. 1.
Foveal avascular zone (FAZ) area on optical coherence tomography angiography (OCTA) in a patient with early proliferative diabetic retinopathy (PDR). (A) The encircled yellow area represents FAZ area obtained by OCTA AngioVue with the scan size of 3 × 3 mm. (B) The retinal superficial capillary plexus at baseline. FAZ area was 0.50 mm2 that decreased to 0.45 mm2 3 months after treatment with panretinal photocoagulation (PRP).
PRP was performed at two to three consecutive sessions with adherence to the guidelines of ETDRS Research Group. Topical anesthesia was applied and 1000 to 1200 spots with an argon laser (532 nm) with the spot size of 500 μm were made at each time point. PRP was applied in all 4 peripheral quadrants in 3 consecutive weekly sessions by an independent ophthalmologist unaware of the study design. OCTA imaging was repeated 3 months following the completion of full PRP.
The patients did not receive any additional therapy including macular photocoagulation or anti-VEGF during the course of the study.
Statistical analysis
All statistical analyses were conducted with SPSS 21 (SPSS, Inc., Chicago, IL, USA). Quantitative variables were tested with Kolmogorov-Smirnov to determine normality and presented as mean with standard deviation. In order to compensate the effect of the neighbor eye, generalized estimating equation (GEE) model was utilized to evaluate the effect of the intervention. A P-value below 0.05 was considered significant level.
Results
Eleven eyes of 6 patients with DM type 2 including 3 males and 3 females were recruited in this pilot study. Mean age was 63.8 ± 6.5 years old. Among them, 4 patients had very severe NPDR, and the remaining 2 had early PDR.
None of the patients with very severe NPDR progressed during the course of study, and the stage of retinopathy regressed in all eyes with proliferative retinopathy.
The choroidal flow area following PRP remained unchanged (15.93 ± 1.39 to 16.35 ± 1.48, P = 0.10).
Retinal thickness consistently increased in all ETDRS 3 mm grids following treatment. Foveal thickness increased significantly from 252 ± 16 μm to 272 ± 13 μm (P = 0.02); however, the rise in other sectors was not statistically significant (Table 1).
Table 1.
Effects of panretinal photocoagulation (PRP) on retinal thickness in Early Treatment Diabetic Retinopathy Study (ETDRS) circles.
| Baseline | After PRP | P valuea | |
|---|---|---|---|
| Fovea (μm) | 252 ± 16 | 272 ± 13 | 0.02 |
| Parafovea (μm) | 333 ± 16 | 350 ± 27 | 0.09 |
| Temporal (μm) | 327 ± 11 | 347 ± 32 | 0.13 |
| Nasal (μm) | 333 ± 24 | 350 ± 32 | 0.07 |
| Superior (μm) | 336 ± 20 | 353 ± 28 | 0.12 |
| Inferior (μm) | 334 ± 18 | 350 ± 31 | 0.10 |
PRP: Panretinal photocoagulation.
By generalized estimating equation (GEE) model.
Vascular density in the foveal area at both superficial and deep levels increased following treatment. The enhancement was more notable in deep layers. Regarding other sectors, vascular density augmented in the temporal area at the deep capillary level (50.8 ± 5.6 to 54.3 ± 2.8; P = 0.05). The FAZ area remained unchanged (Table 2).
Table 2.
Superficial and deep capillary plexus (DCP) vascular density at baseline and following panretinal photocoagulation (PRP) at various Early Treatment Diabetic Retinopathy Study (ETDRS) circles.
| Baseline | After PRP | P valuea | ||
|---|---|---|---|---|
| FAZ | 0.40 ± 0.13 | 0.39 ± 0.13 | 0.75 | |
| SCP vascular density (%) | Fovea | 24.5 ± 4.7 | 26.0 ± 3.3 | 0.007 |
| Para-Fovea | 47.2 ± 3.8 | 47.6 ± 4.6 | 0.80 | |
| Temporal | 47.4 ± 3.5 | 46.1 ± 6.1 | 0.55 | |
| Nasal | 45.0 ± 6.8 | 47.5 ± 4.7 | 0.31 | |
| Superior | 48.4 ± 5.1 | 48.5 ± 4.5 | 0.95 | |
| Inferior | 46.9 ± 4.7 | 48.4 ± 4.8 | 0.35 | |
| DCP vascular density (%) | Fovea | 22.8 ± 7.2 | 26.1 ± 4.9 | 0.03 |
| Para-Fovea | 53.8 ± 2.1 | 53.5 ± 3.2 | 0.79 | |
| Temporal | 50.8 ± 5.6 | 54.3 ± 2.8 | 0.049 | |
| Nasal | 51.6 ± 5.4 | 53.5 ± 2.8 | 0.27 | |
| Superior | 56.0 ± 2.3 | 55.3 ± 3.7 | 0.62 | |
| Inferior | 54.5 ± 3.8 | 54.4 ± 4.1 | 0.85 | |
PRP: Panretinal photocoagulation; FAZ: Foveal avascular zone measure at superficial capillary plexus; SCP: Superficial capillary plexus; DCP: Deep capillary plexus.
By generalized estimating equation (GEE) model.
Discussion
In the present study, an increase in foveal vascular density was observed using OCTA following treatment with PRP in patients with diabetic retinopathy, and other parameters including FAZ area and choroidal flow remained unchanged.
In our study, choroidal flow area in the macular area did not show any significant changes following PRP. This finding is in contrast to previously published investigations. Takahashi et al.19 reported a significant increase in subfoveal choroidal flow one month after PRP in the patients without macular edema, using a laser doppler flowmetry. It was extrapolated that redistribution of choroidal blood flow from obliterated peripheral capillaries to the macula and the inflammatory response triggered by PRP were the main underlying mechanisms for an increment of macular choroidal flow.19 The discrepancy observed between our results and theirs can be due to 3 months interval in post-PRP measurement choroidal flow in our study, during which, the inflammation probably subsides and the autoregulatory functions of retinal and choroidal vasculature come into action. Another possible explanation is different measurement techniques; OCTA vs. doppler flowmetry, between two studies. Flower et al. reported that the effect of coagulating the peripheral retinal area markedly increased the choriocapillaris blood flow in the central area of the fundus relative to that in the periphery in monkey eyes by indocyanine green angiography.20 Augsten et al., using a reflection spectra method, have also reported that peripheral retinal photocoagulation improved the choroidal circulation in the macular area in patients with PDR.21
Numerous preceding studies that focused on the effects of PRP on the ocular circulation reported that PRP reduces retinal blood flow in patients with diabetic retinopathy.22, 23, 24 As expected, we observed a mild increase in macular thickness following PRP. There is substantial evidence in the literature that following PRP, clinical or subclinical macular edema may ensue.25, 26, 27, 28 Interruption of the blood-retinal barrier has been postulated as the main factor contributing to increase in macular thickness after PRP. However, glycemic control and underlying diseases other than DM, also play an important role.28
In our study, vascular density in the foveal area at both superficial and especially deep levels increased following treatment. In addition to redistribution of the circulation, PRP-induced inflammation, causing inducible nitric oxide synthase expression and production, could be a trigger. It is shown that reflow of occluded capillary plexus is possible in the context of diabetic retinopathy.29 We speculate that better oxygenation of retina following PRP and consequent decrease in local VEGF levels,2 and hence reflow of macular microvasculature is the plausible mechanism underlying increase in vascular density after PRP. The intrinsic ability of OCTA in detecting patent vessel with adequate blood flow led to such observation.13
Alterations of FAZ area have been suggested as a marker for screening and monitoring of treatment in diabetic patients.30, 31, 32 OCTA is superior to conventional angiography for delineation of FAZ area.33 In 2015, Takase et al. investigation using OCTA, demonstrated that FAZ enlargement in comparison to healthy subjects may be a universal finding in diabetic patients regardless of the presence or lack of concomitant diabetic retinopathy. Enlargement of FAZ in diabetic patients in comparison with normal healthy patients has been demonstrated in other studies.34, 35 The microvascular abnormalities and subsequent capillary occlusion have been introduced as main factors responsible for FAZ enlargement in diabetic patients.14 Our analyses did not reveal any significant alteration in the FAZ area following PRP. However, the mere non-enlargement of FAZ area can be a sign of controlled ischemia achieve by PRP.
Recently, Lorusso et al. in a similar study investigated the alteration of OCTA parameters following PRP. Despite our results, they did not observe any changes in the vascular density and FAZ area. There are two possible explanations. First, their study period was extended up to six months after PRP comparing to our midterm 3-month finding. Another basis of discrepancy can be the inclusion of patients with very severe NPDR in our study. It can be extrapolated that these patients with a lower stage of diabetic retinopathy as opposed to PDR stage may benefit from earlier PRP treatment. However, the limited number of subjects in each arm precluded us from performing subgroup analysis to further evaluate this theory.
The limited number of patients is a major drawback to this study. A larger number of patients and a longer follow-up period are needed to elucidate the relation between the various studied variables. Lack of control for the metabolic condition of the patients is another concern. However, the relative short follow-up render this less problematic. In addition, failure to perform manual correction of retinal slabs which may affect the vascular density measurement36 is another limitation.
This finding underscores the proficiency of OCTA as a non-invasive method for monitoring macular ischemia and blood flow indices. Further investigations are mandatory to determine the changes in macular vasculature in patients undergoing PRP or determining the patients who might require additional treatments such as anti-VEGF injection.
Footnotes
Conflicts of interest: The authors declare that they have no conflict of interest.
Funding: No funding to declare.
Peer review under responsibility of the Iranian Society of Ophthalmology.
References
- 1.Ruta L.M., Magliano D.J., Lemesurier R., Taylor H.R., Zimmet P.Z., Shaw J.E. Prevalence of diabetic retinopathy in Type 2 diabetes in developing and developed countries. Diabet Med: J Br Diabet Assoc. 2013;30(4):387–398. doi: 10.1111/dme.12119. [DOI] [PubMed] [Google Scholar]
- 2.Stefansson E. Ocular oxygenation and the treatment of diabetic retinopathy. Surv Ophthalmol. 2006;51(4):364–380. doi: 10.1016/j.survophthal.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Mansour A.M., Bynoe L.A., Welch J.C. Retinal vascular events after intravitreal bevacizumab. Acta Ophthalmol. 2010;88(7):730–735. doi: 10.1111/j.1755-3768.2009.01535.x. [DOI] [PubMed] [Google Scholar]
- 4.Shimura M., Yasuda K. Macular ischaemia after intravitreal bevacizumab injection in patients with central retinal vein occlusion and a history of diabetes and vascular disease. Br J Ophthalmol. 2010;94(3):381–383. doi: 10.1136/bjo.2009.160986. [DOI] [PubMed] [Google Scholar]
- 5.Moriarty A.P., Spalton D.J., Shilling J.S., Ffytche T.J., Bulsara M. Breakdown of the blood-aqueous barrier after argon laser panretinal photocoagulation for proliferative diabetic retinopathy. Ophthalmology. 1996;103(5):833–838. doi: 10.1016/s0161-6420(96)30607-6. [DOI] [PubMed] [Google Scholar]
- 6.Mendivil A. Ocular blood flow velocities in patients with proliferative diabetic retinopathy after panretinal photocoagulation. Surv Ophthalmol. 1997;42(Suppl 1):S89–S95. doi: 10.1016/s0039-6257(97)80031-8. [DOI] [PubMed] [Google Scholar]
- 7.Savage H.I., Hendrix J.W., Peterson D.C., Young H., Wilkinson C.P. Differences in pulsatile ocular blood flow among three classifications of diabetic retinopathy. Investig Ophthalmol Vis Sci. 2004;45(12):4504–4509. doi: 10.1167/iovs.04-0077. [DOI] [PubMed] [Google Scholar]
- 8.Bazvand F., Mirshahi R., Fadakar K., Faghihi H., Sabour S., Ghassemi F. The quantitative measurements of vascular density and flow area of optic nerve head using optical coherence tomography angiography. J Glaucoma. 2017;26(8):735–741. doi: 10.1097/IJG.0000000000000722. [DOI] [PubMed] [Google Scholar]
- 9.Ghassemi F., Fadakar K., Bazvand F., Mirshahi R., Mohebbi M., Sabour S. The quantitative measurements of vascular density and flow areas of macula using optical coherence tomography angiography in normal volunteers. Ophthalmic Surg Lasers Imag retin. 2017;48(6):478–486. doi: 10.3928/23258160-20170601-06. [DOI] [PubMed] [Google Scholar]
- 10.Ghassemi F., Mirshahi R., Bazvand F., Fadakar K., Faghihi H., Sabour S. The quantitative measurements of foveal avascular zone using optical coherence tomography angiography in normal volunteers. J Curr Ophthalmol. 2017;29(4):293–299. doi: 10.1016/j.joco.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durbin M.K., An L., Shemonski N.D. Quantification of retinal microvascular density in optical coherence tomographic angiography images in diabetic retinopathy. JAMA Ophthalmol. 2017;135(4):370–376. doi: 10.1001/jamaophthalmol.2017.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schottenhamml J., Moult E.M., Ploner S. An automatic, intercapillary area-based algorithm for quantifying diabetes-related capillary dropout using optical coherence tomography angiography. Retina. 2016;36(suppl 1):S93–S101. doi: 10.1097/IAE.0000000000001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Samara W.A., Shahlaee A., Adam M.K. Quantification of diabetic macular ischemia using optical coherence tomography angiography and its relationship with visual acuity. Ophthalmology. 2017;124(2):235–244. doi: 10.1016/j.ophtha.2016.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Takase N., Nozaki M., Kato A., Ozeki H., Yoshida M., Ogura Y. Enlargement of foveal avascular zone in diabetic eyes evaluated by en face optical coherence tomography angiography. Retina. 2015;35(11):2377–2383. doi: 10.1097/IAE.0000000000000849. [DOI] [PubMed] [Google Scholar]
- 15.Dimitrova G., Chihara E., Takahashi H., Amano H., Okazaki K. Quantitative retinal optical coherence tomography angiography in patients with diabetes without diabetic retinopathy. Investig Ophthalmol Vis Sci. 2017;58(1):190–196. doi: 10.1167/iovs.16-20531. [DOI] [PubMed] [Google Scholar]
- 16.Fingler J., Zawadzki R.J., Werner J.S., Schwartz D., Fraser S.E. Volumetric microvascular imaging of human retina using optical coherence tomography with a novel motion contrast technique. Optic Express. 2009;17(24):22190–22200. doi: 10.1364/OE.17.022190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shahlaee A., Samara W.A., Hsu J. In vivo assessment of macular vascular density in healthy human eyes using optical coherence tomography angiography. Am J Ophthalmol. 2016;165:39–46. doi: 10.1016/j.ajo.2016.02.018. [DOI] [PubMed] [Google Scholar]
- 18.Savastano M.C., Lumbroso B., Rispoli M. In vivo characterization of retinal vascularization morphology using optical coherence tomography angiography. Retina. 2015;35(11):2196–2203. doi: 10.1097/IAE.0000000000000635. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi A., Nagaoka T., Sato E., Yoshida A. Effect of panretinal photocoagulation on choroidal circulation in the foveal region in patients with severe diabetic retinopathy. Br J Ophthalmol. 2008;92(10):1369–1373. doi: 10.1136/bjo.2007.136028. [DOI] [PubMed] [Google Scholar]
- 20.Flower R.W., Fryczkowski A.W., McLeod D.S. Variability in choriocapillaris blood flow distribution. Investig Ophthalmol Vis Sci. 1995;36(7):1247–1258. [PubMed] [Google Scholar]
- 21.Augsten R., Konigsdorffer E., Schweitzer D., Strobel J. Nonproliferative diabetic retinopathy-reflection spectra of the macula before and after laser photocoagulation. Ophthalmologica: J Int d'ophtalmologie – Int J Ophthalmol Zeitschrift fur Augenheilkunde. 1998;212(2):105–111. doi: 10.1159/000027288. [DOI] [PubMed] [Google Scholar]
- 22.Feke G.T., Green G.J., Goger D.G., McMeel J.W. Laser Doppler measurements of the effect of panretinal photocoagulation on retinal blood flow. Ophthalmology. 1982;89(7):757–762. doi: 10.1016/s0161-6420(82)34726-0. [DOI] [PubMed] [Google Scholar]
- 23.Grunwald J.E., Riva C.E., Brucker A.J., Sinclair S.H., Petrig B.L. Effect of panretinal photocoagulation on retinal blood flow in proliferative diabetic retinopathy. Ophthalmology. 1986;93(5):590–595. doi: 10.1016/s0161-6420(86)33691-1. [DOI] [PubMed] [Google Scholar]
- 24.Grunwald J.E., Brucker A.J., Petrig B.L., Riva C.E. Retinal blood flow regulation and the clinical response to panretinal photocoagulation in proliferative diabetic retinopathy. Ophthalmology. 1989;96(10):1518–1522. doi: 10.1016/s0161-6420(89)32697-2. [DOI] [PubMed] [Google Scholar]
- 25.Lee S.B., Yun Y.J., Kim S.H., Kim J.Y. Changes in macular thickness after panretinal photocoagulation in patients with severe diabetic retinopathy and no macular edema. Retina. 2010;30(5):756–760. doi: 10.1097/IAE.0b013e3181c701e0. [DOI] [PubMed] [Google Scholar]
- 26.McDonald H.R., Schatz H. Macular edema following panretinal photocoagulation. Retina. 1985;5(1):5–10. doi: 10.1097/00006982-198500510-00002. [DOI] [PubMed] [Google Scholar]
- 27.Shimura M., Yasuda K., Nakazawa T., Kano T., Ohta S., Tamai M. Quantifying alterations of macular thickness before and after panretinal photocoagulation in patients with severe diabetic retinopathy and good vision. Ophthalmology. 2003;110(12):2386–2394. doi: 10.1016/j.ophtha.2003.05.008. [DOI] [PubMed] [Google Scholar]
- 28.Soman M., Ganekal S., Nair U., Nair K. Effect of panretinal photocoagulation on macular morphology and thickness in eyes with proliferative diabetic retinopathy without clinically significant macular edema. Clin Ophthalmol. 2012;6(1):2013–2017. doi: 10.2147/OPTH.S37340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yamana Y., Oka Y., Ohnishi Y., Ishibashi T., Inoguchi T. Reflow of obstructed capillaries in the maculae of humans with diabetic retinopathy, observed by fluorescein angiography. Br J Ophthalmol. 1988;72(9):660–665. doi: 10.1136/bjo.72.9.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bresnick G.H., Condit R., Syrjala S., Palta M., Groo A., Korth K. Abnormalities of the foveal avascular zone in diabetic retinopathy. Arch Ophthalmol. 1984;102(9):1286–1293. doi: 10.1001/archopht.1984.01040031036019. [DOI] [PubMed] [Google Scholar]
- 31.Conrath J., Giorgi R., Raccah D., Ridings B. Foveal avascular zone in diabetic retinopathy: quantitative vs qualitative assessment. Eye. 2005;19(3):322–326. doi: 10.1038/sj.eye.6701456. [DOI] [PubMed] [Google Scholar]
- 32.Mansour A., Schachat A., Bodiford G., Haymond R. Foveal avascular zone in diabetes mellitus. Retina. 1993;13(2):125–128. doi: 10.1097/00006982-199313020-00006. [DOI] [PubMed] [Google Scholar]
- 33.Khadamy J., Aghdam K.A., Falavarjani K.G. An update on optical coherence tomography angiography in diabetic retinopathy. J Ophthalmic Vis Res. 2018;13(4):487. doi: 10.4103/jovr.jovr_57_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di G., Weihong Y., Xiao Z. A morphological study of the foveal avascular zone in patients with diabetes mellitus using optical coherence tomography angiography. Graefe's Arch Clinical Exp Ophthalmol – Albrecht von Graefes Archiv fur klinische und experimentelle Ophthalmologie. 2016;254(5):873–879. doi: 10.1007/s00417-015-3143-7. [DOI] [PubMed] [Google Scholar]
- 35.Freiberg F.J., Pfau M., Wons J., Wirth M.A., Becker M.D., Michels S. Optical coherence tomography angiography of the foveal avascular zone in diabetic retinopathy. Graefe's Arch Clin Exp Ophthalmol. 2016;254(6):1051–1058. doi: 10.1007/s00417-015-3148-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Falavarjani K.G., Habibi A., Anvari P. Effect of segmentation error correction on optical coherence tomography angiography measurements in healthy subjects and diabetic macular oedema. Br J Ophthalmol. 2019 Apr 29 doi: 10.1136/bjophthalmol-2019-314018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]